Abstract
Background
Oxidative posttranslational modifications (OPTM) impair the function of Sarcoplasmic/endoplasmic reticulum (SR) calcium (Ca2+) ATPase (SERCA) 2 and trigger cytosolic Ca2+ dysregulation. We investigated the extent of OPTM of SERCA2 in patients with non-ischemic cardiomyopathy (NICM).
Methods and results
Endomyocardial biopsy (EMB) was obtained in 40 consecutive patients with NICM. Total expression and OPTM of SERCA2, including sulfonylation at cysteine-674 (S-SERCA2) and nitration at tyrosine-294/295 (N-SERCA2), were examined by immunohistochemical analysis. S-SERCA2 increased in the presence of late gadolinium enhancement on cardiac magnetic resonance imaging. S-SERCA2/SERCA2 and N-SERCA2/SERCA2 correlated with cardiac fibrosis evaluated by Masson’s trichrome staining of EMB. SERCA2 expression modestly increased in parallel with an upward trend in OPTM of SERCA2 with aging. This tendency became prominent only in patients aged >65 years. OPTM of SERCA2 positively correlated with brain natriuretic peptide (BNP) values only in patients aged ≤65 years. Composite major adverse cardiac events (MACE) increased more in the high OPTM group of younger patients; however, MACE-free survival was similar irrespective of the extent of OPTM in older patients.
Conclusions
OPTM of SERCA2 correlate with myocardial fibrosis in NICM. In younger patients, OPTM of SERCA2 correlate with elevated BNP and increased composite MACE.
Keywords: SERCA2, Oxidative posttranslational modification, Myocardial fibrosis, Non-ischemic cardiomyopathy
1. Introduction
Calcium (Ca2+) homeostasis is crucial for cell contraction and relaxation [1]; thus, cytosolic Ca2+ ([Ca2+]c) levels are tightly regulated during the normal contraction-relaxation cycle in the cardiomyocyte. Ca2+ influx through L-type Ca2+ channels during the cardiac action potential primarily triggers Ca2+ release from the sarcoplasmic/endoplasmic reticulum (SR) through the Ca2+ sensitive ryanodine receptor (RyR). Elevated [Ca2+]c leads to myocyte contraction. Alternatively, various pumps and exchangers remove [Ca2+]c to maintain a low level of [Ca2+]c during relaxation. SR Ca2+ ATPase (SERCA) and the sarcolemmal Na+-Ca2+ exchanger (NCX) play major roles in this process, but the balance between SERCA2 and NCX is subject to change in pathological conditions. SERCA2 and NCX produce 70% and 28%, respectively of Ca2+ flux during relaxation in the normal rabbit myocyte [2], but a similar amount (49%) of Ca2+ flux during relaxation in the myocyte of a rabbit heart failure model induced by aortic constriction [3]. Interestingly, mRNA and protein expression levels of SERCA2 in the hearts did not change in this model, indicating that posttranslational regulations of SERCA2 potentially could mediate the decrease in SERCA2 activity [3].
Previous studies on SERCA2 expression in end-stage human heart failure showed controversial results; some studies reported decreased mRNA and/or protein expression of SERCA2, whereas another study reported the decrease in mRNA expression of SERCA2 but not in protein expression [4], [5], [6]. However, historically, studies have showed consistently decreased SERCA activity in patients with heart failure [7], [8], [9].
More recently, SERCA2 targeted gene therapy was evaluated in the Calcium Upregulation by Percutaneous Administration of Gene Therapy in Cardiac Disease (CUPID) trials following the favorable results of experimental models of heart failure demonstrating increased expression of SERCA2 in cardiomyocytes with normalized intracellular Ca2+ cycling and improved cardiac function [10], [11], [12]. In the CUPID1 trial, 39 patients received intracoronary adeno-associated virus type 1 (AAV1)/SERCA2 or placebo. Some beneficial effects, including a reduction in clinical events and hospitalization duration, were observed [13]. However, CUPID2, a multinational, double-blind, placebo-controlled, phase 2b trial that enrolled 250 patients with New York Heart Association functional class II-IV symptoms of heart failure and left ventricular ejection fraction (LVEF) <35% could not demonstrate evidence of improved outcomes at the dose of AAV1/SERCA2 tested [14]. Posttranslational regulatory factors of SERCA2 in human heart failure were considered possible explanations for the failure of the CUPID2 trial.
SERCA2 is subject to a variety of posttranslational modifications, which result in alteration of SERCA2 activity and stability. The small ubiquitin-related modifier (SUMO) can be conjugated to lysine residues (lysine-480 and -585) of SERCA2 to preserve SERCA2 activity and stability. The level of SUMOylated SERCA2 has been shown to decrease significantly in human heart failure [15]. Acetylation and glutathionylation also play an important role to maintain SERCA2 activity [16], [17]. In contrast, several animal studies demonstrated that the decreased SERCA2 activity induced by oxidative posttranslational modifications (OPTM), including sulfonylation at cysteine-674 (Cys-674) and nitration at tyrosine-294/295 (Tyr-294/295), of SERCA2 contributed to the pathophysiology of heart failure [18], [19]. However, the role of OPTM of SERCA2 in human heart failure remains unclear. Therefore, we performed the present study to elucidate the role of OPTM of SERCA2 using human heart tissue from patients with non-ischemic cardiomyopathy (NICM).
2. Methods
The present study was a single-center, retrospective, cohort study investigating OPTM of SERCA2 of the myocardium in patients with NICM. Forty consecutive non-selected patients undergoing endomyocardial biopsies (EMB) for a diagnostic evaluation at our hospital between February 2014 and December 2016 were enrolled. Ischemic heart disease was ruled out before EMB by coronary computed tomography (CTCA), coronary angiography (CAG), or myocardial perfusion scintigraphy (MPS). Patients were classified with NICM if they had no history of myocardial infarction or revascularization and no evidence of >50% stenosis by CAG or CTCA, or no evidence of myocardial ischemia by MPS. This study complies with the Declaration of Helsinki, and our institutional ethics committee approved the protocol. Written informed consent was obtained from all enrolled patients.
2.1. Endomyocardial biopsy
EMB from more than one region of the right ventricular septum were obtained in all patients using a 5.5 F bioptome catheter (Cordis, Miami, FL, USA). The samples were fixed in 10% neutral buffered formalin at room temperature at the time of EMB while minimizing procedure-associated artifacts. The largest EMB tissue was used for analysis.
