Abstract
Objective:
several studies suggested that obsessive-compulsive disorder (OCD) patients display increased impulsivity, impaired decision-making and reward system dysfunction. In a Research Domain Criteria (RDoC) perspective, these findings are prototypical for addiction and have led some authors to view OCD as a behavioural addiction. Thus, the aim of this study was to investigate similarities and differences on impulsivity, decision-making and reward system, as core dimensions of addiction, across OCD and gambling disorder (GD) patients.
Methods:
Forty-four OCD patients, 26 GD patients and 40 healthy controls were included in the study. Impulsivity was assessed through the Barratt Impulsiveness Scale, decision-making through the Iowa Gambling Task and reward system through a self-report clinical instrument (the Shaps-Hamilton Anhedonia Scale) assessing hedonic tone and through an olfactory test assessing hedonic appraisal to odors.
Results:
both OCD and GD patients showed increased impulsivity respect to healthy controls. More specifically, cognitive impulsivity was increased in OCD patients and both cognitive and motor impulsivity were increased in GD patients. Furthermore, both OCD and GD patients showed impaired decision-making performances respect to healthy controls. Finally, GD patients showed increased anhedonia and blunted hedonic response to pleasant odors unrelated to gambling or depression/anxiety symptoms, while OCD patients showed only increased anhedonia levels related to OC and depression/anxiety symptoms.
Conclusion:
OCD patients showed several similarities and some differences with GD patients respect to healthy controls on impulsivity, decision-making and reward system, three core dimensions of addiction. These results could have relevant implications for the search of new treatment targets for OCD.
Introduction
In the last years a growing body of evidence suggested that obsessive-compulsive disorder (OCD) patients display increased impulsivity, impaired decision-making and reward system dysfunction [1–7]. In a Research Domain Criteria (RDoC) (www.nimh.nih.gov) perspective, these findings are prototypical for addiction and recently have led some authors in the last years to view OCD as a behavioral addiction. In this perspective, comparable to addiction, OCD is perceived as process, in which patients with OCD develop over time a dependency upon their compulsive behaviors because of the rewarding effect when performed perfectly or when compulsions reduce obsession-induced anxiety or distress [8]. Indeed, a recent study showed that compulsive behaviors in OCD are reward-driven, as is the case for addictive behaviors [9].
Recently, we investigated the behavioral addiction model of OCD, by assessing two core dimensions of addiction, namely impulsivity and decision-making, in OCD patients and healthy participants. Similar to common findings in addiction, OCD patients demonstrated increased cognitive impulsivity and impaired decision-making compared to healthy controls [2].
Gambling Disorder (GD), which has been included by the DSM-5 (APA, 2013) in the substance-related and addictive disorders chapter, represents the prototype of a behavioral addiction. In a RDoC perspective, increased impulsivity, risky decision-making and reward system dysfunction represents three core dimensions of GD [10]. However, no studies directly compared OCD and GD patients on these three core features of both disorders to date.
Investigating these RDoC networks across OCD and GD could be relevant in moving forward the current OCD models, mainly focused on anxiety and compulsivity, highlighting the relevance on other RDoC dimensions such as inhibitory control networks (impulsivity), decision-making processes and reward system dysfunctions, as putative treatment targets for OCD.
The goal of the present study was to investigate similarities and differences on impulsivity, decision-making and reward system, as core dimensions of addiction, across OCD patients and GD patients. Based on published reports [5;8] and our previous observations [3], we hypothesized that OCD patients, similarly to GD patients, show increased impulsivity, impaired decision-making and reward system dysfunction as compared to healthy controls.
Methods
Participants
Forty-four OCD patients, 26 GD patients and 40 healthy controls (HC) were included in the study. Patients were recruited from the Impulsive-Compulsive Disorders Unit of the University of Florence, between 2014 and 2015. Healthy controls were recruited by advertisements and word of mouth. All groups were matched for sex and age. Both the OCD group and the GD group had a primary diagnosis of OCD and GD respectively.
Inclusion criteria for both patient groups were: 1) presence of DSM-IV criteria for OCD or GD, established by a psychiatrist and confirmed by the Structured Clinical Interview for DSM-IV Axis-I Disorders / Patient Edition (SCID-I/P) [11]; 2) age between 18 and 65 years.
We excluded potential patients with any of the following conditions: 1) current DSM-IV Axis I diagnosis of a mood episode or current substance-related disorders (except for nicotine addiction), schizophrenia or other psychotic disorders, Tourette’s disorder; 2) any primary Axis II clinical diagnosis (established by a clinical interview conducted in accordance to the Structured Clinical Interview for DSM-IV Axis II Personality Disorders (SCID-II) flow-chart) [12]; 3) episodic OCD (this criterium was selected in order to avoid misdiagnosed bipolar spectrum disorder patients); 4) illness duration less than two years; 5) hospitalization in the last 6 months; 6) pharmacological treatment changes in the last 4 weeks; 7) mental disorder due to a general medical condition or history of mental retardation.
Healthy controls did not have any history of psychiatric disorders or a family history of OCD or GD, as confirmed by the SCID-I/NP (Non-patient Edition) [13]. Demographic and clinical variables of all subjects are displayed in Table 1.
Table 1. Clinical and demographic variables.
