Abstract
Endocervical adenocarcinomas (ECAs) are currently classified according to the 2014 World Health Organization (WHO) system, which is predominantly based on descriptive morphologic characteristics, considers factors bearing minimal etiological, clinical or therapeutic relevance, and lacks sufficient reproducibility. The 2017 International Endocervical Adenocarcinoma Criteria and Classification (IECC) system was developed by a group of international collaborators to address these limitations. The IECC system separates ECAs into two major groups—those that are human papillomavirus-associated (HPVA) and those that are non–HPV-associated (NHPVA)—based on morphology (linked to etiology) alone, precluding the need for an expensive panel of immunohistochemical markers for most cases. The major types of HPVA ECA include the usual (with villoglandular and micropapillary architectural variants) and mucinous types (not otherwise specified [NOS], intestinal, signet-ring, and invasive stratified mucin-producing carcinoma). Invasive adenocarcinoma NOS is morphologically uninformative, yet considered part of this group when HPV positive. NHPVA ECAs include gastric, clear cell, endometrioid, and mesonephric types. The IECC system is supported by demographic and clinical features (HPVA ECAs develop in younger patients, are smaller, and are diagnosed at an earlier stage), p16/HPV status (almost all HPVA ECAs are p16 and/or HPV positive), prognostic parameters (NHPVA ECAs more often have lymphovascular invasion, lymph node metastases, and are Silva pattern C), and survival data (NHPVA ECAs are associated with worse survival). A move from the morphology-based WHO system to the IECC system will likely provide clinicians with an improved means to diagnose and classify ECAs, and ultimately, to better personalize treatment for these patients.
Keywords: endocervical adenocarcinoma, classification, International Endocervical Adenocarcinoma Criteria and Classification, HPV
1. Background
The most prevalent cervical malignancy—invasive squamous cell carcinoma (SCC)—is almost always human papillomavirus (HPV) related. The implementation of national screening and vaccination programs in most developed countries has reduced its incidence over the last few decades [1]. In contrast, invasive endocervical adenocarcinoma (ECA) is less common, accounting for ~25% of cervical malignancies; however, its prevalence is rising, particularly among young women in developed countries, even in those with functionable screening programs [2–5]. Most ECAs are HPV related. The HPV 18, 16 and 45 subtypes are equally prevalent, although some epidemiologic studies suggest that the HPV 18 subtype is the most common [1, 3]. Unlike invasive SCCs, a good portion of ECAs (10–15%) are non-HPV related [6–9].
Risk factors associated with ECA are similar to those of SCC and include multiple sexual partners, young age at first intercourse, use of oral contraceptives for more than 10 years, hormonal replacement therapy, and obesity. A genetic predisposition for the development of gastric-type adenocarcinoma (HPV-unassociated) is found in women with Peutz-Jeghers syndrome [10–12].
ECA presents at a mean age of 50 years, often with vaginal bleeding [1]. The malignancy is thought to originate in pluripotential subcolumnar reserve cells. Most ECAs develop within the transformation zone, with a minor proportion of cases located within the endocervical canal, more proximally and adjacent to the lower uterine segment.
Macroscopically, it can appear as an exophytic mass (polypoid, papillary, nodular) or an ulcerated lesion. In rare cases, it forms a “barrel-shaped” cervix, characterized by a thickened cervical wall. In its early stages or when the tumor is located exclusively within the endocervical canal, the tumor can be inconspicuous on clinical examination.
Morphologically, ECAs are a heterogeneous group of tumors, most displaying a mixture of different cell types and patterns. ECAs are currently classified according to the World Health Organization (WHO) system, predominantly based on descriptive morphological characteristics, particularly cytoplasmic features, which are assessed on hematoxylin-eosin (H&E) staining [1]. The WHO system recognizes more than 10 different types of ECAs, with different clinical behaviors, biologic signatures, and treatment outcomes [1, 13]. The classification system is also difficult to apply in daily practice, lacks sufficient reproducibility, and lacks clinical and/or pathogenetic significance, limiting its use for patient management. For example, the WHO system identifies 4 mucinous adenocarcinoma entities, one unassociated with HPV (gastric-type adenocarcinoma) and 3 only variably HPV associated (not otherwise specified [NOS], intestinal and signet-ring cell ECA). Mucinous adenocarcinoma NOS is not clearly defined.
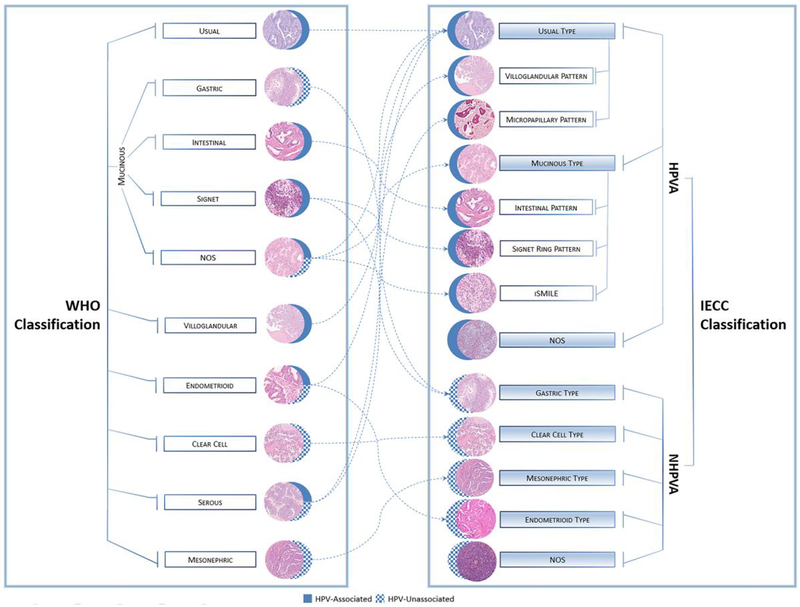
Despite the heterogeneity of ECAs, the National Comprehensive Cancer Network (NCCN) suggests a universal approach to ECA treatment, with no specific treatment strategy based on histologic type or genomic signature as in other gynecologic cancers (e.g., ovarian and endometrial carcinomas) [14–18]. This lack of subtype-specific treatment is due to an erroneous assumption that all ECAs are HPV related and a classification system with many shortcomings. To address this clinical practice gap, a panel of international experts in gynecologic pathology studied more than 400 cases of ECA to develop a new classification system, the International Endocervical Adenocarcinoma Criteria and Classification (IECC) system, which incorporates the use of tumor morphology, HPV testing, and a panel of immunohistochemical markers with clinical correlates [9] (Figure 1).
Figure 1.
Endocervical adenocarcinoma classification systems: The 2014 World Health Organization system and the International Endocervical Adenocarcinoma Criteria and Classification (IECC, 2018) system. The WHO classification system categorizes tumors based primarily on morphology, while the IECC system divides tumors into those that are human papillomavirus associated (HPVA) and those that are not (NHPVA) based on more detailed diagnostic criteria, resulting in the reclassification of many WHO-defined tumors.
2. IECC Classification System
The IECC classification system recognizes two categories of ECAs: HPV-associated (HPVA) and non–HPV-associated (NHPVA) ECAs, a distinction that can be largely achieved with morphologic assessment alone. HPVA tumors demonstrate apical mitotic features and apoptotic bodies at scanning magnification. If not seen, a 200x magnification exam must be performed. HPVA ECAs are subcategorized based on cytoplasmic features, an approach consistent with existing classification schemes. NHPVA ECAs generally lack these aforementioned features and are subclassified based on the criteria described below [9].
IECC classification is supported by demographic and clinical features (HPVA compared with NHPVA ECAs develop in younger patients, are smaller, and are diagnosed at earlier stages), p16/HPV status (almost all HPVAs are p16 and/or HPV positive), and prognostic parameters. HPVA ECAs are less frequently associated with lymphovascular invasion (LVI), lymph node metastasis (LNM), and Silva C pattern. They are also associated with better overall survival (OS), disease-free survival (DFS), and progression-free survival (PFS). They less often recur in the pelvis, and even when they do, they are associated with better OS, DFS, and PFS compared with NHPV ECAs with pelvic recurrence. HPVA ECAs also respond better to adjuvant therapy using conventional agents [9, 19–22]. Importantly, distinguishing HPVA and NHPVA ECAs is more reproducible with the IECC system (kappa=0.51) compared to the WHO classification system (kappa =0.33) [23]. Studying the reproducibility of distinguishing between HPVA and NHPVA ECAs using the IECC system, the authors report a kappa value of 0.45 (moderate/fair agreement), which improved marginally to 0.5 when immunohistochemistry was used as an adjunct to evaluation of H&E-stained slides. These data belie the fact that perfect agreement in diagnosis across a panel of 7 pathologists was reached in 56% of cases and a consensus majority agreement in a further 41%. Although the panel was able to accurately distinguish between types of NHPV carcinomas, this was not true when distinguishing between subsets of HPVA adenocarcinomas, which in many ways recapitulates the imperfect WHO system.