2.2. Immunohistochemical analysis
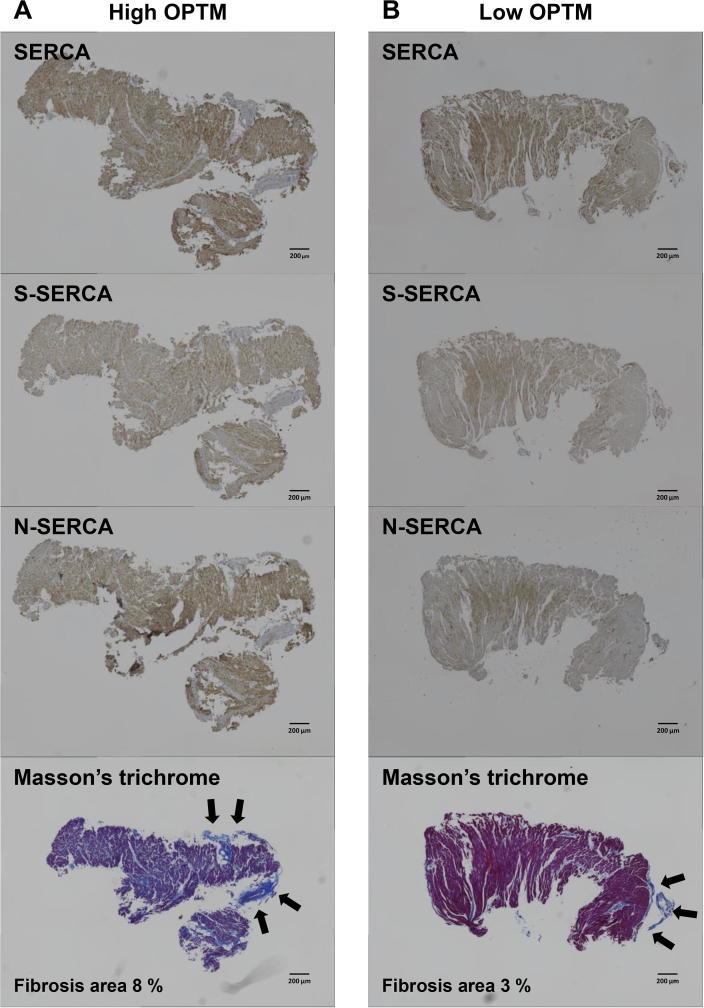
EMB samples were embedded in paraffin. Immunohistochemistry and Masson’s trichrome staining were performed as described previously [20]. The following primary antibodies were used: anti-SERCA2 antibody and site-specific antibodies that detect SERCA2 sulfonylation at Cys-674 [anti-SERCA2 C674-SO3H (S-SERCA2) antibody] or nitrotyrosine at Tyr-294/295 [anti-SERCA2 di-nY-294, 295 (N-SERCA2) antibody] (Bethyl Laboratories, Montgomery, TX, USA) [21], [22], [23]. Briefly, tissue sections (5-μm thick) were deparaffinized and rehydrated in xylene and decreasing concentrations of ethanol and immersed in 3% hydrogen peroxide to quench endogenous peroxidase activity. After an antigen retrieval procedure (heated in 10 mmol/L Tris-buffer [pH 10] for anti-SERCA2 staining; 10 mmol/L sodium citrate buffer [pH 6] for anti-S-SERCA2 antibody and anti-N-SERCA2 antibody), the sections were blocked with 10% bovine serum and subsequently incubated with specific primary antibodies at appropriate dilutions at 4 °C overnight. Then, tissues were incubated with Histofine Simple Stain MAX PO (MULTI) (Nichirei Biosciences Inc., Tokyo, JPN). Staining was revealed using diaminobenzidine (Nichirei Biosciences Inc., Tokyo, JPN), and counterstaining with aqueous hematoxylin was performed. Stained slides were visualized under a microscope (BZ-X700; Keyence, Osaka, JPN). Representative samples of each staining are shown in Fig. 1.
Fig. 1.
Representative pictures of immunohistochemistry (SERCA2, S-SERCA2, and N-SERCA2) and Masson’s trichrome staining, demonstrating high OPTM of SERCA2 (A) and low OPTM of SERCA2 (B) (scale bar = 200 μm). Black arrow indicates superficial fibrosis.
2.3. Evaluation of OPTM of SERCA2
A positively stained area for each primary antibody was quantified using BZ-X analyzer software (Keyence, Osaka, JPN). Percentages of the positively stained area by each antibody were calculated as (%) = (positively stained area)/(entire EMB tissue area), and these values were used as indices of protein expression [24], [25], [26]. OPTM of SERCA2 was determined by (S-SERCA2%)/(SERCA2%), (N-SERCA2%)/(SERCA2%), or (S-SERCA2% + N-SERCA2%)/(SERCA2%).
2.4. Evaluation of myocardial fibrosis
BZ-X analyzer software was used to quantify the total area of fibrosis stained blue with Masson’s trichrome staining. Superficial endocardial fibrosis tissue (Fig. 1, black arrow) was excluded from the analysis. Percentages of myocardial fibrosis was calculated as (%) = (Fibrosis area)/(entire EMB tissue area) [27].
2.5. Blood sampling
Peripheral venous blood was collected on admission within one week prior to EMB sampling for the assessment of serum creatinine and C-reactive protein. Plasma brain natriuretic peptide (BNP) levels were determined in all patients using an immunoenzymometric assay.
2.6. Measurement of cardiac function
Echocardiographic parameters, including the diameters and thickness of the left ventricle (LV), left atrium (LA), and LVEF were measured (EPIQ5/7, Philips Healthcare, Eindhoven, NLD). Further, tricuspid regurgitation pressure gradient, pulmonary regurgitation pressure gradient, mitral valve inflow pattern (E and A wave velocity), early diastolic mean velocity of the mitral annulus (mean e’), and the ratio of the early diastolic velocity of the mitral inflow to e’ (mean E/e’) were recorded. Echocardiography was performed by experienced, certified ultrasonographers or physicians within two weeks before EMB.
2.7. Late gadolinium enhancement and T1 mapping of cardiac magnetic resonance imaging
Cardiac magnetic resonance (CMR) was performed using a 1.5 Tesla system (Ingenia, Philips Healthcare, Eindhoven, NLD) within one month before EMB. A total dose of 0.2 mmol/kg gadolinium was injected, and 10–15 min after contrast injection, short- and long-axis 2D inversion recovery late gadolinium enhancement (LGE) images were obtained. Two physicians individually assessed the presence of LGE visually. The modified Look-Locker Inversion recovery (MOLLI) method was employed to measure T1 relaxation times as previously described [28]. Native and post contrast MOLLI T1 signals of myocardium (T1 myo) and blood (T1 blood) were obtained. Extracellular volume fraction (ECV), an indicator of myocardial fibrosis, was computed from the following formula [29].
ECV was calculated based on the American Heart Association 17 segment model and as the average of whole segments [30].
2.8. Statistical analysis
All values are expressed as means ± standard deviation for normally distributed data, and values with skewed distribution are expressed as medians with interquartile ranges. Baseline demographic and echocardiographic parameters were compared according to the ages ≤65 or >65 years using an unpaired t-test for normally distributed continuous variables, Mann-Whitney U test for non-parametric variables, and chi-squared test for categorical data. Linear regression analysis was performed to identify correlations between two parameters. The associations between parameters were assessed using the Pearson’s correlation test for parametric variables and the Spearman’s rank correlation test for non-parametric variables. Survival curves were constructed with the use of the Kaplan-Meier method for the composite endpoint of all-cause death, hospitalization because of the exacerbation of heart failure, or appropriate implantable cardioverter defibrillator (ICD) therapy for a spontaneous episode of ventricular tachycardia or fibrillation. The differences among groups were analyzed using the log-rank test. All P values <0.05 were considered statistically significant. Univariate and multivariable logistic regression analyses were performed to estimate the independent prognostic power. In multivariable analysis, LVEF and aldosterone blocker use were adjusted. All statistical analyses were performed using JMP pro version 14 software (SAS Institute Inc., Cary, NC, USA).
3. Results
3.1. Patient characteristics
Forty patients whose baseline clinical characteristics, medications, and echocardiographic parameters are shown in Table 1. Patients were stratified by age according to the median (65 years). Baseline characteristics were similar between the two groups. No significant differences in medication use, including angiotensin 2 receptor blockers/angiotensin-converting enzyme inhibitors, beta blockers, Ca2+ channel blockers, diuretics, statins, and nitrates were detected. Only the rate of aldosterone blocker use was higher in younger patients aged ≤65 years (P = 0.03). Echocardiographic parameters demonstrated lower LVEF, larger left ventricular end diastolic diameter (LVEDD), and larger left ventricular end systolic diameter (LVESD) in younger patients than in older patients (P = 0.01, 0.001, and 0.001, respectively).
Table 1.