Data are expressed as percentage for categorical variables or median (interquartile range) for continuous variables.
| OCD | GD | Controls | p | |
|---|---|---|---|---|
| Age | 33 (42.75; 26) | 39 (57.25; 33.75) | 34 (48; 27) | 0.078 |
| Sex (female) | 3/26 (11.5%) | 14/44 (31.8%) | 8/40 (20%) | 0.129 |
| Years of education | 13 (17; 13) | 12.5 (13; 8) | 13 (18; 13) | 0.001 |
| Illness duration | 15.6(±10.4) | 12.1(±9.9) | // | 0.085 |
| OCD or GD symptom dimensions | // | // | ||
| Hoarding 0/26 | Casino 0/26 | |||
| Comorbidities | 12/44 (27.3%) | 15/26 (57.7%) | // | 0.012 |
| Patients taking medications | 41/44 (93.2%) | 16/26 (61.5%) | // | 0.001 |
| IQ verbal score | 108.65 (110.77; 105.59) | 103.66 (108.78; 100.37) | 109.59 (111.22; 105;92) | 0.007 |
| IQ performance | 107.92 (111.57; 105.51) | 106.15 (110.57; 102.19) | 108.10 (111.33; 105.46) | 0.474 |
| IQ total score | 108.45 (111.72; 105.97) | 105.32 (110.35; 101.8) | 108.79 (112.15; 105.95) | 0.085 |
| Fagerstrom score | 0 (0; 0) | 2.5 (5.25; 0) | 0 (1; 0) | 0.003 |
| Y-BOCS score | 24 (28.75; 17.25) | 0 (0; 0) | 0 (0; 0) | <0.001 |
| PG-YBOCS score | 0 (0; 0) | 23 (26.5; 14.9) | 0 (0; 0) | <0.001 |
| HAM-D score | 4 (7.75; 1.5) | 4 (6; 1.75) | 1 (2; 0) | <0.001 |
| HAM-A score | 5 (7.75; 3) | 4 (5; 2) | 1 (3; 0) | <0.001 |
| Odor identification performance | 10.5 (11; 9) | 10 (11; 9) | 11 (12; 11) | <0.001 |
OCD and GD patients with lifetime comorbid psychiatric disorders were not excluded from the study since OCD and GD was respectively the dominant pathology and the reason for seeking treatment. In the OCD group 12 out of 44 patients (27,3%) had a history of lifetime comorbid psychiatric disorders: major depressive disorder (6 patients), anxiety disorders (2 patients panic disorder, 2 patients social anxiety disorder), OCD spectrum disorders (1 patient body dysmorphic disorder and 1 patient hoarding disorder), chronic tic disorders (4 patients). In the GD group 15 out of 26 patients (57.7%) had a history of lifetime comorbid psychiatric disorders: mood disorders (5 patients bipolar spectrum disorders, 4 patients major depressive disorder), substance use disorders (2 patients alcohol use disorder, 1 patient cocaine use disorder), anxiety disorders (2 patients panic disorder, 1 patient social anxiety disorder).
In the OCD group 41 out of 44 patients (93,2% of the total sample) were under ongoing medications. 31 patients were taking SRIs medications only (citalopram, escitalopram, fluvoxamine, fluoxetine, paroxetine, sertraline or clomipramine) and 10 patients were taking SRIs plus antidopaminergic medications (risperidone or aripiprazole). Twenty patients out of the whole OCD sample were undergoing a cognitive-behavioral therapy. In the GD group 16 out of 26 patients (61.5% of the total sample) were under ongoing medications. Six patients were taking SSRIs medications (paroxetine, sertraline and citalopram) while 10 patients were taking mood stabilizers (valproate, lithium, topiramate).
Potential subjects in both groups with conditions that potentially could affect the nasal epithelium functions and therefore odor detection abilities were excluded. Thus, we excluded subjects suffering from any significant olfactory symptoms, such as those associated with the common cold, influenza, nasal allergies, or any organic conditions such as upper respiratory tract infection (rhinosinusitis, polyposis, allergic rhinitis) or patients who had undergone surgery on the nasal septum, turbinates or paranasal sinuses. Also, we excluded patients with a history of any systemic condition that could affect olfaction abilities such as chronic obstructive pulmonary disease, asthma, active hepatitis, cirrhosis, chronic renal failure, vitamin B12 deficiency, cerebral vascular accidents, insulin dependent diabetes mellitus, hypothyroidism and Cushing syndrome.
Smokers were included in the study, although none of the subjects was permitted to smoke in the hour immediately preceding the olfactory examination.
The study procedures were carried out in accordance with the Declaration of Helsinki. The study was approved by the Institutional Review Board of the University of Florence and all participants had to sign the informed consent to be included in the study. All procedures occurred in one day.
Procedures and assessments
Clinical assessments
OCD and GD symptoms severity was assessed by independent evaluators using the Yale-Brown Obsessive-Compulsive Scale (Y-BOCS) [14–15] and the Yale-Brown Obsessive-Compulsive Scale adapted for Pathological Gambling (PG-YBOCS) [16] respectively. Both scales have a score range of 0–40, with higher scores representing greater severity. To assess OCD symptoms subtype we used the Y-BOCS symptoms checklist (Y-BOCS-SC). On the basis of the Y-BOCS-SC, patients’ primary symptoms were classified as one of five a priori dimensions based on factor analysis performed on previous studies [17]: 1) doubt/checking; 2) contamination/cleaning; 3) symmetry/ordering; 4) unacceptable/taboo thoughts; 5) hoarding. GD patients’ primary symptoms were classified according to gambling type as one of the following five dimensions: 1) slot machines, 2) lottery, 3) bets, 4) card games (including online games), 5) casino.
We also performed a clinical interview in order to assess social and demographic variables, duration of illness, current pharmacological or CBT treatments. Clinical variables of OCD and GD subjects are displayed in Table 1.
The presence of anxiety and depression symptoms was assessed through the Hamilton Anxiety Rating Scale (HAM-A) [18] and the Hamilton Depression Rating Scale (HAM-D-17 items) [19] respectively.
Patients’ and controls’ intelligence was assessed through the Italian version of the National Adult Reading Test (Test di Intelligenza Breve, TIB) [20]. The TIB consists of 54 words (34 effective test-words with irregular accent and 20 control-words with high frequency of use), that subjects have to read and pronounce. The total number of mistakes of reading defines the TIB error score. The estimated IQ scores (i.e., performance, verbal and total) are calculated through the regression of equations taking into account sex, age and educational level. The variable we used in the statistical analysis was estimated total IQ, estimated performance IQ and estimated verbal IQ.
In order to control olfaction assessment for smoking status we assessed nicotine dependence through the Fagerstrom test for nicotine dependence (FTND), a widely used test to assess several different nicotine addiction dimensions including the number of smoked cigarettes per day [21].
