Abstract
Pulmonary hypertension (PH) is a debilitating disease characterized by remodeling of the lung vasculature. In rodents, resistin-like molecule-α (RELMα, also known as HIMF or FIZZ1) can induce PH, but the signaling mechanisms are still unclear. In this study, we used human lung samples and a hypoxia-induced mouse model of PH. We found that the human homolog of RELMα, human (h) Resistin, is upregulated in macrophage-like inflammatory cells from lung tissues of patients with idiopathic PH. Additionally, at PH onset in the mouse model, we observed RELMα-dependent lung accumulation of macrophages that expressed high levels of the key damage-associated molecular pattern (DAMP) molecule high-mobility group box 1 (HMGB1) and its receptor for advanced glycation end products (RAGE). In vitro, RELMα/hResistin induced macrophage-specific HMGB1/RAGE expression and facilitated HMGB1 nucleus-to-cytoplasm translocation and extracellular secretion. Mechanistically, hResistin promoted HMGB1 post-translational lysine acetylation by preserving the NAD+-dependent deacetylase sirtuin (Sirt) 1 in human macrophages. Notably, the hResistin-stimulated macrophages promoted apoptosis-resistant proliferation of human pulmonary artery smooth muscle cells in an HMGB1/RAGE-dependent manner. In the mouse model, RELMα also suppressed Sirt1 signal in pulmonary macrophages in the early post-hypoxic period. Notably, recruited macrophages in the lungs of these mice carried the RELMα binding partner Bruton’s tyrosine kinase (BTK). hResistin also mediated the migration of human macrophages by activating BTK in vitro. Collectively, these data reveal a vascular-immune cellular interaction in the early PH stage and suggest that targeting RELMα/DAMP-driven macrophages may offer a promising strategy to treat PH and other related vascular inflammatory diseases.
Keywords: Resistin, HIMF, FIZZ1, DAMP, hypoxia, inflammation
Introduction
Resistin was first identified as an adipokine with insulin resistance properties in mice (1). To date several resistin-like molecule (RELM) family members in rodents and humans have been found to have pro-inflammatory activities (2, 3). Using gene array technology, we have revealed that RELMα (gene symbol: Retnla),which is also known as “hypoxia-induced mitogenic factor (HIMF)” (4) or “found in inflammatory zone (FIZZ) 1” (5), was upregulated in the lungs of mice with hypoxia-induced pulmonary hypertension (PH) (4). RELMα has potent mitogenic effects (4) and is critical to PH development in rodent models (6). Several PH-related downstream vascular and immune processes activated by RELMα or its human (h) homologue resistin (hResistin; gene symbol: RETN) have been identified (4, 6-16), but the manner in which RELMα/hResistin initiates the inflammatory responses under pathologic conditions is unknown. We have showed that the calcium-binding S100 family protein S100A11 can mediate RELMα-induced pulmonary artery smooth muscle cell (PASMC) migration (15). Given that S100 proteins are also known to bind to the receptor for advanced glycation end products (RAGE), a key receptor for mediating the signaling of damage-associated molecular pattern (DAMP), RELMα/hResistin activation might link damage sensing after hypoxic injury to the earliest molecular events in vascular inflammation.
The key DAMP molecule, high-mobility group box (HMGB) 1, is a DNA-binding nuclear protein that is released actively after cytokine stimulation and passively during cell death (17). HMGB1 is a pro-inflammatory cytokine and a pro-angiogenic factor that signals through RAGE (17). Active secretion of HMGB1 requires that it be shuttled from the nucleus into the cytosol. This translocation is dependent on the post-translational acetylation of HMGB1 and results in cytosolic accumulation of the protein (17, 18). Reports have suggested that after secretion, HMGB1 may be an angiogenetic switch molecule induced by hypoxia (19). However, we do not yet know whether and how RELMα/hResistin activates DAMP or regulates its intracellular trafficking and extracellular activities. Nor do we understand the function of the RELMα-DAMP signaling axis in the context of vascular remodeling.
Macrophages/monocytes, the primary source of RELMα/hResistin and HMGB1 under inflammatory conditions (2), are central to the endogenous danger signal response in a variety of angiogenesis-related pathologies, including PH (11, 17, 20). As the innate immune cells, macrophages possess phenotypic plasticity and display specialized functional phenotypes regulated by different environmental cytokines that enable them to play diverse roles during host defense, wound healing, and tissue homeostasis (21, 22). The recent literature has reported that HMGB1 can modulate the polarization of macrophages from a pro-inflammatory to a tissue-healing phenotype (23-25). This information led to the hypothesis that the RELMα/DAMP signaling axis governs macrophages to boost the pro-proliferative environment in PASMCs during vascular inflammation. Our data reveal that RELMα activation is required for hypoxia-induced HMGB1/RAGE expression in pulmonary macrophages. We show that RELMα regulates the post-translational modification of HMGB1 by suppressing the NAD+-dependent deacetylase sirtuin (Sirt) 1, and thereby promotes nucleus-to-cytoplasm translocation and active secretion of this DAMP. By modulating DAMP activities, RELMα mediates the proinflammation-to-proliferation switch of macrophage reprogramming. Thus, these RELMα-licensed macrophages drive vascular remodeling to form the key mechanism that underlies initiation of PH pathogenesis.
Materials and Methods
Human tissue samples
Lung tissues from patients ≥ 18 years old with idiopathic PH (iPAH) were obtained from stored tissue samples through a collaboration with the Johns Hopkins Department of Pathology. To be included in this study, lung slices had to be from subjects who met the inclusion criteria of a mean pulmonary artery pressure > 25 mm Hg proven by right heart catheterization. These iPAH human lung tissues were collected after lung transplantation. For comparison, we also obtained lung tissue from normal patients with no signs of PH. Those normal lung tissues were obtained from either organ donors or resections of lung tumors. The clinical information of these iPAH and control patients, including the right heart catheterization parameters, was published in our previous study (9). Type 4 exemption approval from the Johns Hopkins Medicine Institutional Review Board was obtained before the start of these studies.
In vivo hypoxia mouse model
RELMα (FIZZ1) knockout mice on a C57BL/6 background (≥7 generations) were generated as previously described (26). Sex- and age-matched, 8- to 12-week-old C57BL/6 mice (Charles River Laboratories, Wilmington, MA) were used as wild-type controls for all experiments. Animal housing and experimental protocols were approved by the Animal Care and Use Committee of Johns Hopkins University. For the study on immunoregulation in hypoxic lungs during the early inflammation phase, mice were exposed to 10.0% O2 for 4 days and then sacrificed and processed as previously described (8).
hResistin production and purification
We produced recombinant hResistin protein in our laboratory using a mammalian cell expression system. C-terminal FLAG-tagged hResistin and mouse RELMα were generated by PCR, inserted into pcDNA5/FRT/TO vector, and then integrated in a Flp recombinase-dependent manner into the genome of the Flp-In™ T-Rex™ 293 cell line using the Flp-In™ T-Rex™ kit from Invitrogen as we have described (4). Production of these recombinant proteins was induced by adding tetracycline (1 μg/ml) to the cell culture media. hResistin was purified by anti-FLAG M2 antibody agarose (A2220, Sigma) column chromatography from the 293-cell culture medium with FLAG (0.1 mg/ml) elution. Presence of the protein was determined by SDS-PAGE gel Coomassie staining, and concentration of the protein in the elution was determined by the Bio-Rad Protein Assay. Activity assays were performed on each lot of protein purified. After being serum starved, human (h) PASMCs were treated with 100 nM recombinant hResistin, whereas 3T3 mouse embryonic fibroblasts were treated with the same dosage of recombinant RELMα, all for 15 min. Then cells were collected, lysed, and processed for western blotting. Activity of the purified RELM proteins was tested by assaying their ability to induce Akt phosphorylation (4).
