Abstract
Decompensated cirrhosis is associated with high morbidity and mortality. However, no standardized quality measures (QMs) have yet been adopted widely. The Veterans Affairs (VA) Advanced Liver Disease Technical Advisory Group recently developed a set of six internal QMs to guide quality improvement efforts in cirrhosis in the domains of access to care, hepatocellular carcinoma surveillance, variceal surveillance, quality of inpatient care for upper gastrointestinal (GI) bleeding, and cirrhosis-related rehospitalizations. We aimed to: 1) quantify adherence to cirrhosis QMs and 2) determine whether adherence was associated with all-cause mortality and health-care utilization within a large national cohort of Veterans with cirrhosis. We performed a retrospective study using data from the Veterans Outcomes and Costs Asociated with Liver Disease cohort of 121,129 patients newly diagnosed with cirrhosis from 1/1/2008 to 12/31/16 at 128 VA facilities. The mean follow-up time was 2.7 years (interquartile range 1.1–5.1 years). Adherence to outpatient access to specialty care was 71%, variceal surveillance was 32%, and early post-discharge care was 54%. In adjusted analyses, outpatient access to specialty care (hazard ratio [HR] 0.80; 95% confidence interval [CI]: 0.78–0.82), hepatocellular carcinoma surveillance (HR 0.92, 95% CI: 0.90–0.95), variceal surveillance (HR 0.93; 95% CI: 0.89–0.99), and early post-discharge care (HR 0.57; 95% CI: 0.54–0.60) were associated with lower all-cause mortality. Readmissions after 30 days (HR 1.53; 1.46–1.60) and 90-days (HR 1.88; 95% CI: 1.54–1.70) were associated higher all-cause mortality. Higher adherence to QMs was also associated with lower inpatient healthcare utilization.
Conclusions:
Five of the six proposed VA cirrhosis QMs were measurable using existing data sources, associated with mortality and healthcare utilization, and may be used to guide future quality improvement efforts in cirrhosis.
INTRODUCTION
Cirrhosis is the 12th leading cause of death in the United States resulting in an estimated 36,000–66,000 deaths annually(1) and is the 4th leading cause of mortality among persons ages 45–54 years.(2) Nearly 10% of patients admitted to the hospital with cirrhosis die during their hospitalization and 67% experience a non-elective readmission within 1 year.(3, 4) According to one analysis, the national annual direct costs of cirrhosis were $4 billion in 2002, largely driven by inpatient hospitalizations,(4, 5) whereas the indirect costs from loss of productivity were $10.6 billion in 2004.(6) Despite the recent advent of effective treatment for Hepatitis C virus (HCV), the overall burden of cirrhosis is expected to increase due to the irreversible effects of longstanding HCV infection (including decompensated liver disease and hepatocellular carcinoma (HCC)), the persistent burden of alcoholic liver disease, and the increased prevalence of non-alcoholic fatty liver disease and non-alcoholic steatohepatitis (NAFLD/NASH) related to the obesity epidemic.(1, 7)
Studies suggest there is variability in the quality of cirrhosis care and the adherence guideline-recommended care is often inadequate for ascites, variceal bleeding, hepatocellular carcinoma, and preventive vaccinations. (8–12) Due to the variation in the quality of care for cirrhosis, multiple groups have proposed identifying evidence-based cirrhosis quality measures (QMs) to guide care for all patients, regardless of whether their access to a liver disease specialist, and to evaluate and provide feedback on the quality of care across providers.(13, 14)
In 2017, the Veterans Health Administration, the largest integrated health system providing cirrhosis care in the United States, convened clinical and operations experts in cirrhosis to develop a set of guideline and consensus-based QMs to reduce unwarranted health care variation and improve quality of care for cirrhosis.(15) The QMs address both outpatient and inpatient care of cirrhosis and include the domains of access to care, hepatocellular carcinoma (HCC) surveillance, variceal surveillance, upper gastrointestinal (UGI) bleeding and inpatient healthcare utilization. However, no prior work has evaluated the relationship between adherence to these QMs and to broader patient outcomes. Our objective was to quantify adherence to cirrhosis QMs and investigate whether adherence was associated with lower all-cause mortality and inpatient health-care utilization in a large national cohort of Veterans with cirrhosis.
METHODS
Cohort Identification
The Veterans Outcomes and Costs Associated with Liver disease (VOCAL) cohort was derived from VA’s Corporate Data Warehouse, which includes detailed demographic, clinical, and administrative data using validated methodology.(16) The main inclusion criteria for this retrospective cohort were having 2 outpatient or 1 inpatient International Classification of Diseases version 9 (ICD9-CM) or ICD10-CM codes for cirrhosis (ICD9-CM: 571.2, 571.5, 571.6; ICD10-CM: K74.*, K70.3*) from January 1st, 2010 to December 31th, 2016.(17, 18) The baseline period began on the date the first cirrhosis diagnosis code appeared in the medical record or on the first day of hospitalization. All patients were followed until death, transplantation or December 31st, 2017, whichever came first. Patients were only included in the cohort in each year if they had at least one primary care visit during that year, therefore, could enter and exit the cohort based on receipt of PCP care each year.
Cirrhosis Quality Measures Assessment
The VOCAL cohort was used to assess quality of care for patients with cirrhosis. Table 1 shows a list of VA quality measures with their corresponding domains and definitions. The ascertainment of each QM is described below in detail and shown in Supplementary Table 1. Each QM was benchmarked against prior national estimates of the QM and, if there was a large discrepancy between the measured QM and the benchmark, two experienced adjudicators performed a medical record review to assess the diagnostic accuracy of the QM. All outpatient measures (QM1-QM3) only include Veterans who had at least one outpatient primary care visit in a calendar year. Patients were censored at death or transplantation
Table 1.
Proposed VA cirrhosis quality measures
| Cirrhosis quality measure | Domain measured | Definition | Unit of measurement |
|---|---|---|---|
| QM1. Patients with cirrhosis should be seen by a specialist within 12 months of the cirrhosis diagnosis | Specialty Care Access | Veterans with at least one outpatient gastroenterology or hepatology visit within 12 months of the cirrhosis diagnosis | Patient |
| QM2. Patients with cirrhosis should receive surveillance for hepatocellular carcinoma every 6 months with ultrasound (or other liver imaging), with or without alpha fetoprotein | HCC surveillance | Percentage of time under direct imaging surveillance (PTUDS) with serial abdominal imaging (liver ultrasound, contrast-enhanced CT/MRI) within the first 2 years following the cirrhosis diagnosis | Patient |
| QM3. Patients with cirrhosis and platelet count <150,000/mm3 should receive upper gastrointestinal endoscopy to assess for esophageal varices within 12 months of the initial diagnosis of cirrhosis (excluding patients on non-selective beta blockers). | Variceal surveillance | Veterans with at least one upper endoscopy within 12 months of the cirrhosis diagnosis | Patient |
| QM4. Patients who are discharged after admission for a complication of cirrhosis should be seen in clinic (either specialty or primary care) within 30 days of discharge | Specialty/Primary Care Access | Outpatient primary care, gastroenterology, or hepatology appointment within 30 days of cirrhosis-related admission not resulting in death, transplant, discharge to a facility or hospice | Hospital discharge |
| QM5. Cirrhotic patients admitted to the hospital with upper gastrointestinal bleeding should receive antibiotics in the hospital for ≥ 3 days. | Inpatient upper GI bleeding | --- | |
| QM6. Percent of patients re-hospitalized within 30 days of discharge (and 90 days) | Inpatient utilization | 30 and 90-day cirrhosis-related readmissions not resulting in death, transplant, discharge to a facility or hospice | Hospital discharge |
Abbreviations: HCC=hepatocellular carcinoma, GI=gastrointestinal, PCP=primary care provider, QM=quality measure, VA=Veterans Affairs.
