Abstract
Spinal interneuron (IN) networks can facilitate respiratory motor recovery after spinal cord injury (SCI). We hypothesized that excitatory synaptic connectivity between INs located immediately caudal to unilateral cervical SCI would be most prevalent in a contra- to ipsilateral direction. Adult rats were studied following chronic C2 spinal cord hemisection (C2Hx) injury. Rats were anesthetized and ventilated and a multi-electrode array was used to simultaneously record INs on both sides of the C4–5 spinal cord. The temporal firing relationship between IN pairs was evaluated using cross-correlation with directionality of synaptic connections inferred based on electrode location. During baseline recordings, the majority of detectable excitatory IN connections occurred in a contra- to- ipsilateral direction. However, acute respiratory stimulation with hypoxia abolished this directionality, while simultaneously increasing the detectable inhibitory connections within the ipsilateral cord. We conclude that propriospinal networks caudal to SCI can display a contralateral-to-ipsilateral directionality of synaptic connections and that these connections are modulated by acute exposure to hypoxia.
Keywords: Cervical interneurons, Spinal cord injury, Plasticity, Connectivity
1. Introduction
Cervical interneurons (INs) are of particular interest in the context of spinal cord injury (SCI) (Zholudeva et al., 2018). For example, recruitment of cervical INs to form de novo synaptic pathways can restore connectivity between supraspinal neurons and spinal segments caudal to a spinal lesion (Bareyre et al., 2004). Histological studies suggest that cervical INs are part of the circuitry enabling respiratory motor recovery after SCI (Lane et al., 2008; Zholudeva et al., 2017), and optoand chemogenetic studies have confirmed this hypothesis (Cregg et al., 2017; Satkunendrarajah et al., 2018). However, there is little neurophysiological data available regarding the discharge rates and synaptic connectivity of mid-cervical spinal INs in the injured spinal cord. Here we addressed this issue by using a multi-electrode array (Streeter et al., 2017a) to simultaneously record cervical (C4–5) INs located ipsilateral and contralateral to C2 hemisection (C2Hx). The C2Hx injury transiently eliminates, and chronically reduces, motor output from the ipsilateral phrenic nerve (Goshgarian, 2009). However, bulbo-(Goshgarian, 2003) and corticospinal (Fujiki et al., 2004) synaptic pathways undergo neuroplastic changes after C2Hx that enable neuronal activation in the ipsilateral cord. On the other hand, contralateral neuronal pathways are not directly damaged by C2Hx, and contralateral motor output can show a compensatory increase (Rowley et al., 2005). Therefore, our first purpose was to compare and contrast the discharge rates of mid-cervical INs located ipsilateral vs. contralateral to the C2Hx lesion. We hypothesized that INs recorded contralateral to the lesion would show a greater firing rate as compared to ipsilaterally recorded cells. Our second purpose was to examine synaptic connectivity between INs in the injured cervical spinal cord. To address this goal, we evaluated the temporal firing relationship between IN pairs using cross-correlation analyses (Nuding et al., 2015; Segers et al., 2008). The “directionality” of synaptic connections was determined based on electrode location either ipsi- or contralateral to the C2Hx lesion. Based on observations that INs can “route” descending neural signals around a spinal lesion (Bareyre et al., 2004) and contribute to motor recovery after C2Hx (Satkunendrarajah et al., 2018), we hypothesized that the incidence of excitatory synaptic connectivity between IN pairs would be greatest for the contra- to ipsilateral direction. In other words, we predicated that examples of one IN exciting another would be most prevalent when the “exciting” cell was located in the uninjured side of the cord. Our final purpose was to test the impact of acute exposure to hypoxia on mid-cervical IN firing and synaptic connectivity in the injured spinal cord. Hypoxia is a potent trigger of spinal neuroplasticity (Baker-Herman et al., 2004) and may even have therapeutic value after SCI (Gonzalez-Rothi et al., 2015a). In addition, neurophysiological data from spinal intact animals suggest that cervical INs are part of the neurologic substrate enabling neuroplasticity induced by hypoxia (Sandhu et al., 2015; Streeter et al., 2017a). Thus, we tested the hypothesis that an acute exposure to hypoxia would alter cervical IN burst rate and synaptic connectivity in the chronically injured spinal cord.
2. Materials and methods
2.1. Animals
Adult female Sprague-Dawley rats (n = 8; 279 ± 7 g) from colony 217 at ENVIGO (formally Harlan Laboratories) were studied. Rats were housed in a controlled environment (12 h light/dark cycles) with food and water ad libitum. All experimental protocols were approved by the Institutional Animal Care and Use Committee at the University of Florida.
2.2. Spinal cord injury
Anesthesia was induced with 3% isoflurane in 100% O2, and rats were transferred to a heated pad and anesthesia was maintained through a nose cone with 1.5–2% isoflurane. The surgical area was cleaned with three, alternating rounds of betadine surgical scrub followed by 70% ethanol and a dorsal incision was made over the spinal midline from the base of the skull to the fifth cervical segment. A C2 laminectomy was performed to expose the spinal cord and a left, C2 hemisection (C2Hx) was performed using a micro-scalpel followed by aspiration. The overlying muscles were sutured with sterile 4–0 Vicryl suture and the skin was closed with sterile wound clips. Rats received buprenorphine (0.03 mg/kg, s.q.) for the initial 48 h post-injury, and Lactated Ringer’s solution (10 ml/day, s.q.) and oral Nutri-cal supplements (1–3 ml, Webster Veterinary, MA, USA) until adequate volitional eating and drinking resumed.