Impulsivity assessment
Impulsivity traits were assessed using the Barratt Impulsiveness Scale, version 11 (BIS-11). This scale consists of 30 self-descriptive items, with responses in a four-point Likert-type scale ranging from “Rarely/Never” to “Almost Always/Always.” [22]. It measures the total score (range: 30–120) of impulsivity and three factors: Attentional Impulsiveness (AI), Motor Impulsiveness (MI), and Non-planning Impulsiveness (NPI) with higher scores indicating higher impulsivity. BIS-11 was used in its Italian translation [23].
Decision-making assessment
To assess decision-making, we used the Iowa Gambling Task (IGT), a card game that is widely used to study decision-making under ambiguity conditions (the probability of different outcomes is unknown) [24–25]. Decision making behaviors on the IGT can separate “risky-players” that prefer immediate reward despite negative future consequences, from “risky-avoidant” players that prefer small but long-term rewarding choices [26]. In the IGT the subject must make 100 card selections from four decks (A, B, C, and D) and the objective is the maximum profit. At the beginning of the test the subjects receive a loan of play-money. After turning over each card, subjects are either given money or asked to pay a penalty according to a programmed schedule of reward and punishment. Gains and losses are different for each deck. Decks A and B (disadvantageous decks) are high paying but disadvantageous in the long run, because the penalties are even higher. Decks C and D (advantageous decks), on the other hand, are low paying but advantageous because the penalties are lower, resulting in an overall gain in the long run. In this study, we used a computerized version of the original IGT [27–29]. The performance was measured using the net score, defined by choices from advantageous (C and D) minus disadvantageous (A and B) decks, with higher scores indicating a risk-avoidant pattern of decision making. The net score for each block of 20 cards was also considered in order to evaluate the choice behavior during the task. We considered the 1st and 2nd blocks as decisions under ambiguity (the probability of outcome is unknown during these blocks of choices) while we considered the 3rd, 4th and 5th blocks as decisions under risk (subjects learn how the decks work during the first and second block and thus the probability of outcome is known during these blocks of choices).
Reward system assessment
In order to investigate reward system functioning we assessed hedonic tone through a self-report clinical instrument and through an olfactory test assessing hedonic appraisal to odors. Indeed, several studies suggested that exposure to pleasant and/or aversive odors activate key structures of the human reward system and abnormalities of human reward system functioning may be probed by olfactory stimuli [30–31]. Thus, abnormal hedonic appraisal to odors have been showed in psychiatric disorder characterized by reward system dysfunctions, such as schizophrenia [32].
Anhedonia assessment
To assess hedonic tone, we used the Snaith–Hamilton Pleasure Scale (SHAPS) [33]. The SHAPS is a 14-item self-report instrument designed to assess hedonic tone. Participants are presented with 14 statements of enjoyable activities (e.g., “I would be able to enjoy my favorite meal,” “I would enjoy a warm bath or a refreshing shower.”) and must choose one of four optional responses: Definitely Agree, Agree, Disagree, and Strongly Disagree. Each of the Agree responses receives a score of 0 and each of the Disagree responses receives a score of 1. The SHAPS total score ranges from 0–14 with higher scores reflecting greater anhedonia severity. According to criteria established by Snaith and colleagues, a cutoff score of more than two negative responses determines the presence of clinically significant anhedonia. In the present study, we used the Italian validated version of the SHAPS [34].
Odor hedonic task
Patients were presented with three odor sticks from the 12 sticks of the Burghart’s Sniffin’ Sticks Screening Test [35]. The three sticks represented a “pleasant”, a “neutral” and an “unpleasant” odor respectively. Based on the data of a previous investigation on 10 male and 10 female healthy volunteers aged 18–65 yy, the rose odor stick, the leather odor stick and the fish odor stick were used as the pleasant, neutral and unpleasant odors, respectively. The pleasant odor was always presented first in order to avoid possible carryover effects of the more enduring unpleasant stimulus. Then, they were presented with the neutral odor, followed by the unpleasant odor. Participants were exposed to each odorant for 30 s with 1 minute in between each of the odors. Participants rated the intensity and hedonic valence of each odor by pointing to a specific value on a visual analog scale. The range of the valence scale was from −7 (extremely unpleasant) to +7 (extremely pleasant).
In order to avoid potential confounding effects on pleasantness and unpleasantness patients’ ratings, olfactory identification abilities were assessed using the Burghart’s Sniffin’ Sticks Screening Test [35]. The test employs 12 odorant pens and requires the subject to choose among four written alternatives for each odorant. Reachable scores were 0–12 (higher scores indicate a better ability of odor identification). The order of olfactory testing was the same for all participants. For odor presentation, the cap was removed for 3 s and the pen’s tip was placed 2 cm in front of the nostrils. The inter-stimulus interval was at least 20 s to prevent olfactory desensitization. Olfactory tests were administered birhinally in a quiet, well ventilated room to avoid any background smell interfering with the test odors.
Statistical analyses
Normality of all variables was evaluated using the Shapiro-Wilk test. Non-normally distributed variables were: sex, age, years of education, estimated total IQ, performance IQ and verbal IQ, illness duration, BIS attentional and motor subscale, BIS total score, odor identification score, pleasurable, neutral and aversive odor scorings, IGT netscore 1,2,3,4 and 5, FTND score, HAM-D, HAM-A, PGYBOCS and Y-BOCS. The only normally distributed variables were the BIS Non-planning subscale and the IGT total netscore. Since most of examined variables were not normally distributed, non-parametric tests were used. Primary outcomes were impulsivity score (measured by the BIS-11), decision-making performance (measured by the IGT) and hedonic tone measure (measured by the SHAPS and odor hedonic task). Kruskal-Wallis rank sum test were used to compare continuous or interval variables between the three groups, while chi-square test was used for categorical variables. Bonferroni adjustment was used for all post-hoc analyses. Spearman’s rank correlation test (in order to correlate continuous and dichotomous variables) was used to evaluate the interaction of demographic and clinical variables (age, sex, educational attainment, IQ scores, nicotine dependence, gambling type or OC symptoms subtype, illness duration, history of tics, presence of comorbidity and/or medications) and primary outcome measure (impulsivity, decision-making and hedonic tone measures). Level of significance was set at p = 0.05. All analyses were carried out using the Statistical Package for the Social Sciences v25 (SPSS).
