Highlights
-
•
Opuntia f-i cladodes are used as unique carbon source for wild microorganisms growth.
-
•
A. pittii and K. marxianus had hydrolytic activity on cladode flour, CF.
-
•
K. marxianus was the microorganisms more adequate for hydrolysis and fermentation.
-
•
The CF degradation process will allow bioethanol production with competitive yield.
Abbreviations: CFM, Cladode flour medium; CF, Cladode flour; IU, International unit; FPase, activity Filter paperase activity; CMCase, activity Carboximethylcellulase activity; CMC, Carboxymethylcellulose
Keywords: Opuntia ficus-indica, Acinetobacter pittii, Kluyveromyces marxianus, Nopal cladodes
Abstract
In the present work the effect of three factors: pH, temperature and type of microorganism using a factorial design 32x2 was evaluated on: growth, total cellulases hydrolytic activity (FPases), endoglucanases hydrolytic activity (CMCases), free reducing sugars (FRS), glucose, sucrose, and alcohol production using a culture medium based on mineral salts added with cladodes flour of Opuntia ficus-indica at 20% as the unique carbon source. Two wild microorganisms were used, Acinetobacter pittii a bacteria isolated from decaying cladodes, and Kluyveromyces marxianus a yeast isolated from termite stomach. The maximum hydrolytic activities were obtained with Acinetobacter pittii at 37 °C and pH 6.5 for total cellulases (0.67 ± 0.02 IU/ml) and for endoglucanases (0.23 ± 0.02 IU/ml) at 24 and 4 h. The maximum production of alcohol was 12.98 ± 0.06 g/L obtained with Kluyveromyces marxianus at 4 h with conditions of 40 °C and pH 5.5.
1. Introduction
Lignocellulose is the main component of the cell wall of plants and it is widely distributed on the planet as an abundant carbon source, consisting of three structural polymers: lignin, cellulose and hemi cellulose [1].
Lignocellulosic materials have received special attention as an alternative substrate for the production of ethanol, due to its high content of fermentable sugars and high availability; also this matter does not compete with the use as food. The production of bioethanol from these lignocellulosic materials is called second generation produced and it consists of four steps: pretreatment, sugar hydrolysis, fermentation and product recovery. The highest cost is generated in the steps of pretreatment and the hydrolysis of sugars where commercial enzymes are generally used. The production of third generation bioethanol is obtained from non-food species, through molecular biology techniques; within this group, microalgae stand out today [2]. The main disadvantage of these processes is the low productivity and the high cost of equipment like tubular photobioreactor systems used for this porpouse. Thus, continuity in the study and development of these new types of microorganism cultures is necessary.
The use of microorganisms that produce enzymes capable of hydrolyzing sugars from lignocellulosic materials with little or no prior treatment and / or without the use of commercial enzymes, represents a clear advantage. In nature, a small percentage of microorganisms (bacteria, fungi, yeasts) can degrade cellulose through the expression of cellulases; however, not every microorganism is able to solubilize it completely [3]. Due to the strong hydrogen bonds between the chains the cellulose has crystalline and resistant regions; but when some mechanical damage is applied or by pretreatment processes (chemical or thermal) amorphous regions are formed because the density of the cellulose decreases, which increases the distance between the molecules by varying its orientation [4]; these allows enzymes such as cellulases to degrade the entire complex structure for the use of sugars.
For this reason, studies are carried out to isolate and evaluate wild microorganisms for the production of bioethanol from lignocellulosic biomass and, at the same time, this helps reducing the waste generated by the agricultural industry.
