Abstract
Effector CD4+ T cells with increased IL-17A and reduced IL-2 production contribute to tissue inflammation and organ damage in systemic lupus erythematosus (SLE). Increased expression of the transcription factor cAMP response element modulator (CREM)α promotes altered cytokine expression in SLE. Aim of this study was to investigate CREMα-mediated events favoring effector CD4+ T cells in health and disease. Using CRISPR/Cas9 genome editing and lentiviral transduction we generated CREMα-deficient and CREMα overexpressing Jurkat T cells. Gene expression and regulatory events were assessed using luciferase reporter assays and ChIP. Interaction between CREMα and p300 was investigated using proximity ligation assays, co-immunoprecipitation and knock-down of p300. Gene expression profiles of modified cells were compared to CD4+ T cells from patients with juvenile-onset SLE. We show that CREMα induces dual specificity protein phosphatase (DUSP)4 in effector CD4+ T cells through co-recruitment of p300. The transcriptional co-activator p300 mediates histone acetylation at DUSP4 prompting increased gene expression. Using DUSP4 transfection models and genetically modified CREM-deficient and CREMα overexpressing T cells, we demonstrate the molecular underpinnings whereby DUSP4 induces IL-17A while limiting IL-2 expression. We demonstrate that CD4+ T cells from patients with juvenile-onset SLE share phenotypical features with CREMα overexpressing CD4+ T cells, including increased DUSP4 expression and imbalanced IL-17A and IL-2 production. Taken together, we describe CREMα-mediated mechanisms which involve the transcriptional upregulation of DUSP4 leading to imbalanced cytokine production by effector T cells. Our findings identify the CREMα/DUSP4 axis as a promising candidate in the search for biomarkers and therapeutic targets in SLE.
Keywords: CREM, DUSP4, lupus, epigenetic, cytokine
Introduction
Systemic lupus erythematosus (SLE) is a multi-organ autoimmune/inflammatory condition that is characterized by uncontrolled lymphocyte activation, inappropriate release of inflammatory mediators resulting tissue and organ damage(1–3). Systemic inflammation and organ damage have been linked to increased numbers and activation of effector CD4+ T cells that are characterized by enhanced expression of the pro-inflammatory effector cytokine IL-17A and reduced production of immune regulatory IL-2. Imbalanced expression of IL-17A and IL-2 contribute to the immunological and clinical phenotype of SLE(1, 4, 5). Uncontrolled IL-17A production contributes to immune cell activation and infiltration of inflamed tissues(6, 7). Reduced IL-2 expression contributes to reduced numbers and function of regulatory T cells, increased effector functions, impaired activation-induced cell death contributing to prolonged survival of autoreactive cells and altered function of cytotoxic CD8+ T cells which likely contributes to increased infection rates and severity in SLE patients(4, 8).
Molecular mechanisms which contribute to the uncontrolled effector functions of T cells in SLE have identified the transcription factor cAMP response element modulator (CREM)α as playing an important role(9–11). T cells from patients with SLE express increased levels of CREMα that contribute to antithetically altered cytokine expression. CREMα trans-activates the IL17A proximal promoter and contributes to epigenetic “opening” of the IL17 cluster, spanning IL17A and IL17F, through histone acetylation and DNA demethylation(12–14). The exact underlying molecular events are incompletely understood. Conversely, CREMα trans-represses the IL2 promoter and instructs DNA methylation through its interaction with DNA methyltransferase (DNMT)3a and histone deacetylation through interactions with histone deacetylase (HDAC)1(12, 15–18). To date, it remains elusive as to why CREMα trans-activates one cytokine gene while it trans-represses another(12–15).
Recent studies identified the protein phosphatase dual specificity (dephosphorylates both phospho-tyrosine and phospho-serine/threonine residues) protein phosphatase (DUSP)4 as a regulator of STAT5 phosphorylation, thereby controlling the balance between Treg and IL-17A expressing effector T cells(19, 20). Indeed, primarily IL-17A vs. IL-2 expression in Th17 vs. regulatory (Treg) T cell subpopulations is mediated through a tightly controlled balance between STAT3 and STAT5(21).
In this study, we aimed to investigate i) whether CREMα regulates the expression of DUSP4, ii) the involved mechanisms, and iii) whether this plays a role in effector CD4+ T cell generation and activation in SLE. We chose to investigate cells from jSLE patients, since children with SLE exhibit fewer comorbidities, but increased disease activity and organ damage as compared to patients with adult-onset disease(22).
Materials and Methods:
Study subjects and T cell culture --
T cells from healthy controls, or individuals with jSLE were purified from whole blood as reported previously(12). Juvenile-onset SLE was defined by disease-onset before the 16th birthday and a diagnosis of SLE based on the revised ACR classification criteria(23). Juvenile-onset SLE patients and controls were recruited through the Alder Hey Children’s NHS Foundation trust Department of Rheumatology, and the Alder Hey NIHR Clinical Research Facility. The UK JSLE Cohort Study and Repository was approved by the North West Liverpool East Research Ethics Committee (REC: 6/Q1502/77). Patients, controls or their legal guardians gave written informed assent and/or consent. Age-, sex-, and ethnicity-matched healthy individuals were chosen as controls. Peripheral venous blood was collected in heparin-lithium tubes and CD4+ T cells were purified(12). Epidemiologic and clinical information are displayed in Table S1. For this study, jSLE patients with SLEDAI scores between 4 and 17 were chosen to cover mild and moderate to severe disease. Patients on comparable treatment regimens were chosen for better comparability of results; jSLE patients on high-dose corticosteroid treatment, cyclophosphamide or after B cell depletion were exuded. Primary CD4+ T cells from jSLE patients and matched healthy controls were plated and cultured under resting conditions or stimulated with plate-bound anti-CD3 and anti-CD28 antibodies. For analyses in effector Th populations, T cells were cultured for 5 days in the absence or presence of plate-bound anti-CD3 and anti-CD28 antibodies and lineage-determining cytokines plus cytokine-blocking antibodies (Th0: no cytokines, anti-IL-4 and anti-IFN-γ; Th1: IL-2, IL-12, anti-IL-4; Th17: anti-IL-4, anti-IFN-γ, TGF-β, IL-21). A media change was performed on day 3.
mRNA expression analysis --
Total RNA from Jurkat T cells, control and SLE CD4+ T lymphocytes was isolated using the Qiagen RNeasy Mini Kit (Qiagen). cDNA was generated using a first-strand cDNA synthesis kit (Clontech). For gene expression analyses, cDNA was used for quantitative RT-PCR using TaqMan systems. Primer and probe sequences will be shared upon request. For some experiments, Affymetrix QuantiGene mRNA probes were used and analysed on a LUMINEX200 platform according to manufacturer’s instructions. Messenger-RNA copy numbers were normalised to three housekeeping genes (HPRT1, PPIB, ACTB).
Gene expression plasmids --
Expression plasmids for human CREMα have been described previously(12). Expression plasmids for DUSP4A and DUSP4B were generated on a pcDNA3.1 backbone. Three million primary human CD4+ T lymphocytes were transfected with a total amount of 3 μg of expression plasmid using the Amaxa transfection system (Lonza). Cells were harvested after 24 h.