Baseline characteristics.
| All (N = 40) | Age ≤ 65 (N = 19) | Age > 65 (N = 21) | P value | |
|---|---|---|---|---|
| Clinical parameters | ||||
| Age, yrs | 65.6 ± 11.8 | 59 (50–63) | 74.5 ± 5.2 | |
| Male, n (%) | 25 (62.5) | 11 (57.9) | 14 (66.7) | 0.57 |
| Serum creatinine, mg/dL | 0.89 (0.71–1.1) | 0.76 (0.68–1.12) | 0.93 ± 0.25 | 0.45 |
| eGFR, mL/min/1.73 m2 | 62.5 ± 17.9 | 65.9 ± 21.2 | 59.3 ± 14.1 | 0.25 |
| BNP, pg/mL | 199.6 (95.8–376.1) | 205.3 (96–272.5) | 194.1 (100.4–434.7) | 0.70 |
| CRP, mg/dL | 0.3 (0.3–0.48) | 0.3 (0.3–0.5) | 0.3 (0.3–0.6) | 0.68 |
| Medication | ||||
| ARB/ACEI, n (%) | 31 (77.5) | 16 (84.2) | 15 (71.4) | 0.33 |
| β blocker, n (%) | 22 (55) | 11 (57.8) | 11 (52.4) | 0.73 |
| Aldosterone blocker, n (%) | 16 (40) | 11 (57.8) | 5 (23.8) | 0.03 |
| CCB, n (%) | 8 (20) | 2 (10.5) | 6 (28.6) | 0.15 |
| Diuretics, n (%) | 15 (37.5) | 8 (42.1) | 7 (33.3) | 0.57 |
| statins, n (%) | 10 (25) | 3 (15.8) | 7 (33.3) | 0.20 |
| Nitrate, n (%) | 2 (5) | 1 (5.3) | 1 (4.8) | 0.94 |
| Echocardiographic parameters | ||||
| LVEF, % | 37.4 ± 18.4 | 29.6 ± 13.5 | 44.0 ± 19.7 | 0.01 |
| LVEDD, cm | 5.7 ± 0.8 | 6.2 ± 0.8 | 5.3 ± 0.7 | 0.001 |
| LVESD, cm | 4.7 ± 1.2 | 5.3 ± 1.0 | 4.1 ± 1.1 | 0.001 |
| IVS, cm | 0.9 (0.8–1.1) | 0.9 ± 0.2 | 1.0 ± 0.3 | 0.12 |
| PW, cm | 0.9 ± 0.1 | 0.9 ± 0.2 | 0.9 (0.8–1.1) | 0.80 |
| LAD, cm | 4.4 ± 1.0 | 4.5 ± 0.9 | 4.3 ± 1.0 | 0.46 |
| AOD, cm | 3.0 ± 0.4 | 3.1 ± 0.5 | 2.9 ± 0.3 | 0.3 |
| RVD, cm | 3.0 ± 0.8 | 3.1 ± 0.8 | 2.8 ± 0.8 | 0.55 |
| IVC, cm | 1.4 ± 0.5 | 1.5 ± 0.5 | 1.4 ± 0.5 | 0.56 |
| TRPG, mmHg | 25.4 ± 14.3 | 23.2 ± 14.6 | 27.2 ± 14.2 | 0.42 |
| PRPG, mmHg | 3 (2–6.5) | 3 (2–7.3) | 5 (2–6) | 0.92 |
| E wave velocity, cm/sec | 83.9 ± 26.2 | 85.2 ± 30.9 | 82.8 ± 22.1 | 0.79 |
| A wave velocity, cm/sec | 74.4 ± 26.5 | 74.5 ± 20.0 | 74.4 ± 32.5 | 0.99 |
| DCT, msec | 164 (121–207) | 141 (105–202) | 177 (155–220) | 0.14 |
| E/A | 1.0 (0.7–1.8) | 1.1 (0.6–1.8) | 1.0 (0.7–1.7) | 0.80 |
| E’ velocity, cm/sec | 5.0 ± 2.0 | 5.1 ± 2.4 | 5.0 ± 1.7 | 0.91 |
| E/e’ | 16.5 (12.3–22.3) | 16.1 (11.3–26.2) | 16.9 (13.7–22.2) | 0.95 |
| Immunohistochemical analysis | ||||
| SERCA% | 24.3 ± 8.8 | 23 ± 9.1 | 25.5 ± 8.5 | 0.38 |
| S-SERCA% | 16.5 ± 10.6 | 11.9 ± 5.1 | 20.1 ± 12.6 | 0.007 |
| N-SERCA% | 25.0 ± 10.8 | 21.4 ± 8.7 | 28.1 ± 11.6 | 0.04 |
| S-SERCA%/SERCA% | 0.7 ± 0.3 | 0.6 ± 0.3 | 0.8 ± 0.4 | 0.07 |
| N-SERCA%/SERCA% | 1.1 ± 0.5 | 1.0 ± 0.5 | 1.1 ± 0.4 | 0.51 |
| (S + N)-SERCA%/SERCA% | 1.8 ± 0.8 | 1.6 ± 0.8 | 2.0 ± 0.8 | 0.21 |
Unless otherwise specified, normally distributed values are presented as means ± standard deviations, and values with skewed distribution are expressed as medians with interquartile ranges.
yrs, years old; eGFR, estimated glomerular filtration rate; BNP, brain natriuretic peptide; CRP, C-reactive protein; ARB, angiotensin 2 receptor blocker; ACEI, angiotensin-converting enzyme inhibitor; CCB, calcium channel blocker; LVEF, left ventricular ejection fraction; LVEDD, left ventricular end diastolic diameter; LVESD, left ventricular end systolic diameter; IVS, interventricular septum; PW, posterior wall; LA, left atrium; AO, aorta; RV, right ventricle; IVC, inferior vena cava; TRPG, tricuspid regurgitation pressure gradient; PRPG, pulmonary regurgitation pressure gradient; DCT, deceleration time; SERCA, sarcoplasmic/endoplasmic reticulum Ca2+ ATPase; S-SERCA, sulfonylation at cysteine-674; N-SERCA, nitration at tyrosine-294/295.
3.2. LGE in CMR, myocardial fibrosis, and OPTM of SERCA2
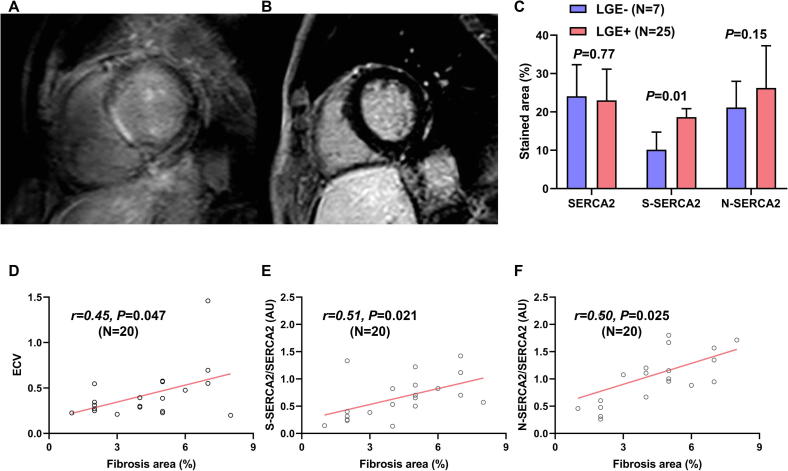
CMR can detect myocardial fibrosis, appearing as LGE. The representative images of LGE-present and -absent hearts are shown in Fig. 2A and B, respectively. The presence of LGE predicts an 8-fold increased risk of adverse cardiac outcomes, including hospitalization for heart failure, appropriate ICD treatment, and cardiac death in NICM [31]. In the present study, 25 of 32 patients who underwent CMR (78.1%) had LGE with the following regional patterns: septal mid-wall (N = 12), subendocardial extending to the epicardial surface (N = 3), and patchy foci (N = 10). We then investigated OPTM of SERCA2 in patients grouped by the presence or absence of CMR LGE as a surrogate marker of worse clinical outcomes in NICM. S-SERCA2 was significantly increased in LGE-present hearts compared to LGE-absent hearts (P = 0.008), whereas N-SERCA2 expression did not reach statistical difference between the LGE-present and -absent groups (P = 0.15) (Fig. 2C). In 32 patients who underwent CMR, T1 mapping was performed in 20 patients. Septal ECV showed a moderate correlation with myocardial fibrosis evaluated by Masson’s trichrome staining of EMB tissues (r = 0.45, P = 0.047) (Fig. 2D). S-SERCA2 and S-SERCA2/SERCA2 correlated with septal ECV (r = 0.67, P = 0.002 for S-SERCA2; r = 0.43, P = 0.066 for S-SERCA2/SERCA2). In order to evaluate the direct correlation between myocardial fibrosis and OPTM of SERCA2 in the same EMB tissue, Masson’s trichrome staining of 20 EMB tissue samples from patients with available T1 mapping data of CMR was performed. Both S-SERCA2/SERCA2 and N-SERCA2/SERCA2 showed similar positive correlation with myocardial fibrosis evaluated by Masson’s trichrome staining of EMB tissues (r = 0.51, P = 0.02 for S-SERCA2/SERCA2; r = 0.50, P = 0.02 for N-SERCA2/SERCA2) (Fig. 2E and F).