2.3. In vivo electrophysiology
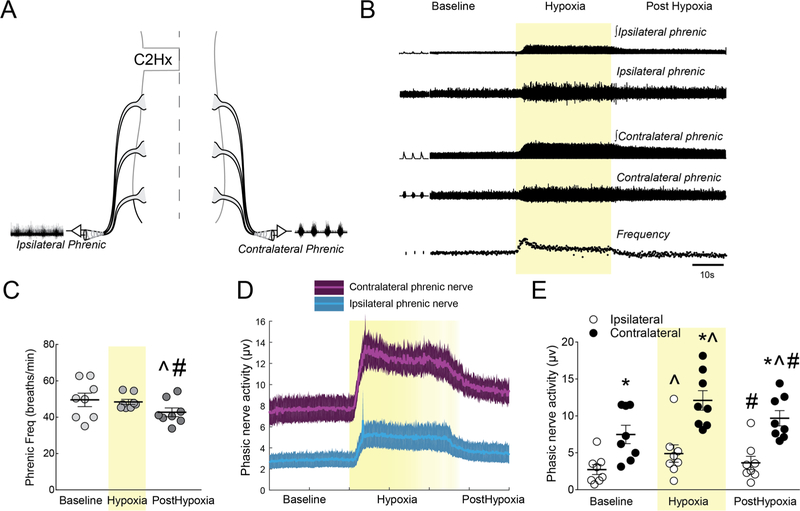
A schematic of the phrenic nerve and spinal IN recording configuration is shown in Fig. 2Figure 1 Throughout the manuscript, the terms ipsilateral and contralateral are used to describe recording position in relation to the C2Hx injury. Rats were studied 12–16 weeks post-injury using established in vivo electrophysiology methods (Streeter et al., 2017a). Our previous work has shown that spontaneous neuroplasticity and therefore phrenic neural output is stable during this time post-injury (Fuller et al., 2008). All rats were initially anesthetized with 3% isoflurane (in 100% O2) and quickly transferred to the surgical station. Rectal temperature was maintained at 37 ± 0.5 °C with a homeothermic heating device (CWE, model 700 TC-1000). A tail vein catheter was placed for intravenous delivery of urethane and fluids. The trachea was cannulated and rats were pump-ventilated (Harvard Apparatus, Rodent Ventilator 683; volume: ˜2.5–3 mL; frequency: 70/minute). Lungs were periodically (˜1/hour) hyperinflated (2–3 breaths) during surgical procedures. Once ventilated, rats were converted (6 ml/hr; Harvard Apparatus syringe pump) to urethane anesthesia (1.7 g/kg, i.v. in distilled water) and isoflurane was slowly withdrawn. CO2 was added (< 3% FiCO2) to the inspiratory line to maintain end-tidal CO2 (ETCO2) between ˜40–50 mmHg throughout the protocol (Capnogard; Respironics, Inc.). A bilateral vagotomy was performed lateral to the trachea and ˜1 cm caudal to the thyroid cartilage. A femoral arterial catheter was placed to monitor blood pressure and sample blood gases (i-STAT1 Analyzer Abbot). Using a dorsal approach, the left and right phrenic nerves were isolated, cut distally, and partially desheathed. A midline incision extending from the base of the skull to mid-thoracic region was made and spinal vertebrae C3–T2 were exposed. Using a nose clamp and a suture tied around the T2 spinous process, the rat was slightly elevated off of the table to reduce motion associated with the process of ventilation and level the spinal cord. A laminectomy was performed from C3–C6, the dura was cut and removed, and the pia was gently teased apart at the multi-electrode insertion point (C4–5 spinal segment). A unilateral pneumothorax was performed to decrease chest wall movement and positive end-expiratory pressure (PEEP) of ˜1–2 cm of water was applied to prevent atelectasis. Animals received the neuromuscular paralytic pancuronium bromide (2.5 mg/kg, i.v., Hospira, Inc.) to eliminate spontaneous breathing efforts. Adequate depth of anesthesia was verified by assessing responses to toe-pinch. Urethane supplements (0.2 mL bolus, i.v.) were given until toe-pinch did not cause an increase in phrenic burst amplitude, frequency, or mean arterial blood pressure. Blood pressure and fluid homeostasis was maintained by a slow infusion of a 1:4 solution (8.4% sodium bicarbonate/Lactated Ringers, i.v.).
Fig. 2.
A. Schematic illustrating bilateral phrenic nerve recordings following C2Hx. B. Representative compressed raw and integrated phrenic neurograms and instantaneous burst frequency before, during, and after hypoxia. C. Average phrenic burst frequency during the center 50 breaths of each experimental time point. D, E. Ipsilateral and contralateral phrenic traces showing the average (center line) and standard error of the mean (SEM; shaded region) and individual data points for phasic inspiratory nerve activity during baseline, hypoxia (shaded yellow) and post-hypoxia. * Significantly different from ipsilateral; ^ significantly different from baseline; # significantly different from hypoxia. All symbols denote P < 0.05.
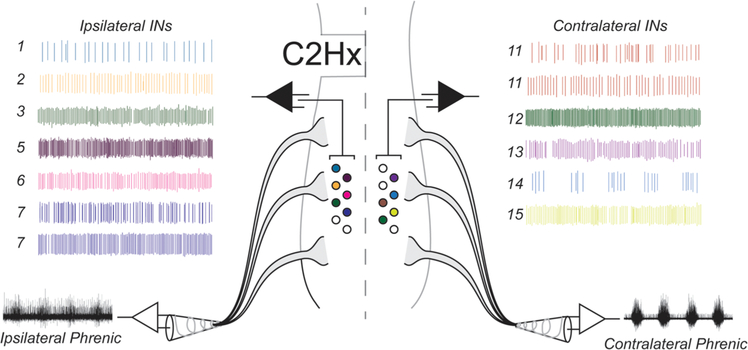
Fig. 1.
Spinal segments C1–C5 illustrating a unilateral C2 hemisection (C2Hx) injury, C3, C4, C5 cervical rootlets, phrenic nerve output and multi-electrode array recordings at C4/C5 (center panel). Representative ipsilateral and contralateral recordings from 13 mid-cervical spinal INs and their corresponding locations within the cervical spinal cord.
Bilateral phrenic nerve output was recorded using custom made bipolar suction electrodes filled with 0.9% saline (Fig. 2A). Compound action potentials were amplified (X10k), band-pass filtered (3 Hz to 3 kHz), digitized (16-bit, 25 kHz/channel; Power1401, Cambridge Electronic Design, Cambridge, UK (CED) in Spike2.v8 software (CED). A custom made multi-electrode recording array used in our previous report (Streeter et al., 2017b) was used to record bilateral mid-cervical (C4/5) spinal activity. The array contained 16 tungsten electrodes coated with Epoxylite Insulation (Frederick Haer & Co., #UEWLEGSE0N1E; impedance: 10 ± 1 MΩ). The array was mounted on a stereotaxic frame and eight electrodes arranged in two staggered rows of four were placed into each hemi-cord caudal to the C2Hx (Fig. 1). The inner distance between the two sets of eight electrodes was approximately 1 mm, while the distance between electrodes within each row was ˜300 μm. Electrode tips were maintained in the x and y plane of this “fixed matrix” by the array guide. One by one, electrodes were advanced into the spinal cord while audio was monitored until a ˜3:1 signal to noise ratio was discriminated. Neural signals from single electrodes were amplified (X5k), band-pass filtered (3–3 kHz), digitized (16-bit, 25 kHz/channel; Power 1401, CED) and recorded with Spike2.v8 software (CED). Once phrenic nerve activity and spinal discharge were stable, “baseline activity” was recorded for 15 min. Rats were exposed to a 5 min period of hypoxia (11% FiO2), and returned to hyperoxia (50% FiO2) for 15 min. A 0.2 ml arterial blood sample was obtained via the femoral artery during the baseline period and the partial pressure of O2, CO2, and pH were measured using a CG4+ i-STAT cartridge and i-STAT1 Analyzer (Abbott Laboratories).