Generation of genetically modified Jurkat CD4+ T cells –
The pL.SSVF_XbaI_GFP_MluI.i_gb3.BspEI.Hygro.str vector was kindly donated by Sebastian Thieme (Department of Pediatrics, Universitätsklinikum Carl Gustav Carus, TU Dresden, Germany). From these, pL.SSVF_XbaI_CREMa_MluI.i_gb3.BspEI.Hygro.str plasmids including the cDNA coding sequence of CREMα were generated using routine cloning techniques. Virus vector particle production was performed as previously described(24). Human Jurkat T cells were cultured for 2–3 days in 24-well plates before and 3–4 days after lentiviral vector transduction in RPMI cell culture media supplemented with 10% FBS and 2 mM L-glutamine. After transduction, 2000 μg/ml hygromycin was added to RPMI media. Copy numbers were tested with qPCR and adjusted numbers of the CREMα transgene were 1/cell. To achieve bi-allelic deletion of the CREM P box region (CREMα subfamily deficient cells; figure S1A), Cas9_T2A_mCherry (Addgene #64324) and px4548_GFP (Addgene #48138) were used. Guide RNA sequences (sgPBox_33_FOR: CACCGTAAGCTAGCCCCTTAGGTAC; sgPBox_33_REV: AAACGTACCTAAGGGGCTAGCTTAC; sgPBox_52_FOR: CACCGAAAGTGCTCCTACGAATCC; sgPBox_52_REV: AAACGGATTCGTAGGAGCACTTTC; sgCas9_1_FOR: CACCGCTTCGAAATGTCCGTTCGGT; sgCas9_1_REV: AAACACCGAACGGACATTTCGAAGC; sgCas9_2_FOR: CACCGATCTTCGACGCAGGTGTCGC; sgCas9_2_REV: AAACGCGACACCTGCGTCGAAGATC) were introduced to plasmids through sub-cloning: Cas9_Cherry_Pbox33 (3’ of P Box) and Cas9_GFP_Pbox52 (5’ of P Box) (25). Next, Jurkat T cells were transfected with Cas9 plasmids (Amaxa Nucleofactor). Cells were rested and mCherry GFP “double positive” cells were sorted and expanded in RPMI media. Generated cell lines where then tested for mono- vs. bi-allelic deletion of the CREM P box region using PCR amplification (Figure S2A–C). CREMα, ICER (Figure S2D,E) and CREMτ (not shown) expression were tested using qRT-PCR.
Western Blot analysis --
For lysis, cells were incubated in 1% Triton lysis buffer. Proteins were then separated using 4–12% gradient Bis-Tris gels (Life Technologies) and transferred on PVDF membranes (Millipore). Membranes were blocked for 1 h with Tris-buffered saline solution containing 0.05% Tween and 5% nonfat dry milk. Membranes were then incubated overnight at 4°C with antibodies indicated, followed by incubation with an HRP-conjugated antibody. Detection was performed with Clarity Western ECL blotting reagents (Bio-Rad), and visualization was achieved with the ChemiDoc XRS+ Molecular Imager (Bio-Rad).
Luciferase assays in Jurkat T cells --
One million CD4+ Jurkat T cells (wild-type, CREM-deficient or CREMα overexpressing as indicated) were transfected with 500 ng/106 cells of plasmid DNA, using the Amaxa transfection system (Lonza). Effector:reporter transfection experiments were performed at a molar ratio of 3:1. Each reporter experiment included 10 ng of Renilla luciferase construct as an internal control. Five hours after transfection, cells were collected, lysed, and luciferase activity was quantified using the Promega Dual Luciferase Assay System following the manufacturer’s instructions.
ChIP assays --
Anti-H3K18ac (Abcam), anti-p300 (Santa Cruz), and anti-HA (EMD Millipore) and non-specific normal rabbit IgG were obtained from EMD Millipore. ChIP assays were carried out according to the manufacturer’s instructions (Invitrogen, Life Technologies). Briefly, cells were cross-linked with 1% formaldehyde, washed with cold PBS, and lysed in buffer containing protease inhibitors (Roche). Cell lysates were sonicated to shear DNA and sedimented, and diluted supernatants were immunoprecipitated with the indicated antibodies. A proportion (10%) of the diluted supernatants was kept as input control. After the recovery of ChIP-DNA, real-time qPCR was performed.
Proximity Ligation Assay --
Primary human T cells (5×106) were cultured for 24 h in the absence or presence of plate-bound anti-CD3 and anti-CD28 antibodies. Then, cells were harvested and subjected to the Duolink proximity ligation assay (PLA) following the manufacturer’s instructions (Olink) and protocols reported previously (26, 27). Briefly, anti-CREM and anti-p300 antibodies were labelled with PLUS and MINUS oligonucleotide tails. Cells were fixed with 3.7% formaldehyde, washed with PBS, permeabilized with Triton X (Life technologies), and incubated with PLUS and MINUS oligonucleotide labelled antibodies. According to the manufacturer’s instructions, cells were washed several times and incubated with ligase (30 min) and polymerase solution (100 min). After all washes and incubations, required according to the manufacturer’s protocol, cells were transferred to microscopy slides using a cytospin centrifuge (Shandon), mounted with DAPI containing medium (Olink), and read on a fluorescence microscope (Zeiss). Three independent experiments were analysed and included 246 unstimulated (experiment 1: 72, experiment 2: 88, experiment 3: 86) and 86 stimulated (experiment 1: 27, experiment 2: 24, experiment 3: 35) Jurkat CD4+ T cells. The number of PLA signals per cell was quantified using ImageJ software (http://rsb.info.nih.gov/ij/disclaimer.html).
Co-immunoprecipitation of p300 with CREMα --
One million HA-CREMα overexpressing or “wild-type” (pLenti as negative control) Jurkat T cells were cultured in the presence or absence of stimulation with plate-bound anti-CD3 and anti-CD28 antibodies, harvested and lysed in 400 μl of RIPA buffer including protease inhibitors (Roche Applied Science). Cell lysates were subjected to centrifugation (14,000 × g, 10 min, 4 °C) and 500 μg of total protein were incubated with anti-HA (directed at HA-CREMα) antibodies at 4 °C overnight and subjected to co-immunoprecipitation with the Pierce Co-IP kit, following the manufacturer’s instructions (Pierce). Co-IP solutions were subjected to SDS-PAGE as described before(26). Proteins were transferred to PVDF membranes and detected by anti-p300 antibodies as indicated, applying suitable secondary peroxidase-linked anti-rabbit antibody (Santa Cruz) and ECL (Amersham Biosciences) as chemiluminescent.
Knock-down of p300 with siRNA --
Three million Jurkat CD4+ T cells were transfected with 30 nM scrambled control siRNA or p300-specific siRNA (OriGene) using Lipofectamine 2000 (Invitrogen, Life Technologies). Cells were collected after overnight culture (24 h) and processed for mRNA analysis as indicated in the qRT-PCR section.
Statistical analysis --
Statistical analysis. The paired two-tailed Student t test was used for statistical analysis. Where indicated, non-parametric Kruskal-Wallis tests were used to determine differences between groups followed by Mann-Whitney tests where statistical significance was reached. The Pearson product moment correlation coefficient (R) was used to determine the correlation between CREM and DUSP4 mRNA levels.
Results
CREMα and DUSP4 expression in effector T cells –
To determine whether CREMα is expressed at increased levels in effector T cells and whether CREMα expression correlates with the expression of the protein phosphatase DUSP4, we differentiated Th0, Th1 and Th17 effector T cells from naïve CD4+ T cells from healthy individuals. Indeed, CREMα mRNA expression was significantly increased in effector T cell populations with highest expression in Th1 and Th17 subsets (Figure 1A). Since reports in mice indicate a role of the short ICER isoform of CREM in Th17 differentiation(28), we monitored its expression in naïve vs. effector T cells in humans, but saw no significant differences in the human system (Figure S1A). Messenger-RNA expression of CREM isoforms CREMτ1 (Figure S1B) and CREMτ2 (not shown were comparable between naive and effector T cells.