2.1. Ancillary Testing to Confirm HPVA ECAs
It is reasonable to consider whether p16 immunohistochemistry should be reflexively performed on all ECAs. We do not necessarily advocate that approach. First, the IECC classification system was designed to be easily applied in any practice setting, without the use of ancillary testing in most cases, and has been shown to be at least moderately reproducible. In the Parra-Herran study [23], kappa values for diagnostic concordance improved only marginally when immunohistochemistry was used alongside traditional morphological interpretation. mRNA HR-HPV ISH and p16 immunostaining performed in our studies were meant to provide an objective validation of morphology-based criteria but were not intended to be used universally for ECA classification. Importantly, many practicing pathologists have yet to master the interpretation of p16 immunohistochemical stains, which in most cases leads to overcalling block-like p16 expression. Furthermore, block-like staining for p16 is not specific for HPVA ECA since high-grade endometrial carcinomas, most notably serous carcinomas and clear cell carcinomas of endometrium and cervix, may show this expression pattern. mRNA HR-HPV ISH would be a far better candidate than p16 for routine application, as this is slightly more sensitive and much more specific than p16 for HPVA ECA. Unfortunately, this test is not available in most laboratories.
2.2. HPVA ECA subtypes of the IECC classification system
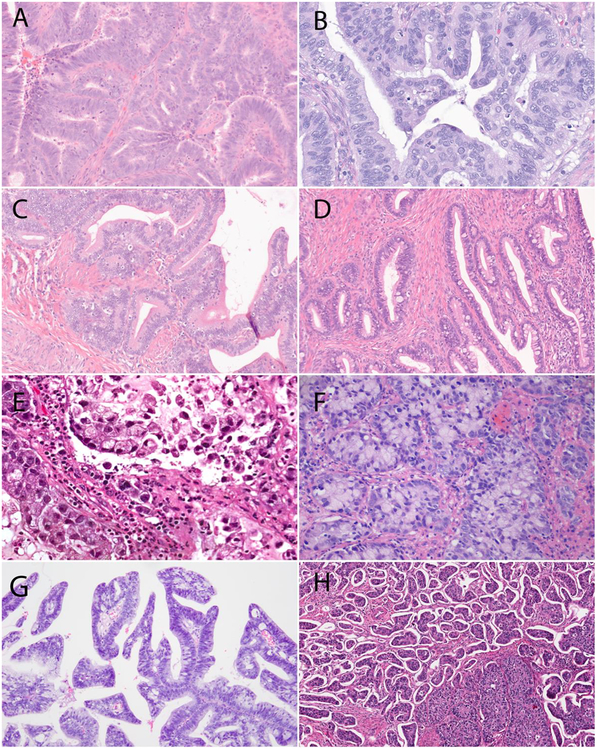
The major IECC HPVA ECA subtypes are the usual type (including the villoglandular and micropapillary architectural variants) and the heterogeneous mucinous type [9]. Invasive stratified mucin-producing carcinoma (iSMILE) is also among this group. Invasive adenocarcinoma NOS is morphologically uninformative, yet considered part of this group when HPV positive [24, 25] (Figure 2).
Figure 2.
Major International Endocervical Adenocarcinoma Criteria and Classification (IECC) HPV-associated types: (A) Usual-type endocervical adenocarcinoma (ECA) exhibiting mucin depletion (<50% of cells) and (B) apical “floating” mitoses and apoptotic debris, (C) mucinous not otherwise specified (NOS)-type ECA exhibiting abundant intracytoplasmic mucin (≥50% of cells), (D) mucinous intestinal-type ECA (goblet cells in ≥50% of cells), (E) mucinous signet-ring type ECA (signet-ring morphology in ≥50% of tumor cells), (F) invasive stratified mucin-producing adenocarcinoma (iSMILE), (G) villoglandular-type ECA and (H) micropapillary-type ECA.
2.2.1. Usual-type HPVA ECA
An estimated 80% of ECAs are usual-type ECAs. The 2014 WHO classification system describes them vaguely as ECAs with little or no cytoplasmic mucin. [1]. To provide a level of reproducibility, the IECC system defines them as ECAs composed 0–50% of cells with appreciable intracytoplasmic mucin assessed on H&E staining.
Architecturally, usual-type ECA is mainly characterized by glands of various shapes and sizes, although papillary, cribriform and solid areas can be encountered. Microglandular, microcystic and macrocystic areas, as well as single-cell patterns, are rare in these tumors. The glands are usually crowded but may be distributed haphzardly in stroma. Large pools of mucin are ocasionally seen within the stroma. The stroma is usually but not always desmoplastic, with inflammatory cells that can form a band-like infiltrate at the base of the tumor [26]. The neoplastic cells contain characteristically pseudostratified, enlarged, elongated, and hypercromatic nuclei. The apical zone of the amphophilic to eosinophilic cytoplasm contains mitotic figures, as well as apoptotic bodies; these features are pathognomonic for this microscopic type (as well as for for other HPVA types). Tumor emboli (LVI) can be found at the tumor’s periphery, especially in large and deeply invasive tumors of Silva C pattern. Usual-type HPVA ECAs have various precursor lesions, including adenocarcinoma in situ (AIS) and stratified mucin-producing intraepithelial lesion (SMILE). High-grade squamous intraepithelial lesion (HSIL) and adenocarcinoma in situ can be seen in combination in 50% of cases [9].
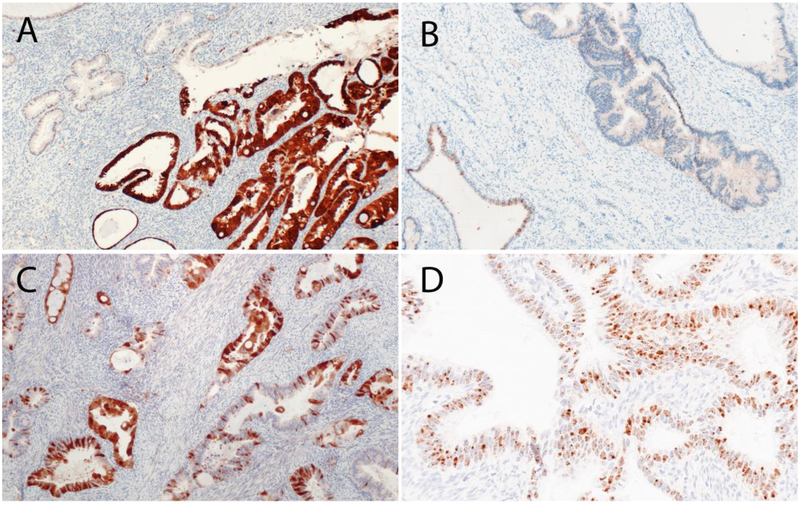
Usual-type ECAs are almost always associated with high-risk HPV (HR-HPV) and are block-positive for p16, a surrogate of HR-HPV infection [1]. In our series, 86.7% of usual-type ECAs were HPV positive using in situ hybridization (ISH), recognizing mRNA of E6 and E7 oncoproteins in 18 types of HR-HPV (mRNA HR-HPV ISH). Furthermore, 83.3% were block-positive for p16 [9] (Figure 3). Similar rates of HPV positivity (~82%) have been reported in the literature [27]. The absence of HPV positivity in rare cases is likely due to different detection sensitivities (polymerase chain reaction [PCR] versus ISH analysis), fixation techniques, or the inability of standard methods to detect rare HPV subtypes [9].
Figure 3.
p16 and HPV. (A) p16 was considered positive if strong, diffuse block-like staining was present. p16 was considered negative if there was non–block-like patchy staining (B) or focal/weak staining (C). (D) HPV RNA-based in situ hybridization showing the presence of high-risk HPV.
The major differential diagnosis consideration for usual-type ECA is endometrioid adenocarcinoma of endometrium. ECAs with “endometrioid” morphology and conspicuous apical mitoses and apoptotic bodies are recategorized as usual-type ECA by the IECC system (discussed below). The distinction between usual-type ECA and true endometrioid adenocarcinoma of uterine corpus is particularly important since the surgical management of the two differs [16]. True endometrioid adenocarcinomas of cervix are very rare, have distinct endometrioid morphology (including endometrioid-associated metaplasias), and may be associated with cervical endometriosis. Immunohistochemistry (IHC) is helpful in differentiating the two, as usual-type ECAs are almost always HPV positive and rarely vimentin (up to 12%), estrogen receptor (ER; up to 5%) and progesterone receptor (PR; up to 20%) positive; true endometrioid adenocarcinomas of both corpus and cervix are HPV negative and frequently contain larger numbers of tumor cells positive for ER and PR. Vimentin and p16 are not always diagnostically helpful, since vimentin can be negative in true endometrioid adenocarcinomas and p16 can be positive in high-grade endometrioid adenocarcinomas [20]. Carcinoembryonic antigen (CEA) has limited diagnostic usefulness, as focal staining can be observed in both tumors. In difficult cases, mRNA HR-HPV ISH together with clinical features can be diagnostically helpful [20].