On the other hand, Nopal cladodes (Mexican cactus) are consumed as fresh vegetables and are used as an herbal remedy for their antioxidant, anti-inflammatory, anti-diabetic and anti-glycemic properties for 12,000 years in Mexico. The production of cactus in Mexico is the most important in the world, reaching values of 812,000 tons per year [5] 70% of this production is sold as fresh or processed food while the 30% remaining represents a prominent excess. This by-product is normally discarded, generating a large amount of waste [6]. Cladodes contains high levels of polysaccharides such as cellulose, hemicellulose and mucilage with the potential to be used as raw material for microbial cultivation, and for methane and bioethanol production [[7], [8], [9], [10]]
Moreover, it has been reported that cladodes can be infected by several types of hydrolytic fungal species and bacteria, which produce the biomass degradation under high humidity conditions [11]. Cladode tissue or stem, first experiences a pectinolytic effect caused by bacteria of the genus Erwinia, due to extracellular pectinases production [12]; two examples are Erwinia chrysanthemi and Erwinia carotovora [13]. In addition, other pectinolytic bacteria of the genus Leuconostoc, Bacillus, Pseudomonas, Micrococcus and Ruminococcus have been identified also in cladodes [13]. Bernabé [14] made the isolation and identification of native bacteria from the rhizosphere of Opuntia ficus-indica obtaining a classification in 16 genera and 48 species that mostly corresponded to the genus Acinetobacter, Bacillus, Enterobacter and Pseudomonas. The aim of this work was to evaluate the effect of pH, temperature and type of pectinolytic microorganisms on sugar release and alcohol production on cladode flour media (CFM), as the unique carbon source.
2. Materials and methods
2.1. Establishment of the experimental design
A factorial design 32 x 2 was established evaluating the following factors (Table 1)
Table 1.
Factorial design 32x2.
| Factors | Level |
||
|---|---|---|---|
| (-) | (0) | (+) | |
| pH | 4.5 | 5.5 | 6.5 |
| Temperature (°C) | 34 | 37 | 40 |
| Microorganisms | 1:Acinetobacter pittii | – | 2:Kluyveromyces marxianus |
The response variables evaluated were: microorganism population (cell/ml), total cellulase activity (FPase, IU/ml), endoglucanase activity (CMCase, IU/ml), free release sugars (g/L), and production of glucose (g/L), sucrose (g/L), and alcohol (g/L).
2.2. Vegetal material
Opuntia ficus-indica (Atlixco variety) cladodes of 12 months were collected in an experimental facility in the central state of Hidalgo, Mexico. The fresh cladodes were cut into 1 cm2 cubes using a vegetable cutter (3/8″blade, Hobart, FP-350, USA) and dried at 80 °C/24 h in a steam trays dryer (Jersa, FT-DCV-02, Mexico). Subsequently, dry cladodes were ground in a pulverizing mill (Pulvex, 200, Mexico) to obtain a flour with a particle size of 1500 μm by sieving. The flour was stored at 25 °C for 1 week in sealed plastic bags for further analysis and culture medium preparation.
2.3. Culture medium preparation
A cladode flour medium (CFM) using a mineral solution with cladodes flour (CF) at 20% of concentration was prepared. The mineral solution was prepared by adding distilled water (135 ml) to a mixture of mineral salts (50:50, 15 ml) consisting of mineral solution I (0.6% K2HPO4) and mineral solution II (1.2% NaCl, 1.2% (NH4)2SO4, 0.6% KH2PO4, 0.12% CaCl2 and 0.25% MgSO4.7H2O) [15]. The ingredients were homogenized by magnetic stirring (3 min) and adjusted to pH according to the experimental design (Table 1) using a potentiometer (Oakton, pH 11 series, Singapore). The mixture was sterilized at 121 °C/15 min.
2.4. Growth of wild hydrolytic microorganisms in cladode flour medium
Acinetobacter pittii a Gram negative bacterium with a cocobacillus form that grows in white circular colonies in nutritious broth medium and Kluyveromyces marxianus an oval-shaped yeast that forms pink circular colonies in peptone yeast extract medium were isolated and identified from Opuntia ficus-indica decayed cladodes and termite stomach, respectively. Both microorganisms grew at 37 °C / 6 h with stirring at 200 rpm from a culture stored in glycerol (-20 °C); then 1 ml of the resulted solution was added to 9 ml of 1% CFM (pH 4.5) as the first step. A second growth pass was made by inoculating 5 ml of the previous culture in 50 ml of 20% CFM (pH 4.5) by incubating at 37 °C / 12 h with stirring at 200 rpm. Finally, the third pass was made in 150 ml of 20% CFM medium (pH 4.5), adjusting the initial cell population to 20 × 10^6 cells / ml from the second pass. This growth was carried out for 72 h at 200 rpm and according to the factorial design.