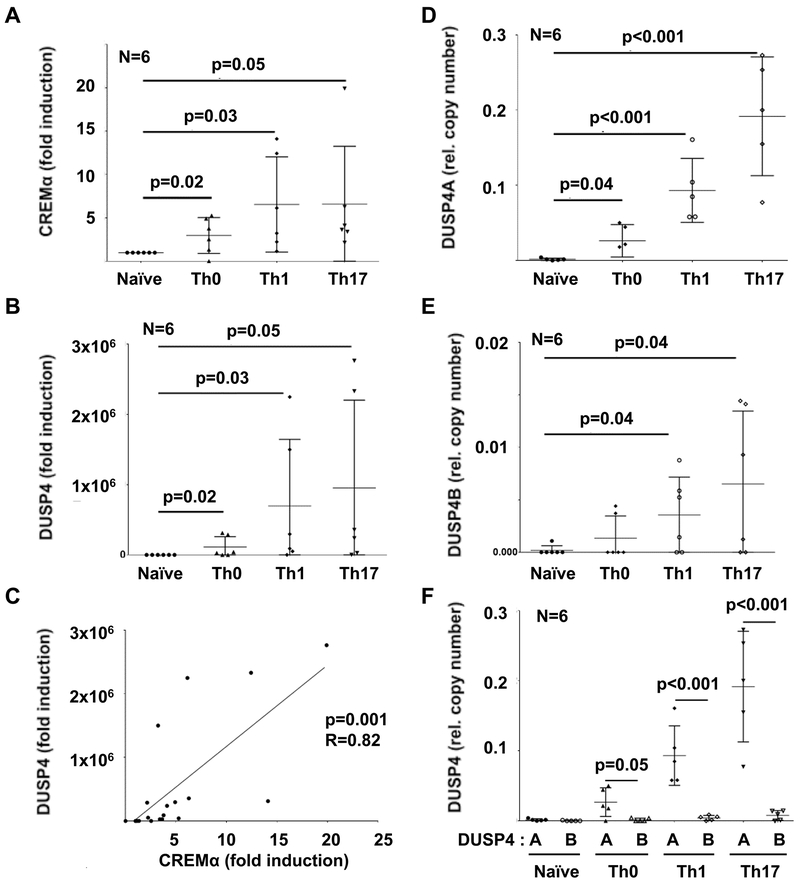
Figure 1: CREMα and DUSP4 expression correlate in effector CD4+ T cells.
A) The expression of CREMα is significantly increased in effector T cell populations with highest expression in Th1 and Th17 subsets. B) Expression of the protein phosphatase (DUSP)4A is significantly increased in effector T cell populations with highest expression in Th1 and Th17 subsets (semi-quantitative qRT-PCR). C) Expression of CREMα and DUSP4 correlate in effector T cells. D-E) DUSP4 has two isoforms, DUSP4A and DUSP4B. DUSP4A (D) and DUSP4B (E) expression follow similar trends (quantitative mRNA probe-based assays). DUSP4B mRNA expression levels are negligible when compared to DUSP4A (F). In figures A, B, D-F means and standard deviations are displayed; figure C displays individual data points, Pearson correlation coefficients (r) and the regression line.
The CREMα isoform is expressed at increased levels in effector CD4+ T cells from patients with SLE(9–11), which reflects CREMα mRNA expression by in vitro differentiated effector CD4+ T cells (Figure 1A). Thus, we focussed on CREMα mRNA expression in effector CD4+ T cells that was mirrored by DUSP4 mRNA expression. DUSP4 was significantly increased in effector CD4+ T cell populations with highest expression in Th1 and Th17 cells and correlated with CREMα expression levels (p=0.001, R=0.82) (Figure 1B,C). DUSP4 has two isoforms, DUSP4A and DUSP4B. Thus, we wondered whether transcripts of both isoforms are expressed in effector CD4+ T cells. DUSP4A (Figure 1D) and DUSP4B (Figure 1E) expression followed similar trends, but DUSP4B mRNA expression levels were significantly lower as compared to DUSP4A mRNA expression (Figure 1F).
CREMα induces DUSP4 expression –
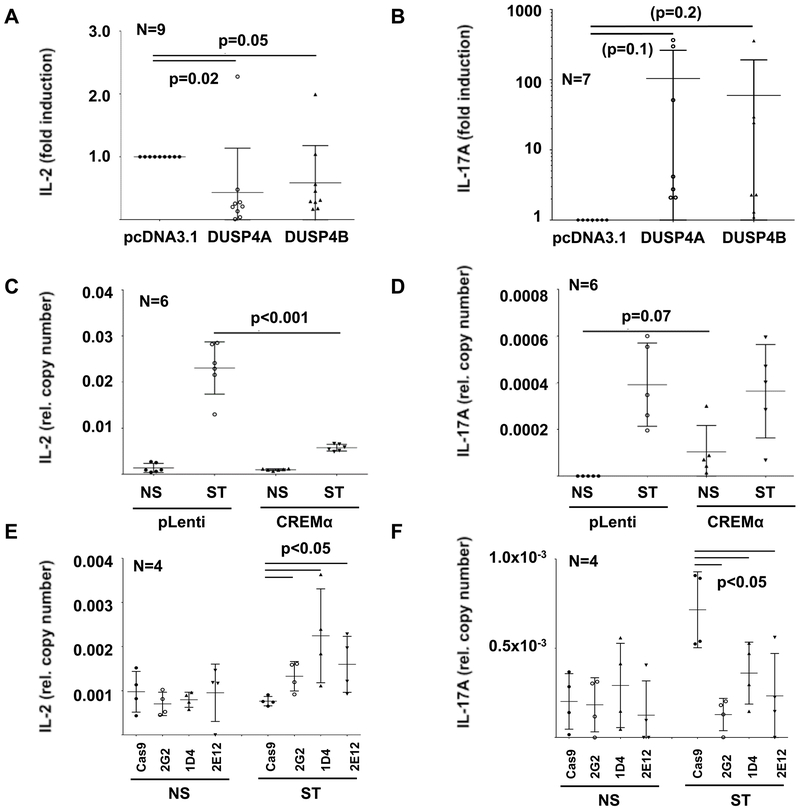
To test whether CREMα influences DUSP4 expression, we overexpressed CREMα in primary human CD4+ T cells through transfection of CREMα expression plasmids (Figure S1C). Indeed, CREMα overexpressing CD4+ T cells express increased levels of DUSP4 mRNA (Figure S1D) when compared to CD4+ T cells transfected with empty control plasmids (pcDNA3.1), and CREMα and DUSP4 mRNA expression closely correlated (p<0.001, R=0.96) (Figure S1E). To produce a reliable and easily accessible tool for subsequent molecular analyses, we generated stable CREMα overexpressing Jurkat CD4+ T cell lines (wild-type CREMα or HA tagged CREMα) using lentiviral transduction techniques. As compared to non-transduced wild-type and additional control cell lines (pLenti: transduced with empty plasmid), genetically modified Jurkat T cells exhibited increased CREMα expression (Figure 2A,B). Furthermore, in line with results from aforementioned transfection experiments in primary human CD4+ T cells, DUSP4 expression mirrored CREMα mRNA expression (Figure 2C, Figure S1F). Of note, DUSP4 mRNA expression reflected mainly DUSP4A expression in CREMα overexpressing cells (Figure 2D).