The second major differential diagnosis consideration for usual-type ECA is serous ECA, although almost all previously reported serous adenocarcinomas are either HPV positive (i.e., not serous) or represent spread of true serous adenocarcinoma from the corpus or adnexa. Usual-type ECA can exhibit extensive papillary architecture and cellular tufting resulting in irregular slit-like luminal spaces, akin to serous adenocarcinomas of endometrial/tubo-ovarian origin (Figure 4). Serous ECA was described by Gilks et al. as a primary malignant tumor of the cervix with a bimodal age distribution, papillary and/or micropapillary architecture, cells showing highly atypical nuclei, a lack of intercellular adhesion, and numerous mitotic figures and apoptotic bodies. Recent papers have shown that after excluding drop metastases and other tumors with papillary architecture, so-called “serous ECAs” are mostly HPV positive [28, 29]. HPV-positive tumors with serous-like morphology are reclassified by the IECC system as usual-type ECAs. In challenging cases, WT1 can help rule out metastasis of tubo-ovarian origin, and HPV ISH and p53 can help differentiate usual-type ECA with serous-like features (i.e., HPV positive, p53 variable) from endometrial serous adenocarcinoma (HPV negative, p53 aberrantly expressed) [29]. Metastasis to the cervix can be be difficult to differentiate from a primary ECA, particularly in the absence of clinical information. IHC for PAX8 and HPV testing can help confirm a primary HPVA ECA, although PAX8 is often negative in iSMILE ECAs (likely due to its reserve cell origin) [20].
Figure 4.
Differential diagnostic considerations for usual-type HPVA. (A) Usual-type endocervical adenocarcinoma can exhibit pseudoendometrioid morphology, mimicking the appearance of (B) endometrioid endometrial adenocarcinoma, but will also have floating mitoses, apoptotic debris, and will be negative for estrogen receptor. Usual-type endocervical adenocarcinoma can also exhibit slit-like spaces (C), thin/fine papillary archiecture (D) or broad papillary architecture (E), mimicking the appearance of endometrial serous adenocarcinoma (F), but it will be negative for p53 and HPV.
Villoglandular pattern HPVA ECA is a rare, well-differentiated, usual-type HPVA ECA with distinct long, thin exophytic papillae lined by columnar cells with mild-to-moderate atypia. It usually occurs in younger women (mean age, 35 years) and is variably associated with oral contraceptive use [1, 3]. Macroscopically, villoglandular HPVA ECA is a well-circumscribed, exophytic, friable, grey tumor of variable size. Like most other usual-type ECAs, it is characterized by luminal mitotic figures and relatively mucin-depleted cells. Invasion is usually superficial but can be deeper, with the deeper component bearing glands rather than papillae. Villoglandular variants of usual-type ECA are rarely associated with LVI and LNM and usually lack or show only superficial stromal invasion.
With only superficial invasion, villoglandular ECAs typically have an excellent prognosis; however, when invasion is deeper and resembles that of standard usual-type ECA, clinical outcomes are similar to those of other usual-type ECAs. Since these tumors can be visualized on gynecologic examination, they are classified as International Federation of Gynecology and Obstetrics (FIGO) stage IB disease, which in many centers, is treated with radical hysterectomy when the tumor measures less than 4 cm. A cone biopsy may be a good strategy to evaluate for stromal invasion, its depth and pattern, and for LVI. A cone biopsy is probably sufficient therapy with negative margins, no or minimal invasion, a Silva pattern A and no LVI, despite the clinical appearance of a FIGO stage IB ECA [30–32].
The main differential diagnosis considerations of villoglandular-pattern HPVA ECAs are drop metastases of tubo-ovarian origin and endometrial serous adenocarcinomas, and the villoglandular type of endometrioid adenocarcinoma of endometrium. Villoglandular ECAs, compared with serous adenocarcinomas, have, by definition, low-grade nuclei and are HPV positive. NHPVA clear cell carcinoma can also display papillary architecture but tends to also exhibit architectural patterns and cytological features similar to those of clear cell adenocarcinoma. Benign cervical lesions, such as papillary adenofibroma and Müllerian papillomas (characteristic for children), usually display papillary architecture but have no atypical features and no association with HPV. Villoglandular ECA can be differentiated from villoglandular endometrial adenocarcinoma via high-resolution imaging, p16 IHC, and mRNA HR-HPV ISH, with or without ER and PR IHC.
Micropapillary pattern HPVA ECA is a newly described histologic pattern (mostly in HPVA ECAs). Microscopically, it is characterized by small, tightly cohesive papillary groups of neoplastic cells, often surrounded by clear spaces resembling vascular channels [33–36]. Macroscopically, it is similar to other types of ECAs; however, on microscopic examination, it is always associated with LVI, often with LNM, and a poor prognosis. A recent, large study of more than 40 cases of pure (comprising >50% of the tumor) or mixed (comprising 10–50% of the tumor) micropapillary pattern ECAs showed that 47.7% of patients with follow-up died of disease and 70% developed recurrent disease or distant metastases, most commonly in the lung, liver, or retroperitoneum [24]. Immunohistochemically, most tumors are p16, MUC1 (reverse polarity staining) and HPV positive, while negative for WT1 and wild-type for p53 [24, 35]. This tumor profile in consideration with clinical data and HPV testing can differentiate micropapillary ECA from metastatic serous adenocarcinoma. The micropapillary pattern is a manifestation of aggressive behavior [24].
2.2.2. Mucinous-type HPVA ECA
WHO-defined mucinous carcinomas are a diverse group of HPV-positive and HPV-negative tumors, unified by the presence of abundant intracytoplasmic mucin. They are categorized as NOS, gastric type and its well-differentiated variant, minimal deviation mucinous adenocarcinoma (HPV negative), intestinal type (HPV-positive and -negative tumors with evidence of intestinal differentiation), and signet-ring type adenocarcinoma (HPV-positive and -negative tumors with signet-ring cells) [1]. The IECC system, which first distinguishes between HPVA and NHPVA ECAs, recognizes the following subcategories of mucinous HPVA ECAs: 1) mucinous NOS (≥50% of tumor cells have intracytoplasmic mucin in a background of usual-type ECA), 2) intestinal (≥50% of cells with goblet morphology in a background of usual-type ECA), 3) signet-ring (≥50% of tumor cells with signet-ring morphology in a background of usual-type ECA) and 4) iSMILE (invasive nests of stratified columnar cells with peripheral palisading and variable amounts of intracytoplasmic mucin, resembling its in situ counterpart). Gastric-type ECA and minimal deviation ECAs are classified as NHPVA ECAs irrespective of intestinal differentiation, signet-ring cells, or a micropapillary pattern [6, 9, 37].
All IECC-classified mucinous HPVA ECAs, irrespective of subcategorization, are HPV positive and most are p16 positive (75% of intestinal types and 62.5% of iSMILEs), in line with the pubished literature [9, 25, 37, 38]. Mucinous NOS, intestinal, signet-ring HPVA, and usual-type HPVA ECAs are immunohistochemically similar, except in terms of MUC6 and HIK1083 (75% of intestinal ECAs are MUC6 positive and 25% are HIK1083 positive). This sometimes complicates the distinction between intestinal-type HPVA and gastric-type adenocarcinomas with intestinal differentiation [20, 25]. p16 and mRNA HR-HPV ISH are more useful for differential diagnosis.
iSMILE is a newly recognized subtype of ECA, first described by Lastra et al. in 2015 as an invasive adenocarcinoma containing nests of stratified columnar epithelium with round to ovoid hyperchromatic nuclei, variable amounts of intracytoplasmic mucin in the form of large mucin droplets, or more delicate and collapsing vacuoles that create spacing between adjacent nuclei and peripheral palisading [37]. Findings from a recent paper demonstrated immunohistochemical differences between iSMILE ECAs and other HPVA ECAs, such as a higher prevalence of p40 and p63 expression and a lower prevalence of PAX8 expression, with possibly more frequent aberrant p53 staining [25]. These data suggest that iSMILE adenocarcinomas diverge from other mucinous HPVA ECAs and could be categorized separately [25]. They were historically considered poorly differentiated adenocarcinomas NOS or adenosquamous carcinomas [25].
