Abstract
Natural Killer (NK) cells can develop cell intrinsic memory-like characteristics. Whether they develop these characteristics during Toxoplasma gondii (T. gondii) infection is unknown. We addressed this question and dissected the mechanisms involved in secondary NK cell responses using a vaccine-challenge mouse model of T. gondii infection. NK cells were required for control of and survival after secondary T. gondii infection. NK cells increased in number at the reinfection site and produced IFNγ. To test if these T. gondii experienced NK cells were intrinsically different from naïve NK cells we performed NK cell adoptive transfer into RAG2/cγ-chain−/− mice, NK-cell fate mapping and RAG1−/− mice vaccine-challenge experiments. Although NK cells contributed to immunity after reinfection, they did not develop cell intrinsic memory-like characteristics after T. gondii vaccination. The mechanisms required for generating these secondary NK cell responses were investigated. Secondary NK cell responses were CD4+ or CD8+ T cell independent. Although IL-12 alone is required for NK cell IFNγ production during primary T. gondii infection, in the absence of IL-12 using IL-12p35−/− mice or anti-IL-12p70 secondary NK cell responses were only partially reduced after reinfection. IL-23 depletion with anti-IL-23p19 in vivo also significantly reduced the secondary NK-cell response. IL-12 and IL-23 blockade with anti-IL-12p40 treatment, completely eliminated secondary NK-cell responses. Importantly, blockade of IL-12, IL-23 or both significantly reduced control of parasite reinfection and increased parasite burden. Our results define a previously unknown protective role for NK cells during secondary T. gondii infection that is dependent on IL-12 and IL-23.
Introduction
Accumulating studies show that NK cells can acquire features of adaptive immune cells and develop immunological memory in response to certain stimuli (1). These memory-like NK cells provide a qualitatively and quantitatively greater response to secondary challenge and are intrinsically different from naïve cells. Antigen-specific memory NK cells are generated after encounters with haptens (2) and viruses, such as murine cytomegalovirus (MCMV) and human cytomegalovirus (HCMV) (3–5). In vitro and in vivo stimulation with certain cytokines, such as IL-12, IL-18 and IL-15, leads to the formation of memory-like features in NK cells that are epigenetically and functionally distinct from naïve cells (6–8). Both antigen specific and cytokine-activated memory-like NK cells are generated after MCMV infection in vivo (9). Whether NK cells develop memory-like characteristics in response to eukaryotic agents has yet to be found.
T. gondii is a food-borne intracellular parasitic protozoan that causes the disease toxoplasmosis. The parasite is present in one-third of the human population worldwide and is a significant health concern for immunocompromised individuals (10–13). At present, there is no vaccine or drug available to prevent or completely cure toxoplasmosis in humans (14, 15). NK cells are involved in innate immunity during acute T. gondii infection and are critical for early protection (16, 17). They mediate protection via IFNγ that is secreted in response to IL-12 provided by innate immune cells such as dendritic cells and macrophages (17, 18). NK-cell IFNγ also facilitates the differentiation of monocytes into inflammatory macrophages and monocyte-derived dendritic cells that then serve as the main source of IL-12 (19). In response to systemic IL-12 production during acute infection, bone marrow NK cells produce IFNγ and prime monocytes for regulatory function (20). NK cells also trigger an adaptive immune cell response to T. gondii. In the absence of CD4+ T cells, IFNγ produced by NK cells promotes CD8+ T-cell activation (21). In the absence of CD8+ T cells, NK-cell IFNγ contributes to the activation of CD4+ T cells (22). In addition to cytokine production, NK cells produce perforin and granzymes in response to the parasite and its subcellular components (23–26).
NK cells are clearly important for early control of T. gondii infection, yet their role in long-term immunity has not been addressed. This is clinically important to understand because there currently is no vaccine that elicits sterilizing immunity to the parasite (15, 27). A vaccine targeting the stimulation of NK cells in addition to CD8+ T cells could therefore be more beneficial long-term. In addition, T. gondii infection causes health complications in immunodeficient patients, many of whom are T-cell deficient (e.g., HIV patients) (11). Discovering new ways to utilize NK cells could be therapeutically beneficial for these patients.
In this study, we aimed to find whether NK cells contribute to long-term immunity against T. gondii in a vaccine challenge setting. We also investigated whether NK cells developed memory-like features in response to this vaccination. Lastly, we tested mechanisms involved in the activation of NK cells during secondary challenge. We demonstrate that NK cells are critical for reducing parasite burdens after lethal challenge. T. gondii infection induces a similar Th1 cytokine milieu as compared to MCMV, however, unlike memory-like NK cells generated by viral infection and cytokine stimulation (3, 9, 28), T. gondii–experienced NK cells did not intrinsically develop memory-like traits. This highlights for the first time that, NK cells are required for control of T. gondii reinfection, but are activated in this capacity by cell extrinsic mechanisms. Our exploration of the mechanisms involved in this secondary NK cell response revealed that their response to reinfection is dependent upon both IL-12 and IL-23. Our results reveal a novel role for NK cells during secondary T. gondii challenge infection in the presence of memory T cells (29–32)that is dependent on IL-12 family cytokine stimulation.
Materials and methods
Mice
C57BL/6 (B6), CBA, B6.129S7-Rag1tm1Mom (Rag1−/−, Rag1 knockout [KO]), B6.129S1-Il12atm1Jm (IL-12p35 KO), B6.129S1-Il12tm1Jm/J (IL-12p40 KO) and B6.129X1-Gt(ROSA)26Sortm1(EYFP)Cos (R26R-EYFP) mice were purchased from The Jackson Laboratory. B10;B6-Rag2tm1Fwa Il2rgtm1Wjl (Rag2−/−γc−/−,) mice were purchased from TACONIC. Transgenic NKp46-CreERT2 mice were kindly provided by Dr. Lewis Lanier (UCSF, CA) and crossed onto ROSA26R-EYFP mice to create inducible reporter mice for fate mapping. All animals were housed under specific pathogen-free conditions at the University of Wyoming Animal Facility.
Ethics Statement
This study was carried out in strict accordance following the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health. The University of Wyoming Institutional Animal Care and Use Committee (IACUC) (PHS/NIH/OLAW assurance number: A3216–01) approved all animal protocols.
T. gondii parasites and infection
Tachyzoites of RH and RHΔcps1–1 (CPS) (kindly provided by Dr. David Bzik, Dartmouth College, NH) were cultured by serial passage in human fetal lung fibroblast (MRC5, ATCC) cell monolayers in complete DMEM (supplemented with 0.2 mM uracil for CPS strain). For mouse infections, parasites were purified by filtration through a 3.0-μm filter (Merck Millipore Ltd.) and washed with phosphate-buffered saline (PBS). Mice were infected intraperitoneally (i.p.) with 1 × 103 or 1 × 106 RH tachyzoites or 1 × 106 CPS tachyzoites. The brains of CBA mice 5 wk after ME49 infection were used as a source of ME49 cysts. Mice were infected i.p. or i.g. (intragastrically) with 10 or 100 ME49 cysts.
Cell depletion and fate mapping
To deplete NK cells, B6 mice were treated i.p. with 200 μg of anti-NK1.1 (PK136, Bio X Cell) 1 d before infection (d −1), on the day of infection (d 0) and every other day after infection for a maximum of 3 wk. To deplete CD8+ or CD4+ T cells, mice were treated i.p. with 200 μg of anti-CD8 T (2.43, Bio X Cell) or anti-CD4 T (GK1.5, Bio X Cell), respectively on d −1 and 0. To neutralize IL-12 family cytokines, mice were treated i.p. with 200 μg of anti-IL-12p70 (R2–9A5, Bio X Cell), anti-IL-12p40 (C17.8, Bio X Cell) and anti-IL-23p19 (MMp19B2, Biolegend) on d −1, 0 and 2. To block DNAM-1, mice were treated i.p. with 100 μg of anti-DNAM1 (480.1, Biolegend) on d −1 and 0. To induce yellow fluorescent protein (YFP) expression on NKp46+ cells, NKp46-CreERT2+/− × R26R-EYFP+/+ mice were treated i.g. with 3.75 mg (males) and 2.5 mg (females) of tamoxifen (Sigma) for five consecutive days, beginning on the day of CPS immunization.
Flow cytometry
Single-cell suspensions of peritoneal exudate cells (PECs), spleen and bone marrow (BM) were harvested from mice. Cells were then plated at 0.5−1.5 × 106 cells/well. For surface staining, cells were washed twice with PBS and stained for viability in PBS using Fixable Live/Dead Aqua (Invitrogen) for 30 min. After the cells were washed with PBS, surface staining was performed using antibodies diluted in stain wash buffer (2% fetal bovine serum in PBS and [2 mM] EDTA) for 25 min on ice. For functional NK-cell assays, cells were stimulated for 4 h with plate bound anti-NK1.1 in the presence of 1× protein transport inhibitor cocktail (PTIC) containing Brefeldin A/Monensin (eBioscience, Thermo Fisher Scientific) and anti-CD107a (eBio1D4B, eBioscience, Thermo Fisher Scientific) in complete Iscove’s DMEM medium (Corning). After fixable live/dead and surface staining, the cells were fixed and permeabilized for 1 h on ice in Fixation/Permeabilization solution (BD Bioscience), followed by intracellular staining in 1× permeabilization wash buffer (BD Bioscience) with anti-IFNγ (XMG1.2, eBioscience, Thermo Fisher Scientific) for 40 min. Additional cells were surface stained with antibodies from Biolegend against CD3 (17A2), CD49b (DX5), NKp46 (29A1.4), NK1.1 , CD4 (RM4–5), CD8b (YTS156.7.7), CD11b (M1/70), CD27 (LG.3A10), KLRG1 (2F1/KLRG1) and DNAM-1 (10E5); antibodies from eBioscience (Thermo Fisher Scientific) against Ly49I (YLI-90), Ly49H (3D10), CD94 (18d3) and CD107a (1D4B) and an antibody from BD Biosciences against Ly49D (4E5). The cells were washed twice with 1× PBS and resuspended in 1× PBS and analyzed using Guava easyCyte 12HT flow cytometer (Millipore) and FlowJo software (Tree Star).
Adoptive transfer
Spleens were harvested from B6 mice 5 wk after immunization with CPS, from mice 5 wk after an initial immunization followed by a second immunization at wk 2 (2×CPS) and from naïve mice, and NK cells were purified by negative selection using EasySep Mouse NK Cell Isolation Kit (STEMCELL TECHNOLOGIES). Normalized NK-cell numbers (1−3 × 106) were injected intravenously (i.v.) into Rag2−/−γc−/− or into naïve B6 mice. Recipient Rag2−/−γc−/− mice were infected with 1 × 103 tach. RH i.p. or 10 ME49 cysts i.g. for the survival assessment. Recipient B6 mice were infected with 1 × 105 tach. RH i.p. or 10 ME49 cysts i.p., and organs were harvested at 4 d after RH and 5 wk after ME49 infection for the parasite burden assessment.
Parasite burden assessment with real-time PCR
DNA was extracted from the entire PEC and spleen sample harvested from infected mice using a DNeasy Blood & Tissue Kit (Qiagen). Parasite DNA from 600 ng of PEC DNA and 800 ng of splenic tissue DNA was amplified using primers specific for the T. gondii B1 gene (forward primer 5′-GGAACTGCATCCGTTCATG-3′ and reverse primer 5′-TCTTTAAAGCGTTCGTGGTC-3′) at 10 pmol of each per reaction (Integrated DNA Technologies) by real-time fluorogenic PCR using SsoAdvanced Universal IT SYBR Green SMx (BIO-RAD) on a CFX Connect Real-Time System cycler (BIO-RAD). Parasite equivalents were determined by extrapolation from a standard curve amplified from purified RH parasite DNA.
Statistical analysis
Statistical analysis was performed using Prism 7.0d (GraphPad) and Microsoft Excel 2011. Significant differences were calculated using either unpaired Student’s t-test with Welch’s correction or analysis of variance (ANOVA). The log-rank (Mantel-Cox) test was used to evaluate survival rate. Data is presented in graphs as the mean± standard deviation (SD). Significance is denoted as follows: ns, not significant (p > 0.05) or significant with a p-value less than 0.05.
