Abstract
Attention-deficit/hyperactivity disorder (ADHD) is a highly heritable neurodevelopmental disorder. However, no study has examined genetic and environmental influences in the longitudinal developmental course of ADHD symptoms in a non-Western population. This study investigated changes of genetic and environmental influences and their contributions to the stability and change of ADHD symptoms of hyperactivity/impulsivity and inattention in Chinese adolescent twins. A prospective sample of 602 twin pairs (48% male) self-reported both DSM-IV ADHD symptom subscales three times at the approximate age of 12, 13, and 15 years. Longitudinal multivariate genetic analyses through structural equation modeling examined genetic and environmental contributions to the developmental course of ADHD symptoms. From early (time 1 & 2) to middle adolescence (time 3), both symptoms showed modest and non-significant genetic influences that became substantial and significant, whereas shared environmental influences were substantial and significant and became modest and non-significant. The same genetic factors influenced ADHD symptoms throughout adolescence, while shared and non-shared environmental influences largely came from new emerging factors. In early adolescence, genetic factor contributed to the stability of inattention, whereas shared environmental factor contributed to the stability of hyperactivity/impulsivity. Genetic influences of ADHD tended to be smaller, whereas shared environmental influences tended to be larger in Chinese than in Western populations. Genetic factors played a large role in the stability of ADHD throughout adolescence, while shared and non-shared environment primarily contributed to its change. Findings highlight the importance of shared family, neighborhood, and community experiences on child psychopathology in a collectivistic culture such as the Chinese society.
Keywords: adolescent, ADHD, Chinese, genetic influences, twins
Introduction
As a neurodevelopmental disorder, attention-deficit/hyperactivity disorder (ADHD) is one of the most common psychiatric disorders among children and adolescents, with an estimated prevalence of 3.4% worldwide [1–3]. The prevalence of ADHD is comparable in developing countries such as China [4–7]. Males have higher prevalence than females [1, 4–6, 8]. Despite a decreasing prevalence [8, 9], childhood ADHD persists into adolescence and adulthood substantially, with up to 78% of children with ADHD continuing to meet full or subthreshold of DSM diagnostic criteria or fail to attain functional remission by age 25–30 years [10, 11]. Furthermore, hyperactivity/impulsivity tends to decrease from childhood to adolescence, whereas inattention tends to remain stable [12, 13]. Both the clinical disorder and subclinical symptoms of ADHD, as well as the developmental course (i.e., systematic changes in symptoms), are associated with a wide range of concurrent and long-term functional impairments, such as low academic performance, social dysfunction, criminal behavior, substance abuse/dependency, and mental health problems [12–15].
Twin studies have demonstrated that ADHD in children and adolescents are predominantly under genetic influences (approximately 70–80%), with the remaining variance explained by child-specific environmental experiences (experiences not shared between siblings that make them different from each other) [16, 17]. Few studies have found shared environmental experiences (experiences shared by siblings that make them similar to each other) [18–22], and a meta-analysis suggested negligible magnitude [17], although a more conservative analysis suggested modest shared environmental effects (22%) [23]. Longitudinal twin studies can facilitate our understanding of the developmental changes of genetic and environmental influences on child and adolescent ADHD. Particularly, they can reveal the change in the magnitudes of genetic and environmental influences over time, and unravel genetic and environmental contributions to the stability and change of ADHD, hence showing their dynamic developmental patterns [24]. For example, genetic attenuation is suggested when genetic factors at previous times explain less phenotypic variance at later times, while genetic innovation is suggested when new genetic factors emerge at later times that explain phenotypic variance [25].
Several longitudinal twin studies have examined genetic and environmental etiology of the development of ADHD using large Western population-based samples. For instance, among Swedish girls and boys, genetic influences were 68% and 35% at age 8–9 years, 61% and 74% at age 13–14 years, respectively. At age 16–17 years, genetic influences were between 62–73% and similarly in young adulthood (19–20 years) [19, 20, 26]. Shared environmental influences decreased from modest to moderate at 8–9 years (12% for girls and 40% for boys) to negligible in later times [19, 20, 26]. Substantial genetic influences (72–86%) were also found in UK children from age 2 to 8 years, with modest shared environmental influences (14%) found only at age 8 years [27, 28]. Similar findings on genetic influences were also reported among Dutch twins from age 3 to 12 years (70–74%) [29–31], while adolescent (12–18 years) and adult (>18 years) twins showed lower genetic influences (40–56%) [32]. However, no shared environmental influences were revealed at any age in Dutch studies [29–32]. In all Swedish, UK, and Netherland studies, common genetic factors largely explained the stability over development, although new genetic factors also emerged over time (i.e., genetic innovation). Furthermore, genetic factors also explained systematic changes (e.g., increasing or decreasing symptoms) over time [33]. A short-term (19-month) longitudinal study among 8–16 years old US twins also supported common as well as new genetic factors over time [34]. However, another study [21] using US adolescent twins found that genetic influences decreased, whereas shared environmental influences increased from age 12 to 16 years, and both genetic and shared environmental factors largely contributed to stability. All previous studies consistently demonstrated that non-shared environmental influences were largely time-specific (i.e., environmental innovation) in that different non-shared environmental factors affected ADHD symptoms at different times (i.e., low stability), hence primarily contributing to the change of ADHD symptoms over time.
Despite the cumulative evidence of genetic etiology of child and adolescent ADHD, almost all previous twin studies used samples from developed Western countries. Therefore, the generalization of these findings to non-Western developing countries such as China is largely unknown. Different genetic backgrounds and socialcultural differences between Western and non-Western societies could bring out different genetic and environmental influences on ADHD. Notably, recent Chinese twin studies on other psychiatric symptoms (e.g., depression) suggest that genetic influences are smaller, whereas shared environmental influences are larger in Chinese children and adolescents than in Western populations [35–38]. Scarce twin studies have examined genetic etiology of ADHD in Chinese children and adolescents, and all were cross-sectional. One study reported 70% and 82% genetic influences in 279 pairs of twins and same-sex sibling-pairs of adolescent girls and boys (12–16 years), respectively, without shared environmental influences [39], whereas another study reported similar genetic influences (72%) but significant shared (20%) and modest non-shared (8%) environmental influences in 1316 6–18 years old Chinese twins [36]. Another small study of 66 pairs of 12–18 years old Chinese adolescent twins nevertheless reported modest genetic influences (26%) without shared environmental influences [40]. Another study using 662 pairs of Korean twins 3–13 years old also found that genetic influences on ADHD symptoms were lower than those typically found in Western populations, without shared environmental influences [41]. Therefore, current evidence of genetic and environmental influences on ADHD in Chinese children and adolescents remains inconclusive, whereas genetic and environmental contributions to the stability and change of ADHD in the Chinese population are unknown.
