Abstract
Background:
Identifying neural characteristics that predict cannabis initiation is important for prevention efforts. The orbitofrontal cortex (OFC) is critical for reward response and may be vulnerable to substance-induced alterations.
Aims:
We measure OFC thickness (CT), surface area (SA), and volume prior to the onset of use to predict cannabis involvement during an average 9-year follow-up.
Methods:
Adolescents (N=118) aged 12–15 completed baseline behavioral assessment and magnetic resonance imaging scans, then were followed up to 13 years with annual substance use interviews. Logistic regression examined baseline (pre-substance use) bilateral medial and lateral OFC characteristics (volume, SA, or CT) as predictors of regular cannabis use by follow-up. Post-hoc multinomial logistic regression assessed whether OFC characteristics significantly predicted either alcohol use alone or cannabis+alcohol co-use. Brain-behavior relationships were assessed through follow-up correlations of baseline relationships between OFC and executive functioning, reward responsiveness, and behavioral approach traits.
Results:
Larger left lateral OFC (LOFC) volume predicted classification as cannabis user by follow-up (p=.025, OR=1.808). LOFC volume also predicted cannabis+alcohol co-user status (p=.008, OR=2.588), but not alcohol only status. Larger LOFC volume positively correlated with greater baseline reward responsiveness (p=.030, r=.348). There were no significant results by SA or CT (ps>.05).
Conclusions:
Larger left LOFC measured from ages 12–15 and prior to initiation of substance use was related to greater reward responsiveness at baseline and predicted classification as a cannabis user and cannabis+alcohol co-user by final follow-up. Larger LOFC volume may represent aberrant OFC maturation and increasing vulnerability for later substance use.
Keywords: Cannabis, Orbitofrontal Cortex, Cannabis Use Onset, Alcohol, Reward Response
Introduction
By 12th grade (typically age 17–18), 45% of U.S. students have tried cannabis, yet only 29% report that regular cannabis use is harmful (Schulenberg et al., 2018). While longitudinal studies are limited, regular cannabis use is associated with neurocognitive performance decrements and alterations in brain morphometry and neural functioning (for review, see (Gonzalez et al., 2017; Meier et al., 2012; Jacobus et al., 2015a), though genetics, socioeconomic status, and recency of use may play a significant role in behavioral outcomes (Meier et al., 2018; Scott et al., 2018). Adolescents may be more susceptible to neurotoxic influences (Spear, 2000; Schneider, 2008) as the brain, and the endocannabinoid system in particular (Mechoulam and Parker, 2013), undergoes vast change during this developmental period (Giedd and Rapoport, 2010; Shaw et al., 2008; Gogtay et al., 2004). Despite the high prevalence of cannabis use in youth and growing efforts to understand the effect of cannabis on neurodevelopment and cognition in adolescents and young adults, knowledge of neurobiological predictors of cannabis use onset remains scarce.
The orbitofrontal cortex (OFC) is postulated as vital to reward sensitivity and impulsivity (Dom et al., 2005; Costumero et al., 2013) and implicated at all stages of addiction (Volkow and Fowler, 2000). Sensitivity to reward, in turn, is strongly associated with substance use behaviors (Grant and Chamberlain, 2014). The OFC has multiple hypothesized roles in reward processing, including encoding rewards, reappraisal of stimuli, controlling inhibitory response, emotional appraisal, and decision making (Fettes et al., 2017; Walton et al., 2011). It is subdivided into medial and lateral sections that are anatomically and functionally distinct, as lateral OFC contributes to choice value, prediction errors, extinction and devaluation, while medial OFC is active in estimating relative subjective value and responding to reward (Tekin and Cummings, 2002; Fettes et al., 2017).
In cross-sectional studies of adolescents and emerging adults, cannabis use has been linked to smaller OFC volume (Battistella et al., 2014; Price et al., 2015; Churchwell et al., 2010), though not consistently (Lorenzetti et al., 2015; Chye et al., 2017). Teen cannabis users have similarly shown thinner cortices in frontal regions (Lopez-Larson et al., 2011). Other studies have found thicker OFC in cannabis users, though some of these findings have not survived correction for multiple comparisons (Mashhoon et al., 2015; Levar et al., 2018). Continued use of cannabis over time has also been linked to increased cortical thickness in frontal regions (Jacobus et al., 2015b; Epstein and Kumra, 2015). Functionally, adolescents with history of cannabis use disorder (CUD) show different OFC resting state connectivity patterns (Camchong et al., 2017), including hypoactivation to rewarded outcomes after making risky decisions and hyperactivation to no-reward-outcomes compared to non-users (De Bellis et al., 2013). OFC activation patterns during a psychotherapy intervention have also been shown to predict substance-related behavioral change (Feldstein Ewing et al., 2017).
Early features of the OFC, then, may be potential biomarkers of risk for cannabis use onset, as has been argued by others (Whelan et al., 2012). Indeed, smaller OFC volumes in pre-teens predicted cannabis initiation four years later (Cheetham et al., 2012), and volumetric differences in OFC and other frontal regions predicted later problematic drinking and substance use disorder (Cheetham et al., 2014; Cheetham et al., 2017).
Three primary cortical components are often considered as measures of neuroanatomy: cortical thickness, surface area, and volume. Each component demonstrates a different developmental trajectory (Ostby et al., 2009; Wierenga et al., 2014). Cortical thickness follows a linear curve, surface area is cubic, and volume (the product of thickness and area) is quadratic (Wierenga et al., 2014). Cortical thickness and surface area differ in neuroarchitecture (Wierenga et al., 2014), as it has been suggested that surface area is determined by the number of cortical columns while the number of cells within a column determines cortical thickness (Rakic, 1995). As each component may uniquely stand as a biomarker, each measurement is individually considered in this investigation.