2.3. HPVA ECA prognostic indicators
In the IECC panel’s multivariate analysis, FIGO stage, Silva invasion pattern, and possibly mucinous differentiation were found to be independently associated with clinical outcomes [22]. In the IECC panel’s analysis of significant prognostic parameters, HPVA ECAs with mucinous differentiation, compared with those lacking mucin, stood out as a possible indicator of worse PFS [9]. Furthermore, a recent study [39] reported iSMILE is a more aggressive adenocarcinoma than other HPVA ECAs. A micropapillary pattern of invasion was recently identified as an aggressive phenotype [24]. Although there are no large clinicopathological studies of HPVA signet-ring cell adenocarcinomas, intuitively, they are likely aggresive tumors [40].
There is no specific grading system for ECA. Implementing the FIGO grading system (as used for endometrial adenocarcinomas), there were no statistically significant differences in OS, DFS or PFS between FIGO grade 1, 2, and 3 HPVA ECAs [22]. Stage (including depth of stromal invasion) is a better prognosticator, but the depth of invasion in ECAs can be difficult to assess. Alternatively, pattern of cervical stromal invasion (Silva patterns A, B, and C) may best assess LNM risk. This approach has been validated by several investigators, and although now used internationally, Silva pattern is not yet included in the FIGO staging system [41, 42].
Silva A tumors exhibit well-demarcated glands with a lobular architecture, without evidence of destructive stromal or lymphovascular invasion. These tumors are associated with a negligible risk of pelvic LNM and local recurrence. Silva B tumors are similar to Silva A tumors but with focal destructive invasion. Silva C tumors exhibit diffusely infiltrative glands with associated extensive desmoplasia. These tumors can be associated with LNM, local recurrence, and death. Although the Silva system was originally intended only for usual-type HPVA ECAs, a recent study reported statistically significant associations between Silva pattern and LNM and clinical outcomes in HPVA ECAs, regardless of mucin content; 14.7% of HPVA ECAs were Silva A, 9.3% were Silva B, and 76% were Silva C. This suggests the Silva system might work as an adjunct to or surrogate for HPVA ECA grade [21]. Furthermore, the presence of LVI and number of vessels with LVI are associated with pelvic recurrence [22, 26].
2.4. NHPVA ECA subtypes of the IECC classification system
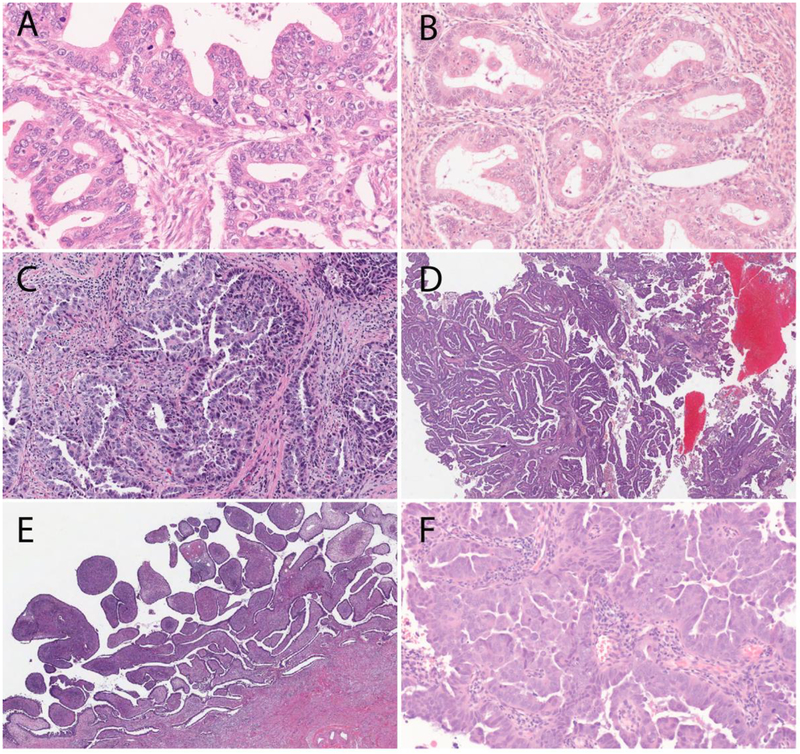
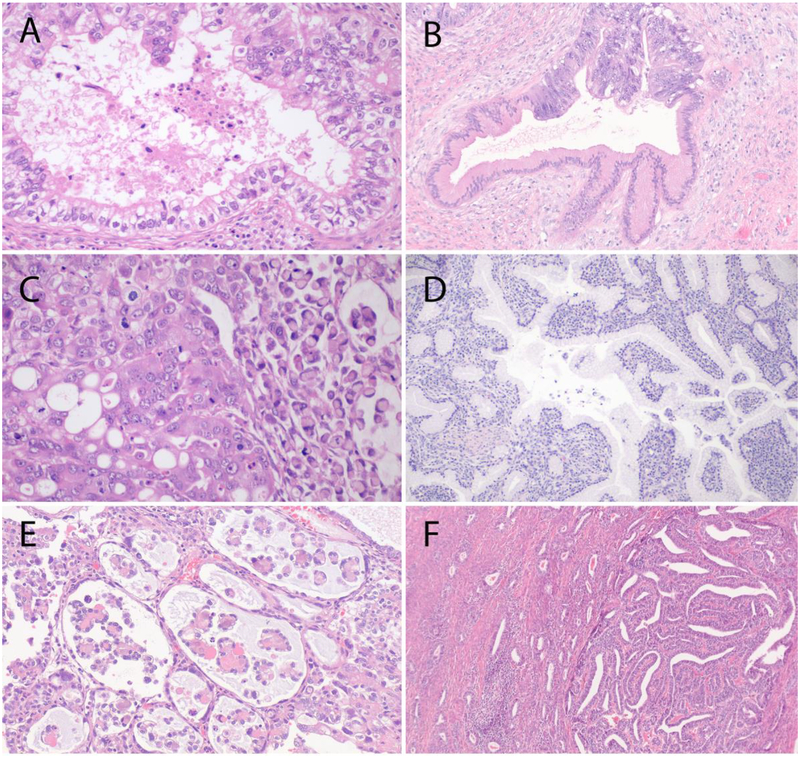
The 4 major IECC NHPVA ECA subtypes are gastric, clear cell, endometrioid, and mesonephric adenocarcinomas [9] (Figure 5). The gastric type is the most common, accounting for 5–25% of all ECAs, depending on geographic location (higher frequency among Japanese patients) [43]; the IECC panel reported an incidence rate of 10%. The 3 other NHPVA ECA types are very rare.
Figure 5.
Major International Endocervical Adenocarcinoma Criteria and Classification (IECC) non–HPV-associated (NHPVA) types: (A) Gastric-type adenocarcinoma, which can be associated with gastric-type adenocarcinoma in situ colonizing normal glands (B), signet-ring cell morphology (C) lobular endocervical glandular hyperplasia (LEGH), E) clear cell, and F) mesonephric types.
2.4.1. Gastric-type NHPVA ECA
Gastric-type ECA represents a spectrum of lesions, from well-differentiated minimal deviation adenocarcinoma of mucinous type (also known as adenoma malignum) to moderately to poorly differentiated ECA containing gastric-type mucin [1, 44]. Adenoma malignum is characterized by benign-appearing, irregularly shaped glands that often deeply infiltrate cervical stroma, usually without overt stromal desmoplasia or easily appreciated mitotic activity. Gastric-type adenocarcinoma (including adenoma malignum) contains cells with abundant clear, foamy or pale eosinophilic cytoplasm, distinct cytoplasmic borders, generally low nuclear-cytoplasmic ratios and irregular basally located nuclei, with no or limited HPVA-like features (no mitotic figures or apoptotic bodies) [9]. These tumors may contain intestinal differentiation in the form of goblet cells and neuroendocrine-like eosinophilic granular cytoplasm, and occasionally, solid areas, micropapillae, and stromal mucin pools. All gastric-type ECAs contain gastric-type mucin, which is easily identified with magenta-colored cytoplasmic staining using Alcian Blue/PAS stains. Although the immunohistochemical antibody HIK1083 marks many of these tumors with good specificity, it is not widely commercially available [20]. MUC6 staining has been suggested as a reasonable alternative to HIK1083, but it lacks specificity [20]. Several benign cervical lesions, such as gastric metaplasia, tunnel clusters type A and diffuse laminar endocervical glandular hyperplasia, also contain pyloric-type mucin.
Compared with HPVA ECAs, gastric-type ECAs are often larger, occur in older patients, are diagnosed at higher stages [9], and present with watery or mucinous vaginal discharge (one case in association with hyponatremia from excesive watery discharge) and often a negative Pap test [45]. Macroscopically, these tumors are white/tan/yellow and firm, but sometimes friable and mucoid. They can form a “barrel-shape” cervix, as well as polypoid and ulcerative forms. It is unclear whether gastric-type carcinoma originates from the transformation zone, but the proximal endocervix is not an uncommon site of origin, making it difficult to detect in its earliest stages.
