Abstract
Age-associated B cells (ABCs) are a unique subset of B cells defined by surface CD11b and CD11c expression. Although ABC expansion has been observed in both human and animal studies in the setting of advanced age, during humoral autoimmunity and following viral infection, the functional properties of this cellular subset remain incompletely defined. In the current study, we demonstrate that ABCs fulfill the criteria for memory B cells, based on evidence of antigen-dependent expansion and persistence in a state poised for rapid differentiation into antibody-secreting plasma cells during secondary responses. First, we show that a majority of ABCs are not actively cycling but exhibit an extensive replication history consistent with prior antigen engagement. Second, despite unswitched surface IgM expression, ABCs show evidence of activation-induced cytidine deaminase (AID)-dependent somatic hypermutation. Third, B cells receptors (BCR) cloned from sorted ABCs exhibit broad autoreactivity and polyreactivity. Although the overall level of ABC self-reactivity was not increased relative to naïve B cells, ABCs lacked features of functional anergy characteristic of autoreactive B cells. Fourth, ABCs express memory B cell surface markers consistent with being poised for rapid plasma cell differentiation during recall responses. Finally, in a murine model of viral infection, adoptively transferred CD11c+ B cells rapidly differentiated into class-switched antibody-secreting cells (ASCs) upon antigen rechallenge. In summary, we phenotypically and functionally characterize ABCs as IgM-expressing memory B cells, findings that together implicate ABCs in the pathogenesis of systemic autoimmunity.
INTRODUCTION
Immunologic memory is a defining feature of the adaptive immune system. During a humoral immune response, the activation of antigen-specific B cells results in the generation of plasma cells and memory B cells (MBCs). While plasma cells provide long-term protection via the production of specific antibodies, MBCs persist in a quiescent state for prolonged periods. Relative to naïve B cells, MBCs exhibit a lower threshold for antigen stimulation resulting in rapid cell cycle entry, and differentiation into antibody-secreting plasma cells or seeding of secondary germinal centers (GCs). In this manner, the generation of long-lived MBCs allows efficient recall responses to secondary antigen challenge (1, 2).
In addition to protective roles during infection, B cells promote the pathogenesis of systemic autoimmunity. In this context, the presence of autoreactive MBC likely contributes to long-term disease persistence and represents an important barrier to immunologic cure. However, the study of MBCs in autoimmunity is hampered by the lack of uniform surface markers to identify MBC subsets. Whereas MBCs in infectious and candidate antigen models can be identified by antigen-specificity and efficient secondary responses, the diversity of disease-associated autoantigen epitopes and ongoing nature of autoimmune inflammation prevents the ready identification of autoreactive MBCs.
In 2011, independent groups identified a novel B cell subset, now termed age-associated B cells (ABCs), characterized by lack of surface CD21 and CD23, or expression of integrins CD11b and CD11c (3, 4). Importantly, several lines of evidence linked this B cell subpopulation to the pathogenesis of systemic autoimmunity, including ABC accumulation in diverse murine lupus models and human subjects with autoimmunity (4–10), and the ex vivo production of anti-nuclear antibodies by Toll-like receptor (TLR)-stimulated ABCs (4). Since ABC do not spontaneously secrete antibodies but increase in number with age, ABCs have been hypothesized to represent a new MBC subset (11–13). However, a definitive functional characterization of this B cell subset is lacking. In the current study, we present functional and phenotypic evidence that ABCs are a population of IgM+ MBCs. Using a surface marker agnostic definition of B cell memory, we demonstrate that ABCs are antigen-experienced B cells with an extensive replicative history, that persist in a resting state but can rapidly differentiate into antibody-secreting plasma cells following secondary antigen challenge.
MATERIALS AND METHODS
Mice
Wild-type (WT), μMT (14), Was−/− (15), CD11c-Cre-GFP mice (16), and Ai14 tdTomato reporter (17) mice on the C57BL/6 background were obtained from Jackson Laboratory (Bar Harbor, ME) and subsequently bred and maintained in the specific pathogen-free (SPF) animal facility of Seattle Children’s Research Institute (Seattle, WA). All animal studies were conducted in accordance with Seattle Children’s Research Institute IACUC approved protocols.
WAS chimera model
WAS chimeras were generated as described (9, 18–20). Briefly, bone marrow (BM) was harvested from Was−/− mice and depleted of CD138+ plasma cells (Miltenyi Biotec, 130-098-257). Donor BM was mixed with B cell-deficient μMT BM (20:80 ratio, 6 × 106 total cells) and injected retro-orbitally into lethally irradiated (450cGy x 2 doses) μMT recipients. Resulting chimeras were sacrificed at 24 weeks post-transplant, with data representative of ≥ 2 independent cohorts
Flow cytometry and cell sorting
Flow cytometry of single cell suspensions was performed as described (9, 20), using the following anti-murine antibodies: B220 (RA3-6B2), CD80 (16-10A1), from BD Biosciences; B220 (RA3-6B2), CD11c (N418), CD11b (M1/70), CD38 (90), CD73 (TY/23) from Thermo Fisher; B220 (RA3-6B2), PD-L2 (TY25), CD19 (ID3), from BioLegend; PNA (Fl-1071) from Vector Labs; and Fas (Jo2) from BD Pharmingen. Cell sorting was performed on surface stained murine cells, using a BD FACSAria (BD Biosciences). Tetramer positive anti-MSP1 MBC were sorted from C57BL/6 mice infected 100 days prior with Plasmodium chabaudi, as previously described (36). Ca2+ flux was measured by flow cytometry using splenic B cells incubated with Indo-1 (Invitrogen), surface stained, and then washed and stimulated with a stimulatory anti-IgM F(ab)2 (18).
κ-deleting recombination excision circle (KREC) analysis
Naïve (CD19+PNA−FAS−CD11b−CD11c−), CD19+PNA+FAS+ GC, and CD19+CD11b+CD11c+ ABC B cells were sorted and total DNA was isolated using an AllPrep Micro kit (Qiagen). Replication history of sorted B cell subsets was subsequently determined by KREC analysis, as previously described (21).
BrdU incorporation
Aged C57BL/6 female (18-months-old) and WAS chimera mice were injected intraperitoneally with 1μg BrdU (BD Biosciences #42530) 24 hours prior to sacrifice, and BrdU uptake by distinct B cell populations identified by flow cytometry.
Single cell BCR cloning
Single-cell BCR cloning was performed as described (22). Briefly, Ig heavy and light (κ and λ) gene transcripts from sorted single CD19+CD11b+CD11c+ ABCs from autoimmune WAS chimeras and CD19+CD21intCD24int follicular mature (FM) cells from 3-month-old C57BL/6 mice were cloned into human IGG1, IGK, or IGL expression vectors, transfected into HEK293T cells, and monoclonal antibodies purified from culture supernatants using protein A–agarose beads.
Measurement of autoantibodies
ELISAs were performed using 96 well Nunc-Immuno MaxiSorp plates (Thermo Fisher) coated with dsDNA (Sigma-Aldrich), phosphorylcholine (PC)-10 (Sigma-Aldrich), Sm/RNP (Arotec Diagnostic), or Qβ-VLP (1μg/ml in PBS) (23). Plates were blocked with 1% BSA in PBS prior to incubation with diluted serum or supernatant. Specific antibodies were detected using goat anti-mouse IgM-, IgG-, or IgG2c -HRP (SouthernBiotech) and peroxidase reactions were developed using OptEIA TMB substrate (BD Biosciences) and stopped with 2N H2SO4. Absorbance at 450nm was read using a SpectraMax 190 microplate reader (Molecular Devices) and data analyzed using GraphPad Prism (GraphPad Software, Inc.). Autoantigen microarrays were performed at the UT Southwestern Medical Center Microarry Core Facility, Dallas, TX (24).
Qβ-VLP Memory Experiment
3-month-old C57BL/6 mice were immunized intraperitoneally with 2μg ssRNA-Qβ-VLP or empty-Qβ-VLP, prior to magnetic microbead (Miltenyi Biotec #130-108-338) purification of splenic CD11c+ cells at 16 days post-immunization. 1.5 × 106 CD11c+ cells and corresponding controls were transferred to 3-month-old C57BL/6 recipient mice by intravenous injection. 4 days post-transfer, mice were challenged with empty-Qβ-VLP and serum collected at 2 day intervals until 6 days after secondary immunization.
Statistical Evaluation
P-values were calculated by the Mann-Whitney and one-way ANOVA tests (GraphPad Software, Inc).