QM5 was not able to be validated, therefore, no association with clinical outcomes is reported
QM1: Patients with cirrhosis should be seen by a specialist within 12 months of the cirrhosis diagnosis.
Data on completed outpatient visits were obtained from the outpatient visit data from the CDW. Patients were classified as having seen a gastroenterology or hepatology physician or advanced practice provider (nurse practitioner, physician’s assistant) within 12 months before or after the date the cirrhosis diagnosis code was first entered in the medical record. Patients coded as having seen a provider in a GI clinic or hepatology clinic at least once were classified as receiving specialty care.
QM2: Patients with cirrhosis should receive surveillance for hepatocellular carcinoma (HCC) every 6 months with ultrasound (or other liver imaging), with or without alphafetoprotein (AFP).
Imaging data were obtained from the radiology database in the Corporate Data Warehouse and were supplemented from the “fee-basis” database which contains additional data on studies performed outside of VA facilities that are paid for by VA funds; 95% of fee basis claims are processed within 200 days.(19) Imaging adherence was measured over the first 2 years of follow-up after the cirrhosis diagnosis and was assessed as the percentage of time under direct surveillance (PTUDS), as previously reported.(19) Patients were only eligible for this measure if alive for at least 2 years following the cirrhosis diagnosis. Patients were considered under direct surveillance if they had an imaging test within 7 months of a prior study (a 1-month grace period to the usual 6 months was given to account for small scheduling delays in clinical practice settings). The 7-month surveillance interval restarted on the date of re-imaging if imaging was performed before the completion of the previous time interval. For example, patients who received no imaging were included in the calculation with a PTUDS of 0% and those with 2 abdominal imaging studies 1 year apart would have PTUDS of 50%.
The main analysis included outpatient or inpatient abdominal ultrasounds, contrast-enhanced computed tomography (CT) scans or magnetic resonance imaging (MRI) with gadolinium irrespective of imaging intent.(19) The justification to include all studies was based on the pragmatic consideration that if a patient received adequate quality imaging for a reason other than HCC surveillance, the treating clinician would “credit them” as having received appropriate surveillance in routine clinical practice. A sensitivity analysis evaluated only outpatient studies. We did not include AFP in this calculation because it was considered optional.
QM3. Patients with cirrhosis and platelet count <150,000/mm3 should receive upper gastrointestinal endoscopy to assess for esophageal varices within 12 months of the initial diagnosis of cirrhosis (excluding patients on non-selective beta blockers).
Data for upper endoscopy were obtained from outpatient data and supplemented with fee-basis data to capture studies performed outside of the VA (using CPT codes 43191–43232, 43235–43259).(13, 14, 19) Procedures for upper endoscopy with stent placement, foreign body extraction, balloon dilation, and guidewire insertion were specifically excluded.(13, 14) Patients were considered eligible for upper endoscopy if they were not prescribed propranolol, nadolol, or carvedilol within +/−90 days of the cirrhosis diagnosis (because these patients were assumed to have appropriate primary variceal prophylaxis). Patients were considered adherent with upper endoscopy if it was performed within 12 months before or after the first cirrhosis diagnosis code in the medical record.
QM4. Patients who are discharged after admission for a complication of cirrhosis should be seen in clinic (either specialty or primary care) within 30 days of discharge.
Inpatient admission data were obtained, which included all admission and discharge diagnoses, admission and discharge dates, and discharge disposition (home, rehabilitation, hospice etc.). Cirrhosis-related admissions were identified using ICD-9-CM and ICD-10-CM codes for common liver-related complications such as ascites, variceal bleeding, spontaneous bacterial peritonitis, hepatorenal syndrome, etc. (detailed categorization of liver-related admissions is provided in the Supplementary Table 2). The diagnosis codes were additionally supplemented with Clinical Classification Software (CCS), a diagnosis categorization scheme which collapses ICD-9-CM and ICD-10-CM codes into fewer clinically relevant categories and has been widely used to investigate inpatient healthcare utilization. (10, 20, 21) CCS categories related to bacterial infections, electrolyte disturbances, gastrointestinal bleeding, liver cancer, biliary disorders, and alcohol-related disorders were used to more broadly capture potential cirrhosis-related admissions. Patients were considered adherent if they had a post-discharge visit to primary care, gastroenterology, or hepatology providers (physicians or advance practice practitioners) within 30 days of discharge, obtained from the outpatient data from the CDW as for QM1. Using CPT codes for elective procedures within CCS, all elective admissions were excluded from the analysis. Discharges that resulted in death within 30 days, hospice care within 30 days, or were to locations other than home were likewise excluded, which accounted for 1.8% of admissions.
QM5. Cirrhotic patients admitted to the hospital with upper gastrointestinal (GI) bleeding should receive antibiotics in the hospital for ≥3 days.
Variceal and non-variceal upper GI bleeding were ascertained using ICD-9-CM and ICD-10-CM codes linked to inpatient admissions (Supplementary Table 3). Antibiotic prescriptions were obtained from the inpatient pharmacy barcoded medication administration table, which includes all inpatient oral and intravenous medications. Patients were considered adherent if they received any antibiotic with gram negative coverage that could serve as effective prophylaxis for spontaneous bacterial peritonitis (SBP) for three or more days during the hospitalization; see Supplementary Table 4.
After initial descriptive analysis, the calculated adherence was only 10%, raising concern for misclassification bias. A medical record review was performed by two hepatologists (MS and DEK) on 280 inpatient admission records to independently assess the performance characteristics of ICD9-CM and ICD-10-CM codes for upper GI bleeding such as sensitivity, specificity, positive predictive values (PPVs), and area under the receiver operator curve (AUROC). CPT codes for upper endoscopy and data on packed red blood cell transfusions were added to test whether this would improve the positive predictive value. A medical record review showed that the PPV for UGI bleed using ICD-9-CM/ICD-10-CM codes was only 46%. This increased to 61% and 75% when adding PRBC transfusions and PRBC transfusions with inpatient EGD, respectively, however, at the higher PPV, sensitivity for capturing GI bleed was only 2.3% (Supplementary Table 5). Multivariable models for mortality were, therefore, not performed due to insufficient diagnostic accuracy of this measure using administrative data.
QM6. Percent of patients re-hospitalized within 30 days of discharge (and 90 days) should be quantified.
Readmissions data for cirrhosis-related admissions were obtained using the same methodology as for QM4. All rehospitalizations that occurred within 30 days (or 90 days) of discharge for any reason were counted as a readmission. Admissions that were elective or that resulted in death, hospice care, or non-home discharge were excluded from the QM adherence calculation.