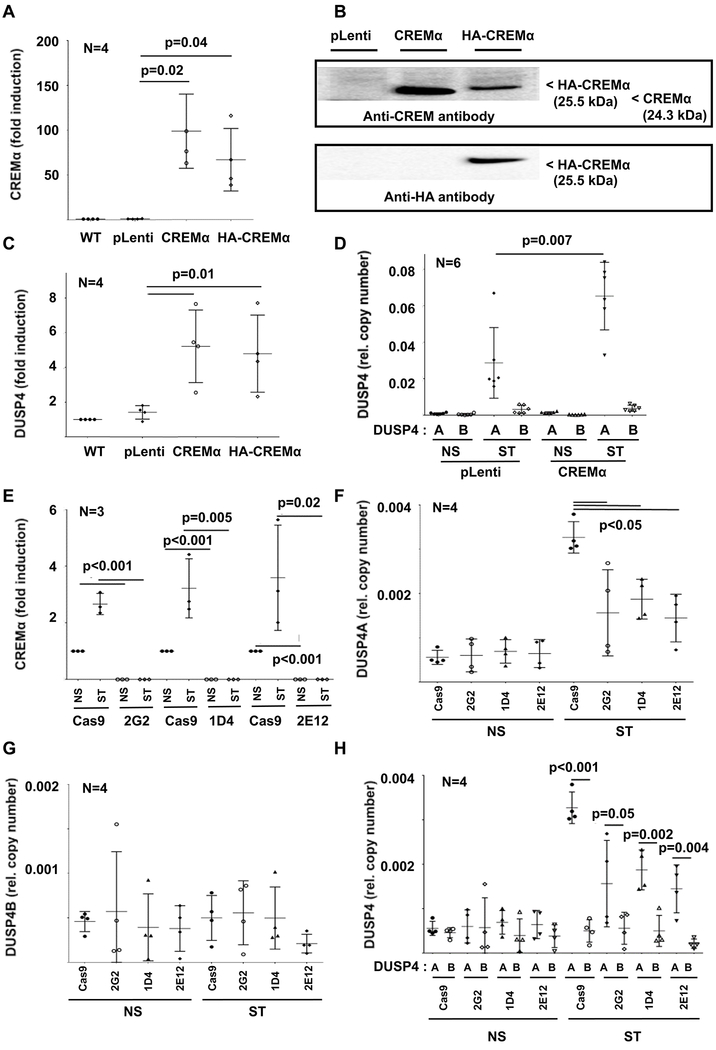
Figure 2: Effects of CREMα expression on DUSP4.
Using lentiviral transduction, CREMα overexpressing cell lines (wild-type CREMα or HA tagged CREMα) and control cell lines (pLenti: transduced with empty plasmid; pLenti_GFP: GPF expression plasmid) were generated. CREMα mRNA (qRT-PCR) (A) and protein (B) expression is increased in both CREMα overexpressing cell lines as compared to controls. Western blots show variable signals or band size depending on molecular weight (in kDa) or targeted epitope (CREM vs HA). Total DUSP4 (qRT-PCR) (C), mainly reflecting DUSP4A (quantitative mRNA probe-based assays) (D) expression is increased in CREMα overexpressing cells (NS: not stimulated; ST: stimulated with plate-bound anti-CD3 and anti-CD28 antibodies). E) In response to stimulation (NS: not stimulated; ST: stimulated) with plate-bound anti-CD3 and anti-CD28 antibodies, CREMα is expressed in increased levels in Cas9 control Jurkat T cells (qRT-PCR). CREM-deficient clones (2G2, 1D4 and 2E12), however, fail to express CREMα under all tested conditions (qRT-PCR). F) Expression of DUSP4A is reduced in CREM-deficient cell lines, while the low expression of DUSP4B (G) is comparable between all cell lines. H) As in primary human CD4+ T cells, DUSP4A expression (black symbols) was significantly higher when compared to DUSP4B (open symbols) (quantitative mRNA probe-based assays). In figures A and C-H means and standard deviations are displayed.
Deletion of the CREM P box region alters DUSP4 mRNA expression –
The CREM gene encodes for more than 50 isoforms which are subject to alternative promoter regions and splicing variants(10). The CREM promoter P1 regulates the expression of “long” CREM isoforms, including CREMα. The ICER promoter governs expression of the short ICER isoform (Figure S2A). The two exons spanning P box region (red box) is shared by the CREMα subfamily (CREMα, CREMτ, CREMβ), but not the short ICER isoform. Thus, we chose to delete the P box section of the gene using CRISPR/Cas9 genome editing technology (Figure S2B–E) and generated three CREM-deficient CD4+ Jurkat T cell lines: 2G2, 1D4, and 2E12. Indeed, under resting conditions and in response to stimulation with plate-bound CD3 and CD28 antibodies, CREMα was expressed in control Jurkat T cells (Cas9), while CREM-deficient clones 2G2, 1D4 and 2E12 failed to express CREMα mRNA (Figure 2E). Furthermore, also DUSP4A mRNA expression was reduced in CREM-deficient cell lines after stimulation (Figure 2F), while DUSP4B (which is only expressed at low copy numbers in T cells) expression was not significantly affected (Figure 2G,H).
CREMα fails to trans-activate DUSP4 –
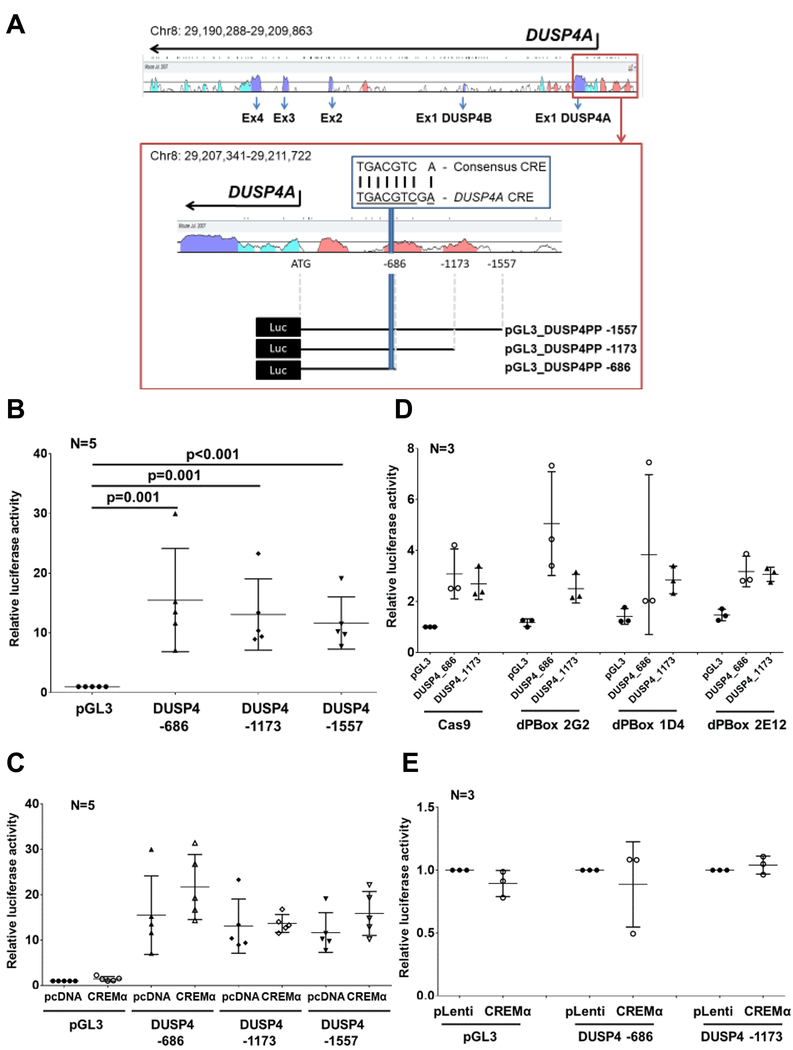
CREMα is a transcription factor that trans-regulates a number of genes involved in the molecular pathophysiology of SLE. Thus, we aimed to test whether CREMα trans-activates DUSP4. The DUSP4 gene is located on chromosome 8 and contains 5 exons (Figure 3A). As a result of alternative splicing, DUSP4A and DUSP4B consist of 4 exons with an alternative exon 1 specific to each isoform. The DUSP4 proximal promoter harbors one almost completely conserved consensus CREM recruitment element (CRE). Based on these observations, we generated three luciferase reporter constructs of different lengths, all including the CRE element. All reported constructs exhibited increased luciferase activity when compared to empty plasmids (Figure 3B). Overexpression of CREMα through co-transfection of CREMα expression plasmids, however, did not affect promoter activity of any of the reporter constructs (Figure 3C), and luciferase activity of DUSP4 promoter constructs was not altered when transfected in CREM-deficient (Figure 3D) or CREMα overexpressing (Figure 3E) cell lines as compared to controls. These observations suggest that CREMα does not trans-regulate the DUSP4 proximal promoter, but rather induces DUSP4 mRNA expression through other mechanisms.
Figure 3: CREMα does not trans-activate the DUSP4 promoter.