2.5. Analytical determinations
All analytical determinations were performed in culture medium supernatant with cladode flour. Free reducing sugars was carried out using the DNS method at 550 nm [16]. Alcohol determination was carried out by the method of potassium dichromate at 585 nm [17]; glucose and sucrose determination was performed by a biochemical analyzer (YSI, model 2900D) using selective membranes with immobilized enzymes (YSI 2703/YSI 2365).
2.6. Enzymatic assay
The enzymatic activity was determined according to the methods recommended by IUPAC [18]. The activity of total cellulases and endoglucanases was determined by quantifying the reducing sugars from a filter paper (Whatman No.1) and with soluble cellulose (carboxymethylcellulose, CMC) respectively, as substrate. For total cellulase activity (FPase), 0.5 ml of the culture supernatant (crude enzyme extract) was incubated for 1 h at 50 °C with 1 ml of 0.05 M sodium citrate buffer (pH 4.8) containing 50 mg of filter paper Whatman No. 1 (1 x 6 cm strip). To determine the activity of endoglucanases (CMCase), 0.5 ml of the enzyme extract was incubated with 0.5 ml of 2% carboxymethylcellulose in 0.05 M sodium citrate buffer (pH 4.8) at 50 °C for 30 min. After incubation, the samples were centrifuged at 7000 rpm for 10 min and 4 °C, and an aliquot of 0.5 ml was taken. The free reducing sugars were quantified by the DNS method; controls of sole substrate, enzyme and a reference solution of glucose (1%) were also included. One cellulase/ endoglucanase unit (IU/ml) was defined as the amount of enzyme that produces 1 μmol equivalent of glucose per minute.
2.7. Statistical analysis
Based on laboratory tests conducted in duplicate, the effect of pH (three levels), temperature (three levels), microorganisms (two levels) and their interaction were statistically analyzed. All response variables were examined by an analysis of variance ANOVA with a statistical software (Statgraphics Centurion XVI, USA) with a 5% of significance.
3. Results and discussions
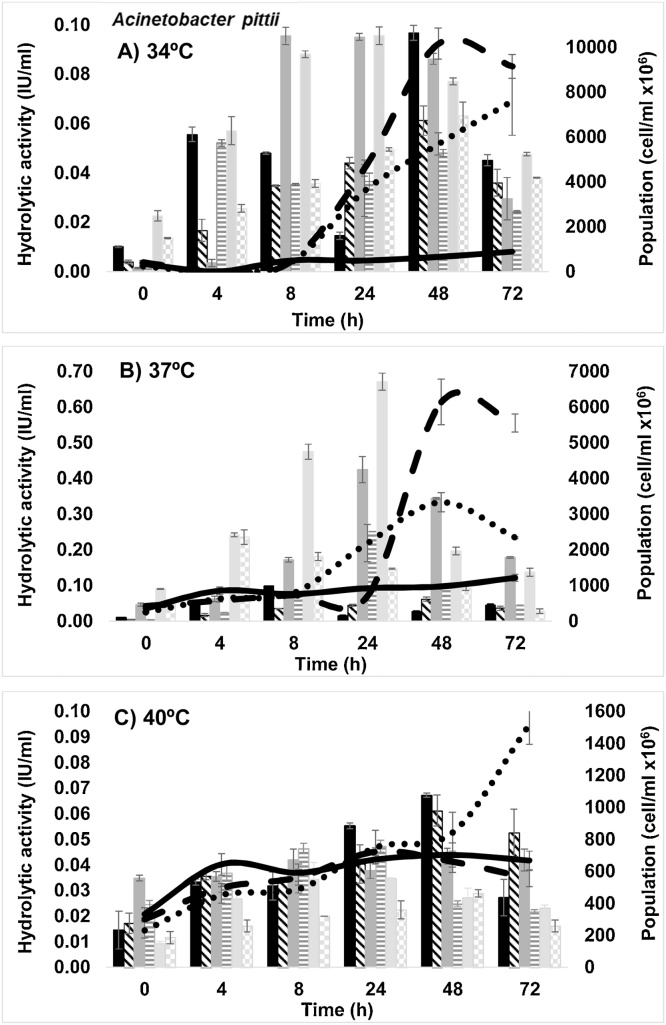
Acinetobacter pittii and Kluyveromyces marxianus had the capacity to grow and hydrolyze liquid culture medium added with cladode flour in the three pH and temperatures tested. K. marxianus presented fermentative capacity using released sugars on cladode flour medium (CFM). Fig. 1 shows the growth curves, FPase (total cellulases) and CMCase (endoglucanases) activity of A. pittii at each temperatures and pH proposed.