RESULTS:
ABCs represent a non-cycling population with an extensive replication history
To fulfill a functional definition of B cell memory, we predicted that ABCs should have expanded following initial antigen engagement, but returned to a quiescent state while awaiting secondary challenge. However, whereas CD11c+ ABC numbers increase over time and correlate with disease flares in SLE (3, 4, 25, 26), it remains unclear whether ABCs are a continually renewing population which turns over rapidly, or a stable, long-lived subset that is largely quiescent and expands progressively following antigen challenge. To distinguish these possibilities, we examined the replicative history and proliferative state of ABCs sorted from both aged female mice and a well-characterized murine lupus model termed the Wiskott-Aldrich syndrome (WAS) chimera model (9, 18–20). Importantly, in addition to spontaneous autoantibody production and the development of immune-complex glomerulonephritis, autoimmune WAS chimeras exhibit a prominent expansion of CD11c+ ABCs (9, 27) suggesting a contribution of ABCs to disease pathogenesis in this model.
First, we sorted naïve, GC B cells and ABCs from aged female C57BL/6 mice and diseased WAS chimeras and assessed replication history by κ-deleting recombination excision circle (KREC) analysis (21). Notably, ABCs derived from both aged wild-type (WT) and autoimmune WAS chimera mice exhibited extensive KREC dilution relative to naïve B cells, with the number of cellular divisions broadly equivalent to cycling GC B cells (Fig. 1A, B).
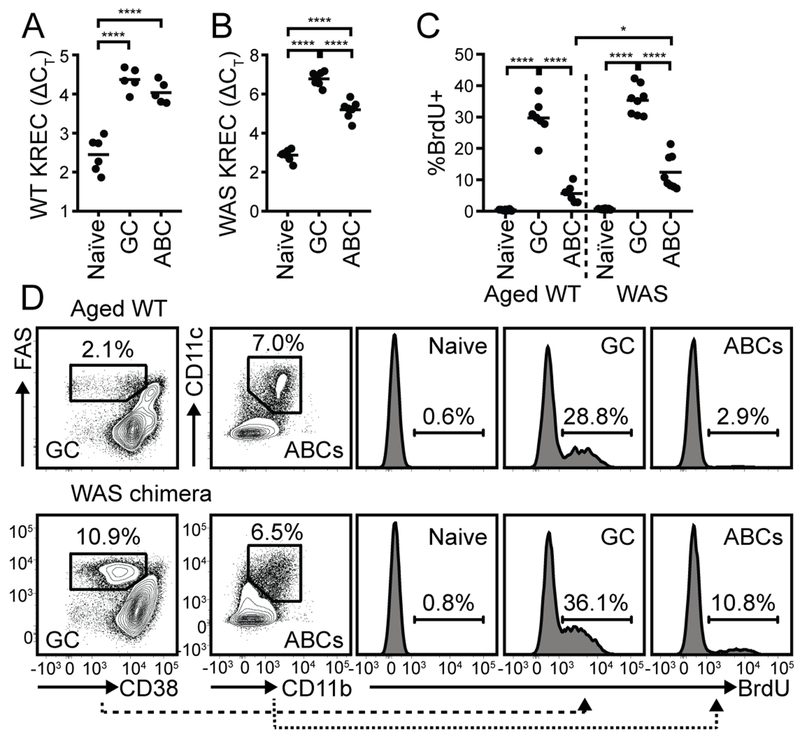
Figure 1: ABCs are a predominantly quiescent population with an extensive replication history.
(A, B) KREC analysis of sorted naïve B cells (CD19+CD11b−CD11c−FAS−), CD38loFAS+ GC B cells, and CD11b+CD11c+ ABCs from 12-month-old C57BL/6 female (A) and autoimmune WAS chimera (B) mice. (C) % BrdU positive B cells in naïve, GC, and ABC compartments in 18-month-old aged female (left) and WAS chimera (right) mice. (A-C) Each data point indicates an individual animal, pooled from two independent experiments. *, P<0.05; ****, P<0.0001; by one-way ANOVA, followed by Tukey’s multiple comparison test. (D) FACS plots showing gating of GC B cells and ABCs (left panels), and histograms of BrdU labeling in indicated subsets (right panels) in representative 18-month-old C57BL/6 female (upper panels) and WAS chimera (lower panels) mouse. Number indicates % within gate.
Based on this evidence of prior cellular proliferation, we next assessed whether ABCs were actively cycling. To do this, we injected 18-month-old female C57BL/6 mice and diseased WAS chimeras with BrdU 24 hours prior to sacrifice. As predicted, ~35% of pro-/pre-B cells (B220+IgM−IgD−) in the bone marrow were BrdU+ (Supplemental Fig. 1), confirming successful in vivo labeling of proliferating B cells. Strikingly, despite a broadly similar replicative history, the proportion of ABCs that had incorporated BrdU was markedly lower than GC B cells (~33% BrdU+ GC B cells vs. ~9% BrdU+ ABCs) (Fig. 1C, D). Together, these data support a model wherein ABCs are memory B cells, based on a predominantly quiescent state together with evidence of extensive previous replication. Importantly, a subset of ABCs had incorporated BrdU in the 24 hours prior to sacrifice, suggesting that these cells were either recently generated or re-entered the cell cycle following antigen reengagement during an ongoing immune response. Consistent with this idea, the proportion of cycling ABCs was higher in WAS chimeras than aged female WT mice, suggesting that active autoimmunity drives the continuous generation and/or reactivation of ABCs (Fig. 1C, D).
ABCs express a diverse, somatically-mutated BCR repertoire
During an adaptive immune response, the pool of antigen-specific memory B cells is expanded above the baseline frequency within the pre-immune B cell repertoire. Based on this model, ABCs might exhibit oligoclonal B cell receptor (BCR) variable (V) gene segment usage. Alternatively, since a large number of autoantigens can facilitate autoreactive B cell activation in SLE, diverse BCR specificities could be recruited into the ABC compartment. Consistent with the former hypothesis, sequence analysis of bulk sorted ABCs (defined as CD21lo splenic B cells lacking the GC marker GL7) derived from autoimmune surrogate light chain deficient (Slc−/−) mice showed enrichment for specific H-CDR3 clones (11). In contrast, Russell Knode, et al. reported that ABCs from old C57BL/6 mice (age 18-22 months old) express a diverse BCR repertoire largely equivalent to FM and marginal zone (MZ) B cells (28). Whether this discrepancy is explained by differences in surface markers used to identify ABCs, specific alterations in the BCR repertoire in the absence of surrogate light chain expression (29), or the analysis of lupus-prone (i.e. Slc−/−) vs. non-autoimmune WT mice is unclear. For this reason, we sorted single ABCs and GC B cells from autoimmune WAS chimeras, ABCs from 8-month-old female WT mice, as well as control FM B cells from 3-month-old WT animals, for BCR sequencing. Consistent with prior studies (3, 4, 10, 27, 28), a majority of ABCs from both WT animals and WAS chimera mice expressed unswitched IgM heavy chains, although a small percentage of WAS chimera ABCs (<5%) expressed switched IgG transcripts (data not shown), as was previously reported in Swap70−/−.Def6−/− lupus-prone mice (10). Importantly, in keeping with bulk sequencing analysis by Russell Knode, et al. (28), ABCs exhibited diverse VH-family usage without significant enrichment for individual BCR clones (Fig. 2A).
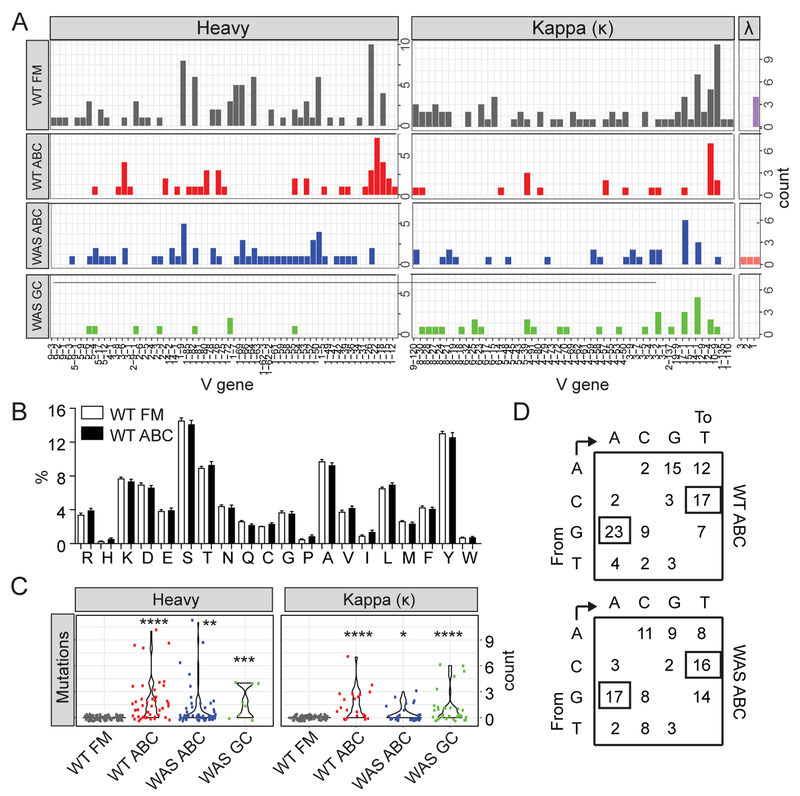
Figure 2: ABCs express a diverse, somatically-mutated BCR repertoire.