A) The DUSP4 gene is located on chromosome 8 and contains 5 exons. As a result of alternative splicing, DUSP4A and DUSP4B consist of 4 exons. The highly conserved DUSP4 proximal promoter (red box) harbors one almost complete consensus CREM recruitment element (CRE). We generated three luciferase reporter constructs of different lengths, all including the CRE. B) All reported constructs exhibit increased luciferase activity when compared to empty plasmids. C) Overexpression of CREMα through co-transfection of CREMα expression plasmids does not affect promoter activity of any of the three constructs. D) Luciferase activity of DUSP4 promoter constructs was not altered in CREM-deficient (D) or CREMα overexpressing (E) cell lines as compared to controls. In figures B-E means and standard deviations are displayed.
CREMα induces histone acetylation at the DUSP4 promoter –
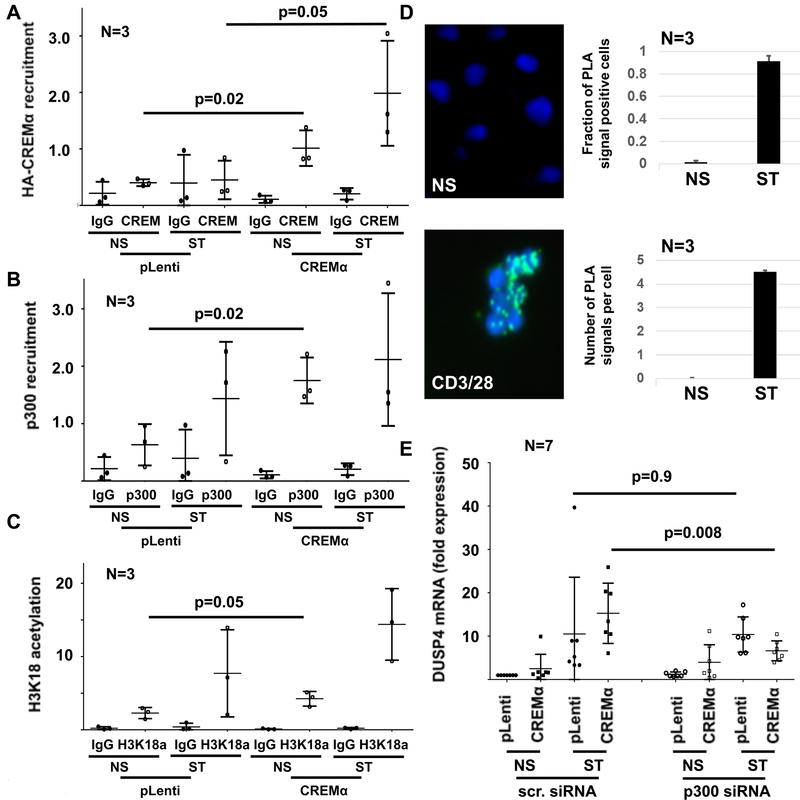
Because CREMα can interact with other proteins thereby orchestrating epigenetic events that regulate gene expression, we asked whether CREMα interacts with the transcriptional co-activator p300 and thereby induces histone H3K18 acetylation(4). Indeed, we found that the transcriptional co-activator p300 is recruited to the same region in the DUSP4 promoter as CREMα (Figure 4A,B), and overexpression of CREMα coincided with increased H3K18ac (Figure 4C). To further establish an interaction between CREMα and p300, we performed proximity ligation assays and protein co-immunoprecipitation, which identified interactions between CREMα and p300 in response to stimulation of primary human CD4+ T cells with plate-bound CD3 and CD28 antibodies (Figure 4D, figure S2F). To determine the functional interactions between CREMα and p300, we transfected wild-type (pLenti) and CREMα overexpressing cells with control (scrambled) siRNA or p300 siRNA and monitored DUSP4 mRNA expression (Figure 4E). Silencing of p300 in CREMα overexpressing cells resulted in reduced expression of DUSP4 when compared to control (untransfected or transfected with scrambled control siRNA) cells further confirming the interaction between CREMα and p300.
Figure 4: CREMα recruits p300 to the DUSP4 promoter.
A) Overexpression of HA-CREMα results in recruitment to the DUSP4 promoter as assessed by ChIP (NS: not stimulated; ST: stimulated with plate-bound andi-CD3 and anti-CD28 antibodies). B) The transcriptional co-activator p300 recruits to the same region in the DUSP4 promoter. C) Since p300 acts as histone acetyl transferase that can mediate H3K18 acetylation, this epigenetic modification was tested in the same region. CREMα overexpressing cells exhibit increased H3K18ac at the DUSP4 promoter. D) Proximity ligation assays (PLA) indicate interactions between CREMα and p300 in response to stimulation of primary human CD4+ T cells with plate-bound anti-CD3 and anti-CD28 antibodies. Displayed are results from 3 independent experiments representing a total of 246 unstimulated (NS) and 86 stimulated (ST) cells. The left panel shows PLA signals (green) in NS and CD3/CD28 ST CD4+ T cells as indicated; the right panel displays the fraction of PLA signal positive out of all CD4+ T cells (top) and the median number of signals per cell (bottom). E) Knock-down of p300 in CREMα overexpressing cells resulted in reduced mRNA expression of DUSP4 when compared to controls (untransfected or transfected with scrambled control siRNA; scr. siRNA) suggesting functional interactions between CREMα and p300 (qRT-PCR). In all figures mean values and standard deviations are displayed.
CREMα and DUSP4 control cytokine expression in an antithetic manner –
To establish whether increased DUSP4 expression indeed contributes to altered cytokine expression, we overexpressed DUSP4A or DUSP4B in Jurkat CD4+ T cells and in primary human CD4+ T cells using expression plasmids (Figure S3A). Increased expression of DUSP4 resulted in significantly reduced IL-2 mRNA expression and a trend towards increased IL-17A expression (Figure 5A,B, figure S3B,C) without reaching statistical significance. This was reflected by IL-2 and IL-17A mRNA expression patterns in CREMα overexpressing CD4+ Jurkat T cell lines (Figure 5C,D). In agreement with these observations, CREM-deficient cell lines 2G2, 1D4 and 2E12 exhibited increased IL-2 and reduced IL-17A mRNA expression (Figure 5E,F) indicating that both CREMα and, most likely as a direct result, DUSP4 are involved in the regulation of IL-2 and IL-17A expression.
Figure 5: Altered CREMα or DUSP4 expression affect cytokine expression.
Overexpression of DUSP4A or DUSP4B using expression plasmids result in significantly reduced IL-2 (A) and a trend towards increased IL-17A (B) mRNA expression in Jurkat CD4+ T cells (qRT-PCR). Because of variation of results, IL-17A mRNA expression is displayed using a logarithmic scale. This is reflected by IL-2 (C) and IL-17A (D) expression patterns in lentiviral cells with stable CREMα overexpression (NS: not stimulated; ST: stimulated with plate-bound anti-CD3 and anti-CD28 antibodies) (quantitative mRNA probe-based assays). CREM-deficient cells on the other hand exhibit increased IL-2 (E) and reduced IL-17A (F) expression (quantitative mRNA probe-based assays). In all figures means and standard deviations are displayed.