Understanding neurobiological factors that predict cannabis use onset in adolescents may focus the development of prevention and intervention efforts (Volkow et al., 2015; Volkow et al., 2016). To this end, we aim to investigate whether OFC estimates of thickness, surface area, and volume prior to substance use initiation (ages 12–15) predict cannabis use over a nine-year follow-up period. Based on previous findings (Cheetham et al., 2017; Cheetham et al., 2012), we hypothesize that smaller surface area, smaller volume, and thinner OFC at baseline will predict increased probability of classification as a regular (weekly) cannabis user at a follow-up appointment (ages 14–26). In addition, we expected smaller surface area, smaller volume, and thinner OFC would predict broader substance use, including heavy alcohol use, as has been found previously (Cheetham et al., 2017).
Methods
Participants.
One hundred and eighteen participants were selected from a larger prospective study of adolescent substance use (N=295). All participants were between the ages of 12–15 at baseline, recruited from local San Diego area schools and were followed for up to 13 years. At baseline, participants underwent structural and functional brain magnetic resonance imaging (MRI) scanning, neuropsychological assessment, and detailed assessment of substance use, mental health, and other life events, with annual follow-up consisting of detailed substance use assessment. Here, we focus on follow-up substance use information collected 9 years post baseline assessment (on average) as the outcome of interest. Structural imaging data collected at baseline (cortical thickness, area, and volume) from medial and lateral OFC were examined as predictors of substance use outcomes. All participants underwent written informed consent (or assent if under age 18 and consent from their guardians) in accordance with the University of California, San Diego Human Research Protections Program.
Baseline Exclusion Criteria and Groups at Follow-Up.
Exclusion criteria for all participants at baseline included: more than two drinks of alcohol per week in their lifetime; any history of illicit drug use; history of substance use disorder; diagnosis of a primary DSM-IV Axis I psychiatric disorder other than conduct disorder; left-handedness; learning disability; history of head trauma or serious neurological disorder; serious physical health problems; use of psychotropic medications that alter brain function and/or blood flow; family history of bipolar I disorder or schizophrenia within a first-degree relative; antisocial personality disorder in either parent; color blindness; prenatal medical issues or exposure to substance use; claustrophobia; metal implants; or pregnancy.
All follow-up substance use data was examined and participants were categorized to clearly differentiate regular cannabis initiators from those with minimal to no use of cannabis, which resulted in two sub-samples: 1) cannabis users (CU, n=50), defined as endorsing one year of regular cannabis use (≥50 past-year cannabis use episodes); and 2) those with minimal to no cannabis use (MCU n=68), defined as having no more than 5 past-year cannabis use episodes at any follow-up interview, never having engaged in regular weekly cannabis use, and having fewer than 50 lifetime cannabis use episodes. Neither group could have more than 50 lifetime other drug use episodes. Alcohol use was not considered in cannabis group categorization.
Measures
Substance Use.
The Customary Drinking and Drug Use Record (CDDR) was used to assess lifetime alcohol, cannabis, cigarette, and other drug use (Brown et al., 1998) defined as cumulative use (e.g., alcohol, cannabis) and episodes (i.e., number of days) reported at study entry. For other substance use, participants were individually asked about each of the following: Amphetamines, barbiturates, hallucinogens, inhalants, benzodiazepines, opiates, ecstasy, ketamine, PCP, GHB, or “any other drug” which was then recoded into the appropriate category. Substance use patterns were recorded at baseline and each annual follow-up. Consistent with prior work (Jacobus et al., 2016; Silins et al., 2015; Guttmannova et al., 2017; Pfefferbaum et al., 2016), subjects were classified as CU or MCU, based on past-year and lifetime cannabis consumption.
Demographics, Emotional and Executive Functioning.
To identify and exclude those individuals with Axis-I disorders other than conduct disorder, the Diagnostic Interview Schedule for Children (DISC) Predictive Scales (DPS; Lucas et al., 2001; Shaffer et al., 1996) was administered to youth and parent at screening. During baseline study participation, parental income was reported during a clinical interview prior to the baseline imaging session. Parents also completed the Family History Assessment Module (Rice et al., 1995), which assessed family history of psychiatric and substance use disorders. Participants completed the Behavioral Inhibition System and Behavioral Approach System (BIS/BAS; (Carver and White, 1994)). BIS/BAS measures approach and avoidance behaviors of moving towards/away from appetitive or unpleasant stimuli, respectively, through five subscales: total approach, total avoidance, reward responsiveness, drive, and fun seeking. In addition, participants completed subtests from the Delis-Kaplan Executive Function System (D-KEFS; (Delis et al., 2001)), including Color-Word Interference, Trails, and Tower Task, as measures of executive functioning. The Wide Range Achievement Test (WRAT-3) Word Reading subtest was included as an estimate of premorbid intellectual functioning (Wilkinson, 1993).
MRI Acquisition and Processing.
Prior publications detail MRI acquisition and processing (Jacobus et al., 2014); a brief description also follows. All processing was completed within our laboratory. A 3.0 Tesla CXK4 short bore Excite-2 magnetic resonance system (General Electric, Milwaukee, WI) with an eight-channel phase array head coil was used to acquire each scan at the University of California San Diego Center. A high-resolution T1-weighted anatomical spoiled gradient recall (SPGR) scan was acquired (TE/TR=min full, field of view=24 cm, resolution=1mm3, 170 continuous slices).