To our knowledge, no study has investigated the changes of genetic and environmental influences on child and adolescent ADHD with a prospective longitudinal twin design in a non-Western population. Therefore, our primary goal was to examine the changes in the magnitudes of genetic and environmental influences on ADHD symptoms from early (approximately 12 and 13 years) to middle (approximately 15 years) adolescence in a Chinese twin sample. The second goal was to investigate genetic and environmental contributions to the stability and change of ADHD throughout adolescence, more specifically, genetic and environmental innovation and attenuation in the phenotypic stability and change of ADHD symptoms. The prevalence and levels of ADHD decrease during the transition from childhood to adolescence, as well as during adolescence (e.g., from 9–12/13 years to 14–17/18 years) [8, 9]. Hence, the time period followed in this study could potentially reveal stability as well as change of ADHD. Because previous studies have shown that inattention and hyperactivity/impulsivity show different genetic etiology and developmental courses [5, 12, 13, 16, 17], we investigated these two dimensions separately. We first hypothesized that, as the pattern generally observed in Western populations, the magnitude of genetic influences would increase, whereas the magnitude of shared environmental influences would decrease, from early to middle adolescence. However, based on extant Chinese twin studies on other psychiatric symptoms, we hypothesized that the magnitude of genetic influences could be smaller, whereas shared environmental influences could be larger in the current Chinese sample, compared to common estimates from Western populations. Third, we also hypothesized that genetic factors would contribute to both stability and change, while environment would primarily contribute to the change, of ADHD throughout adolescence.
Methods
Participants and procedure
Participants in the study were drawn from the Qingdao Twin Registry (QTR) [42, 43]. Twins were recruited to join through medical records, schools, and media outreach campaigns. The QTR includes approximately 74% of all twins living in the city of Qingdao, China as of 2005. More details on recruitment and survey procedures were described elsewhere [42, 43]. Zygosity was determined by DNA testing using 16 short tandem repeat markers in blood samples, with an over 0.996 probability of correctly identifying monozygosity. The current sample comprised of the adolescent cohort who were first contacted and asked to take part in 2006 (n = 1204) and were followed up approximately one year and three months later in 2007 (n = 1106), and again approximately two years later in 2009 (n = 907). The sample comprised of 602 pairs of twins 9–16 years old in 2006 (M = 12.16 years, SD = 1.93, 58.1% between 10 and 12 years old, males = 48.4%, 99% Han ethnicity), with 321 monozygotic (MZ) and 281 dizygotic (DZ) twin pairs. In 2009 the mean age of the sample was 14.99 years (SD = 1.88, 63.1% between 13 and 15 years old, males = 48.2%). Informed consent was obtained from participants and their parents. All procedures were approved from the University of Southern California institutional review board.
Measures
Attention-Deficit/Hyperactivity Disorder was measured with six items derived from the DSM-IV diagnostic criteria [44] by self-reports through paper-and-pencil questionnaire, with three items each for inattentive and hyperactive/impulsive symptoms. Items for inattention included: have difficulty keeping attention on tasks or activities; avoid, dislike, or put off tasks requiring mental efforts over a long period of time; and do not seem to listen when spoken to directly. Items for hyperactivity/impulsivity included: have difficulty doing activities quietly; have feelings of restlessness; and “on the go” or often act as if “driven by a motor”. Participants rated each item on a five-point scale (0 = no or never; 1 = mildly or rarely; 2 = moderately; 3 = quite a lot; 4 = very often). A total score was created by summing all items, for inattention and hyperactivity/impulsivity separately. Both symptoms were normally distributed. Across three time points, skewness ranged from 0.70 to 1.14, while kurtosis ranged from 0.13 to 1.62. Hence, no further transformation was made to these scores. Cronbach’s α over three times ranged from 0.81 to 0.82 and from 0.72 to 0.82 for inattention and hyperactivity/impulsivity, respectively.
Statistical analysis
Twin analyses make use of the difference in genetic relatedness between MZ twins, who share all of their alleles, and DZ twins, who share on average half of their segregating alleles. In univariate models, the total phenotypic variance is divided into three independent components: additive genetic factor (A) that represents the sum of effects of alleles at different loci that affect a phenotype, shared environmental factor (C) that represents non-genetic factors shared by family members making twins similar to each other, and non-shared environmental factor (E) that represents unique environmental influences making twins different from each other, as well as measurement error. The correlation between twins for A is 1.0 for MZ twins and 0.5 for DZ twins, reflecting their genetic resemblance. The correlation for C is 1.0 for both MZ and DZ twins growing up in the same household. Non-shared environmental factors are not correlated between twins [45]. Higher MZ correlation than for DZ twins suggests A. Shared environmental influences can be inferred from the remaining familial resemblance not explained by A and can be estimated by subtracting estimated A from MZ correlation. Non-shared environmental influences can be inferred by the extent to which the MZ correlation is less than 1.0.
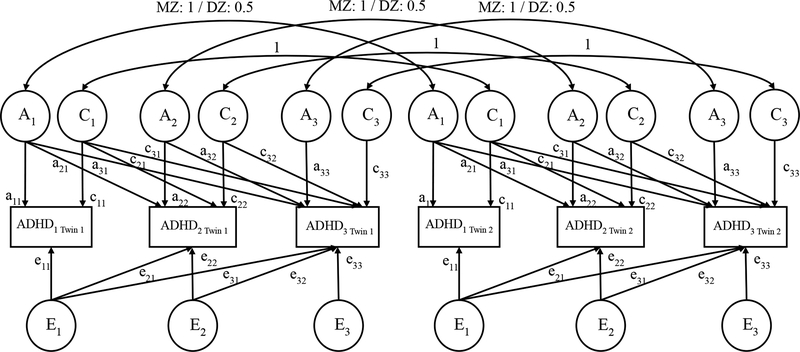
Longitudinal multivariate twin analysis focuses on the covariance between variables. Cross-twin crosstime correlations between MZ and DZ twins are compared to decompose the covariance into A, C, and E components. Larger MZ correlations than for DZ twins suggest genetic contributions to the phenotypic stability over time. We used the Cholesky decomposition to examine longitudinal genetic and environmental influences on the developmental course of ADHD symptoms (see Figure 1). For instance, at time 1, ADHD symptoms of each twin (ADHD1Twin1 and ADHD1Twin2) were explained by latent genetic (A1), shared environmental (C1), and non-shared environmental (E1) factors through their respective paths (a11, c11, e11). The correlations between A1 (1.0 for MZ and 0.5 for DZ twins) and C1 (1.0 for both MZ and DZ twins) factors for twins in the same pair were specified according to the above description. Influences from previous times can directly affect all later assessments (e.g., A1 also loads on time 2 and 3 symptoms through paths a21 and a31). New influences also emerge at each subsequent assessment (e.g., time 2 symptoms are influenced by A2 through path a22), and these influences can also affect all later assessments (e.g., A2 also loads on time 3 symptoms through path a32). Cholesky model can straightforwardly illustrate genetic innovation and attenuation and thus is especially suited for longitudinal data.
Figure 1.