Results
Demographic and Clinical Characteristics
There were no significant differences between the three groups with respect to sex and age (see table 1). The three groups differed on years of education (χ2(2) =14.364, p=0.001). Post-hoc test revealed that GD patients have a lower educational attainment (median 12.5) than OCD patients (median 13, p=0.004) and healthy controls (median 13, p=0.001) while no significant differences were found between OCD patients and healthy controls (see table 1). However, the three groups did not differ on total IQ measures (p=0.085) and performance IQ (p=0.474), and they only differed on verbal IQ (χ2(2) =9.903), p=0.007) (GD patients showed significantly lower scores (median 103.66) than OCD patients (median 108.65, p=0.034) and healthy controls (median 109.59, p=0.007), while no difference were observed between OCD patients and healthy controls). The three groups differed on Fagerstrom test scores (χ2(2) =11.954, p=0.003) and GD patients (median 2.5) showed significant higher scores than OCD patients (median 0, p=0.003) and healthy controls (median 0, p=0.013), while OCD patients and healthy controls did not show significant differences (see table 1).
The three groups differed on odor identification abilities (χ2(2) =20.564, p<0.001). Both OCD (median 10.5) and GD (median 10) showed significantly lower scores than healthy controls (median 11) (p=0.019 for OCD vs HCs and p<0.001 for GD vs HCs), while OCD and GD did not differ (p=0.096).
As expected the three groups differed on anxiety (χ2(2) =39.638, p<0.001) and depressive symptoms (χ2(2) =42.031, p<0.001) (see table 1). GD and OCD patients showed higher scores on both HAM-D and HAM-A scales respect to healthy controls (p<0.001 for both tests), but no statistically significant difference was found between them. Also, we found a significant difference between the three groups on obsessive-compulsive symptoms assessed with the Y-BOCS and gambling symptoms assessed with the PG-YBOCS (χ2(2) =89.675, p<0.001 and χ2(2) =86.479, p<0.001 respectively). As expected, GD patients showed significantly higher PG-YBOCS scores than the other groups (p<0.001), while OCD and HCs did not show differences and OCD patients showed higher Y-BOCS score than the groups (p<0.001), while GD and HCs did not differ.
OCD and GD patients did not differ on illness duration (χ2(2) = 2.969, p=0.085), while they differed on comorbidity rates (χ2(2) = 6.383, p=0.012) and proportion of medicated patients (χ2(2) = 10.821, p=0.001) (see table 1).
Impulsivity measure results
The three groups significantly differed on BIS-11 total score and all the BIS-11 subscales (χ2(2) =23.007, p<0.001 for BIS-11 total score, χ2(2) =28.049, p<0.001 for BIS-11 Attentional subscale, χ 2(2) =11.487, p=0.003 for BIS-11 motor subscale and χ2(2) =9.989, p=0.007 for BIS-11 Non-planning subscale) (see table 2). Post-hoc test revealed that both OCD (median 67, p<0.001) and GD (median 68, p<0.001) patients scored significantly higher that HCs (median 58) on BIS-11 total score, while no statistically significant difference were found between OCD and GD patients. Concerning BIS-11 subscales, post-hoc test showed that both OCD and GD patients showed significant higher scores on BIS-11 attentional subscale (median 19, p<0.001 for OCD and 13 for GD, p=0.006) than HCs (median 13), but no differences were found between OCD and GD groups (p=0.4). On the other hand, GD patients showed significantly higher scores on BIS-11 motor subscale (median 22.5) than HCs (median 19, p=0.002), while no differences were observed between GD and OCD (median 21, p=0.122) and OCD and HCs (p=0.335). Also, GD patients showed significantly higher scores on BIS-11 non-planning subscale (median 31.5) than HCs (median 25, p=0.007), while no differences were observed between GD and OCD (median 27, p=0.761) and OCD and HCs (p=0.085).
Table 2. Impulsivity, decision-making and reward measures across OCD, GD and HCs.
Data are expressed as percentage or median (interquartile range) for all variables.
| OCD | GD | Controls | p | |
|---|---|---|---|---|
| BIS-11 total | 67 (73; 16) | 68 (84; 60.75) | 58 (61; 56) | <0.001 |
| BIS-11 attentional | 19 (21.75; 16) | 17 (20; 13) | 13 (15.75; 11) | <0.001 |
| BIS-11 motor | 21 (23.75; 17) | 22.5 (29.25; 19) | 19 (21; 17) | 0.003 |
| BIS-11 Nonplanning | 27 (32; 24.25) | 31.5 (32.25; 24.25) | 25 (28; 23.25) | 0.007 |
| Netscore 1 | −2 (0; −4) | −2 (2.5; −4.5) | 0 (2; −4) | 0.309 |
| Netscore 2 | 0 (4; −2) | 0 (4; −4) | 2 (6; 0) | 0.196 |
| Netscore 3 | 0 (7.5; −3.5) | 0 (2; −2.5) | 4 (12; 2) | <0.001 |
| Netscore 4 | 2 (7.5; −4) | 2 (6; −6) | 6 (15.5; 2) | 0.002 |
| Netscore 5 | 0 (3.5; −4) | 2 (6; 0) | 7 (18; 2) | <0.001 |
| Netscore total | 9(17.5; −15.5) | 3 (14.5; −8.5) | 22 (41.5; 8) | <0.001 |
| SHAPS | 1.25 (5; 0) | 3 (5; 1) | 0 (1; 0) | <0.001 |
| Pleasant odor | 4 (5; 2) | 3 (5; 0.5) | 5 (6; 3.25) | 0.003 |
| Neutral odor | −1.5 (1; −4) | 0 (2; −2) | 0 (1; −1.75) | 0.062 |
| Unpleasant odor | −6 (1; −4) | −7 (−5.75; −7) | −6 (−5; −7) | 0.219 |
Decision-making performance results
The three groups showed a significant difference on IGT total Net Score (χ2(2) =24.877, p<0.001). Post-hoc test revealed that IGT total Net Score was statistically significantly lower in OCD (median 9, p<0.001) and GD patients (median 3, p<0.001) compared to HCs (median 22) (see table 2), while there was no statistically significant difference between OCD and GD groups. Regarding the five IGT blocks series the three groups did not show significant differences on block 1 and 2 (putatively representing decision-making performance under ambiguous conditions), while they differed on blocks 3, 4 and 5 (putatively representing decision-making performance under risky conditions) (see table 2). On block 3, 4 and 5 both OCD (median 0, p=0.013, 2, median 2, p=0.008 and median 0, p<0.001, respectively) and GD patients (median 0, p=0.013, median 2, p=0.006 and median 2, p=0.014, respectively) showed significantly lower net scores than HCs (median 4, 6 and 7 respectively) (see table 2). However, OCD and GD patients did not differ on blocks 3 (p=0.401), 4 (p=1.000) and 5 (p=0.241) (see table 2).