Neuroimaging data processing used FreeSurfer software (version 5.1, surfer.nmr.mgh.harvard.edu) to compute cortical surface reconstruction, volume, and thickness estimates (Dale et al., 1999). Standard preprocessing steps were conducted, including motion correction and averaging of T1 weighted images, removal of non-brain tissue and transformation to standardized space, segmentation of subcortical white and deep gray matter structures, intensity normalization, and tessellation of the gray/white matter boundary. A surface deformation algorithm places smooth borders differences in tissues classes, as indicated by the greatest shift in intensity and as it is guided by local MRI intensity gradients (Dale et al., 1999). Thus, submillimeter group differences are quantified (Fischl and Dale, 2000).
Distance from the gray/white matter boundary to the gray matter/cerebral spinal fluid boundary at each cortical surface vertex was used to calculate cortical thickness (Fischl and Dale, 2000). This process of cortical thickness measurement has been deemed valid and verified using histological analysis and manual measurements (Kunerberg et al., 2003). The entire cortex was also parcellated by gyral and sulcal regions to calculate surface area.
While blind to participant characteristics, one rater (JJ) followed reconstruction edit procedures to correct errors made during cortical reconstruction, including verifying the automated skull stripping and conducting a coronal plane slice-by-slice inspection of gray/white matter and gray matter/cerebral spinal fluid surfaces. Tissue misclassifications, such as residual dura matter classified as cortex, were modified as needed for correction.
Data Analysis.
Primary Analyses.
Analyses of variance (ANOVA) were run between groups to evaluate differences on demographic and substance use variables. CU and MCU differed by age at baseline, which was included as covariates in regression analyses. In the primary analysis, logistic regression predicted cannabis group classification at follow-up by bilateral medial and lateral OFC volume, surface area, and cortical thickness, controlling for age at baseline, time to follow-up, intracranial volume (ICV), and family history of substance/alcohol use disorder. Twelve logistic regressions analyses in total were conducted to assess each hemisphere (right and left), subregion (medial and lateral region), and structural characteristics (volume, surface area, and cortical thickness).
Selection of Covariates.
To account for other factors that may be significantly related to OFC metrics and/or substance use onset, four covariates were selected. Family history of SUD was included as it has previously been associated with altered neuromaturation, even in substance-naïve adolescents (Cservenka, 2016) and increased risk of substance initiation (Gray and Squeglia, 2018). As adolescence marks a unique period of neurodevelopment (Giedd et al., 2015; Gogtay et al., 2004) and participants were between the ages of 12 and 15 at baseline with varying lengths of follow-up, both age at baseline and time to follow-up were also included as covariates. ICV was included as a proxy for headsize, as this may influence volumetric differences in some individuals (Barnes et al., 2010).
Post-hoc Cannabis Analyses.
To assess the potential for significant OFC metrics as a predictor not just of use but of level of use, correlational analyses were used to determine whether significant OFC metrics (i.e., baseline left lateral OFC volume) correlated with number of either past-year or lifetime cannabis use episodes.
Post-hoc Alcohol Analyses.
Multinomial regression analyses examined the influence of significant baseline OFC regional predictors on alcohol use status in these groups by follow-up. For these analyses, three-groups (n=96) were subsequently defined for the alcohol status outcome variable and included: 1) a control group (n=23) that consisted of individuals who had used cannabis less than 5 times in the past year, had not binged nor had used alcohol more than 12 times in the past year, 2) an alcohol only group (n=25), where individuals had engaged in at least one binge episode and drank alcohol at least every other week and who had not used cannabis more than 5 times in the past year, and 3) a cannabis and alcohol group, consisting of individuals who, in the past year, had used cannabis at least 50 times, had engaged in binge drinking at least once, and drank alcohol at least every other week on average (n=48). Participants who did not fall into one of the three groups (n=22) were not included in the alcohol post-hoc analyses. For example, those individuals reporting some infrequent alcohol use were not able to be classified as a control or regular alcohol user based on our criteria above and therefore were not included in these analyses.
Results
Demographics.
Mean age at baseline assessment was 13.48±0.71 years and mean age at last follow-up assessment was 22.22±2.03 years, with an average of 8.74±1.93 total years in the study (see Table 1). Cannabis Users (n=50) included those who initiated regular (≥50 past year episodes, averaging at least weekly use for a year) cannabis use by final follow-up. MCU (n=68) were cannabis-naïve at baseline and had used cannabis less than 5 times in the last year at their final follow-up and used cannabis less than 50 times in their lifetime. CU differed significantly from MCU in lifetime alcohol use [F(1,116)=23.55, p<.001], lifetime cannabis use [F(1,116)=84.69, p<.001], past year cannabis use [F(1,116)=200.54, p<.001], and lifetime other drug use [F(1,116)=41.50, p<.001] by follow-up. They also differed by baseline age [F(1,116)=8.73, p=.004]. Baseline OFC surface area, volume, and cortical thickness did not significantly differ between Cannabis Users and MCU.
Table 1.