(A) Heavy chain (left; μ FM and ABC, γ WAS GC) and light chain (right; κ and λ) variable gene family usage among single sorted 3-month-old WT FM B cells (CD19+CD21intCD24int), ABCs from 8-month-old WT female mice, ABCs from autoimmune WAS chimeras (CD19+CD11b+CD11c+), and GC B cells (CD19+PNA+FAS+) from WAS chimera mice. (B) Amino acid usage in heavy and light chain variable genes from sorted ABCs from aged female mice, compared with control FM B cells from 3-month-old WT animals. (C) Mutation count in heavy and κ light chain variable genes in sorted single B cells from indicated genotypes. *, P<0.05; **, P<0.01; ***, P<0.001; ****, P<0.0001 for each experimental population compared with WT FM control mutational frequency, by Kruskal-Wallis one-way ANOVA test. (D) Nucleotide substitution patterns in mutated sequences from sorted aged WT and autoimmune WAS chimera ABCs. Numbers indicate percentage of each specific type of substitution among mutated BCRs, demonstrating bias for G to A and C to T transitions.
The basic amino acids, arginine (R) and lysine (K), are enriched within the H-CDR3 of anti-dsDNA reactive autoantibodies (30). However, we observed no change in amino acid usage or significant enrichment for basic amino acids (arginine (R), histidine (H), or lysine (K)) in WT ABCs relative to FM B cells (Fig. 2B). Finally, we assessed whether sorted ABCs from aged WT and autoimmune WAS chimera mice exhibited evidence of antigen-driven somatic mutation. Despite expression of predominantly unswitched IgM+ BCRs, an increased proportion of WT and WAS ABCs expressed mutated BCRs (Fig. 2C), in keeping with prior bulk sequencing analysis (28). The overall mutational frequency of IgM+ ABCs was similar to that of IgG+ GC B cells sorted from disease WAS chimeras, whereas mutations were absent in control FM B cells from naïve WT mice. Observed mutations were biased towards G to A and C to T nucleotide substitutions (Fig. 2D), consistent with activation-induced cytidine deaminase (AID) enzymatic activity (31).
In summary, ABCs from lupus prone mice express a diverse, but somatically mutated, BCR repertoire without evidence for oligoclonal expansion or increased usage of basic amino acids. Thus, ABC repertoire diversity in the WAS chimera lupus model mirrors that of ABCs from aged WT animals, without evidence of the clonal expansion or autoreactive BCR characteristics observed in Slc−/− mice (11, 28).
Single-cell cloning demonstrates similar autoreactivity of lupus ABCs and naïve follicular B cells
Since ABCs accumulate in both murine lupus models and in human subjects with autoimmunity, this B cell subpopulation is predicted to express predominantly autoreactive B cell receptors (BCRs). Consistent with this idea, ex-vivo sorted ABCs from female NZB/NZW F1 lupus mice secrete anti-chromatin autoantibodies following R848 stimulation (4). In addition, IgM hybridomas generated from bulk CD21low B cells from surrogate light chain deficient (Slc−/−) mice produce antibodies which bind diverse nuclear autoantigens (11). However, while these data generally support a model wherein autoreactive B cells are enriched within the ABC compartment, there are several important caveats to this interpretation. Specifically, a substantial fraction of naïve B cells in the pre-immune repertoire express polyreactive BCRs binding diverse self-antigens (32–34). However, since naïve follicular mature (FM) and marginal zone (MZ) B cells do not produce significant IgG in response to ex-vivo R848 stimulation (4), anti-chromatin reactivity of ABC supernatants might be explained by enhanced TLR7-driven antibody production rather than a greater relative self-reactivity of this subpopulation. Similarly, although CD21low hybridomas from Slc−/− mice exhibit autoreactivity, no corresponding population of naïve B cells was included in this analysis; an important control given that surrogate light chain deletion is known to alter negative selection during B cell development (29).
For these reasons, we directly assessed the BCR specificity of ABCs expanding in autoimmunity. To do this, we cloned BCRs from sorted CD11b+CD11c+ ABCs from autoimmune WAS chimeras, as well as control FM B cells from naïve WT animals, and generated recombinant monoclonal antibodies (mAb). Since the majority of ABCs are unswitched, we limited our analyses to IgM+ clones. Antibody specificity was first evaluated by ELISA, which demonstrated that a subset of WAS chimera ABCs exhibited reactivity to self-antigens phosphorylcholine (PC), DNA, and the RNA-associated autoantigen Sm/RNP (Fig. 3A). However, the degree of self-reactivity was not increased relative to WT FM B cells, whether assessed by the proportion of positive clones or by relative binding affinity quantified by the area under the curve (AUC) (Fig. 3B, C). These findings suggested that, contrary to prior reports, ABCs are not self-reactive. However, since tolerance mechanisms are known to limit entry of autoreactive B cells into the memory compartment (35), an alternate interpretation is residual self-reactivity of cloned ABCs represents a failure of negative selection. For this reason, we contrasted these findings with the autoreactivity of IgM+ MBC generated during a murine model of malaria infection. To do this, IgM+ B cells reactive against the Plasmodium antigen, Merozoite Surface Protein 1 (MSP1), were cloned from mice infected 100 days prior with Plasmodium chabaudi (36). As predicted, MSP1+ MBC exhibited markedly reduced self-antigen reactivity compared with naïve FM B cells (Supplemental Fig. 2), findings which contrasted with the persistent autoreactivity of cloned ABCs.
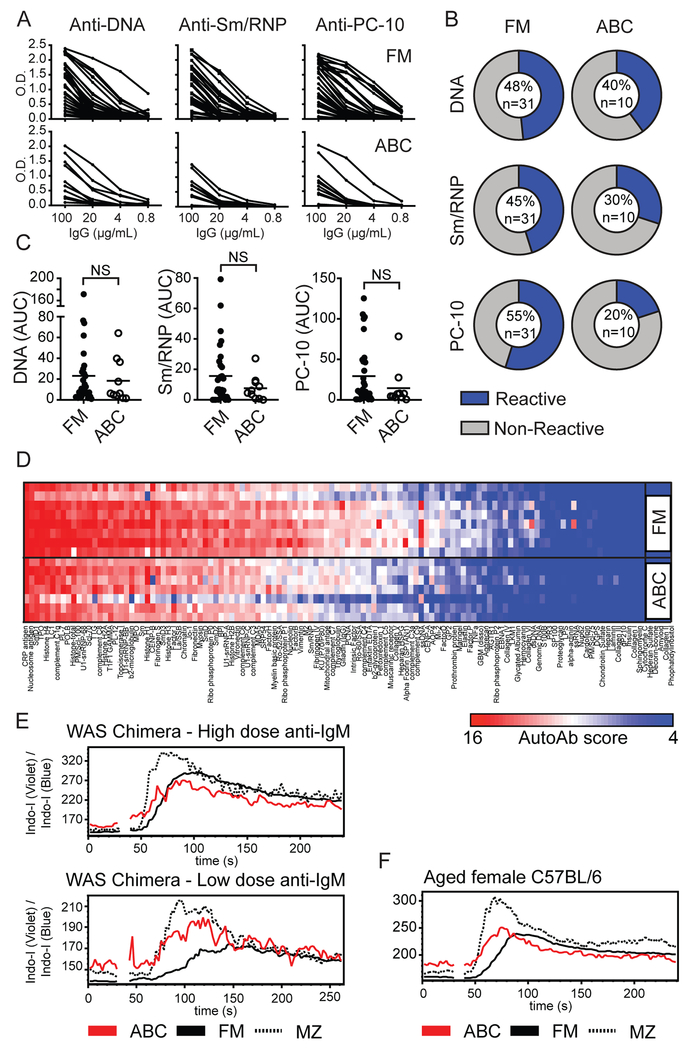
Figure 3: Self-reactivity of ABC repertoire is broadly similar to naïve FM B cells.
