Abstract
Actinomycosis is a rare granulomatous disease caused by commensal bacteria (Actinomycetaceae family) of the oropharynx, gastrointestinal, and urogenital tract. Infection most commonly involves the cervicofacial region but less frequently the abdominal region, typically secondary to a disruption of normal gastrointestinal mucosa. We present a patient with vague symptoms of fevers and myalgias and a recent diagnosis of rectal cancer. On CT, there were multiple centrally hypoattenuating hepatic lesions suspicious for metastasis vs abscesses, also confirmed by ultrasound. Initial image guided biopsy was non-diagnostic. Laparoscopic resection of one of the hepatic lesions showed pus consistent with an abscess. No organisms were identified by culture and a sample was sent to an outside laboratory for genomic polymerase chain reaction (PCR) analysis where Actinomyces DNA was isolated. This case report highlights a rare presentation of primary hepatic Actinomycosis and some of the challenges in diagnosing Actinomycosis due to its variable clinical and radiological manifestations and lack of diagnostic sensitivity by traditional microscopy and culture based techniques.
Keywords: Liver, Actinomycosis, Abscess, Metastasis, PCR
Introduction
Actinomycosis is a rare granulomatous disease caused by filamentous Gram-positive anaerobic bacteria from the Actinomycetaceae family (genus Actinomyces). These bacteria are primarily human commensal flora of the oropharynx, gastrointestinal tract, and urogenital tract. When tissue integrity is disrupted, local invasion and subsequent endogenous infection can occur. [1,2] Actinomyces israeli is the most common species isolated in humans but more than 30 species have been identified. Infection typically affects the cervicofacial region (approximately 50%), abdominopelvic region (approximately 20%), and thoracic region (approximately 15%-20%). [3] The prevalence of endogenous infection is not well established due to a paucity of data but has been quoted between 1:300,000 and 1:1,000,000. [2] In this case report, we discuss a patient presenting with primary hepatic actinomycosis mimicking metastatic disease.
Case report