Reward system results
The three groups significantly differed on SHAPS scores (χ2(2) = 31.876, p<0.001). Post hoc test revealed that both OCD (median 1.25, p<0.001) and GD (median 3, p<0.001) patients showed higher anhedonia scores than HCs (median 0), while no significant differences emerged between OCD and GD patients (p=0.333). Percentage of presence of significant anhedonia (SHAPS score higher than 2) were 53.8% for GD (14 out of 26 patients), 40.9% for OCD (18 out of 44 patients) and 0% for HCs (0 out of 40 subjects).
Depressive, anxiety and OC or GD symptoms scores did not correlate with SHAPS scores for GD patients, while moderate positive correlations were found between depressive, anxiety, OC symptoms and SHAPS scores for OCD patients (for HAM-D rs =.358, p=.017; for HAM-A rs =.384, p=.010; for Y-BOCS rs =.389, p=.049).
Concerning subjects’ response to pleasant, neutral and unpleasant odors, the three groups significantly differed on pleasant odor ratings (χ2(2) = 11.856, p=0.003), while they did not differ on both neutral (χ2(2) = 5.573, p=0.062) and unpleasant (χ2(2) = 3.036, p=0.219) odors ratings. Post-hoc test showed that GD patients showed significantly lower ratings (median 3, p0.002) for the pleasant odor than HCs (median 5), while no difference where observed between OCD (median 4, p=0.110) and healthy controls and OCD and GD patients (p=0.327). Both odor identification abilities and smoking status/nicotine dependence did not correlate with odor pleasantness rating for OCD, GD and HCs groups.
Correlation analyses
When we look to correlation between clinical demographic and clinical characteristics and primary outcome measures we found several correlations. GD and OCD patients differed on comorbidity rates and medicated patient’s rates. However, the presence of comorbidity did not correlate with any primary outcome measure for both OCD and GD patients and the presence of ongoing medications only correlated with unpleasant odor rating but patients did not differ from controls on this measure. The most clinically relevant correlations observed in the patients’ groups were as follow. In the OCD group, for impulsivity measures, BIS-11 total score positively correlated with HAM-D scores (rs =.350, p=.020), HAM-A scores (rs =.319, p=.035) and SHAPS score (rs =.402, p=.007), while in the GD group, BIS-11 total score positively correlated with PG-YBOCS score (rs =.453, p=.020). Depression, anxiety and OC symptoms positively correlated with SHAPS score in the OCD group (for HAM-D rs =.358, p=.017; for HAM-A rs =.384, p=.010; for Y-BOCS rs =.389, p=.049), while this was not the case in the GD group.
Although antidopaminergic medications could affect reward and decision-making networks, when we compared OCD patients treated with antidopaminergic agents (10 patients) and OCD patients treated with SRIs and/or CBT only we did not find any difference on primary outcome measures.
Discussion
The main finding of this study is that OCD patients display several similarities and some differences with GD patients on impulsivity, decision-making and reward functioning, three core dimensions of substance and behavioural addictions, respect to healthy controls. Specifically, both OCD and GD patients showed increased impulsivity respect to healthy controls but for OCD patients this difference was driven by increased cognitive impulsivity while for GD patients was driven by both cognitive and motor impulsivity. Furthermore, both OCD and GD patients showed impaired decision-making performances respect to healthy controls. Finally, GD patients showed increased anhedonia and blunted hedonic response to pleasant odors unrelated to gambling or depression/anxiety symptoms, while OCD patients showed only increased anhedonia levels related to OC and depression/anxiety symptoms. To the best of our knowledge, this is the first study investigating impulsivity, decision-making and reward system across OCD and GD patients.
Our impulsivity results are in line with previous literature showing that OCD patients display higher cognitive impulsivity than healthy controls on the BIS-11 scale [2; 4; 36], and with previous studies showing both cognitive and motor impulsivity as core features of GD patients [37]. Indeed, both OCD and GD patients showed increased impulsivity respect to HC but differently driven by cognitive and both cognitive and motor impulsivity respectively. Of note, some studies assessing motor impulsivity in OCD patients through a behavioral task (stop signal task) showed increased motor impulsivity respect to healthy controls [7]. Therefore, it could be hypothesized that the BIS-11 scale is not sensitive or specific enough to capture motor impulsivity features in OCD patients. Thus, further studies comparing OCD and GD patients on motor impulsivity through behavioral tasks are needed in order to elucidate similarities and differences between OCD and GD on motor impulsivity. Decision-making results in our study are largely in line with previous literature showing impaired performances on the IGT respect to healthy controls for both OCD and GD patients [2–3; 6; 26; 38]. In our sample both OCD and GD patients showed impaired decision-making performances respect to healthy controls and specifically they performed worse than controls on the last three blocks of cards, putatively assessing decision-making under risky conditions. However, it is still debated if the IGT is really able to distinguish decision-making under ambiguity and under risk and therefore it is mainly considered a decision-making task under ambiguous conditions. Moreover, previous studies on OCD patients comparing decision-making performances under ambiguity and under risk with different tasks did not find impaired performances under risky conditions [39].