2.4. Analysis of electrophysiological signals
All electrophysiological data were initially analyzed using Spike2.v8 software (CED). Extracellular action potentials from individual neurons were extracted from continuous recordings and converted to waveforms using “spike-sorting” tools from the CED acquisition software. Spikes were determined to represent a single neuron based on criteria which included: 1) at least 80% template matching, 2) assessment of inter-spike interval, and 3) principle component analysis. The sorted spike waveforms and phrenic nerve output were analyzed with custom MATLAB software (The MathWorks R2015a). All data were averaged over the center 50 breaths of each experimental period (25 breaths before and after the center of the experimental time point). Baseline data were taken during the five minutes immediately preceding the hypoxic challenge and the post-hypoxia data were taken ten minutes after hypoxia (during the last five minutes of the post-hypoxia recording). In regards to hypoxia, our a priori hypothesis focused on the acute hypoxic response rather than the persistent changes in bursting that may be evoked by hypoxia. Statistical analyses were performed in GraphPad Prism 7 (GraphPad Software, Inc.). The statistical significance level was set to 0.05. Results are shown as means ± standard error.
Phrenic burst frequency was expressed as neural breaths per minute. Integrated burst amplitude was analyzed as, 1) absolute voltage (i.e., μV.) and 2) normalized to baseline amplitude (% baseline), Phasic (inspiratory) activity was quantified as the peak of the integrated inspiratory burst amplitude relative to the amplitude of the expiratory phase preceding it. A two-way analysis of variance (ANOVA) with repeated measures design was used for statistical comparisons. A one-way ANOVA with repeated measures design was used to analyze phrenic burst frequency. For all tests, individual time point comparisons were made using Fisher’s Least Significant Difference (LSD) post hoc test.
Spike-triggered averaging (STA) of the phrenic nerve activity in relation to spinal neuron discharge was used to examine the temporal relationship of neuronal spikes and phrenic motor output (Lipski et al., 1983). Phrenic nerve activity was filtered with a 2-pole Butterworth filter (250–3,000 Hz), signals were rectified and integrated (time constant: 50 ms) for all subsequent analyses. Left and right phrenic nerves were averaged separately using the sorted spikes. Short-latency (e.g., < 1.0 ms) peaks > 5 standard deviations from the average background (calculated over 6 ms prior to the trigger) were taken as evidence that the recorded neuron was a phrenic motoneuron (Baekey et al., 2004; Sandhu et al., 2015; Streeter et al., 2017b). Identified phrenic motoneurons were not included in the subsequent analysis since our a priori intent was to study INs.
In our previous work (Sandhu et al., 2015; Streeter et al., 2017a), features in the phrenic nerve STA occurring with a lag time > 1.0 ms were taken as evidence that the recorded cell was synaptically antecedent to the phrenic motoneuron pool (i.e., pre-phrenic interneuron). In this data set, no pre-phrenic interneurons were identified. Thus, all non-phrenic motoneurons were classified as cervical interneurons (INs). Discharge frequency for all INs was compared between the ipsilateral and contralateral hemicords using three separate two-way ANOVAs (e.g., one for all neurons, one for hypoxia activated neurons, one for hypoxia inhibited neurons). Some neurons were not active throughout the protocol, and accordingly a repeated measures statistical design could not be used for the analysis. Individual time point comparisons were determined by Fisher’s LSD post hoc test.
To evaluate functional connectivity, cross-correlation histograms were constructed for all possible pairs of simultaneously recorded neurons using a bin width of 0.2 ms (Moore et al., 1970). The detectability index (DI) (Aertsen and Gerstein, 1985) was calculated for each correlogram as the peak (or trough) relative to average background activity (calculated over 12 ms prior to the trigger), divided by the standard deviations. Features were considered significant if the DI was > 3 (Aertsen and Gerstein, 1985; Aertsen et al., 1989; Melssen and Epping, 1987), and only significant features in the positive direction were counted. Peaks or troughs are consistent with functional excitation or inhibition between the trigger and target neurons, respectively (Aertsen and Gerstein, 1985; Kirkwood, 1979). Summary graphs were expressed as the number of positive cross-correlogram features (CCs) relative to the total number of possible features (i.e., the value if every neuronal pair had shown a correlogram feature). For statistical comparisons of the excitatory connections, Chi-square tests with Yates’ correction (Hazra and Gogtay, 2016) was used to analyze differences in the proportion of CCs between the ipsilateral and contralateral hemi-cords. Since fewer inhibitory connections were detected, the proportion of CCs between the ipsilateral and contralateral hemicords were analyzed with Fisher’s exact test which provides an exact p value (which is more appropriate for small sample sizes).
3. Results
3.1. Phrenic nerve burst amplitude and frequency
Baseline blood samples indicated that rats were well oxygenated (PaO2 = 198 ± 13 mmHg) and normocapnic (PaCO2 = 45 ± 2 mmHg). The mean arterial blood pressure at baseline was 107 ± 11 mmHg. Representative, compressed phrenic neurograms and the associated inspiratory burst frequency are shown in Fig. 2B. Inspiratory burst frequency was different across baseline, hypoxia, and post-hypoxia (F7,14 = 5.41, P = 0.0397; Fig. 2C). Post-hoc analysis confirmed the presence of post-hypoxia burst frequency depression (Bach et al., 1999) with lower phrenic burst rate compared to baseline values (P = 0.0140).
Phrenic neurograms illustrating ipsilateral and contralateral phasic inspiratory bursting, and mean data are provided in Fig. 2D and E. A distinct inspiratory burst was present in the ipsilateral phrenic nerve during baseline conditions in all experiments. Statistical analyses of phasic burst amplitude revealed an interaction between the condition (baseline, hypoxia, post-hypoxia) and the side of the recording (F2,28 = 5.892, P = 0.0073; Fig. 2E). Post-hoc tests indicated reduced phasic bursting in the ipsilateral vs. contralateral neurogram during baseline (P = 0.0032), hypoxia (P < 0.0001), and post-hypoxia (P = 0.0003). There was also a robust increase in both the ipsilateral (P = 0.0002) and contralateral (P < 0.0001) inspiratory burst amplitude during the acute hypoxic exposure (Fig. 2E).
Following hypoxia there was evidence for short-term potentiation (STP) of burst amplitude in both the ipsilateral and contralateral phrenic nerves (Lee et al., 2015). The raw amplitude (μv) of the contralateral phrenic burst was robustly elevated post-hypoxia (P = 0.0001 vs. baseline). The STP of ipsilateral burst amplitude (μv) was not statistically significant (P = 0.0787, Fig. 2E). When burst amplitude data were normalized to the baseline burst (%baseline, data not shown) an effect of condition (baseline, hypoxia, post-hypoxia) was evident (F2,28 = 34.23, P < 0.0001), and post-hoc analysis confirmed STP of both ipsilateral (40 ± 9%, P = 0.0119) and contralateral burst amplitude (45 ± 17%, P = 0.0051).
3.2. Spike triggered averaging of IN and phrenic nerve activity
Spike triggered averaging (STA) was used to determine if IN discharge was temporally related to phrenic motor output (Lipski et al., 1983). This approach can identify cervical INs which are synaptically coupled to phrenic motoneurons, often termed pre-phrenic INs (Sandhu et al., 2015; Streeter et al., 2017a, b). In the current data set there was no evidence that the recorded INs had direct synaptic connections to the phrenic motoneuron pool. Specifically, we were unable to detect peaks in the phrenic nerve STA occurring with a lag time greater than 1 ms, which would indicate direct synaptic connectivity to phrenic motoneurons.