Fig. 1.
Growth at pH 4.5 (continuous line), 5.5 (dashed line) and 6.5 (dotted line). FPase activity (solid bars) at pH 4.5 (black), 5.5 (dark gray) and 6.5 (light gray). CMCase activity at pH 4.5 (bars whit inclined lines), 5.5 (bars whit vertical lines) and 6.5 (bars whit squares) for Acinetobacter pittii at A) 34 °C, B) 37 °C and C) 40 °C.
The major growth of Acinetobacter pittii was obtained at 34 °C and pH 5.5 (1.02 ± 0.7 × 1010 cell/ml) at 48 h, with a short lag stage and an exponential stage from 8 h, indicating a better adaptation of the microorganism to these conditions. According to Vegasa and Nieves [19] Acinetobacter genus is strictly aerobic and grows easily in a temperature range of 33–37 °C and at pH from 5.5 to 6.0. In this study conducted at 37 °C, the highest growth also occurred at pH 5.5 but with a longer lag stage (24 h). At both, pH of 5.5 and 6.5, the FPase and CMCase activity increased over time. Maximum FPase activity (0.67 ± 0.02 IU/ml) was obtained at pH 6.5 at 24 h and 37 °C while a low activity (0.23 ± 0.02 IU/ml) was observed at 4 h. Moreover, at pH 5.5 at 24 h a low activity presented was 0.42 ± 0.3 IU/ml at 37 °C. This lasts results were similar to the reported for Acinetobacter anitratus grown in salts medium added with carboxymethylcellulose (CMC) and glucose as the sole carbon sources separately, where the FPase of the enzyme extracts (supernatant) exhibited activity values of 0.48 and 0.24 IU/ml for CMC and glucose, respectively [20]. A maximum value of CMCase activity (0.23 ± 0.02 IU / ml) was obtained at a temperature of 37 °C and pH 5.5 at 24 h and was similar to that obtained at pH 6.5 at 4 h at this same temperature. Deka et al. [21] reported a value of 0.43 IU/ml of CMCase activity for Bacillus subtilis, while Irfan et al. [22] reported a value of 0.45 IU/ml for a Cellulomonas strain. The results presented in this work showed that A. pittii had a lower CMCase activity than Bacillus and Cellulomonas strains, this difference may be due to the source of isolation and differences on physiology of the species or even to grow culture conditions. In this sense, Alcarraz et al. [23] mentioned that it is essential to consider the type of sample and the source of isolation, as an important condition to obtain bacteria with high hydrolytic capacity on cellulose residues. Relating the hydrolytic activity with the growth, it was observed that under the same conditions the maximum activity was obtained in the middle of the exponential phase when maximum growth has not yet been reached, so it is inferred that the microorganism used the substrate for production of cellulolytic enzymes rather than for cell growth. Poszytek et al. [24] determined the optimum conditions to develop hydrolytic activity of strains isolated from different sources. The analyzes in media added with CMC showed that all the bacteria tested were able to grow and presented a hydrolytic activity in a wide range of temperatures (22–45 °C) and pH (4–10). At 40 °C the growth increased slightly during the first 24 h, showing a steady state after at both pH 4.5 and 5.5. Moreover, at pH of 6.5 a considerable population increase was observed from the 48 h, however the population obtained at 72 h at 40 °C was lower than the obtained at the same growth time at 34 °C. Fig. 2 shows free reducing sugars (FRS) and alcohol production for each condition tested.