Human cell culture and in vitro treatment
Cultured hPASMCs (CC-2581, Lonza) were used within passages 5-9. THP-1 monocytes (88081201, Sigma) cultured in RPMI-1640 medium (R8758, Sigma) were stimulated with 50 ng/ml phorbol myristate acetate (P1585, Sigma) for 48 h to induce differentiation into macrophages. The differentiated human macrophages were treated with 200 ng/ml lab-made recombinant hResistin protein. Some of these cells were pretreated with Sirt1 activator resveratrol (20 μM, R5010, Sigma) or SRT1720 (1 μM, A4180, ApexBio Technology). We selected the concentrations based on previous tests of these two Sirt1 enhancers in macrophages (27). Resveratrol concentrations of 1-100 μM also have been evaluated in endothelial cells (28) and neural progenitor cells (29). Those studies showed that 50 μM (or lower) resveratrol and 1 μM SRT1720 can activate Sirt1 function without causing cytotoxic effects. Peripheral blood CD14+ monocytes (2W-400, Lonza) were cultured in RPMI medium with 25 ng/ml rHU M-CSF (78057, StemCell Technologies, Canada) to produce human primary macrophages. They were exposed to hResistin in stimulation studies. To prepare conditioned medium, we serum- and growth factor-starved differentiated THP-1 macrophages for 24 h and then treated them with 200 ng/ml lab-made recombinant hResistin or vehicle. After a 16-h incubation, the medium was concentrated with an Amicon Ultra-2.0 Centrifugal Filter (Millipore Sigma) and used for western blotting or applied to the starved human primary PASMCs. Recombinant Box-A, the truncated N-terminal fragment of HMGB1, efficiently interacts with RAGE without functional stimulation (17). This HMGB1 antagonist can prevent the intracellular nuclear-to-cytoplasmic translocation, attenuate the extracellular release of HMGB1, and downregulate the HMGB1-RAGE axis (30). We have cloned, expressed, and purified the recombinant Box A protein and assessed its activity. We found that 2 μg/ml was effective to inhibit the full-length HMGB1 and truncated Box B proteins in monocytes (31). FPS-ZM1 is a specific RAGE antagonistic peptide shown not to cause cytotoxicity at concentrations as high as 10 μM (32). In some experiments, hPASMCs were pretreated with the Box-A protein (2 μg/ml, REHM012, Tecan) or FPS-ZM1 peptide (553030, EMD Millipore, 200 nM) before being incubated with the conditioned medium. The conditioned medium-treated hPASMCs were collected after 16 h for western blot analysis or flow cytometry as described below. In some experiments, cells seeded on the fibronectin-coated coverslips (12 mm round, Neuvitro, GG-12-fibronectin) were treated as described above and then fixed with frozen methanol and used for immunocytochemistry.
Bronchoalveolar lavage fluid (BALF)-derived cell collection and analysis
After mice were euthanized, their lungs were lavaged in three flushes by delivering 800 μl of sterile Ca2+- and Mg2+-free PBS supplemented with 0.1 mM EDTA. Cells collected in the three flushes were pelleted by centrifugation at 1000g for 10 min and then resuspended and pooled in 100–150 μl PBS supplemented with 2% fetal bovine serum. An automated cell counter (Bio-Rad, Hercules, CA) was used to count cell numbers in the BALF. Cells were then seeded on the fibronectin-coated coverslips (Neuvitro) and treated with 200 ng/ml lab-made murine RELMα recombinant protein. After incubation, cells were fixed with frozen methanol. For immunocytochemistry staining, samples were incubated with anti-HMGB1 (ab18256, Abcam) and anti-F4/80 (ab6640, Abcam) antibodies, stained by the corresponding secondary antibodies conjugated with fluorescent dyes (Jackson ImmunoResearch, West Grove, PA), counterstained with DAPI, and mounted on slides (P36935, Life Technologies).
Immunofluorescence analyses
Sections of lung tissues were blocked and then incubated with anti-hResistin (AF1359, R&D Systems), anti-Sirt1 (ab110304, Abcam), anti-F4/80 (ab6640, Abcam), anti-RAGE (ab3611, Abcam), anti-HMGB1 (ab18256, Abcam), or anti-(total) Bruton’s tyrosine kinase (BTK; 8547, Cell Signaling) antibodies, or a combination of two antibodies for double immunofluorescence labeling. Then the sections were incubated with the appropriate fluorochrome-coupled secondary antibodies (Jackson ImmunoResearch) and mounted with ProLong Gold anti-fade reagent with DAPI (P36935, Thermo-Fisher). Staining was imaged and tissue sections were analyzed by confocal microscopy (Leica SPE DMI8). For quantitative analysis, the proportion of area with positive staining was determined with Adobe Photoshop software (Creative Suite 5). Alternatively, positive cells in lung sections from each animal were counted on five randomly chosen high-power fields at 200- or 400-fold magnification. For the study with human tissue samples, quantitative analysis was performed as previously described (33, 34). Briefly, hResistin-positive cells were counted on 10 randomly chosen visual fields of lung sections in each patient at 400-fold magnification, and the average number of cells in 10 fields was calculated.
Western blot analysis
Mouse lung tissues or cultured cell pellets in RIPA buffer (Sigma) [supplemented with 1 mM PMSF, 1 mM Na4VO3, and protease inhibitor mixture (116974980011, Roche)] were lysed with homogenization beads (0.9-2.0 mm; SSB14B, Bullet Blender) in a Bullet Blender at 4°C before being vortexed and centrifuged. The concentration of isolated proteins was measured with a BCA kit (Bio-Rad). The supernatants were mixed in SDS sample loading buffer (NuPAGE, Invitrogen) at 99°C for 10 min and then subjected to SDS-PAGE. After electrophoresis, proteins were transferred onto polyvinylidene difluoride membranes and blotted with anti-HMGB1, anti-RAGE, anti-Sirt1, or anti-acetyl lysine (ab22550) (from Abcam) with anti-total-BTK (8547, Cell Signaling), anti-phospho-BTK (87141, Cell Signaling), or anti-β-actin (A1978, Sigma) overnight at 4°C. Membranes were then probed with HRP-conjugated secondary antibody for 2 h at room temperature. Protein bands were visualized by chemiluminescence (ECL; Amersham Pharmacia Biotech, Arlington Heights, IL). In some experiments, the subcellular fractions were isolated with the ReadyPrep Protein Extraction Kit (1632089, Bio-Rad) to separate cytoplasmic proteins from intact nuclei before being processed for western blotting.
Flow cytometry-based assay
Proliferation of hPASMCs was assessed by flow cytometry with the CellTrace CFSE Cell Proliferation Kit (C34554, Molecular Probes) according to the manufacturer’s instructions. Analysis gates were set on live cells defined by scatter characteristics. The discrete peaks (proliferating smooth muscle cells) representing successive generations were gated for comparison to the undivided parent generation. For apoptosis assays, treated hPASMCs were processed with Annexin V-fluorescein isothiocyanate (FITC) apoptosis detection kit (APOAF-50TST, Sigma). Cells that were positive for Annexin V FITC staining and negative for propidium iodide staining were gated as the early apoptotic smooth muscle cells. Data were acquired by flow cytometry using CELLQuest software (Becton-Dickinson, Bedford, MA) and analyzed with FlowJo Software (FlowJo, Ashland, OR).
Immunoprecipitation
Immunoprecipitation was carried out with the Pierce Classic IP Kit (26146, Thermo Fisher) according to the manufacturer’s instructions. Briefly, 100 μg cell lysates were incubated with 2 μg of HMGB1 antibody overnight at 4°C. Then, these immune complexes were incubated with 20 μl of protein A/G Agarose in the spin column. Samples were washed and eluted with loading buffer and analyzed for acetylated lysines and HMGB1 loading by western blotting.
Scratch wound healing model
The in vitro wound-healing assays were carried out as we have previously published (35). Briefly, we created a gap in the monolayer of the attached THP-1-differentiated macrophages. Cells were treated with lab-made hResistin recombinant protein with or without the specific BTK inhibitor LFM-A13 (CAS 62004-35-7, Calbiochem) at concentrations of 25 or 50 μM for 16 h. Honda et al. reported that 50 μM of LFM-A13 does not inhibit other protein tyrosine kinases in human immune cells (36), and we previously showed that 25 μM of this BTK inhibitor prevents RELMα-induced migration of myeloid cells (37). Cell movement phase images were subsequently captured under light microscopy. The results were quantified by counting the number of migrated cells in the scratched (gate) area.
Statistical analysis
The means and SEM were calculated on all parameters determined in the study. Data were analyzed with Student’s t test for comparisons between two groups, or with one-way ANOVA followed by the Newman-Keuls post-hoc test for multiple comparisons. All analyses were carried out with Prism 7.0e (GraphPad Software, La Jolla, CA). A p < 0.05 was accepted as statistically significant.