3.3. Cervical IN discharge rates
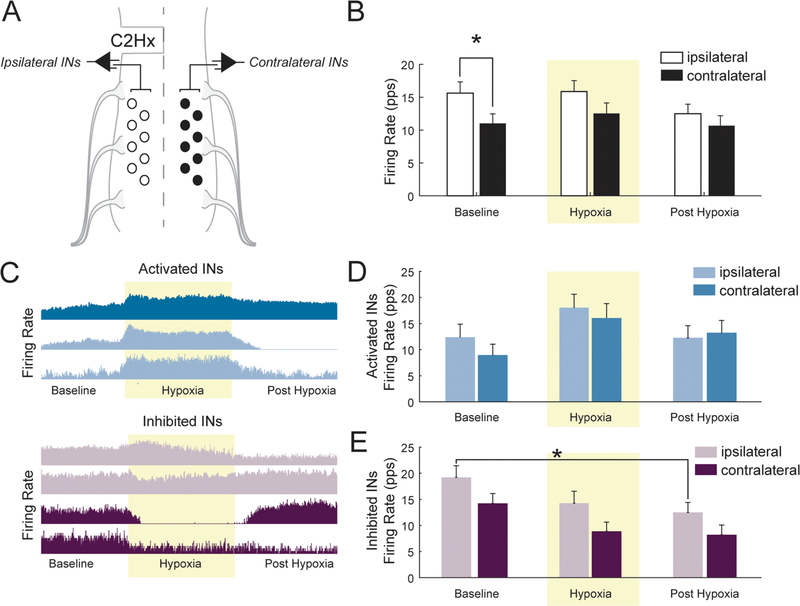
A schematic of the spinal IN recording approach is shown in Fig. 3A. The majority of the recorded INs discharged across both the inspiratory and expiratory phase, and were therefore considered to have tonic firing pattern. The average firing rates (pulses per second) are provided in Fig. 3B. Analyses of IN discharge revealed a main effect (F1,240 = 6.275, P = 0.0129) for the side of the recording (i.e., ipsi-lateral vs. contralateral). The difference in firing rate was most prominent at baseline, with ipsilateral firing rate significantly higher than contralateral (P = 0.0440; Fig. 3B). Discharge rates were further evaluated by stratifying INs based on their response to hypoxia: ‘activated’ cells increased discharge rate during hypoxia and ‘inhibited’ cells decreased firing (Fig. 3C). With this assessment, there was no apparent effect of the side of the recording for hypoxia activated INs (Fig. 3D, F1,129 = 1.093, P = 0.2979). In contrast, an effect of side (F1,105 = 8.714, P = 0.0039) and condition (F1,105 = 4.49, P = 0.0135) was detected for hypoxia inhibited INs. The overall statistical effect of side indicates that ipsilaterally and contralaterally located INs that were inhibited by hypoxia had different burst rates and this can be appreciated when viewing Fig. 3E. The post-hoc tests did not identify a specific condition during which the difference in burst rates were statistically different, but the P-values for each condition approached the0.05 threshold (baseline: P = 0.0702; hypoxia: P = 0.0742; post-hypoxia: P = 0.1413; Fig. 3E). The overall effect of condition indicates that hypoxia inhibited cells on both sides of the spinal cord decreased firing rate as the protocol progressed from baseline to post-hypoxia (Fig. 3E).
Fig. 3.
A. A schematic illustrating the method for multi-electrode array recordings of spinal INs following C2Hx. B. Average firing rate (pulses per second) of all ipsilateral and contralateral spinal INs before, during (shaded yellow), and after hypoxia. C. Representative compressed firing rate traces, from one experiment illustrating hypoxia-activated neurons (i.e. those which increase firing during hypoxia) and hypoxia-inhibited INs (i.e. those which decrease firing during hypoxia). D. Averaged firing rate of activated and E. inhibited INs before, during (shaded yellow), and after hypoxia. Post-hoc analysis indicated ipsilateral INs had a lower burst rate during the post-hypoxic period as compared to baseline (P = 0.0195, indicated by *).
3.4. Cross correlation of IN spikes
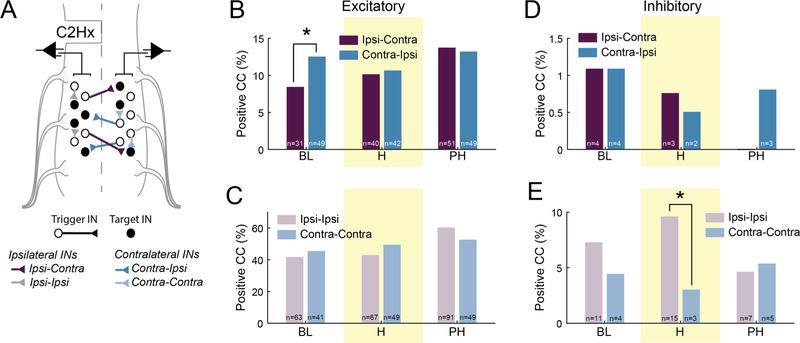
Cross-correlation analysis (Nuding et al., 2015; Segers et al., 2008) was used to examine the temporal relationship of action potential discharge between cervical IN pairs during all recording conditions. This analysis determines if an action potential spike from a “trigger” IN (open circles in Fig. 4A) will be followed by an action potential spike from a second “target” IN (filled circles in Fig. 4A). Positive (peaks) and negative (troughs) features in the resulting correlogram indicate excitatory or inhibitory synaptic connections, respectively (Aertsen and Gerstein, 1985; Kirkwood, 1979). The “directionality” of the inferred synaptic connection was determined based on the location of the recording electrodes. For example, if a trigger IN in the contralateral spinal cord produced a correlogram peak when “targeting” an ipsi-lateral IN, this was considered as an excitatory “contralateral to ipsi-lateral” connection. The observed number of IN pairs that produced a correlogram feature was expressed relative to the total number of possible IN connections (i.e., based on the total number of recorded cells at that time point). This analysis provided the proportion of IN pairs showing apparent synaptic connectivity and is provided in Fig. 4B–E.
Fig. 4.
A. Schematic illustrating cross-correlation analysis of bilateral multi-electrode array recordings of spinal INs following C2Hx. For each pair of neurons, the trigger cell (open circle) was used to form the correlation histogram, and the target cell (filled circle) fired in synchrony or after the trigger cell. Based on the recording location of the cell soma, a directionality (ex: ipsi to contra) of the connection was assigned. B, C. The proportion of excitatory, and D, E. inhibitory connections (as a % of possible connections) during baseline, hypoxia (shaded yellow) and post-hypoxia across the hemicord (ipsi-contra; contra-ipsi) and within each hemicord (ipsi-ipsi; contra-contra). The number of excitatory/inhibitory connections present at each time is noted in each bar. Note: the total number of possible connections varies within each group at each time point. * Denotes P < 0.05.