(A) ELISA serial dilution curves showing reactivity of mAb cloned from single FM and WAS ABCs towards autoantigens DNA, Sm/RNP, and phosphorylcholine (PC-10). (B) Percentage of mAb reactive to specific autoantigens (blue=reactive clones based on AUC>10, gray=nonreactive clones; numbers within pie chart indicate percentage reactive clones and total number of clones tested). (C) Cloned mAb reactivity by calculated AUC of autoantigen ELISA dilution curves. Each data point indicates an individual mAb. NS=not significant, by Mann-Whitney test. (D) mAb reactivity by autoantigen microarray. Specific autoantigens are ordered from left to right based on intensity of reactivity. Each row represents a pool of 4 randomly selected FM and WAS ABC cloned mAb. (E) Calcium flux following anti-IgM stimulation of CD11b+CD11c+ ABCs (red), FM (solid line), and MZ B cells (dashed line) from representative WAS chimera mouse at 24 weeks post-transplant. Upper panel: High dose anti-IgM (10μg/mL); Lower panel: Low-dose anti-IgM (1μg/mL). (F) Calcium flux in 17-month-old female WT mouse stimulated with 10μg/mL anti-IgM.
To confirm these findings, we examined the breadth of ABC self-reactivity using a microarray panel consisting of 128 autoantigens. Strikingly, recombinant mAb from both WAS ABCs and control wild-type FM B cells exhibited polyreactive binding to diverse disease-associated autoantigens, although without any increase in autoreactivity in the ABC compartment (Fig. 3D). Rather, there was a trend towards reduced self-reactivity in ABCs based on both the ELISA and microarray analyses (Fig. 3A–D).
Although the mature B cell repertoire is characterized by prevalent poly / autoreactive B cell (32–34), functional anergy limits the inappropriate activation of these clones (37). Moreover, in the setting of chronic infectious disease and systemic autoimmunity, exhausted CD21lo atypical memory B cells are expanded in peripheral blood of human subjects (38–42). These data suggest that, rather than being a pathogenic memory population, CD11c+CD21lo ABCs might represent functionally anergic autoreactive B cells. Therefore, we measured calcium mobilization upon anti-IgM BCR cross-linking. Notably, ABCs exhibited rapid BCR-dependent calcium flux, with an amplitude similar to FM B cells with high-dose anti-IgM, and increased flux amplitude relative to FM B cells after low-dose anti-IgM stimulation (Fig. 3E). Importantly, Wiskott-Aldrich syndrome protein deficiency is associated with modestly hyper-responsive BCR signaling (18, 43). Thus, we examined whether this BCR signaling profile was also observed CD11b+CD11c+ ABCs from aged WT female mice. Notably, WT ABCs exhibited similar anti-IgM calcium flux with earlier peak calcium response relative to FM B cells, and an amplitude between FM and MZ B cell subsets (Fig. 3F). In summary, ABCs cloned from WT and lupus-prone mice express polyreactive BCRs binding diverse disease-associated autoantigens, with an overall degree of self-reactivity that is similar to the naïve FM B cell repertoire. However, we observed no functional evidence of BCR anergy in the ABC compartment, suggesting that this population may directly contribute to the pathogenesis of systemic autoimmunity.
Age-associated B cells express memory B cell surface markers indicative of propensity for plasma cell differentiation
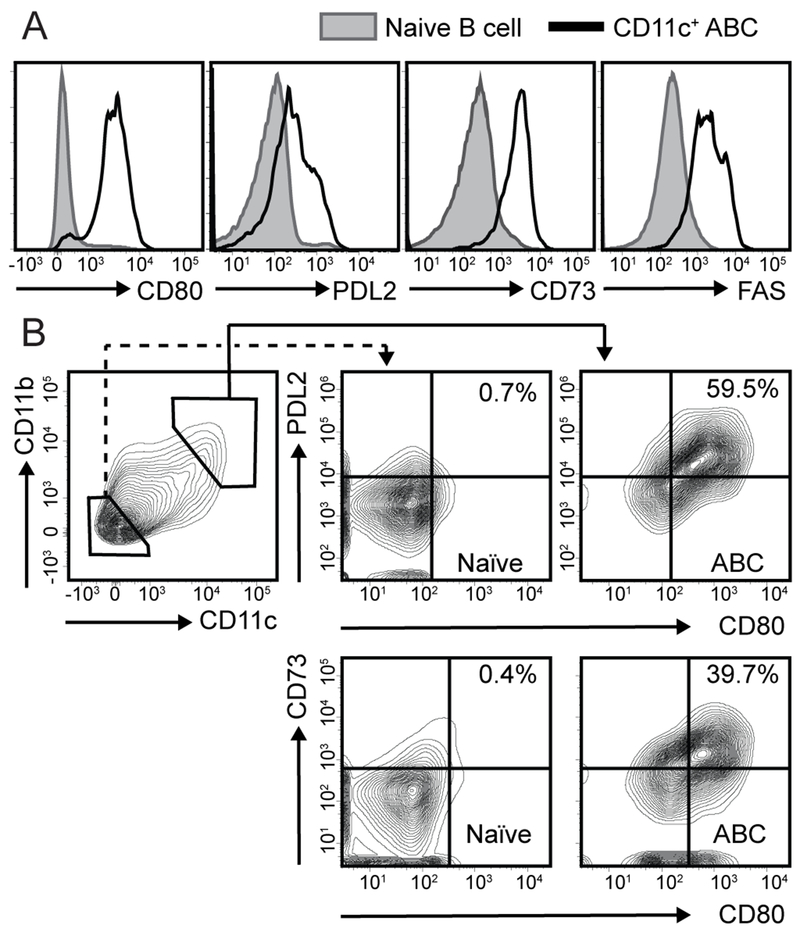
Functionally, MBCs can either differentiate into plasma cells or reseed germinal centers (GC) during a secondary immune response (1). Whereas distinct effector responses of MBC were initially attributed to surface isotype expression, with IgM+ MBC re-entering GCs and switched IgG+ MBCs undergoing plasma cell differentiation (44, 45), subsequent studies have uncovered a diversity of IgM+ MBC responses (36, 46). Although the study of B cell memory is hampered by a paucity of surface markers able to identify all memory B cell subsets, murine studies have indicated that MBC exhibit heterogeneous expression of surface markers associated with T cell interactions, including CD73, CD80, and PD-L2 (CD273) (46–49). Based on these data, we quantified expression of known memory markers on ABCs and assessed whether this surface phenotype predicted a likely functional outcome during putative recall responses. As predicted, a majority of splenic CD11b+CD11c+ ABCs from both aged WT mice and autoimmune WAS chimeras expressed CD80, PD-L2 (CD273), CD73, and FAS, consistent with a MBC phenotype (46–49) (Fig. 4A; and not shown). Interestingly, despite surface IgM expression, a majority of splenic ABCs fell within the CD80+PD-L2+ and CD73+CD80+ “double-positive” gates (Fig. 4B), a surface phenotype associated with secondary plasma cell differentiation (46). Thus, whereas IgM+ memory B cells developing after T-dependent antigen immunization are predominantly CD80−PD-L2− “double-negative” (46), unswitched ABCs express a surface phenotype indicating a propensity for rapid class-switched recombination and ASC differentiation during autoimmunity.
Figure 4: ABCs express B cell memory markers.
(A) Histograms showing expression of MBC markers CD80, PD-L2, CD73, and FAS on CD11b+CD11c+ ABCs (black line), compared with CD11b−CD11c− naïve B cells (gray), from representative 19-month-old female WT mouse. (B) Left panel: FACS plots showing gating of CD11b+CD11c+ ABCs and CD11b−CD11c− naïve B cells from representative WAS chimera mouse. (Middle, left panels) Surface CD80/PD-L2 and CD80/CD73 expression on naïve B cells (middle) and ABCs (left). Number indicates % within CD80+PD-L2+ and CD80+CD73+ gates.
CD11c+ B cells mount rapid, secondary responses to virus-like particle (VLP) immunization
A defining characteristic of MBCs is the ability to efficiently respond to secondary antigen challenge (1, 2). However, whereas the kinetic of the MBC response can be readily characterized in immunization and infection models, the likely continuous activation of self-reactive naïve and memory B cells clones during autoimmunity renders a similar assessment of MBC fate in SLE more challenging. Thus, we hypothesized that expression of defined ABC surface markers could provide the unique opportunity to track MBCs differentiation during established autoimmunity. For this reason, we crossed CD11c-Cre-GFP mice (16) with the Ai14 tdTomato reporter strain (17) to irreversibly “fate map” B cells that had ever entered the ABC compartment. Unfortunately, although CD11c has been proposed as a marker of the ABC phenotype within the B cell compartment, we observed tdTomato expression in a significant proportion of naïve and GC B cells in young wild-type mice examined prior to ABC development (tdTomato+: ~25-50% in naïve B cells; >90% in GC B cells; data not shown); an observation likely explained by low-level CD11c expression during B cell development (50). Thus, although we had hoped that this reporter strategy could have been used to track the contribution of ex-ABCs to ongoing GCs vs. the plasma cell compartment in SLE, limited reporter specificity prevented this approach.