Outcomes
We assessed the relationship between each quality metric and all-cause mortality at the patient level. All-cause mortality was evaluated using the VA Vital Status Master File, which is highly sensitive for capturing death.(22, 23) Detailed information on the index date and mortality definition for each measure is described in Supplementary Table 1. For several of the QMs we also assessed the relationship between the QM and healthcare utilization, defined as the number of cirrhosis-related hospitalizations within 2 years of the quality measure index date as shown in Table 6. Cirrhosis-related hospitalizations were defined using the same methodology as for QM1 and QM4.
Table 6.
Multivariable models of the associations between adherence to cirrhosis quality measures and the number of cirrhosis-related hospitalizations within 2 years
| Practice quality measure | Cirrhosis-related admissions Unadjusted IRR (95% CI) |
Cirrhosis-related admissions Adjusted IRR (95% CI) |
|---|---|---|
| QM1. Gastroenterology/hepatology appointment within 12 months of cirrhosis diagnosisa | 1.16 (1.11–1.22) | 1.24 (1.18–1.32) |
| QM2. HCC surveillance – percentage of time under direct surveillance (PTUDS) ≥ 50% versus < 50%a | 0.81 (0.77–0.86) | 0.85 (0.80–0.90) |
| QM3. Upper endoscopy within 12 months of cirrhosis diagnosis to assess for varices if platelet count <150 and not on not on prophylactic beta blocker | 1.36 (1.25–1.49) | 1.44 (1.33–1.56) |
| QM4. Primary or specialty care appointment within 30 days of discharge from cirrhosis-related hospitalizationb,c | 0.96 (0.92–1.00)* | 0.97 (0.94–1.02)** |
Abbreviations: CT=computed tomography, HCC=hepatocellular carcinoma, IRR=incidence rate ratio, MRI=magnetic resonance imaging,
All models were adjusted for patient factors: age, sex, race, service connectedness, patient distance to VA transplant center, diabetes, substance abuse, alcohol use, etiology of liver disease, cirrhosis comorbidity index, MELD score, Child Turcotte Pugh Score, VA facility factors: academic facility, volume of outpatient cirrhosis visits, VA region, rurality
p=0.066,
p=0.226
Measure includes patients with at least 1 primary care appointment in a calendar year
Measure excludes patients with elective admissions, in-hospital death, non-home discharge, and hospice discharge
Cirrhosis-related admission defined as admissions with the diagnosis codes for cirrhosis, cirrhosis complications (variceal bleeding, hepatic encephalopathy, ascites, liver cancer), electrolyte abnormalities, infections, and alcohol abuse)
Covariates
Patient-level covariates included demographics (age, sex, race) and percent service-connectedness, which is measure of VA benefits coverage; patients with >50% service connectedness generally have lower copayments. Distance between the VA facility where the patient was receiving care and the nearest VA transplant center was asessed using facility zip codes. Obesity was defined as with body mass index >30 kg/m2 at least once during the baseline period. Diabetes was defined using a previously validated VA algorithm.(24) Alcohol use was ascertained using the Alcohol Use Disorders Identification Test (AUDIT-C), which is annually administered in VA primary care clinics; a score of 5 or greater for males and 4 or greater for females was considered to be significant alcohol use. (25) Data were also obtained regarding the etiology of liver disease, model for end stage liver disease (MELD) score and Child-Turcotte-Pugh (CTP) score using previously validated methodology.(7, 26, 27) Comorbidity adjustment was performed with the cirrhosis comorbidity index, which includes ICD9/10 diagnosis codes for acute myocardial infarction, peripheral arterial disease, epilepsy, substance abuse other than alcoholism, heart failure, cancer, and chronic kidney disease.(28) VA facility characteristics included geographic region, academic affiliation, rurality and whether the facilty had a high number of outpatient cirrhosis-related visits per year (volume). High volume was defined as being above the 50th percentile of outpatient visits. Rurality codes were derived from the U.S. Department of Agriculture 2013 Rural-Urban Continuum Codes.(29) All patient-level covariates were measured at baseline (the time of cirrhosis diagnosis) with the exception of MELD and CTP scores, which were used as time-updating covariates in cases where the exposures were time-updating (see details below).
Statistical analysis
Multivariable models for all-cause mortality
For QM1 (specialty care access within 12 months of the cirrhosis diagnosis) and QM3 (receipt of upper endoscopy within 12 months of the cirrhosis diagnosis) we used Cox proportional hazards models censored at 2 years (or for death, liver transplantation). Receipt of the quality measure was specified as a time-updating exposure to address possible immortal time bias.(30) For example, patients not receiving specialty care were analyzed as unexposed until the specialty care appointment occurred. Secondary analyses for QM1 and QM3 excluded patients who died within 30 days of the cirrhosis diagnosis to account for possible late referral bias.(31) For QM2 (HCC surveillance with abdominal imaging) we used a Cox model with the exposure being PTUDS categorized as a dichotomous variable (greater than or equal to 50% of time under surveillance versus less than 50%) as the primary exposure. The PTUDS threshold was chosen for ease of measurement and interpretation (19) For QM4 (post-discharge specialty/primary care access) and QM6 (30-day, 90-day rehospitalizations) Cox models were fit with hospital discharge as the unit of analysis. Details regarding analyses for each QM are in Supplementary Table 1.
All models were adjusted for patient and facility-level variables (listed in covariates) and for each individual VA facility. Robust standard errors were calculated, accounting for correlation of observations within patients. Models with time-updating exposures (imaging studies, hospitalizations) were fit with time-updating MELD and Child-Turcotte-Pugh (CTP) scores; values closest to the exposure date were used. Analyses for all QMs were repeated fitting multilevel mixed-effects survival models with a Weibull distribution (using the Stata mestreg command) to account for cluster-specific random effects.
Multivariable models for the number of cirrhosis-related hospitalizations
The associations between adherence to QM1-QM4 and healthcare utilization, defined as the number of cirrhosis-related admissions within 2 years of cohort entry (QM1-QM3) or for 2 years after the index cirrhosis-related hospitalization (QM4), were evaluated with negative binomial regression models, which are used for count outcomes in instances of overdispersion (in this case due to a large proportion of patients who were never hospitalized). The covariate adjustment approach was similar to models for all-cause mortality. The primary analyses excluded patients who died within a 2-year period; secondary analyses were conducted including all patients.