CD4+ T cells from patients with jSLE exhibit effector phenotypes –
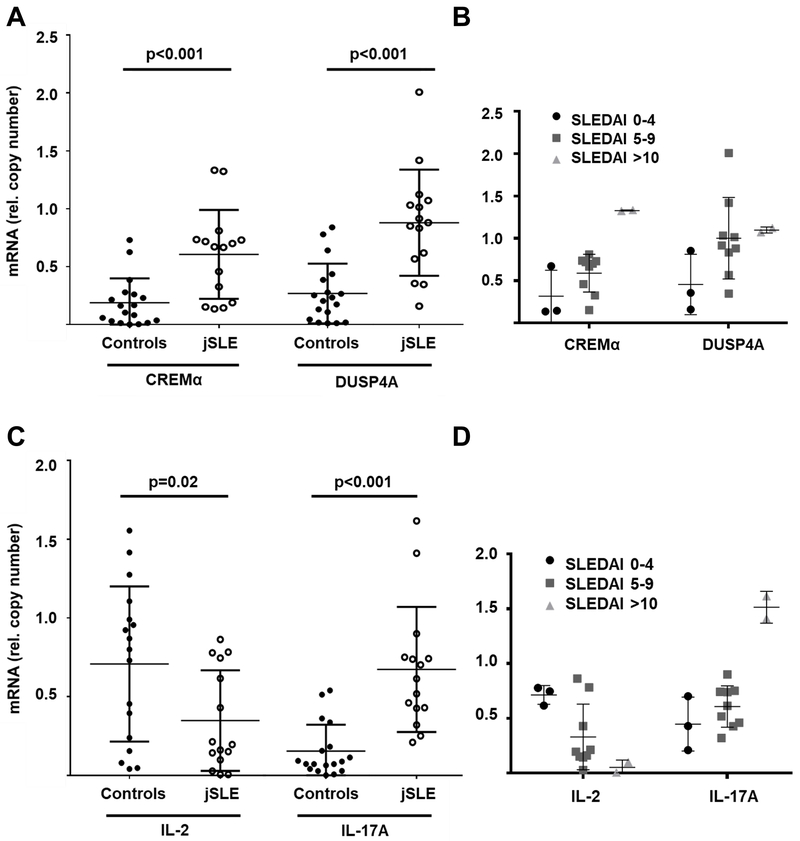
To investigate whether the aforementioned mechanisms have translational value, we isolated CD4+ T cells from patients with juvenile-onset SLE (Median age: 16 years; N=14) and age and gender matched healthy controls (Median age: 15 years; N=17) (Table S1). Ex vivo isolated CD4+ T cells exhibit increased expression of CREM, DUSP4A, IL-17A mRNA as assessed by Affymetrix QuantiGene assays, while failing to express IL-2 (Figure 6A,C). Grouping of patient samples based on SLEDAI scores (“low”: 0–4; “medium”: 5–9; “high”: >10) suggests associations of higher disease activity with increased CREMα and DUSP4 expression, reduced IL-2 and increased IL-17A expression (Figure 6B,D).
Figure 6: CD4+ T cells from jSLE patients exhibit altered gene expression.
Ex vivo isolated CD4+ T cells from post pubertal patients with jSLE (N=14) exhibit increased expression of CREM isoforms (A), DUSP4A (A), and IL-17A (C) when compared to cells from matched healthy controls (N=17). At the same time, CD4+ T cells from jSLE patients fail to express IL-2 (C), reflecting effector T cell phenotypes previously reported in adult SLE cohorts (quantitative mRNA probe-based assays). Grouping of patient samples based on SLEDAI scores (B,D) (“low”: 0–4; “medium”: 5–9; “high”: >10) suggests associations of higher disease activity with increased CREMα and DUSP4 expression, reduced IL-2 and increased IL-17A expression.
Discussion
The CREM transcription factor family includes more than 50 known isoforms that are involved in various biological functions, including spermatogenesis, adrenal/pituitary cell physiology and T lymphocyte differentiation and activation(10). We and others reported previously that the CREMα isoform is expressed at increased levels in T cells from patients with SLE(9, 11, 29, 30). Indeed, CREMα expression follows disease activity and contributes to altered T cell phenotypes in SLE, including increased IL-17A and reduced IL-2 expression(9–15, 29). CREMα instructs trans-activation of the IL17A promoter and epigenetic “opening” of the IL17 cluster(12–14), while it trans-represses IL2 and mediates epigenetic silencing through its interactions with HDAC1 and DNMT3a(12, 15–18). However, details of molecular events mediated by CREMα, particularly the exact reasons for diametric effects on IL-2 and IL17A expression, remain unknown. Advance on this question is complicated by the multitude of aforementioned CREM isoforms and the fact that knock-out of CREM in animals affected all isoforms, including CREMτ which is essential for reproduction resulting in significant breeding problems(10, 31, 32). To overcome some of these limitations, we generated genetically modified Jurkat CD4+ T cells. CREMα overexpressing cells were generated using stable lentiviral transduction; CREMα-deficient cells were generated through CRISPR/Cas9-mediated biallelic deletion of the CREM P box region(10). The constructed novel Jurkat CD4+ T cells represent valuable new tools for detailed investigation of CREMα and its molecular effects on T cell biology and gene regulation.
Results from this study link increased expression of CREMα with the promoter activity of the protein phosphatase DUSP4. The protein phosphatase DUSP4 is involved in fine tuning of STAT5 phosphorylation and activity, and DUSP4 deficiency mediates increased STAT5 phosphorylation, IL-2 expression, and regulatory T cell functions(33). Furthermore, DUSP4 affects T helper cell polarization, away from Treg and towards Th17 phenotypes(19, 20). This may be mediated through destabilization of STAT5 by DUSP4 and result in an imbalance between phosphorylated STAT5 and STAT3(19), a hallmark of T cells from SLE patients(1, 12–15). In agreement with these reports, increased expression of DUSP4 in genetically modified (CREMα overexpressing) cells and in response to transfection of primary human CD4+ T cells with DUSP4 expression plasmids correlated with significantly reduced IL-2 and a trend towards increased Il-17A expression, hallmarks of effector T cells. Lastly, increased DUSP4 expression promotes T cell senescence and lymphopenia(34) which represents an additional cellular feature in SLE(35–37). These observations in conjunction with findings from this study add DUSP4 to the growing list of genes and molecules involved in effector T cell development and activation, and the pathophysiology of SLE.
The association of CREMα with the DUSP4 promoter and resulting molecular events are of particular interest, since they provide another example for the transcription factor CREMα orchestrating epigenetic events in a region- and target-specific manner rather than exercising “classical” transcription factor effects, namely trans-activation. Indeed, DUSP4 promoter activity is not regulated through CREMα-mediated trans-activation, but the induction of epigenetic remodeling through the targeted recruitment of the transcriptional co-activator p300, a histone acetyltransferase. Indeed, interactions between several transcription factors and p300 have been suggested and discussed at other genes involved in the molecular pathophysiology of SLE, including the immune regulatory cytokine IL-10(27), IL-2(11), and IL-17A(4). Thus, data presented support the hypothesis that CREMα itself as well as other previously reported transcription factors involved in the molecular pathophysiology of SLE, including the STAT transcription factor family, interact with epigenetic modifiers thereby orchestrating epigenetic remodeling, chromatin accessibility and gene expression(27). These observations deliver arguments for the development and investigation of inhibitors of epigenetic modifiers and/or transcription factors, or medications that inhibit interactions between transcription factors and epigenetic modifiers for the treatment of autoimmune/inflammatory conditions.
The question of whether CREMα-mediated mechanisms are involved in physiological effector T cell differentiation or whether they are limited to not naïve effector T cells in systemic autoimmune/inflammatory conditions that are characterized by increased CREMα expression (such as SLE) remains incompletely answered and is beyond the scope of the this project. The observation that effects of increased CREMα as well as DUSP4 expression on reduced IL-2 production are more striking as compared to their capacity to increase IL-17A expression in Jurkat CD4+ T cells may be discussed as an argument for their effects non-naïve CD4+ T cells that already exhibit effector phenotypes and allow some extent of IL-17A expression. On the other hand, CREMα and DUSP4 expression are increased in effector T helper cell populations when compared to naïve CD4+ T cells and closely correlate suggest that CREMα-mediated upregulation of DUSP4 may be involved in CD4+ T helper cell differentiation. Final evidence for or against the hypothesis that CREMα may play a role also during Th17 (or other effector T cell subset, such as Th1) differentiation from naïve T cells, however, cannot be provided using the CD4+ Jurkat T cell system generated in this study, and new CREMα subfamily specific constitutional knock-out systems, e.g. in mice, may be necessary to reliably answer this question.