Results
hResistin signal in human iPAH lungs
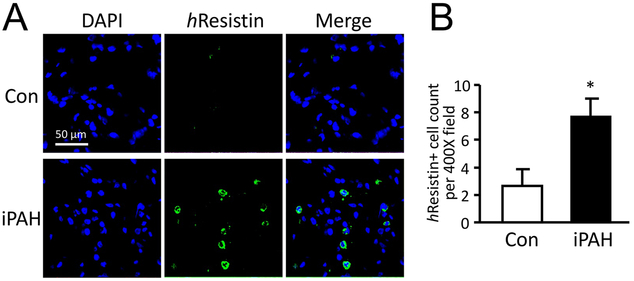
Our previous findings of RELMα-induced PH (8) strongly suggested that hResistin signaling might be clinically significant in human PH. These findings provided a strong impetus for us to analyze whether hResistin is activated in PH patient lung tissues. Here we evaluated lung samples from patients with iPAH by histological staining and compared the results with those from patients with no history of PH. hResistin expression was weakly detectable by immunofluorescence analysis in the lung specimens of control patients but was upregulated in patients with iPAH (Fig. 1A and B). Closer analysis revealed that hResistin expression was localized to the leukocyte-like immune cells of human iPAH lungs (Fig. 1A). These clinical findings strengthen the argument for an etiologic role of RELMα in animal PH models, and further underpin the relationship between hResistin and human PH clinically.
Figure 1.
Expression of hResistin in lungs from patients with iPAH. (A) Paraffin-embedded sections of lungs from normal subjects (Con, n = 6) and patients with iPAH (n = 6) were stained with anti-hResistin Abs (green) and counter-stained with DAPI (blue). Immune cells producing hResistin were observed in iPAH lungs but were infrequent in control tissues. Magnification: 400X. (B) hResistin-expressing cells in the lungs were quantified. Positive cells were counted on 10 randomly chosen fields of lung sections in each patient at 400-fold magnification. Data are presented as means ± SEM, *p < 0.05. Immunofluorescence experiments and analyses were repeated twice.
RELMα/hResistin induces HMGB1/RAGE activation in macrophages
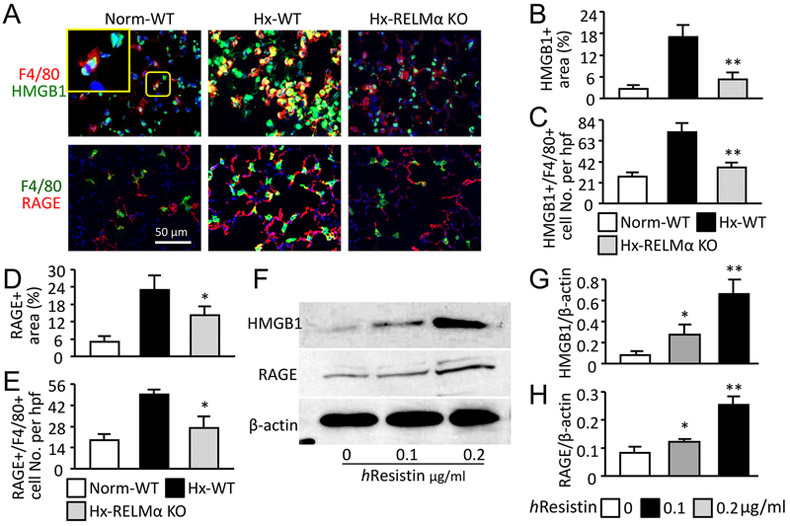
The presence of hResistin-expressing inflammatory cells in human PH lungs prompted us to interrogate the mechanism whereby RELMα/hResistin activates macrophages and mediates vascular inflammation. We focused on DAMP signaling as proposed above. In the mouse hypoxia model, HMGB1 and RAGE were highly induced in the lungs (Fig. 2A-E), and both co-localized with the accumulated F4/80+ macrophages at post-hypoxic day-4 during the early inflammatory stage (Fig. 2A). In knockout mice that lacked RELMα, HMGB1/RAGE signals were suppressed (Fig. 2A, B, and D) and the number of F4/80+ macrophages, particularly the DAMP-producing macrophages in hypoxic lung tissue, declined (Fig. 2A, C, and E). To further validate the RELMα-activated DAMPs in macrophages, we used human macrophages differentiated from a THP-1 monocyte cell line for an in vitro study. In line with in vivo observations, stimulation with hResistin dose-dependently upregulated the protein expression of HMGB1 and RAGE in human macrophages (Fig. 2F-H). These data suggest that RELMα plays a crucial role in macrophage-induced vascular inflammation and PH development by triggering DAMP signaling in these immune cells.
Figure 2.
RELMα/hResistin activates HMGB1/RAGE axis in pulmonary macrophages in vivo and in vitro. (A) Double-color immunofluorescence analysis of lung tissues after 4 days of hypoxia (Hx). Sections were stained with anti-F4/80 Abs and co-stained with anti-HMGB1 or anti-RAGE Abs. The high-magnification inset depicts HMGB1 nuclear localization in the normoxic wild-type (WT) mouse lung. Photographs shown are representative of three independent experiments with four animals per group. Magnification: 400X. Norm, Normoxia; KO, knockout. (B-E) Quantitative analysis of data in (A). Percentage of areas positive for HMGB1 (B) or RAGE (D) in wound sites was determined with Adobe Photoshop software. The F4/80+ pulmonary macrophages expressing HMGB1 (C) or RAGE (E) were also counted and expressed as numbers per high-power field (hpf). Data are presented as means ± SEM (n = 4 animals). *p < 0.05 vs Hx WT mice. (F) Western blot analysis of HMGB1/RAGE expression in the hResistin-treated macrophages differentiated from THP-1 monocytes. (G, H) Quantitative analysis of data in (F). The ratios of HMGB1 (G) and RAGE (H) to β-actin were determined. Data are presented as means ± SEM (n = 6). *p < 0.05, ** p < 0.01 vs control vehicle treatment. Data are representative of at least three independent experiments.
hResistin facilitates HMGB1 subcellular translocation and secretion by regulating its acetylation in macrophages
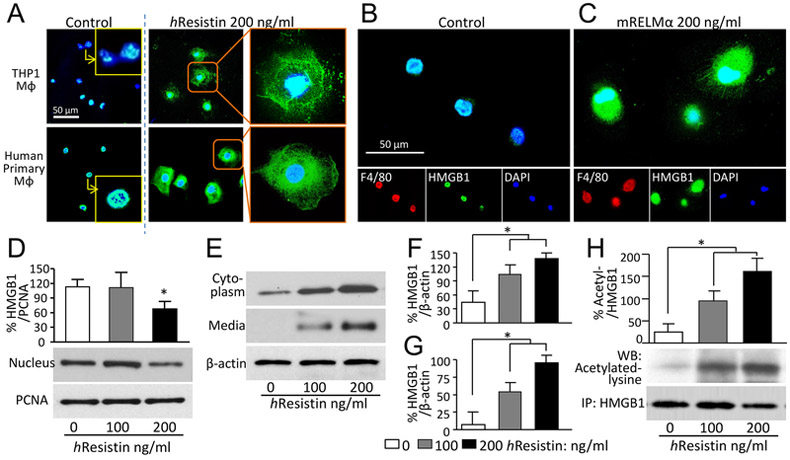
Given the RELMα-activated HMGB1 in pulmonary macrophages, we next tested whether RELMα/hResistin mediates HMGB1 intracellular trafficking, which is a vital step for the extracellular secretion and function of this DAMP. Using immunocytochemistry, we observed hResistin-induced HMGB1 cytoplasmic accumulation in the macrophages differentiated from THP-1 cells (Fig. 3A, upper panels). We also verified that RELMα/hResistin induced HMGB1 nucleus-to-cytoplasm translocation in primary human macrophages produced from peripheral blood CD14+ monocytes (Fig. 3A, lower panels) and in mouse F4/80-positive macrophages isolated from BALF (Fig. 3B). We used western blot analysis to test subcellular cytoplasmic/nuclear fractions and cell-culture supernatant of the hResistin-treated human macrophages. Quantification confirmed that hResistin promoted HMGB1 nuclear export, cytoplasmic accumulation (Fig. 3D-F), and subsequent secretion into the extracellular milieu (Fig. 3E and G).
Figure 3.