A contralateral-to-ipsilateral directionality was prevalent during the baseline recording conditions (Fig. 4B). Specifically, the proportion of detectable excitatory connections originating from the contralateral spinal cord was greater than the number that could be detected from the ipsi-lateral cord (X2(1, n = 737) = 3.899, P = 0.0483). Respiratory stimulation with hypoxia completely abolished the directionality of the connections. Thus, during hypoxia the relative proportion of excitatory connections was similar for contralateral-to-ipsilateral and ipsilateral-to-contralateral directions (X2(1, n = 788) = 0.013, P = 0.9071). This was also true during the post-hypoxia recording period (X2(1, n = 742) = 0.011 P = 0.9144). No indication of differences in the relative proportion of excitatory connections were observed within the ipsilateral (i.e., ipsilateral:ipsilateral) or contralateral spinal cord (i.e., contralateral:contralateral) during any of the recording conditions (Fig. 4C; baseline, X2(1, n = 241) = 0.199, P = 0.6550; hypoxia, X2(1, n = 255) = 0.799, P = 0.3713; post-hypoxia, X2(1, n = 244) = 1.059, P = 0.3035).
The cross-correlation analyses also revealed inhibitory synaptic connections (Fig. 4D–E). There was no evidence for a difference in the proportion of inhibitory ipsilateral:contralateral or contralateral:ipsilateral projections during any of the recording conditions (baseline: P > 0.9999; hypoxia: P > 0.9999; post-hypoxia: P = 0.2490). Differences were noted, however, regarding the inhibitory connectivity within each hemicord (Fig. 4E). Specifically, during the hypoxic exposure, the number of inhibitory connections were greater within the ipsilateral spinal cord (ipsilateral:ipsilateral; P = 0.0482) as compared to those detected opposite to the spinal lesion (contralateral:contralateral). Similar differences between the ipsilateral and contralateral recordings were not detected at baseline (P = 0.4251) or post-hypoxia (Fig. 4E, P = 0.7705). Overall, we detected approximately 10-fold fewer inhibitory connections as compared to the number of excitatory connections. However, inhibitory synaptic connections are more difficult to detect using cross-correlation analyses (Aertsen and Gerstein, 1985), and accordingly this result should not be taken as evidence that the cervical spinal cord has fewer inhibitory than excitatory propriospinal connections.
4. Discussion
Our findings reveal differences in discharge rates and connectivity between cervical INs located ipsilateral versus contralateral to a uni-lateral cervical SCI. Interneurons which were recorded ipsilateral and immediately caudal to C2Hx (i.e., cells deprived of the majority of descending synaptic input) fire at higher rates when compared to INs in the contralateral spinal cord and the majority of INs on both sides of the cord changed firing rate during acute exposure to hypoxia. Evaluation of temporal relationships between IN pairs with cross-correlation revealed a “directionality” of their synaptic connectivity. Specifically, there was a higher incidence of excitatory connections in a contralateral-to-ipsilateral direction as compared to the inverse. Our data also confirm that the capacity for hypoxia-induced dynamic remodeling of mid-cervical IN networks (Streeter et al., 2017a) is preserved in the chronically injured spinal cord. Collectively, the current data support the long-standing hypothesis (Moreno et al., 1992; Porter, 1895) that synaptic information “flows” across the spinal midline after hemisection injury (i.e., moving contralateral-to-ipsilateral). The results are also consistent with the hypothesis that cervical INs are part of the substrate enabling neuroplasticity in response to hypoxia (Sandhu et al., 2015; Streeter et al., 2017a) and/or spinal cord pathology (Romer et al., 2017; Zholudeva et al., 2018).
4.1. Cervical INs and phrenic motor output
The neural drive to breathe is generated in the brainstem and relayed to phrenic motoneurons via monosynaptic bulbospinal projections (Duffin and Iscoe, 1996; Ellenberger and Feldman, 1988; Tian and Duffin, 1996). There is also a significant role for cervical INs in inspiratory activation of phrenic motoneurons (Zaki Ghali et al., 2018). Upper (C1–C2) and mid-cervical (C3–C6) INs are synaptically coupled to phrenic motoneurons (Douse et al., 1992; Lane et al., 2008; Streeter et al., 2017a), and INs throughout the cervical cord have respiratory-related firing patterns (Bellingham and Lipski, 1990; Iscoe and Duffin, 1996; Palisses et al., 1989; Zaki Ghali et al., 2018). Subsequent studies in rats and cats have confirmed that INs can modulate phrenic motoneuron output (Bellingham and Lipski, 1990; Davies et al., 1985; Sandhu et al., 2015; Streeter et al., 2017a). In addition, several publications indicate an increase in synaptic connectivity between phrenic motoneurons and cervical INs after C2Hx (Buttry and Goshgarian, 2014; Zholudeva et al., 2017). In the current study, however, none of the recorded INs showed evidence of a monosynaptic connection with phrenic motoneurons as determined using established spike triggered averaging methods (Baekey et al., 2004; Sandhu et al., 2015; Streeter et al., 2017a). Accordingly, from the current data we draw no conclusions regarding the prevalence and/or importance of “prephrenic” INs following chronic C2Hx injury. However, the absence of evidence of such cells is not, in fact, evidence of absence. Although the sample size (92 recorded INs) and recording locations were similar to our previous study which detected pre-phrenic INs, it remains possible that the reduction in phasic bursting/increased tonic activity on the ipsilateral phrenic nerve as result of the C2Hx injury may have limited the ability to detect pre-phrenic INs.
4.2. Implications for motor control post-SCI
Cross-correlation analyses of IN firing indicated the predominance of excitatory connections projected from the contralateral to the ipsilateral cord. Beginning in the late 1800s, many authors speculated that after hemisection, synaptic information moves across the spinal midline in this direction (e.g., contralateral-to-ipsilateral) (Porter, 1895). Commissural phrenic motoneuron dendrites were the original hypothesis put forth (Porter, 1895) to explain what was later termed the “crossed phrenic phenomenon” (Goshgarian, 2003); but dendritic commissural projections, appear to be rare in adult animals (Prakash et al., 2000). On the other hand, bulbospinal fibers crossing the spinal midline caudal to C2Hx have been very clearly demonstrated (Goshgarian et al., 1991; Moreno et al., 1992). Accordingly, it seems highly likely that bulbospinal neurons contribute to activation of phrenic motoneurons located ipsilateral to C2Hx, although unequivocal neurophysiological proof is lacking.