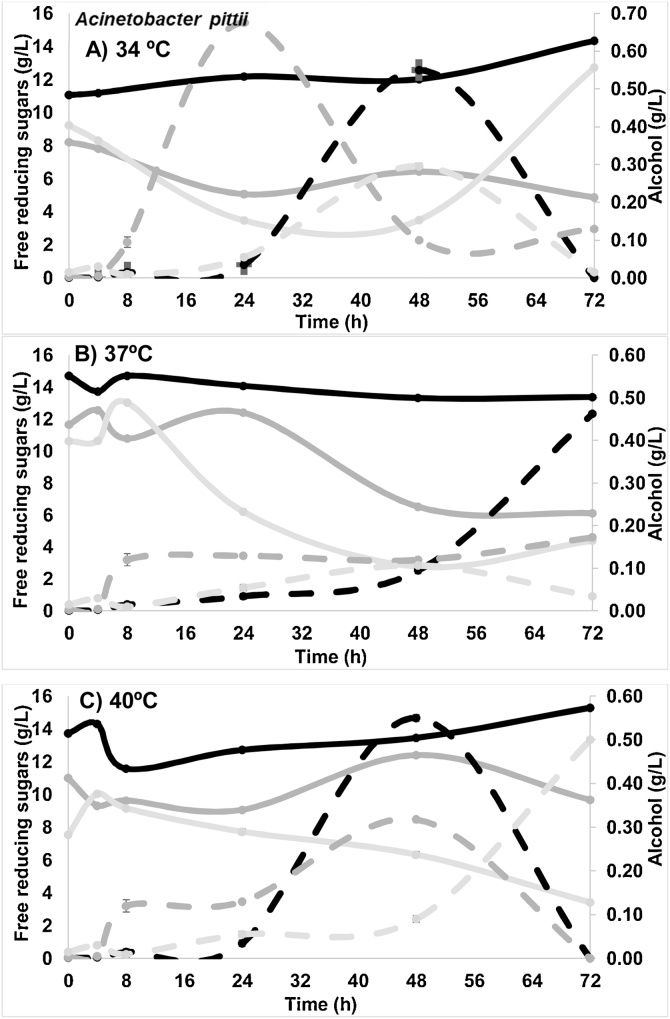
Fig. 2.
Free reducing sugars (continue lines) and alcohol (dashed lines) for Acinetobacter pittii at pH 4.5 (black), 5.5 (dark gray) and 6.5 (light gray) at A).
There was an increase in FRS of 9 g/L (from 4 ± 0.04 to 13 ± 0.04 g/L) with increasing time (48 h to 72 h) at pH 6.5 and 34 °C. Similar behavior of an increase in FRS of 2.4 g/L (from 10.62 ± 0.04 to 13.03 ± 0.04 g/L) was observed at 37 °C during first 8 h. This indicates a culture medium hydrolysis and showed a positive significant difference with the values obtained for pH of 4.5 and 5.5. Significant alcohol production was not found; until now bacteria have not been attributed with fermentative capacities. The sugars present in the medium were used for bacterial growth and for cellulases production. With respect to glucose and sucrose concentration, a similar behavior was observed in the three temperatures and pH. During first 8 h an increase in the concentration of both sugars were observed, which subsequently decreased due to the consumption. Maximum sugars release (4 g/L) was observed at pH 5.5 at 37 °C from 5.8 ± 0.02 to 9.80 ± 0.01 g/L (glucose plus saccharose). Fig. 3 shows the growth curves of K. marxianus yeast.