RELMα/hResistin induces acetylation, nucleus-to-cytoplasm translocation, and secretion of HMGB1 in macrophages. (A) THP-1-differentiated macrophages (upper panels) and human primary macrophages differentiated from CD14+ peripheral blood monocytes (lower panels) were treated with hResistin and immunofluorescently stained with anti-HMGB1 Abs (green). n = 6. Magnification: 400X. Higher magnification images in the insets show control vehicle-treated macrophages with HMGB1 nuclear retention. MΦ, macrophages. (B, C) Mouse macrophages collected from bronchoalveolar lavage were treated with control medium (B) or lab-made recombinant mouse (m) RELMα protein (C). The RELMα-facilitated HMGB1 intracellular mobilization was determined by immunocytochemistry with anti-F4/80 (red) and anti-HMGB1 (green) Abs. Representative results from three independent experiments with 4-6 animals in each group are shown. Magnification: 400X. (D) Western blot analysis of nuclear HMGB1 expression in hResistin-treated THP-1-differentiated human macrophages. Proliferating cell nuclear antigen (PCNA), a nuclear housekeeping protein, served as a control. Data represent means ± SEM (n = 3). *p < 0.05 vs control medium. (E) Cytoplasmic proteins extracted from THP-1-differentiated macrophages, as well as secreted proteins in concentrated medium, were subjected to western blotting with anti-HMGB1 Abs. As a constitutively expressed housekeeping protein, β-actin was used as a loading control. (F, G) Quantitative analysis of data in (E) for HMGB1 expression in cytoplasm (F) and in concentrated medium (G). Data are presented as means ± SEM (n = 3-4). *p < 0.05. (H) THP-1-differentiated macrophages were treated with hResistin and subjected to immunoprecipitation (IP) with anti-HMGB1 Abs. The HMGB1 acetylation was measured by using specific Abs against acetylated lysine. Data are presented as means ± SEM (n = 6). *p < 0.05.
Lysine residues on HMGB1 must be acetylated before it can be mobilized intracellularly and released to the extracellular space (18). Therefore, we sought to determine the potential effect of hResistin on HMGB1 acetylation. Immunoprecipitation experiments showed that hResistin treatment dose-dependently increased the acetylation of HMGB1 in human macrophages (Fig. 3H). This finding indicates that hResistin modulates the post-translational modification of HMGB1 to promote nuclear translocation and active secretion.
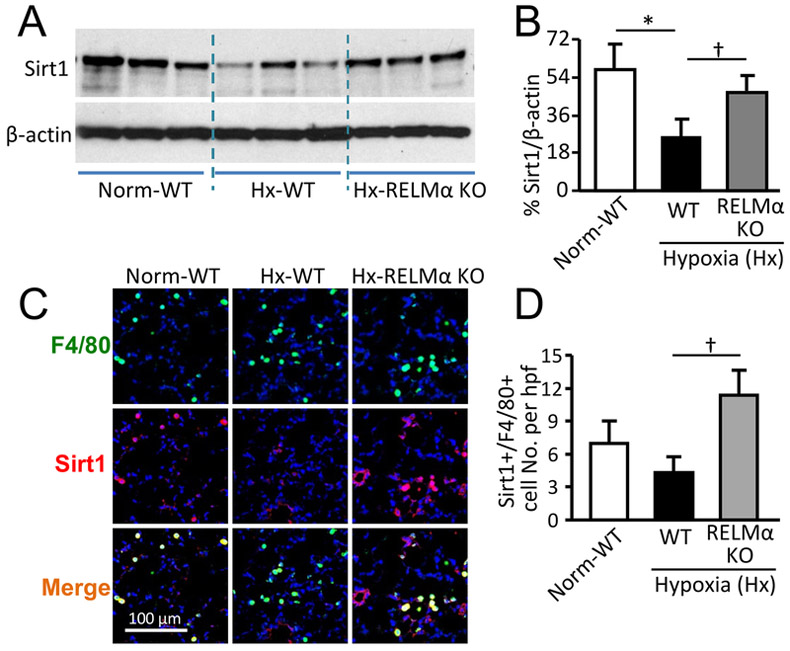
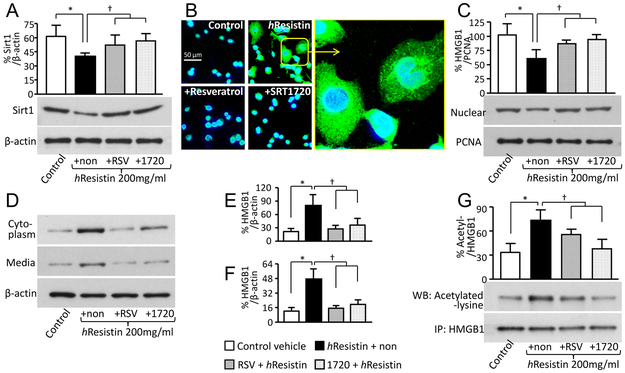
RELMα promotes HMGB1 acetylation by suppressing Sirt1
Because acetylation is required for HMGB1 secretion, we hypothesized that Sirt1, an NAD+-dependent deacetylase, may participate in the cellular pathologic process by which RELMα/hResistin causes PH development. Western blot analysis revealed that hypoxia impeded Sirt1 signal in injured mouse lungs (Fig. 4A and B). RELMα gene deficiency rescued the dysregulation of Sirt1 expression in hypoxic lungs (Fig. 4A and B). Immune staining of mouse lung tissues in the early inflammatory phase showed that hypoxia exposure weakened Sirt1 activation in macrophages in a RELMα-dependent manner (Fig. 4C and D). Similarly, in vitro, hResistin stimulation significantly reduced Sirt1 expression in human macrophages (Fig. 5A), further suggesting a role of Sirt1 in the RELMα-regulated HMGB1 activities. For validation, we used two selective Sirt1 activators, the chemical compound SRT1720 and the natural polyphenol resveratrol (38). We showed that these two compounds prevented hResistin-induced downregulation of Sirt1 expression in human macrophages (Fig. 5A). When immunoprecipitated acetylated HMGB1 from human macrophages was pre-incubated with these Sirt1 inducers, the hResistin-promoted HMGB1 acetylation was significantly curtailed (Fig. 5G). As assessed by western blotting of subcellular fractions, pharmacologic enhancement of Sirt1 signal restored HMGB1 nuclear retention (Fig. 5C), suppressed HMGB1 cytoplasmic enrichment in macrophages (Fig. 5D and E), and reduced extracellular secretion (Fig. 5D and F). Immunocytochemistry confirmed that Sirt1 reconstitution by SRT1720 or resveratrol reversed the hResistin-potentiated HMGB1 translocation in human macrophages (Fig. 5B). These results shed light on the immunomodulatory properties of RELMα/hResistin in initiating the active secretion of hyper-acetylated HMGB1 from macrophages in PH lungs.
Figure 4.
RELMα impairs Sirt1 signaling in lung macrophages of mice exposed to 4 days of hypoxia. (A) Western blot analysis of mouse lung lysates for Sirt1 expression. (B) Quantitative analysis of data in (A). *p < 0.05 vs normoxic (Norm) wild-type (WT) group. †p < 0.05 vs hypoxic (Hx) WT group. Data are presented as means ± SEM and are representative of two independent experiments with n = 6 mice per group. (C) Mouse lung sections were stained with anti-F4/80 (green) and anti-Sirt1 (red) Abs. Photographs shown are representative of 4 individual animals per group. Magnification: 400X. (D) Number of pulmonary F4/80+ macrophages expressing Sirt1 per high-power field (hpf). Data are presented as means ± SEM and are representative of two independent experiments with n = 4 mice per group. †p < 0.05 vs Hx WT group. KO, knockout.
Figure 5.
hResistin signaling suppresses Sirt1 to promote the hyper-acetylation, intracellular relocation, and secretion of macrophage HMGB1. (A) Western blot analysis of Sirt1 expression in hResistin-treated THP-1-differentiated macrophages. Human macrophages were pretreated with vehicle (hResistin + non), 20 μM Sirt1 activator resveratrol (hResistin + RSV), or 1 μM Sirt1 activator SRT1720 (hResistin + 1720) before being stimulated with 200 ng/ml hResistin. The non- hResistin (Control) and non-pretreatment (hResistin + non) cells served as negative controls. Data are presented as means ± SEM (n = 3-4). *p < 0.05 vs control (medium)-treated group. † p < 0.05 vs the group treated with hResistin alone (+non). (B) Immunocytochemical analysis of HMGB1 signal (green) in the hResistin -treated THP-1 macrophages with or without Sirt1 activators. n = 4. Original magnification: 400X. The boxed area in the image showing macrophages treated with hResistin alone is enlarged in the right panel to show cytoplasmic accumulation of HMGB1. (C) Western blot analysis of nuclear HMGB1 expression in THP-1-differentiated macrophages treated with hResistin and Sirt1 activators. Proliferating cell nuclear antigen (PCNA), a nuclear housekeeping protein, served as a control. Data are presented as means ± SEM (n = 3-4). *p < 0.05 vs control (medium)-treated group. † p < 0.05 vs the group treated with hResistin alone (+non). (D) Cytoplasmic proteins extracted from THP-1 macrophages and proteins secreted into concentrated medium were subjected to western blotting with anti-HMGB1 Abs. β-actin was used as a loading control. (E, F) Quantitative analysis of data in D for HMGB1 expression in cytoplasm (E) and in concentrated medium (F). Data are presented as means ± SEM (n = 4). *p < 0.05 vs control (medium)-treated group. † p < 0.05 vs the group treated with hResistin alone (+non). (G) hResistin-stimulated THP-1 macrophages were pretreated with Sirt1 activators and subjected to immunoprecipitation (IP) with anti-HMGB1 Abs. The HMGB1 acetylation was assessed by western blotting (WB) with specific Abs against acetylated lysine. Data are presented as means ± SEM (n = 4). *p < 0.05 vs control (medium)-treated group. † p < 0.05 vs the group treated with hResistin alone (+non). Data are representative of at least three independent experiments.