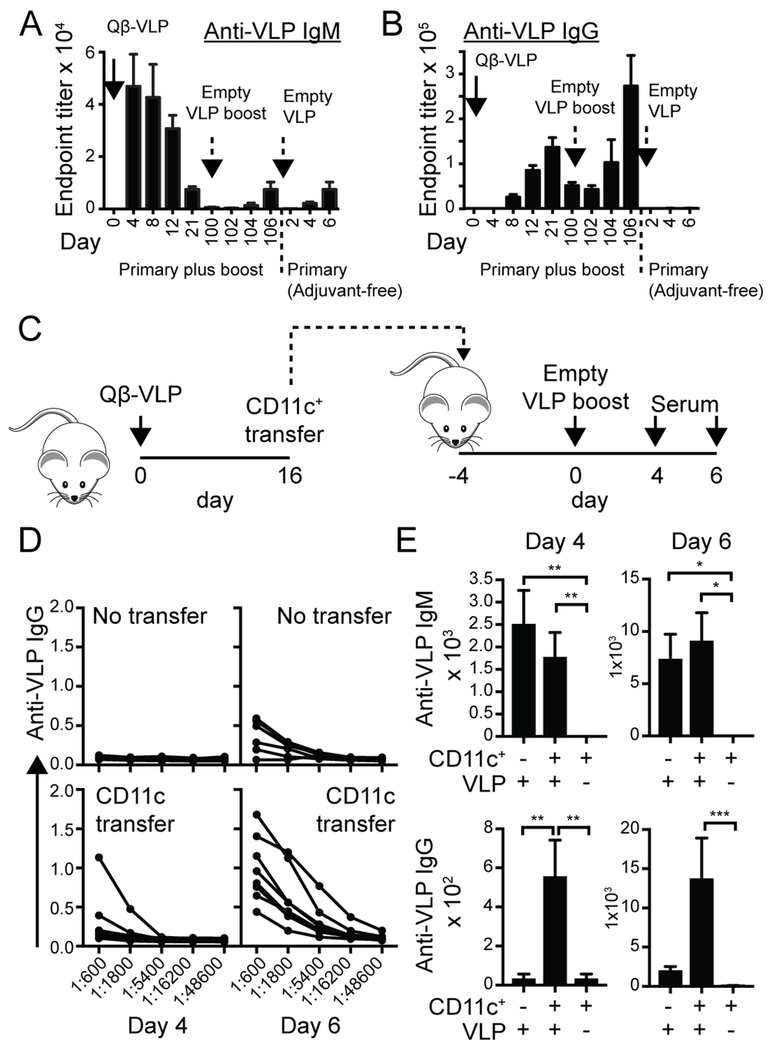
As an alternate strategy, we examined the contribution of CD11c+ ABCs to memory B cell responses following Qβ virus-like particle (VLP) immunization. Since ABC expansion and B cell responses to Qβ-VLP immunization both require B cell-intrinsic Myd88 and TLR7 signals (4, 9, 23), we hypothesized that this approach could be used to model the requirement for dual BCR/TLR signals in both pathogen and autoimmune responses. In addition, a significant advantage of the Qβ-VLP strategy is that the nucleic acid incorporated within capsid particles can be manipulated to target specific B cell endosomal TLRs, including TLR7 activating single-stranded RNA (ssRNA)-VLP, and empty VLP lacking TLR ligands (23). We first examined the kinetic of VLP-specific antibody responses following primary and secondary VLP immunizations. As predicted, primary immunization with ssRNA-VLP resulted in both IgM and IgG anti-VLP antibodies, while boosting with empty (ssRNA-free) VLP induced a rapid and enhanced secondary anti-VLP IgG response. In contrast, empty VLP alone (without primary immunization) resulted in minimal anti-VLP IgM or IgG antibodies, consistent with the requirement for B cell-intrinsic TLR engagement for class-switched autoantibody production to VLP antigens (23) (Fig. 5A, B). Thus, the absolute requirement for TLR ligands in facilitating primary, but not secondary, B cell responses suggested that the VLP model is a useful strategy to dissect the contribution of CD11c+ ABCs to anti-viral memory responses.
Figure 5: Adoptive transfer model confirms memory phenotype of CD11c+ B cells.
(A, B) VLP Memory model: Anti-VLP IgM (A) and IgG (B) titers after immunization of WT mice with Qβ-VLP, followed by empty Qβ-VLP (ssRNA-free) booster at 100 days. As predicted, secondary immunization with empty VLP induced an enhanced anti-VLP IgG response, but no significant increase in IgM titers. Empty Qβ-VLP alone (without primary immunization) resulted in minimal anti-VLP IgM or IgG. (C) Diagram of experimental strategy showing timing of primary Qβ-VLP immunization, adoptive transfer of CD11c+ B cells into naïve recipients, and secondary immunization with empty Qβ-VLP. (D) ELISA dilution curves showing rapid appearance of anti-VLP IgG titers between days 4 and 6 after empty Qβ-VLP immunization, in animals adoptively transferred with VLP-primed CD11c+ B cells (lower panels). Control animals without CD11c+ transfer developed minimal anti-VLP IgG Ab (upper panels). (E) Anti-VLP IgM (upper) and IgG (lower) titers on indicated days after empty Qβ-VLP immunization. Error bars indicate S.E.M. *, P<0.05; **, P<0.01; by Kruskal-Wallis one-way ANOVA test.
Wild-type C57BL/6 mice were immunized with ssRNA-Qβ-VLP to model primary viral challenge. At day 16 post-immunization, splenic CD11c+ cells were isolated by magnetic microbead separation. 1.5 × 106 total cells from the CD11c+ fraction (encompassing ~15,000 VLP-specific ABCs) were then transferred into naïve C57BL/6 recipients. Analysis of the post-enrichment fraction confirmed successful enrichment for CD11c+ B cells with ~75% of VLP-specific B cells expressing CD11b and CD11c. In addition, transferred CD11c+CD11b+ B cells exhibited features consistent with an ABC phenotype, including increased cell size and granularity, and reduced surface CD21 and CD23 expression (Supplemental Fig 3). Recipient animals were subsequently immunized with empty (ssRNA-free) VLP, serum was collected at 2 day intervals, and mice were sacrificed at day 6 for analysis of memory response (Fig. 5C). Strikingly, adoptive transfer of CD11c+ B cells resulted in a rapid and enhanced production of class-switched IgG and IgG2c anti-VLP antibody following empty (ssRNA-free) VLP immunization, without significantly impacting low-titer anti-IgM responses to this adjuvant-free viral antigen (Fig. 5D, E; and not shown). Importantly, adoptive transfer of CD11c-enriched splenocytes without secondary empty VLP immunization did not result in spontaneous anti-VLP antibody production, indicating that the CD11c+-fraction is not contaminated by antigen-specific ASCs (Fig. 5E). In summary, these data indicate that ABCs do not constitutively secrete antibodies but are able to mount rapid secondary immune responses following recognition of their cognate antigen.
DISCUSSION
By persisting for prolonged periods in a state poised for rapid effector responses, MBCs provide an additional layer of protection to the host against secondary infectious challenge. However, these functional characteristics of MBC likely also contribute to the pathogenesis of systemic autoimmunity. Although treatment outcomes for patients with diverse autoimmune diseases have markedly improved in recent decades, discontinuation of therapy frequently results in disease relapse, emphasizing the importance of immune memory as a barrier to cure. In the current study, we functionally classify CD11b+CD11c+ ABCs as an MBC population that accumulates during systemic autoimmunity. Using a surface marker agnostic approach, we demonstrate that: i) ABCs are an extensively divided B cell subset, that remains largely quiescent in the absence of secondary antigen challenge; ii) antigen receptors cloned from this population show evidence of somatic hypermutation and broad poly- and autoreactivity, consistent with antigen-driven expansion; and iii) adoptively-transferred IgM+ CD11c+ B cells rapidly differentiate into IgG+ and IgM+ ASCs during a recall response, consistent with the pattern of MBC surface markers expressed by this population. Together, our data indicate that ABCs are a MBC population, findings which both advance our understanding disease pathogenesis and suggest that targeting ABCs may be an effective therapeutic strategy in humoral autoimmunity.
Rather than a uniform B cell population, MBCs can be subdivided into distinct subsets, differing by survival kinetics, surface isotype expression, extent of somatic hypermutation, and the outcome of secondary antigen challenge (2). Amongst heterogeneous subpopulations of IgM+ MBCs, the expression of effector molecules CD73, CD80, and PD-L2, identifies cells that are poised for rapid differentiation into antibody-secreting plasmablasts (2, 36, 46). Based on extensive BCR mutation and the rapid differentiation of adoptively transferred VLP-specific ABCs into ASCs, our data indicate that CD11c+ ABCs are phenotypically and functionally most similar to CD73+CD80+PD-L2+ unswitched MBCs identified after immunization with T-dependent antigen NP-CGG, and following Ehrlichia muris or malaria infection (36, 46, 51).