Results
NK cells are required for protection during secondary T. gondii infection
NK cells have been shown in several infection models to develop memory-like characteristics, including the ability to contribute to protection against secondary challenge of infection (3, 33–35). NK cells are critical for protection during acute T. gondii infection (16); however, whether they can also contribute to secondary infection by this parasite in the presence of T-cell memory is not known(29–32). A previously published report suggested that NK1.1+ and ASGM1+ cells helped provide immunity against T. gondii reinfection in the absence of CD8+ T cells (36). Therefore, we used a vaccine challenge model to test whether NK cells also helped provide immunity against T. gondii in the presence of memory T cells(29–32). For the primary infection, B6 mice were infected i.p. with tachyzoites of live attenuated uracil auxotroph strain CPS. These parasites are able to invade cells in vivo but do not replicate in the absence of uracil and get cleared within a week (37). Infection with this vaccine strain induces localized inflammation and leads to the generation of memory CD8+ T cells that protect mice against lethal reinfections regardless of the infecting strain or route of infection (31, 38). To determine if NK cells were important during secondary infection in vaccinated animals,,vaccinated mice were treated or not with anti-NK1.1 then reinfected with lethal doses of highly virulent type I RH tachyzoites.and parasite burdens measured by semi-quantitative real-time PCR for the T. gondii–specific B1 gene. As shown in Figure 1A, parasite burdens were significantly higher early after challenge in NK cell−depleted mice as compared with the controls. To further investigate the importance of secondary NK cell responses beyond the acute stage and during long-term infection, vaccinated animals were reinfection orally with the chronic infection forming type II parasite strain ME49. Challenged immunized and naïve mice were treated with anti-NK1.1 for 3 weeks and brain cyst burdens were assayed. Measuring cysts burdens in the brain is an effective method to measure long-term outcomes of T. gondii infection when control of acute infection is reduced. The cyst counts in the brain clearly showed elevated cyst numbers in anti-NK1.1−treated mice as compared with non-depleted controls (Fig. 1B). To test whether secondary NK cell responses were important for survival of vaccinated animals after lethal challenge separate groups of mice were separate animals were orally challenged with 100 cysts of the ME49 strain, treated or not with anti-NK1.1 and mouse survival assayed. Vaccinated animals whose NK cells were depleted during ME49 challenge had significantly reduced survival (Fig. 1C) as compared to non-treatment controls. These data indicate that NK cells play a protective role in secondary infections both acute and long term after vaccination and in the presence of T-cell memory during type I (RH) and type II (ME49) T. gondii infection.
FIGURE 1.
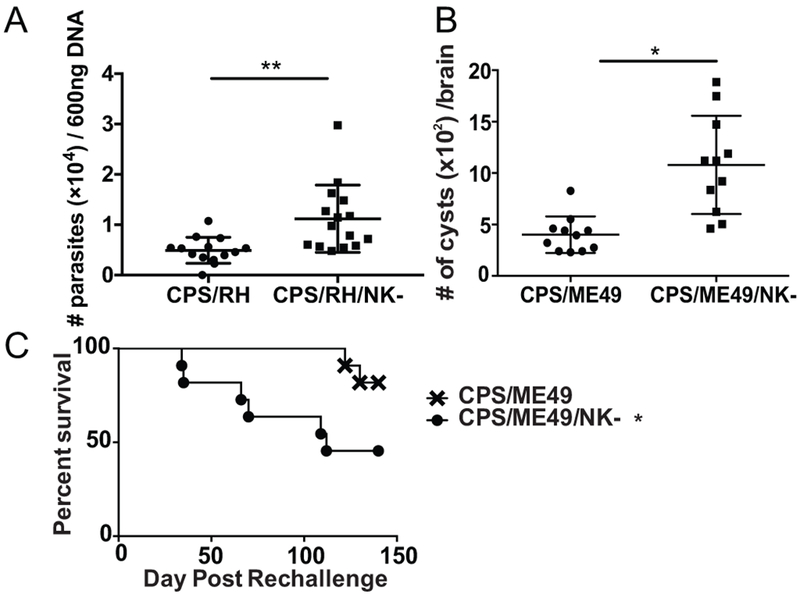
NK cells are required for protection during secondary T. gondii infection. (A - C) B6 mice were infected i.p. with 1 × 106 CPS. Five weeks later, mice were infected i.p. with (A) 1 × 106 RH tachyzoites or (B) 100 cysts of ME49 and were treated with anti-NK1.1 (PK136) or were untreated. (A) Two days after RH infection, the number of parasites was compared among PEC DNA (600 ng/sample) based on semiquantitative real-time PCR for the T. gondii B1. The graph presents all data points from 4 independent experiments with an n = 3 or 4 mice/group/experiment. (B) T. gondii cysts were quantified in the brains of B6 mice 5 wk after ME49 infection with or without anti-NK1.1 treatment. The graph presents all data points from 3 independent experiments with an n = 3−5 mice/group/experiment. Data are the mean ± SD with individual data points. Unpaired Student’s t-test with Welch’s correction, *p < 0.05, **p < 0.01. (C) CPS vaccinated animals were treated or not with anti-NK1.1 starting 5 weeks after immunization. Mice were then challenged orally with 100 cysts of ME49 and survival was monitored. Data presented is pooled from 3 independent experiments with an n=4/group/experiment. * denotes significance after analysis using Log-rank (Mantel-Cox) test with a p≤0.04.
NK cells become activated during an adaptive recall response
We showed in Figure 1 that, after vaccination and in the presence of memory T cells, NK cells contributed to the control of a challenge infection. How the NK cells were responding to the secondary infection was not, however, known. Therefore, we next determined whether NK cells were activated during secondary challenge and defined how they responded. We measured NK-cell frequency, number and functionality in the peritoneum, the site of infection, and in the spleen by flow cytometry. B6 mice were infected with CPS i.p. and 5 to 6 wk later were challenged i.p. with type I RH parasites. NK1.1+CD3− lymphocytes, which included NK cells and other innate lymphoid cells (ILCs) that express the NK1.1 receptor, increased in frequency and number at the infection site as early as 1 d after reinfection, remained there through d 5 and gradually declined by d 10 (Fig. 2A, 2B and 2C). Over the course of the secondary infection, NK1.1+CD3− cells constituted a large proportion of the lymphocytes with frequencies and numbers comparable to those of CD8+ T cells (Fig. 2B, 2C). To differentiate between NK cells and other NK1.1+ ILCs, NK cells were further defined as CD49b+NKp46+ cells (gated on CD3− live lymphocytes) (Fig. 2D). Only CD49b+NKp46+ cells increased in frequency and number at the site of reinfection, suggesting that ILC1 (CD49b−NKp46+) did not mount a secondary response (Fig. 2D, 2E). NK cells did not increase in the spleen after challenge, suggesting that the secondary NK-cell response was localized to the site of infection (Supplemental Fig. 1A, 1B). In the peritoneum, the NK-cell increase in frequency and number after secondary infection was similar to that which occurred during primary infection (Supplemental Fig. 1C).
FIGURE 2.
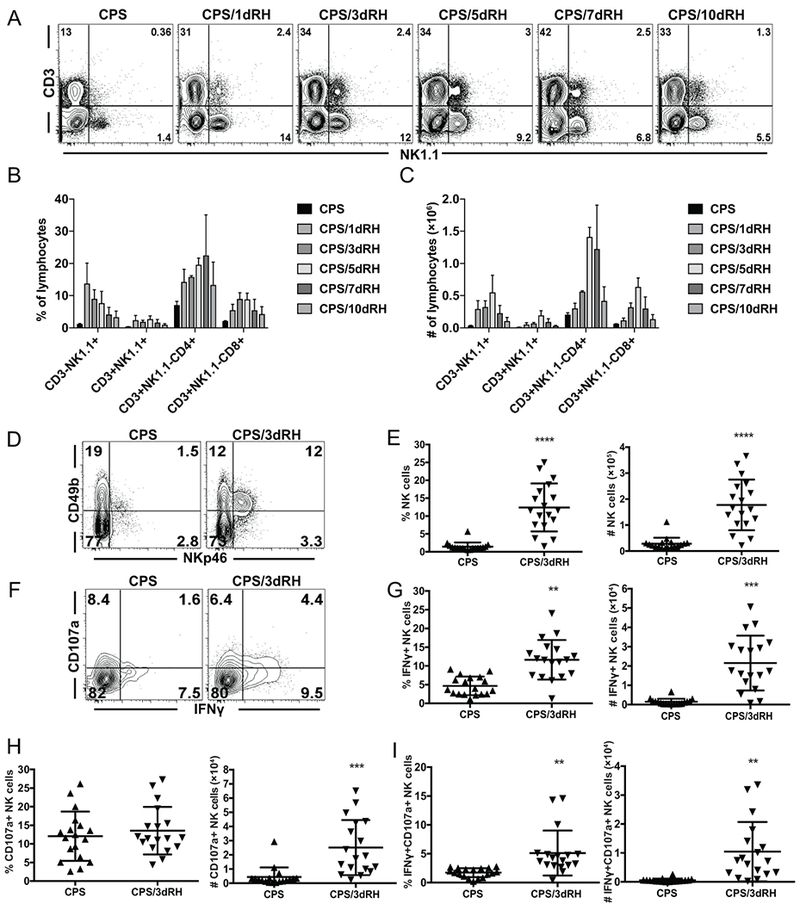
NK cells become activated during an adaptive recall response. (A-I) B6 mice were infected i.p. with 1 × 106 CPS tachyzoites and were then reinfected i.p. with 1 × 103 RH tachyzoites 5–6 wk later. PECs were analyzed by flow cytometry at d 1, 3, 5, 7 and 10 (A, B and C) or d 3 (D-I) after RH infection. (A) Representative contour plots of the cells expressing NK1.1 and CD3 in live lymphocytes. (B) The percentages and (C) numbers per PEC of CD3−NK1.1+ (ILCs), CD3+NK1.1+ (NK T cells), CD3+NK1.1–CD4+ (CD4+ T cells) and CD3+NK1.1–CD8b+ (CD8+ T cells) among live lymphocytes. The graphs present data from one experiment repeated independently twice with an n = 3 or 4 mice/group/per experiment. Data are the mean ± SD. (D, E) (D) Representative contour plots of CD49b X NKp46 are presented showing frequencies of cells. (E) The percentages and numbers of NK cells (CD49b+NKp46+) among CD3− live lymphocytes are presented in the column graphs. (F-I) (F) Representative contour plots of NK cells CD107a X IFNγ are presented showing frequencies of cells. (G)The percentages and numbers of IFNγ+, CD107a+ and IFNγ+CD107a+ NK cells are presented in the column graphs. (E, G, H and I) The graphs present all data points from 4 independent experiments with an n = 3 or 4 mice/group. Data are the mean ± SD. **p < 0.01, ***p < 0.001, ****p < 0.0001, one-way ANOVA.
The main function provided by NK cells during acute T. gondii infection is IFNγ production, which is critical for protection (16, 39). To assess how NK cells were responding, we measured their IFNγ production. NK cells produced IFNγ during secondary infection, and the frequency and number of IFNγ+ NK cells increased at the site of infection (Fig. 2F, 2G and Supplemental Fig. 1D). In addition to the cytokine production, NK cells develop a cytotoxic response after stimulation with T. gondii parasites and their subcellular components (23, 25). We measured NK-cell cytotoxicity during secondary challenge using the surrogate marker CD107a (40). The frequency of cytotoxic CD107a+ NK cells did not significantly change, whereas their absolute cell numbers increased (Fig. 2F, 2H and Supplemental Fig. 1D). NK cells also developed a polyfunctional response (CD107a+IFNγ+), which was significantly increased during secondary challenge (Fig. 2F, 2I and Supplemental Fig. 1D). These findings indicate that NK cells become rapidly activated during the response to secondary T. gondii infection to provide effector functions constituting a substantial portion of the total lymphocytes during a recall response.