Fig. 2.
Representative cardiac magnetic resonance late gadolinium enhancement (LGE) images of LGE-present (A) and LGE-absent hearts (B). (C) The S-SERCA2 stained area was significantly increased in CMR LGE-present hearts (Unpaired t-test P = 0.008). N-SERCA2 tended to increase in CMR LGE-present hearts, but this was not statistically significant (Unpaired t-test P = 0.15). (D) Septal extracellular volume fraction showed significant positive correlation with fibrosis area (%) evaluated by Masson’s trichrome staining of the same tissue (Pearson’s correlation test r = 0.45, P = 0.047 [N = 20]). S-SERCA2/SERCA2 (E) and N-SERCA2/SERCA2 (F) showed significant positive correlation with fibrosis area (%) evaluated by Masson’s trichrome staining of the same EMB tissue (Pearson’s correlation test r = 0.51, P = 0.02 for S-SERCA2/SERCA2 [N = 20]; r = 0.50, P = 0.02 for N-SERCA2/SERCA2 [N = 20]).
3.3. Changes in the SERCA2 expression and the OPTM of SERCA2 with aging
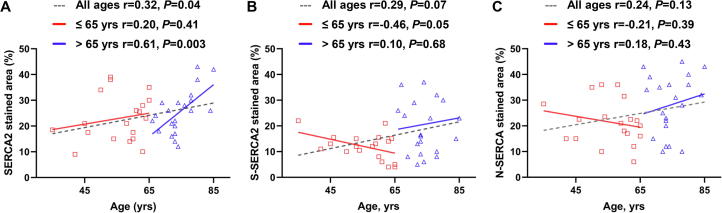
Based on previous studies with respect to aging and SERCA2 activity in rodents, the OPTM of SERCA2 was shown to increase with aging, leading to partial inactivation of the SR Ca2+ uptake activity in senescent hearts [18]. Therefore, the association between aging and the expression and OPTM of SERCA2 was investigated. Interestingly, SERCA2 expression modestly, but significantly, increased with aging (r = 0.32, P = 0.04) (Fig. 3A). Furthermore, in older patients aged >65 years, the impact of aging on SERCA2 expression became remarkably conspicuous (r = 0.61, P = 0.003), whereas in younger patients aged ≤65 years, aging did not affect SERCA2 expression (Fig. 3A), consistent with a previous report [32]. S-SERCA2 and N-SERCA2 tended to increase with aging, but did not reach statistical significance (r = 0.29, P = 0.071 for S-SERCA2; r = 0.24, P = 0.13 for N-SERCA2) (Fig. 3B and C, respectively).
Fig. 3.
A, SERCA2 modestly increased with aging in patients with non-ischemic cardiomyopathy (Pearson’s correlation test r = 0.32, P = 0.044). This association became prominent in older patients aged >65 years (Pearson’s correlation test r = 0.61, P = 0.003), but was not observed in younger patients aged ≤65 years (Pearson’s correlation test r = 0.20, P = 0.41). B and C, OPTM of SERCA2 tended to increase with aging, though this was not statistically significant (Pearson’s correlation test r = 0.29, P = 0.071 for S-SERCA2; r = 0.24, P = 0.13 for N-SERCA).
3.4. Cardiac function, brain natriuretic peptide values, and OPTM of SERCA2
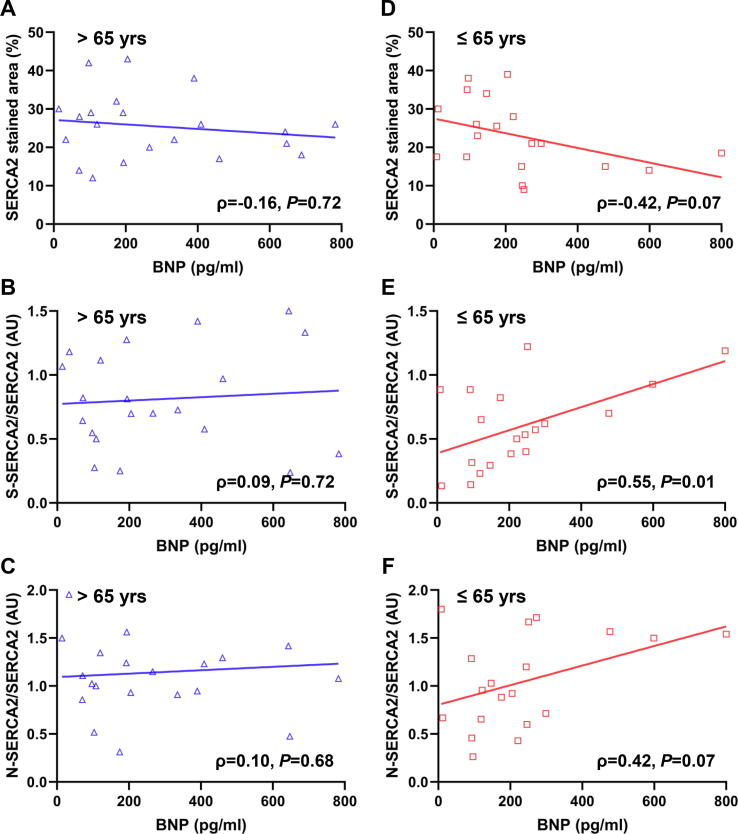
In the present study, neither LVEF nor E/e’ were significantly correlated with OPTM of SERCA2 in all age groups (data not shown). To further elucidate the effect of OPTM of SERCA2 on heart failure, we investigated the association between BNP values and SERCA2 expression, and the association between BNP values and OPTM of SERCA2 based on age groups of ≤65 or >65 years. SERCA2 expression did not show significant correlation with BNP value in all ages (Fig. 4A and D). In older patients (>65 years old), no correlation was found between BNP values and OPTM of SERCA2 (ρ = 0.09, P = 0.72 for S-SERCA2; ρ = 0.10, P = 0.68 for N-SERCA2) (Fig. 4B and C). In contrast, in younger patients (≤65 years old), a strong positive correlation was observed between BNP values and OPTM of SERCA2, especially in S-SERCA2/SERCA2 (ρ = 0.55, P = 0.01 for S-SERCA2; ρ = 0.42, P = 0.07 for N-SERCA2) (Fig. 4E and F). BNP values were not different between patients with aldosterone blocker treatment and those without (119.0 [119.6–568.4] pg/mL vs. 119.6 [93.8–317.7] pg/mL, P = 0.35 in all ages; 205.3 [118.9–477.0] pg/mL vs.184.0 [93.8–245.8] pg/mL, P = 0.32 in patients ≤65 years; 192.7 [135.9–647.5] pg/mL vs. 199.6 [79.5–404.3] pg/mL, P = 0.62 in patients >65 years).
Fig. 4.
A, SERCA2 expression did not correlate with BNP values in older patients >65 years (Spearman’s rank correlation test ρ = −0.16, P = 0.72). B and C, OPTM of SERCA2 did not correlate with BNP values in older patients >65 years (Spearman’s rank correlation test ρ = 0.09, P = 0.72 for S-SERCA2; ρ = 0.10, P = 0.68 for N-SERCA2). D, SERCA2 did not correlate with BNP values in younger patients ≤65 years (Spearman’s rank correlation test ρ = −0.42, P = 0.07). E and F, Association between OPTM of SERCA2 and BNP values showed a positive correlation (Spearman’s rank correlation test ρ = 0.55, P = 0.01 for S-SERCA2; ρ = 0.42, P = 0.07 for N-SERCA2) in younger patients ≤65 years old with non-ischemic cardiomyopathy.