A 59-year-old male presented to the emergency department (ED) with a 10-day history of intermittent fevers, chills, and myalgias. Two months prior he had been diagnosed with rectal adenocarcinoma on colonoscopy at an outside institution and was awaiting further staging with an endoscopic ultrasound. Additional past medical history includes diabetes, hypertension, and benign prostatic hyperplasia. He has a 40 pack-year smoking history.
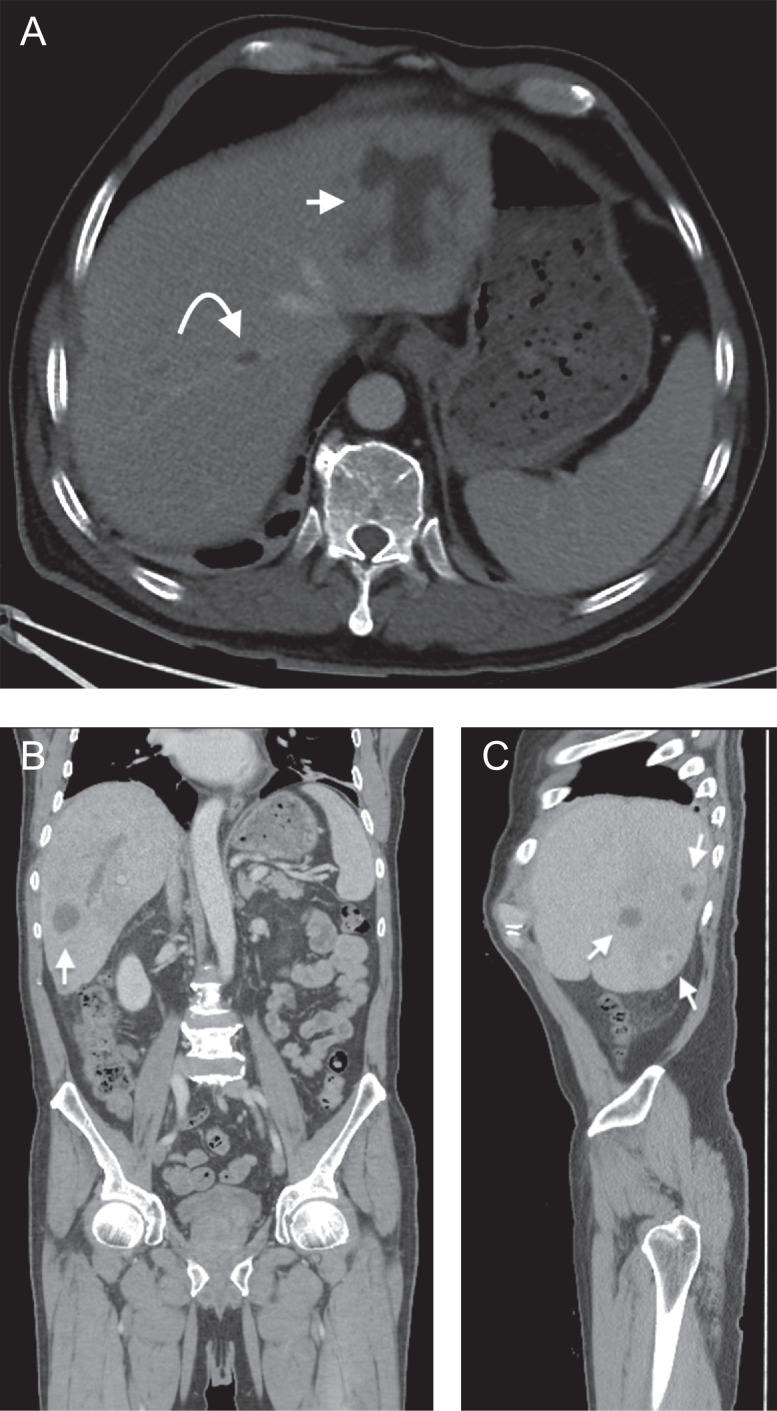
During the emergency department admission, the patient was evaluated with a computed tomography scan (CT) of the abdomen/pelvis which demonstrated numerous, approximately 8, hypoattenuating lesions within both hepatic lobes, suspicious for either malignancy or abscess formation (Fig. 1). The patient was admitted and further evaluated with ultrasound which confirmed both complex cystic and mixed cystic/solid lesions within the liver (Fig. 2A, B). Given the patients recent rectal cancer diagnosis, these were favored to represent metastases and further staging imaging of the brain and chest was performed which showed no additional suspicious metastatic lesions. Ultrasound guided liver biopsy was performed with pathology reporting no evidence of malignancy, but instead fibrous tissue with acute and chronic inflammation (Fig. 2C).
Fig. 1.
Axial (1A), coronal (1B) and sagittal (1C) contrast enhanced CT images demonstrating several of the largest hepatic lesions (arrows), many of which are centrally hypoattenuating. Some of these hypoattenuating central foci measure fluid density (<20 Hounsfield units). A right hepatic vein thrombus is also shown and likely represents septic thrombophlebitis (curved arrow).
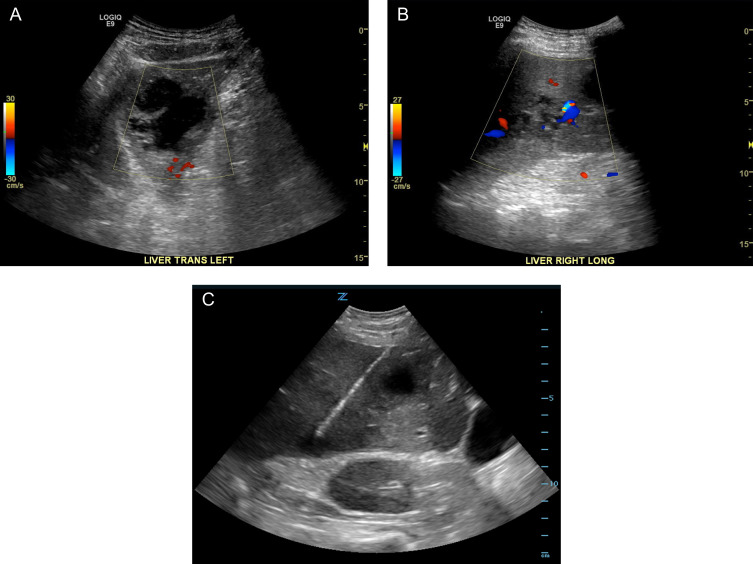
Fig. 2.
A, B – Color Doppler sonographic images of the left and right hepatic lobes demonstrating two lesions of varying complexity. On the left (A) a hypoechoic complex cystic mass with thickened irregular walls and septations is demonstrated. On the right (B) is a heterogeneously hypoechoic predominantly solid lesion with scattered areas of vascularity. C – Gray scale sonographic image acquired during subsequent fine needle aspiration targeting the peripherally solid portions of two of these lesions.