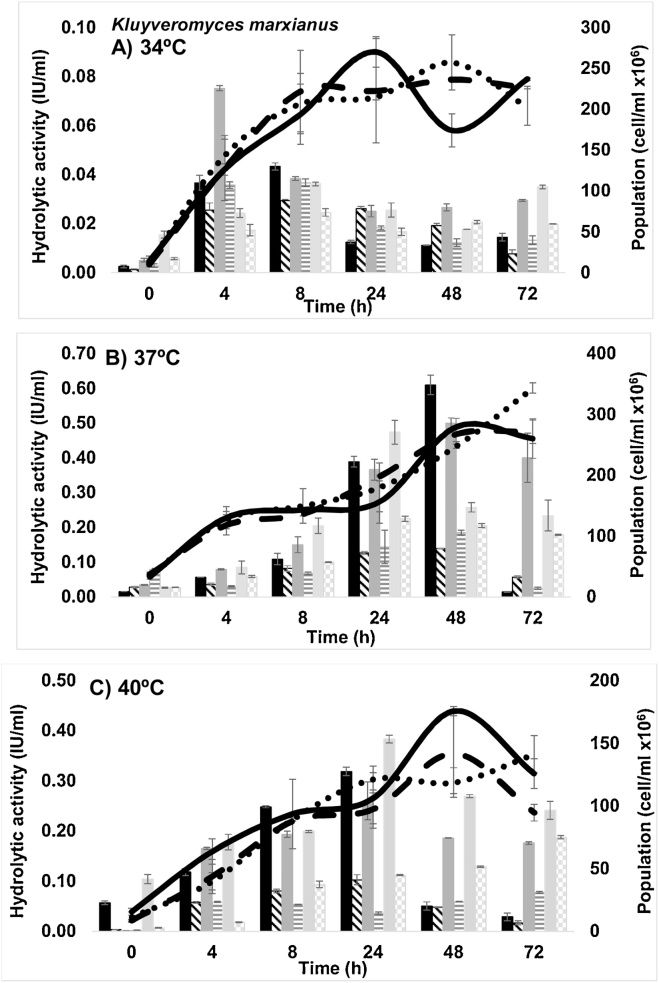
Fig. 3.
Growth at pH 4.5 (continuous line), 5.5 (dashed line) and 6.5 (dotted line). FPase activity (solid bars) at pH 4.5 (black), 5.5 (dark gray) and 6.5 (light gray). CMCase activity at pH 4.5 (bars whit inclined lines), 5.5 (bars whit vertical lines) and 6.5 (bars whit squares) for Kluyveromyces marxianus at A) 34 °C, B).
A similar growth was observed in the three conditions of pH, obtaining the largest population at 37 °C (3.43 ± 0.08 cell/ml x108) at 72 h. In addition, it exhibited an exponential growth after 4 h. At 34 °C the exponential growth covered the first 8 h to later remain a steady state, while at 40 °C the growth was the lowest reported. Raimondi et al. [25] indicates that K. marxianus is able to grow efficiently in a wide range of temperatures (4–45 °C) and pH between 4 and 7, the results obtained in the present work are within is the reported by literature [25]. The yeast K. marxianus also had hydrolytic activity. In a previous work [26], the hydrolytic activity of the yeast was verify, by the presence of halos of degradation in solid medium added with cladode flour. In this study, the hydrolytic activity was quantified and the values were very similar to those obtained for the bacterium, which makes both microorganisms promising for hydrolysis and fermentation. The maximum FPase was 0.67 ± 0.03 IU/ml at pH 6.5 and of 0.40 ± 0.01 IU/ml (both at 24 h and 37 °C) at pH 5.5, with significant differences. The maximum activity of CMCase was obtained at 37 °C at pH of 5.5 while at 6.5 of pH this activity was 0.22 ± 0.007 IU / ml, at 24 h and 4 h, respectively. It has been reported by Yanase et al. [27] that an endoglucanase activity was performed on the cell surface of a recombinant yeast of K. marxianus and also in the extracellular supernatant. Using a cell pellet fraction, a 0.12 IU/OD600 was obtained when the temperature used was 40 °C; in contrast, a value of 7.02 IU/OD600 was observed at 50 °C. It is important to clarify that a mixture of live cells (collected after growth in YPD for 48 h at 30 °C) with an adjusted optical density and mixed with a solution of 1% CMC in 0.1 M citric acid buffer (pH 5) was used to quantify the enzymatic activity of the cell surface with obvious higher activity. Although, these conditions were not the same, that study was compared with the results obtained for the wild yeast of this work, without any genetic modification, as a reference. K. marxianus has been isolated from a wide variety of habitats, resulting in a high metabolic diversity. As a result, biotechnological applications with this yeast as the production of enzymes as β-galactosidase, β-glucosidase, polygalacturonase, inulinase, among others, have been investigated. The yeast K. marxianus presented a contrasting behavior to that shown by the bacteria. Fig. 4 shows the free reducing sugars and alcohol produced in each of the conditions tested.