Finally, results from reward system assessment (self-report of anhedonia symptoms and odor hedonic task) showed that both GD e OCD showed the presence of a reward dysfunction. However, this reward dysfunction seems to be differently mediated in GD and OCD. Indeed, both OCD and GD patients display higher anhedonia symptoms scores than healthy controls. However, while GD patients’ anhedonia symptoms were independent by gambling symptoms, depression and anxiety symptoms, OCD patients’ anhedonia symptoms were correlated to OC symptoms, depression and anxiety symptoms. These results on OCD patients are in line with a previous study showing high anhedonia ratings in OCD positively correlated with OC symptoms severity. However, contrary to our results, in this previous study OCD patients’ anhedonia scores were not correlated to depressive symptoms [40]. In that study, the authors used our same instruments (the HAM-D and the SHAPS) to assess anhedonia and depressive symptoms, however the sample was larger than our sample (more than hundred patients). Thus, we cannot rule out the hypothesis that our results differed from the study because we were underpowered on that outcome.
Also, in the odor hedonic task, GD patients showed significantly lower hedonic ratings to the pleasant odor respect to both OCD and healthy controls, while OCD patients did not differ from controls. These results seem to be in line with neuroimaging studies investigating reward system in OCD and GD. In fact, while imaging study consistently showed both anticipatory and consummatory reward dysfunction in GD [41], imaging studies on OCD showed mainly a reward anticipation dysfunction [5]. Thus, since our instruments assessed mainly consummatory reward (hedonic tone and hedonic response to pleasant odors), further investigations of both anticipatory and consummatory reward functioning in GD and OC are needed in order to elucidate commonalities and differences.
Several limitations are worth of mentioning. First, we investigated reward system by two instruments (a self-report anhedonia symptoms scale and the odor hedonic task) that putatively assess only consummatory reward, instead of both reward anticipation and reward consumption. Thus, we are not able to further investigate putative similarities and differences between different reward circuitries across these two disorders. Also, we did not use any behavioral measure of impulsivity and therefore we cannot exclude the presence of some commonalities on behavioral inhibition that the BIS-11 did not capture between OCD and GD patients. Finally, we did not assess patients’ functioning and disability and thus we cannot speculate on how differently the presence of impulsivity, impaired decision-making and reward dysfunction could impact on OCD and GD patients.
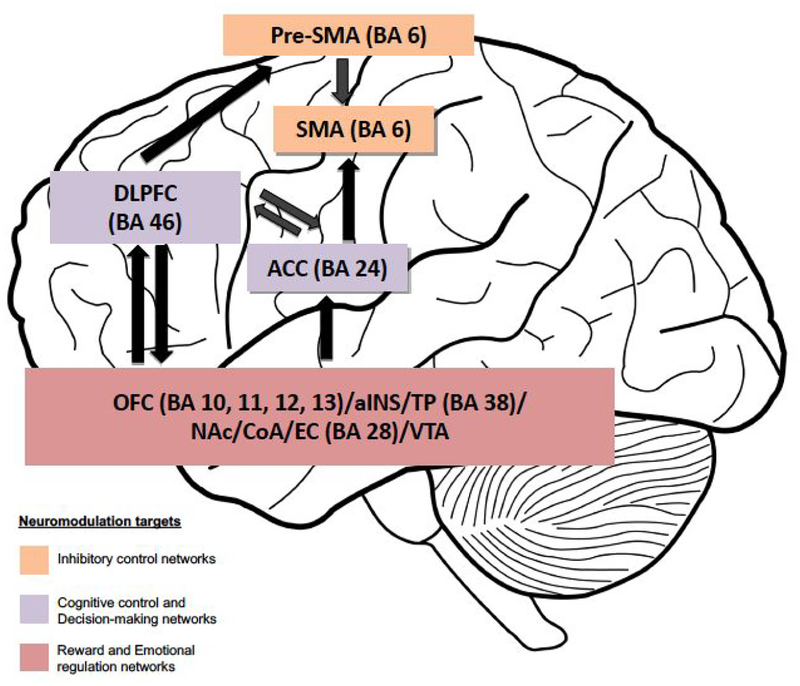
Our results could be relevant in moving forward the current OCD models, mainly focused on anxiety and compulsivity, highlighting the relevance on other RDoC dimensions such as inhibitory control networks (impulsivity), decision-making processes and reward system dysfunctions, as putative treatment targets for OCD. Indeed, our results are in line with several neuromodulation studies that in the last years targeted these networks and showed promising results. For instance, deep brain stimulation of the reward system (targeting the nucleus accumbens and the ventral internal capsule) showed promising results in several studies [42–43], and transcranic magnetic stimulation of inhibitory control networks (targeting the supplementary motor area) also showed promising results [44–46] (in figure 1 are presented the anatomical targets for neuromodulation of decision-making, impulsivity and reward networks in OCD; in table 3 are presented the RDoC dimensions and constructs investigated in the study).
Figure 1. Anatomical targeting of impulsivity, decision-making and reward networks in OCD.
Abbreviations: ACC: anterior cingulate cortex; aINS: anterior insular cortex; BA: Brodmann’s area; CoA: cortical amygdala; DLPFC: dorsolateral prefrontal cortex; EC: enthorinal cortex; NAc: nucleus accumbens; OFC: orbitofrontal cortex; SMA: supplementary motor area; TP: temporopolar area. Brain areas involved in the same networks are grouped together. Different colors indicate different neuromodulation targets. The arrows indicate connections between brain networks.
Table 3.
RDoC systems and constructs of impulsivity, decision-making and anhedonia in OCD
| Clinical Dimension | RDoC System | RDoC Construct/Sub-Constract |
|---|---|---|
| Impulsivity | Cognitive Systems | Cognitive Control: Inhibition/Suppression |
| Decision-making | Cognitive Systems/Positive Valence Systems | Goal Selection / Flexible Updating / Reward Learning and Valuation |
| Anhedonia | Positive Valence Systems | Reward Responsiveness |
Conclusion
In conclusion, OCD patients showed several similarities and some differences compared to GD patients as well as with healthy controls on impulsivity, decision-making and reward system, three core dimensions of addiction. These results could have relevant implication for the search of new treatment targets for OCD and thus deserve further investigation.