Lobular endocervical glandular hyperplasia (LEGH) has been suggested as a precursor lesion [46, 47]. Atypical LEGH and ”gastric-type adenocarcinoma in situ” [48] are the intermediary lesions linking LEGH and invasive gastric-type ECA. In the IECC study, up to 15% of gastric-type ECAs were associated with a gastric-type adenocarcinoma in situ [9]. There is accumulating evidence that gastric metaplasia evolves to non-atypical and then atypical LEGH, and subsequently gastric-type ECA, with or without the features of minimal deviation adenocarcinoma of mucinous type, via an HPV-independent molecular pathway (driven by TP53, STK11, GNAS, and KRAS mutations) [49]. Additional studies exploring the molecular underpinnings of gastric-type ECA have revealed mutations mostly in TP53, followed by MSH6, SLX4, POLE, CDKN2A/B, FANCA, ARID1A, STK11, BRCA2 and MSH2, a molecular profile that is similar to that of pancreatic ductal adenocarcinoma [50, 51]. Very rare cases of mismatch repair deficiency due to germline alterations (i.e., attributable to Lynch syndrome) have been reported [52]. In the context of Peutz-Jeghers syndrome (autosomal dominant mutation of STK11/LKB1), gastric-type ECA, including adenoma malignum, may coexist with an ovarian mucinous neoplasm or, in rare cases, ovarian sex cord stromal tumor(s) with annular tubules (SCTAT) [53].
In the IECC study, all gastric-type ECAs were HPV-negative [9], but greater than 30% were block-positive for p16 [6, 20], which is on the high end of previously reported rates. Gastric-type ECA is rarely positive for vimentin and hormone receptors, and has variable positivity for MUC6 (45.8%) and HIK1083 (41.7%) [6, 20, 54]. These tumors are often MUC6 and HNF-1β positive, but their histological mimics can be positive as well, rendering these markers useless for differential diagnosis. Recent work by our group and others has shown promise in using trefoil factor family 2 (TFF2) protein expression for differential diagnosis, as it is positive by IHC in many gastric-type ECAs and its precursors while also negative in all clear cell and almost all usual-type and mucinous NOS ECAs [55]. In our study, predictive biomarkers were mostly negative (3.8% of cases were HER2 positive and none were androgen receptor positive) [20].
All gastric-types ECAs, including adenoma malignum, are potentially aggressive tumors associated with high-risk clinical features such as LVI, LNM, and metastasis to the ovaries, other pelvic/abdominal organs, and distant sites (liver, lung, bone, and brain). Compared with usual-type ECAs, they have a worse prognosis (shorter OS and DFS) and a higher mortality rate [1, 6, 21, 43, 56].
The differential diagnosis of adenoma malignum usually can be made with the identification of various benign glandular lesions. When distinguishing between adenoma malignum and a benign entity, ER/PR IHC is useful, as gastric-type ECAs (and adenoma malignum) are negative. The claw-like shapes and deep placement of glands along with at least mild nuclear atypia and the presence of at least focal stromal desmoplasia are also more typical of adenoma malignum. Some gastric-type ECAs can have admixed usual-like components, and in these cases, ancillary testing for p53 and HPV may be needed [57]. In rare cases, a metastasis, especially from the pancreas, needs to be ruled out with clinical correlation, since the morphology and immunophenotype of gastric-type ECAs and pancreatic ductal adenocarcinomas can be very similar, if not identical.
2.4.2. Clear cell type NHPVA ECA
Clear cell carcinoma of the lower genital tract has been linked to in utero exposure to diethylstilbestrol (young patients, exophytic/endophytic appearance, developing in the transformation zone or vagina), but now, nearly all cases are sporadic (older patients, higher up in the endocervical canal, not visible on colposcopic examination) [58, 59]. Some clear cell ECAs are related to vaginal adenosis or gross structural cervicovaginal mesenchymal abnormalities [60]. The IECC panel recognized that its appearance and criteria for diagnosis are identical to those of other Müllerian clear cell adenocarcinomas.
Although some older reports in the literature have shown an association between clear cell ECAs and HPV, the IECC and other studies have shown they are HPV negative [6–9]. Despite this, up to one-third of cases are p16 positive [6, 20]. The main differential diagnosis consideration is gastric-type ECA, especially in a biopsy, as both can have optically clear cytoplasm. HNF-1β, Napsin A, MUC6, and HIK1083 positivity, as well as aberrant p53 staining, can be seen in both tumor types [20] and are not diagnostically useful. TFF2 may have diagnostic value, as it is positive in many gastric-type ECAs and negative in clear cell adenocarcinomas (unpublished data). Arias Stella reaction [61] and florid microglandular hyperplasia [62] can mimic clear cell ECA, but they are typically strongly ER positive, whereas clear cell ECA is usually negative.
There is a paucity of data in the literature regarding the prognostic indicators and clinical outcomes of these tumors, as they are rare. Some studies suggest stage as a prognosticator, showing FIGO stage I and II disease have a better prognosis than stage III and IV disease [63].
2.4.3. Mesonephric-type NHPVA ECA
This extremely rare NHPVA ECA typically arises from mesonephric remnants, which is why it is often located in the lateral and deep cervical walls [1]. Mucosal/luminal involvement may also be seen.
The 2014 WHO and IECC classification systems use identical diagnostic criteria for mesonephric ECA. Mesonephric ECAs frequently contain a mixture of growth patterns (ductal, tubular [with eosinophilic luminal secretions], papillary, slit-like, cord-like, spindle cell, and glomeruloid), although one pattern can be the dominant or only pattern in a tumor [1, 9]. Mesonephric ECA tumor cells have scant cytoplasm, with rare mitoses. The nuclei are ovoid and often resemble those of papillary thyroid carcinomas; chromatin clearing and nuclear overlap are common. The presence of benign mesonephric remnants is helpful for an accurate diagnosis. These tumors are HPV, p16, MUC6, HIK1083, ER, and PR negative, and usually GATA3, Calretinin, CD10, TTF1, and HNF-1β positive [9, 64]. Recent molecular studies have shown alterations in KRAS and NRAS in up to 81% of cases, as well as mutations in chromation remodelling genes (ARID1A, ARID1B, SMARCA4) in two-thirds of cases [15].
The main differential diagnosis consideration is usual-type ECA. HPV and GATA3 testing can be diagnostically useful. In contrast to mesonephric ECAs, endometrioid carcinomas of endometrium are mostly ER/PR positive and show confirmatory endometrioid features. Florid mesonephric hyperplasia can mimic mesonephric ECA, but it usually does not form a mass lesion or have atypical nuclei, LVI, or morphologic patterns other than small tubules [65].
Due to the rarity of the tumor, little is known about its prognosis, although small series have reported both local recurrences and distant metastases (bone, lung, pleura, abdomen, and liver) [64, 66].
2.4.4. Endometrioid-type NHPVA ECA
Endometrioid-type ECA is a confusing entity for clinicians and pathologists. Its reported prevalence varies greatly (7–50% of all ECAs), likely due to a lack of clear-cut diagnostic criteria. According to the 2014 WHO classification system, “endometrioid” ECAs can be HPVA (and similar to usual type) or NHPVA, the latter reportedly developing from cervical endometriosis [1]. In the IECC study, WHO criteria were initially applied to the examination of more than 400 ECAs, and the majority ended up being classified as “endometrioid.” With the possible exception of 3 cases, all were HPV negative, none showed “confirmatory endometrioid features”, and all were easily classified as HPVA ECAs [9]. Confirmatory endometrioid features are recognized in adenocarcinomas containing at least focal low-grade endometrioid glands lined by columnar cells, with pseudostratified nuclei, no more than moderate nuclear atypia, with or without squamous differentiation and/or endometriosis. Unlike HPVA ECAs, these tumors lack easily appreciated apical mitoses and karyorrhexis. Cribriform architecture is diagnostically uninformative. The majority of cases meeting the 2014 WHO criteria for an “endometrioid” diagnosis were block-positve for p16 and HR-HPV positive [9]. As such, almost all WHO-defined “endometrioid” adenocarcinomas are reclassified as usual-type ECAs by the IECC, which is more in line with their biology.
The major differential diagnosis consideration is usual-type ECA. The other major differential diagnosis consideration is endometrioid adenocarcinoma extending from the corpus. Correlation with clinical and imaging findings are most helpful in this scenario. In rare cases, usual-type ECA can be associated with bland, metaplastic-appearing squamous differentiation covering the surface of the tumor, which should not be confused with either endometrioid adenocarcinoma with squamous differentiation or cervical adenosquamous carcinoma [25]. The prognosis of endometrioid-type ECA is unclear due to the lack of uniform criteria in previous studies and the limited number of cases in the IECC study.