The patient continued to be febrile during the admission and a repeat CT of the abdomen/pelvis showed hyperenhancement of a markedly enlarged prostate in addition to the previous liver findings. While these findings are nonspecific, the patient additionally complained of increased urinary frequency and demonstrated tenderness to palpation on a digital rectal examination. His fevers were therefore attributed to prostatitis and he was discharged home on a short course of antibiotics with scheduled follow-up as an outpatient.
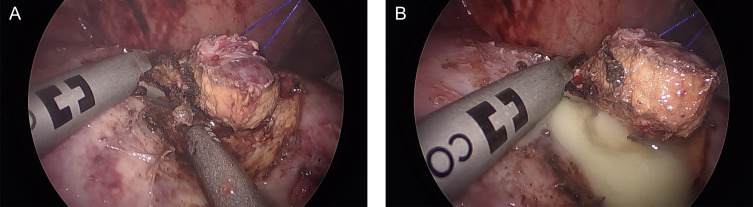
Over the next 2 months, the patient was admitted multiple times with continuous fevers and a greater than 20-pound weight loss. Eventually the patient was admitted to the ICU with sepsis. After he was stabilized, the decision was made to perform a diagnostic laparoscopy with excisional biopsy of a lesion. It was noted during extraction of a hepatic segment II lesion that pus was seen oozing from the sample (Fig. 3).
Fig. 3.
A – Laparoscopic image showing excision of hepatic segment II lesion with harmonic shears. This lesion was localized using an intraoperative ultrasound probe (not shown). B – Subsequent expulsion of pus from the excised lesion consistent with an abscess.
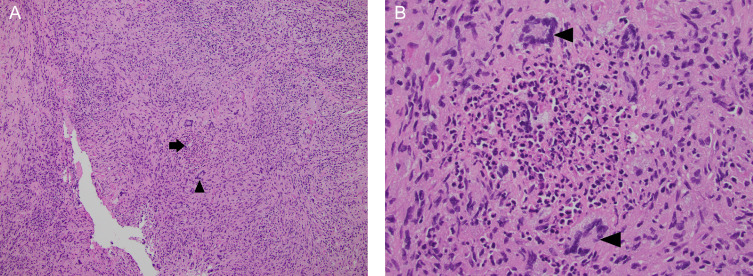
Pathology results were again negative for malignancy but demonstrated focal caseating granulomatous inflammation (Fig. 4). Tissue samples were sent to an offsite laboratory, and the infectious disease service was consulted. It was identified that the patient had a positive Quantiferon-TB test with no history of treatment for tuberculosis. The liver lesions were therefore thought secondary to disseminated reactivated tuberculosis and he was initiated on RIPE therapy and discharged for follow up.
Fig. 4.
Hematoxylin and eosin-stained sections of the liver wedge biopsy. Low power (4A, 10X) magnification reveals areas of liver architecture completely replaced by inflammatory cells and a central granuloma (arrow), characterized by multinucleated giant cells (arrowhead) surrounding an area of necrosis. At high power (4B, 40x) magnification, the necrotizing granuloma is seen as a central collection of neutrophils and cellular debris surrounded by histiocytes and abundant pink cytoplasm. Several multinucleated giant cells are also present (arrowheads). (Color version of figure is available online.)
Shortly after, external laboratory PCR results came back positive for Actinomyces DNA (A. naeslundii or A. viscosus species, not differentiated by the 16s rRNA primer set). The PCR results were negative for tuberculosis. RIPE therapy was discontinued and the patient was initiated on a typical treatment regimen of oral Amoxicillin for 6 months. [1]
Follow-up imaging demonstrated a decrease in the size and number of lesions at 3 months with near complete resolution at 6 months. The patient completed his antibiotic course with plans for additional imaging at 9 months. He eventually underwent repeat colonoscopy and MRI which revealed a T3N0 rectal tumor and was initiated on neoadjuvant radiation therapy towards the end of his 6-month antibiotic course. There was initially concern for chemotherapy induced immunosuppression with the active actinomycosis infection but the patient ultimately refused chemotherapy which was offered after completion of the antibiotic treatment regimen.