Cholesky Model of ADHD Symptoms over Three Times. A: additive genetic influence. C: shared environmental influences. E: non-shared environmental influences. MZ: monozygotic twins. DZ: dizygotic twins
All twin analyses were done using the structural equation modeling (SEM) package OpenMx [46] with raw data maximum likelihood estimation to handle missing data. Parameter estimates, 95% confidence intervals (CIs), and model fit statistics were provided. Model goodness of fit was assessed with minus twice the log likelihood (−2LL). Difference in −2LL between a full model and a nested submodel (reduced model with fewer parameters) was assessed by χ2 tests, with the degrees of freedom equal to the difference in the number of parameters estimated between the full and the reduced model. A non-significant χ2 test suggests the reduced model as a more parsimonious model. SEM is a unified platform for path analysis and variance component models. Through matrix calculation and numerical optimization by minimizing a goodness-of-fit function between observed and predicted covariance matrices, SEM can test hypothesized relationships between observed (e.g., ADHD symptoms) and latent (e.g., A, C, and E factors) variables, estimate model parameters, compare the fit of different models. Hence, one can infer the relative contributions (i.e., importance) of latent (i.e., genetic and environmental) factors through modeling observed covariance matrices of both MZ and DZ twins simultaneously [45]. Consistent with previous studies [21, 26, 32, 47, 48], preliminary analyses suggested no sex difference in genetic and environmental influences on ADHD symptoms. Therefore, analyses were conducted with males and females included together.
Results
Descriptive Statistics and Correlations
As shown in Table 1, males consistently showed higher levels of all symptoms at each time than females. SEM constraining means to be the same across males and females at each time demonstrated that females showed significantly lower levels of inattentive and hyperactivity/impulsivity symptoms at time 1, χ2(1) = 6.07 and 10.28, ps = 0.01 and 0.00, respectively, and at time 3, χ2(1) = 6.69 and 7.95, ps = 0.01 and 0.00, respectively. No sex difference was revealed for either symptom in time 2, χ2(1) = 2.76 and 1.10, ps = 0.10 and 0.29, respectively. From time 1 to 2, hyperactivity/impulsivity significantly decreased, χ2(1) = 7.22, p = 0.01, whereas inattention remained stable, χ2(1) = 0.80, p = 0.37. From time 2 to 3, neither symptoms showed significant decrease, χ2(1) = 0.32 and 3.00, ps = 0.57 and 0.08, respectively. Both symptoms showed moderate stability: between time 1 and 2, Pearson’s rs = 0.34 (95% CI: 0.28–0.39) and 0.34 (95% CI: 0.28–0.40) for inattention and hyperactivity/impulsivity, respectively, and between time 2 and 3, rs = 0.25 (95% CI: 0.18–0.31) and 0.23 (95% CI: 0.17–0.30). Following standard practice in twin analyses, all symptoms scores were regressed on age and sex and their residuals were used in following genetic analyses.
Table 1.
Means and Standard Deviations (SD) of ADHD Symptoms by Sex and Time
| 1 | 2 | 3 | ||||
|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | |
| Inattention | ||||||
| Males | 2.63a | 2.44 | 2.49 | 2.31 | 2.74a | 2.36 |
| Females | 2.34 | 2.21 | 2.29 | 2.26 | 2.40 | 2.21 |
| Overall | 2.48 | 2.33 | 2.39 | 2.28 | 2.57 | 2.29 |
| Hyperacti vity/Impul si vity | ||||||
| Males | 2.61a | 2.44 | 2.18 | 2.09 | 2.39a | 2.28 |
| Females | 2.21 | 2.18 | 2.10 | 2.10 | 2.02 | 2.16 |
| Overall | 2.40b | 2.32 | 2.14 | 2.10 | 2.21 | 2.22 |
Significant mean difference between males and females at each time for χ2 test with 1 degree of freedom.
Significant mean difference between time 1 and time 2 for χ2 test with 1 degree of freedom.
At both time 1 and 2 (see Table 2), MZ correlations were very similar to DZ twins (e.g., 0.39 vs. 0.35 for time 1 hyperactivity/impulsivity), suggesting minimal to modest genetic influences. At time 3, however, MZ correlations were consistently larger than DZ correlations for both symptoms (e.g., 0.52 vs. 0.32 for inattention), suggesting increasing genetic influences. Between time 2 and 3, MZ and DZ cross-time correlations were very close (e.g., 0.18 and 0.16 for hyperactivity/impulsivity) for both symptoms, indicating that shared environmental effects were mostly responsible for stability. The pattern was different between time 1 and 2 for inattention, where MZ cross-time correlation was larger than DZ twins (0.26 vs. 0.13), suggesting that both genetic and shared environmental influences were responsible for its stability during this period.
Table 2.
Within- and Cross-Time Twin Correlations (95% Confidence Intervals) of ADHD Symptoms by Zygosity
| Within-time | 1 | 2 | 3 | |||
|---|---|---|---|---|---|---|
| r | 95% Cl | r | 95% Cl | r | 95% Cl | |
| Inattention | ||||||
| MZ | 0.36 | 0.26–0.44 | 0.57 | 0.48–0.63 | 0.52 | 0.42–0.60 |
| DZ | 0.33 | 0.22–0.43 | 0.53 | 0.45–0.61 | 0.32 | 0.19–0.43 |
| Hyperacti vity/Impul si vity | ||||||
| MZ | 0.39 | 0.29–0.47 | 0.44 | 0.35–0.53 | 0.54 | 0.45–0.62 |
| DZ | 0.35 | 0.25–0.45 | 0.51 | 0.41–0.58 | 0.33 | 0.20–0.44 |
| Cross-time | Time 1 and 2 | Time 2 and 3 | Time 1 and 3 | |||
| r | 95% Cl | r | 95% Cl | r | 95% Cl | |
| Inattention | ||||||
| MZ | 0.26 | 0.18–0.32 | 0.14 | 0.16–0.22 | 0.12 | 0.04–0.20 |
| DZ | 0.13 | 0.05–0.21 | 0.14 | 0.05–0.22 | 0.07 | −0.02–0.16 |
| Hyperacti vity/Impul si vity | ||||||
| MZ | 0.16 | 0.08–0.24 | 0.18 | 0.10–0.26 | 0.13 | 0.04–0.20 |
| DZ | 0.20 | 0.11–0.27 | 0.16 | 0.07–0.25 | 0.07 | −0.02–0.16 |
Note. All 95% confidence intervals are two-tailed. MZ: monozygotic twins, DZ: dizygotic twins.