An interneuronal relay may also be part of the anatomical substrate for “bridging” excitatory bulbospinal commands to phrenic motoneurons caudal and ipsilateral to hemisection (Lane et al., 2008; Zholudeva et al., 2018). For example, activation of upper cervical (C1–C2) INs with local application of glutamate following C1 transection increases phrenic motor output (Lu et al., 2004). Additionally, activation of glutamatergic INs, including Chx10-positive V2a (Romer et al., 2017) and Vglut2-positive (Cregg et al., 2017) neurons enhance respiratory motor output and have synaptic connections to phrenic motoneurons (Zholudeva et al., 2017). Our cross-correlation data (e.g., Fig. 4) suggest functional synaptic connectivity among commissural pathways caudal to injury. The time synchrony of discharge was consistent with INs on one side of the spinal cord having an excitatory influence on contralateral cells. Moreover, there was a predominance of contralateral-to-ipsilateral connectivity, at least during the baseline condition. Furthermore, recent evidence suggests that activation of excitatory INs after acute SCI is sufficient to restore breathing (Cregg et al., 2017; Satkunendrarajah et al., 2018). Although we discuss our results in the context of respiratory motor output, it should be noted that cervical INs are also part of the substrate that contributes to recovery of the upper limb muscles after SCI (Gonzalez-Rothi et al., 2015b).
Another observation from the current work was that acute exposure to hypoxia caused dynamic changes in IN burst rate, as well as functional connectivity. This is interesting to consider in regard to hypoxia-induced neuroplastic changes in respiratory, autonomic or somatic motor output (Dick et al., 2007; Fuller and Mitchell, 2016; Lovett-Barr et al., 2012). Acute exposure to mild, repeated episodes of hypoxia can also be a therapeutic approach to promote motor recovery following SCI (Gonzalez-Rothi et al., 2015a; Hayes et al., 2014; Tester et al., 2014; Trumbower et al., 2012). The overwhelming majority of prior mechanistic studies related to hypoxia-induced respiratory neuroplasticity have focused on motoneurons (for review (Devinney et al., 2013)). A few recent studies from our group, however, show that cervical IN networks respond to brief hypoxic exposure (Sandhu et al., 2015; Streeter et al., 2017b). For example, cervical IN connectivity is persistently altered after intermittent hypoxia exposure (Streeter et al., 2017a). The current study shows that the chronically injured spinal cord retains the capacity for dynamic modulation of spinal INs and accordingly, activation of spinal INs in response to a hypoxic challenge may be relevant as a means of enhancing motor output below the level of injury (Gonzalez-Rothi et al., 2015a). This is not limited to restoring respiratory motor output, as it is well documented that hypoxia can enhance somatic motor function (Lovett-Barr et al., 2012), voluntary ankle strength (Trumbower et al., 2012), and locomotor outcomes (Hayes et al., 2014) which may involve cervical INs (Gonzalez-Rothi et al., 2015b).
Lastly, we noted differences in the discharge rates of INs recorded ipsilateral (vs. contralateral) to C2Hx injury. Counter to our original hypothesis, ipsilateral INs had a higher firing rate than contralateral INs during the baseline recording period. A similar increase in discharge rate has been observed in phrenic motoneurons recorded ipsilateral to C2Hx (el-Bohy et al., 1998). There are multiple mechanisms that could underlie differences in ipsilateral vs. contralateral neuronal firing. Perhaps the simplest explanation is the release from a descending inhibitory influence (i.e., following axotomy of bulbospinal axons) manifested as in increase in IN discharge. Another possibility is that descending synaptic pathways which cross the spinal midline caudal to the lesion (Goshgarian et al., 1991; Lane et al., 2009) may be excitatory and result in an increase in IN activity ipsilateral to injury. A final consideration is that compensatory neuroplastic changes within the INs may be occurring in response to reduced levels of activity/inactivity. Changes in synaptic morphology (i.e., increase in synapses, dendrodendritic appositions, and active zones) in identified phrenic motoneurons can rapidly occur after disruption of descending inputs (Castro-Moure and Goshgarian, 1997) and C2Hx (Sperry and Goshgarian, 1993), and similar changes may occur in INs caudal to injury. In addition, brief or prolonged periods of reduced activity in the phrenic motor pool can activate complex mechanisms which lead to “inactivity-induced inspiratory motor facilitation” upon restoration of synaptic inputs (Streeter and Baker-Herman, 2014). Thus, initial periods of inactivity in phrenic motoneurons or cervical INs after C2Hx could trigger cellular mechanisms of homeostatic plasticity that lead to subsequent increases in neuronal discharge rates (Braegelmann et al., 2017). To our knowledge, however, the potential for inactivity to induce plasticity in mid-cervical INs has not been formally studied.
4.3. Conclusion
Recent work has emphasized the “neuroplastic potential” of segmental and propriospinal INs in neurological disorders such as SCI (Bareyre et al., 2004; Cregg et al., 2017; Jiang et al., 2018; Lane et al., 2008; May et al., 2017; Rank et al., 2018; Romer et al., 2017; Satkunendrarajah et al., 2018; Zholudeva et al., 2017, 2018). Spinal INs provide a substrate for restorative and/or compensatory neuroplasticity and accordingly, may serve as a therapeutic target after SCI. The current results provide additional insights by demonstrating a “directionality” of segmental IN connections consistent with the hypothesis that synaptic information may be relayed across the spinal midline to activate silent circuits after unilateral SCI (Goshgarian, 2003, 2009). These data also confirm that the capacity for hypoxia-induced dynamic remodeling of mid-cervical networks (Streeter et al., 2017a) is preserved in the chronically injured spinal cord. Spinal INs may thus be relevant in the context of neurorehabilitation strategies that utilize brief exposures to hypoxia as a means of enhancing respiratory, somatic, and sympathetic motor output.
Acknowledgements
This work was supported by funding from the National Institute of Health, grant numbers: RO1NS080180-01A1 (DDF), R01HL139708-01A1 (DDF), F32NS095620-01 (KAS), K99HL143207-01 (KAS) and T32-ND043730 (MDS). Additional support was provided by the State of Florida Brain and Spinal Cord Injury Research Trust Fund (DDF and PJR) awarded through the McKnight Brain Institute at the University of Florida and The Department of Defense, grant number W81XWH-14-1-0625 (PJR and DDF).
Footnotes
Declaration of Competing Interest
The authors declare no competing financial interests.