Discussion
This case represents a prime example of the difficulty in diagnosing primary actinomycosis and the multiple factors that may confound physicians. Hepatic presentation is varied with 3 general patterns: solitary, multiple, or disseminated. As in this case, these may mimic tumors or TB and generally demonstrate a hypo-enhancing center with a thickened enhancing wall. [4] Radiologic imaging can be of limited value in distinguishing between these entities. While it has been shown that infection often invades across tissue planes and boundaries in Actinomycosis, which may aid in diagnosis, this was not seen in the present case. [5]
Tissue sampling is therefore the preferred method of diagnosis. Given the patient's recent diagnosis of rectal cancer, the initial fine needle aspiration (FNA) biopsy results (inflammation without evidence of malignancy) were thought to be discordant and contributed to a delay in diagnosis of his underlying infection. And while granulomatous inflammation is a hallmark of Actinomycosis, it is nonspecific and the lack of a positive culture or specific histopathologic finding (ie “sulfur granules”) on excisional biopsy led to inappropriate treatment with antitubercular therapy. However, it has been noted that isolation and identification of Actinomyces occurs only in a minority of cases. The failure rate of culture is high often due to recent antibiotic therapy, inadequate culture conditions, or inadequate incubation time. [1] In fact, even in culture proven cases of Actinomyces, the number of sulfur granules identified on histopathologic examination is often few (1-3 granules identified in 56% of cases) to none (in approximately 4% of cases). [6]
Since granulomatous inflammation was seen in this case, samples were sent to an outside lab to further evaluate for causative organisms via PCR. The presumption was that this was tuberculosis even though acid-fast staining was negative. While this was ultimately disproven, it demonstrates the emergence of molecular techniques in diagnosing otherwise occult pathogens. 16s rRNA sequencing has proven effective in this regard and is also superior in distinguishing between the various Actinomyces species. This may have future implications regarding patient outcomes and therapies. For example, A. radingae has been associated with recurrent abscesses of the chest, back, and breast. [1, 7] However, no such specific link has been reported between the species identified in our report (A. Naeslundii vs A. Viscosus), other than their generalized association with actinomycosis.
In conclusion, this report highlights the difficulty facing physicians with making a diagnosis of hepatic actinomycosis. Even in retrospect, it is unlikely that a different treatment/diagnostic course would be recommended in this case, other than earlier performance of a diagnostic laparoscopy. This presents a conundrum for an illness with significant morbidity. Data remains limited due to the rarity of Actinomycosis, but one study reports mortality rates ranging between 0 and 28% depending on the site of infection and timing of treatment, with involvement of the CNS system reporting the worst outcomes. [2] And while this patient faced no such consequence, there was postponed treatment of his known rectal cancer with radiation therapy due to the concern for metastatic disease and delayed staging. Even after the causative organism was identified, it is worth noting that antibiotic therapy for actinomycosis is typically recommended for over 6 months.[1] There was concern for concomitant treatment with antibiotics and systemic chemotherapy due to immunosuppression with an active infection. The patient was therefore offered chemotherapy after resolution of his hepatic actinomycosis, which he ultimately refused.
Footnotes
Competing Interests: None.
References
- 1.Valour F., Senechal A., Dupieux C., Karsenty J., Lustig S., Breton P. Actinomycosis: etiology, clinical features, diagnosis, treatment, and management. Infect Drug Resist. 2014;7:183–197. doi: 10.2147/IDR.S39601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wong V.K., Turmezei T.D., Weston V.C. Actinomycosis. BMJ. 2011;343:d6099. doi: 10.1136/bmj.d6099. [DOI] [PubMed] [Google Scholar]
- 3.Weese W.C., Smith I.M. A study of 57 cases of actinomycosis over a 36-year period. A diagnostic 'failure' with good prognosis after treatment. Arch Intern Med. 1975;135(12):1562–1568. [PubMed] [Google Scholar]
- 4.Liu C.H., Yu C.Y., Catalano O., Mueller P. Radiological reasoning: imaging differentiation of a solitary hepatic mass. AJR Am J Roentgenol. 2008;190(6 Suppl):S57–S61. doi: 10.2214/AJR.07.7005. [DOI] [PubMed] [Google Scholar]
- 5.Ha H.K., Lee H.J., Kim H., Ro H.J., Park Y.H., Cha S.J. Abdominal actinomycosis: CT findings in 10 patients. AJR Am J Roentgenol. 1993;161(4):791–794. doi: 10.2214/ajr.161.4.8372760. [DOI] [PubMed] [Google Scholar]
- 6.Brown J.R. Human actinomycosis. A study of 181 subjects. Hum Pathol. 1973;4(3):319–330. doi: 10.1016/s0046-8177(73)80097-8. [DOI] [PubMed] [Google Scholar]
- 7.Clarridge J.E., 3rd, Zhang Q. Genotypic diversity of clinical Actinomyces species: phenotype, source, and disease correlation among genospecies. J Clin Microbiol. 2002;40(9):3442–3448. doi: 10.1128/JCM.40.9.3442-3448.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]