3.5. Composite MACE and OPTM of SERCA2
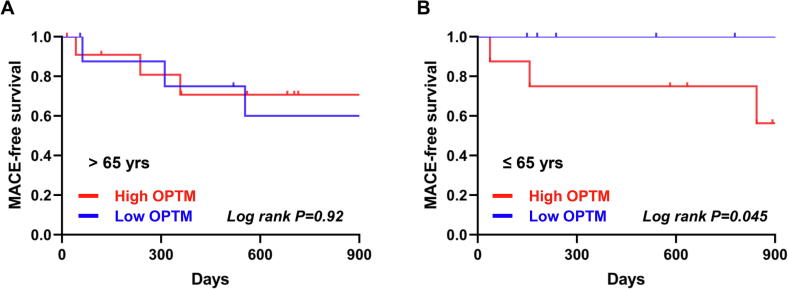
Finally, we investigated the association of composite MACE and OPTM of SERCA2 based on age groups of ≤65 or >65 years. We divided patients into two groups according to median of S-SERCA2 + N-SERCA2, defined as the low and high OPTM groups. Three of 8 patients (37.5%) in younger patients ≤65 years with high OPTM had MACE, whereas 3 of 12 patients (25.0%) in older patients >65 years with high OPTM had MACE. In older patients (>65 years old), no significant differences were detected between the low and high OPTM groups (Log rank P = 0.92) (Fig. 5A). In contrast, in younger patients (≤65 years old), MACE-free survival was significantly lower in the high OPTM group versus the low OPTM group (Log rank P = 0.045) (Fig. 5B), which corresponded to the association between BNP values and OPTM of SERCA2. In univariate analysis, high OPTM was significantly associated with MACE in patients ≤65 years old (P = 0.03) (Table 2). High OPTM was independently associated with MACE after adjustment for LVEF and aldosterone blocker use in patients ≤65 years old (P = 0.02) (Table 3).
Fig. 5.
Kaplan-Meier plot showing MACE-free survival in patients (A) >65 years and (B) ≤65 years with non-ischemic cardiomyopathy. The rate of composite MACE including cardiovascular death, appropriate ICD therapy, and hospitalization due to exacerbation of heart failure was significantly higher in the high OPTM (>median) group compared to the low OPTM (≤median) group in younger patients (Log rank P = 0.045), whereas the extent of OPTM did not affect MACE-free survival in older patients (Log rank P = 0.92).
Table 2.
Association between high OPTM and MACE with stratification by age, sex, and LVEF.
| High OPTM | MACE | Odds ratio | 95% CI | P value | |
|---|---|---|---|---|---|
| Age | |||||
| ≤65 years | 8/19 (42.1) | 3/19 (15.8) | * | * | 0.01 |
| >65 years | 12/21 (57.1) | 6/21 (28.6) | 0.67 | [0.10–4.48] | 0.68 |
| Sex | |||||
| Male | 10/25 (40.0) | 5/25 (20.0) | 2.79 | [0.37–20.82] | 0.31 |
| Female | 10/15 (66.7) | 4/15 (26.7) | 1.71 | [0.13–22.51] | 0.68 |
| LVEF | |||||
| <40% | 10/23 (43.5) | 1/23 (4.4) | * | * | 0.24 |
| ≥40% | 10/16 (62.5) | 8/16 (50.0) | 1.00 | [0.13–7.57] | 1.00 |
Odds ratio and 95% CI were blank (*) in patients ≤65 years and patients with LVEF <40% because all MACE were observed in patients with high OPTM. OPTM, oxidative posttranslational modifications; MACE, major cardiovascular events; LVEF, left ventricular ejection fraction; CI, confidence interval.
Table 3.
Association between high OPTM and MACE after LVEF in multivariate analysis.
| Adjusted for LVEF |
Adjusted for LVEF and aldosterone blocker use |
|||||
|---|---|---|---|---|---|---|
| Odds ratio | 95% CI | P Value | Odds ratio | 95% CI | P Value | |
| All ages | 2.0 | [0.4–11.2] | 0.42 | 7.7 | [0.64–91.2] | 0.11 |
| ≤65 years | * | * | 0.02 | * | * | 0.02 |
| >65 years | 0.5 | [0.1–5.4] | 0.57 | 5.5 | [0.1–414.3] | 0.40 |
Odds ratio and 95% CI were blank (*) in patients ≤65 years because all MACE was observed in patients with high OPTM. LVEF, left ventricular ejection fraction; CI, confidence interval.
4. Discussion
SERCA2 uptakes [Ca2+]c into the SR in order to maintain low levels of [Ca2+]c, especially during the diastolic phase. SERCA2 is predominantly expressed in the myocardium for the control of systolic and diastolic properties of the heart. OPTM impairs the function of SERCA2, resulting in dysregulation of intracellular Ca2+ concentration and cardiac function. In the present study, we demonstrated that OPTM of SERCA2 was associated with elevated BNP and increased composite MACE in patients ≤65 years old.
Nitric oxide (NO) stimulates SERCA2 activity by S-glutathiolation at Cys-674. Sulfonylation at Cys-674 prevents NO-induced activation of SERCA2; thus, NO-induced arterial relaxation is impaired in a pathological condition with excessive oxygen stress, such as atherosclerosis [17]. Similarly, Cys-674 sulfonylation was found to decrease SERCA2 activity and impair myocyte relaxation in senescent hearts in mice [18]. Tyrosine nitration also plays a vital role in posttranslational modification of proteins in a variety of disease processes. Tyrosine residues of SERCA2 Tyr-294/295 were identified as central targets for tyrosine nitration, acting to downregulate SERCA2 activity in aging skeletal and cardiac muscle [19]. Lancel et al. reported that OPTM of SERCA2, including sulfonylation at Cys-674 and nitration at Tyr-294/295, partly mediated Gaq-induced cardiac contractile dysfunction, a model of NICM, and Ca2+ dysregulation induced by decreased SERCA2 activity [22]. In contrast, the overexpression of catalase protected from OPTM of SERCA2 restored SERCA2 activity and improved cardiac function without any changes in SERCA2 protein levels [22]. Previous animal studies suggested that OPTM of SERCA2 contributed to the pathophysiology of myocardial dysfunction in heart failure, but the role of OPTM of SERCA2 in humans has yet to be determined. To elucidate this question, we performed the present study using EMB of patients with NICM.
First, we showed that OPTM of SERCA2, especially S-SERCA2, was significantly increased in the CMR LGE-present hearts (Fig. 2C), which denotes the presence of fibrosis and correlates with a higher risk of cardiovascular events in NICM. In fact, OPTM of SERCA2 was significantly correlated with myocardial fibrosis evaluated by Masson’s trichrome staining of the same EMB tissue (Fig. 2E and F). Though a causal link between OPTM of SERCA2 and myocardial fibrosis could not be drawn from the present study, these results indicate that OPTM of SERCA2 and subsequent [Ca2+]c dysregulation are involved in human heart failure. This association between OPTM of SERCA2 and myocardial fibrosis is consistent with the results of a previous study showing that myocardial fibrosis was mitigated by upregulation of SERCA2 in mice [33]. Further, a recent study reported that fibroblasts exacerbate [Ca2+]c dysregulation in failing myocytes by reducing SR Ca2+ content [34]. Thus, [Ca2+]c dysregulation by fibroblast-myocyte coupling might further aggravate [Ca2+]c dysregulation by OPTM of SERCA2.