The role of imbalanced IL-2 and IL-17A expression in the pathophysiology of adult-onset SLE and resulting tissue damage has been established(1, 12–15). Here, we chose to analyze CD4+ T cells from patients with juvenile-onset SLE, since pediatric patients exhibit more severe phenotypes with increased tissue and organ damage already at the time of diagnosis, higher need for immune suppressive treatment, and lastly exhibit fewer co-existing morbidities (such as arteriosclerosis, hypercholesterinemia, etc.) which may affect effector T cell phenotypes and inflammatory responses(22). Patients enrolled exhibited SLEDAI scores between 4 and 17 to cover a population with mild to severe disease activity. Patients enrolled were treated low-dose corticosteroids, hydroxychloroquine (HCQ), and or azathioprine (AZA) or mycophenolate mofetil (MMF). Indeed, MMF can inhibit HDAC activity thereby potentially contributing to increased histone acetylation(38). In breast cancer cell lines, HCQ can induce histone acetylase activity thereby increasing histone acetylation(39). Since healthy controls were not treated with these mediactions, it cannot be excluded completely that these medications partially contributed to histone acetylation and increased gene expression of DUSP4. To avoid further potential bias, patients on treatment with cyclophosphamide or B cell depleting drugs were excluded. Of note, in the predominantly Caucasian UK jSLE cohort, cyclophosphamide is used less frequently in children with lupus-nephritis as compared to adult populations(40). To our knowledge, this is the first study to investigate molecular mechanisms contributing to effector T cell phenotypes and reduced IL-2 and increased IL-17A expression in patients with jSLE. We limited this study to jSLE patients with disease-onset after puberty, who, based on the similar gender distribution and auto-antibody patterns in this age group when compared to adults, likely share molecular lymphocyte phenotypes with adult-onset patients. Direct comparisons between samples from patients with “post-pubertal” SLE vs. patients with very early disease-onset in the first decade of life vs. adult-onset SLE as well as healthy and disease controls in all age groups are currently pending and will be the focus of future studies. While such efforts are challenging, they will help to better understand the contribution of effector T cells and differential pathogenetic mechanisms across age groups.
Observations from the study presented suggests the previously not reported CREMα/DUSP4A axis as central regulatory mechanisms in effector T cell activation and (potentially) differentiation. This makes both molecules promising candidates in the search for disease biomarkers and therapeutic targets in SLE, but also other disorders characterized by increased CREMα expression and effector T cell involvement.
Supplementary Material
Key messages.
CREMα induces DUSP4 phosphatase expression in CD4+ T cells
DUSP4 promotes IL-17A and reduces IL-2 production by effector T cells
The CREMα/DUSP4 axis is involved in the pathophysiology of SLE
Acknowledgements
We wish to thank patients, families and healthy controls for their support and consent to donating blood for experiments. The authors thank Sebastian Thieme, Department of Pediatrics, Universitätsklinikum Carl Gustav Carus, TU Dresden, Germany, for helpful discussion and donation of pL.SSVF_XbaI_GFP_MluI.i_gb3.BspEI.Hygro.str vectors. Financial support was provided by the Fritz-Thyssen Foundation and the Institute of Translational Medicine, University of Liverpool (to C.M.H.). This work was supported by the UK’s Experimental Arthritis Treatment Centre for Children (supported by Versus Arthritis, Alder Children’s NHS Foundation Trust, the Alder Hey Charity, and the University of Liverpool) and partially carried out at the National Institute for Health Research (NIHR) Alder Hey Clinical Research Facility. The views expressed are those of the authors and not necessarily those of the Fritz-Thyssen Foundation, NHS, the NIHR or the Department of Health.
Funding information
The main body of work was supported by the Fritz-Thyssen-Foundation (to C.M.H.). C.M.H. received additional support from LUPUS UK, FAIR (funding autoimmune research), the Hugh Greenwood Legacy Fund, and the Institute of Translational Medicine, University of Liverpool. S.R.H. received support from the German Research Foundation (DFG-KFO249, HO4510/1–2 and the Roland Ernst Stiftung für Gesundheitswesen (3/17). G.T.C was supported by the National Institutes of Health (R37 AI 49954).
Abbreviations
- cAMP
Cyclic adenosine monophosphate
- CD
Cluster of differentiation
- ChIP
Chromatin immunoprecipitation
- Co-IP
Co-immunoprecipitation
- CyTOF
Cytometry time of flight mass spectrometry
- CREM
cAMP response element modulator
- DNA
Deoxynucleic acid
- DNMT
DNA methyltransferase
- DUSP4
dual specificity protein phosphatase 4
- H3K18ac
Histone H3 Lysine 18 acetylation
- HDAC
Histone deacetylase
- ICER
Inducible cAMP early repressor
- IL
Interleukin
- jSLE
juvenile-onset systemic lupus erythematosus
- RNA
Ribonucleic acid
- siRNA
short inhibiting RNA
- SLE
Systemic lupus erythematosus
- STAT
Signal transducer and activator of transcription
- Th
T helper cell
Footnotes
Competing interests
The authors report no conflict of interest relevant to this manuscript.
Ethical approval:
The UK JSLE Cohort Study and Repository was approved by the North West Liverpool East Research Ethics Committee (REC: 6/Q1502/77). Patients and controls were recruited through the Alder Hey Children’s NHS Foundation trust Department of Rheumatology. Patients, controls or their legal guardians gave written informed assent and/or consent.
References
- 1.Crispin JC, Hedrich CM, Suarez-Fueyo A, Comte D, Tsokos GC. SLE-Associated Defects Promote Altered T Cell Function. Crit Rev Immunol. 2017;37(1):39–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tsokos GC. Systemic lupus erythematosus. N Engl J Med. 2011;365(22):2110–21. [DOI] [PubMed] [Google Scholar]
- 3.Tsokos GC, Lo MS, Costa Reis P, Sullivan KE. New insights into the immunopathogenesis of systemic lupus erythematosus. Nat Rev Rheumatol. 2016;12(12):716–30. [DOI] [PubMed] [Google Scholar]
- 4.Hedrich CM. Mechanistic aspects of epigenetic dysregulation in SLE. Clin Immunol. 2018;196:3–11. [DOI] [PubMed] [Google Scholar]
- 5.Hedrich CM, Crispin JC, Tsokos GC. Epigenetic regulation of cytokine expression in systemic lupus erythematosus with special focus on T cells. Autoimmunity. 2014;47(4):234–41. [DOI] [PubMed] [Google Scholar]
- 6.Apostolidis SA, Crispin JC, Tsokos GC. IL-17-producing T cells in lupus nephritis. Lupus. 2011;20(2):120–4. [DOI] [PubMed] [Google Scholar]
- 7.Crispin JC, Tsokos GC. Interleukin-17-producing T cells in lupus. Curr Opin Rheumatol. 2010;22(5):499–503. [DOI] [PubMed] [Google Scholar]
- 8.Lieberman LA, Tsokos GC. The IL-2 defect in systemic lupus erythematosus disease has an expansive effect on host immunity. J Biomed Biotechnol. 2010;2010:740619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kyttaris VC, Wang Y, Juang YT, Weinstein A, Tsokos GC. CAMP response element modulator a expression in patients with systemic lupus erythematosus. Lupus. 2006;15(12):840–4. [DOI] [PubMed] [Google Scholar]
- 10.Rauen T, Hedrich CM, Tenbrock K, Tsokos GC. cAMP responsive element modulator: a critical regulator of cytokine production. Trends Mol Med. 2013;19(4):262–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Solomou EE, Juang YT, Gourley MF, Kammer GM, Tsokos GC. Molecular basis of deficient IL-2 production in T cells from patients with systemic lupus erythematosus. J Immunol. 2001;166(6):4216–22. [DOI] [PubMed] [Google Scholar]