Multiple NK-cell subpopulations are activated during secondary T. gondii infection
Inflammatory cytokines and/or activating receptor engagement with cognate ligands can activate NK cells (41, 42). After MCMV infection, both cytokine-activated and ligand-driven NK-cell responses occur (9). During some viral infections, specific NK-cell subpopulations, defined by their receptor expression, dominate the response (43, 44). During acute T. gondii infection, we and others have not observed a dominant responding NK-cell population, suggesting that NK cells are driven by a cytokine-dependent process, rather than by a process that depends on activating receptor ligands (22, 45). To further define how NK cells were responding to secondary T. gondii infection, we measured whether a dominant NK-cell population developed in the context of immune memory to the parasite. Mice were immunized with CPS and 5 to 6 wk later were infected with type I RH. The expression of inhibitory receptors, including Ly49I and CD94, and activating receptors, including Ly49H and Ly49D, was assessed on the total NK-cell population as well as on IFNγ+ NK cells by flow cytometry. Total and IFNγ+ NK cells were similarly distributed within the subsets of lymphocytes most of which showed the expected activation in the RH-infected mice relative to the CPS-only mice, and there was no dominant population observed after reinfection (Fig. 3A, 3B). Interestingly, the fold increase in the CD94+ subset was higher than that of Ly49I+, Ly49H+ and Ly49D+ NK cells.
FIGURE 3.
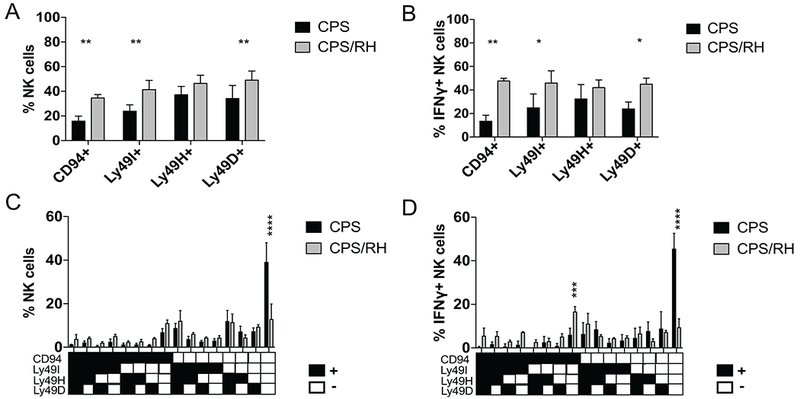
Multiple NK-cell subpopulations are activated during secondary T. gondii infection. (A-D) B6 mice were infected i.p. with 1 × 106 CPS and 5–6 wk later were infected i.p. with 1 × 103 RH tachyzoites. PECs were analyzed by flow cytometry at d 3 after RH infection. (A and B) Bar graphs present the frequency of CD94+, Ly49I+, Ly49H+ and Ly49D+ cells within the (A) total NK cell population and (B) IFNγ+ NK cells. (C and D) Graphs present the frequency of NK cells expressing combinations of the receptors (CD94, Ly49I, Ly49H, Ly49D) within the (C) total NK cell population and (D) IFNγ+ NK cells. Representative graph present data from one of two independent experiments with n = 4 mice/group. Data are the mean ± SD. *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001, one-way and two-way ANOVA.
As NK cells stochastically express an array of receptors, the dominant NK-cell population might express multiple receptors rather than a single receptor. We therefore next analyzed the phenotype of NK cells expressing combinations of NK-cell receptors in CPS-immunized mice compared with mice that were immunized with CPS and later challenged with RH 3 d before analysis. Among the total NK cells and IFNγ+ NK cells, we observed a significant decrease in the frequency of CD94−Ly49I−Ly49H−Ly49D− NK cells in the RH-challenged mice (Fig. 3C, 3D). This reduction of the receptor-negative NK cells was associated with an increase in NK cells that expressed multiple combinations of the receptors and that were widely distributed. Interestingly, the combinations that included CD94 receptor had the greatest increase in frequency (Fig. 3D). This included NK cells that were CD94+Ly49I−Ly49H−Ly49D−. Nevertheless, compared with viral infections, a distinct, dominant responding NK-cell population did not stand out during secondary T. gondii infection, similar to our observations during acute T. gondii infection.
Activated NK cells during secondary T. gondii infection are mature
MCMV-primed Ly49H+ and alloantigen-primed Ly49D+ ligand-driven NK-cell subsets that develop intrinsic memory develop a mature phenotype marked by being CD11b+CD27−KLRG1highDNAM-1low on their surface (3, 46, 47). Maturation of NK cells then seems to be critical for their ability to acquire features of adaptive immune cells. Therefore, measuring whether NK cell maturation was altered during secondary challenge after vaccination is important to better understand NK-cell biology in this setting. We next defined how vaccination and the presence of immune memory impacted NK-cell maturation after secondary T. gondii infection. As NK cells mature in the periphery, they progress from the least mature stage, R0 (CD27−CD11b−); to stage R1 (CD27+CD11b−); followed by stage R2 (CD27+CD11b+) and the terminally mature stage, R3 (CD27−CD11b+) (48). Mice were immunized with CPS as described above, and NK-cell maturation was measured at the site of reinfection based on expression of CD27 and CD11b. RH infection of CPS-vaccinated animals significantly elevated NK-cell maturation to stage R2 (Fig. 4A). This was even more evident when we measured NK cell maturation (CD27−CD11b+) in IFNγ+ NK-cell populations (Fig. 4B). Immature NK cells (CD27−CD11b−, R0) decreased, intermediately mature NK cells (CD27+CD11b+, R2) increased and other subsets (R1, CD27+CD11b+ and R3, CD27−CD11b+) did not change after secondary infection.
FIGURE 4.
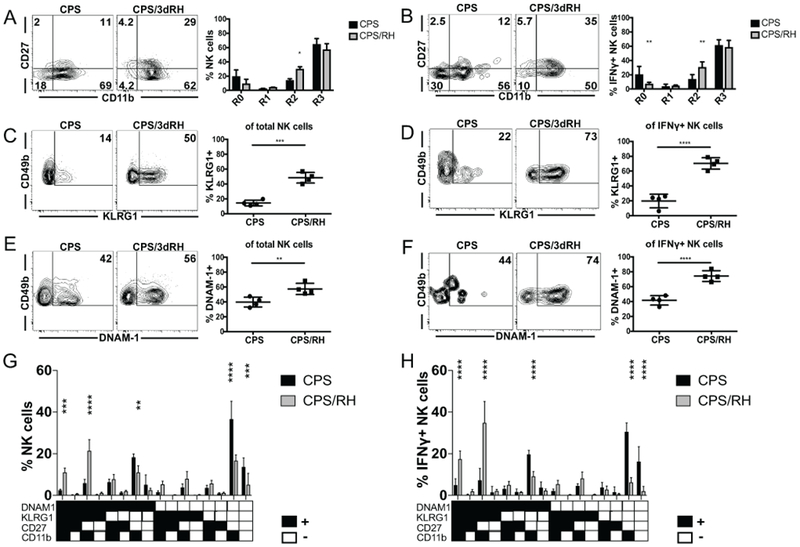
Activated NK cells during secondary T. gondii infection are mature. (A-H) B6 mice were infected i.p. with 1 × 106 CPS and 5–6 wk later were infected i.p. with 1 × 103 RH tachyzoites. PECs were analyzed by flow cytometry at d 3 after RH infection. (A and B) The frequency of R0 (CD27–CD11b–), R1 (CD27+CD11b−), R2 (CD27+CD11b+), R3 (CD27−CD11b+) NK cells within the (A) total and (B) IFNγ+ NK cells. (C and D) The frequency of KLRG1+ NK cells within (C) total and (D) IFNγ+ NK cells. (E and F) The frequency of DNAM-1+ NK cells within the (E) total and (F) IFNγ+ NK cells. (G and H) The frequency of NK cells expressing the combinations of the receptors (KLRG1, DNAM1, CD27, CD11b) within the (G) total and (H) IFNγ+ NK cells. The graphs present data from 1 of 2 independently repeated experiments with an n = 4 mice/group. Data are the mean ± SD. *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001, one-way and two-way ANOVA.
In addition to conventional maturation markers, we also measured expression of the NK-cell activation marker KLRG1 and the costimulatory molecule DNAM-1. Adaptive NK cells have high KLRG1 and low DNAM-1 expression after MCMV and alloantigen stimulation (3, 46, 47). NK cells from CPS-immunized and naïve mice did not significantly differ in the expression of these markers (data not shown). There was a significant increase in KLRG1+ and DNAM-1+ subsets after reinfection (Fig. 4C–F). By measuring the expression of these markers in combination with each other, we found that NK cells of the memory cell phenotype (KLRG1highDNAM-1lowCD11b+CD27−) did not significantly change and constituted a small portion of the total and IFNγ+ NK cells before and after reinfection (Fig. 4G, 4H). The population that increased and produced the most IFNγ was KLRG1+DNAM-1+CD11b+CD27−, followed by KLRG1+DNAM-1+CD11b+CD27+. IFNγ was mainly produced by mature DNAM-1+ rather than mature DNAM-1− NK cells (Fig. 4H). This suggested that DNAM-1 could be involved in NK-cell responses to secondary T. gondii infection.
We addressed this possibility by blocking DNAM-1 signaling in vivo using anti-DNAM-1 in CPS-immunized and RH-challenged mice. Anti-DNAM-1 treatment appeared to possibly deplete DNAM-1high NK cells, however, treatment did not result in a reduction of total NK cell and IFNγ-producing NK cell frequency or number or frequency (Supplemental Fig. 2 A,B and C). Based on this result, treatment most likely did not deplete DNAM-1high cells and simply blocked the receptor resulting in decreased staining ex vivo (Supplemental Fig. 2C). In summary, there does not appear to be a specific NK-cell subpopulation that develops in response to T. gondii vaccination.
T. gondii−experienced and naïve NK cells are not intrinsically different
Accumulating studies indicate that NK cells can further differentiate after primary stimulation (cytokine stimulation, hapten exposure or viral infection) to acquire features of adaptive immune cells and develop memory-like abilities in a cell-intrinsic manner (2, 3, 35). However, the development of cell-intrinsic NK-cell memory-like features in response to eukaryotic pathogens has yet to be discovered. To address whether T. gondii infection−experienced NK cells were intrinsically different from naïve cells (without parasite experience) in their ability to protect against secondary infection, we used T and B cell−deficient Rag1−/− mice (49). One group of Rag1−/− mice were immunized with CPS parasites, whereas a second was not. Both groups were challenge infected with type I RH (i.p.) or type II ME49 cysts (i.p. and i.g.) 5 to 6 wk later, and their survival was monitored CPS-immunized Rag1−/− mice were not protected better than non-immunized controls (Fig. 5A–C). The administration of the parasites via different routes also did not affect this result (Fig. 5B, C).
FIGURE 5.