Genetic Model-fitting Analyses
As shown in Tables 3 & 4, at time 1, inattention and hyperactivity/impulsivity were explained by modest genetic influences that were not significant (0.16, 95% CI: 0.00–0.40, and 0.09, 95% CI: 0.00–0.34). However, both symptoms were explained by moderate and significant shared environmental influences (0.22, 95% CI: 0.01–0.37, and 0.30, 95% CI: 0.09–0.43) and substantial and significant non-shared environmental influences (0.62, 95% CI: 0.54–0.70, and 0.61, 95% CI: 0.53–0.69). The pattern was very similar at time 2, with negligible to modest and non-significant genetic influences (0.13, 95% CI: 0.00–0.32, and 0.01, 95% CI: 0.00–0.15), but substantial and significant shared environmental influences (0.45, 95% CI: 0.28–0.57, and 0.47, 95% CI: 0.34–0.54), and significant non-shared environmental influences (0.42, 95% CI: 0.36–0.49, and 0.52, 95% CI: 0.46–0.59). There was a drastic change at time 3, where genetic influences became substantial and significant (0.40, 95% CI: 0.13–0.59, and 0.43, 95% CI: 0.15–0.60), whereas shared environmental influences became modest and non-significant (0.12, 95% CI: 0.00–0.35, and 0.12, 95% CI: 0.00–0.35). Non-shared environmental influences generally remained the same (0.48, 95% CI: 0.40–0.57, and 0.46, 95% CI: 0.38–0.55).
Table 3.
Cholesky Decomposition of Additive Genetic (A), Shared Environmental (C), and Non-Shared Environmental (E) Influences (95% Confidence Intervals) for Inattention across time
| Time | 1 | 2 | 3 | Total | ||||
|---|---|---|---|---|---|---|---|---|
| 95% Cl | 95% Cl | 95% Cl | 95% Cl | |||||
| Additive genetic influences | A1 | A2 | A3 | A | ||||
| 1 | 0.16 | 0.00–0.40 | 0.16 | 0.00–0.40 | ||||
| 2 | 0.13 | 0.00–0.32 | 0.00 | 0.00–0.12 | 0.13 | 0.00–0.32 | ||
| 3 | 0.03 | 0.00–0.49 | 0.37 | 0.00–0.56 | 0.00 | 0.00–0.55 | 0.40 | 0.13–0.59 |
| Shared environmental influences | Cl | C2 | C3 | C | ||||
| 1 | 0.22 | 0.01–0.37 | 0.22 | 0.01–0.37 | ||||
| 2 | 0.04 | 0.00–0.16 | 0.41 | 0.28–0.49 | 0.45 | 0.28–0.57 | ||
| 3 | 0.01 | 0.00–0.21 | 0.02 | 0.00–0.13 | 0.08 | 0.00–0.30 | 0.12 | 0.00–0.35 |
| Non-shared environmental influences | El | E2 | E3 | E | ||||
| 1 | 0.62 | 0.54–0.70 | 0.62 | 0.54–0.70 | ||||
| 2 | 0.02 | 0.01–0.04 | 0.40 | 0.34–0.46 | 0.42 | 0.36–0.49 | ||
| 3 | 0.00 | 0.00–0.02 | 0.02 | 0.00–0.05 | 0.46 | 0.38–0.55 | 0.48 | 0.40–0.57 |
Note. Estimates whose lower limit of 95% confidence interval (two-tailed) is larger than 0.01 are bolded. Across each row, total A, C, and E equal to the sum of estimated A, C, and E across all time, respectively, representing the relative contributions of genetic and environmental influences cumulated from previous times. For instance, for total A in time 3 (0.40), it equals to the sum of A of all three time points (0.40 = 0.03 + 0.37 + 0.00). In the last column for total A, C, and E, their estimates sum to 1 for each time. For instance, for time 2, A (0.13) + C (0.45) + E (0.42) = 1.
Table 4.
Cholesky Decomposition of Additive Genetic (A), Shared Environmental (C), and Non-Shared Environmental (E) Influences (95% Confidence Intervals) for Hyperactivity/Impulsivity across time
| Time | 1 | 2 | 3 | Total | ||||
|---|---|---|---|---|---|---|---|---|
| 95% Cl | 95% Cl | 95% Cl | 95% Cl | |||||
| Additive genetic influences | A1 | A2 | A3 | A | ||||
| 1 | 0.09 | 0.00–0.34 | 0.09 | 0.00–0.34 | ||||
| 2 | 0.00 | 0.00–0.12 | 0.01 | 0.00–0.15 | 0.01 | 0.00–0.15 | ||
| 3 | 0.12 | 0.00–0.60 | 0.31 | 0.00–0.57 | 0.00 | 0.00–0.55 | 0.43 | 0.15–0.60 |
| Shared environmental influences | Cl | C2 | C3 | C | ||||
| 1 | 0.30 | 0.09–0.43 | 0.30 | 0.09–0.43 | ||||
| 2 | 0.11 | 0.02–0.38 | 0.36 | 0.09–0.45 | 0.47 | 0.34–0.54 | ||
| 3 | 0.00 | 0.00–0.13 | 0.05 | 0.00–0.24 | 0.07 | 0.00–0.30 | 0.12 | 0.00–0.35 |
| Non-shared environmental influences | El | E2 | E3 | E | ||||
| 1 | 0.61 | 0.53–0.69 | 0.61 | 0.53–0.69 | ||||
| 2 | 0.04 | 0.02–0.08 | 0.48 | 0.42–0.55 | 0.52 | 0.46–0.59 | ||
| 3 | 0.00 | 0.00–0.01 | 0.00 | 0.00–0.02 | 0.45 | 0.38–0.54 | 0.46 | 0.38–0.55 |
Note. Estimates whose lower limit of 95% confidence interval (two-tailed) is larger than 0.01 are bolded. Across each row, total A, C, and E equal to the sum of estimated A, C, and E across all time, respectively, representing the relative contributions of genetic and environmental influences cumulated from previous times. For instance, for total A in time 3 (0.43), it equals to the sum of A of all three time points (0.40 = 0.12 + 0.31 + 0.00). In the last column for total A, C, and E, their estimates sum to 1 for each time. For instance, for time 2, A (0.01) + C (0.47) + E (0.53) = 1.
Genetic influences on inattention at time 2 largely came from time 1 genetic factor (0.13), but not for hyperactivity/impulsivity. At time 3, similarly, no new genetic factor emerged for either symptoms, while most genetic influences were from time 2 genetic factors (0.37 and 0.31). However, at time 2, shared and non-shared environmental influences largely came from new emerging factors. For instance, 0.40 out of the total 0.42 non-shared environmental influences on inattention came from time 2 new factor. One notable exception was shared environmental influences on hyperactivity/impulsivity (0.47), of which about a quarter (0.11) were from time 1 shared environmental factor. The same pattern remained for non-shared environmental influences at time 3, and to a lesser degree for shared environmental influences: 0.08 out of 0.12 for inattention, and 0.07 out of 0.11 for hyperactivity/impulsivity were from time 3 new factors.