Missing values
Missing laboratory values were imputed using chained regression equations with the mice package, R programming environment using age, race, BMI, diabetes, liver disease etiology, alcohol, liver decompensation diagnosis codes, and future laboratory values.(32) Missing AUDIT-C values (15%) were analyzed as 0 (no alcohol abuse)(33); in sensitivity analyses regression models for all QMs and outcomes were fit with missing AUDIT-C as a separate category. All other analyses were conducted using Stata 15.0 (StataCorp, College Station, TX)
RESULTS
The baseline characteristics of the 121,129 patients who were diagnosed with cirrhosis from 2010–2016 and had at least one primary care appointment in that calendar year are shown in Table 2. The mean age was 62.1 years (standard deviation [SD]=8.7). Greater than half of the patients were white, 15.4% were black, and 9.1% were Hispanic. About one third of patients were ≥50% service-connected, indicating higher degree of VA benefits coverage. At baseline, 51.5% of the cohort had diabetes and 67.6% had obesity. Greater than half of the cohort had a past reported history of substance abuse and 43.6% had a prior alcohol abuse at baseline. The most common cirrhosis etiologies were HCV and/or alcohol, together accounting for 72.3% of cases, and 18.1% had NAFLD/NASH. The mean MELD score at cohort entry was 6 (interquartile range [IQR]:6–12); 64.3% were Child-Turcotte-Pugh (CTP)-A, 29.8% were CTP-B, and 5.9% were CTP-C. The median follow-up time was 2.7 years (IQR: 1.1–5.1), median time to death was 1.8 years (IQR:0.6–3.6) and the median time to transplantation was 2.0 years (IQR: 0.9–3.7). The median time to decompensation was 1.3 years (IQR:0.3–2.9).. Nearly half of the patients died during follow-up, 12.0% developed HCC, and about half developed hepatic decompensation.
Table 2.
Baseline characteristics of veterans diagnosed with cirrhosis from 2010–2016.
| Variable | N=121,129 |
|---|---|
| Age, M (SD) | 62.1 (8.7) |
| Male, n (%) | 118,318 (2.4) |
| Race, n (%) | |
| White | 68,286 (56.4) |
| Black | 16,689 (15.4) |
| Hispanic | 11,064 (9.1) |
| Asian/PI/Alaskan | 2,961 (2.4) |
| Unknown/other | 20,170 (16.7) |
| Service connected, n (%) | |
| 50–100% | 35,944 (29.7) |
| 0–50% | 30,339 (25.0) |
| Other | 54,887 (45.3) |
| Comorbidities | |
| Diabetes, n (%) | 62,401 (51.5) |
| Obesity (body mass index >30), n (%) | 81,880 (67.6) |
| Substance abuse history*, n (%) | 76,031 (62.8) |
| Alcohol misuse (AUDIT-C), n (%) | 52,792 (43.6) |
| Cirrhosis comorbidity index* n (%) | |
| zero | 73,165 (60.4) |
| 1+0 | 25,363 (20.9) |
| 1+1 | 8,203 (88.1) |
| 3+0 | 4,122 (3.4) |
| 3+1 | 9,953 (8.2) |
| 5+0 | 17 (0.01) |
| 5+1 | 347 (0.29) |
| Liver disease etiology, n (%) | |
| HCV | 18,454 (15.2) |
| HCV and alcohol | 29,858 (24.6) |
| Alcohol | 39,428 (32.5) |
| Non-alcoholic fatty liver disease | 21,873 (18.1) |
| Hepatitis B virus | 2,931 (2.4) |
| Cryptogenic | 4,918 (4.1) |
| Primary Biliary Cholangitis | 1,372 (1.1) |
| Primary Sclerosing Cholangitis | 649 (0.5) |
| Hemochromatosis | 1,332 (1.1) |
| Autoimmune | 355 (0.3) |
| MELD score, median (IQR) | 6 (6–12) |
| Child-Turcotte-Pugh score, n (%) | |
| A (5–6) | 77,966 (64.3) |
| B (7–9) | 36,069 (29.8) |
| C (10–15) | 7,135 (5.9) |
| Follow-up time (years), median (IQR) | 2.7 (1.1–5.1) |
| Died during follow-up, n (%) | 58,006 (47.9) |
| Time to death (years), median (IQR) | 1.8 (0.6–3.6) |
| Transplanted during follow-up, n (%) | 2,975 (2.5) |
| Time to transplantation (years), median (IQR) | 2.0 (0.9–3.7) |
| Developed hepatic decompensation**, n (%) | 12,992 (16.7) |
| Time to hepatic decompensation (years), median (IQR) | 1.3 (0.3–2.9) |
Abbreviations: BMI=body mass index, IQR=interquartile range, HCV=hepatitis C virus, MELD=model for end stage liver disease; AUDIT-C=Alcohol Use Disorder Identification Test (threshold score for identifying alcohol use disorder: ≥4 for males and ≥3 for females). Hepatic decompensation was defined as ascites, spontaneous bacterial peritonitis, or variceal hemorrhage.
Comorbidity index includes acute myocardial infarction, peripheral arterial disease, epilepsy, substance abuse other than alcoholism, heart failure, cancer, and chronic kidney disease38
Hepatic decompensation calculated among patients with Child-Turcotte-Pugh A cirrhosis (n=77,966). Missing laboratory values were imputed using chained equations, mice package in R. Missing AUDIT-C values were coded as 0 (no alcohol abuse)
Facility characteristics of the study cohort (based on the first facility where patients received the incident cirrhosis diagnosis code) are described in Table 3. A total of 84.3% of patients received care at urban facilities and 57.7% received care at academically-affiliated facilities. Only 17.6% of Veterans with cirrhosis received care at a VA within a 200-mile radius of a VA transplant center, 75.7% of patients were at high-volume facilities.
Table 3.
Characteristics of 121,129 patients diagnosed with cirrhosis at 128 VA facilities
| Variable, n (%) | |
|---|---|
| Region | |
| Northeast | 17,036 (14.1) |
| Southeast | 29,072 (24.0) |
| Midsouth | 27,805 (23.0) |
| Central | 18,867 (15.6) |
| West | 28,349 (23.4) |
| Rurality | |
| Metropolitan | 102,121 (84.3) |
| Non-metropolitan, 20,000 or more | 5,943 (4.9) |
| Non-metropolitan up to 19,999 | 13,065 (10.8) |
| Academic VA facility | 69,930 (57.7) |
| Facility <200 miles from a VA transplant center | 21,352 (17.6) |
| High volume facility | 90,434 (75.7) |
Characteristics reported for the VA facility with the incident cirrhosis diagnosis
High volume facility defined as having greater than the median number of annual outpatient specialty care cirrhosis visits (median number of cirrhosis visit=616; 1809 patients at 7 facilities had no specialty care visits).
Table 4 shows the percent adherence to QM1-QM4 as well as 30- and 90-day readmission rates. Among the 121,129 patients newly diagnosed with cirrhosis, 70.5% were seen by a specialist within 12 months of the cirrhosis diagnosis (QM1). For QM2, among 50,434 eligible patients, the median percentage of time under surveillance (PTUDS) for HCC was 37.2% with 42.0% of the cohort having PTUDS ≥ 50% in the two years after cirrhosis diagnosis. Among the 50,079 patients with platelet counts less than 150,000 who were not taking a prophylactic beta blocker (QM3), 31.9% of patients underwent upper endoscopy for variceal surveillance. Among the 46,821 eligible cirrhosis-related hospitalizations, 53.6% had early outpatient follow-up visits with primary care or gastroenterology/hepatology within 30 day of hospital discharge (QM4). The percent of cirrhosis-related hospitalizations with 30-day and 90-day readmissions (QM6) was 34.0% and 53.4%, respectively.
Table 4.