Despite this putative MBC phenotype, an unanticipated aspect of our study is the lack of enrichment for autoreactive BCR specificities within the ABC compartment. Although multiple studies have confirmed autoantibody production by ex vivo stimulated CD11c+ B cells (4, 11, 25), the overall degree of autoreactivity of ABCs cloned from autoimmune WAS chimeras was broadly similar to naïve FM B cells. Thus, although CD11c+ B cell expansion correlates with lupus development, our data raise the possibility that the contribution of ABCs to autoimmune disease is relatively limited. However, an alternate interpretation of these apparently contradictory findings is implied by the observation that entry of autoreactive B cells into the memory and plasma cell compartments is restricted by tolerance mechanisms in healthy subjects (35, 52–54). In this model, rather than the positive selection of autoreactive specificities, the failure to censor self-reactive clones from entry into the ABC compartment underlies lupus pathogenesis. Indeed, our findings are consistent with data from human lupus patients. Whereas B cells expressing the intrinsically-autoreactive VH4.34 heavy chain (identifiable using 9G4 idiotype antibody) are censored at the pre-GC stage in healthy subjects, 9G4+ B cells are able to enter the MBC and plasma cell compartments in lupus patients. However, the proportion of 9G4+ MBCs in SLE remains largely equivalent to the 9G4 percentage in the naïve B cell repertoire (35, 53). Moreover, the observation that ABCs exhibit a lower threshold for activation, and lack features of B cell anergy characteristic of autoreactive B cells in the naïve repertoire suggests a propensity for rapid plasma cell differentiation. Thus, we propose a model wherein CD11c+ ABCs express polyreactive and autoreactive antigen receptors and can thereby serve as the source for diverse autoantibody specificities in human autoimmune diseases, such as SLE.
These observations in murine models concur with recent phenotypic characterization of CD11c+ and/or T-bet+ B cell subsets in human SLE. Although different studies use distinct gating strategies to identify this B cell subpopulation, several defining features are emerging as characteristic features of “ABC-like” cells. For example, ABCs in mouse and man are characterized by: i) CD11b and/or CD11c expression (4, 25–27); ii) reduced surface CD21 and CD23 levels (3, 27), and in human cells absence of the prototypic memory marker CD27 (25, 26); iii) Fc receptor-like protein 5 (FcRL5) expression (25, 26, 55); iv) generation in vitro in response to combined TLR7, IFN-γ, and IL-21 stimulation (25–27, 56); v) increased levels of the transcription factor, T-bet (25–27, 56, 57); and, vi) a lack of spontaneous antibody production, but the propensity for rapid plasmablast differentiation in response to cytokine and/or TLR stimulation ex vivo (3, 4, 25, 26). One notable distinction is that, whereas the bulk of CD11b+CD11c+ B cells in murine lupus models express unswitched BCRs (3, 4, 10, 27, 28), a sizeable proportion of ABC-like cells in human SLE are IgG+ (25, 26). Based on these phenotypic and functional characteristics, ABCs have been described either as MBC (11, 12, 58, 59) or pre-plasma cells (26), a distinction which may be largely semantic since both describe antigen-experienced cells poised for rapid differentiation into antibody-producing effectors.
Importantly, recent data indicated that CD11c+ ABCs directly contribute to the pathogenesis of human SLE. For example, Wang et al. (25) reported that human lupus patients exhibit an expansion of CD11chiT-bet+ B cells, in particular those subjects with increased disease severity and active lupus nephritis. Functionally, despite lacking the human MBC marker CD27, the frequency of CD11chi B cells correlated with circulating plasma cell numbers and serum autoantibody titers, and this B cell subset was poised to differentiate into autoantibody-producing ASCs ex vivo (25). Similarly, the Sanz group has delineated a distinct extrafollicular B cell activation pathway driving pathogenic ASC expansion in human SLE. In a predominantly African American lupus cohort, an expanded frequency of active naïve (aNAV; defined as IgD+CD27−CXCR5−CD11c+) and double-negative 2 (DN2; IgD−CD27−CXCR5− CD11c +) B cells was observed, which correlated with disease activity and autoantibody titers (26, 60). Importantly, clonal analysis and transcriptional phenotyping demonstrated an in vivo developmental link between unswitched IgM+ aNAV B cells, switched IgG+ DN2 and circulating ASCs (26). Similar to our findings, DN2 B cells exhibited conserved BCR signaling (26), suggesting distinct functional characteristics of this subset compared with “exhausted” CD21lo B cells observed during chronic infection (38–42).
In summary, the current study advances our understanding of CD11c+ ABC biology by functionally characterizing this B cell subset as an MBC population. Based on these data and parallel studies in human lupus (25, 26, 60), we predict that ABCs are an important source for pathogenic ASCs in SLE, likely generated via a continuous, extrafollicular B cell activation pathway. More importantly, this MBC population may drive disease relapse following treatment discontinuation. Although B cell depletion with rituximab (Rituxan) induces rapid depletion of circulating CD20+ B cells, diverse tissue-resident B cell subpopulations are known to resist anti-CD20 B cell depletion (61–64). Clinical data indicate that MBC are included among the rituximab-resistant B cell populations based on evidence of rapid “memory-like” antibody responses to secondary vaccination after anti-CD20 B cell depletion (65, 66). By characterizing CD11c+ ABCs as an MBC population, our findings, in combination with recent reports (10, 11, 25, 26, 28, 60), suggest that ABCs likely contribute to disease pathogenesis and to frequent clinical relapses in human autoimmunity. More importantly, we predict that more effective targeting of CD11c+ ABCs may be an effective therapeutic strategy in SLE, by eliminating an important source of pathogenic ASCs.
Supplementary Material
Key points:
CD11c+ ABCs exhibit functional characteristics of memory B cells.
ABCs express polyreactive B cell receptors binding diverse self-antigens.
Adoptively-transferred ABCs contribute to rapid recall responses to viral antigens.
Acknowledgments
The authors thank Jit Khim and Karen Sommer for assistance with murine studies and laboratory management, and Dr. Marion Pepper (University of Washington) for kindly providing mAb cloned from malaria-specific MBC. This work was supported by the National Institutes of Health under award numbers: P01HL053749-21A1 (DJR), DP3DK111802 (DJR), R21AI123818 (DJR), K08AI112993 (SWJ) and R01AR073938 (SWJ). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. Additional support provided by the Benaroya Family Gift Fund (DJR); by the ACR REF Rheumatology Scientist Development Award (SWJ); by the American College of Rheumatology (ACR) Rheumatology Research Foundation (RRF) Career Development K Supplement (SWJ); by the Arthritis National Research Foundation (ANRF) Eng Tan Scholar Award (SWJ); by a Lupus Research Alliance, Novel Research Grant (SWJ); and by the Arnold Lee Smith Endowed Professorship for Research Faculty Development (SWJ).