Discussion
This study used a prospective longitudinal design to follow a group of Chinese twins through adolescence and investigated the changes in the magnitudes of genetic and environmental influences on selfreported ADHD symptoms, as well as genetic and environmental innovation and attenuation in the phenotypic stability and change of ADHD symptoms. At the phenotypic level, consistent with the literature [5, 12, 13], hyperactivity/impulsivity decreased during early adolescence and remained stable into middle adolescence. On the contrary, inattention remained stable from early to middle adolescence. Both symptoms showed moderate stability, such that adolescents with higher levels of inattentive and/or hyperactive/impulsive symptoms tended to show higher levels over three years, supporting the persistence of ADHD symptoms during childhood and adolescence [10, 11]. However, the stability for both symptoms tended to be smaller than in previous Western studies. This is possibly due to the limited measures (only three items each) of ADHD symptoms, which could not cover the full range of symptoms as the full DSM diagnostic criteria or the Conner’s index. The wide age range in the sample might also partly explain the smaller phenotypic correlations as ADHD symptoms in general tend to decline from childhood to adolescence, hence making it particularly less correlated or stable among older children [8, 9, 12, 13]. Males consistently showed higher levels of both symptoms than females at time 1 and 3, consistent with previous Chinese studies [4–6]. Both symptoms showed modest genetic influences in early adolescence in time 1 and 2 (average age 12 and 13 years old), close to the estimate of a Chinese study [40]. Together with the findings from another Korean study [41], this finding put the genetic influences of ADHD at the lower end compared to those from Western studies.
Moderate shared environmental influences were found in early adolescence, similar to another Chinese study [36]. Shared environmental influences were rarely found in studies using Western samples [18–22], and the estimates tend to be low [23]. Shared environmental influences encompass the cumulative effects of shared family, neighborhoods, and communities in which families are embedded. Therefore, the substantial shared environmental influences as compared to Western populations suggest that socialization experiences of Chinese children and adolescents both inside and outside their homes are particularly important during this developmental transition period. Given that previous studies have also found profound shared environmental influences in Chinese samples on other psychiatric symptoms [35–38], this particular finding has implications for the development of child and adolescent psychopathology in Asian societies. As opposed to the individualistic culture in most Western societies, Asian collectivistic cultures emphasize more family, parentchild, and interpersonal relationships, and parents have different parenting behaviors (e.g., corporal punishment is more commonly adopted) [49, 50]. Therefore, shared environmental influences might be more pronounced during this developmental transition period that is also accompanied by drastic environmental changes.
Self-reports may partly explain the lower estimates of genetic influences, as estimates of genetic influences on ADHD symptoms are influenced by rater effects and assessment instruments [51]. Self-reports are more likely than parent-reports to be affected by increased unreliability and idiosyncratic interpretations of the questions, which would contribute to measurement error, decrease twin similarity, and thus increase the estimates of non-shared environmental influences. In contrast, parent- reports could possibly have shared method variance because a parent typically reports on both twins, which may produce higher correlations between twins. More specifically, among Western studies, different measures (e.g., DSM diagnostic criteria, Strength and Difficulty Questionnaire [SDQ], Conners’ rating scale, Child Behavior Checklist [CBCL]) generally provide comparable estimates of genetic influences [28, 31, 32, 52]. However, self-reports (e.g., Youth Self-Reports [YSR]) or measures on full spectrum of activity (e.g., Strengths and Weakness of ADHD symptoms and Normal behavior rating scale [SWAN]) typically produce lower genetic influences and higher shared environmental influences than parent- or teach-reports [18, 22, 23, 48]. For instance, Kan et al. [32] found that genetic influences decreased from 70–74% in childhood when using maternal reports to 51–56% in adolescence and 40– 54% in adulthood when using youth self-reports. Notably, they did not find any shared environmental influences.
Nevertheless, current genetic estimates are still lower than in Western studies that have used self-reports, especially in early adolescence (e.g., 45–53% in [26], 51–56% in [32], 48% in [48]). The other Chinese study [40] that has also used self-reports (i.e., YSR) similarly reported lower genetic influences (26%). Furthermore, compared to other Western studies using parent-reports, the Korean study [41] used maternal reported SDQ and found lower genetic influences (33–51%), while one Chinese study [36] using parent reported CBCL found comparable genetic influences (72%) but also significant shared environmental influences (20%). Therefore, measurement differences do not seem to entirely explain the differences in genetic and shared environmental influences between the current study and Western studies. Collectively, the current findings joined an emerging body of empirical evidence and literature suggesting that genetic influences might be smaller whereas shared environmental influences might be larger in Asian populations than in Western populations. Nevertheless, we acknowledge the scarcity of current studies focusing on Asian populations and highlight the preliminary nature of our findings and tentative conclusions. More studies using non-Western samples are needed to replicate the current findings and to further examine cross-cultural similarities and differences in genetic and environmental influences on child and adolescent psychopathology.
From early to middle adolescence in time 3 (average age 15 years old), genetic influences changed from modest and non-significant to substantial and became significant, whereas shared environmental influences changed from moderate and significant to modest and non-significant. Non-shared environmental influences generally remained the same. Given the overlapping confidence intervals of all estimates over times, which is possibly due to the relatively smaller sample size compared to other Western samples from large twin registries, one cannot confidently conclude that genetic influences increased and shared environmental influences decreased from early to middle adolescence. However, the general patterns of the current findings are notably consistent with previous studies using Western samples [19, 20], thus joining the congruence of a large body of literature demonstrating the increasing importance of genes over development.
Notably, genetic influences in middle adolescence came largely from genetic factors in early adolescence without the emergence of new genetic factors, consistent with previous studies using Western samples in showing that common genetic factors largely explained ADHD symptoms over development [19, 20, 26, 28, 30, 32, 34], but different in that we found no evidence of genetic innovation. Instead, we found evidence of genetic amplification [24], such that the influences of the same genes became larger in middle adolescence. The change from time 1 (age 12) to time 3 (age 15) is approximately consistent with the transition from early to middle adolescence, which is accompanied by the onset of puberty, as well as drastic social and interpersonal changes in adolescents’ lives. For Chinese adolescents in particular, this transition period is typically accompanied by the transition from primary school to secondary or middle school, which usually results in drastic changes in school and classroom settings and structures, classmate and friend networks and compositions, as well as a more structured curriculum. Hence, it is possible all these biological and social changes during this transition period could lead to amplified genetic influences whereas diminished shared environmental influences. Gene-environment correlation [53] could also partly explain the increase of genetic influences, where adolescents with ADHD symptoms either actively seek out environmental experiences consistent with their genetic predispositions, or that their peers, teachers, and families respond to them in a way that further potentiates their genetic liability.
Different from genetic factors, shared and non-shared environmental influences in adolescence were primarily due to new emerging factors, suggesting environmental innovation. This finding, especially regarding non-shared environmental factors, are largely consistent with previous studies [12, 13, 26, 28, 30, 32, 34], highlighting that non-shared environmental factors are mostly time-specific and contribute to the change of ADHD symptoms. The finding on shared environmental factors might be more unique to the Chinese population and its collectivistic culture. Hence, stability of ADHD symptoms throughout adolescence was primarily due to the same genes influencing over time, whereas change in ADHD symptoms was mainly due to both shared and child-specific environmental factors continuously emerging during adolescence.