hResistin-driven macrophages induce the proliferative phenotype of PASMCs
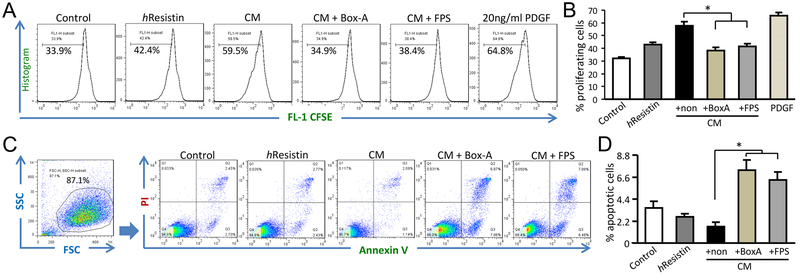
By using primary hPASMCs, we further investigated the function of hResistin -potentiated macrophages in the context of vascular remodeling and PH development. When evaluated by FACS-CFSE labeling and tracing, hPASMCs directly incubated with hResistin alone exhibited a proliferative phenotype. However, hPASMCs incubated with conditioned medium collected from hResistin-treated macrophages exhibited more potent cell mitogenesis and growth (Fig. 6A and B). The conditioned medium also weakened the apoptotic responses of hPASMCs after starvation, as detected by Annexin-V FITC assay (Fig. 6C and D). Intriguingly, pretreating the hPASMCs with HMGB1 antagonist Box-A recombinant protein, or RAGE inhibitor FPS-ZM1, reversed the enhanced proliferation and alleviated apoptosis promoted by the conditioned medium (Fig. 6). These observations indicate that the HMGB1/RAGE axis is pivotal for RELMα/hResistin-driven macrophages to induce the pro-PH phenotype of PASMCs.
Figure 6.
hResistin /HMGB1 signaling activation in macrophages induces apoptosis-resistant proliferation of hPASMCs. (A) PASMC proliferation was determined by flow cytometry analysis. Primary hPASMCs were treated with 1 μg/ml hResistin or with conditioned medium (CM) from hResistin-stimulated THP-1 macrophages (200 ng/ml, 16 h). For the HMGB1/RAGE inhibitor application, hPASMCs were pretreated for 1 h with 2 μg/ml Box-A recombinant protein or 200 nM FPS-ZM1 (FPS) before treatment with CM. Before treatment, hPASMCs were labeled with CFSE for tracing proliferation. Treatment with platelet-derived growth factor (PDGF, 20 ng/ml) served as a positive control. (B) Quantitative analysis of the data in (A). Data are presented as means ± SEM (n = 4-5). *p < 0.05. (C) Flow cytometry analysis of hPASMC apoptosis after starvation and treatments as described in (A). hPASMC apoptosis was tested with the Annexin V-FITC kit. Early apoptotic cells that were positive for Annexin V FITC stain and negative for propidium iodide were gated. (D) Quantitative analysis of the data in (C). Data represent means ± SEM (n = 4). *p < 0.05. Data are representative of at least two independent experiments.
RELMα-induced recruitment amplifies macrophage activation
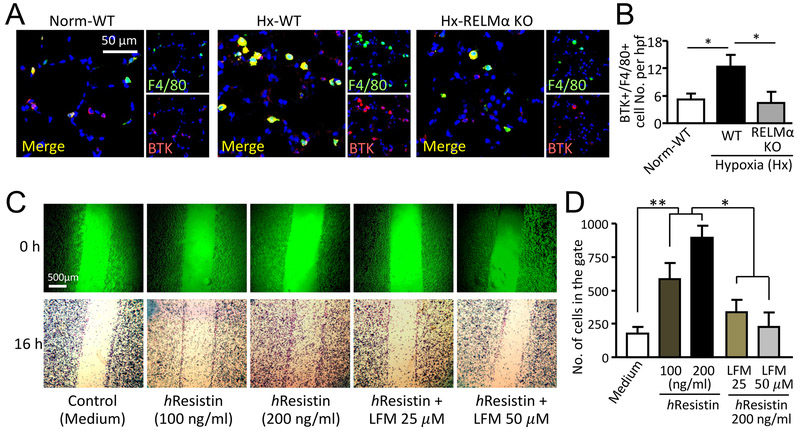
The increase in HMGB1/RAGE-expressing pulmonary macrophages (Fig. 2) induced by RELMα in vivo suggested that RELMα activation also boosts macrophage recruitment to the hypoxic lungs. Our previous study showed that recombinant murine RELMα mediates chemotaxis of primary mouse bone marrow myeloid cells through the BTK pathway in vitro, and identified BTK as a functional RELMα binding partner (37). Here we tested BTK involvement in an in vivo hypoxia study. Immunofluorescent analysis showed that hypoxia induced accumulation of BTK-expressing immune cells in the inflamed lungs and that the major fraction of these infiltrating cells co-stained for the macrophage marker F4/80 (Fig. 7A), indicating the main cellular source of this RELMα binding partner. Loss of RELMα in the knockout mice significantly dampened this hypoxia-promoted enrichment of BTK+F4/80+ cells in mouse lungs (Fig. 7A and B), further supporting the premise that a RELMα-dependent mechanism underlies the macrophage-mediated vascular inflammation and remodeling.
Figure 7.
Macrophage migration is mediated by hResistin-BTK. (A) Immunofluorescence staining for total BTK and F4/80 in lungs of mice after 4 days of hypoxia. Digitally merged signals are shown in the left panel, and separate channels are displayed in the right panels. Photographs shown are representative of three independent experiments with four individual lung samples. Magnification: 400X. (B) The total number of F4/80+ pulmonary macrophages that expressed BTK per high-power field (hpf, 400X) in (A). Data are presented as means ± SEM (n = 4 animals). *p < 0.05. (C) In vitro wound healing assay. Representative microphotographs show motility of human macrophages in scratch wound-healing model. THP-1-differentiated macrophages were treated with medium (control) or hResistin (100 and 200 ng/ml) for 16 h. In parallel, 200 ng/ml hResistin-stimulated macrophages were pretreated with LFM-A13 for 30 min. (D) Quantitative assessment of data from (C). Data are presented as means ± SEM (n = 6) and are representative of two independent experiments. *p < 0.05, **p < 0.01.
Next, by using the in vitro scratch wound healing model, we showed that hResistin treatment dose-dependently induced the migration of human macrophages (Fig. 7C and D). This migration was reversed by preincubation with BTK inhibitor LFM-A13. The data signify that hResistin’s ability to induce macrophage migration is BTK-dependent, in line with our previous observation of RELMα/BTK-mediated mouse bone marrow cell chemotaxis (37). Thus, in the context of hypoxic inflammation, BTK-mediated macrophage recruitment might augment and amplify the RELMα/DAMP-potentiated macrophage proliferation, thereby contributing to pulmonary vascular remodeling during PH development.
Discussion
Accumulating evidence suggests that the vascular remodeling of PH may be reversed by approaches that address critical immune processes (39). Here we identified a core role of the RELMα/DAMP signaling axis in PH pathogenesis. The RELMα-activated macrophages produced and secreted HMGB1 to trigger and amplify vascular inflammation. RELMα/hResistin also may mediate the inflammation-to-proliferation function switch of macrophages by modulating HMGB1 activity, thereby contributing to apoptosis-resistant smooth muscle cell growth. Thus, the RELMα-trained macrophages drive the immune-vascular interaction required for persistent vascular remodeling. Uncovering this signaling hub will enable us to dissect the complicated PH initiation and progression process and ultimately allow us to overcome critical barriers of developing novel effective anti-PH therapy.