In the present study, we found an interesting association between aging and SERCA2 expression in patients with NICM (Fig. 3A). Younger patients aged ≤65 years did not show a positive correlation between age and SERCA2 expression, whereas older patients aged >65 years demonstrated a strong positive correlation between age and SERCA2 expression. Based on these data, we hypothesize that SERCA2 expression increases with aging in order to compensate for the decreased SERCA2 activity caused by the aging-related increase in SERCA2 OPTM. If this is true, it could explain why OPTM of SERCA2 demonstrated a significant positive correlation with BNP values and composite MACE only in younger patients, because decreased SERCA2 activity induced by OPTM of SERCA2 was well compensated by increased SERCA2 expression in older patients, whereas decreased SERCA2 activity induced by OPTM of SERCA2 was not fully offset by increasing SERCA2 expression in younger patients. However, the explanation might instead be a complex regulatory structure of SERCA2 expression or the difference in the etiologies of NICM between young and old patients.
Most of the MACE occurring in the present study was hospitalization due to the exacerbation of heart failure, but it should be mentioned that we observed one patient with sudden cardiac death and one patient with appropriate ICD therapy, both from younger patients in the higher OPTM group. Abnormal SR Ca2+ leak from RyR2 has been shown to be a cause of arrhythmogenic disorders, such as catecholaminergic polymorphic ventricular tachycardia and arrhythmogenic right ventricular cardiomyopathy type 2 [35], [36]. [Ca2+]c dysregulation induced by decreased SERCA2 activity also might influence arrhythmogenicity in patients with heart failure.
[Ca2+]c is the primary determinant of myocardial contractile properties. Therefore, multiple ion channels, transporters, and pumps are required to work in a highly coordinated manner. In failing hearts, OPTM may alter the functions of other [Ca2+]c handling proteins besides SERCA2. OPTM of the ryanodine receptor induced by increased oxidative stress has been shown to increase SR Ca2+ leak, leading to fatal arrhythmias [37], [38]. Phospholamban, a reversible inhibitor of SERCA2, also is a target of functional modulation through posttranslational modifications. Phospholamban phosphorylation leads to a decrease in its inhibitory effect on SERCA2 and to increased SERCA2 activity [39]. However, elevated protein phosphatase activity induces dephosphorylation of phospholamban in failing hearts [40]. Oxidative stress has been indicated to play a key role in multiple processes leading to decreased SERCA2 activity [41]. In this pilot study, we focused on the OPTM of SERCA2. However, we cannot exclude a possible alteration of other Ca2+ handling proteins, which will require future investigations.
The present study has several limitations. First, this investigation was a retrospective study based on histological analysis of EMB. Therefore, the results of this study should be considered as hypothesis generating rather than conclusive. We could not analyze the regulation of SERCA2 at the protein or mRNA levels, and we could not measure SR Ca2+ uptake and/or ATPase activity of SERCA2 in this study. Direct measurement of the protein or SERCA2 activities would strengthen the findings of this study. Second, the lack of oxidative stress markers and other biomarkers such as Troponin and H-FABP limits our ability to evaluate the possible mechanistic link of oxidative stress and myocardial injury to OPTM of SERCA2 and its association with MACE. Third, we could not observe the association between OPTM of SERCA2 and systolic and diastolic functions of the left ventricles evaluated by echocardiography at rest. Stress echocardiography is necessary to conclude the association between OPTM of SERCA2 and cardiac function, and to demonstrate the effect of OPTM of SERCA2 on MACE. Further, we only had EMB tissues from the right ventricles. Given the diagnostic accuracy of right ventricular EMB is substantially lower than left ventricular EMB when the structural and functional abnormalities affect exclusively left ventricles, the association between OPTM of SERCA2 and left ventricular function might be underestimated [42]. Fourth, LVEF was significantly different between age groups, and higher LVEF correlated with a higher rate of composite MACE in this cohort (data not shown). However, high OPTM of SERCA2 is an independent predictor of MACE after adjustment for LVEF in younger patients aged ≤65 years (Table 3). Finally, we enrolled a relatively small number of patients with a variety of cardiomyopathies. However, in the cohort including different types of non-ischemic cardiomyopathies, consistent data with respect to the association between OPTM of SERCA2 and clinical worsening was observed utilizing various observation methods, including CMR imaging, laboratory markers, and composite MACE, thereby indicating that OPTM of SERCA2 might be a universal target for the treatment of heart failure due to diverse causes in younger patients ≤65 years old.
Thus far, SUMOylation of lysine residues of SERCA2 has been validated as an effective treatment among the therapies targeting posttranslational modifications of SERCA2 [43]. Further, histone deacetylase inhibition improved efficiency of [Ca2+]c removal by increasing SERCA2 acetylation with a concomitant increase in SERCA2 activity in rats [16]. Also, histone deacetylase inhibition prevented ventricular arrhythmias in dystrophic mice [44]. The results of these studies provide us with the full range of possibilities for the protection of SERCA2 from OPTM as a feasible therapeutic target for heart failure.
In summary, we identified the OPTM of SERCA2 potentially to be involved in the pathogenesis of heart failure in patients with NICM on the basis of immunohistological analysis using EMB. Therapy targeting OPTM of SERCA2 might be a more effective treatment for younger patients with NICM. Direct assessment of SERCA activity in the myocardium will reinforce our findings of this study. Additionally, future research utilizing stress echocardiography needs to be performed to demonstrate how OPTM of SERCA2 affects cardiac function. Also, further studies are necessary to address the impact of OPTM of SERCA2 in patients with ischemic cardiomyopathy for future application of therapy targeting OPTM of SERCA2 in this population.
Grants
This work was supported by a grant from the Ministry of Defense (Japan). The funding source had no involvement in the study design, analysis, and interpretation of the data.
Declaration of Competing Interest
None
Acknowledgements
We would like to thank members of the Department of Pathology and Laboratory Medicine, National Defense Medical College for assistance with pathology.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ijcha.2019.100437.
Appendix A. Supplementary material
The following are the Supplementary data to this article:
References
- 1.Bers D.M. Calcium fluxes involved in control of cardiac myocyte contraction. Circ. Res. 2000;87(4):275–281. doi: 10.1161/01.res.87.4.275. [DOI] [PubMed] [Google Scholar]