Notably, in addition to different developmental courses in early adolescence, inattention and hyperactivity/impulsivity also differed in the etiology of their phenotypic stability: the former was largely explained by the genetic factor, whereas the latter was not explained by the genetic factor at all but primarily by the shared environmental factor. This finding suggests that different underlying genetic and shared environmental mechanisms could partly explain the different developmental courses of inattention and hyperactivity/impulsivity, thus validating these two symptoms as separate dimensions of ADHD in Chinese populations [12, 13]. The findings are congruent with the dual pathway theory that posits inattention and hyperactivity/impulsivity map onto the neural systems of executive functions (e.g., prefrontal-striatal circuitry) and reward/motivation (e.g., frontal-limbic circuitry) respectively and involve different neuropsychological performances [54, 55].
Limitations and Strengths
A few limitations should be considered when interpreting the current findings and should be addressed in future research. First, our sample is not a cohort sample but covers a wide age range. Therefore, changes across the three time points may not exactly reflect changes along the developmental course. Notably, in a follow-up sensitivity analysis where only 10–12 years old twins at time 1 were selected (i.e., no age overlap between time 1 and 3), estimates of genetic and environmental influences at each time changed for both symptoms, but all within reasonable ranges and fell within the 95% confidence intervals of the estimates from the full sample. The general pattern of increasing genetic influences and decreasing shared environmental influences remained for hyperactivity/impulsivity, but to a lesser degree for inattention. Following twins of the same age could better depict developmental changes in genetic and environmental influences, and can particularly examine genetic influences on the systematic change of ADHD over time [33]. However, it is important to note that we controlled for age effect and the general pattern regarding genetic and environmental influences is consistent with previous findings. Second, our sample only represents adolescents from one Chinese province. Therefore, the findings may not be generalizable to twins of other Chinese provinces with different ethnicity composition and socioeconomic environments. National twin registry, collaboration initiatives, and consortium are needed to build national representative twin samples. Relatedly, based on statistical simulations [56], the current sample size has adequate power to detect genetic and environmental influences at each time as small as estimated in the current findings, regardless of genetic and environmental correlations over time. However, our sample size is underpowered to accurately estimate genetic and environmental correlations, hence their contributions to the stability and change over development. Larger Chinese sample sizes, preferably from a cohort sample through national twin registry, are much needed to provide more refined estimates.
Third, we only investigated genetic and environmental influences on the developmental course of ADHD symptoms. Future studies could also examine the overlap of genetic etiology of inattentive and hyperactive/impulsive dimensions [57] and their associations over time [47], and the comorbidity of ADHD with other disorders, such as autistic spectrum disorders [58], externalizing problems [34], and internalizing problems [36, 59, 60]. Fourth, ADHD symptoms were self-reported. As aforementioned, different informants (e.g., parents, teachers, children) can produce different genetic influences estimates, although the overlap of different informants is largely influenced by one common genetic factor [31, 34, 48, 52]. Future studies should use a multi-informant approach to capture common and unique information from different raters. Relatedly, we only measured ADHD symptoms with a subset of items from DSM-IV within a community sample rather than clinically diagnosed ADHD disorder using full criteria. Therefore, the findings may not generalize to clinical disorder or clinically referred populations. However, the few items included in the study were also included in the ADHD index of Conners’ rating scales-revised, particularly inattentive symptoms [31, 61], and previous studies have shown high genetic overlaps between the Conners’ rating scale-revised and DSM items [31].
Limitations notwithstanding, this study has a few notable strengths. Our sample is among the largest of all studies that have examined genetic and environmental influences on ADHD symptoms in Chinese twins. The only study [36] with slightly larger sample nevertheless included twins of a much larger age range (6–18 years). Our study is also the first to employ a prospective longitudinal design to investigate genetic and environmental contributions to the stability and change of ADHD symptoms using a Chinese sample, and to our best knowledge even among all those using Asian samples. Hence, the current findings contribute unique knowledge to the literature on the cross-cultural similarities and differences regarding genetic and environmental etiology of the development of child and adolescent ADHD symptoms.
Conclusion
This study found that genetic influences of ADHD symptoms tended to be smaller, whereas shared environmental influences tended to be larger in a Chinese population than in Western populations. Nevertheless, genetic and environmental influences showed a similar pattern as commonly found in Western populations in that from early to middle adolescence genetic influences changed from modest and non-significant to substantial and significant, whereas shared environmental influences changed from moderate and significant to modest and non-significant. Genetic factors played a large role in the stability of ADHD symptoms throughout adolescence, whereas shared and non-shared environmental influences were largely time-specific. Finding shared environmental influences highlights the importance of shared family, neighborhood, and community experiences on child psychopathology in Chinese and possibly other Asian societies with a collectivistic culture. For clinicians, school-based and family-focused therapy and interventions against ADHD might be more effective in Chinese populations.
Acknowledgements
This research was supported by the National Cancer Institute grant #5P50CA084735 (to Dr. Unger).
Footnotes
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
Ethical standards
This work was approved by the appropriate ethics committee. The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national and institutional committees on human experimentation and with the Helsinki Declaration of 1975, as revised in 2008. All participants and their parents gave their informed consent prior to their inclusion in the study.
Conflict of Interest
The authors declare that they have no conflict of interest.