RELMα/hResistin-facilitated secretion of HMGB1 by macrophages is the primary step of RELMα-triggered inflammation. Post-translational acetylation of HMGB1 abolishes its ability to bind to DNA and redirects it toward cytoplasm and the secretory pathway (18, 40). In our study, we found that suppression of Sirt 1 by RELMα permitted HMGB1 intracellular translocation and subsequent secretion, as the de-acetylation capacity of Sirt1 failed. Interestingly, a previous study showed that duodenal Sirt1 activation by resveratrol reversed insulin resistance through a gut-brain-liver neuronal axis (38), and here we explored the modulatory effects of resistin family members on Sirt1 signal. Sirt1 expression has been reported to decrease in PH animal models (41-43). We showed that Sirt1 exerts an anti-inflammatory effect in quenching HMGB1 activation. Future investigation is warranted to characterize the acetylation status of cysteine residues in the RELMα-regulated HMGB1. In addition to the epigenetic-dependent pathway, HMGB1 release may also be mediated by inflammasome activation (44). This possibility points to the RELMα binding partner BTK, which negatively regulates the production of reactive oxygen species (36) and is essential for NLRP3 inflammasome activation (45). BTK was shown to mediate the immune cell cross-talk to promote Th2-type macrophage programming during tumor growth (46). Given the RELMα-dependent accumulation of BTK-carrying macrophages in the hypoxic lungs and the RELMα/BTK-mediated macrophage migration observed in our current study, a follow-up study is needed to further clarify the role of BTK in the RELMα/HMGB1 immune axis.
HMGB1 secreted extracellularly may further strengthen the RELMα-triggered vascular inflammation through feed-forward macrophage activation. Deletion of the RELMα gene attenuated hypoxia-induced HMGB1 expression in pulmonary macrophages at an early inflammatory phase, indicating that RELMα functions in an autocrine loop to actively increase HMGB1 release from macrophages. Thus, cooperation between RELMα and hypoxia might provide the two signals needed to induce HMGB1 secretion: the signal to elicit HMGB1 trafficking from the nucleus and the signal to initiate release of hyper-acetylated HMGB1. The hypoxia-induced recruitment of more inflammatory cells that express RELMα and BTK (37) enhances these circuits for further amplification of DAMP signaling. In addition to this direct pro-migratory effect, RELMα might also upregulate the production of chemokines such as CXCL12/SDF-1 and MCP-1 to indirectly induce macrophage infiltration in lungs during PH development (12). Thus, by initiating and expediting macrophage recruitment, RELMα/hResistin activation not only ignites but also continuously fuels the vascular inflammatory milieu during the onset and progression of PH. RELMα (FIZZ1) is also known to be a marker for alternatively activated (M2) macrophages (21), and we recently showed that RELMα promotes activation and recruitment of IL-6-expressing macrophages (11). These data suggest that RELMα may also orchestrate the macrophage phenotype shift by regulating DAMP signaling for immune response to tissue damage.
As a DAMP receptor, RAGE is essential for the HMGB1-sustained positive-feedback loop during inflammation (47). Our findings further identified the pivotal role of this DAMP receptor in the self-replenishing mechanism required for vascular remodeling. RAGE is localized predominantly in lung and has been considered as an eligible biomarker for evaluating anti-PH therapy in patients (48). Activation of RAGE can mitigate reactive oxygen species-induced oxidative injury and suppress apoptosis during oxidative stress (49). More intriguingly, RAGE is the DAMP receptor selected by non-oxidative HMGB1 (50). Reduced HMGB1 binds to RAGE, but not to another putative HMGB1 receptor, the toll-like receptor (TLR) 4, (51) to exert its pro-autophagic and pro-proliferative functions (50). Moreover, it has been reported that the reduced form of HMGB1 is more effectively able to establish a tissue-healing and pro-proliferative microenvironment through engaging RAGE (25, 52), whereas the disulfide oxidative HMGB1 exacerbates inflammation and suppresses regeneration exclusively via activation of TLR4 (25, 52). Thus, as BTK also negatively regulates reactive oxygen species (36), RAGE may cooperate with this RELMα binding partner to maintain the non-oxidative form of HMGB1 after its extracellular secretion. This RAGE function may be crucial for RELMα-modified macrophages to switch from a pro-inflammatory to a pro-proliferative phenotype.
Jia et al. (53) recently reported that the interaction affinity between HMGB1 and RAGE, but not TLR4, was enhanced in hypoxic and normoxic mouse and hPASMCs. Additionally, TLR4 knockdown did not affect the resistin-induced monocyte activation (54), further supporting a predominant role of RAGE in the RELMα/HMGB1-driven PH phenotype of smooth muscle cells in the context of hypoxia. However, the possibility that TLR4 might participate cannot be completely excluded. Further investigation is warranted to better understand the implication of these DAMP receptors in RELMα-induced PH. Nevertheless, our findings suggest that a DAMP-dependent mechanism underlies the pro-proliferative and anti-apoptotic environment in PASMCs driven by the RELMα-modulated macrophages.
The clinical significance of our experimental findings is further underpinned by our demonstration that hResistin signal is upregulated in the lungs of patients diagnosed with iPAH. To the best of our knowledge, expression levels and patterns of hResistin in human iPAH lungs have not been reported previously. Our findings suggest that hResistin might serve as a potential biomarker of PH progression and therapeutic outcome. Whether hResistin level can be used as a measure of disease severity is being further evaluated in clinical trials. In addition, given the correlation between insulin aberration and PH pathogenesis (55), the insulin resistance properties of resistin (1), and the connection between HMGB1 and insulin sensitivity (56), whether and how this RELMα/HMGB1 axis bridges the gap between insulin dysregulation and vascular inflammation in PH requires further study.
In sum, we identified that RELMα acts as a master regulator for the pro-PH properties of macrophages by modulating DAMP activities. The RELMα/HMGB1 signaling cascade is vital to governing the role of macrophages in tissue remodeling. This immunoregulation is triggered by injury-initiated RELMα production and leads to activation and secretion of HMGB1, which functions through RAGE. In turn, macrophages create an autocrine positive-feedback loop that perpetuates the vascular inflammatory state. Thus, blocking RELMα/hResistin might not only target the ignition source to quench the flame of endogenous danger signals, but also impede the continual replenishment of macrophages to block the PH proliferative phenotype in PASMCs. Our ongoing efforts to develop anti- RELMα/hResistin antibodies may support our findings, as some of these antibodies have shown promise in preventing and reversing PH in rodent models (unpublished data and related manuscript submission). Because PH is a complex and multifactorial disease with a network of interacting inflammatory signals, sorting out these interconnected pathways in PH is critical to defining an effective therapy that addresses the mechanism of disease and not the symptoms. In this context, uncovering the essential role of the RELMα/HMGB1 signaling axis in PH development is moving a step in that direction.
Key Points:
RELMα (hResistin) activates pulmonary macrophages in PAH patients and hypoxic mice.
RELMα (hResistin) induces HMGB1 acetylation by suppressing Sirt1 in macrophages.
The RELMα-HMGB1 axis instigates macrophage-mediated SMC proliferation.
Acknowledgments:
We thank Claire F. Levine for editing the article in manuscript form.
Sources of Funding: This work was supported by National Institutes of Health (NIH) Centers for Advanced Diagnostics and Experimental Therapeutics in Lung Diseases Stage II (CADET II) 5UH2HL123827-02 and NIH 1R01HL138497-01 grants (to R.A.J.), as well as a Stimulating and Advancing ACCM Research (StAAR) grant (to Q.L.) from the Department of Anesthesiology and Critical Care Medicine, Johns Hopkins University.
Abbreviations
- BALF
bronchoalveolar lavage fluid
- DAMP
damage-associated molecular pattern
- FIZZ1
found in inflammatory zone-1
- HIMF
hypoxia-induced mitogenic factor
- HMGB1
high-mobility group box 1; h, human; hResistin, human resistin
- PH
pulmonary hypertension
- RAGE
receptor for advanced glycation end products
- RELM
resistin-like molecule
- PASMC
pulmonary artery smooth muscle cell
Footnotes
Disclosures: The authors have no financial conflicts of interest.