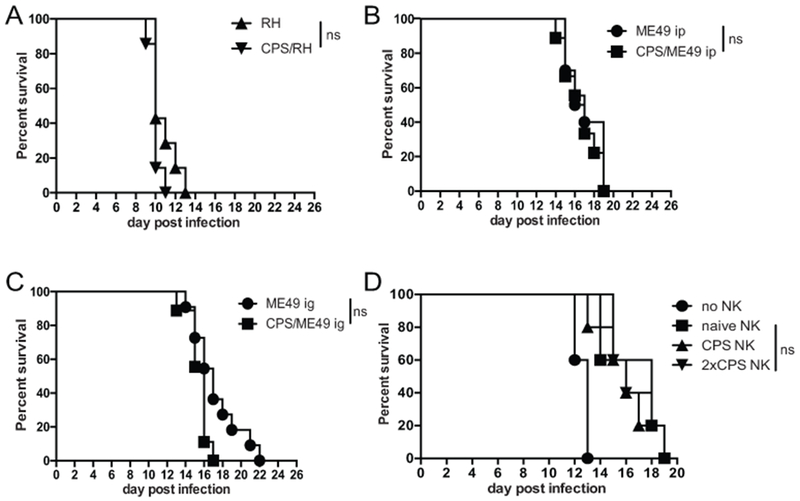
T. gondii–experienced and naïve NK cells are not intrinsically different with respect to their ability to protect against subsequent infection. (A-C) Survival after infection with (A) 1 × 103 RH tachyzoites i.p. or with (B) 10 brain ME49 cysts i.p. or (C) i.g. was compared between Rag1−/− mice 5–6 wk after CPS immunization and non-immunized Rag1−/− mice. Each graph presents pooled data from two independent experiments, n = 3–6 mice/group. (D) NK cells were purified from spleens of naïve B6 mice (naive NK) and from mice 5 wk after CPS immunization (CPS NK) and from mice 5 wk after initial CPS immunization that was followed by a second round of immunization at wk 2 (2×CPS NK) B6 mice. Purified NK cells were then transferred i.v. into Rag2−/−γc−/− mice (1.4 × 106 NK cells/mouse). Recipient mouse survival after i.g. infection with 10 ME49 cysts was determined. The data presented are from 2 independent experiments, n = 5 mice per experiment. ns, not significant. The log-rank (Mantel-Cox) test was used to evaluate survival rates.
NK cells do not express RAG recombinase once they begin to develop (50). However, in Rag1−/− mice there is impaired NK-cell expansion and differentiation of memory-like characteristics after MCMV infection (51). One reason we may not have observed a survival difference between CPS-immunized Rag1−/− and non-immunized Rag1−/− mice could be due to the RAG1 deficiency and a consequent loss of the development of CPS immunization−induced NK cell−intrinsic memory-like features. Therefore, to further test whether cell-intrinsic differences existed between naïve and CPS-experienced NK cells, we performed NK-cell adoptive transfer into Rag2−/−γc−/− mice, which lack T, B and NK cells (52). Wild-type (WT) B6 animals were immunized with CPS parasites or were not immunized. Bulk NK cells were purified by negative selection from the spleens of 5-wk CPS-immunized mice or non-immunized age-matched controls. NK cells were transferred i.v. into the Rag2−/−γc−/− recipient mice. Those mice were then challenged with type II ME49 cysts, and their survival was measured. As in immunized Rag1−/− animals, Rag2−/−γc−/− recipients of CPS-experienced NK cells did not survive any longer than did recipients of naïve NK cells (Fig. 5D). This result indicated that T. gondii–experienced NK cells were not intrinsically different in their ability to protect immunodeficient mice as compared with naïve NK cells.
In Rag2−/−γc−/− mice, adoptively transferred NK cells undergo homeostatic proliferation, resulting in their spontaneous activation and production of IFNγ (53). This could have been why we could not detect a cell-intrinsic difference between T. gondii–experienced and naïve NK cells. Therefore, to limit homeostatic proliferation-associated cell activation, WT mice were used as recipients of CPS-experienced and naïve NK cells to see if parasite-experienced NK cells were more protective. Purified NK cells from the spleens of CPS-immunized or naïve B6 mice were transferred into WT recipients. Recipient animals were then challenged with either type I RH parasites or type II ME49 cysts. The parasite burdens were measured by real-time PCR for the parasite-specific B1 gene at 4 d after RH and by brain cyst counts 5 wk after ME49 infection. We did not observe any significant differences in parasite burdens between the recipients of CPS-immunized and naïve NK cells (Supplemental Fig. 3A, 3B).
In addition to conveying more-efficient protection in response to secondary challenge with the same stimuli, memory lymphocytes develop the ability to persist longer than naïve cells (35). For the above adoptive transfer experiments, NK cells were purified from B6 mice 5 wk after immunization. These bulk NK cells contained cells that had experienced primary T. gondii infection as well as newly generated naïve cells. To differentiate between long-lived and naïve NK cells and to assess their ability to persist, the tamoxifen-inducible reporter strain NKp46-CreERT2 × Rosa26-YFP was used to fate map NK cells activated during immunization and not newly generated NK cells (9). To label and track whether NK cells were long-lived after T. gondii infection, reporter mice were immunized with CPS and at the same time treated with tamoxifen. Tamoxifen treatment was continued for five consecutive days after immunization, and mice were harvested 5 wk later. Control reporter mice that were not immunized with CPS were treated with tamoxifen for 5 d and harvested 5 wk later. The flow cytometry analysis revealed that the frequency and number of YFP+ NK cells, which represented long-lived persistent cells, was not different between CPS-immunized and naïve mice in the peritoneum (the site of infection) (Fig. 6A), spleen (periphery) (Fig. 6B) and the bone marrow (the site of NK-cell generation) (Fig. 6C). Interestingly, there was a trend toward a reduction in the frequency of YFP+ NK cells in immunized mice as compared with naïve mice. Taken together, these results indicate that T. gondii−experienced and naïve murine NK cells are not intrinsically different in their ability to protect against or persist after T. gondii infection.
FIGURE 6.
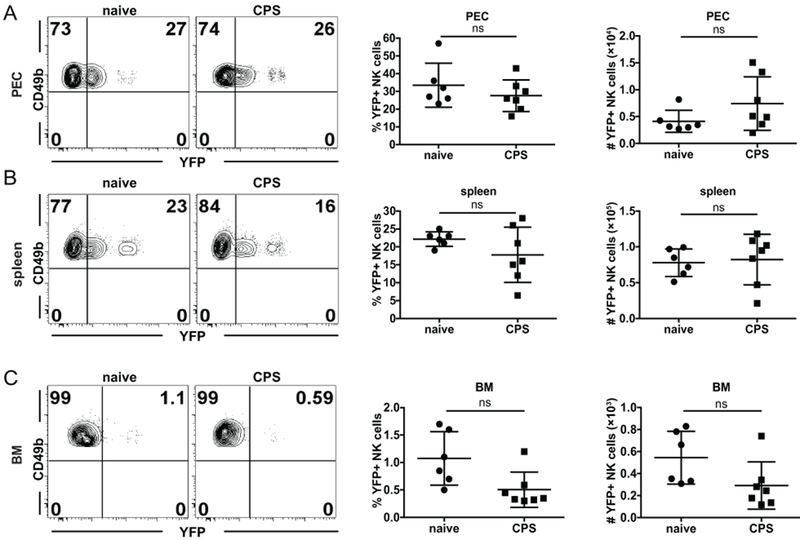
T. gondii–experienced and naïve NK cells are not intrinsically different in their ability to persist. (A-C) NKp46-CreERT2 × Rosa26-YFP mice were i.g. treated with tamoxifen for five consecutive days beginning on the day of CPS immunization. Five weeks after tamoxifen treatment, the frequency and number of YFP+ NK cells (gated as CD49b+NKp46+ within CD3− live lymphocytes) were compared between CPS-immunized and non-immunized mice by flow cytometry in the (A) PEC, (B) spleen and (C) BM. (A-C) Representative contour plots present Frequency of YFP+ vs. YFP – NK cells (CD49b+). The graphs present frequency and absolute number of YFP+ NK cells. The graphs include all data points from 2 independent experiments with an n = 3-4 mice/group/experiment. Data are the mean ± SD. ns, not significant. Statistical analysis was performed using one-way ANOVA.
NK cells get activated independently of T cells
Intrinsic NK-cell memory does not develop in all disease situations (54). NK cells can still contribute to adaptive immune recall responses even if they are not intrinsically different from naïve cells. Studies suggest that antigen-specific CD4+ T cells rapidly produce IL-2 and can activate NK cells (54–57). We found that NK cells responded to secondary T. gondii infection, but they were not intrinsically different from naïve NK cells. Therefore, we next examined whether memory CD4+ or CD8+ T cells were required for helping to activate NK cells in response to secondary T. gondii infection. To test the role of CD4+ and CD8+ T cells in NK-cell activation, mice were immunized with CPS and 5 to 6 wk later depleted of CD4+ or CD8+ T cells. Depleted and non-depleted animals were then challenged with RH, and NK-cell responses were measured at the site of infection. NK cells increased in frequency and number and produced IFNγ even when CD4+ or CD8+ T cells were absent (Fig. 7). Interestingly, after the treatment with anti-CD4, NK cells produced more IFNγ than non-treated mice. This could be explained by a concurrent depletion of CD4+ Treg cells by the antibody (58). Overall, the above result showed that, in contrast to other infection models in which NK-cell responses depended on antigen-specific T cells, CD4+ and CD8+ T cells were not required for NK-cell activation during secondary T. gondii infection.
FIGURE 7.
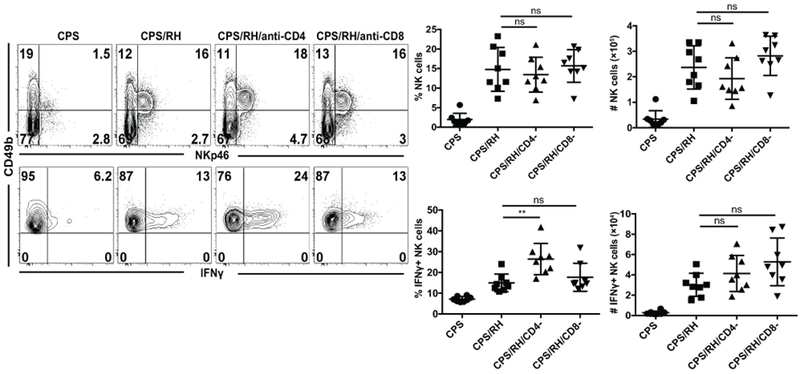
NK cells are activated independently of T cells. B6 mice were infected i.p. with 1 × 106 CPS tachyzoites. Five to 6 weeks later, mice were infected i.p. with 1 × 103 RH tachyzoites and treated with antibodies that deplete CD4+ or CD8+ T cells. Three days after RH infection, the frequency and number of NK cells (CD49b+NKp46+CD3− live lymphocytes) and their IFNγ production in PECs were measured by flow cytometry. Representative contour plots from one of two independent experiments are presented. The graphs present the frequency and absolute number of total NK cells (top row) and IFNγ+ NK cells (bottom row). The graphs include all data points from two independent experiments, n = 4 mice/group. Data are the mean ± SD. ns, not significant; **p < 0.01, one-way ANOVA.
IL-12 and IL-23 are required for the NK-cell response to secondary infection
T. gondii infection was one of the first systems in which the IL-12/IFNγ axis was demonstrated (17). More specifically, IL-12−dependent NK-cell IFNγ production was shown to protect against acute T. gondii infection (16–18). Thus, IL-12 could be required for NK-cell IFNγ production during secondary infection with the parasite in the presence of immune memory. To test whether IL-12 was involved in secondary NK-cell responses, CPS-immunized mice were treated with anti-IL-12p40 or were untreated and then were challenged with RH tachyzoites. The frequency and absolute number of total NK cells and activated IFNγ+ NK cells were measured 3 d later. The frequency of NK cells was not affected by anti-IL-12p40, but their numbers were significantly lower than in untreated mice (Fig. 8A). Consistent with the previously shown role of IL-12p40 during acute infection (17, 18), the absence of IL-12p40 during secondary infection led to a dramatic decrease in IFNγ production by NK cells. Both the frequency and number of IFNγ+ NK cells were comparable to non-challenged control mice. These data show that after secondary T. gondii infection, IL-12p40 is essential for NK-cell IFNγ production.
FIGURE 8.