Estimates of adherence to cirrhosis quality measures in Veterans Affairs from 2008 to 2016
| Cirrhosis quality measure (QM) | Unit of analysis (N) | QM adherence |
|---|---|---|
| QM1. Seen by a specialist within 12 months of cirrhosis diagnosisa, % adherent | Patient (N=121,129) |
70.5% |
| QM2. HCC surveillance – percentage of time under direct surveillance (PTUDS) ≥ 50%a | Patient (N=50,434) |
42.0% |
| QM3. Upper endoscopy within 12 months of cirrhosis diagnosis to assess for varices if platelet count <150 and not on not on prophylactic beta blocker, % adherent | Patient (N=50,079) |
31.9% |
| QM4. Primary or specialty care appointment within 30 days of discharge from cirrhosis-related VA hospitalizationb,c,, % adherent | Discharge (N=46,821) |
53.6% |
| QM5. Percent hospitalizations for upper GI bleed should receiving antibiotics for at least 3 daysd | Discharge (N=15,105) |
--- |
| QM6a. Percent of 30-day of cirrhosis-related VA hospital readmissions with 1 year of index admissionb,c,% | Discharge (N=46,821) |
34.0% |
| QM6b. Percent of 90-day of cirrhosis-related VA hospital readmissions within 1 year of index admissionb,c,% | Discharge (N=46,821) |
53.4% |
Abbreviations:HCC=hepatocellular carcinoma, NSBB=non-selective beta blocker, QM=quality measure
Measure includes patients with at least 1 primary care appointment in a calendar year
Measure excludes patients with elective admissions, in-hospital death, non-home discharge, and hospice discharge
Cirrhosis-related admission defined as admissions with the diagnosis codes for cirrhosis, cirrhosis complications (variceal bleeding, hepatic encephalopathy, ascites, liver cancer), electrolyte abnormalities, infections, and alcohol abuse)
Measure not found not be valid after medical record review
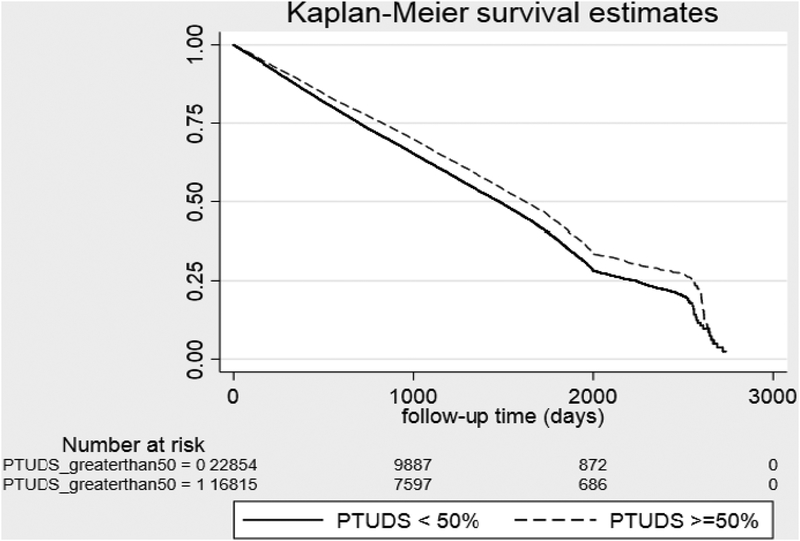
Unadjusted Kaplan Meier curves for QMs 2 and 4are shown in Figures 1 &2; unadjusted and adjusted results of multivariable models for with all-cause mortality are presented in Table 5. In analyses adjusted for patient demographics, liver disease etiology and severity, medical comorbidity, and facility-level characteristics, higher adherence to QM1 (gastroenterology/hepatology appointment within 12 months of the cirrhosis diagnosis [HR:0.80; 95% CI:0.78–0.82] and QM3 (variceal surveillance within q3 month of the cirrhosis diagnosis in the presence of portal hypertension: [HR:0.93; 95% CI:0.89–0.99] were associated with lower 2-year mortality. After excluding patients who died within 30 days of the cirrhosis diagnosis, the adjusted associations remained significant (QM1: HR 0.90, 95% CI 0.87–0.93; QM3:HR 0.85, 95% CI:0.82–0.90). With regards to QM2 (HCC surveillance with liver ultrasound, or contrast-enhanced CT/MRI), percentage of time under direct surveillance (PTUDS) ≥50% with any of these imaging studies was associated with lower mortality [HR: 0.92; 95% CI:0.90–0.95]. Figure 1 shows the unadjusted Kaplan Meier curves for the association between PTUDS and all-cause mortality stratified by Child-Turcotte-Pugh (CTP) class A and B (Panel A) versus CTP C cirrhosis (Panel B). The unadjusted association between PTUDS and lower mortality was limited to CTP classes A and B and was not significant for CTP class C. Receipt of a specialty or primary care appointment within 30 days of hospital discharge (QM4) was associated with lower mortality [HR:0.57; 95% CI:0.54–0.60] whereas readmission (QM6) within 30 days [HR:1.53; 95% CI:1.46–1.60] and 90 days [HR:1.62; 95% CI:1.54–1.70] were associated with higher mortality.
Figure 1.
QM2. Unadjusted Kaplan-Meier survival estimates stratified by proportion of time under direct surveillance (PTUDS) for Hepatocellular Carcinoma (HCC) stratified by PTUDS >=50 versus < 50%. (A) HCC surveillance among patients with Child-Turcotte-Pugh A & B cirrhosis. Log rank: P < 0.001. Index date starts at 2 years after the cirrhosis diagnosis. (B) HCC surveillance among patients with Child-Turcotte-Pugh C cirrhosis. Log rank: P = 0.15.
Figure 2.
QM4. Unadjusted 90-day Kaplan-Meier survival estimates stratified by receipt of primary or specialty care appointment within 30 days of discharge from a cirrhosis-related hospitalization. Log rank P < 0.001.
Table 5.