References
- 1.Steppan CM, Bailey ST, Bhat S, Brown EJ, Banerjee RR, Wright CM, Patel HR, Ahima RS, and Lazar MA. 2001. The hormone resistin links obesity to diabetes. Nature 409: 307–312. [DOI] [PubMed] [Google Scholar]
- 2.Fan C, Johns BA, Su Q, Kolosova IA, and Johns RA. 2013. Choosing the right antibody for resistin-like molecule (RELM/FIZZ) family members. Histochem Cell Biol 139: 605–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yang RZ, Huang Q, Xu A, McLenithan JC, Eisen JA, Shuldiner AR, Alkan S, and Gong DW. 2003. Comparative studies of resistin expression and phylogenomics in human and mouse. Biochem Biophys Res Commun 310: 927–935. [DOI] [PubMed] [Google Scholar]
- 4.Teng X, Li D, Champion HC, and Johns RA. 2003. FIZZ1/RELMalpha, a novel hypoxia-induced mitogenic factor in lung with vasoconstrictive and angiogenic properties. Circ Res 92: 1065–1067. [DOI] [PubMed] [Google Scholar]
- 5.Holcomb IN, Kabakoff RC, Chan B, Baker TW, Gurney A, Henzel W, Nelson C, Lowman HB, Wright BD, Skelton NJ, Frantz GD, Tumas DB, Peale FV Jr., Shelton DL, and Hebert CC. 2000. FIZZ1, a novel cysteine-rich secreted protein associated with pulmonary inflammation, defines a new gene family. EMBO J 19: 4046–4055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Angelini DJ, Su Q, Yamaji-Kegan K, Fan C, Skinner JT, Champion HC, Crow MT, and Johns RA. 2009. Hypoxia-induced mitogenic factor (HIMF/FIZZ1/RELMalpha) induces the vascular and hemodynamic changes of pulmonary hypertension. Am J Physiol Lung Cell Mol Physiol 296: L582–593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Angelini DJ, Su Q, Kolosova IA, Fan C, Skinner JT, Yamaji-Kegan K, Collector M, Sharkis SJ, and Johns RA. 2010. Hypoxia-induced mitogenic factor (HIMF/FIZZ1/RELM alpha) recruits bone marrow-derived cells to the murine pulmonary vasculature. PLoS One 5: e11251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Angelini DJ, Su Q, Yamaji-Kegan K, Fan C, Skinner JT, Poloczek A, El-Haddad H, Cheadle C, and Johns RA. 2013. Hypoxia-induced mitogenic factor (HIMF/FIZZ1/RELMalpha) in chronic hypoxia- and antigen-mediated pulmonary vascular remodeling. Respir Res 14: 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Angelini DJ, Su Q, Yamaji-Kegan K, Fan C, Teng X, Hassoun PM, Yang SC, Champion HC, Tuder RM, and Johns RA. 2009. Resistin-like molecule-beta in scleroderma-associated pulmonary hypertension. Am J Respir Cell Mol Biol 41: 553–561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Johns RA 2010. Th2 inflammation, hypoxia-induced mitogenic factor/FIZZ1, and pulmonary hypertension and vascular remodeling in schistosomiasis. Am J Respir Crit Care Med 181: 203–205. [DOI] [PubMed] [Google Scholar]
- 11.Johns RA, Takimoto E, Meuchel LW, Elsaigh E, Zhang A, Heller NM, Semenza GL, and Yamaji-Kegan K. 2016. Hypoxia-Inducible Factor 1alpha Is a Critical Downstream Mediator for Hypoxia-Induced Mitogenic Factor (FIZZ1/RELMalpha)-Induced Pulmonary Hypertension. Arterioscler Thromb Vasc Biol 36: 134–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yamaji-Kegan K, Su Q, Angelini DJ, Champion HC, and Johns RA. 2006. Hypoxia-induced mitogenic factor has proangiogenic and proinflammatory effects in the lung via VEGF and VEGF receptor-2. Am J Physiol Lung Cell Mol Physiol 291: L1159–1168. [DOI] [PubMed] [Google Scholar]
- 13.Yamaji-Kegan K, Su Q, Angelini DJ, Myers AC, Cheadle C, and Johns RA. 2010. Hypoxia-induced mitogenic factor (HIMF/FIZZ1/RELMalpha) increases lung inflammation and activates pulmonary microvascular endothelial cells via an IL-4-dependent mechanism. J Immunol 185: 5539–5548. [DOI] [PubMed] [Google Scholar]
- 14.Yamaji-Kegan K, Takimoto E, Zhang A, Weiner NC, Meuchel LW, Berger AE, Cheadle C, and Johns RA. 2014. Hypoxia-induced mitogenic factor (FIZZ1/RELMalpha) induces endothelial cell apoptosis and subsequent interleukin-4-dependent pulmonary hypertension. Am J Physiol Lung Cell Mol Physiol 306: L1090–1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fan C, Fu Z, Su Q, Angelini DJ, Van Eyk J, and Johns RA. 2011. S100A11 mediates hypoxia-induced mitogenic factor (HIMF)-induced smooth muscle cell migration, vesicular exocytosis, and nuclear activation. Mol Cell Proteomics 10: M110 000901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fan C, Su Q, Li Y, Liang L, Angelini DJ, Guggino WB, and Johns RA. 2009. Hypoxia-induced mitogenic factor/FIZZ1 induces intracellular calcium release through the PLC-IP(3) pathway. Am J Physiol Lung Cell Mol Physiol 297: L263–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sims GP, Rowe DC, Rietdijk ST, Herbst R, and Coyle AJ. 2010. HMGB1 and RAGE in inflammation and cancer. Annu Rev Immunol 28: 367–388. [DOI] [PubMed] [Google Scholar]
- 18.Bonaldi T, Talamo F, Scaffidi P, Ferrera D, Porto A, Bachi A, Rubartelli A, Agresti A, and Bianchi ME. 2003. Monocytic cells hyperacetylate chromatin protein HMGB1 to redirect it towards secretion. EMBO J 22: 5551–5560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schlueter C, Weber H, Meyer B, Rogalla P, Roser K, Hauke S, and Bullerdiek J. 2005. Angiogenetic signaling through hypoxia: HMGB1: an angiogenetic switch molecule. Am J Pathol 166: 1259–1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vergadi E, Chang MS, Lee C, Liang OD, Liu X, Fernandez-Gonzalez A, Mitsialis SA, and Kourembanas S. 2011. Early macrophage recruitment and alternative activation are critical for the later development of hypoxia-induced pulmonary hypertension. Circulation 123: 1986–1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mosser DM, and Edwards JP. 2008. Exploring the full spectrum of macrophage activation. Nat Rev Immunol 8: 958–969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Weavers H, Evans IR, Martin P, and Wood W. 2016. Corpse Engulfment Generates a Molecular Memory that Primes the Macrophage Inflammatory Response. Cell 165: 1658–1671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schaper F, de Leeuw K, Horst G, Bootsma H, Limburg PC, Heeringa P, Bijl M, and Westra J. 2016. High mobility group box 1 skews macrophage polarization and negatively influences phagocytosis of apoptotic cells. Rheumatology (Oxford) 55: 2260–2270. [DOI] [PubMed] [Google Scholar]
- 24.Son M, Porat A, He M, Suurmond J, Santiago-Schwarz F, Andersson U, Coleman TR, Volpe BT, Tracey KJ, Al-Abed Y, and Diamond B. 2016. C1q and HMGB1 reciprocally regulate human macrophage polarization. Blood 128: 2218–2228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tirone M, Tran NL, Ceriotti C, Gorzanelli A, Canepari M, Bottinelli R, Raucci A, Di Maggio S, Santiago C, Mellado M, Saclier M, Francois S, Careccia G, He M, De Marchis F, Conti V, Ben Larbi S, Cuvellier S, Casalgrandi M, Preti A, Chazaud B, Al-Abed Y, Messina G, Sitia G, Brunelli S, Bianchi ME, and Venereau E. 2018. High mobility group box 1 orchestrates tissue regeneration via CXCR4. J Exp Med 215: 303–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu T, Yu H, Ullenbruch M, Jin H, Ito T, Wu Z, Liu J, and Phan SH. 2014. The in vivo fibrotic role of FIZZ1 in pulmonary fibrosis. PLoS One 9: e88362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yoshizaki T, Schenk S, Imamura T, Babendure JL, Sonoda N, Bae EJ, Oh DY, Lu M, Milne JC, Westphal C, Bandyopadhyay G, and Olefsky JM. 2010. SIRT1 inhibits inflammatory pathways in macrophages and modulates insulin sensitivity. Am J Physiol Endocrinol Metab 298: E419–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chen F, Qian LH, Deng B, Liu ZM, Zhao Y, and Le YY. 2013. Resveratrol protects vascular endothelial cells from high glucose-induced apoptosis through inhibition of NADPH oxidase activation-driven oxidative stress. CNS Neurosci Ther 19: 675–681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kumar V, Pandey A, Jahan S, Shukla RK, Kumar D, Srivastava A, Singh S, Rajpurohit CS, Yadav S, Khanna VK, and Pant AB. 2016. Differential responses of Trans-Resveratrol on proliferation of neural progenitor cells and aged rat hippocampal neurogenesis. Sci Rep 6: 28142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ulloa L, Ochani M, Yang H, Tanovic M, Halperin D, Yang R, Czura CJ, Fink MP, and Tracey KJ. 2002. Ethyl pyruvate prevents lethality in mice with established lethal sepsis and systemic inflammation. Proc Natl Acad Sci U S A 99: 12351–12356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lin Q, Fang J, Fang D, Li B, Zhou H, and Su SB. 2011. Production of recombinant human HMGB1 and anti-HMGB1 rabbit serum. Int Immunopharmacol 11: 646–651. [DOI] [PubMed] [Google Scholar]