- 12.Hedrich CM, Crispin JC, Rauen T, Ioannidis C, Apostolidis SA, Lo MS, et al. cAMP response element modulator alpha controls IL2 and IL17A expression during CD4 lineage commitment and subset distribution in lupus. Proc Natl Acad Sci U S A. 2012;109(41):16606–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hedrich CM, Rauen T, Kis-Toth K, Kyttaris VC, Tsokos GC. cAMP-responsive element modulator alpha (CREMalpha) suppresses IL-17F protein expression in T lymphocytes from patients with systemic lupus erythematosus (SLE). J Biol Chem. 2012;287(7):4715–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rauen T, Hedrich CM, Juang YT, Tenbrock K, Tsokos GC. cAMP-responsive element modulator (CREM)alpha protein induces interleukin 17A expression and mediates epigenetic alterations at the interleukin-17A gene locus in patients with systemic lupus erythematosus. J Biol Chem. 2011;286(50):43437–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hedrich CM, Rauen T, Tsokos GC. cAMP-responsive element modulator (CREM)alpha protein signaling mediates epigenetic remodeling of the human interleukin-2 gene: implications in systemic lupus erythematosus. J Biol Chem. 2011;286(50):43429–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tenbrock K, Juang YT, Gourley MF, Nambiar MP, Tsokos GC. Antisense cyclic adenosine 5’-monophosphate response element modulator up-regulates IL-2 in T cells from patients with systemic lupus erythematosus. J Immunol. 2002;169(8):4147–52. [DOI] [PubMed] [Google Scholar]
- 17.Tenbrock K, Juang YT, Leukert N, Roth J, Tsokos GC. The transcriptional repressor cAMP response element modulator alpha interacts with histone deacetylase 1 to repress promoter activity. J Immunol. 2006;177(9):6159–64. [DOI] [PubMed] [Google Scholar]
- 18.Tenbrock K, Juang YT, Tolnay M, Tsokos GC. The cyclic adenosine 5’-monophosphate response element modulator suppresses IL-2 production in stimulated T cells by a chromatin-dependent mechanism. J Immunol. 2003;170(6):2971–6. [DOI] [PubMed] [Google Scholar]
- 19.Hsiao WY, Lin YC, Liao FH, Chan YC, Huang CY. Dual-Specificity Phosphatase 4 Regulates STAT5 Protein Stability and Helper T Cell Polarization. PLoS One. 2015;10(12):e0145880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yan D, Farache J, Mingueneau M, Mathis D, Benoist C. Imbalanced signal transduction in regulatory T cells expressing the transcription factor FoxP3. Proc Natl Acad Sci U S A. 2015;112(48):14942–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Laurence A, Tato CM, Davidson TS, Kanno Y, Chen Z, Yao Z, et al. Interleukin-2 signaling via STAT5 constrains T helper 17 cell generation. Immunity. 2007;26(3):371–81. [DOI] [PubMed] [Google Scholar]
- 22.Hedrich CM, Smith EMD, Beresford MW. Juvenile-onset systemic lupus erythematosus (jSLE) - Pathophysiological concepts and treatment options. Best Pract Res Clin Rheumatol. 2017;31(4):488–504. [DOI] [PubMed] [Google Scholar]
- 23.Hochberg MC. Updating the American College of Rheumatology revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. 1997;40(9):1725. [DOI] [PubMed] [Google Scholar]
- 24.Ugarte F, Ryser M, Thieme S, Fierro FA, Navratiel K, Bornhauser M, et al. Notch signaling enhances osteogenic differentiation while inhibiting adipogenesis in primary human bone marrow stromal cells. Exp Hematol. 2009;37(7):867–75 e1. [DOI] [PubMed] [Google Scholar]
- 25.Ran FA, Hsu PD, Wright J, Agarwala V, Scott DA, Zhang F. Genome engineering using the CRISPR-Cas9 system. Nat Protoc. 2013;8(11):2281–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hedrich CM, Crispin JC, Rauen T, Ioannidis C, Koga T, Rodriguez Rodriguez N, et al. cAMP responsive element modulator (CREM) alpha mediates chromatin remodeling of CD8 during the generation of CD3+ CD4- CD8- T cells. J Biol Chem. 2014;289(4):2361–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hedrich CM, Rauen T, Apostolidis SA, Grammatikos AP, Rodriguez Rodriguez N, Ioannidis C, et al. Stat3 promotes IL-10 expression in lupus T cells through trans-activation and chromatin remodeling. Proc Natl Acad Sci U S A. 2014;111(37):13457–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yoshida N, Comte D, Mizui M, Otomo K, Rosetti F, Mayadas TN, et al. ICER is requisite for Th17 differentiation. Nat Commun. 2016;7:12993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rauen T, Benedyk K, Juang YT, Kerkhoff C, Kyttaris VC, Roth J, et al. A novel intronic cAMP response element modulator (CREM) promoter is regulated by activator protein-1 (AP-1) and accounts for altered activation-induced CREM expression in T cells from patients with systemic lupus erythematosus. J Biol Chem. 2011;286(37):32366–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang Q, Ding S, Zhang HL. [Effect of aberrant H3K27me3 modification in promoter regions on cAMP response element modulator alpha expression in CD4(+) T cells from patients with systemic lupus erythematosus]. Nan Fang Yi Ke Da Xue Xue Bao. 2017;37(12):1597–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhou Y, Sun Z, Means AR, Sassone-Corsi P, Bernstein KE. cAMP-response element modulator tau is a positive regulator of testis angiotensin converting enzyme transcription. Proc Natl Acad Sci U S A. 1996;93(22):12262–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nagamori I, Yomogida K, Adams PD, Sassone-Corsi P, Nojima H. Transcription factors, cAMP-responsive element modulator (CREM) and Tisp40, act in concert in postmeiotic transcriptional regulation. J Biol Chem. 2006;281(22):15073–81. [DOI] [PubMed] [Google Scholar]
- 33.Huang CY, Lin YC, Hsiao WY, Liao FH, Huang PY, Tan TH. DUSP4 deficiency enhances CD25 expression and CD4+ T-cell proliferation without impeding T-cell development. Eur J Immunol. 2012;42(2):476–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bignon A, Regent A, Klipfel L, Desnoyer A, de la Grange P, Martinez V, et al. DUSP4-mediated accelerated T-cell senescence in idiopathic CD4 lymphopenia. Blood. 2015;125(16):2507–18. [DOI] [PubMed] [Google Scholar]
- 35.Calhoun C, Shivshankar P, Saker M, Sloane LB, Livi CB, Sharp ZD, et al. Senescent Cells Contribute to the Physiological Remodeling of Aged Lungs. J Gerontol A Biol Sci Med Sci. 2016;71(2):153–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chen Y, Gorelik GJ, Strickland FM, Richardson BC. Decreased ERK and JNK signaling contribute to gene overexpression in “senescent” CD4+CD28- T cells through epigenetic mechanisms. J Leukoc Biol. 2010;87(1):137–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liu Y, Chen Y, Richardson B. Decreased DNA methyltransferase levels contribute to abnormal gene expression in “senescent” CD4(+)CD28(−) T cells. Clin Immunol. 2009;132(2):257–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yang Y, Tang Q, Zhao M, Liang G, Wu H, Li D, et al. The effect of mycophenolic acid on epigenetic modifications in lupus CD4+T cells. Clin Immunol. 2015;158(1):67–76. [DOI] [PubMed] [Google Scholar]
- 39.Rahim R, Strobl JS. Hydroxychloroquine, chloroquine, and all-trans retinoic acid regulate growth, survival, and histone acetylation in breast cancer cells. Anticancer Drugs. 2009;20(8):736–45. [DOI] [PubMed] [Google Scholar]
- 40.Smith E, Al-Abadi E, Armon K, Bailey K, Ciurtin C, Davidson J, et al. Outcomes following mycophenolate mofetil versus cyclophosphamide induction treatment for proliferative juvenile-onset lupus nephritis. Lupus. 2019;28(5):613–20. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.