References
- 1.Merikangas KR, He JP, Burstein M, Swanson SA, Avenevoli S, Cui L, Benjet C, Georgiades K, Swendsen J (2010) Lifetime prevalence of mental disorders in US adolescents: results from the National Comorbidity Survey Replication–Adolescent Supplement (NCS-A). J Am Acad of Child Adolesc Psychiatry 49:980–989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Polanczyk GV, Salum GA, Sugaya LS, Caye A, Rohde LA (2015) Annual Research Review: a meta analysis of the worldwide prevalence of mental disorders in children and adolescents. J Child Psychol Psychiatry 56:345–365. [DOI] [PubMed] [Google Scholar]
- 3.Polanczyk GV, Willcutt EG, Salum GA, Kieling C, Rohde LA (2014) ADHD prevalence estimates across three decades: an updated systematic review and meta-regression analysis. Int J Epidemiol 43:434–442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Leung PW, Hung SF, Ho TP, Lee CC, Liu WS, Tang CP, Kwong SL (2008) Prevalence of DSM-IV disorders in Chinese adolescents and the effects of an impairment criterion. Eur Child Adolesc Psychiatry 17:452–461. [DOI] [PubMed] [Google Scholar]
- 5.Huang Y, Zheng S, Xu C, Lin K, Wu K, Zheng M, Zhang J, Xu H (2017) Attention-deficit hyperactivity disorder in elementary school students in Shantou, China: prevalence, subtypes, and influencing factors. Neuropsychiatric Dis Treat 13:785–792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jin W, Du Y, Zhong X, Coghill D (2014) Prevalence and contributing factors to attention deficit hyperactivity disorder: a study of five- to fifteen- year- old children in Zhabei District, Shanghai. Asia Pac Psychiatry 6:397–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tong L, Shi HJ, Zang JJ (2013) Prevalence of ADHD in children of China: a systematic review and meta analysis. Chinese J Public Health 29:1279–1283. [In Chinese]. [Google Scholar]
- 8.Ramtekkar UP, Reiersen AM, Todorov AA, Todd RD (2010) Sex and age differences in attention deficit/hyperactivity disorder symptoms and diagnoses: implications for DSM-V and ICD-11. J Am Acad Child Adolesc Psychiatry 49:217–228. [PMC free article] [PubMed] [Google Scholar]
- 9.Costello EJ, Copeland W, Angold A (2011) Trends in psychopathology across the adolescent years: what changes when children become adolescents, and when adolescents become adults? J Child Psychol Psychiatry 52:1015–1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Biederman J, Petty CR, Evans M, Small J, Faraone SV (2010) How persistent is ADHD? A controlled 10- year follow-up study of boys with ADHD. Psychiatry Res 177:299–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Faraone SV, Biederman J, Mick E (2006) The age-dependent decline of attention deficit hyperactivity disorder: a meta-analysis of follow-up studies. Psychol Med 36:159–165. [DOI] [PubMed] [Google Scholar]
- 12.Willcutt EG, Nigg JT, Pennington BF, Solanto MV, Rohde LA, Tannock R, Loo SK, Carlson CL, McBurnett K, Lahey BB (2012) Validity of DSM-IV attention deficit/hyperactivity disorder symptom dimensions and subtypes. J Abnorm Psychol 121:991–1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Willoughby MT (2003) Developmental course of ADHD symptomatology during the transition from childhood to adolescence: a review with recommendations. J Child Psychol Psychiatry 44:88–106. [DOI] [PubMed] [Google Scholar]
- 14.Pingault JB, Tremblay RE, Vitaro F, Carbonneau R, Genolini C, Falissard B, Côté SM (2011) Childhood trajectories of inattention and hyperactivity and prediction of educational attainment in early adulthood: a 16-year longitudinal population-based study. Am J Psychiatry 168:1164–1170. [DOI] [PubMed] [Google Scholar]
- 15.Pingault JB, Côté SM, Galéra C, Genolini C, Falissard B, Vitaro F, Tremblay RE (2013) Childhood trajectories of inattention, hyperactivity and oppositional behaviors and prediction of substance abuse/dependence: a 15-year longitudinal population-based study. Mol Psychiatry 18:806–812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Faraone SV, Larsson H (2018) Genetics of attention deficit hyperactivity disorder. Mol Psychiatry 10.1038/s41380-018-0070-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nikolas MA, Burt SA (2010) Genetic and environmental influences on ADHD symptom dimensions of inattention and hyperactivity: a meta-analysis. J Abnorm Psychol 119:1–17. [DOI] [PubMed] [Google Scholar]
- 18.Hay DA, Bennett KS, Levy F, Sergeant J, Swanson J (2007) A twin study of attention-deficit/hyperactivity disorder dimensions rated by the strengths and weaknesses of ADHD-symptoms and normal-behavior (SWAN) scale. Biol Psychiatry 61:700–705. [DOI] [PubMed] [Google Scholar]
- 19.Larsson JO, Larsson H, Lichtenstein P (2004) Genetic and environmental contributions to stability and change of ADHD symptoms between 8 and 13 years of age: a longitudinal twin study. J Am Acad Child Adolesc Psychiatry 43:1267–1275. [DOI] [PubMed] [Google Scholar]
- 20.Larsson H, Lichtenstein P, Larsson JO (2006) Genetic contributions to the development of ADHD subtypes from childhood to adolescence. J Am Acad Child Adolesc Psychiatry 45:973–981. [DOI] [PubMed] [Google Scholar]
- 21.Peng CZ, Grant JD, Heath AC, Reiersen AM, Mulligan RC, Anokhin AP (2016) Familial influences on the full range of variability in attention and activity levels during adolescence: a longitudinal twin study. Dev Psychopathol 28:517–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wood AC, Saudino KJ, Rogers H, Asherson P, Kuntsi J (2007) Genetic influences on mechanically assessed activity level in children. J Child Psychol Psychiatry 48:695–702. [DOI] [PubMed] [Google Scholar]
- 23.Wood AC, Buitelaar J, Rijsdijk F, Asherson P, Kuntsi J (2010) Rethinking shared environment as a source of variance underlying attention-deficit/hyperactivity disorder symptoms: comment on Burt (2009). Psychol Bull 136:331–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Plomin R (1986) Multivariate analysis and developmental behavioral genetics: developmental change as well as continuity. Behav Genet 16:25–43. [DOI] [PubMed] [Google Scholar]
- 25.Kendler KS, Gardner CO, Lichtenstein P (2008) A developmental twin study of symptoms of anxiety and depression: evidence for genetic innovation and attenuation. Psychol Med 38:1567–1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chang Z, Lichtenstein P, Asherson PJ, Larsson H (2013) Developmental twin study of attention problems: high heritabilities throughout development. JAMA Psychiatry 70:311–318. [DOI] [PubMed] [Google Scholar]
- 27.Price TS, Simonoff E, Asherson P, Curran S, Kuntsi J, Waldman I, Plomin R (2005) Continuity and change in preschool ADHD symptoms: longitudinal genetic analysis with contrast effects. Behav Genet 35:121–132. [DOI] [PubMed] [Google Scholar]
- 28.Kuntsi J, Rijsdijk F, Ronald A, Asherson P, Plomin R (2005) Genetic influences on the stability of attention deficit/hyperactivity disorder symptoms from early to middle childhood. Biol Psychiatry 57:647–654. [DOI] [PubMed] [Google Scholar]
- 29.Rietveld MJ, Hudziak JJ, Bartels M, van Beijsterveldt CEM, Boomsma DI (2003) Heritability of attention problems in children: I. cross sectional results from a study of twins, age 3–12 years. Am J Med Genet B 117:102–113. [DOI] [PubMed] [Google Scholar]