References
- Aertsen AM, Gerstein GL, 1985. Evaluation of neuronal connectivity: sensitivity of cross-correlation. Brain Res. 340, 341–354. [DOI] [PubMed] [Google Scholar]
- Aertsen AM, Gerstein GL, Habib MK, Palm G, 1989. Dynamics of neuronal firing correlation: modulation of “effective connectivity”. J. Neurophysiol 61, 900–917. [DOI] [PubMed] [Google Scholar]
- Bach KB, Kinkead R, Mitchell GS, 1999. Post-hypoxia frequency decline in rats: sensitivity to repeated hypoxia and alpha2-adrenoreceptor antagonism. Brain Res. 817, 25–33. [DOI] [PubMed] [Google Scholar]
- Baekey DM, Morris KF, Nuding SC, Segers LS, Lindsey BG, Shannon R, 2004. Ventrolateral medullary respiratory network participation in the expiration reflex in the cat. J. Appl. Physiol. (1985) 96, 2057–2072. [DOI] [PubMed] [Google Scholar]
- Baker-Herman TL, Fuller DD, Bavis RW, Zabka AG, Golder FJ, Doperalski NJ, Johnson RA, Watters JJ, Mitchell GS, 2004. BDNF is necessary and sufficient for spinal respiratory plasticity following intermittent hypoxia. Nat. Neurosci 7, 48–55. [DOI] [PubMed] [Google Scholar]
- Bareyre FM, Kerschensteiner M, Raineteau O, Mettenleiter TC, Weinmann O, Schwab ME, 2004. The injured spinal cord spontaneously forms a new intraspinal circuit in adult rats. Nat. Neurosci 7, 269–277. [DOI] [PubMed] [Google Scholar]
- Bellingham MC, Lipski J, 1990. Respiratory interneurons in the C5 segment of the spinal cord of the cat. Brain Res. 533, 141–146. [DOI] [PubMed] [Google Scholar]
- Braegelmann KM, Streeter KA, Fields DP, Baker TL, 2017. Plasticity in respiratory motor neurons in response to reduced synaptic inputs: a form of homeostatic plasticity in respiratory control? Exp. Neurol 287, 225–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buttry JL, Goshgarian HG, 2014. Injection of WGA-Alexa 488 into the ipsilateral hemidiaphragm of acutely and chronically C2 hemisected rats reveals activity-dependent synaptic plasticity in the respiratory motor pathways. Exp. Neurol 261, 440–450. [DOI] [PubMed] [Google Scholar]
- Castro-Moure F, Goshgarian HG, 1997. Morphological plasticity induced in the phrenic nucleus following cervical cold block of descending respiratory drive. Exp. Neurol 147, 299–310. [DOI] [PubMed] [Google Scholar]
- Cregg JM, Chu KA, Hager LE, Maggard RSJ, Stoltz DR, Edmond M, Alilain WJ, Philippidou P, Landmesser LT, Silver J, 2017. A latent propriospinal network can restore diaphragm function after high cervical spinal cord injury. Cell Rep. 21, 654–665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies JG, Kirkwood PA, Sears TA, 1985. The distribution of monosynaptic connexions from inspiratory bulbospinal neurones to inspiratory motoneurones in the cat. J. Physiol 368, 63–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devinney MJ, Huxtable AG, Nichols NL, Mitchell GS, 2013. Hypoxia-induced phrenic long-term facilitation: emergent properties. Ann. N. Y. Acad. Sci 1279, 143–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dick TE, Hsieh YH, Wang N, Prabhakar N, 2007. Acute intermittent hypoxia increases both phrenic and sympathetic nerve activities in the rat. Exp. Physiol 92, 87–97. [DOI] [PubMed] [Google Scholar]
- Douse MA, Duffin J, Brooks D, Fedorko L, 1992. Role of upper cervical inspiratory neurons studied by cross-correlation in the cat. Exp. Brain Res 90, 153–162. [DOI] [PubMed] [Google Scholar]
- Duffin J, Iscoe S, 1996. The possible role of C5 segment inspiratory interneurons investigated by cross-correlation with phrenic motoneurons in decerebrate cats. Exp. Brain Res 112, 35–40. [DOI] [PubMed] [Google Scholar]
- el-Bohy AA, Schrimsher GW, Reier PJ, Goshgarian HG, 1998. Quantitative assessment of respiratory function following contusion injury of the cervical spinal cord. Exp. Neurol 150, 143–152. [DOI] [PubMed] [Google Scholar]
- Ellenberger HH, Feldman JL, 1988. Monosynaptic transmission of respiratory drive to phrenic motoneurons from brainstem bulbospinal neurons in rats. J. Comp. Neurol 269, 47–57. [DOI] [PubMed] [Google Scholar]
- Fujiki M, Kobayashi H, Inoue R, Ishii K, 2004. Immediate plasticity in the motor pathways after spinal cord hemisection: implications for transcranial magnetic motor-evoked potentials. Exp. Neurol 187, 468–477. [DOI] [PubMed] [Google Scholar]
- Fuller DD, Doperalski NJ, Dougherty BJ, Sandhu MS, Bolser DC, Reier PJ, 2008. Modest spontaneous recovery of ventilation following chronic high cervical hemisection in rats. Exp. Neurol 211, 97–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuller DD, Mitchell GS, 2016. Respiratory neuroplasticity - Overview, significance and future directions. Exp. Neurol 287, 144–152. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Rothi EJ, Lee KZ, Dale EA, Reier PJ, Mitchell GS, Fuller DD, 2015a. Intermittent hypoxia and neurorehabilitation. J. Appl. Physiol. (1985) 119, 1455–1465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Rothi EJ, Rombola AM, Rousseau CA, Mercier LM, Fitzpatrick GM, Reier PJ, Fuller DD, Lane MA, 2015b. Spinal interneurons and forelimb plasticity after incomplete cervical spinal cord injury in adult rats. J. Neurotrauma 32, 893–907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goshgarian HG, 2003. The crossed phrenic phenomenon: a model for plasticity in the respiratory pathways following spinal cord injury. J. Appl. Physiol 94, 795–810. [DOI] [PubMed] [Google Scholar]
- Goshgarian HG, 2009. The crossed phrenic phenomenon and recovery of function following spinal cord injury. Respir. Physiol. Neurobiol 169, 85–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goshgarian HG, Ellenberger HH, Feldman JL, 1991. Decussation of bulbospinal respiratory axons at the level of the phrenic nuclei in adult rats: a possible substrate for the crossed phrenic phenomenon. Exp. Neurol 111, 135–139. [DOI] [PubMed] [Google Scholar]
- Hayes HB, Jayaraman A, Herrmann M, Mitchell GS, Rymer WZ, Trumbower RD, 2014. Daily intermittent hypoxia enhances walking after chronic spinal cord injury: a randomized trial. Neurology 82, 104–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hazra A, Gogtay N, 2016. Biostatistics series module 4: comparing groups – categorical variables. Indian J. Dermatol 61, 385–392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iscoe S, Duffin J, 1996. Effects of stimulation of phrenic afferents on cervical respiratory interneurones and phrenic motoneurones in cats. J. Physiol 497 (Pt 3), 803–812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang YQ, Sarkar A, Amer A, Martin JH, 2018. Transneuronal downregulation of the premotor cholinergic system after corticospinal tract loss. J. Neurosci 38, 8329–8344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkwood PA, 1979. On the use and interpretation of cross-correlation measurements in the mammalian central nervous system. J. Neurosci. Methods 1, 107–132. [DOI] [PubMed] [Google Scholar]