2.5. NHPVA prognostic indicators
In the IECC multivariate analysis, patient age, FIGO stage, and tumor size were significantly associated with survival [22]. Silva pattern is uninformative, as nearly all NHPVA ECAs are Pattern C [21, 39]. Of note, the IECC study included mainly gastric-type ECAs [22, 39], making it unclear if the determined prognostic indicators and clinical outcomes apply to all NHPVA ECAs.
3. Conclusions
The IECC classification system divides ECAs into HPVA and NHPVA ECAs on histologic examination alone, without the need for IHC markers. The system is easy to use in daily practice, has good reproducibility, and excellent prediction of HPV status [9, 23]. Cancer care has shifted from a purely morphologic diagnosis and ‘one size fits all’ approach to one that is personalized and tumor specific, integrating etiologic, molecular, and clinical characteristics. As such, the IECC panel recommends a move from the 2014 WHO classification system to the proposed 2019 IECC system (Figure 1) [22, 39].
Funding:
This study was funded in part through the NIH/NCI Support Grant P30 CA008748 (Dr. Soslow).
Footnotes
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of a an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
Dislcosure of Potential Conflicts of Interest: The authors have no conflicts of interest to disclose
Research Involving Human Participants and/or Animals: Ethical standards of research involving human participants were followed.
References
- 1.Kurman RJ, Carcangiu ML, Herrington CS, Young RH (2014) WHO Classification of Tumours of the Female Reproductive Organs, 4th Edition IARC, Lyon [Google Scholar]
- 2.Bray F, Carstensen B, Moller H, Zappa M, Zakelj MP, Lawrence G, Hakama M, Weiderpass E (2005) Incidence trends of adenocarcinoma of the cervix in 13 European countries. Cancer Epidemiol Biomarkers Prev 14:2191–2199. doi: 10.1158/1055-9965.epi-05-0231 [DOI] [PubMed] [Google Scholar]
- 3.Loureiro J, Oliva E (2014) The spectrum of cervical glandular neoplasia and issues in differential diagnosis. Arch Pathol Lab Med 138:453–483. doi: 10.5858/arpa.2012-0493-RA [DOI] [PubMed] [Google Scholar]
- 4.Smith HO, Tiffany MF, Qualls CR, Key CR (2000) The rising incidence of adenocarcinoma relative to squamous cell carcinoma of the uterine cervix in the United States--a 24-year population-based study. Gynecol Oncol 78:97–105. doi: 10.1006/gyno.2000.5826 [DOI] [PubMed] [Google Scholar]
- 5.Vesterinen E, Forss M, Nieminen U (1989) Increase of cervical adenocarcinoma: a report of 520 cases of cervical carcinoma including 112 tumors with glandular elements. Gynecol Oncol 33:49–53 [DOI] [PubMed] [Google Scholar]
- 6.Park KJ, Kiyokawa T, Soslow RA, Lamb CA, Oliva E, Zivanovic O, Juretzka MM, Pirog EC (2011) Unusual endocervical adenocarcinomas: an immunohistochemical analysis with molecular detection of human papillomavirus. Am J Surg Pathol 35:633–646. doi: 10.1097/PAS.0b013e31821534b9 [DOI] [PubMed] [Google Scholar]
- 7.Pirog EC, Kleter B, Olgac S, Bobkiewicz P, Lindeman J, Quint WG, Richart RM, Isacson C (2000) Prevalence of human papillomavirus DNA in different histological subtypes of cervical adenocarcinoma. Am J Pathol 157:1055–1062. doi: 10.1016/s0002-9440(10)64619-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pirog EC, Lloveras B, Molijn A, Tous S, Guimera N, Alejo M, Clavero O, Klaustermeier J, Jenkins D, Quint WG, Xavier Bosch F, Alemany L, de Sanjose S (2014) HPV prevalence and genotypes in different histological subtypes of cervical adenocarcinoma, a worldwide analysis of 760 cases. Mod Pathol 27:1559–1567. doi: 10.1038/modpathol.2014.55 [DOI] [PubMed] [Google Scholar]
- 9.Stolnicu S, Barsan I, Hoang L, Patel P, Terinte C, Pesci A, Aviel-Ronen S, Kiyokawa T, Alvarado-Cabrero I, Pike MC, Oliva E, Park KJ, Soslow RA (2018) International Endocervical Adenocarcinoma Criteria and Classification (IECC): A New Pathogenetic Classification for Invasive Adenocarcinomas of the Endocervix. Am J Surg Pathol 42:214–226. doi: 10.1097/pas.0000000000000986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Castellsague X, Diaz M, de Sanjose S, Munoz N, Herrero R, Franceschi S, Peeling RW, Ashley R, Smith JS, Snijders PJ, Meijer CJ, Bosch FX (2006) Worldwide human papillomavirus etiology of cervical adenocarcinoma and its cofactors: implications for screening and prevention. J Natl Cancer Inst 98:303–315. doi: 10.1093/jnci/djj067 [DOI] [PubMed] [Google Scholar]
- 11.Lacey JV Jr., Brinton LA, Barnes WA, Gravitt PE, Greenberg MD, Hadjimichael OC, McGowan L, Mortel R, Schwartz PE, Kurman RJ, Hildesheim A (2000) Use of hormone replacement therapy and adenocarcinomas and squamous cell carcinomas of the uterine cervix. Gynecol Oncol 77:149–154. doi: 10.1006/gyno.2000.5731 [DOI] [PubMed] [Google Scholar]
- 12.Lacey JV Jr., Swanson CA, Brinton LA, Altekruse SF, Barnes WA, Gravitt PE, Greenberg MD, Hadjimichael OC, McGowan L, Mortel R, Schwartz PE, Kurman RJ, Hildesheim A (2003) Obesity as a potential risk factor for adenocarcinomas and squamous cell carcinomas of the uterine cervix. Cancer 98:814–821. doi: 10.1002/cncr.11567 [DOI] [PubMed] [Google Scholar]
- 13.Rose PG (2012) Are the differences in treatment outcome for adenocarcinoma of the cervix different enough to change the treatment paradigm? Gynecol Oncol 125:285–286. doi: 10.1016/j.ygyno.2012.03.010 [DOI] [PubMed] [Google Scholar]
- 14.Cancer Genome Atlas Research Network (2017) Integrated genomic and molecular characterization of cervical cancer. Nature 543:378–384. doi: 10.1038/nature21386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mirkovic J, Sholl LM, Garcia E, Lindeman N, MacConaill L, Hirsch M, Dal Cin P, Gorman M, Barletta JA, Nucci MR, McCluggage WG, Howitt BE (2015) Targeted genomic profiling reveals recurrent KRAS mutations and gain of chromosome 1q in mesonephric carcinomas of the female genital tract. Mod Pathol 28:1504–1514. doi: 10.1038/modpathol.2015.103 [DOI] [PubMed] [Google Scholar]
- 16.Network NCC (2018) NCCN Clinical Practice Guidelines in Oncology: Cervical Cancer Version 3.2019. https://www.nccn.org/professionals/physician_gls/pdf/cervical.pdf. Accessed March 5, 2019 [DOI] [PubMed]
- 17.Ojesina AI, Lichtenstein L, Freeman SS, Pedamallu CS, Imaz-Rosshandler I, Pugh TJ, Cherniack AD, Ambrogio L, Cibulskis K, Bertelsen B, Romero-Cordoba S, Trevino V, Vazquez-Santillan K, Guadarrama AS, Wright AA, Rosenberg MW, Duke F, Kaplan B, Wang R, Nickerson E, Walline HM, Lawrence MS, Stewart C, Carter SL, McKenna A, Rodriguez-Sanchez IP, Espinosa-Castilla M, Woie K, Bjorge L, Wik E, Halle MK, Hoivik EA, Krakstad C, Gabino NB, Gomez-Macias GS, Valdez-Chapa LD, Garza-Rodriguez ML, Maytorena G, Vazquez J, Rodea C, Cravioto A, Cortes ML, Greulich H, Crum CP, Neuberg DS, Hidalgo-Miranda A, Escareno CR, Akslen LA, Carey TE, Vintermyr OK, Gabriel SB, Barrera-Saldana HA, Melendez-Zajgla J, Getz G, Salvesen HB, Meyerson M (2014) Landscape of genomic alterations in cervical carcinomas. Nature 506:371–375. doi: 10.1038/nature12881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wright AA, Howitt BE, Myers AP, Dahlberg SE, Palescandolo E, Van Hummelen P, MacConaill LE, Shoni M, Wagle N, Jones RT, Quick CM, Laury A, Katz IT, Hahn WC, Matulonis UA, Hirsch MS (2013) Oncogenic mutations in cervical cancer: genomic differences between adenocarcinomas and squamous cell carcinomas of the cervix. Cancer 119:3776–3783. doi: 10.1002/cncr.28288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kojima A, Shimada M, Mikami Y, Nagao S, Takeshima N, Sugiyama T, Teramoto N, Kiyokawa T, Kigawa J, Nishimura R (2018) Chemoresistance of Gastric-Type Mucinous Carcinoma of the Uterine Cervix: A Study of the Sankai Gynecology Study Group. Int J Gynecol Cancer 28:99–106. doi: 10.1097/igc.0000000000001145 [DOI] [PubMed] [Google Scholar]