Demographics and Substance Use Characteristics
| CU (n=50) M (SD) range |
MCU (n=68) M (SD) range |
|
|---|---|---|
| Age, Baseline* | 13.70 (.69) 12.39–15.07 |
13.32 (.68) 12.13–14.71 |
| Age, Follow-Up | 22.11 (2.01) 16.11–26.84 |
22.30 (2.06) 14.40–26.52 |
| Est. IQ (WRAT-3), Baseline | 111.54 (10.26) 80–132 |
112.50 (10.02) 83–132 |
| % Female | 66% | 50% |
| % Hispanic | 24% | 24% |
| % Caucasian | 64% | 69% |
| Total length of follow-up interval in years | 8.41 (2.00) 2.99–12.02 |
8.98 (1.86) 1.98–13.14 |
| Number of follow-up visits | 9.02 (2.05) 4–13 |
9.65 (1.73) 4–13 |
| BDI, Baseline | 1.26 (2.28) 0–9 |
1.28 (2.47) 0–15 |
| Family History of SUD/AUD | .23 (.28) 0–1 |
.17 (.29) 0–1 |
| Cannabis use episodes, Baseline | -- | -- |
| Lifetime cannabis use episodes, Follow-Up* | 624.18 (555.44) 50–2518 |
5.24 (8.78) 0–39 |
| Past year cannabis use episodes, Follow-Up* | 197.20 (114.66) 50–381 |
.59 (1.11) 0–4 |
| % CUD Diagnosis, Follow-Up | 6% | -- |
| Age of Onset of Regular Cannabis Use | 18.78 (1.90) 15–23 |
-- |
| Length of Regular Cannabis Use, Years | 3.33 (2.09) .04–8.64 |
-- |
| Alcohol use days, Baseline | .08 (.40) 0–2 |
.02 (.12) 0–1 |
| Lifetime alcohol use days, Follow-Up* | 437.88 (354.80) 40–1427 |
170.43 (243.92) 0–1113 |
| Past year alcohol use days, Follow-Up* | 118.80 (100.95) 7–443 |
57.04 (71.29) 0–337 |
| Past year binge episodes, Follow-Up* | 27.70 (48.37) 0–206 |
6.00 (11.92) 0–72 |
| Lifetime other drug Use, Follow-Up* | 13.50 (15.79) 0–49 |
0.87 (3.09) 0–19 |
| Left Lateral OFC Volume | 9498.18 (1089.36) 7317–11361 |
9136.40 (1128.65) 6946–11423 |
| Right Lateral OFC Volume | 9640.84 (1230.05) 6287–11400 |
9378.12 (1187.16) 6785–12458 |
| Left Medial OFC Volume | 6384 (1139.00) 4608–8754 |
6407.78 (958.78) 4587–8790 |
| Right Medial OFC Volume | 6370.28 (861.95) 4635–8493 |
6338.26 (683.23) 5179–8239 |
| Left Lateral OFC Cortical Thickness | 2.80 (.18) 2.39–3.15 |
2.77 (.18) 2.32–3.10 |
| Right Lateral OFC Cortical Thickness | 2.83 (.22) 2.19–3.33 |
2.81 (.16) 2.42–3.18 |
| Left Medial OFC Cortical Thickness | 2.73 (.19) 2.22–3.06 |
2.74 (.17) 2.38–3.27 |
| Right Medial OFC Cortical Thickness | 2.72 (.21) 2.18–3.08 |
2.73 (.17) 2.34–3.18 |
| Left Lateral OFC Surface Area | 2867.94 (325.99) 2169–3382 |
2777.13 (316.04) 2099–3478 |
| Right Lateral OFC Surface Area | 2938.92 (329.38) 2263–3383 |
2870.28 (330.56) 2193–3682 |
| Left Medial OFC Surface Area | 1966.020 (344.28) 1506–2661 |
1961.50 (286.80) 1329–2716 |
| Right Medial OFC Surface Area | 1945.36 (275.00) 1418–2618 |
1945.35 (248.55) 1529–2658 |
Notes:
p<.05
CU=Cannabis Users; MCU=Minimal Cannabis User; OFC=Orbitofrontal Cortex; BDI=Beck Depression Inventory; SUD=Substance Use Disorder; AUD=Alcohol Use Disorder; CUD=Cannabis Use Disorder; Regular Cannabis Use is defined as weekly cannabis use
Primary Aims.
Baseline left lateral OFC volume (Wald’s χ2 = 5.012, p = .025; OR=1.808, CI: 1.077–3.036; see Figure 1) predicted cannabis group classification, such that individuals with larger volume at baseline were more likely to have initiated regular cannabis use by follow up, controlling for age at baseline, time to follow-up, ICV, and family history of SUD/AUD. In addition, being older at baseline predicted cannabis group status (Wald’s χ2 = 8.635, p = .003; OR=2.510, CI: 1.359–4.636). Neither surface area nor cortical thickness significantly predicted cannabis use status by follow-up.
Figure 1.
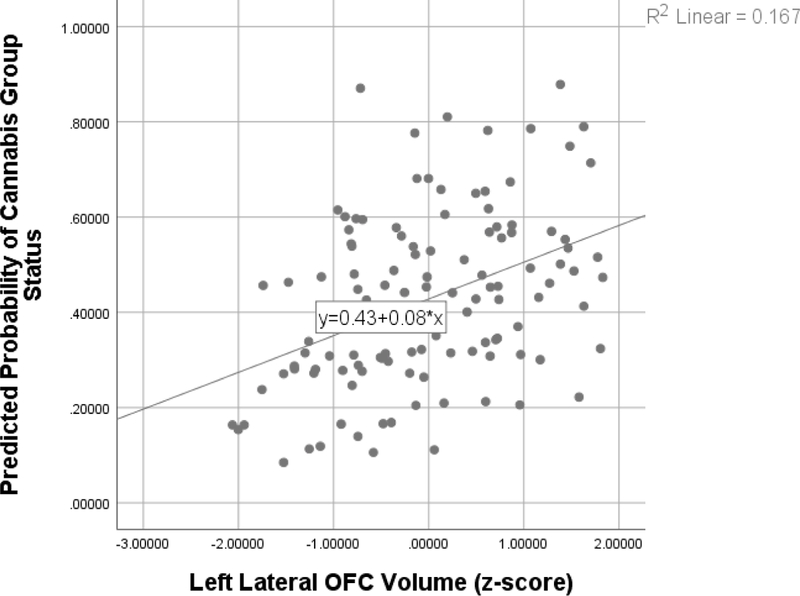
Scatterplot of relationship between baseline left lateral OFC volume and predicted odds (as determined through logistic regression) of initiating regular cannabis use by average 9-year follow-up.
Post-hoc Analyses.