- 2.Bassani J.W., Bassani R.A., Bers D.M. Relaxation in rabbit and rat cardiac cells: species-dependent differences in cellular mechanisms. J. Physiol. 1994;476(2):279–293. doi: 10.1113/jphysiol.1994.sp020130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pogwizd S.M., Qi M., Yuan W., Samarel A.M., Bers D.M. Upregulation of Na(+)/Ca(2+) exchanger expression and function in an arrhythmogenic rabbit model of heart failure. Circ. Res. 1999;85(11):1009–1019. doi: 10.1161/01.res.85.11.1009. [DOI] [PubMed] [Google Scholar]
- 4.Mercadier J.J., Lompre A.M., Duc P., Boheler K.R., Fraysse J.B., Wisnewsky C., Allen P.D., Komajda M., Schwartz K. Altered sarcoplasmic reticulum Ca2(+)-ATPase gene expression in the human ventricle during end-stage heart failure. J. Clin. Investig. 1990;85(1):305–309. doi: 10.1172/JCI114429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Takahashi T., Allen P.D., Izumo S. Expression of A-, B-, and C-type natriuretic peptide genes in failing and developing human ventricles. Correlation with expression of the Ca(2+)-ATPase gene. Circ. Res. 1992;71(1):9–17. doi: 10.1161/01.res.71.1.9. [DOI] [PubMed] [Google Scholar]
- 6.Linck B., Boknik P., Eschenhagen T., Muller F.U., Neumann J., Nose M., Jones L.R., Schmitz W., Scholz H. Messenger RNA expression and immunological quantification of phospholamban and SR-Ca(2+)-ATPase in failing and nonfailing human hearts. Cardiovasc. Res. 1996;31(4):625–632. [PubMed] [Google Scholar]
- 7.Schwinger R.H., Bohm M., Schmidt U., Karczewski P., Bavendiek U., Flesch M., Krause E.G., Erdmann E. Unchanged protein levels of SERCA II and phospholamban but reduced Ca2+ uptake and Ca(2+)-ATPase activity of cardiac sarcoplasmic reticulum from dilated cardiomyopathy patients compared with patients with nonfailing hearts. Circulation. 1995;92(11):3220–3228. doi: 10.1161/01.cir.92.11.3220. [DOI] [PubMed] [Google Scholar]
- 8.Hasenfuss G., Reinecke H., Studer R., Meyer M., Pieske B., Holtz J., Holubarsch C., Posival H., Just H., Drexler H. Relation between myocardial function and expression of sarcoplasmic reticulum Ca(2+)-ATPase in failing and nonfailing human myocardium. Circ. Res. 1994;75(3):434–442. doi: 10.1161/01.res.75.3.434. [DOI] [PubMed] [Google Scholar]
- 9.Limas C.J., Olivari M.T., Goldenberg I.F., Levine T.B., Benditt D.G., Simon A. Calcium uptake by cardiac sarcoplasmic reticulum in human dilated cardiomyopathy. Cardiovasc. Res. 1987;21(8):601–605. doi: 10.1093/cvr/21.8.601. [DOI] [PubMed] [Google Scholar]
- 10.Hajjar R.J., Kang J.X., Gwathmey J.K., Rosenzweig A. Physiological effects of adenoviral gene transfer of sarcoplasmic reticulum calcium ATPase in isolated rat myocytes. Circulation. 1997;95(2):423–429. doi: 10.1161/01.cir.95.2.423. [DOI] [PubMed] [Google Scholar]
- 11.Davia K., Bernobich E., Ranu H.K., del Monte F., Terracciano C.M., MacLeod K.T., Adamson D.L., Chaudhri B., Hajjar R.J., Harding S.E. SERCA2A overexpression decreases the incidence of aftercontractions in adult rabbit ventricular myocytes. J. Mol. Cell. Cardiol. 2001;33(5):1005–1015. doi: 10.1006/jmcc.2001.1368. [DOI] [PubMed] [Google Scholar]
- 12.Kawase Y., Ly H.Q., Prunier F., Lebeche D., Shi Y., Jin H., Hadri L., Yoneyama R., Hoshino K., Takewa Y., Sakata S., Peluso R., Zsebo K., Gwathmey J.K., Tardif J.C., Tanguay J.F., Hajjar R.J. Reversal of cardiac dysfunction after long-term expression of SERCA2a by gene transfer in a pre-clinical model of heart failure. J. Am. Coll. Cardiol. 2008;51(11):1112–1119. doi: 10.1016/j.jacc.2007.12.014. [DOI] [PubMed] [Google Scholar]
- 13.Jessup M., Greenberg B., Mancini D., Cappola T., Pauly D.F., Jaski B., Yaroshinsky A., Zsebo K.M., Dittrich H., Hajjar R.J. Calcium upregulation by percutaneous administration of gene therapy in cardiac disease (CUPID): a phase 2 trial of intracoronary gene therapy of sarcoplasmic reticulum Ca2+-ATPase in patients with advanced heart failure. Circulation. 2011;124(3):304–313. doi: 10.1161/CIRCULATIONAHA.111.022889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Greenberg B., Butler J., Felker G.M., Ponikowski P., Voors A.A., Desai A.S., Barnard D., Bouchard A., Jaski B., Lyon A.R., Pogoda J.M., Rudy J.J., Zsebo K.M. Calcium upregulation by percutaneous administration of gene therapy in patients with cardiac disease (CUPID 2): a randomised, multinational, double-blind, placebo-controlled, phase 2b trial. Lancet (London, England) 2016;387(10024):1178–1186. doi: 10.1016/S0140-6736(16)00082-9. [DOI] [PubMed] [Google Scholar]
- 15.Kho C., Lee A., Jeong D., Oh J.G., Chaanine A.H., Kizana E., Park W.J., Hajjar R.J. SUMO1-dependent modulation of SERCA2a in heart failure. Nature. 2011;477(7366):601–605. doi: 10.1038/nature10407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Meraviglia V., Bocchi L., Sacchetto R., Florio M.C., Motta B.M., Corti C., Weichenberger C.X., Savi M., D'Elia Y., Rosato-Siri M.D., Suffredini S., Piubelli C., Pompilio G., Pramstaller P.P., Domingues F.S., Stilli D., Rossini A. HDAC inhibition improves the sarcoendoplasmic reticulum Ca(2+)-ATPase activity in cardiac myocytes. Int. J. Mol. Sci. 2018;19(2):419. doi: 10.3390/ijms19020419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Adachi T., Weisbrod R.M., Pimentel D.R., Ying J., Sharov V.S., Schoneich C., Cohen R.A. S-Glutathiolation by peroxynitrite activates SERCA during arterial relaxation by nitric oxide. Nat. Med. 2004;10(11):1200–1207. doi: 10.1038/nm1119. [DOI] [PubMed] [Google Scholar]
- 18.Qin F., Siwik D.A., Lancel S., Zhang J., Kuster G.M., Luptak I., Wang L., Tong X., Kang Y.J., Cohen R.A., Colucci W.S. Hydrogen peroxide-mediated SERCA cysteine 674 oxidation contributes to impaired cardiac myocyte relaxation in senescent mouse heart. J. Am. Heart Assoc. 2013;2(4) doi: 10.1161/JAHA.113.000184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Knyushko T.V., Sharov V.S., Williams T.D., Schoneich C., Bigelow D.J. 3-Nitrotyrosine modification of SERCA2a in the aging heart: a distinct signature of the cellular redox environment. Biochemistry. 2005;44(39):13071–13081. doi: 10.1021/bi051226n. [DOI] [PubMed] [Google Scholar]
- 20.Hakuno D., Kimura N., Yoshioka M., Mukai M., Kimura T., Okada Y., Yozu R., Shukunami C., Hiraki Y., Kudo A., Ogawa S., Fukuda K. Periostin advances atherosclerotic and rheumatic cardiac valve degeneration by inducing angiogenesis and MMP production in humans and rodents. J. Clin. Investig. 2010;120(7):2292–2306. doi: 10.1172/JCI40973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xu S., Ying J., Jiang B., Guo W., Adachi T., Sharov V., Lazar H., Menzoian J., Knyushko T.V., Bigelow D., Schoneich C., Cohen R.A. Detection of sequence-specific tyrosine nitration of manganese SOD and SERCA in cardiovascular disease and aging. Am. J. Physiol. Heart Circ. Physiol. 2006;290(6):H2220–H2227. doi: 10.1152/ajpheart.01293.2005. [DOI] [PubMed] [Google Scholar]