- 20.Stolnicu S, Barsan I, Hoang L, Patel P, Chiriboga L, Terinte C, Pesci A, Aviel-Ronen S, Kiyokawa T, Alvarado-Cabrero I, Pike MC, Oliva E, Park KJ, Soslow RA (2018) Diagnostic Algorithmic Proposal Based on Comprehensive Immunohistochemical Evaluation of 297 Invasive Endocervical Adenocarcinomas. Am J Surg Pathol 42:989–1000. doi: 10.1097/pas.0000000000001090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stolnicu S, Barsan I, Hoang L, Patel P, Terinte C, Pesci A, Aviel-Ronen S, Kiyokawa T, Alvarado-Cabrero I, Oliva E, Park KJ, Abu-Rustum NR, Pike MC, Soslow RA (2018) Stromal invasion pattern identifies patients at lowest risk of lymph node metastasis in HPV-associated endocervical adenocarcinomas, but is irrelevant in adenocarcinomas unassociated with HPV. Gynecol Oncol 150:56–60. doi: 10.1016/j.ygyno.2018.04.570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stolnicu S, Hoang L, Chiu D, Hanko-Bauer O, Terinte C, Pesci A, Aviel-Ronen S, Kiyokawa T, Alvarado-Cabrero I, Oliva E, Park KJ, Abu-Rustum NR, Soslow RA (2019) Clinical Outcomes of HPV-associated and Unassociated Endocervical Adenocarcinomas Categorized by the International Endocervical Adenocarcinoma Criteria and Classification (IECC). Am J Surg Pathol. doi: 10.1097/pas.0000000000001224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hodgson A, Park KJ, Djordjevic B, Howitt BE, Nucci MR, Oliva E, Stolnicu S, Xu B, Soslow RA, Parra-Herran C (2019) International Endocervical Adenocarcinoma Criteria and Classification: Validation and Interobserver Reproducibility. Am J Surg Pathol 43:75–83. doi: 10.1097/pas.0000000000001095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Alvarado-Cabrero I, McCluggage WG, Estevez-Castro R, Perez-Montiel D, Stolnicu S, Ganesan R, Vella J, Castro R, Canedo-Matute J, Gomez-Cifuentes J, Rivas-Lemus V, Park K, Soslow RA, Oliva E, Valencia-Cedillo R (2019) Micropapillary cervical adenocarcinoma: a clinicopathologic study of 44 cases. Am J Surg Pathol:In press [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stolnicu S, Hoang L, Hanko-Bauer O, Barsan I, Terinte C, Pesci A, Aviel-Ronen S, Kiyokawa T, Alvarado-Cabrero I, Oliva E, Park KJ, Soslow RA (2019) Cervical adenosquamous carcinoma: detailed analysis of morphology, immunohistochemical profile, and clinical outcomes in 59 cases. Mod Pathol 32:269–279. doi: 10.1038/s41379-018-0123-6 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 26.Alvarado-Cabrero I, Roma AA, Park KJ, Rutgers JKL, Silva EG (2017) Factors Predicting Pelvic Lymph Node Metastasis, Relapse, and Disease Outcome in Pattern C Endocervical Adenocarcinomas. Int J Gynecol Pathol 36:476–485. doi: 10.1097/pgp.0000000000000357 [DOI] [PubMed] [Google Scholar]
- 27.Molijn A, Jenkins D, Chen W, Zhang X, Pirog E, Enqi W, Liu B, Schmidt J, Cui J, Qiao Y, Quint W (2016) The complex relationship between human papillomavirus and cervical adenocarcinoma. Int J Cancer 138:409–416. doi: 10.1002/ijc.29722 [DOI] [PubMed] [Google Scholar]
- 28.Gilks CB, Clement PB (1992) Papillary serous adenocarcinoma of the uterine cervix: a report of three cases. Mod Pathol 5:426–431 [PubMed] [Google Scholar]
- 29.Togami S, Sasajima Y, Kasamatsu T, Oda-Otomo R, Okada S, Ishikawa M, Ikeda S, Kato T, Tsuda H (2015) Immunophenotype and human papillomavirus status of serous adenocarcinoma of the uterine cervix. Pathol Oncol Res 21:487–494. doi: 10.1007/s12253-014-9854-y [DOI] [PubMed] [Google Scholar]
- 30.Jones MW, Silverberg SG, Kurman RJ (1993) Well-differentiated villoglandular adenocarcinoma of the uterine cervix: a clinicopathological study of 24 cases. Int J Gynecol Pathol 12:1–7 [DOI] [PubMed] [Google Scholar]
- 31.Kaku T, Kamura T, Shigematsu T, Sakai K, Nakanami N, Uehira K, Amada S, Kobayashi H, Saito T, Nakano H (1997) Adenocarcinoma of the uterine cervix with predominantly villogladular papillary growth pattern. Gynecol Oncol 64:147–152. doi: 10.1006/gyno.1996.4539 [DOI] [PubMed] [Google Scholar]
- 32.Korach J, Machtinger R, Perri T, Vicus D, Segal J, Fridman E, Ben-Baruch G (2009) Villoglandular papillary adenocarcinoma of the uterine cervix: a diagnostic challenge. Acta Obstet Gynecol Scand 88:355–358. doi: 10.1080/00016340902730359 [DOI] [PubMed] [Google Scholar]
- 33.Kajiyama A, Ishii E, Nakagawa M, Hara J, Honda T, Ikegami A, Teramoto K, Oyama T (2013) Adenocarcinoma of uterine cervix with micropapillary pattern - a case report. J Japan Soc Clin Cyto 3:231–236 [Google Scholar]
- 34.Munakata S, Hosoi A, Yamamoto T (2018) Invasive Micropapillary Carcinoma of the Uterine Cervix: Case Report of a Rare Entity. Int J Gynecol Pathol 37:368–371. doi: 10.1097/pgp.0000000000000432 [DOI] [PubMed] [Google Scholar]
- 35.Stewart CJR, Koay MHE, Leslie C, Acott N, Leung YC (2018) Cervical carcinomas with a micropapillary component: a clinicopathological study of eight cases. Histopathology 72:626–633. doi: 10.1111/his.13419 [DOI] [PubMed] [Google Scholar]
- 36.Toyoda S, Kita T, Sugiura A, Itani Y, Okada H, Nakamura S, Ohbayashi C (2016) Cervical adenocarcinoma with stromal micropapillary pattern. Diagn Cytopathol 44:133–136. doi: 10.1002/dc.23393 [DOI] [PubMed] [Google Scholar]
- 37.Lastra RR, Park KJ, Schoolmeester JK (2016) Invasive stratified mucin-producing carcinoma and stratified mucin producing intraepithelial lesion (SMILE): 15 cases presenting a spectrum of cervical neoplasia with description of a distinctive variant of invasive adenocarcinoma. Am J Surg Pathol 40:262–269 [DOI] [PubMed] [Google Scholar]
- 38.Balci S, Saglam A, Usubutun A (2010) Primary signet-ring cell carcinoma of the cervix: case report and review of the literature. Int J Gynecol Pathol 29:181–184. doi: 10.1097/PGP.0b013e3181b70176 [DOI] [PubMed] [Google Scholar]
- 39.Hodgson A, Olkhov-Mitsel E, Howitt BE, Nucci MR, Parra-Herran C (2019) International Endocervical Adenocarcinoma Criteria and Classification (IECC): correlation with adverse clinicopathological features and patient outcome. J Clin Pathol. doi: 10.1136/jclinpath-2018-205632 [DOI] [PubMed] [Google Scholar]
- 40.Wang YC, Yu YL, Fan CW, Huang SY (2018) Primary signet ring cell carcinoma of the cervix: a case report with review of the literature. Taiwan J Obstet Gynecol 57:862–866 [DOI] [PubMed] [Google Scholar]