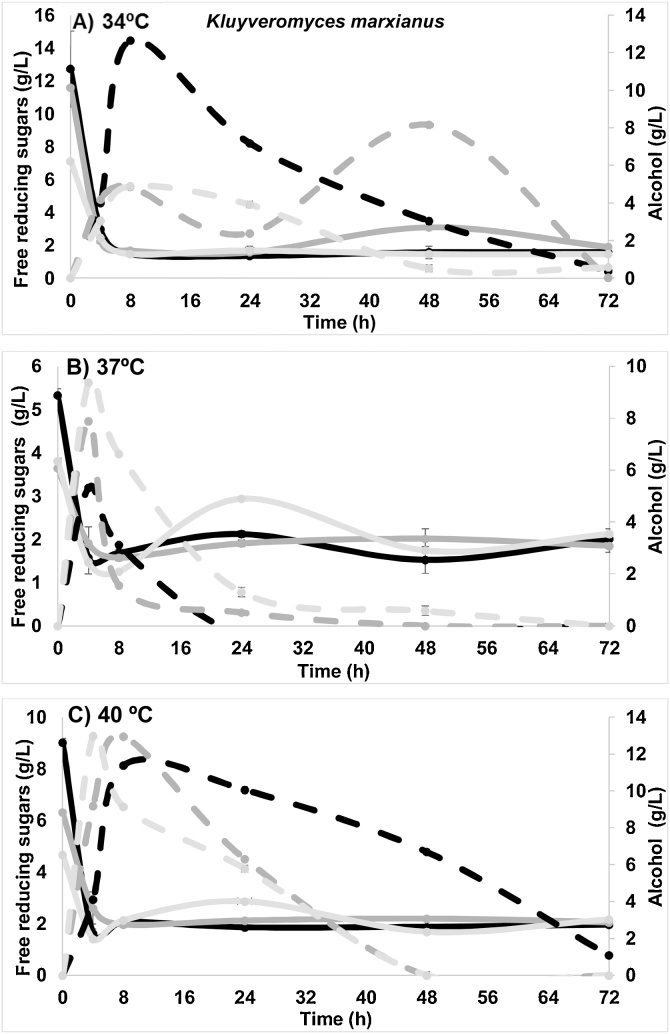
Fig. 4.
Free reducing sugars (continue lines) and alcohol (dashed lines) for Kluyveromyces marxianus at pH 4.5 (black), 5.5 (dark gray) and 6.5 (light gray) at A) 34 °C, B) 37 °C and C) 40 °C.
The FRS decreased in the first 4 h at all temperatures and pH, reflecting a consume of these sugars to achieve alcohol production, obtaining the maximum alcohol concentration (12.95 ± 0.03 g/L) at pH 5.5, 40 °C and 8 h; although, none significant differences were found with pH of 6.5. Similar alcohol production was obtained with pH of 4.5 at 34 °C. This maximum value obtained corresponds to half of that reported by Kuloyo et al. [10]. The authors made a fermentation of cladodes of Opuntia ficus-indica also using K. marxianus. They obtained a value of 25 g/L of alcohol at 40 °C after 48 h of fermentation. It is important to note that the raw material was previously hydrolyzed with dilute acid and commercial enzymes, which allowed the release of higher concentrations of fermentable sugars while in this work none previous hydrolysis was carried out. Alencar et al. [28] also obtained a very similar alcohol value, 26.4 g/L using O. ficus-indica and K. marxianus (with previous acid and enzymatic hydrolysis). The hydrolysis process in the present work was exclusively biological since it was carried out by the yeast itself (with a previous thermal pretreatment, consisting of a sterilization of the medium of 121 °C, 15 min), which demonstrated hydrolytic activity on the raw material. The yeast consumed all the available glucose at the initial time to carry out the conversion to alcohol, while the sucrose remains the same throughout the first 48 h at both temperatures (34 and 37 °C) and for both pH (5.5 and 6.5), subsequently the concentration decreases considerably. On the contrary, at pH 4.5 the concentration increased to a maximum of 12 g/L after 48 h. The yeast assimilated the FRS and glucose presented in the medium followed by the Embden-Meyerhof-Parnas route for the subsequent production of alcohol. Similar to the bacterium, K. marxianus metabolized the carbon source for the production of bioethanol instead of growing, since the process was carried out at 40 °C where the lowest population was reported. Based on the results, 37 °C and pH 6.5 were selected as the best conditions to obtain the maximum hydrolytic activities while 37 °C and pH 5.5 were selected for sugars released (glucose plus saccharose) for later fermentation, using in both cases to A. pittii (highest hydrolytic activity). The microorganism was the factor that showed the greatest effect on both response variables FRS and alcohol. The best conditions to obtain the highest concentration of alcohol with K. marxianus was a pH of 5.5 and 40 °C, obtaining a concentration of 12.95 ± 0.03 g/L (without significant difference between the three pH).