Correlational analyses assessed whether either lifetime or past year cannabis use was associated with baseline left lateral OFC volume. Neither relationship was significant (lifetime: p=.44, r=.07; past year: p=.11, r=.15).
A multinomial logistic regression was performed to assess the relationship between baseline left lateral OFC volume and membership in an alcohol-use group as defined above (i.e., controls, alcohol only, and alcohol+cannabis). Larger baseline left lateral OFC volume predicted alcohol+cannabis group status relative to controls [Wald’s χ2 =7.102, p = 0.008; OR = 2.588, CI: 1.286–5.207], with no significant results between controls and alcohol only [Wald’s χ2 = 2.4111, p = 0.120; OR = 1.796, CI: .858–3.760] or alcohol only and alcohol+cannabis [Wald’s χ2 = 1.120, p = 0.290; OR = .694, CI: .353–1.365]. Older age at baseline [Wald’s χ2 = 5.869, p = 0.015; OR = 2.987, CI: 1.232–7.238] also predicted alcohol+cannabis status relative to controls. Results remain the same whether or not other substance use is included as a covariate.
Exploratory Analysis.
Bivariate correlations were conducted between left lateral OFC volume estimates and measures of cognitive and behavioral control (i.e., DKEFS subtests and BIS/BAS) in the Cannabis Users group. Full results are presented in Table 2. Baseline left lateral OFC positively correlated with baseline BIS/BAS Reward Responsiveness in the Cannabis Users (r=.348, p=.030) in that greater volume was associated with greater reward responsiveness. No significant relationships were found between left lateral OFC volume and other BIS/BAS or executive functioning measures at baseline (ps>.05).
Table 2.
Exploratory Correlations Between Baseline Left Lateral OFC Volume and Cognitive and Behavioral Control Factors in the Cannabis Users Group
| p | r | |
|---|---|---|
| D-KEFS | ||
| Towers – Total Achievement | .57 | .08 |
| Color-Word Interference – Inhibition | .51 | .10 |
| Color-Word Interference – Inhibition/Switching | .17 | .20 |
| Trails – Switching | .26 | .16 |
| BIS/BAS | ||
| Drive | .87 | .03 |
| Fun Seeking | .60 | .09 |
| Reward Responsiveness | .03 | .35 |
| BAS Total | .20 | .21 |
| BIS Total | .42 | .13 |
Note: All D-KEFS scores represent scaled score value, while BIS/BAS scores represent total raw scores for each subcategory
Discussion
This study investigated structural characteristics of orbitofrontal regions from adolescents ages 12–15 prior to substance use initiation (i.e., cannabis and alcohol) as a predictor of regular cannabis use onset monitored over a period of nine years, on average. Novel findings indicate larger left lateral OFC volume predicted cannabis use group status (individuals who used cannabis at least weekly) in later adolescence/young adulthood, in contrast to prior studies of smaller OFC volume predicting cannabis use (Cheetham et al., 2017; Cheetham et al., 2012). Further, larger left lateral OFC volume uniquely predicted classification as a heavier substance user (i.e., cannabis and alcohol co-use), and was not related to classification as an alcohol user only. Baseline left lateral OFC volume also positively correlated with baseline reward responsiveness. However, neither surface area nor cortical thickness predicated cannabis use group status. The odds ratios presented here suggest small to medium effect sizes; together these results suggest lateral OFC volume may be one biomarker of vulnerability to regular use of cannabis and alcohol, or heavier substance use patterns in general.
The lateral OFC contains axonal projections to the striatum, as well as connections with sensory networks (Fettes et al., 2017). Functionally, lateral OFC plays a greater role in cognitive control regions and reversal learning, while the medial OFC is central in evaluating and assigning hedonic value, key for reward encoding (Fettes et al., 2017). It is possible that lateral OFC characteristics measured prior to substance use initiation may predict reward-based learning for substance use behaviors.
Our results are consistent with findings linking neurostructural characteristics of the OFC to substance use behaviors in adolescents and young adults (Cheetham et al., 2014; Cheetham et al., 2017; Cheetham et al., 2012), albeit directionally different. These results are also unique in that volume, surface area, and cortical thickness were simultaneously assessed, rather than only examining volume, as other studies have done (Cheetham et al., 2014; Cheetham et al., 2017; Cheetham et al., 2012). For example, a similar study found smaller lateral OFC volume predicted cannabis use onset in adolescents over a 4-year follow up (Cheetham et al., 2012), with onset defined as any cannabis use by follow-up. A more recent study by the same research team also found that smaller OFC volume predicted lifetime history of substance use disorder diagnosis by age 18, including cannabis use disorder (Cheetham et al., 2017). In both of these studies, only 22–23% of the sample had transitioned to substance use/disorder and the baseline time point was restricted to only 12-year-olds. In contrast, 42% of our sample transitioned to cannabis use and only 6% of users had a diagnosis of CUD (3% of total sample). Our inclusion of a broader baseline age range prior to substance use initiation (up to age 15) may provide new insight into how alterations in the pattern of cortical development beyond age 12 may relate to neural vulnerability and behavioral outcomes. For example, decreases in estimates of cortical volume are typically expected during neuromaturation (Giedd and Rapoport, 2010). Given prefrontal volume peaks around age 10.5–11 for girls and 11.5–12 for boys and then declines (Lenroot et al., 2007; Pfefferbaum et al., 2016; Vijayakumar et al., 2016), our findings suggest that youth who go on to regularly use cannabis use may have a different neuromaturational trajectory in volumetric growth or may begin neuronal pruning processes later than optimal, putting them at greater risk of less efficient neural processing in reward networks and thus substance use initiation.