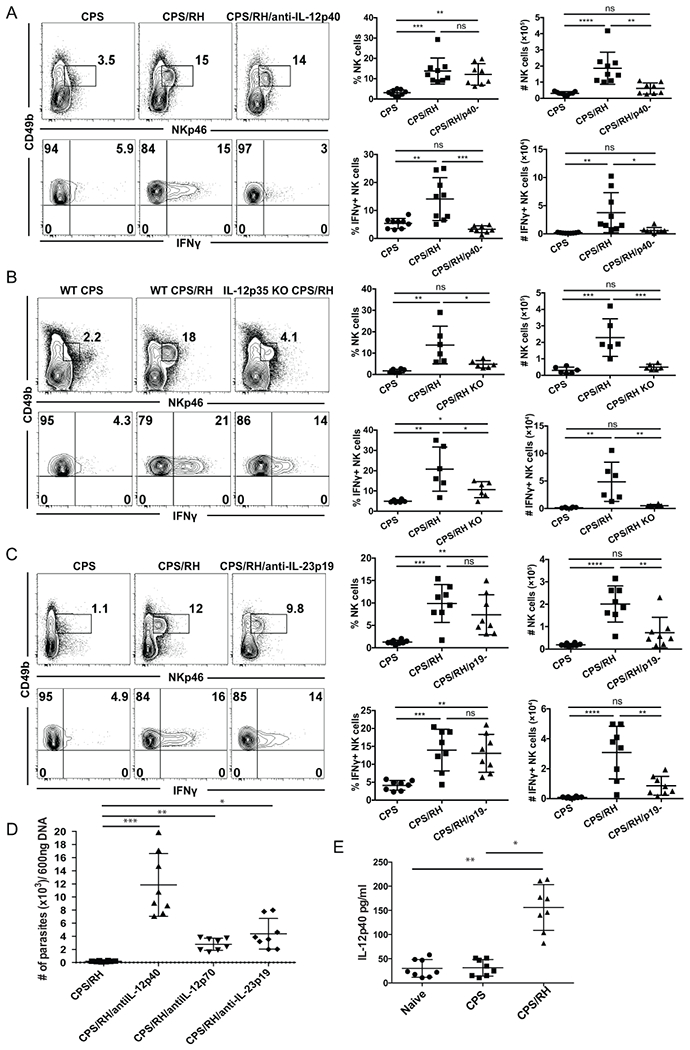
IL-12 and IL-23 are required for the NK-cell response to secondary T. gondii infection. (A-E) B6 (A and C) and IL-12p35 KO (B) mice were i.p. infected with 1 × 106 CPS and 5–6 wk later were i.p. infected with 1 × 103 RH tachyzoites. NK cells (CD49b+NKp46+CD3− lymphocytes) and their IFNγ production were analyzed in PECs at 3 d after RH infection by flow cytometry. (A) Mice were treated with anti-IL-12p40 blocking antibody during RH reinfection. Representative contour plots are shown from one of two independent experiments. The graphs present all data points acquired from two independent experiments, n = 3–5 mice/group/experiment. (B) NK-cell frequency and IFNγ production in IL-12p35 KO mice. Representative contour plots from one of two independent experiments are presented. The graphs present all data points acquired from two independent experiments, n = 3 mice/group/experiment. (C) Mice were treated with anti-IL-23p19 during RH reinfection. Representative contour plots are presented from one of two independent experiments. The graphs present all data points acquired from two independent experiments , n = 4 mice/group/experiment. Data are the mean ± SD. ns, not significant; *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001, ordinary one-way ANOVA. (D) 5 weeks after CPS vaccination mice were treated with anti-IL-12p40, anti-IL-12p70 or anti-IL-23 i.p. Mice were then challenged i.p. with 1 X 106 RH tachyzoites and 3 days later PEC were harvested and assayed for parasite burden by real time PCR. The graph presents all data from 2 independent repeated experiments. Data are the mean ± SD *p<0.004 **p<0.0001 ***p< 0.0006 ,ordinary one-way ANOVA. (E) The level of IL-12p40 produced by the PEC in naïve, CPS immunized for 5 weeks and CPS immunized and RH challenged for 3 days was measured by ELISA and is presented. The graph presents all data from 2 independent repeated experiments. Data are the mean ± SD *p<0.0001 **p< 0.0001,ordinary one-way ANOVA.
In the majority of studies, the role of IL-12 in acute T. gondii infection has been assayed by measuring the p40 subunit of IL-12 (17, 18). However, p40 is a subunit of multiple cytokines and can be biologically active as a subunit of heterodimers IL-12p70 and IL-23 as well as mono- and homodimers (59–61). Therefore, we more specifically addressed the role of IL-12 in NK-cell activation during secondary infection by neutralizing IL-12p70 by antibody treatment during RH reinfection of CPS-immunized mice. Consistent with the anti-IL12p40 data, the blockade of IL-12p70 did not significantly affect NK-cell frequency, but NK-cell numbers were reduced (Supplemental Fig. 4). In contrast to anti-IL-12p40, anti-IL-12p70 treatment did not lead to a significant decrease in the frequency or number of IFNγ-producing NK cells. As this was an unexpected result, we further tested the role of IL-12p70 in NK-cell activation in IL-12p35 KO mice. Although T cells do not develop memory in the absence of IL-12 in IL-12p35 KO mice (30), we could use this mouse model because the NK-cell response to secondary T. gondii infection did not require T-cell help (Fig. 7). Flow cytometry analysis revealed that both the frequency and number of total and IFNγ-producing NK cells in IL-12p35 KO mice were lower than in WT mice in response to secondary infection (Fig. 8B). The differences between the antibody treatment and KO mice could be explained by an incomplete neutralization of IL-12p70 by the antibody in vivo. Nevertheless, both approaches showed that IL-12p70 was essential for increased NK-cell numbers and IFNγ production during secondary T. gondii infection.
In contrast to a complete absence of NK-cell IFNγ production after anti-IL-12p40 blockade, some percentage of NK cells still produced IFNγ in mice treated with anti-IL-12p70 and in IL-12p35 KO mice. One potential explanation for this is that there is an IL-12p70−independent and p40-dependent mechanism involved in NK-cell activation and IFNγ production during secondary infection. Because IL-12p40 is also a subunit of the cytokine IL-23, we tested whether IL-23 could also activate NK cells during secondary T. gondii infection. IL-23 extends the life of p40 KO mice during acute T. gondii infection (62). To find the role of IL-23 in activation of NK cells during reinfection, B6 mice were treated with anti-IL-23p19 5 to 6 wk after CPS immunization during secondary infection with RH. The number of NK cells was significantly lower in mice treated with anti-IL-23p19 as compared with control mice (Fig. 8C). The frequency of IFNγ+ NK cells after reinfection was not affected by IL-23p19 blockade. However, the absolute number of IFNγ-producing NK cells was reduced. These data show that during secondary infection IL-23 was essential for the increase in NK-cell numbers rather than IFNγ production. To further test how IL-12 and IL-23 contribute to secondary NK cell response control of reinfection, vaccinated mice were treated or not with anti-IL-12p40 to block both IL-12 and IL-23, anti-IL-12p70 to only block IL-12 and anti-IL-23p19 to block only IL-23. These mice then were given a lethal dose of Type I RH tachyzoites i.p. On day 3-post infection the PEC were harvested and parasite burdens measured by real time PCR. As shown in figure 8D, blockade of IL-12 and IL-23, IL-12 alone and IL-23 alone all resulted in decreased control of the parasite as indicated by an increase in parasite burden in the PEC when compared to no treatment controls. Importantly, blockade of IL-12 and IL-23 resulted in the greatest loss of control compared to blockade of IL-12 or IL-23 alone. Lastly, we measured whether IL-12p40 was produced during T. gondii reinfection (Fig. 8E) to validate our blockade findings. PEC cells were harvested 3 days after RH challenge infection and their IL-12p40 production compared to PEC harvested from naïve and CPS immunized non-reinfected mice. IL-12p40 was significantly increased in production after reinfection of vaccinated animals (Fig. 8E) indicating that both IL-12 and IL-23 could be produced during secondary infection. These data suggest that the NK-cell response to secondary T. gondii infection is dependent on IL-12 and IL-23. IL-12 is essential for the NK-cell increase in numbers and for IFNγ production, and IL-23 is essential for the NK-cell increase in numbers.
NK-cell maturation is reduced in the absence of IL-12 and IL-23
Based on the phenotypic analysis of NK cells responding to the secondary T. gondii infection, the majority (~73%) of IFNγ-producing NK cells expressed KLRG1 (Fig. 4D). KLRG1 expression is dependent on IL-12, which also induces expression of the transcription factor T-bet and IFNγ (29, 63). As IL-12p70 and IL-23 were required for NK-cell IFNγ production during secondary T. gondii infection, we next asked if these cytokines were also involved in the maturation of NK cells. The frequency of KLRG1+ NK cells was significantly lower in the absence of IL-12p40 and IL-23 after anti-IL-12p40 treatment and in the absence of IL-12p70 in IL-12p35 KO mice (Fig. 9A, 9B). In addition, the KLRG1+ NK cells were also reduced in the absence of IL-23 after anti-IL-23p19 treatment (Fig. 9C). Thus, NK cell maturation during secondary infection is also dependent on both IL-12p70 and IL-23.
FIGURE 9.
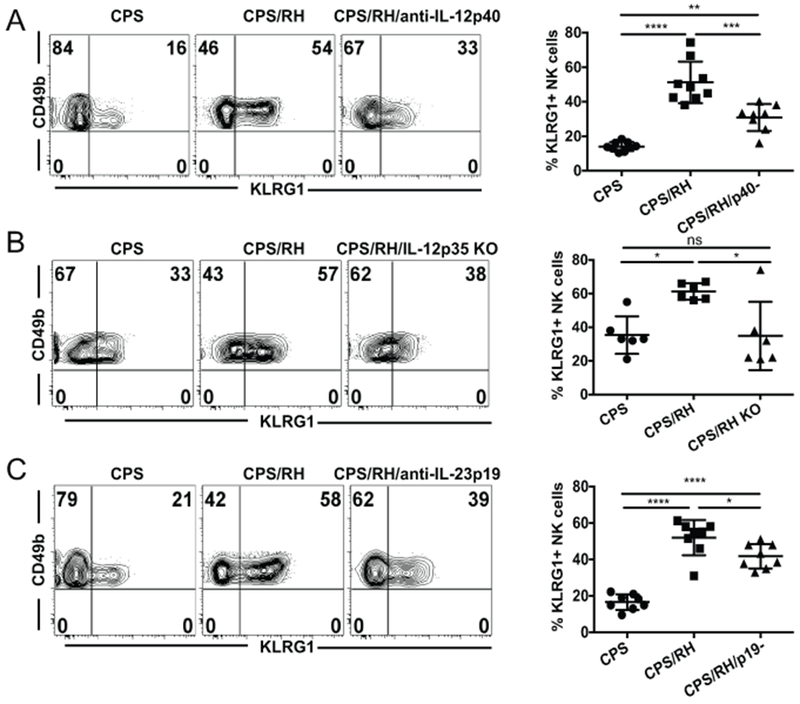
NK-cell maturation is reduced in the absence of IL-12 and IL-23 during secondary T. gondii infection. (A-C) B6 (A and C) and IL-12p35 KO (B) mice were i.p. infected with 1 × 106 CPS1–1 and 5−6 wk later were i.p. infected with 1 × 103 RH tachyzoites. KLRG1 expression on NK cells (CD49b+NKp46+CD3− live lymphocytes) was analyzed by flow cytometry in PECs at 3 d after RH infection. (A) Mice were treated with anti-IL-12p40 blocking antibody during RH infection. Representative contour plots and graphs from one of two independent experiments are presented. The graphs present all data points gathered from two independent experiments, n = 3−5 mice/group/experiment. (B) NK-cell KLRG1 expression in IL-12p35 KO mice. Representative contour plots from one of two independent experiments. The graphs present all data points gathered from two independent experiments, n = 3 mice/group/experiment. (C) Mice were treated with anti-IL-23p19 antibody during RH infection. Representative plots and graphs from one of two independent experiments are presented. The graphs present all data points from two independent experiments, n = 3 or 4 mice/group/experiment. Data are the mean ± SD. ns, not significant; *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001, one-way ANOVA.