Associations between adherence to quality measures and all-cause mortality outcomes
| Practice quality measure | Outcome | Unadjusted HR (95% CI) |
Adjusted HR (95% CI) |
|---|---|---|---|
| QM1. Seen by a specialist within 12 months of cirrhosis diagnosisa | 2-year mortality | 0.63 (0.61–0.64) | 0.80 (0.78–0.82) |
| QM2. HCC surveillance – percentage of time under direct surveillance (PTUDS) ≥ 50% versus < 50%a | Overall mortalitye | 0.89 (0.87–0.92) | 0.92 (0.90–0.95) |
| QM3. Upper endoscopy within 12 months of cirrhosis diagnosis to assess for varices if platelet count <150 and not on not on prophylactic beta blocker, % adherent | 2-year mortality | 0.82 (0.78–0.86) | 0.93 (0.89–0.99) |
| QM4. Primary or specialty care appointment within 30 days of discharge from cirrhosis-related hospitalizationc,d | 90-day mortality | 0.67 (0.65–0.70) | 0.57 (0.54–0.60) |
| QM6a. Readmission within 30 days of cirrhosis-related hospitalizationc,d | 2-year mortality | 1.70 (1.62–1.78) | 1.53 (1.46–1.60) |
| QM6b. Readmission within 90 days of cirrhosis-related hospitalizationc,d | 2-year mortality | 1.88 (1.80–1.97) | 1.62 (1.54–1.70) |
Abbreviations: CI=confidence interval, HCC=hepatocellular carcinoma, HR=hazard ratio, NSBB=non-selective beta blocker
All models were adjusted for patient factors: age, sex, race, service connectedness, patient distance to VA transplant center, diabetes, substance abuse, alcohol use, etiology of liver disease, cirrhosis comorbidity index, MELD score, Child Turcotte Pugh Score, VA facility factors: academic facility, volume of outpatient cirrhosis visits, VA region, rurality
Measure includes only routine users of VA healthcare defined as at least one outpatient primary care appointment in the calendar year that the measure was assessed
a 30-day grace period was given for surveillance, studies considered adherent if 7 months apart
Measure excludes patients with elective admissions, in-hospital death, non-home discharge, and hospice discharge
Cirrhosis-related admission defined as admissions with the diagnosis codes for cirrhosis, cirrhosis complications (variceal bleeding, hepatic encephalopathy, ascites, liver cancer), electrolyte abnormalities, infections, and alcohol abuse)
Mortality was measured until end of follow-up
Results of the multivariable models for associations between inpatient healthcare utilization and the number of cirrhosis-related hospitalizations within 2 years are shown in Table 6. Gastroenterology/hepatology appointment within 12 months of the cirrhosis diagnosis (QM1) and variceal surveillance (QM3) were associated with higher 2-year inpatient utilization (QM1: incidence rate ratio [IRR] 1.24, 95% CI:1.18–1.32; QM2: IRR 1.44, 95% CI: 1.33–1.56). HCC surveillance (QM2) was associated with lower inpatient utilization (IRR: 0.85, 95% CI: 0.80–0.90). Receipt of a specialty or primary care appointment within 30 days of hospital discharge (QM4) was not associated with the number of subsequent cirrhosis-related readmissions within a 2-year period (IRR 0.97, 95% CI: 0.97–1.02) or the odds of readmission (30-day readmission: OR 1.04, 95% CI: 0.98–1.12, p=0.20; 90-day readmission: OR 0.98, 95% CI: 0.93–1.04, p=0.55). Results were similar when including patients who died within 2 years of cohort entry.
Sensitivity Analyses
In secondary analyses for mortality fitting multivariable mixed-effects models to account for cluster-specific random effects (e.g. VA facilities for all measures and individual patients for hospitalization-related measures), effect estimates were similar to our primary analyses (Supplementary Table 6). Additional detailed descriptive data regarding adherence to cirrhosis QMs and clinical outcomes stratified by age, liver disease severity, liver disease etiology, and geographic region are presented in Supplementary Tables 7–10.
DISCUSSION
In this study of the proposed cirrhosis QMs among a large national cohort of Veterans with cirrhosis, we found that five the six proposed VA QMs generally had higher adherence than reported in non-VA settings. We also found that adherence to the QMs was generally associated with better patient outcomes. Specifically, after adjusting for multiple patient-level and facility-level characteristics, we found lower mortality among patients with access to liver disease specialists within 1 year of the cirrhosis diagnosis (20% lower 2-year mortality), with EGD surveillance for patients with portal hypertension (7% lower mortality 2-year mortality), and with early post-discharge care after a cirrhosis-related hospitalization with a primary or specialty care provider (43% lower 90-day mortality).
It is not surprising that timely access to specialty care was associated with lower mortality as multiple previous studies, including those by our team, have shown higher receipt of guideline-recommended care for HBV, HCC, variceal surveillance, and management of ascites among patients who were seen by a gastroenterologist or hepatologist.(8, 10, 11, 19, 34) Although our estimate of 71% access to specialty care within 1 year of the cirrhosis diagnosis is higher than previously reported, the relationship with mortality is nearly identical to a recent smaller study by Mellinger et al., which showed that access to subspecialty care for cirrhosis among Veterans within the VA’s Midwestern US network was associated with a 19% lower mortality.(31) The reasons for a higher proportion of receiving specialty care may be due to our cohort having a new cirrhosis diagnosis prompting a specialty consult and the fact that we credited patients with a specialist visit even if this occurred within a year prior to the cirrhosis diagnosis. Furthermore, a recent single-center study reported that hepatology telemedicine consultation among 513 patients with liver disease was associated with improved survival.(35) With regards to the QM of variceal surveillance for patients with portal hypertension (not taking a prophylactic beta blocker) our estimated adherence was 32%, which is within range of the previously reported data among small patient samples.(10)
Adherence to QM2, which was defined HCC surveillance at 6-month intervals (as recommended by the AASLD) with a 1-month grace period, was 37% when measured as the percentage of time under direct imaging surveillance (PTUDS) with 42% of patients having PTUDS of 50% or greater; on average 60% of the patients had one abdominal imaging study per year. Our estimates are generally higher than those noted in a systematic review of 9 US HCC surveillance observational studies where the pooled adherence was 19%. However, adherence ranged from 11% to 78%. The variation might be explained by the heterogeneity of the studies, including that the definition of HCC surveillance varied from one-time to biannual imaging with or without AFP and the fact that we restricted our sample to individuals who survived at least 2 years after the diagnosis (36).
In the analysis of HCC surveillance on overall mortality, we noted that PTUDS was associated with lower mortality. Our results need to be interpreted in context of the fact that the majority of cirrhosis patients do not die of HCC, but rather die of other cirrhosis-related or unrelated complications. Previous retrospective studies have generally shown a mortality benefit with greater HCC surveillance; however, the greatest benefits of surveillance were apparent among patients with well-compensated liver disease.(37) Similar findings were noted in this study whereby in unadjusted analyses, lower mortality was apparent for patients with CTP-A/B cirrhosis, but not with CTP-C (although the sample size was lower for CTP-C patients increasing the potential for type II error). Despite these noted associations between higher HCC surveillance and lower mortality, the mechanism for these findings is not well-understood. For example, a recent methodologically rigorous matched case-controlled VA study showed no association between HCC surveillance and HCC-related mortality. (38) Despite the lack of association between surveillance and early cancer detection in other studies, effective HCC surveillance is likely a surrogate of healthcare quality whether via healthcare engagement, improved access or provider vigilance. It has a plausible role in identifying early-stage HCC and as such, is a reasonable quality indicator.
We also confirmed that early post-discharge care after a cirrhosis-related admission was associated with lower mortality whereas greater inpatient utilization was associated with higher mortality.(9) The adherence to early post-discharge follow-up was only 52%. Given its strong association with lower mortality, this QM is a highly viable target for local as well as national quality improvement efforts in cirrhosis. We found that 30-day and 90-day readmissions were associated with higher mortality representing a measure associated with higher risks of a poor outcome. Although we do not make specific recommendations regarding readmission-rate benchmarks, identifying patients with 30-day and 90-day readmissions may help guide hospital quality improvement efforts whether by focusing on preventable readmissions or identifying patients in need of social services, transplantation referral, or palliative care. Future, in-depth investigations of determining readmission targets and mitigating potentially preventable readmissions (e.g., routine paracentesis or hepatic encephalopathy) should be conducted.