Funding:
“Research reported in this publication was supported by the National Institute On Drug Abuse of the National Institutes of Health under Award Number R21DA042271 (NM and SP). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.”
Footnotes
Conflict of Interest: The authors declare that they have no conflict of interest.
References
- [1].Prochazkova L, Parkes L, Dawson A et al. Unpacking the role of self-reported compulsivity and impulsivity in obsessive-compulsive disorder. CNS Spectr. 2018; 23(1): 51–58. [DOI] [PubMed] [Google Scholar]
- [2].Grassi G, Figee M, Ooms P et al. Impulsivity and decision-making in obsessive-compulsive disorder after effective deep brain stimulation or treatment as usual. CNS Spectr. 2018; 4:1–7. [DOI] [PubMed] [Google Scholar]
- [3].Grassi G, Pallanti S, Righi L et al. Think twice: Impulsivity and decision making in obsessive-compulsive disorder. J Behav Addict. 2015; 4(4): 263–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Benatti B, Dell’Osso B, Arici C et al. Characterizing impulsivity profile in patients with Obsessive-Compulsive Disorder. Int J Psychiatry Clin Pract. 2014; 18(3): 156–160. [DOI] [PubMed] [Google Scholar]
- [5].Figee M, Vink M, de Geus F et al. Dysfunctional reward circuitry in obsessive-compulsive disorder. Biol Psychiatry. 2011; 69(9): 867–74. [DOI] [PubMed] [Google Scholar]
- [6].Cavedini P, Zorzi C, Piccini M et al. Executive dysfunctions in obsessive-compulsive patients and unaffected relatives: searching for a new intermediate phenotype. Biol Psychiatry. 2010; 67(12): 1178–84. [DOI] [PubMed] [Google Scholar]
- [7].Chamberlain SR, Fineberg NA, Menzies LA et al. Impaired cognitive flexibility and motor inhibition in unaffected first-degree relatives of patients with obsessive-compulsive disorder. Am J Psychiatry. 2007; 164(2): 335–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Denys D. Obsessionality and compulsivity: A phenomenology of obsessive compulsive disorder. Philosophy, Ethics, and Humanities in Medicine, 2011; 6–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Ferreira GM, Yücel M, Dawson A et al. Investigating the role of anticipatory reward and habit strength in obsessive-compulsive disorder. CNS Spectr. 2017; 9: 1–10. [DOI] [PubMed] [Google Scholar]
- [10].Grant JE, Odlaug BL, Chamberlain SR. Neural and psychological underpinnings of gambling disorder: A review. Prog Neuropsychopharmacol Biol Psychiatry. 2016; 65: 188–93. [DOI] [PubMed] [Google Scholar]
- [11].First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Research Version, Patient Edition (SCID-I/P). New York: Biometrics Research, New York State Psychiatric Institute; 2002. [Google Scholar]
- [12].First MB, Gibbon M, Spitzer RL et al. Structured Clinical Interview for DSM-IV Axis II Personality Disorders (SCID-II). Washington, DC: American Psychiatry Press Inc; 1997. [Google Scholar]
- [13].First MB, Spitzer RL, Gibbon M et al. Structured Clinical Interview for DSM-IV TR Axis I Disorders, Research Version, Non-patient Edition (SCID-I/NP). New York: Biometrics Research, New York State Psychiatric Institute; 2002. [Google Scholar]
- [14].Goodman WK, Price LH, Rasmussen SA et al. The Yale-Brown Obsessive-Compulsive Scale (Y-BOCS), part I: development, use, and reliability. Arch Gen Psychiatry. 1898; 46: 1006–11. [DOI] [PubMed] [Google Scholar]
- [15].Goodman WK, Price LH, Rasmussen SA et al. The Yale-Brown Obsessive-Compulsive Scale (Y-BOCS), part II: validity. Arch Gen Psychiatry. 1989; 46: 1012–16. [DOI] [PubMed] [Google Scholar]
- [16].Pallanti S, DeCaria CM, Grant JE et al. Reliability and validity of the pathological gambling adaptation of the Yale-Brown Obsessive-Compulsive Scale (PG-YBOCS). J Gambl Stud. 2005; 21(4): 431–43. [DOI] [PubMed] [Google Scholar]
- [17].Brakoulias V, Starcevic V, Berle D et al. Further support for five dimensions of obsessive-compulsive symptoms. J Nerv Ment Dis. 2013; 201: 452–59. [DOI] [PubMed] [Google Scholar]
- [18].Hamilton M The assessment of anxiety states by rating. Br J Med Psychol. 1959; 32: 50–55. [DOI] [PubMed] [Google Scholar]
- [19].Hamilton M Development of a rating scale for primary depressive illness. Br J Soc Clin Psychol. 1967; 6(4): 278–96. [DOI] [PubMed] [Google Scholar]
- [20].Sartori G, Colombo L, Vallar G, et al. T.I.B.: Test di Intelligenza Breve per la valutazione del quoziente intellettivo attuale e pre-morboso. La Professione di Psicologo. 1997; 1: II–XXIV. [Google Scholar]
- [21].Heatherton TF, Kozlowski LT, Frecker RC et al. The Fagerström test for nicotine dependence: a revision of the Fagerström tolerance questionnaire. Br J Addict. 1991; 86: 1119–27. [DOI] [PubMed] [Google Scholar]
- [22].Patton JH, Stanford MS, Barratt ES. Factor structure of the Barratt Impulsiveness Scale. Journal of Clinical Psychology. 1995; 51: 768–774. [DOI] [PubMed] [Google Scholar]
- [23].Fossati A, Di Ceglie A, Acquarini et al. Psychometric properties of an Italian version of the Barratt Impulsiveness Scale-11 (BIS-11) in nonclinical subject. Journal of Clinical Psychology. 2001; 57: 815–828. [DOI] [PubMed] [Google Scholar]