Discussion
Recent evidence suggests that NK cells can participate in adaptive immunity by developing memory-like features or as immune effector cells regulated by memory T cells (3, 28, 35, 42, 54, 57). Whether NK cells contribute to long-term adaptive immune responses to T. gondii infection is not known. In this study we used a vaccine challenge model of T. gondii infection and showed that NK cells were required for protection during secondary infection when T-cell memory was present. However, adoptive transfer and fate mapping experiments indicated that NK cells did not develop cell-intrinsic memory-like characteristics and did not persist after primary T. gondii infection. NK-cell responses to secondary infection were dependent on new NK-cell generation and required cell-extrinsic factors IL-12/23p40, IL-12p70 and IL-23 cytokines to become activated and to become mature. Although NK cells did not develop memory-like features during T. gondii infection, they were essential for control of secondary parasite infection in an IL-12p70− and IL-23−dependent manner. Our results demonstrate a novel role for NK cells as innate immune cells that contribute significantly to secondary T. gondii infection, and both IL-12p70 and IL-23 are involved in this process. This study highlights the potential for NK cells to boost adaptive immunity against secondary infection in individuals whose immune systems become compromised.
Protection against secondary T. gondii infection is provided mainly by memory CD8+ T cells and was defined using vaccine and challenge approaches (38, 64, 65). How other immune cells, including NK cells, might contribute to this protection has not been clear. In a previous T. gondii vaccine challenge study performed in mice deficient for CD8+ T cells, cells expressing markers of NK cells including NK1.1 and ASGM1 provided protection against secondary infection (36). This study suggested that NK cells could substitute for CD8+ T cells in immunocompromised mice during an adaptive immune recall response. Whether this was also occurring in immunocompetent mice was not known. In our study, we demonstrated that NK1.1+ cells are critical for protection in immunocompetent mice in the presence of memory CD4+ and CD8+ T cells. Anti-NK1.1 treatment of vaccinated animals prior to lethal challenge resulted in significantly increased parasite burdens both in short-term and long-term infection. Anti-NK1.1 treatment of vaccinated mice also reduced long-term survival during lethal challenge with the Type II strain ME49. In addition, NK1.1+CD3− NK cells increased with a similar kinetic profile as compared with CD8+ T cells at the site of reinfection. Thus, we found that in addition to CD8+ T cells, NK cells contributed to control of secondary T. gondii infection in the presence of intact T-cell memory.
NK cells protect against primary T. gondii infection by producing IFNγ rather than by direct lysis of infected cells (16, 17, 26). We found that NK cells produce IFNγ, become cytotoxic (CD107a+) and are polyfunctional (IFNγ+CD107a+) in response to secondary infection. However, whereas the frequency of IFNγ-producing NK cells increased, the level of NK-cell cytotoxicity did not increase after reinfection. This suggests that IFNγ production could be the main NK-cell function necessary for protection during secondary parasite challenge in a manner similar to that which occurs during primary T. gondii infection (16–21).
A hallmark of NK cells that develop memory-like features is their ability to respond more efficiently and robustly to a secondary challenge similar to memory CD8+ T cells (2, 3, 28). For example, in a contact hypersensitivity model, hapten-sensitized Rag2−/− mice, which have neither T cells nor B cells, are more sensitive to secondary challenge than non-sensitized mice (2). Ly49H+ NK cells in mice infected with MCMV develop a more robust response to secondary MCMV infection (3). T and B cell−deficient mice immunized with influenza or HIV-1 virus-like particles (VLPs) have an increased response to secondary exposure to the corresponding VLPs (33). NK cells cultured with IL-12, IL-15 and IL-18 also develop the ability to respond more robustly to secondary stimulation (28). Whether primary T. gondii infection induces NK cells to develop a more efficient response to secondary parasite infection is not known. Our data indicate that T. gondii immunization of WT mice did not result in a more robust or efficient secondary NK-cell response during challenge infection. T. gondii immunization of T and B cell−deficient (Rag1−/−) mice did not lead to the development of stronger NK cell−dependent protection against secondary parasite infection. Expression of RAG1 early during NK-cell differentiation in the bone marrow has been suggested to be required for development of memory-like features in response to MCMV (51). Therefore, this may be one reason why we did not observe better NK cell−dependent protection in the Rag1−/− mice. However, there was no increase in protection against parasite challenge in immunodeficient Rag2−/−γc−/− or WT mice that received purified T. gondii–experienced NK cells from immunized WT mice. Thus, our data suggest that although NK cells are an important component for the control of T. gondii challenge infection in the presence of T-cell memory, they do not appear to acquire cell-intrinsic features of a memory-like phenotype.
NK cells that develop memory-like features also acquire the ability to persist long-term(3, 35). They can also have specific tissue tropism (2, 3, 35). For example, liver NK cells develop a hapten-specific response (2). Liver and lung NK cells from influenza-immunized and liver NK cells from attenuated VSV−infected Rag1−/− donors mediate protection in Rag2−/−γc−/− recipients (33). MCMV- and SIV-specific NK cells are found in the spleen and other organs (3, 35). Our fate mapping studies using reporter NKp46-CreERT2 × Rosa26-YFP mice indicate that, similar to their protective qualities, T. gondii–experienced and naïve NK cells were not intrinsically different in their ability to persist, in contrast to the MCMV-mediated increase in the number of long-lived YFP+ NK cells (9). We also did not observe any increase in YFP+ NK cells after T. gondii immunization in the peritoneum, spleen or bone marrow. This further supported the finding that NK cells do not develop cell-intrinsic characteristics of immune memory in response to T. gondii infection. One possible explanation for our not observing NK cell intrinsic characteristics of memory-like responses in our studies could be because of the genetic background of the parasite strain we used, which was an attenuated Type I (virulent) strain. Whether infection of mice with Type II strain parasites generates memory like NK cell responses is unclear. Results of a recent study suggest that NK cells differentiate into a long lived liver resident ILC1 population after repeated T. gondii vaccination or persistent Type II Prugniaud strain infection. This study suggests that repeated or persistent exposure to the parasite may be required (66). However, the importance of these NK cell derived ILC1 during T. gondii infection was not determined in this study and in our hands repeated CPS immunization of RAG1 KO mice did not generate NK cell intrinsic memory-like features (Fig. 5D). Therefore our results raise an important question: Why do NK cells not further differentiate and acquire memory-like characteristics during T. gondii infection as compared with hapten, virus or cytokine stimulation? Answering this question could explain why this parasite can escape and persist and be a continual problem for the host and also could help improve vaccine design.
NK-cell memory might not be generated in response to T. gondii infection for several reasons. These include the nature of NK-cell recognition of T. gondii infection or the lack thereof, co-stimulatory factors and the cytokine milieu. The development of memory-like features in NK cells in response to MCMV and HCMV infections requires signal 1 (antigen), signal 2 (co-stimulation) and signal 3 (cytokines) (3, 44, 46, 67, 68). One explanation for NK cells not developing intrinsic memory to T. gondii infection could be that the parasite does not itself express or does not induce a host cell to express a specific activating ligand for NK cells, and thus activation of NK cells lacks signal 1. In support of this possibility, our data indicate that after T. gondii immunization or after immunization and challenge no dominant NK-cell receptor was enriched within the responding population of total NK cells. We also do not observe any specific NK−cell receptor enrichment among the total responding NK-cell population during acute T. gondii infection, regardless of parasite virulence (45). Overall, we observed a wide array of NK-cell populations based on NK-cell receptor expression, indicating that there was a global response to infection. This could be common among apicomplexan infections because the NK-cell response to Plasmodium falciparum infection is also cell extrinsic (56). It is possible that NK cells evolved specific recognition mechanisms for viruses and bacteria but not for protozoan pathogens.
NK cells might not develop a memory-like response to T. gondii because they do not receive the necessary co-stimulatory signals or combination of cytokine stimulation. In response to MCMV and HCMV, specific co-stimultatory molecules and their interactions with their cognate ligands are essential for the generation of memory NK cells (46, 68, 69). DNAM-1 is important for memory NK-cell development during MCMV infection (46). We found that DNAM-1 co-stimulation was not required for the NK-cell response because its blockade did not alter NK-cell expansion or IFNγ production during secondary T. gondii infection. Other costimulatory molecules could also be involved. CD28 is important for maximizing the NK-cell responses to acute T. gondii infection (70). In addition to a potential alteration of co-stimualtory signals, T. gondii could induce higher expression of co-inhibitory molecules TIGIT and CD96 (71), which could then inhibit NK-cell memory formation; however, we did not observe any significant increases in the expression of these molecules during secondary T. gondii infection (data not shown).
Type I IFN and IL-12 are essential for expansion and survival of MCMV-specific NK cells (67, 72). Cytokine-induced memory-like NK cells can be generated in mice and humans after stimulation with IL-12, IL-18 and IL-15 (6, 28). All of these cytokines are produced during acute T. gondii infection (17, 45, 73, 74), with the exception of Type I IFNs (75). Therefore, it is surprising that NK cells do not further differentiate into memory-like cells. The development of NK-cell memory-like responses may require an activating receptor, costimulatory molecules and the correct combination of cytokines during T. gondii infection. Dissecting the reasons why NK cells do not develop memory-like features to T. gondii in a cell-intrinsic manner will be important future questions to address.
Because a second wave of NK-cell responses was critical for the control of secondary T. gondii infection in the presence of memory T cells, we addressed how the NK-cell extrinsic immune environment of vaccinated animals regulated their response. After Rabies virus vaccination secondary NK-cell responses depend on the presence of memory T cells (54). During Rabies virus secondary challenge, IL-2 producing antigen-specific memory CD4+ T cells in combination with IL-12 and IL-18 from accessory cells reactivated NK-cells. NK-cell responses in PBMCs of patients vaccinated with a Plasmodium falciparum specific vaccine correlated with IL-2 production by T cells (56, 57). These studies suggest that memory T cells could provide cell extrinsic signals important for secondary NK cell responses to these infections. Unlike these previous studies, we did not find a role for antigen-specific memory CD4+ or CD8+ T cells in helping NK-cell responses to secondary infection. Activation of NK cells that is independent of memory T cells may have important implications for individuals that are T-cell deficient.
Multiple studies demonstrate that IL-12p40 is essential for protective immunity against T. gondii infection, including for NK cell IFNγ production during primary infection (17, 18, 76–78). Our data indicate that NK-cell activation in the presence of memory T cells during secondary infection of immunized animals was also dependent on the IL-12 subunit p40. IL-12p40 is a subunit of bioactive IL-12p70 (IL-12) and also of IL-23 (79). IL-12 and IL-23 are both capable of inducing IFNγ production by NK and T cells (79–81). During primary T. gondii infection, IL-12 is the main mediator of protection, and IL-23 provides protection if IL-12 is absent (62). Surprisingly, that study also demonstrated that IL-12p35 KO mice survived longer than IL-12/23p40 KO mice during T. gondii infection. The difference in protection was independent of T cells, raising an interesting question about the role of each of these cytokines in activating the non−T cell compartment to control primary T. gondii infection. Our results demonstrated that both IL-12 and IL-23 contribute to NK-cell responses during secondary T. gondii infection. NK-cell numbers were reduced in the absence of IL-12 or IL-23 during secondary T. gondii infection. However, NK-cell IFNγ was only partially reduced in the absence of IL-12, and depletion of IL-23 did not affect the frequency of IFNγ+ NK cells at the site of infection. Using anti-IL-12p40 treatment, we observed a near complete elimination of the secondary NK-cell response during parasite challenge. Thus, IL-12 is important in activating NK cells to produce IFNγ, whereas IL-23 may be more important for increasing the number of NK cells at the site of secondary infection. Treatment of vaccinated animals with anti-IL-12p40, anti-IL-12p70 or anti-IL-23 during lethal challenge also reduced control of the parasite and increased parasite burdens at the site of infection. The mechanisms by which IL-12 and IL-23 work together to regulate NK-cell numbers and their IFNγ production in a T. gondii vaccine challenge situation could be direct via inducing NK-cell proliferation, migration and the ability to survive after infection. However, the mechanism of IL-12 and IL-23 action could also be indirect via signaling in other cell types that may be recruited to the site of secondary infection.