The data in this study are an important early step towards establishing baseline rates of adherence to the proposed QMs and informing ongoing efforts to improve quality of care in cirrhosis. As an integrated system of care, the VA has proactively funded and spearheaded quality improvement efforts in advanced liver disease. For example, the VA HIV, Hepatitis, & Related Conditions Programs (HHRC) within the Office Specialty Care Services has established Hepatic Innovation Teams within each Veteran Integrated Service Network to improve care in viral hepatitis, HIV and cirrhosis. In 2017, the HRRC created the Advanced Liver Disease Technical Advisory Group, and in addition to identifying cirrhosis QMs, has developed a working prototype of a cirrhosis dashboard based on the VA electronic heath record, which facilitates population management within each VA facility and allows for assessment of adherence to cirrhosis quality measures.(15) Investigators at Michael E. DeBakey VA Medical Center in Houston recently developed and implemented a population-based cirrhosis identification and management system, which allowed for identification and linkage to care of 30% of cirrhosis patients who were previously lost to follow-up.(39) The AASLD Practice Metrics Committee has also focused on developing evidence-based cirrhosis quality which include important clinical and patient-reported outcomes.(40) Future studies will need to investigate how these QMs and EHR-based tools can be leveraged in routine clinical care as well as the barriers and facilitators to their adoption.
We must acknowledge several study limitations. This was a retrospective cohort study with the potential for misclassification bias as cirrhosis was captured using diagnosis codes, which may have underestimated its true prevalence. While our multivariable models robustly adjust for the measurable patient and facility-level characteristics, the remaining differences in mortality could nonetheless be explained by other unmeasured patient and health-system factors… We did not specifically assess VA facility variation in care although we adjusted for hospitals and hospital characteristics; this requires further, in-depth investigation. Future studies using mixed methodology (including patient and provider interviews) should focus on reasons for low and high adherence that cannot be obtained using administrative data. In addition, studies in the Veteran population may not be readily generalizable due to the predominantly male population, the single integrated system of care, and the fact that recent internal VA initiatives have been targeting population-based cirrhosis management and quality improvement. Our estimates likely underestimate readmissions as these could have occurred at non-VA hospitals. Finally, this study excluded transplant recipients and did not evaluate referral for transplantation as a quality measure.
While further work is needed to test these quality measures in VA and non-VA populations, this study sheds important light on the quality of care provided to Veterans with cirrhosis, highlights areas for improvement within the VA and, perhaps most importantly, provides some evidence that these quality measures capture important information about the quality of care among patients with cirrhosis. Future studies should investigate how earlier referral either for transplantation, active HCC therapy, or palliative care when appropriate should be instituted. Additionally, prospective implementation studies of how quality improvement can best be integrated into clinical practice are needed. The VA, as an integrated national system of health care and an early adopted of telemedicine technology to improve access to care, is well-poised to lead these efforts.
Supplementary Material
Acknowledgement:
This research was supported by the National Institute of Diabetes and Digestive and Kidney Diseases, award #1K23DK115897–01. Rachel Werner is supported in part by K24-AG047908 from the National Institute on Aging.
Abbreviations:
- AUDIT-C
Alcohol Use Disorder Identification Test
- BMI
body mass index
- CPT
current procedural terminology
- CTP
Child-Turcotte-Pugh score
- CT
computed tomography
- HCC
hepatocellular carcinoma
- HCV
hepatitis C virus
- ICD
international classification of disease
- IQR
interquartile range
- M
mean
- MELD
model for end stage liver disease
- MRI
magnetic resonance imaging
- NAFLD
non-alcoholic fatty liver disease
- NASH
non-alcoholic steatohepatitis
- NSBB
non-selective beta blocker
- PTUDS
percentage of time under direct surveillance
- SD
standard deviation
- QM
quality measure
- VA
veterans affairs
Footnotes
Publisher's Disclaimer: Disclaimer: The views expressed in this article are those of the authors and do not necessarily represent the views of the U.S. Department of Veterans Affairs of the U.S. Government. The content is the responsibility of the authors alone and does not necessarily reflect the views of or imply endorsement by the U.S. Government.
Disclosures: none
References
- 1.Setiawan VW, Stram DO, Porcel J, Lu SC, Le Marchand L, Noureddin M. Prevalence of chronic liver disease and cirrhosis by underlying cause in understudied ethnic groups: the multiethnic cohort. Hepatology. 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kim WR, Brown RS Jr., Terrault NA, El-Serag H. Burden of liver disease in the United States: summary of a workshop. Hepatology. 2002;36(1):227–42. [DOI] [PubMed] [Google Scholar]
- 3.Mellinger JL, Richardson CR, Mathur AK, Volk ML. Variation among United States hospitals in inpatient mortality for cirrhosis. Clinical gastroenterology and hepatology : the official clinical practice journal of the American Gastroenterological Association. 2015;13(3):577–84; quiz e30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Volk ML, Tocco RS, Bazick J, Rakoski MO, Lok AS. Hospital readmissions among patients with decompensated cirrhosis. Am J Gastroenterol. 2012;107(2):247–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Talwalkar JA. Prophylaxis with beta blockers as a performance measure of quality health care in cirrhosis. Gastroenterology. 2006;130(3):1005–7. [DOI] [PubMed] [Google Scholar]
- 6.Neff GW, Duncan CW, Schiff ER. The current economic burden of cirrhosis. Gastroenterology & hepatology. 2011;7(10):661–71. [PMC free article] [PubMed] [Google Scholar]
- 7.Beste LA, Leipertz SL, Green PK, Dominitz JA, Ross D, Ioannou GN. Trends in burden of cirrhosis and hepatocellular carcinoma by underlying liver disease in US veterans, 2001–2013. Gastroenterology. 2015;149(6):1471–82 e5; quiz e17–8. [DOI] [PubMed] [Google Scholar]
- 8.Serper M, Taddei TH, Mehta R, D’Addeo K, Dai F, Aytaman A, et al. Association of Provider Specialty and Multidisciplinary Care With Hepatocellular Carcinoma Treatment and Mortality. Gastroenterology. 2017;152(8):1954–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kanwal F, Asch SM, Kramer JR, Cao Y, Asrani S, El-Serag HB. Early outpatient follow-up and 30-day outcomes in patients hospitalized with cirrhosis. Hepatology. 2016;64(2):569–81. [DOI] [PubMed] [Google Scholar]
- 10.Buchanan PM, Kramer JR, El-Serag HB, Asch SM, Assioun Y, Bacon BR, et al. The quality of care provided to patients with varices in the department of Veterans Affairs. Am J Gastroenterol. 2014;109(7):934–40. [DOI] [PubMed] [Google Scholar]
- 11.Kanwal F, Kramer JR, Buchanan P, Asch SM, Assioun Y, Bacon BR, et al. The quality of care provided to patients with cirrhosis and ascites in the Department of Veterans Affairs. Gastroenterology. 2012;143(1):70–7. [DOI] [PubMed] [Google Scholar]
- 12.El-Serag HB, Alsarraj A, Richardson P, Davila JA, Kramer JR, Durfee J, et al. Hepatocellular carcinoma screening practices in the Department of Veterans Affairs: findings from a national facility survey. Digestive diseases and sciences. 2013;58(11):3117–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kanwal F, Volk M, Singal A, Angeli P, Talwalkar J. Improving quality of health care for patients with cirrhosis. Gastroenterology. 2014;147(6):1204–7. [DOI] [PubMed] [Google Scholar]
- 14.Tapper EB. Building Effective Quality Improvement Programs for Liver Disease: A Systematic Review of Quality Improvement Initiatives. Clinical gastroenterology and hepatology : the official clinical practice journal of the American Gastroenterological Association. 2016;14(9):1256–65 e3. [DOI] [PubMed] [Google Scholar]
- 15.HIV, Hepatitis, & Related Conditions Programs, Office of Specialty Care Services, Annual Report 2017. https://www.hiv.va.gov/pdf/HHRC-annual-report-2017.pdf, Accessed November 1st, 2018
- 16.Kaplan DE, Serper M, Mehta R, Fox R, John B, Aytaman A, et al. Effects of Hypercholesterolemia and Statin Exposure on Survival in a Large National Cohort of Patients With Cirrhosis. Gastroenterology. 2019. [DOI] [PubMed] [Google Scholar]
- 17.VA Information Resources Center (VIReC) Intranet. Accessed April 23rd, 2016.