- 22.Lancel S., Qin F., Lennon S.L., Zhang J., Tong X., Mazzini M.J., Kang Y.J., Siwik D.A., Cohen R.A., Colucci W.S. Oxidative posttranslational modifications mediate decreased SERCA activity and myocyte dysfunction in Galphaq-overexpressing mice. Circ. Res. 2010;107(2):228–232. doi: 10.1161/CIRCRESAHA.110.217570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ying J., Sharov V., Xu S., Jiang B., Gerrity R., Schoneich C., Cohen R.A. Cysteine-674 oxidation and degradation of sarcoplasmic reticulum Ca(2+) ATPase in diabetic pig aorta. Free Radic. Biol. Med. 2008;45(6):756–762. doi: 10.1016/j.freeradbiomed.2008.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rizzardi A.E., Johnson A.T., Vogel R.I., Pambuccian S.E., Henriksen J., Skubitz A.P., Metzger G.J., Schmechel S.C. Quantitative comparison of immunohistochemical staining measured by digital image analysis versus pathologist visual scoring. Diagn. Pathol. 2012;7 doi: 10.1186/1746-1596-7-42. 42 42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Verhaart I.E.C., Putker K., van de Vijver D., Tanganyika-de Winter C.L., Pasteuning-Vuhman S., Plomp J.J., Aartsma-Rus A.M., van Putten M. Cross-sectional study into age-related pathology of mouse models for limb girdle muscular dystrophy types 2D and 2F. PloS One. 2019;14(8) doi: 10.1371/journal.pone.0220665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sakaue T., Shikata F., Utsunomiya K., Fukae S., Kurata M., Nakaoka H., Okazaki M., Kawanishi Y., Kojima A., Higashiyama S., Izutani H. Proteomics-based analysis of lung injury–induced proteins in a mouse model of common bile duct ligation. Surgery. 2017;161(6):1525–1535. doi: 10.1016/j.surg.2016.12.017. [DOI] [PubMed] [Google Scholar]
- 27.Matundan H.H., Sin J., Rivas M.N., Fishbein M.C., Lehman T.J., Chen S., Gottlieb R.A., Crother T.R., Abe M., Arditi M. Myocardial fibrosis after adrenergic stimulation as a long-term sequela in a mouse model of Kawasaki disease vasculitis. JCI Insight. 2019;4(3) doi: 10.1172/jci.insight.126279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Messroghli D.R., Radjenovic A., Kozerke S., Higgins D.M., Sivananthan M.U., Ridgway J.P. Modified Look-Locker inversion recovery (MOLLI) for high-resolution T1 mapping of the heart. Magn. Reson. Med. 2004;52(1):141–146. doi: 10.1002/mrm.20110. [DOI] [PubMed] [Google Scholar]
- 29.Haaf P., Garg P., Messroghli D.R., Broadbent D.A., Greenwood J.P., Plein S. Cardiac T1 mapping and extracellular volume (ECV) in clinical practice: a comprehensive review. J. Cardiovasc. Magn. Reson. 2016;18(1):89. doi: 10.1186/s12968-016-0308-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cerqueira M.D., Weissman N.J., Dilsizian V., Jacobs A.K., Kaul S., Laskey W.K., Pennell D.J., Rumberger J.A., Ryan T., Verani M.S. Standardized myocardial segmentation and nomenclature for tomographic imaging of the heart. A statement for healthcare professionals from the Cardiac Imaging Committee of the Council on Clinical Cardiology of the American Heart Association. Circulation. 2002;105(4):539–542. doi: 10.1161/hc0402.102975. [DOI] [PubMed] [Google Scholar]
- 31.Wu K.C., Weiss R.G., Thiemann D.R., Kitagawa K., Schmidt A., Dalal D., Lai S., Bluemke D.A., Gerstenblith G., Marban E., Tomaselli G.F., Lima J.A. Late gadolinium enhancement by cardiovascular magnetic resonance heralds an adverse prognosis in nonischemic cardiomyopathy. J. Am. Coll. Cardiol. 2008;51(25):2414–2421. doi: 10.1016/j.jacc.2008.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kaplan P., Jurkovicova D., Babusikova E., Hudecova S., Racay P., Sirova M., Lehotsky J., Drgova A., Dobrota D., Krizanova O. Effect of aging on the expression of intracellular Ca(2+) transport proteins in a rat heart. Mol. Cell. Biochem. 2007;301(1–2):219–226. doi: 10.1007/s11010-007-9414-9. [DOI] [PubMed] [Google Scholar]
- 33.Katz M.G., Brandon-Warner E., Fargnoli A.S., Williams R.D., Kendle A.P., Hajjar R.J., Schrum L.W., Bridges C.R. Mitigation of myocardial fibrosis by molecular cardiac surgery-mediated gene overexpression. J. Thoracic Cardiovasc. Surg. 2016;151(4) doi: 10.1016/j.jtcvs.2015.11.031. 1191 200.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mora M.T., Ferrero J.M., Gomez J.F., Sobie E.A., Trenor B. Ca2+ Cycling impairment in heart failure is exacerbated by fibrosis: insights gained from mechanistic simulations. Front. Physiol. 2018;9(1194) doi: 10.3389/fphys.2018.01194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Xiong J., Liu X., Gong Y., Zhang P., Qiang S., Zhao Q., Guo R., Qian Y., Wang L., Zhu L., Wang R., Hao Z., Wen H., Zhang J., Tang K., Zang W.F., Yuchi Z., Chen H., Chen S.R.W., Zheng W., Wang S.Q., Xu Y.W., Liu Z. Pathogenic mechanism of a catecholaminergic polymorphic ventricular tachycardia causing-mutation in cardiac calcium release channel RyR2. J. Mol. Cell. Cardiol. 2018;117:26–35. doi: 10.1016/j.yjmcc.2018.02.014. [DOI] [PubMed] [Google Scholar]
- 36.Roux-Buisson N., Gandjbakhch E., Donal E., Probst V., Deharo J.C., Chevalier P., Klug D., Mansencal N., Delacretaz E., Cosnay P., Scanu P., Extramiana F., Keller D., Hidden-Lucet F., Trapani J., Fouret P., Frank R., Fressart V., Faure J., Lunardi J., Charron P. Prevalence and significance of rare RYR2 variants in arrhythmogenic right ventricular cardiomyopathy/dysplasia: results of a systematic screening. Heart rhythm. 2014;11(11):1999–2009. doi: 10.1016/j.hrthm.2014.07.020. [DOI] [PubMed] [Google Scholar]
- 37.Yang J., Zhang R., Jiang X., Lv J., Li Y., Ye H., Liu W., Wang G., Zhang C., Zheng N., Dong M., Wang Y., Chen P., Santosh K., Jiang Y., Liu J. Toll-like receptor 4-induced ryanodine receptor 2 oxidation and sarcoplasmic reticulum Ca(2+) leakage promote cardiac contractile dysfunction in sepsis. J. Biol. Chem. 2018;293(3):794–807. doi: 10.1074/jbc.M117.812289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bovo E., Mazurek S.R., Zima A.V. Oxidation of ryanodine receptor after ischemia-reperfusion increases propensity of Ca(2+) waves during beta-adrenergic receptor stimulation. Am. J. Physiol. Heart Circ. Physiol. 2018;315(4):H1032–H1040. doi: 10.1152/ajpheart.00334.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mattiazzi A., Mundina-Weilenmann C., Guoxiang C., Vittone L., Kranias E. Role of phospholamban phosphorylation on Thr17 in cardiac physiological and pathological conditions. Cardiovasc. Res. 2005;68(3):366–375. doi: 10.1016/j.cardiores.2005.08.010. [DOI] [PubMed] [Google Scholar]
- 40.Gupta R.C., Mishra S., Rastogi S., Imai M., Habib O., Sabbah H.N. Cardiac SR-coupled PP1 activity and expression are increased and inhibitor 1 protein expression is decreased in failing hearts. Am. J. Physiol.-Heart Circ. Physiol. 2003;285(6):H2373–H2381. doi: 10.1152/ajpheart.00442.2003. [DOI] [PubMed] [Google Scholar]
- 41.Balderas-Villalobos J., Molina-Munoz T., Mailloux-Salinas P., Bravo G., Carvajal K., Gomez-Viquez N.L. Oxidative stress in cardiomyocytes contributes to decreased SERCA2a activity in rats with metabolic syndrome. Am. J. Physiol. Heart Circ. Physiol. 2013;305(9):H1344–H1353. doi: 10.1152/ajpheart.00211.2013. [DOI] [PubMed] [Google Scholar]
- 42.Chimenti C., Frustaci A. Contribution and risks of left ventricular endomyocardial biopsy in patients with cardiomyopathies. Circulation. 2013;128(14):1531–1541. doi: 10.1161/CIRCULATIONAHA.13.001414. [DOI] [PubMed] [Google Scholar]
- 43.Kho C., Lee A., Jeong D., Oh J.G., Gorski P.A., Fish K., Sanchez R., DeVita R.J., Christensen G., Dahl R., Hajjar R.J. Small-molecule activation of SERCA2a SUMOylation for the treatment of heart failure. Nat. Commun. 2015;6:7229. doi: 10.1038/ncomms8229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Colussi C., Berni R., Rosati J., Straino S., Vitale S., Spallotta F., Baruffi S., Bocchi L., Delucchi F., Rossi S., Savi M., Rotili D., Quaini F., Macchi E., Stilli D., Musso E., Mai A., Gaetano C., Capogrossi M.C. The histone deacetylase inhibitor suberoylanilide hydroxamic acid reduces cardiac arrhythmias in dystrophic mice. Cardiovasc. Res. 2010;87(1):73–82. doi: 10.1093/cvr/cvq035. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.