In summary, we demonstrated that NK cells play an important protective role beyond primary T. gondii infection and in the presence of memory T cells after vaccination and during a challenge infection. NK cells become activated and help control the parasite during secondary infection. In their absence, parasite burdens are increased both in short-term and long-term challenge infection. NK cells do not develop cell-intrinsic characteristics of memory and rely on cell-extrinsic IL-12 and IL-23 to respond during secondary challenge. Thus, NK cells provide protection in the presence of memory T cells and should be taken into consideration in vaccination and immunotherapy strategies to combat T. gondii infection.
Supplementary Material
Key points.
NK cells are required for immune control of secondary T. gondii infection.
NK cells do not develop instrinsic memory-like features during T. gondii infection.
Secondary NK cell responses depend upon IL-12 and IL-23.
Acknowledgments
This work is supported by grants from the American Heart Association AHA 17GRNT33700199 and University of Wyoming INBRE P20 GM103432 DRPP awarded to JPG. DLI is a University of Wyoming INBRE P20 GM103432 Graduate Assistantship recipient. T.M.M. is a University of Wyoming INBRE P20 GM103432 supported undergraduate fellow. This project is supported in part by a grant from the National Institute of General Medical Sciences (2P20GM103432) from the National Institutes of Health. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health
Abbreviations used in this article:
- BM
bone marrow
- HCMV
human cytomegalovirus
- i.g.
intragastrically
- MCMV
murine cytomegalovirus
References
- 1.Beaulieu AM. 2018. Memory responses by natural killer cells. J Leukoc Biol doi: 10.1002/JLB.1RI0917-366R. [DOI] [PubMed] [Google Scholar]
- 2.O’Leary JG, Goodarzi M, Drayton DL, von Andrian UH. 2006. T cell- and B cell-independent adaptive immunity mediated by natural killer cells. Nat Immunol 7:507–516. [DOI] [PubMed] [Google Scholar]
- 3.Sun JC, Beilke JN, Lanier LL. 2009. Adaptive immune features of natural killer cells. Nature 457:557–561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Arase H, Mocarski ES, Campbell AE, Hill AB, Lanier LL. 2002. Direct recognition of cytomegalovirus by activating and inhibitory NK cell receptors. Science 296:1323–1326. [DOI] [PubMed] [Google Scholar]
- 5.Lopez-Botet M, Muntasell A, Vilches C. 2014. The CD94/NKG2C+ NK-cell subset on the edge of innate and adaptive immunity to human cytomegalovirus infection. Semin Immunol 26:145–151. [DOI] [PubMed] [Google Scholar]
- 6.Romee R, Schneider SE, Leong JW, Chase JM, Keppel CR, Sullivan RP, Cooper MA, Fehniger TA. 2012. Cytokine activation induces human memory-like NK cells. Blood 120:4751–4760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cooper MA, Fehniger TA, Ponnappan A, Mehta V, Wewers MD, Caligiuri MA. 2001. Interleukin-1beta costimulates interferon-gamma production by human natural killer cells. Eur J Immunol 31:792–801. [DOI] [PubMed] [Google Scholar]
- 8.Ni J, Miller M, Stojanovic A, Garbi N, Cerwenka A. 2012. Sustained effector function of IL-12/15/18-preactivated NK cells against established tumors. J Exp Med 209:2351–2365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nabekura T, Lanier LL. 2016. Tracking the fate of antigen-specific versus cytokine-activated natural killer cells after cytomegalovirus infection. J Exp Med 213:2745–2758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hill DE, Chirukandoth S, Dubey JP. 2005. Biology and epidemiology of Toxoplasma gondii in man and animals. Anim Health Res Rev 6:41–61. [DOI] [PubMed] [Google Scholar]
- 11.Kodym P, Maly M, Beran O, Jilich D, Rozsypal H, Machala L, Holub M. 2015. Incidence, immunological and clinical characteristics of reactivation of latent Toxoplasma gondii infection in HIV-infected patients. Epidemiol Infect 143:600–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Flegr J, Prandota J, Sovickova M, Israili ZH. 2014. Toxoplasmosis--a global threat. Correlation of latent toxoplasmosis with specific disease burden in a set of 88 countries. PLoS One 9:e90203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jones JL, Dubey JP. 2012. Foodborne toxoplasmosis. Clin Infect Dis 55:845–851. [DOI] [PubMed] [Google Scholar]
- 14.Coppens I 2014. Exploitation of auxotrophies and metabolic defects in Toxoplasma as therapeutic approaches. Int J Parasitol 44:109–120. [DOI] [PubMed] [Google Scholar]
- 15.Radke JB, Burrows JN, Goldberg DE, Sibley LD. 2018. Evaluation of Current and Emerging Antimalarial Medicines for Inhibition of Toxoplasma gondii Growth in Vitro. ACS Infect Dis 4:1264–1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sher A, Oswald IP, Hieny S, Gazzinelli RT. 1993. Toxoplasma gondii induces a T-independent IFN-gamma response in natural killer cells that requires both adherent accessory cells and tumor necrosis factor-alpha. J Immunol 150:3982–3989. [PubMed] [Google Scholar]
- 17.Gazzinelli RT, Hieny S, Wynn TA, Wolf S, Sher A. 1993. Interleukin 12 is required for the T-lymphocyte-independent induction of interferon gamma by an intracellular parasite and induces resistance in T-cell-deficient hosts. Proc Natl Acad Sci U S A 90:6115–6119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hunter CA, Subauste CS, Van Cleave VH, Remington JS. 1994. Production of gamma interferon by natural killer cells from Toxoplasma gondii-infected SCID mice: regulation by interleukin-10, interleukin-12, and tumor necrosis factor alpha. Infect Immun 62:2818–2824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Goldszmid RS, Caspar P, Rivollier A, White S, Dzutsev A, Hieny S, Kelsall B, Trinchieri G, Sher A. 2012. NK cell-derived interferon-gamma orchestrates cellular dynamics and the differentiation of monocytes into dendritic cells at the site of infection. Immunity 36:1047–1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Askenase MH, Han SJ, Byrd AL, Morais da Fonseca D, Bouladoux N, Wilhelm C, Konkel JE, Hand TW, Lacerda-Queiroz N, Su XZ, Trinchieri G, Grainger JR, Belkaid Y. 2015. Bone-Marrow-Resident NK Cells Prime Monocytes for Regulatory Function during Infection. Immunity 42:1130–1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Combe CL, Curiel TJ, Moretto MM, Khan IA. 2005. NK cells help to induce CD8(+)-T-cell immunity against Toxoplasma gondii in the absence of CD4(+) T cells. Infect Immun 73:4913–4921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Goldszmid RS, Bafica A, Jankovic D, Feng CG, Caspar P, Winkler-Pickett R, Trinchieri G, Sher A. 2007. TAP-1 indirectly regulates CD4+ T cell priming in Toxoplasma gondii infection by controlling NK cell IFN-gamma production. J Exp Med 204:2591–2602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hauser WE Jr., Sharma SD, Remington JS 1983. Augmentation of NK cell activity by soluble and particulate fractions of Toxoplasma gondii. J Immunol 131:458–463. [PubMed] [Google Scholar]
- 24.Kamiyama T, Hagiwara T. 1982. Augmented followed by suppressed levels of natural cell-mediated cytotoxicity in mice infected with Toxoplasma gondii. Infect Immun 36:628–636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sharma SD, Verhoef J, Remington JS. 1984. Enhancement of human natural killer cell activity by subcellular components of Toxoplasma gondii. Cell Immunol 86:317–326. [DOI] [PubMed] [Google Scholar]
- 26.Denkers EY, Yap G, Scharton-Kersten T, Charest H, Butcher BA, Caspar P, Heiny S, Sher A. 1997. Perforin-mediated cytolysis plays a limited role in host resistance to Toxoplasma gondii. J Immunol 159:1903–1908. [PubMed] [Google Scholar]
- 27.Roozbehani M, Falak R, Mohammadi M, Hemphill A, Razmjou E, Meamar AR, Masoori L, Khoshmirsafa M, Moradi M, Gharavi MJ. 2018. Characterization of a multi-epitope peptide with selective MHC-binding capabilities encapsulated in PLGA nanoparticles as a novel vaccine candidate against Toxoplasma gondii infection. Vaccine 36:6124–6132. [DOI] [PubMed] [Google Scholar]
- 28.Cooper MA, Elliott JM, Keyel PA, Yang L, Carrero JA, Yokoyama WM. 2009. Cytokine-induced memory-like natural killer cells. Proc Natl Acad Sci U S A 106:1915–1919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wilson DC, Matthews S, Yap GS. 2008. IL-12 signaling drives CD8+ T cell IFN-gamma production and differentiation of KLRG1+ effector subpopulations during Toxoplasma gondii Infection. J Immunol 180:5935–5945. [DOI] [PubMed] [Google Scholar]
- 30.Wilson DC, Grotenbreg GM, Liu K, Zhao Y, Frickel EM, Gubbels MJ, Ploegh HL, Yap GS. 2010. Differential regulation of effector- and central-memory responses to Toxoplasma gondii Infection by IL-12 revealed by tracking of Tgd057-specific CD8+ T cells. PLoS Pathog 6:e1000815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gigley JP, Fox BA, Bzik DJ. 2009. Cell-mediated immunity to Toxoplasma gondii develops primarily by local Th1 host immune responses in the absence of parasite replication. J Immunol 182:1069–1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jordan KA, Wilson EH, Tait ED, Fox BA, Roos DS, Bzik DJ, Dzierszinski F, Hunter CA. 2009. Kinetics and phenotype of vaccine-induced CD8+ T-cell responses to Toxoplasma gondii. Infect Immun 77:3894–3901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Paust S, Gill HS, Wang BZ, Flynn MP, Moseman EA, Senman B, Szczepanik M, Telenti A, Askenase PW, Compans RW, von Andrian UH. 2010. Critical role for the chemokine receptor CXCR6 in NK cell-mediated antigen-specific memory of haptens and viruses. Nat Immunol 11:1127–1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Abdul-Careem MF, Lee AJ, Pek EA, Gill N, Gillgrass AE, Chew MV, Reid S, Ashkar AA. 2012. Genital HSV-2 infection induces short-term NK cell memory. PLoS One 7:e32821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Reeves RK, Li H, Jost S, Blass E, Li H, Schafer JL, Varner V, Manickam C, Eslamizar L, Altfeld M, von Andrian UH, Barouch DH. 2015. Antigen-specific NK cell memory in rhesus macaques. Nat Immunol 16:927–932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Denkers EY, Gazzinelli RT, Martin D, Sher A. 1993. Emergence of NK1.1+ cells as effectors of IFN-gamma dependent immunity to Toxoplasma gondii in MHC class I-deficient mice. J Exp Med 178:1465–1472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fox BA, Bzik DJ. 2002. De novo pyrimidine biosynthesis is required for virulence of Toxoplasma gondii. Nature 415:926–929. [DOI] [PubMed] [Google Scholar]
- 38.Gigley JP, Fox BA, Bzik DJ. 2009. Long-term immunity to lethal acute or chronic type II Toxoplasma gondii infection is effectively induced in genetically susceptible C57BL/6 mice by immunization with an attenuated type I vaccine strain. Infect Immun 77:5380–5388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Suzuki Y, Orellana MA, Schreiber RD, Remington JS. 1988. Interferon-gamma: the major mediator of resistance against Toxoplasma gondii. Science 240:516–518. [DOI] [PubMed] [Google Scholar]