Footnotes
The authors have declared that no conflict of interest exists
REFERENCES:
- 1.Weisel F, and Shlomchik M. 2017. Memory B Cells of Mice and Humans. Annu Rev Immunol 35: 255–284. [DOI] [PubMed] [Google Scholar]
- 2.Harms Pritchard G, and Pepper M. 2018. Memory B cell heterogeneity: Remembrance of things past. J Leukoc Biol 103: 269–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hao Y, O’Neill P, Naradikian MS, Scholz JL, and Cancro MP. 2011. A B-cell subset uniquely responsive to innate stimuli accumulates in aged mice. Blood 118: 1294–1304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rubtsov AV, Rubtsova K, Fischer A, Meehan RT, Gillis JZ, Kappler JW, and Marrack P. 2011. Toll-like receptor 7 (TLR7)-driven accumulation of a novel CD11c(+) B-cell population is important for the development of autoimmunity. Blood 118: 1305–1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Koh YT, Scatizzi JC, Gahan JD, Lawson BR, Baccala R, Pollard KM, Beutler BA, Theofilopoulos AN, and Kono DH. 2013. Role of nucleic acid-sensing TLRs in diverse autoantibody specificities and anti-nuclear antibody-producing B cells. J Immunol 190: 4982–4990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rowland SL, Riggs JM, Gilfillan S, Bugatti M, Vermi W, Kolbeck R, Unanue ER, Sanjuan MA, and Colonna M. 2014. Early, transient depletion of plasmacytoid dendritic cells ameliorates autoimmunity in a lupus model. The Journal of experimental medicine 211: 1977–1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Maine CJ, Marquardt K, Scatizzi JC, Pollard KM, Kono DH, and Sherman LA. 2015. The effect of the autoimmunity-associated gene, PTPN22, on a BXSB-derived model of lupus. Clin Immunol 156: 65–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dai X, James RG, Habib T, Singh S, Jackson S, Khim S, Moon RT, Liggitt D, Wolf-Yadlin A, Buckner JH, and Rawlings DJ. 2013. A disease-associated PTPN22 variant promotes systemic autoimmunity in murine models. J Clin Invest 123: 2024–2036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jackson SW, Scharping NE, Kolhatkar NS, Khim S, Schwartz MA, Li QZ, Hudkins KL, Alpers CE, Liggitt D, and Rawlings DJ. 2014. Opposing impact of B cell-intrinsic TLR7 and TLR9 signals on autoantibody repertoire and systemic inflammation. J Immunol 192: 4525–4532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Manni M, Gupta S, Ricker E, Chinenov Y, Park SH, Shi M, Pannellini T, Jessberger R, Ivashkiv LB, and Pernis AB. 2018. Regulation of age-associated B cells by IRF5 in systemic autoimmunity. Nat Immunol 19: 407–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Aranburu A, Hook N, Gerasimcik N, Corleis B, Ren W, Camponeschi A, Carlsten H, Grimsholm O, and Martensson IL. 2018. Age-associated B cells expanded in autoimmune mice are memory cells sharing H-CDR3-selected repertoires. Eur J Immunol 48: 509–521. [DOI] [PubMed] [Google Scholar]
- 12.Rubtsova K, Rubtsov AV, Cancro MP, and Marrack P. 2015. Age-Associated B Cells: A T-bet-Dependent Effector with Roles in Protective and Pathogenic Immunity. J Immunol 195: 1933–1937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Karnell JL, Kumar V, Wang J, Wang S, Voynova E, and Ettinger R. 2017. Role of CD11c(+) T-bet(+) B cells in human health and disease. Cell Immunol 321: 40–45. [DOI] [PubMed] [Google Scholar]
- 14.Kitamura D, Roes J, Kuhn R, and Rajewsky K. 1991. A B cell-deficient mouse by targeted disruption of the membrane exon of the immunoglobulin mu chain gene. Nature 350: 423–426. [DOI] [PubMed] [Google Scholar]
- 15.Snapper SB, Rosen FS, Mizoguchi E, Cohen P, Khan W, Liu CH, Hagemann TL, Kwan SP, Ferrini R, Davidson L, Bhan AK, and Alt FW. 1998. Wiskott-Aldrich syndrome protein-deficient mice reveal a role for WASP in T but not B cell activation. Immunity 9: 81–91. [DOI] [PubMed] [Google Scholar]
- 16.Stranges PB, Watson J, Cooper CJ, Choisy-Rossi CM, Stonebraker AC, Beighton RA, Hartig H, Sundberg JP, Servick S, Kaufmann G, Fink PJ, and Chervonsky AV. 2007. Elimination of antigen-presenting cells and autoreactive T cells by Fas contributes to prevention of autoimmunity. Immunity 26: 629–641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Madisen L, Zwingman TA, Sunkin SM, Oh SW, Zariwala HA, Gu H, Ng LL, Palmiter RD, Hawrylycz MJ, Jones AR, Lein ES, and Zeng H. 2010. A robust and high-throughput Cre reporting and characterization system for the whole mouse brain. Nat Neurosci 13: 133–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Becker-Herman S, Meyer-Bahlburg A, Schwartz MA, Jackson SW, Hudkins KL, Liu C, Sather BD, Khim S, Liggitt D, Song W, Silverman GJ, Alpers CE, and Rawlings DJ. 2011. WASp-deficient B cells play a critical, cell-intrinsic role in triggering autoimmunity. The Journal of experimental medicine 208: 2033–2042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Arkatkar T, Du SW, Jacobs HM, Dam EM, Hou B, Buckner JH, Rawlings DJ, and Jackson SW. 2017. B cell-derived IL-6 initiates spontaneous germinal center formation during systemic autoimmunity. J Exp Med 214: 3207–3217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jackson SW, Jacobs HM, Arkatkar T, Dam EM, Scharping NE, Kolhatkar NS, Hou B, Buckner JH, and Rawlings DJ. 2016. B cell IFN-gamma receptor signaling promotes autoimmune germinal centers via cell-intrinsic induction of BCL-6. The Journal of experimental medicine 213: 733–750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.van Zelm MC, Szczepanski T, van der Burg M, and van Dongen JJ. 2007. Replication history of B lymphocytes reveals homeostatic proliferation and extensive antigen-induced B cell expansion. The Journal of experimental medicine 204: 645–655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jacobs HM, Thouvenel CD, Leach S, Arkatkar T, Metzler G, Scharping NE, Kolhatkar NS, Rawlings DJ, and Jackson SW. 2016. Cutting Edge: BAFF Promotes Autoantibody Production via TACI-Dependent Activation of Transitional B Cells. J Immunol 196: 3525–3531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hou B, Saudan P, Ott G, Wheeler ML, Ji M, Kuzmich L, Lee LM, Coffman RL, Bachmann MF, and DeFranco AL. 2011. Selective utilization of Toll-like receptor and MyD88 signaling in B cells for enhancement of the antiviral germinal center response. Immunity 34: 375–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li QZ, Zhou J, Wandstrat AE, Carr-Johnson F, Branch V, Karp DR, Mohan C, Wakeland EK, and Olsen NJ. 2007. Protein array autoantibody profiles for insights into systemic lupus erythematosus and incomplete lupus syndromes. Clin Exp Immunol 147: 60–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang S, Wang J, Kumar V, Karnell JL, Naiman B, Gross PS, Rahman S, Zerrouki K, Hanna R, Morehouse C, Holoweckyj N, Liu H, T. Autoimmunity Molecular Medicine, Manna Z, Goldbach-Mansky R, Hasni S, Siegel R, Sanjuan M, Streicher K, Cancro MP, Kolbeck R, and Ettinger R. 2018. IL-21 drives expansion and plasma cell differentiation of autoreactive CD11c(hi)T-bet(+) B cells in SLE. Nat Commun 9: 1758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jenks SA, Cashman KS, Zumaquero E, Marigorta UM, Patel AV, Wang X, Tomar D, Woodruff MC, Simon Z, Bugrovsky R, Blalock EL, Scharer CD, Tipton CM, Wei C, Lim SS, Petri M, Niewold TB, Anolik JH, Gibson G, Lee FE, Boss JM, Lund FE, and Sanz I. 2018. Distinct Effector B Cells Induced by Unregulated Toll-like Receptor 7 Contribute to Pathogenic Responses in Systemic Lupus Erythematosus. Immunity 49: 725–739 e726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Du SW, Arkatkar T, Jacobs HM, Rawlings DJ, and Jackson SW. 2019. Generation of functional murine CD11c(+) age-associated B cells in the absence of B cell T-bet expression. Eur J Immunol 49: 170–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Russell Knode LM, Naradikian MS, Myles A, Scholz JL, Hao Y, Liu D, Ford ML, Tobias JW, Cancro MP, and Gearhart PJ. 2017. Age-Associated B Cells Express a Diverse Repertoire of VH and Vkappa Genes with Somatic Hypermutation. J Immunol 198: 1921–1927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Keenan RA, De Riva A, Corleis B, Hepburn L, Licence S, Winkler TH, and Martensson IL. 2008. Censoring of autoreactive B cell development by the pre-B cell receptor. Science 321: 696–699. [DOI] [PubMed] [Google Scholar]
- 30.Radic MZ, and Weigert M. 1994. Genetic and structural evidence for antigen selection of anti-DNA antibodies. Annu Rev Immunol 12: 487–520. [DOI] [PubMed] [Google Scholar]
- 31.Di Noia JM, and Neuberger MS. 2007. Molecular mechanisms of antibody somatic hypermutation. Annu Rev Biochem 76: 1–22. [DOI] [PubMed] [Google Scholar]
- 32.Yurasov S, Wardemann H, Hammersen J, Tsuiji M, Meffre E, Pascual V, and Nussenzweig MC. 2005. Defective B cell tolerance checkpoints in systemic lupus erythematosus. J Exp Med 201: 703–711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wardemann H, Yurasov S, Schaefer A, Young JW, Meffre E, and Nussenzweig MC. 2003. Predominant autoantibody production by early human B cell precursors. Science 301: 1374–1377. [DOI] [PubMed] [Google Scholar]