- 32.Deane R, Singh I, Sagare AP, Bell RD, Ross NT, LaRue B, Love R, Perry S, Paquette N, Deane RJ, Thiyagarajan M, Zarcone T, Fritz G, Friedman AE, Miller BL, and Zlokovic BV. 2012. A multimodal RAGE-specific inhibitor reduces amyloid beta-mediated brain disorder in a mouse model of Alzheimer disease. J Clin Invest 122: 1377–1392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lin Q, Fang D, Fang J, Ren X, Yang X, Wen F, and Su SB. 2011. Impaired wound healing with defective expression of chemokines and recruitment of myeloid cells in TLR3-deficient mice. J Immunol 186: 3710–3717. [DOI] [PubMed] [Google Scholar]
- 34.Lin Q, Yang XP, Fang D, Ren X, Zhou H, Fang J, Liu X, Zhou S, Wen F, Yao X, Wang JM, and Su SB. 2011. High-mobility group box-1 mediates toll-like receptor 4-dependent angiogenesis. Arterioscler Thromb Vasc Biol 31: 1024–1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang L, Wang H, Yang T, Su Z, Fang D, Wang Y, Fang J, Hou X, Le Y, Chen K, Wang JM, Su SB, Lin Q, and Zhou Q. 2016. Formylpeptide receptor 1 mediates the tumorigenicity of human hepatocellular carcinoma cells. Oncoimmunology 5: e1078055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Honda F, Kano H, Kanegane H, Nonoyama S, Kim ES, Lee SK, Takagi M, Mizutani S, and Morio T. 2012. The kinase Btk negatively regulates the production of reactive oxygen species and stimulation-induced apoptosis in human neutrophils. Nat Immunol 13: 369–378. [DOI] [PubMed] [Google Scholar]
- 37.Su Q, Zhou Y, and Johns RA. 2007. Bruton’s tyrosine kinase (BTK) is a binding partner for hypoxia induced mitogenic factor (HIMF/FIZZ1) and mediates myeloid cell chemotaxis. FASEB J 21: 1376–1382. [DOI] [PubMed] [Google Scholar]
- 38.Cote CD, Rasmussen BA, Duca FA, Zadeh-Tahmasebi M, Baur JA, Daljeet M, Breen DM, Filippi BM, and Lam TK. 2015. Resveratrol activates duodenal Sirt1 to reverse insulin resistance in rats through a neuronal network. Nat Med 21: 498–505. [DOI] [PubMed] [Google Scholar]
- 39.Simonneau G, Hoeper MM, McLaughlin V, Rubin L, and Galie N. 2016. Future perspectives in pulmonary arterial hypertension. Eur Respir Rev 25: 381–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lu B, Antoine DJ, Kwan K, Lundback P, Wahamaa H, Schierbeck H, Robinson M, Van Zoelen MA, Yang H, Li J, Erlandsson-Harris H, Chavan SS, Wang H, Andersson U, and Tracey KJ. 2014. JAK/STAT1 signaling promotes HMGB1 hyperacetylation and nuclear translocation. Proc Natl Acad Sci U S A 111: 3068–3073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ding M, Lei J, Qu Y, Zhang H, Xin W, Ma F, Liu S, Li Z, Jin F, and Fu E. 2015. Calorie Restriction Attenuates Monocrotaline-induced Pulmonary Arterial Hypertension in Rats. J Cardiovasc Pharmacol 65: 562–570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhou S, Li MT, Jia YY, Liu JJ, Wang Q, Tian Z, Liu YT, Chen HZ, Liu DP, and Zeng XF. 2015. Regulation of Cell Cycle Regulators by SIRT1 Contributes to Resveratrol-Mediated Prevention of Pulmonary Arterial Hypertension. Biomed Res Int 2015: 762349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lim JH, Lee YM, Chun YS, Chen J, Kim JE, and Park JW. 2010. Sirtuin 1 modulates cellular responses to hypoxia by deacetylating hypoxia-inducible factor 1alpha. Mol Cell 38: 864–878. [DOI] [PubMed] [Google Scholar]
- 44.Lu B, Nakamura T, Inouye K, Li J, Tang Y, Lundback P, Valdes-Ferrer SI, Olofsson PS, Kalb T, Roth J, Zou Y, Erlandsson-Harris H, Yang H, Ting JP, Wang H, Andersson U, Antoine DJ, Chavan SS, Hotamisligil GS, and Tracey KJ. 2012. Novel role of PKR in inflammasome activation and HMGB1 release. Nature 488: 670–674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ito M, Shichita T, Okada M, Komine R, Noguchi Y, Yoshimura A, and Morita R. 2015. Bruton’s tyrosine kinase is essential for NLRP3 inflammasome activation and contributes to ischaemic brain injury. Nat Commun 6: 7360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gunderson AJ, Kaneda MM, Tsujikawa T, Nguyen AV, Affara NI, Ruffell B, Gorjestani S, Liudahl SM, Truitt M, Olson P, Kim G, Hanahan D, Tempero MA, Sheppard B, Irving B, Chang BY, Varner JA, and Coussens LM. 2016. Bruton Tyrosine Kinase-Dependent Immune Cell Cross-talk Drives Pancreas Cancer. Cancer Discov 6: 270–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.van Beijnum JR, Buurman WA, and Griffioen AW. 2008. Convergence and amplification of toll-like receptor (TLR) and receptor for advanced glycation end products (RAGE) signaling pathways via high mobility group B1 (HMGB1). Angiogenesis 11: 91–99. [DOI] [PubMed] [Google Scholar]
- 48.Suzuki S, Nakazato K, Sugimoto K, Yoshihisa A, Yamaki T, Kunii H, Suzuki H, Saitoh S, and Takeishi Y. 2016. Plasma Levels of Receptor for Advanced Glycation End-Products and High-Mobility Group Box 1 in Patients With Pulmonary Hypertension. Int Heart J 57: 234–240. [DOI] [PubMed] [Google Scholar]
- 49.Kang R, Tang D, Lotze MT, and Zeh HJ 3rd. 2011. RAGE regulates autophagy and apoptosis following oxidative injury. Autophagy 7: 442–444. [DOI] [PubMed] [Google Scholar]
- 50.Janko C, Filipovic M, Munoz LE, Schorn C, Schett G, Ivanovic-Burmazovic I, and Herrmann M. 2014. Redox modulation of HMGB1-related signaling. Antioxid Redox Signal 20: 1075–1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Park JS, Svetkauskaite D, He Q, Kim JY, Strassheim D, Ishizaka A, and Abraham E. 2004. Involvement of toll-like receptors 2 and 4 in cellular activation by high mobility group box 1 protein. J Biol Chem 279: 7370–7377. [DOI] [PubMed] [Google Scholar]
- 52.Yamasoba D, Tsubota M, Domoto R, Sekiguchi F, Nishikawa H, Liu K, Nishibori M, Ishikura H, Yamamoto T, Taga A, and Kawabata A. 2016. Peripheral HMGB1-induced hyperalgesia in mice: Redox state-dependent distinct roles of RAGE and TLR4. J Pharmacol Sci 130: 139–142. [DOI] [PubMed] [Google Scholar]
- 53.Jia D, He Y, Zhu Q, Liu H, Zuo C, Chen G, Yu Y, and Lu A. 2017. RAGE-mediated extracellular matrix proteins accumulation exacerbates HySu-induced pulmonary hypertension. Cardiovasc Res 113: 586–597. [DOI] [PubMed] [Google Scholar]
- 54.Lee S, Lee HC, Kwon YW, Lee SE, Cho Y, Kim J, Lee S, Kim JY, Lee J, Yang HM, Mook-Jung I, Nam KY, Chung J, Lazar MA, and Kim HS. 2014. Adenylyl cyclase-associated protein 1 is a receptor for human resistin and mediates inflammatory actions of human monocytes. Cell Metab 19: 484–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.West J, Niswender KD, Johnson JA, Pugh ME, Gleaves L, Fessel JP, and Hemnes AR. 2013. A potential role for insulin resistance in experimental pulmonary hypertension. Eur Respir J 41: 861–871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ingels C, Derese I, Wouters PJ, Van den Berghe G, and Vanhorebeek I. 2015. Soluble RAGE and the RAGE ligands HMGB1 and S100A12 in critical illness: impact of glycemic control with insulin and relation with clinical outcome. Shock 43: 109–116. [DOI] [PubMed] [Google Scholar]