- 30.Rietveld MJ, Hudziak JJ, Bartels M, van Beijsterveldt CEM, Boomsma DI (2004) Heritability of attention problems in children: longitudinal results from a study of twins, age 3 to 12. J Child Psychol Psychiatry 45:577–588. [DOI] [PubMed] [Google Scholar]
- 31.Derks EM, Hudziak JJ, Dolan CV, van Beijsterveldt TC, Verhulst FC, Boomsma DI (2008) Genetic and environmental influences on the relation between attention problems and attention deficit hyperactivity disorder. Behav Genet 38:11–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kan KJ, Dolan CV, Nivard MG, Middeldorp CM, van Beijsterveldt CEM, Willemsen G, Boomsma DI (2013) Genetic and environmental stability in attention problems across the lifespan: evidence from the Netherlands Twin Register. J Am Acad Child Adolesc Psychiatry 52:12–25. [DOI] [PubMed] [Google Scholar]
- 33.Pingault J-B, Viding E, Galéra C, Greven CU, Zheng Y, Plomin R, Rijsdijk F (2015) Genetic and environmental influences on the developmental course of attention-deficit/hyperactivity disorder symptoms from childhood to adolescence. JAMA Psychiatry 72:651–658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nadder TS, Rutter M, Silberg JL, Maes HH, Eaves LJ (2002) Genetic effects on the variation and covariation of attention deficit-hyperactivity disorder (ADHD) and oppositional-defiant disorder/conduct disorder (ODD/CD) symptomatologies across informant and occasion of measurement. Psychol Med 32:39–53. [DOI] [PubMed] [Google Scholar]
- 35.Chen J, Yu J, Zhang J, McGue M (2015) Investigating genetic and environmental contributions to adolescent externalizing behavior in a collectivistic culture: a multi-informant twin study. Psychol Med 45:1989–1997. [DOI] [PubMed] [Google Scholar]
- 36.Chen TJ, Ji CY, Wang SS, Lichtenstein P, Larsson H, Chang Z (2016) Genetic and environmental influences on the relationship between ADHD symptoms and internalizing problems: a Chinese twin study. Am J Med Genet B Neuropsychiatr Genet 171:931–937. [DOI] [PubMed] [Google Scholar]
- 37.Chen J, Li X (2013) Genetic and environmental influences on adolescent rumination and its association with depressive symptoms. J Abnorm Child Psychol 41:1289–1298. [DOI] [PubMed] [Google Scholar]
- 38.Zheng Y, Rijsdijk F, Pingault J-B, McMahon RJ, Unger JB (2016) Developmental changes in genetic and environmental influences on Chinese child and adolescent anxiety and depression. Psychol Med 46:1829–1838. [DOI] [PubMed] [Google Scholar]
- 39.Kuo PH, Lin CC, Yang HJ, Soong WT, Chen WJ (2004) A twin study of competence and behavioral/emotional problems among adolescents in Taiwan. Behav Genet 34:63–74. [DOI] [PubMed] [Google Scholar]
- 40.Zhu W, Bo Y, Li T, Deng W, Wang Y (2017) A twin study of behavioral problems and relations to genetic and environmental factors in adolescents. Chinese Ment Health J 31:127–132. [In Chinese] [Google Scholar]
- 41.Hur YM (2014) Increasing phenotypic and genetic variations in hyperactivity/inattention problems from age 3 to 13 years: a cross-sectional twin study. Twin Res Hum Genet 17:545–552. [DOI] [PubMed] [Google Scholar]
- 42.Pang Z, Ning F, Unger J, Johnson CA, Wang S, Guo Q, Cao W, Lee L (2006) The Qingdao Twin Registry: a focus on chronic disease research. Twin Res Hum Genet 9:758–762. [DOI] [PubMed] [Google Scholar]
- 43.Duan H, Ning F, Zhang D, Wang S, Zhang D, Tan Q, Tian X, Pang Z (2013) The Qingdao twin registry: a status update. Twin Res Hum Genet 16:79–85. [DOI] [PubMed] [Google Scholar]
- 44.American Psychiatric Association (2000) Diagnostic and statistical manual of mental disorders. 4th ed American Psychiatric Association, Washington, DC. [Google Scholar]
- 45.Rijsdijk FV, Sham PC (2002) Analytic approaches to twin data using structural equation models. Brief Bioinform 3:119–133. [DOI] [PubMed] [Google Scholar]
- 46.Boker S, Neale M, Maes HH, Wilde M, Spiegel M, Brick T, Spies J, Estabrook R, Kenny S, Bates T, Mehta P, Fox J (2011) OpenMx: an open source extended structural equation modeling framework. Psychometrika 76:306–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Greven CU, Asherson P, Rijsdijk FV, Plomin R (2011) A longitudinal twin study on the association between inattentive and hyperactive-impulsive ADHD symptoms. J Abnorm Child Psychol 39:623–632. [DOI] [PubMed] [Google Scholar]
- 48.Merwood A, Greven CU, Price TS, Rijsdijk F, Kuntsi J, McLoughlin G, Larsson H, Asherson PJ (2013) Different heritabilities but shared etiological influences for parent, teacher and self-ratings of ADHD symptoms: an adolescent twin study. Psychol Med 43:1973–1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chen X, Chen H (2010) Children’s social functioning and adjustment in the changing Chinese society In: Silbereisen RK, Chen X (eds) Social change and human development: Concepts and results. Sage, London, pp 209–226. [Google Scholar]
- 50.Chen X, French D (2008) Children’s social competence in cultural context. Annu Rev Psychol 59:591–616. [DOI] [PubMed] [Google Scholar]
- 51.Freitag CM, Rohde LA, Lempp T, Romanos M (2010) Phenotypic and measurement influences on heritability estimates in childhood ADHD. Eur Child Adolesc Psychiatry 19:311–323. [DOI] [PubMed] [Google Scholar]
- 52.Nadder TS, Silberg JL, Rutter M, Maes HH, Eaves LJ (2001) Comparison of multiple measures of ADHD symptomatology: a multivariate genetic analysis. J Child Psychol Psychiatry 42:475–486. [PubMed] [Google Scholar]
- 53.Knafo A, Jaffee SR (2013) Gene–environment correlation in developmental psychopathology. Dev Psychopathol 25:1–6. [DOI] [PubMed] [Google Scholar]
- 54.Sonuga-Barke EJ (2003) The dual pathway model of AD/HD: an elaboration of neuro-developmental characteristics. Neurosci Biobehav Rev 27:593–604. [DOI] [PubMed] [Google Scholar]
- 55.Sonuga-Barke EJ (2005) Causal models of attention-deficit/hyperactivity disorder: from common simple deficits to multiple developmental pathways. Biol Psychiatry 57:1231–1238. [DOI] [PubMed] [Google Scholar]
- 56.Verhulst B (2017) A power calculator for the classical twin design. Behav Genet 47:255–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Greven CU, Rijsdijk FV, Plomin R (2011) A twin study of ADHD symptoms in early adolescence: hyperactivity-impulsivity and inattentiveness show substantial genetic overlap but also genetic specificity. J Abnorm Child Psychol 39:265–275. [DOI] [PubMed] [Google Scholar]
- 58.Lichtenstein P, Carlström E, Råstam M, Gillberg C, Anckarsäter H (2010) The genetics of autism spectrum disorders and related neuropsychiatric disorders in childhood. Am J Psychiatry 167:1357–1363. [DOI] [PubMed] [Google Scholar]
- 59.Michelini G, Eley TC, Gregory AM, McAdams TA (2015) Aetiological overlap between anxiety and attention deficit hyperactivity symptom dimensions in adolescence. J Child Psychol Psychiatry 56:423–431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Merwood A, Chen W, Rijsdijk F, Skirrow C, Larsson H, Thapar A, Kuntsi J, Asherson P (2014) Genetic associations between the symptoms of attention-deficit/hyperactivity disorder and emotional lability in child and adolescent twins. J Am Acad Child Adolesc Psychiatry 53:209–220. [DOI] [PubMed] [Google Scholar]
- 61.Hudziak JJ, Derks EM, Althoff RR, Rettew DC, Boomsma DI (2005) The genetic and environmental contributions to attention deficit hyperactivity disorder as measured by the Conners’ Rating Scales-Revised. Am J Psychiatry 162:1614–1620. [DOI] [PubMed] [Google Scholar]