Further, consistent with proposed theories of OFC function (Dom et al., 2005; Costumero et al., 2013; Fettes et al., 2017), our results suggest a significant correlation between larger left lateral OFC volume and reward responsiveness at baseline. The combined results may represent a vulnerability of the OFC to both sensitivity to reward and substance use onset which may combine to increase risk of substance use disorder, consistent with theories of addiction (Koob and Volkow, 2010; Volkow and Fowler, 2000; Jordan and Andersen, 2017).
Here we did not find surface area or cortical thickness of the OFC to be significant predictors of cannabis use onset, despite the fact that the product of these two characteristics (i.e., volume) predicted onset. Volume may be accounting for cellular (Rakic, 1995) and genetic (Panizzon et al., 2009) determinants that are not readily captured by surface area or cortical thickness alone. Volume, then, may be a sensitive measure to underlying histological changes that nuanced factors of surface area and cortical thickness do not reveal when assessed alone. Thus, as surface area, cortical thickness, and volume may elucidate unique patterns that will not always be captured by just one factor (Raznahan et al., 2011; Winkler et al., 2010; Infante et al., 2018), consideration of all neuroanatomical structural characteristics is warranted.
As in prior studies (Cheetham et al., 2017; Cheetham et al., 2012), the present sample of cannabis users also had high levels of alcohol, cigarette, and other drug use. This raises the question of whether the findings better explain general vulnerability to substance use, alcohol use, or cannabis use alone. Given post-hoc analysis of left lateral OFC predicting alcohol and cannabis group status relative to controls, with no significant prediction of the alcohol only group, it appears that, at minimum, larger left lateral OFC predicts heavy substance use if not cannabis use in particular.
Our findings should be interpreted in light of several considerations. Cannabis users had higher levels of both alcohol use and lifetime other drug use by follow-up, though both groups were naïve at baseline. Nevertheless, it cannot be ruled out that lateral OFC volume predicts overall substance use behaviors, rather than uniquely being related to cannabis use onset. Here we investigated aspects of neuroanatomical structure in one specific brain region, OFC, finding a modest effect; other regions and functional relationships are also likely important factors in substance use onset. Future research should include multi-modal imaging techniques. While all twelve primary analyses were planned a priori, it is important to note that multiple comparisons corrections were not applied and therefore replication is needed. Neurodevelopment and substance use risk are both influenced by many environmental and genetic factors (Fjell et al., 2015; Creze et al., 2014; Gray and Squeglia, 2018; Jordan and Andersen, 2017; Giedd et al., 2015), and how these factors may directly or indirectly influence neuromaturation trajectories and behavioral outcomes associated with the OFC will be further investigated in future work (Jernigan et al., 2018). Finally and consistent with other studies (Rosen et al., 2018), participants in this study were relatively high-functioning healthy individuals (e.g., non-treatment seeking).
Our study builds on the existing literature, suggesting larger lateral OFC volume prior to substance use initiation related to early reward responsiveness traits and future cannabis use onset. Specifically, larger left lateral OFC volume predicted onset of regular cannabis use and heavier substance use patterns in general (cannabis+alcohol) in adolescents followed over an average of nine years. Future studies are needed to provide additional information on biological and psychological risk factors that predispose adolescents to initiating cannabis use. Such information will inform novel prevention and intervention efforts that aim to prevent and/or reduce problematic substance use.
Acknowledgements
The authors extend appreciation to the San Diego Unified School District and to the families who participated.
Funding Acknowledgements
Research reported in this publication was supported by the National Institute on Alcohol Abuse and Alcoholism of the National Institutes of Health under Award Numbers: R01 AA13419, T32 AA13525 (PI: Riley/Tapert to Wade), KL2 TR001444 (PI: Jacobus), 5K12 DA000357 (PI: Bagot), K23 AA025399 (PI: Squeglia).
Footnotes
The Authors declare that there is no conflict of interest.
References
- Barnes J, Ridgway GR, Bartlett J, et al. (2010) Head size, age and gender adjustment in MRI studies: a necessary nuisance? Neuroimage 53: 1244–1255. [DOI] [PubMed] [Google Scholar]
- Battistella G, Fornari E, Annoni JM, et al. (2014) Long-term effects of cannabis on brain structure. Neuropsychopharmacology 39: 2041–2048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown, Myers MG, Lippke K, et al. (1998) Psychometric evaluation of the customary drinking and durg use record (CDDR): A measure of adolescent alcohol and drug involvement. Journal of Studies on Alcohol 59: 427–438. [DOI] [PubMed] [Google Scholar]
- Camchong J, Lim KO and Kumra S. (2017) Adverse Effects of Cannabis on Adolescent Brain Development: A Longitudinal Study. Cereb Cortex 27: 1922–1930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carver CS and White TL. (1994) Behavioral inhibition, behavioral activation, and affective responses to impending reward and punishment: The BIS/BAS scales. Journal of Personality and Social Psychology 67: 319–333. [Google Scholar]
- Cheetham A, Allen NB, Whittle S, et al. (2014) Volumetric differences in the anterior cingulate cortex prospectively predict alcohol-related problems in adolescence. Psychopharmacology (Berl) 231: 1731–1742. [DOI] [PubMed] [Google Scholar]
- Cheetham A, Allen NB, Whittle S, et al. (2017) Orbitofrontal Cortex Volume and Effortful Control as Prospective Risk Factors for Substance Use Disorder in Adolescence. Eur Addict Res 23: 37–44. [DOI] [PubMed] [Google Scholar]