- 34.Metzler G, Dai X, Thouvenel CD, Khim S, Habib T, Buckner JH, and Rawlings DJ. 2017. The Autoimmune Risk Variant PTPN22 C1858T Alters B Cell Tolerance at Discrete Checkpoints and Differentially Shapes the Naive Repertoire. J Immunol 199: 2249–2260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pugh-Bernard AE, Silverman GJ, Cappione AJ, Villano ME, Ryan DH, Insel RA, and Sanz I. 2001. Regulation of inherently autoreactive VH4-34 B cells in the maintenance of human B cell tolerance. J Clin Invest 108: 1061–1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Krishnamurty AT, Thouvenel CD, Portugal S, Keitany GJ, Kim KS, Holder A, Crompton PD, Rawlings DJ, and Pepper M. 2016. Somatically Hypermutated Plasmodium-Specific IgM(+) Memory B Cells Are Rapid, Plastic, Early Responders upon Malaria Rechallenge. Immunity 45: 402–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cambier JC, Gauld SB, Merrell KT, and Vilen BJ. 2007. B-cell anergy: from transgenic models to naturally occurring anergic B cells? Nat Rev Immunol 7: 633–643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Moir S, Ho J, Malaspina A, Wang W, DiPoto AC, O’Shea MA, Roby G, Kottilil S, Arthos J, Proschan MA, Chun TW, and Fauci AS. 2008. Evidence for HIV-associated B cell exhaustion in a dysfunctional memory B cell compartment in HIV-infected viremic individuals. The Journal of experimental medicine 205: 1797–1805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Joosten SA, van Meijgaarden KE, Del Nonno F, Baiocchini A, Petrone L, Vanini V, Smits HH, Palmieri F, Goletti D, and Ottenhoff TH. 2016. Patients with Tuberculosis Have a Dysfunctional Circulating B-Cell Compartment, Which Normalizes following Successful Treatment. PLoS Pathog 12: e1005687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Weiss GE, Clark EH, Li S, Traore B, Kayentao K, Ongoiba A, Hernandez JN, Doumbo OK, Pierce SK, Branch OH, and Crompton PD. 2011. A positive correlation between atypical memory B cells and Plasmodium falciparum transmission intensity in cross-sectional studies in Peru and Mali. PloS one 6: e15983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Weiss GE, Crompton PD, Li S, Walsh LA, Moir S, Traore B, Kayentao K, Ongoiba A, Doumbo OK, and Pierce SK. 2009. Atypical memory B cells are greatly expanded in individuals living in a malaria-endemic area. J Immunol 183: 2176–2182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Isnardi I, Ng YS, Menard L, Meyers G, Saadoun D, Srdanovic I, Samuels J, Berman J, Buckner JH, Cunningham-Rundles C, and Meffre E. 2010. Complement receptor 2/CD21- human naive B cells contain mostly autoreactive unresponsive clones. Blood 115: 5026–5036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kolhatkar NS, Scharping NE, Sullivan JM, Jacobs HM, Schwartz MA, Khim S, Notarangelo LD, Thrasher AJ, Rawlings DJ, and Jackson SW. 2015. B-cell intrinsic TLR7 signals promote depletion of the marginal zone in a murine model of Wiskott-Aldrich syndrome. Eur J Immunol 45: 2773–2779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dogan I, Bertocci B, Vilmont V, Delbos F, Megret J, Storck S, Reynaud CA, and Weill JC. 2009. Multiple layers of B cell memory with different effector functions. Nat Immunol 10: 1292–1299. [DOI] [PubMed] [Google Scholar]
- 45.Pape KA, Taylor JJ, Maul RW, Gearhart PJ, and Jenkins MK. 2011. Different B cell populations mediate early and late memory during an endogenous immune response. Science 331: 1203–1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zuccarino-Catania GV, Sadanand S, Weisel FJ, Tomayko MM, Meng H, Kleinstein SH, Good-Jacobson KL, and Shlomchik MJ. 2014. CD80 and PD-L2 define functionally distinct memory B cell subsets that are independent of antibody isotype. Nat Immunol 15: 631–637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Anderson SM, Tomayko MM, Ahuja A, Haberman AM, and Shlomchik MJ. 2007. New markers for murine memory B cells that define mutated and unmutated subsets. J Exp Med 204: 2103–2114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tomayko MM, Steinel NC, Anderson SM, and Shlomchik MJ. 2010. Cutting edge: Hierarchy of maturity of murine memory B cell subsets. J Immunol 185: 7146–7150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yates JL, Racine R, McBride KM, and Winslow GM. 2013. T cell-dependent IgM memory B cells generated during bacterial infection are required for IgG responses to antigen challenge. J Immunol 191: 1240–1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Abram CL, Roberge GL, Hu Y, and Lowell CA. 2014. Comparative analysis of the efficiency and specificity of myeloid-Cre deleting strains using ROSA-EYFP reporter mice. J Immunol Methods 408: 89–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kenderes KJ, Levack RC, Papillion AM, Cabrera-Martinez B, Dishaw LM, and Winslow GM. 2018. T-Bet(+) IgM Memory Cells Generate Multi-lineage Effector B Cells. Cell Rep 24: 824–837 e823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Brink R, and Phan TG. 2018. Self-Reactive B Cells in the Germinal Center Reaction. Annu Rev Immunol 36: 339–357. [DOI] [PubMed] [Google Scholar]
- 53.Cappione A 3rd, Anolik JH, Pugh-Bernard A, Barnard J, Dutcher P, Silverman G, and Sanz I. 2005. Germinal center exclusion of autoreactive B cells is defective in human systemic lupus erythematosus. J Clin Invest 115: 3205–3216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Reed JH, Jackson J, Christ D, and Goodnow CC. 2016. Clonal redemption of autoantibodies by somatic hypermutation away from self-reactivity during human immunization. The Journal of experimental medicine 213: 1255–1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kim CC, Baccarella AM, Bayat A, Pepper M, and Fontana MF. 2019. FCRL5(+) Memory B Cells Exhibit Robust Recall Responses. Cell Rep 27: 1446–1460 e1444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Naradikian MS, Myles A, Beiting DP, Roberts KJ, Dawson L, Herati RS, Bengsch B, Linderman SL, Stelekati E, Spolski R, Wherry EJ, Hunter C, Hensley SE, Leonard WJ, and Cancro MP. 2016. Cutting Edge: IL-4, IL-21, and IFN-gamma Interact To Govern T-bet and CD11c Expression in TLR-Activated B Cells. J Immunol 197: 1023–1028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rubtsova K, Rubtsov AV, Thurman JM, Mennona JM, Kappler JW, and Marrack P. 2017. B cells expressing the transcription factor T-bet drive lupus-like autoimmunity. J Clin Invest 127: 1392–1404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Winslow GM, Papillion AM, Kenderes KJ, and Levack RC. 2017. CD11c+ T-bet+ memory B cells: Immune maintenance during chronic infection and inflammation? Cell Immunol 321: 8–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Knox JJ, Myles A, and Cancro MP. 2019. T-bet(+) memory B cells: Generation, function, and fate. Immunol Rev 288: 149–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tipton CM, Fucile CF, Darce J, Chida A, Ichikawa T, Gregoretti I, Schieferl S, Hom J, Jenks S, Feldman RJ, Mehr R, Wei C, Lee FE, Cheung WC, Rosenberg AF, and Sanz I. 2015. Diversity, cellular origin and autoreactivity of antibody-secreting cell population expansions in acute systemic lupus erythematosus. Nat Immunol 16: 755–765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kamburova EG, Koenen HJ, Borgman KJ, ten Berge IJ, Joosten I, and Hilbrands LB. 2013. A single dose of rituximab does not deplete B cells in secondary lymphoid organs but alters phenotype and function. Am J Transplant 13: 1503–1511. [DOI] [PubMed] [Google Scholar]
- 62.Ahuja A, Shupe J, Dunn R, Kashgarian M, Kehry MR, and Shlomchik MJ. 2007. Depletion of B cells in murine lupus: efficacy and resistance. J Immunol 179: 3351–3361. [DOI] [PubMed] [Google Scholar]
- 63.Teng YK, Levarht EW, Toes RE, Huizinga TW, and van Laar JM. 2009. Residual inflammation after rituximab treatment is associated with sustained synovial plasma cell infiltration and enhanced B cell repopulation. Ann Rheum Dis 68: 1011–1016. [DOI] [PubMed] [Google Scholar]
- 64.Pijpe J, Meijer JM, Bootsma H, van der Wal JE, Spijkervet FK, Kallenberg CG, Vissink A, and Ihrler S. 2009. Clinical and histologic evidence of salivary gland restoration supports the efficacy of rituximab treatment in Sjogren’s syndrome. Arthritis Rheum 60: 3251–3256. [DOI] [PubMed] [Google Scholar]
- 65.Bingham CO 3rd, Looney RJ, Deodhar A, Halsey N, Greenwald M, Codding C, Trzaskoma B, Martin F, Agarwal S, and Kelman A. 2010. Immunization responses in rheumatoid arthritis patients treated with rituximab: results from a controlled clinical trial. Arthritis Rheum 62: 64–74. [DOI] [PubMed] [Google Scholar]
- 66.Cho A, Bradley B, Kauffman R, Priyamvada L, Kovalenkov Y, Feldman R, and Wrammert J. 2017. Robust memory responses against influenza vaccination in pemphigus patients previously treated with rituximab. JCI Insight 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.