- 40.Alter G, Malenfant JM, Altfeld M. 2004. CD107a as a functional marker for the identification of natural killer cell activity. J Immunol Methods 294:15–22. [DOI] [PubMed] [Google Scholar]
- 41.Hammer Q, Ruckert T, Romagnani C. 2018. Natural killer cell specificity for viral infections. Nat Immunol 19:800–808. [DOI] [PubMed] [Google Scholar]
- 42.Romee R, Rosario M, Berrien-Elliott MM, Wagner JA, Jewell BA, Schappe T, Leong JW, Abdel-Latif S, Schneider SE, Willey S, Neal CC, Yu L, Oh ST, Lee YS, Mulder A, Claas F, Cooper MA, Fehniger TA. 2016. Cytokine-induced memory-like natural killer cells exhibit enhanced responses against myeloid leukemia. Sci Transl Med 8:357ra123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dokun AO, Kim S, Smith HR, Kang HS, Chu DT, Yokoyama WM. 2001. Specific and nonspecific NK cell activation during virus infection. Nat Immunol 2:951–956. [DOI] [PubMed] [Google Scholar]
- 44.Hammer Q, Ruckert T, Borst EM, Dunst J, Haubner A, Durek P, Heinrich F, Gasparoni G, Babic M, Tomic A, Pietra G, Nienen M, Blau IW, Hofmann J, Na IK, Prinz I, Koenecke C, Hemmati P, Babel N, Arnold R, Walter J, Thurley K, Mashreghi MF, Messerle M, Romagnani C. 2018. Peptide-specific recognition of human cytomegalovirus strains controls adaptive natural killer cells. Nat Immunol 19:453–463. [DOI] [PubMed] [Google Scholar]
- 45.Ivanova DL, Fatima R, Gigley JP. 2016. Comparative Analysis of Conventional Natural Killer Cell Responses to Acute Infection with Toxoplasma gondii Strains of Different Virulence. Frontiers in Immunology 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nabekura T, Kanaya M, Shibuya A, Fu G, Gascoigne NR, Lanier LL. 2014. Costimulatory molecule DNAM-1 is essential for optimal differentiation of memory natural killer cells during mouse cytomegalovirus infection. Immunity 40:225–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nabekura T, Lanier LL. 2014. Antigen-specific expansion and differentiation of natural killer cells by alloantigen stimulation. J Exp Med 211:2455–2465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chiossone L, Chaix J, Fuseri N, Roth C, Vivier E, Walzer T. 2009. Maturation of mouse NK cells is a 4-stage developmental program. Blood 113:5488–5496. [DOI] [PubMed] [Google Scholar]
- 49.Mombaerts P, Iacomini J, Johnson RS, Herrup K, Tonegawa S, Papaioannou VE. 1992. RAG-1-deficient mice have no mature B and T lymphocytes. Cell 68:869–877. [DOI] [PubMed] [Google Scholar]
- 50.Kondo M, Weissman IL, Akashi K. 1997. Identification of clonogenic common lymphoid progenitors in mouse bone marrow. Cell 91:661–672. [DOI] [PubMed] [Google Scholar]
- 51.Karo JM, Schatz DG, Sun JC. 2014. The RAG recombinase dictates functional heterogeneity and cellular fitness in natural killer cells. Cell 159:94–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mazurier F, Fontanellas A, Salesse S, Taine L, Landriau S, Moreau-Gaudry F, Reiffers J, Peault B, Di Santo JP, de Verneuil H. 1999. A novel immunodeficient mouse model--RAG2 x common cytokine receptor gamma chain double mutants--requiring exogenous cytokine administration for human hematopoietic stem cell engraftment. J Interferon Cytokine Res 19:533–541. [DOI] [PubMed] [Google Scholar]
- 53.Sun JC, Beilke JN, Bezman NA, Lanier LL. 2011. Homeostatic proliferation generates long-lived natural killer cells that respond against viral infection. J Exp Med 208:357–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Horowitz A, Behrens RH, Okell L, Fooks AR, Riley EM. 2010. NK cells as effectors of acquired immune responses: effector CD4+ T cell-dependent activation of NK cells following vaccination. J Immunol 185:2808–2818. [DOI] [PubMed] [Google Scholar]
- 55.Bihl F, Pecheur J, Breart B, Poupon G, Cazareth J, Julia V, Glaichenhaus N, Braud VM. 2010. Primed antigen-specific CD4+ T cells are required for NK cell activation in vivo upon Leishmania major infection. J Immunol 185:2174–2181. [DOI] [PubMed] [Google Scholar]
- 56.Horowitz A, Newman KC, Evans JH, Korbel DS, Davis DM, Riley EM. 2010. Cross-talk between T cells and NK cells generates rapid effector responses to Plasmodium falciparum-infected erythrocytes. J Immunol 184:6043–6052. [DOI] [PubMed] [Google Scholar]
- 57.Horowitz A, Hafalla JC, King E, Lusingu J, Dekker D, Leach A, Moris P, Cohen J, Vekemans J, Villafana T, Corran PH, Bejon P, Drakeley CJ, von Seidlein L, Riley EM. 2012. Antigen-specific IL-2 secretion correlates with NK cell responses after immunization of Tanzanian children with the RTS,S/AS01 malaria vaccine. J Immunol 188:5054–5062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yi H, Zhen Y, Zeng C, Zhang L, Zhao Y. 2008. Depleting anti-CD4 monoclonal antibody (GK1.5) treatment: influence on regulatory CD4+CD25+Foxp3+ T cells in mice. Transplantation 85:1167–1174. [DOI] [PubMed] [Google Scholar]
- 59.Cooper AM, Khader SA. 2007. IL-12p40: an inherently agonistic cytokine. Trends Immunol 28:33–38. [DOI] [PubMed] [Google Scholar]
- 60.Abdi K, Singh NJ, Spooner E, Kessler BM, Radaev S, Lantz L, Xiao TS, Matzinger P, Sun PD, Ploegh HL. 2014. Free IL-12p40 monomer is a polyfunctional adaptor for generating novel IL-12-like heterodimers extracellularly. J Immunol 192:6028–6036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gounder AP, Yokoyama CC, Jarjour NN, Bricker TL, Edelson BT, Boon ACM. 2018. Interferon induced protein 35 exacerbates H5N1 influenza disease through the expression of IL-12p40 homodimer. PLoS Pathog 14:e1007001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lieberman LA, Cardillo F, Owyang AM, Rennick DM, Cua DJ, Kastelein RA, Hunter CA. 2004. IL-23 provides a limited mechanism of resistance to acute toxoplasmosis in the absence of IL-12. J Immunol 173:1887–1893. [DOI] [PubMed] [Google Scholar]
- 63.Joshi NS, Cui W, Chandele A, Lee HK, Urso DR, Hagman J, Gapin L, Kaech SM. 2007. Inflammation directs memory precursor and short-lived effector CD8(+) T cell fates via the graded expression of T-bet transcription factor. Immunity 27:281–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Suzuki Y, Remington JS. 1988. Dual regulation of resistance against Toxoplasma gondii infection by Lyt-2+ and Lyt-1+, L3T4+ T cells in mice. J Immunol 140:3943–3946. [PubMed] [Google Scholar]
- 65.Splitt SD, Souza SP, Valentine KM, Castellanos BE, Curd AB, Hoyer KK, Jensen KDC. 2018. PD-L1, TIM-3, and CTLA-4 blockade fail to promote resistance to secondary infection with virulent strains of Toxoplasma gondii. Infect Immun doi: 10.1128/IAI.00459-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Park E, Patel S, Wang Q, Andhey P, Zaitsev K, Porter S, Hershey M, Bern M, Plougastel-Douglas B, Collins P, Colonna M, Murphy KM, Oltz E, Artyomov M, Sibley LD, Yokoyama WM. 2019. Toxoplasma gondii infection drives conversion of NK cells into ILC1-like cells. Elife 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sun JC, Madera S, Bezman NA, Beilke JN, Kaplan MH, Lanier LL. 2012. Proinflammatory cytokine signaling required for the generation of natural killer cell memory. J Exp Med 209:947–954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Liu LL, Landskron J, Ask EH, Enqvist M, Sohlberg E, Traherne JA, Hammer Q, Goodridge JP, Larsson S, Jayaraman J, Oei VYS, Schaffer M, Tasken K, Ljunggren HG, Romagnani C, Trowsdale J, Malmberg KJ, Beziat V. 2016. Critical Role of CD2 Co-stimulation in Adaptive Natural Killer Cell Responses Revealed in NKG2C-Deficient Humans. Cell Rep 15:1088–1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Rolle A, Halenius A, Ewen EM, Cerwenka A, Hengel H, Momburg F. 2016. CD2-CD58 interactions are pivotal for the activation and function of adaptive natural killer cells in human cytomegalovirus infection. Eur J Immunol 46:2420–2425. [DOI] [PubMed] [Google Scholar]
- 70.Hunter CA, Ellis-Neyer L, Gabriel KE, Kennedy MK, Grabstein KH, Linsley PS, Remington JS. 1997. The role of the CD28/B7 interaction in the regulation of NK cell responses during infection with Toxoplasma gondii. J Immunol 158:2285–2293. [PubMed] [Google Scholar]
- 71.Kim N, Kim HS. 2018. Targeting Checkpoint Receptors and Molecules for Therapeutic Modulation of Natural Killer Cells. Front Immunol 9:2041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Madera S, Rapp M, Firth MA, Beilke JN, Lanier LL, Sun JC. 2016. Type I IFN promotes NK cell expansion during viral infection by protecting NK cells against fratricide. J Exp Med 213:225–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Villegas EN, Elloso MM, Reichmann G, Peach R, Hunter CA. 1999. Role of CD28 in the generation of effector and memory responses required for resistance to Toxoplasma gondii. J Immunol 163:3344–3353. [PubMed] [Google Scholar]
- 74.Schulthess J, Meresse B, Ramiro-Puig E, Montcuquet N, Darche S, Begue B, Ruemmele F, Combadiere C, Di Santo JP, Buzoni-Gatel D, Cerf-Bensussan N. 2012. Interleukin-15-dependent NKp46+ innate lymphoid cells control intestinal inflammation by recruiting inflammatory monocytes. Immunity 37:108–121. [DOI] [PubMed] [Google Scholar]
- 75.Pierog PL, Zhao Y, Singh S, Dai J, Yap GS, Fitzgerald-Bocarsly P. 2018. Toxoplasma gondii Inactivates Human Plasmacytoid Dendritic Cells by Functional Mimicry of IL-10. J Immunol 200:186–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Scharton-Kersten TM, Yap G, Magram J, Sher A. 1997. Inducible nitric oxide is essential for host control of persistent but not acute infection with the intracellular pathogen Toxoplasma gondii. J Exp Med 185:1261–1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Yap G, Pesin M, Sher A. 2000. Cutting edge: IL-12 is required for the maintenance of IFN-gamma production in T cells mediating chronic resistance to the intracellular pathogen, Toxoplasma gondii. J Immunol 165:628–631. [DOI] [PubMed] [Google Scholar]
- 78.Khan IA, Matsuura T, Kasper LH. 1994. Interleukin-12 enhances murine survival against acute toxoplasmosis. Infect Immun 62:1639–1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Oppmann B, Lesley R, Blom B, Timans JC, Xu Y, Hunte B, Vega F, Yu N, Wang J, Singh K, Zonin F, Vaisberg E, Churakova T, Liu M, Gorman D, Wagner J, Zurawski S, Liu Y, Abrams JS, Moore KW, Rennick D, de Waal-Malefyt R, Hannum C, Bazan JF, Kastelein RA. 2000. Novel p19 protein engages IL-12p40 to form a cytokine, IL-23, with biological activities similar as well as distinct from IL-12. Immunity 13:715–725. [DOI] [PubMed] [Google Scholar]
- 80.van de Wetering D, de Paus RA, van Dissel JT, van de Vosse E. 2009. IL-23 modulates CD56+/CD3- NK cell and CD56+/CD3+ NK-like T cell function differentially from IL-12. Int Immunol 21:145–153. [DOI] [PubMed] [Google Scholar]
- 81.Ziblat A, Nunez SY, Raffo Iraolagoitia XL, Spallanzani RG, Torres NI, Sierra JM, Secchiari F, Domaica CI, Fuertes MB, Zwirner NW. 2017. Interleukin (IL)-23 Stimulates IFN-gamma Secretion by CD56(bright) Natural Killer Cells and Enhances IL-18-Driven Dendritic Cells Activation. Front Immunol 8:1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