- Cheetham A, Allen NB, Whittle S, et al. (2012) Orbitofrontal volumes in early adolescence predict initiation of cannabis use: a 4-year longitudinal and prospective study. Biol Psychiatry 71: 684–692. [DOI] [PubMed] [Google Scholar]
- Churchwell JC, Lopez-Larson M and Yurgelun-Todd DA. (2010) Altered frontal cortical volume and decision making in adolescent cannabis users. Front Psychol 1: 225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chye Y, Solowij N, Suo C, et al. (2017) Orbitofrontal and caudate volumes in cannabis users: a multi-site mega-analysis comparing dependent versus non-dependent users. Psychopharmacology (Berl) 234: 1985–1995. [DOI] [PubMed] [Google Scholar]
- Costumero V, Barros-Loscertales A, Bustamante JC, et al. (2013) Reward sensitivity modulates connectivity among reward brain areas during processing of anticipatory reward cues. Eur J Neurosci 38: 2399–2407. [DOI] [PubMed] [Google Scholar]
- Creze M, Versheure L, Besson P, et al. (2014) Age- and gender-related regional variations of human brain cortical thickness, complexity, and gradient in the third decade. Hum Brain Mapp 35: 2817–2835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cservenka A (2016) Neurobiological phenotypes associated with a family history of alcoholism. Drug Alcohol Depend 158: 8–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale AM, Fischl B and Sereno MI. (1999) Cortical surface-based analysis. Neuroimage 9: 179–194. [DOI] [PubMed] [Google Scholar]
- De Bellis MD, Wang L, Bergman SR, et al. (2013) Neural mechanisms of risky decision-making and reward response in adolescent onset cannabis use disorder. Drug Alcohol Depend 133: 134–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delis DC, Kaplan E and Kramer JH. (2001) Delis-Kaplan Executive Function System (D-KEFS). , San Antonio, TX: The Psychological Corporation. [Google Scholar]
- Dom G, Sabbe B, Hulstijn W, et al. (2005) Substance use disorders and the orbitofrontal cortex. British Journal of Psychiatry 187: 209–220. [DOI] [PubMed] [Google Scholar]
- Epstein KA and Kumra S. (2015) Altered cortical maturation in adolescent cannabis users with and without schizophrenia. Schizophr Res 162: 143–152. [DOI] [PubMed] [Google Scholar]
- Feldstein Ewing SW, Chung T, Caouette JD, et al. (2017) Orbitofrontal cortex connectivity as a mechanism of adolescent behavior change. Neuroimage 151: 14–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fettes P, Schulze L and Downar J. (2017) Cortico-Striatal-Thalamic Loop Circuits of the Orbitofrontal Cortex: Promising Therapeutic Targets in Psychiatric Illness. Front Syst Neurosci 11: 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl B and Dale AM. (2000) Measuring the thickness of the human cerebral cortex from magnetic resonance imaging. PNAS 97: 11050–11055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fjell AM, Grydeland H, Krogsrud SK, et al. (2015) Development and aging of cortical thickness correspond to genetic organization patterns. Proc Natl Acad Sci U S A 112: 15462–15467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giedd JN and Rapoport JL. (2010) Structural MRI of pediatric brain development: what have we learned and where are we going? Neuron 67: 728–734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giedd JN, Raznahan A, Alexander-Bloch A, et al. (2015) Child psychiatry branch of the National Institute of Mental Health longitudinal structural magnetic resonance imaging study of human brain development. Neuropsychopharmacology 40: 43–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gogtay N, Giedd JN, Lusk L, et al. (2004) Dynamic mapping of human cortical development during childhood through early adulthood. PNAS 101: 8174–8179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez R, Pacheco-Colon I, Duperrouzel JC, et al. (2017) Does Cannabis Use Cause Declines in Neuropsychological Functioning? A Review of Longitudinal Studies. J Int Neuropsychol Soc 23: 893–902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant JE and Chamberlain SR. (2014) Impulsive action and impulsive choice across substance and behavioral addictions: cause or consequence? Addict Behav 39: 1632–1639. [DOI] [PubMed] [Google Scholar]
- Gray KM and Squeglia LM. (2018) Research Review: What have we learned about adolescent substance use? J Child Psychol Psychiatry 59: 618–627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guttmannova K, Kosterman R, White HR, et al. (2017) The association between regular marijuana use and adult mental health outcomes. Drug Alcohol Depend 179: 109–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Infante MA, Courtney KE, Castro N, et al. (2018) Adolescent brain surface area pre- and post-cannabis and alcohol initiation. J Stud Alcohol Drugs 79: 835–843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobus J, Castro N, Squeglia LM, et al. (2016) Adolescent cortical thickness pre- and post marijuana and alcohol initiation. Neurotoxicol Teratol 57: 20–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobus J, Squeglia LM, Infante MA, et al. (2015a) Neuropsychological performance in adolescent marijuana users with co-occurring alcohol use: A three-year longitudinal study. Neuropsychology 29: 829–843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobus J, Squeglia LM, Meruelo AD, et al. (2015b) Cortical thickness in adolescent marijuana and alcohol users: A three-year prospective study from adolescence to young adulthood. Dev Cogn Neurosci 16: 101–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobus J, Squeglia LM, Sorg SF, et al. (2014) Coritcal thickness and neurocognition in adolescent marijuana and alcohol users following 28 days of monitored abstinence. Journal of Studies on Alcohol and Drugs 75: 729–743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jernigan TL, Brown SA and Coordinators AC. (2018) Introduction. Dev Cogn Neurosci 32: 1–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordan CJ and Andersen SL. (2017) Sensitive periods of substance abuse: Early risk for the transition to dependence. Dev Cogn Neurosci 25: 29–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF and Volkow ND. (2010) Neurocircuitry of Addiction. Neuropharmacology 35: 217–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenroot RK, Gogtay N, Greenstein DK, et al. (2007) Sexual dimorphism of brain developmental trajectories during childhood and adolescence. Neuroimage 36: 1065–1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levar N, Francis AN, Smith MJ, et al. (2018) Verbal Memory Performance and Reduced Cortical Thickness of Brain Regions Along the Uncinate Fasciculus in Young Adult Cannabis Users. Cannabis Cannabinoid Res 3: 56–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Larson MP, Bogorodzki P, Rogowska J, et al. (2011) Altered prefrontal and insular cortical thickness in adolescent marijuana users. Behav Brain Res 220: 164–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorenzetti V, Solowij N, Whittle S, et al. (2015) Gross morphological brain changes with chronic, heavy cannabis use. Br J Psychiatry 206: 77–78. [DOI] [PubMed] [Google Scholar]
- Mashhoon Y, Sava S, Sneider JT, et al. (2015) Cortical thinness and volume differences associated with marijuana abuse in emerging adults. Drug Alcohol Depend 155: 275–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mechoulam R and Parker LA. (2013) The endocannabinoid system and the brain. Annu Rev Psychol 64: 21–47. [DOI] [PubMed] [Google Scholar]
- Meier MH, Caspi A, Ambler A, et al. (2012) Persistent cannabis users show neuropsychological decline from childhood to midlife. Proc Natl Acad Sci U S A 109: E2657–2664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meier MH, Caspi A, Danese A, et al. (2018) Associations between adolescent cannabis use and neuropsychological decline: a longitudinal co-twin control study. Addiction 113: 257–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostby Y, Tamnes CK, Fjell AM, et al. (2009) Heterogeneity in subcortical brain development: A structural magnetic resonance imaging study of brain maturation from 8 to 30 years. J Neurosci 29: 11772–11782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panizzon MS, Fennema-Notestine C, Eyler LT, et al. (2009) Distinct genetic influences on cortical surface area and cortical thickness. Cereb Cortex 19: 2728–2735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfefferbaum A, Rohlfing T, Pohl KM, et al. (2016) Adolescent Development of Cortical and White Matter Structure in the NCANDA Sample: Role of Sex, Ethnicity, Puberty, and Alcohol Drinking. Cereb Cortex 26: 4101–4121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price JS, McQueeny T, Shollenbarger S, et al. (2015) Effects of marijuana use on prefrontal and parietal volumes and cognition in emerging adults. Psychopharmacology (Berl) 232: 2939–2950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rakic P (1995) A small step for the cell, a giant leap for mankind: a hypothesis of neocortical expansion during evolution. Trends Neurosci 18: 383–388. [DOI] [PubMed] [Google Scholar]
- Raznahan A, Shaw P, Lalonde F, et al. (2011) How does your cortex grow? J Neurosci 31: 7174–7177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen AS, Sodos LM, Hirst RB, et al. (2018) Cream of the Crop: Clinical Representativeness of Eligible and Ineligible Cannabis Users in Research. Subst Use Misuse 53: 1937–1950. [DOI] [PubMed] [Google Scholar]
- Schneider M (2008) Puberty as a highly vulnerable developmental period for the consequences of cannabis exposure. Addict Biol 13: 253–263. [DOI] [PubMed] [Google Scholar]
- Scott JC, Slomiak ST, Jones JD, et al. (2018) Association of Cannabis With Cognitive Functioning in Adolescents and Young Adults: A Systematic Review and Meta-analysis. JAMA Psychiatry 75: 585–595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw P, Kabani NJ, Lerch JP, et al. (2008) Neurodevelopmental trajectories of the human cerebral cortex. J Neurosci 28: 3586–3594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silins E, Fergusson DM, Patton GC, et al. (2015) Adolescent substance use and educational attainment: An integrative data analysis comparing cannabis and alcohol from three Australasian cohorts. Drug Alcohol Depend 156: 90–96. [DOI] [PubMed] [Google Scholar]
- Spear LP. (2000) The adolescent brain and age-related behavioral manifestations. Neuroscience and Biobehavioral Reviews 24: 417–463. [DOI] [PubMed] [Google Scholar]
- Tekin S and Cummings JL. (2002) Frontal-subcortical neuronal circuits and clinical neuropsychiatry: An update. Journal of Psychosomatic Research 53: 647–654. [DOI] [PubMed] [Google Scholar]
- Vijayakumar N, Allen NB, Youssef G, et al. (2016) Brain development during adolescence: A mixed-longitudinal investigation of cortical thickness, surface area, and volume. Hum Brain Mapp 37: 2027–2038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND and Fowler JS. (2000) Addiction, a disease of compusion and drive: Involvement of the orbitofrontal cortex. Cereb Cortex 10: 318–325. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Koob G and Baler R. (2015) Biomarkers in substance use disorders. ACS Chem Neurosci 6: 522–525. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Swanson JM, Evins AE, et al. (2016) Effects of Cannabis Use on Human Behavior, Including Cognition, Motivation, and Psychosis: A Review. JAMA Psychiatry 73: 292–297. [DOI] [PubMed] [Google Scholar]
- Walton ME, Behrens TE, Noonan MP, et al. (2011) Giving credit where credit is due: orbitofrontal cortex and valuation in an uncertain world. Ann N Y Acad Sci 1239: 14–24. [DOI] [PubMed] [Google Scholar]
- Whelan R, Weierstall K and Garavan H. (2012) The orbitofrontal cortex, drug use and impulsivity: Can teenage rebellion be predicted through neural correlates? Future Neurology 7. [Google Scholar]
- Wierenga LM, Langen M, Oranje B, et al. (2014) Unique developmental trajectories of cortical thickness and surface area. Neuroimage 87: 120–126. [DOI] [PubMed] [Google Scholar]
- Winkler AM, Kochunov P, Blangero J, et al. (2010) Cortical thickness or grey matter volume? The importance of selecting the phenotype for imaging genetics studies. Neuroimage 53: 1135–1146. [DOI] [PMC free article] [PubMed] [Google Scholar]