- [24].Brand M, Labudda K, Markowitsch H et al. Neuropsychological correlates of decision-making in ambiguous and risky situations. Neural Networks. 2007; 19: 1266–76. [DOI] [PubMed] [Google Scholar]
- [25].Brand M, Recknor EC, Grabenhorst F et al. Decision under ambiguity and decision under risk: Correlations with executive functions and comparisons of two different gambling tasks with implicit and explicit rules. Journal of Clinical and Experimental Neuropsychology. 2007; 29(1): 86–99. [DOI] [PubMed] [Google Scholar]
- [26].Cavedini P, Zorzi C, Baraldi C et al. The somatic marker affecting decisional processes in obsessive-compulsive disorder. Cognitive Neuropsychiatry. 2012; 17(2): 177–190. [DOI] [PubMed] [Google Scholar]
- [27].Bechara A, Damasio AR, Damasio H et al. Insensitivity to future consequences following damage to human prefrontal cortex. Cognition. 1994; 50: 7–15. [DOI] [PubMed] [Google Scholar]
- [28].Struglia F, Stratta P, Gianfelice D et al. Decision making impairment in schizophrenia: Relationships with positive symptomatology. Neuroscience Letter. 2011; 502: 80–83. [DOI] [PubMed] [Google Scholar]
- [29].Tomassini AR, Struglia F, Spaziani D et al. Decision making, impulsivity and personality traits in alcohol dependent subjects. American Journal of Addictions. 2012; 21: 263–267. [DOI] [PubMed] [Google Scholar]
- [30].Anderson AK, Christoff K, Stappen I et al. Dissociated neural representations of intensity and valence in human olfaction. Nature Neuroscience. 2003; 6(2): 196–202. [DOI] [PubMed] [Google Scholar]
- [31].Fagundo AB, Jiménez-Murcia S, Giner-Bartolomé C et al. Modulation of higher-order olfaction components on executive functions in humans. PLoS One. 2015; 17: 10(6). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Mesholam-Gately RI, Gibson LE, Seidman LJ et al. Schizophrenia and co-occurring substance use disorder: reward, olfaction and clozapine. Schizophrenia Research. 2014; 155(1–3): 45–51. [DOI] [PubMed] [Google Scholar]
- [33].Snaith RP, Hamilton M, Morley S et al. A scale for the assessment of the hedonic tone: the Snaith–Hamilton Pleasure Scale. British Journal of Psychiatry. 1995; 167: 99–103. [DOI] [PubMed] [Google Scholar]
- [34].Santangelo G, Morgante L, Savica R et al. Anhedonia and cognitive impairment in Parkinson’s disease: Italian validation of the Snaith-Hamilton Pleasure Scale and its application in the clinical routine practice during the PRIAMO study. Parkinsonism Relat Disord. 2009; 15(8): 576–81. [DOI] [PubMed] [Google Scholar]
- [35].Steinbach S, Hummel T, Böhner C et al. Qualitative and quantitative assessment of taste and smell changes in patients undergoing chemotherapy for breast cancer or gynecologic malignancies. J Clin Oncol. 2009; 10; 27(11): 1899–905. [DOI] [PubMed] [Google Scholar]
- [36].Ettelt S, Ruhrmann S, Barnow S, et al. Impulsiveness in obsessive-compulsive disorder: Results from a family study. Acta Psychiatrica Scandinavica. 2007; 115(1): 41–47. [DOI] [PubMed] [Google Scholar]
- [37].Chowdhury NS, Livesey EJ, Blaszczynski A et al. Pathological Gambling and Motor Impulsivity: A Systematic Review with Meta-Analysis. J Gambl Stud. 2017; 33(4): 1213–39. [DOI] [PubMed] [Google Scholar]
- [38].Kovács I, Richman MJ, Janka Z et al. Decision making measured by the Iowa Gambling Task in alcohol use disorder and gambling disorder: a systematic review and meta-analysis. Drug Alcohol Depend. 2017; 181: 152–61. [DOI] [PubMed] [Google Scholar]
- [39].Zhang L, Dong Y, Ji Y et al. Trait-related decision-making impairment in obsessive-compulsive disorder: evidence from decision making under ambiguity but not decision making under risk. Sci Rep. 2015; 5:1731–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Abramovitch A, Pizzagalli DA, Reuman L et al. Anhedonia in obsessive-compulsive disorder: beyond comorbid depression. Psychiatry Res. 2014; 216(2): 223–29. [DOI] [PubMed] [Google Scholar]
- [41].Luijten M, Schellekens AF, Kühn S et al. Disruption of Reward Processing in Addiction: An Image-Based Meta-analysis of Functional Magnetic Resonance Imaging Studies. JAMA Psychiatry. 2017; 74(4): 387–98. [DOI] [PubMed] [Google Scholar]
- [42].Denys D, Mantione M, Figee M et al. Deep brain stimulation of the nucleus accumbens for treatment-refractory obsessive-compulsive disorder. Arch Gen Psychiatry. 2010; 67(10): 1061–68. [DOI] [PubMed] [Google Scholar]
- [43].Figee M, Luigjes J, Smolders R et al. Deep brain stimulation restores frontostriatal network activity in obsessive-compulsive disorder. Nat Neurosci. 2013; 16(4): 386–7 [DOI] [PubMed] [Google Scholar]
- [44].Pallanti S, Marras A, Grassi G. Outcomes with Neuromodulation in Obsessive-Compulsive Disorder. Psychiatric Annals. 2015; 45(6): 316–20 [Google Scholar]
- [45].Pallanti S, Marras A, Salerno L et al. Better than treated as usual: Transcranial magnetic stimulation augmentation in selective serotonin reuptake inhibitor-refractory obsessive-compulsive disorder, mini-review and pilot open-label trial. J Psychopharmacol. 2016; 30(6): 568–78. [DOI] [PubMed] [Google Scholar]
- [46].Pallanti S, Hollander E. Pharmacological, experimental therapeutic, and transcranial magnetic stimulation treatments for compulsivity and impulsivity. CNS Spectr. 2014; 19(1) :50–61. [DOI] [PubMed] [Google Scholar]