- 18.Kramer JR, Davila JA, Miller ED, Richardson P, Giordano TP, El-Serag HB. The validity of viral hepatitis and chronic liver disease diagnoses in Veterans Affairs administrative databases. Aliment Pharmacol Ther. 2008;27(3):274–82. [DOI] [PubMed] [Google Scholar]
- 19.Goldberg DS, Taddei TH, Serper M, Mehta R, Dieperink E, Aytaman A, et al. Identifying barriers to hepatocellular carcinoma surveillance in a national sample of patients with cirrhosis. Hepatology. 2017;65(3):864–74. [DOI] [PubMed] [Google Scholar]
- 20.Mellinger JL, Volk ML. Multidisciplinary management of patients with cirrhosis: a need for care coordination. Clinical gastroenterology and hepatology : the official clinical practice journal of the American Gastroenterological Association. 2013;11(3):217–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Machnicki G, Pinsky B, Takemoto S, Balshaw R, Salvalaggio PR, Buchanan PM, et al. Predictive ability of pretransplant comorbidities to predict long-term graft loss and death. Am J Transplant. 2009;9(3):494–505. [DOI] [PubMed] [Google Scholar]
- 22.Sohn MW, Arnold N, Maynard C, Hynes DM. Accuracy and completeness of mortality data in the Department of Veterans Affairs. Popul Health Metr. 2006;4:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Arnold N, Sohn M, Maynard C, Hynes DM. VIReC Technical Report 2: VA-NDI Mortality Data Merge Project. Hines, IL: Hines Edward J. Jr. VA Hospital–Veterans Affairs Information Resource Center;2006. [Google Scholar]
- 24.Butt AA, McGinnis K, Rodriguez-Barradas MC, Crystal S, Simberkoff M, Goetz MB, et al. HIV infection and the risk of diabetes mellitus. AIDS. 2009;23(10):1227–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bush K, Kivlahan DR, McDonell MB, Fihn SD, Bradley KA. The AUDIT alcohol consumption questions (AUDIT-C): an effective brief screening test for problem drinking. Ambulatory Care Quality Improvement Project (ACQUIP). Alcohol Use Disorders Identification Test. Archives of internal medicine. 1998;158(16):1789–95. [DOI] [PubMed] [Google Scholar]
- 26.Kaplan DE, Dai F, Aytaman A, Baytarian M, Fox R, Hunt K, et al. Development and Performance of an Algorithm to Estimate the Child-Turcotte-Pugh Score From a National Electronic Healthcare Database. Clinical gastroenterology and hepatology : the official clinical practice journal of the American Gastroenterological Association. 2015;13(13):2333–41 e1-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kamath PS, Wiesner RH, Malinchoc M, Kremers W, Therneau TM, Kosberg CL, et al. A model to predict survival in patients with end-stage liver disease. Hepatology. 2001;33(2):464–70. [DOI] [PubMed] [Google Scholar]
- 28.Jepsen P, Vilstrup H, Lash TL. Development and validation of a comorbidity scoring system for patients with cirrhosis. Gastroenterology. 2014;146(1):147–56; quiz e15–6. [DOI] [PubMed] [Google Scholar]
- 29.Groeneveld PW, Kruse GB, Chen Z, Asch DA. Variation in cardiac procedure use and racial disparity among Veterans Affairs Hospitals. American heart journal. 2007;153(2):320–7. [DOI] [PubMed] [Google Scholar]
- 30.Jones M, Fowler R. Immortal time bias in observational studies of time-to-event outcomes. J Crit Care. 2016;36:195–9. [DOI] [PubMed] [Google Scholar]
- 31.Mellinger JL, Moser S, Welsh DE, Yosef MT, Van T, McCurdy H, et al. Access to Subspecialty Care And Survival Among Patients With Liver Disease. Am J Gastroenterol. 2016;111(6):838–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.van Buuren S, Groothuis-Oudshoorn K. mice: Multivariate Imputation by Chained Equations in R. 2011. 2011;45(3):67. [Google Scholar]
- 33.Knox J, Wall M, Witkiewitz K, Kranzler HR, Falk D, Litten R, et al. Reduction in Nonabstinent WHO Drinking Risk Levels and Change in Risk for Liver Disease and Positive AUDIT-C Scores: Prospective 3-Year Follow-Up Results in the U.S. General Population. Alcohol Clin Exp Res. 2018;42(11):2256–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Serper M, Choi G, Forde KA, Kaplan DE. Care delivery and outcomes among U.S. veterans with hepatitis B: A national cohort study. Hepatology. 2015. [DOI] [PubMed] [Google Scholar]
- 35.Su GL, Glass L, Tapper EB, Van T, Waljee AK, Sales AE. Virtual Consultations Through the Veterans Administration SCAN-ECHO Project Improves Survival for Veterans With Liver Disease. Hepatology. 2018. [DOI] [PubMed] [Google Scholar]
- 36.Singal AG, Yopp A, C SS, Packer M, Lee WM, Tiro JA. Utilization of hepatocellular carcinoma surveillance among American patients: a systematic review. Journal of general internal medicine. 2012;27(7):861–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Singal AG, Pillai A, Tiro J. Early detection, curative treatment, and survival rates for hepatocellular carcinoma surveillance in patients with cirrhosis: a meta-analysis. PLoS Med. 2014;11(4):e1001624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Moon AM, Weiss NS, Beste LA, Su F, Ho SB, Jin GY, et al. No Association Between Screening for Hepatocellular Carcinoma and Reduced Cancer-Related Mortality in Patients With Cirrhosis. Gastroenterology. 2018;155(4):1128–39 e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kanwal F, Mapaskhi S, Smith D, Taddei T, Hussain K, Madu S, et al. Implementation of a Population-Based Cirrhosis Identification and Management System. Clinical gastroenterology and hepatology : the official clinical practice journal of the American Gastroenterological Association. 2018;16(8):1182–6 e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kanwal F, Tapper EB, Ho C, Asrani SK, Ovchinsky N, Poterucha J, et al. Development of Quality Measures in Cirrhosis by the Practice Metrics Committee of the American Association for the Study of Liver Diseases. Hepatology. 2018. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.