- Lane MA, Lee KZ, Fuller DD, Reier PJ, 2009. Spinal circuitry and respiratory recovery following spinal cord injury. Respir. Physiol. Neurobiol 169, 123–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane MA, White TE, Coutts MA, Jones AL, Sandhu MS, Bloom DC, Bolser DC, Yates BJ, Fuller DD, Reier PJ, 2008. Cervical prephrenic interneurons in the normal and lesioned spinal cord of the adult rat. J. Comp. Neurol 511, 692–709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee KZ, Sandhu MS, Dougherty BJ, Reier PJ, Fuller DD, 2015. Hypoxia triggers short term potentiation of phrenic motoneuron discharge after chronic cervical spinal cord injury. Exp. Neurol 263, 314–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipski J, Kubin L, Jodkowski J, 1983. Synaptic action of R beta neurons on phrenic motoneurons studied with spike-triggered averaging. Brain Res. 288, 105–118. [DOI] [PubMed] [Google Scholar]
- Lovett-Barr MR, Satriotomo I, Muir GD, Wilkerson JE, Hoffman MS, Vinit S, Mitchell GS, 2012. Repetitive intermittent hypoxia induces respiratory and somatic motor recovery after chronic cervical spinal injury. J. Neurosci 32, 3591–3600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu F, Qin C, Foreman RD, Farber JP, 2004. Chemical activation of C1–C2 spinal neurons modulates intercostal and phrenic nerve activity in rats. Am. J. Physiol. Regul. Integr. Comp. Physiol 286, R1069–1076. [DOI] [PubMed] [Google Scholar]
- May Z, Fenrich KK, Dahlby J, Batty NJ, Torres-Espín A, Fouad K, 2017Following spinal cord injury transected reticulospinal tract axons develop new collateral inputs to spinal interneurons in parallel with locomotor recovery. Neural Plast. 1932875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melssen WJ, Epping WJM, 1987. Detection and estimation of neural connectivity based on crosscorrelation analysis. Biol. Cybern 57, 403–414. [DOI] [PubMed] [Google Scholar]
- Moore GP, Segundo JP, Perkel DH, Levitan H, 1970. Statistical signs of synaptic interaction in neurons. Biophys. J 10, 876–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno DE, Yu XJ, Goshgarian HG, 1992. Identification of the axon pathways which mediate functional recovery of a paralyzed hemidiaphragm following spinal cord hemisection in the adult rat. Exp. Neurol 116, 219–228. [DOI] [PubMed] [Google Scholar]
- Nuding SC, Segers LS, Iceman KE, O’Connor R, Dean JB, Bolser DC, Baekey DM, Dick TE, Shannon R, Morris KF, Lindsey BG, 2015. Functional connectivity in raphé-pontomedullary circuits supports active suppression of breathing during hypocapnic apnea. J. Neurophysiol 114, 2162–2186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palisses R, Persegol L, Viala D, 1989. Evidence for respiratory interneurones in the C3–C5 cervical spinal cord in the decorticate rabbit. Exp. Brain Res 78, 624–632. [DOI] [PubMed] [Google Scholar]
- Porter WT, 1895. The path of the respiratory impulse from the bulb to the phrenic nuclei. J. Physiol 17, 455–485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prakash YS, Mantilla CB, Zhan WZ, Smithson KG, Sieck GC, 2000. Phrenic motoneuron morphology during rapid diaphragm muscle growth. J. Appl. Physiol 89, 563–572. [DOI] [PubMed] [Google Scholar]
- Rank MM, Galea MP, Callister R, Callister RJ, 2018. Is more always better? How different’ doses’ of exercise after incomplete spinal cord injury affects the membrane properties of deep dorsal horn interneurons. Exp. Neurol 300, 201–211. [DOI] [PubMed] [Google Scholar]
- Romer SH, Seedle K, Turner SM, Li J, Baccei ML, Crone SA, 2017. Accessory respiratory muscles enhance ventilation in ALS model mice and are activated by excitatory V2a neurons. Exp. Neurol 287, 192–204. [DOI] [PubMed] [Google Scholar]
- Rowley KL, Mantilla CB, Sieck GC, 2005. Respiratory muscle plasticity. Respir. Physiol. Neurobiol. 147, 235–251. [DOI] [PubMed] [Google Scholar]
- Sandhu MS, Baekey DM, Maling NG, Sanchez JC, Reier PJ, Fuller DD, 2015. Midcervical neuronal discharge patterns during and following hypoxia. J. Neurophysiol 113, 2091–2101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satkunendrarajah K, Karadimas SK, Laliberte AM, Montandon G, Fehlings MG, 2018. Cervical excitatory neurons sustain breathing after spinal cord injury. Nature 562, 419–422. [DOI] [PubMed] [Google Scholar]
- Segers LS, Nuding SC, Dick TE, Shannon R, Baekey DM, Solomon IC, Morris KF, Lindsey BG, 2008. Functional connectivity in the pontomedullary respiratory network. J. Neurophysiol 100, 1749–1769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sperry MA, Goshgarian HG, 1993. Ultrastructural changes in the rat phrenic nucleus developing within 2 h after cervical spinal cord hemisection. Exp. Neurol 120, 233–244. [DOI] [PubMed] [Google Scholar]
- Streeter KA, Baker-Herman TL, 2014. Decreased spinal synaptic inputs to phrenic motor neurons elicit localized inactivity-induced phrenic motor facilitation. Exp. Neurol 256, 46–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streeter KA, Sunshine MD, Patel S, Gonzalez-Rothi EJ, Reier PJ, Baekey DM, Fuller DD, 2017a. Intermittent hypoxia enhances functional connectivity of midcervical spinal interneurons. J. Neurosci 37, 8349–8362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streeter KA, Sunshine MD, Patel SR, Liddell SS, Denholtz LE, Reier PJ, Fuller DD, Baekey DM, 2017b. Coupling multielectrode array recordings with silver labeling of recording sites to study cervical spinal network connectivity. J. Neurophysiol 117, 1014–1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tester NJ, Fuller DD, Fromm JS, Spiess MR, Behrman AL, Mateika JH, 2014. Long-term facilitation of ventilation in humans with chronic spinal cord injury. Am. J. Respir. Crit. Care Med 189, 57–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian GF, Duffin J, 1996. Spinal connections of ventral-group bulbospinal inspiratory neurons studied with cross-correlation in the decerebrate rat. Exp. Brain Res 111, 178–186. [DOI] [PubMed] [Google Scholar]
- Trumbower RD, Jayaraman A, Mitchell GS, Rymer WZ, 2012. Exposure to acute intermittent hypoxia augments somatic motor function in humans with incomplete spinal cord injury. Neurorehabil. Neural Repair 26, 163–172. [DOI] [PubMed] [Google Scholar]
- Zaki Ghali MG, Britz G, Lee KZ, 2018. Pre-phrenic interneurons: characterization and role in phrenic pattern formation and respiratory recovery following spinal cord injury. Respir. Physiol. Neurobiol 265, 24–31. [DOI] [PubMed] [Google Scholar]
- Zholudeva LV, Karliner JS, Dougherty KJ, Lane MA, 2017. Anatomical recruitment of spinal V2a interneurons into phrenic motor circuitry after high cervical spinal cord injury. J. Neurotrauma 34, 3058–3065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zholudeva LV, Qiang L, Marchenko V, Dougherty KJ, Sakiyama-Elbert SE, Lane MA, 2018. The neuroplastic and therapeutic potential of spinal interneurons in the injured spinal cord. Trends Neurosci. 41, 625–639. [DOI] [PMC free article] [PubMed] [Google Scholar]