- 41.Bhatla N, Aoki D, Sharma DN, Sankaranarayanan R (2018) Cancer of the cervix uteri. Int J Gynaecol Obstet 143 Suppl 2:22–36. doi: 10.1002/ijgo.12611 [DOI] [PubMed] [Google Scholar]
- 42.Diaz De Vivar A, Roma AA, Park KJ, Alvarado-Cabrero I, Rasty G, Chanona-Vilchis JG, Mikami Y, Hong SR, Arville B, Teramoto N, Ali-Fehmi R, Rutgers JK, Tabassum F, Barbuto D, Aguilera-Barrantes I, Shaye-Brown A, Daya D, Silva EG (2013) Invasive endocervical adenocarcinoma: proposal for a new pattern-based classification system with significant clinical implications: a multi-institutional study. Int J Gynecol Pathol 32:592–601. doi: 10.1097/PGP.0b013e31829952c6 [DOI] [PubMed] [Google Scholar]
- 43.Kojima A, Mikami Y, Sudo T, Yamaguchi S, Kusanagi Y, Ito M, Nishimura R (2007) Gastric morphology and immunophenotype predict poor outcome in mucinous adenocarcinoma of the uterine cervix. Am J Surg Pathol 31:664–672. doi: 10.1097/01.pas.0000213434.91868.b0 [DOI] [PubMed] [Google Scholar]
- 44.Ishii K, Hosaka N, Toki T, Momose M, Hidaka E, Tsuchiya S, Katsuyama T (1998) A new view of the so-called adenoma malignum of the uterine cervix. Virchows Arch 432:315–322 [DOI] [PubMed] [Google Scholar]
- 45.Mills KE, Shuen P, Zolis L (2015) Adenoma Malignum Presenting With Profound Hyponatremia. J Obstet Gynaecol Can 37:624–627. doi: 10.1016/s17012163(15)30200-0 [DOI] [PubMed] [Google Scholar]
- 46.Mikami Y, Hata S, Fujiwara K, Imajo Y, Kohno I, Manabe T (1999) Florid endocervical glandular hyperplasia with intestinal and pyloric gland metaplasia: worrisome benign mimic of “adenoma malignum”. Gynecol Oncol 74:504–511. doi: 10.1006/gyno.1999.5462 [DOI] [PubMed] [Google Scholar]
- 47.Nucci MR, Clement PB, Young RH (1999) Lobular endocervical glandular hyperplasia, not otherwise specified: a clinicopathologic analysis of thirteen cases of a distinctive pseudoneoplastic lesion and comparison with fourteen cases of adenoma malignum. Am J Surg Pathol 23:886–891 [DOI] [PubMed] [Google Scholar]
- 48.Talia KL, Stewart CJR, Howitt BE, Nucci MR, McCluggage WG (2017) HPV-negative Gastric Type Adenocarcinoma In Situ of the Cervix: A Spectrum of Rare Lesions Exhibiting Gastric and Intestinal Differentiation. Am J Surg Pathol 41:1023–1033. doi: 10.1097/pas.0000000000000855 [DOI] [PubMed] [Google Scholar]
- 49.Mikami Y, McCluggage WG (2013) Endocervical glandular lesions exhibiting gastric differentiation: an emerging spectrum of benign, premalignant, and malignant lesions. Adv Anat Pathol 20:227–237. doi: 10.1097/PAP.0b013e31829c2d66 [DOI] [PubMed] [Google Scholar]
- 50.Nagaria T, Garg S, Stockley T, Clarke B, Bernardini MQ, Rouzbahman M (2018) Molecular landscape of gastric-type endocervical adenocarcinomas (GAS)-next generation sequencing of 14 cases. Mod Pathol 31:1238. [DOI] [PubMed] [Google Scholar]
- 51.Winter JM, Maitra A, Yeo CJ (2006) Genetics and pathology of pancreatic cancer. HPB (Oxford) 8:324–336. doi: 10.1080/13651820600804203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Moat M, O’Donnell RL, McCluggage WG, Ralte A, Edmondson RJ (2014) Gastric-type adenocarcinoma of the cervix in a patient with Lynch syndrome: a case report. Gynecol Oncol Rep 10:41–3. doi: 10.1016/j.gynor.2014.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ito M, Minamiguchi S, Mikami Y, Ueda Y, Sekiyama K, Yamamoto T, Takakura K (2012) Peutz-Jeghers syndrome-associated atypical mucinous proliferation of the uterine cervix: a case of minimal deviation adenocarcinoma (‘adenoma malignum’) in situ. Pathol Res Pract 208:623–627. doi: 10.1016/j.prp.2012.06.008 [DOI] [PubMed] [Google Scholar]
- 54.Carleton C, Hoang L, Sah S, Kiyokawa T, Karamurzin YS, Talia KL, Park KJ, McCluggage WG (2016) A Detailed Immunohistochemical Analysis of a Large Series of Cervical and Vaginal Gastric-type Adenocarcinomas. Am J Surg Pathol 40:636–644. doi: 10.1097/pas.0000000000000578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Asaka S, Nakajima T, Momose M, Miyamoto T, Uehara T, Ota H (2019) Trefoil factor family 2 protein: a potential immunohistochemical marker for aiding diagnosis of lobular endocervical glandular hyperplasia and gastric-type adenocarcinoma of the uterine cervix. Virchows Arch 474:79–86. doi: 10.1007/s00428-018-2469-z [DOI] [PubMed] [Google Scholar]
- 56.Karamurzin YS, Kiyokawa T, Parkash V, Jotwani AR, Patel P, Pike MC, Soslow RA, Park KJ (2015) Gastric-type Endocervical Adenocarcinoma: An Aggressive Tumor With Unusual Metastatic Patterns and Poor Prognosis. Am J Surg Pathol 39:1449–1457. doi: 10.1097/pas.0000000000000532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wada T, Ohishi Y, Kaku T, Aman M, Imamura H, Yasutake N, Sonoda K, Kato K, Oda Y (2017) Endocervical Adenocarcinoma With Morphologic Features of Both Usual and Gastric Types: Clinicopathologic and Immunohistochemical Analyses and High-risk HPV Detection by In Situ Hybridization. Am J Surg Pathol 41:696–705. doi: 10.1097/pas.0000000000000833 [DOI] [PubMed] [Google Scholar]
- 58.Matias-Guiu X, Lerma E, Prat J (1997) Clear cell tumors of the female genital tract. Semin Diagn Pathol 14:233–239 [PubMed] [Google Scholar]
- 59.Robboy SJ, Young RH, Welch WR, Truslow GY, Prat J, Herbst AL, Scully RE (1984) Atypical vaginal adenosis and cervical ectropion. Association with clear cell adenocarcinoma in diethylstilbestrol-exposed offspring. Cancer 54:869–875 [DOI] [PubMed] [Google Scholar]
- 60.Kurman RJ, Ronnett BM, Sherman ME, Wilkinson EJ (2010) Tumors of the cervix, vagina and vulvaAFIP Atlas of Tumor Pathology. Armed Forces Institute of Pathlogy, Washington D.C., pp. 197 [Google Scholar]
- 61.Nucci MR, Young RH (2004) Arias-Stella reaction of the endocervix: a report of 18 cases with emphasis on its varied histology and differential diagnosis. Am J Surg Pathol 28:608–612 [DOI] [PubMed] [Google Scholar]
- 62.Nucci MR (2014) Pseudoneoplastic glandular lesions of the uterine cervix: a selective review. Int J Gynecol Pathol 33:330–338. doi: 10.1097/pgp.0000000000000139 [DOI] [PubMed] [Google Scholar]
- 63.Thomas MB, Wright JD, Leiser AL, Chi DS, Mutch DG, Podratz KC, Dowdy SC (2008) Clear cell carcinoma of the cervix: a multi-institutional review in the post-DES era. Gynecol Oncol 109:335–339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kenny SL, McBride HA, Jamison J, McCluggage WG (2012) Mesonephric adenocarcinomas of the uterine cervix and corpus: HPV-negative neoplasms that are commonly PAX8, CA125, and HMGA2 positive and that may be immunoreactive with TTF1 and hepatocyte nuclear factor 1-beta. Am J Surg Pathol 36:799–807. doi: 10.1097/PAS.0b013e31824a72c6 [DOI] [PubMed] [Google Scholar]
- 65.Mirkovic J, Schoolmeester JK, Campbell F, Miron A, Nucci MR, Howitt BE (2017) Cervical mesonephric hyperplasia lacks KRAS/NRAS mutations. Histopathology 71:1003–1005. doi: 10.1111/his.13307 [DOI] [PubMed] [Google Scholar]
- 66.Silver SA, Devouassoux-Shisheboran M, Mezzetti TP, Tavassoli FA (2001) Mesonephric adenocarcinomas of the uterine cervix: a study of 11 cases with immunohistochemical findings. Am J Surg Pathol 25:379–387 [DOI] [PubMed] [Google Scholar]