According to the statistical analysis (Table 2), all the factors and their interactions had a significant effect on the alcohol production and hydrolytic activities (p = 0.0000), except for the binary interaction pH-microorganism (for CMCase activity with p = 0.2094). For both activities, the interaction temperature-microorganism presented the greatest effect while for alcohol and population the microorganism factor had the greatest effect.
Table 2.
P-values obtained from ANOVA for the evaluated responses.
| Source | Population | Total Activity (FPU) | Endo Activity (CMCasas) | Glucose | Saccharose | Alcohol | FRS* |
|---|---|---|---|---|---|---|---|
| MAIN EFFECTS | |||||||
| A:pH | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 |
| B:Temperatura | 0.0000 | 0.0000 | 0.0000 | 0.2686 | 0.0000 | 0.0000 | 0.0000 |
| C:Microorganismo | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 |
| INTERACTIONS | |||||||
| AB | 0.0000 | 0.0000 | 0.0000 | 0.0302 | 0.0000 | 0.0000 | 0.0002 |
| AC | 0.0000 | 0.0000 | 0.2094 | 0.0000 | 0.0001 | 0.0000 | 0.0000 |
| BC | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 |
| ABC | 0.0000 | 0.0000 | 0.0000 | 0.0003 | 0.0003 | 0.0000 | 0.0000 |
Free Reductor Sugars.
4. Conclusions
The two microorganisms analyzed grew satisfactorily in the culture medium added with cladode flour. It was observed that Acinetobacter pittii had the highest growth compared to yeast. Likewise, the bacteria had the highest activity of total cellulases and endoglucanases at 37 °C and pH 6.5, as a result of the enzymatic induction, due to the cellulose present in the culture medium added with cladode flour, considered a universal inducer of cellulases. Hydrolysis of the carbon source was utilized to obtain free reducing sugars, these sugars were used as a substrate for microorganisms growth and enzymes production. The Kluyveromyces marxianus also exhibited total cellulase and endoglucanase activity, and the decrease in glucose concentration along the kinetics was associated with an effective fermentation for conversion to alcohol (this yeast presented the maximum alcohol production at 40 °C and pH 5.5 at 4 h). Based on the results obtained, it is suggest the use of Acinetobacter pittii under the best conditions for carbohydrates release and after that, the used of the yeast Kluyveromyces marxianus for alcoholic fermentation in a simultaneous or semi-simultaneous process.
Declaration of Competing Interest
The authors declare that there are no conflicts of interests
Acknowledgments
This work was supported by the National Council of Science and Technology of Mexico (CONACYT) and Secretariat of Agriculture, Livestock, Rural Development, Fisheries and Food (SAGARPA) with project No. 195157.
The author López-Domínguez thanks to National Council of Science and Technology of Mexico (CONACYT) for scholarship No. 265844.
References
- 1.Barnette A.L., Bradley L.C., Verest B.D., Schereiner E.P., Park Y.B., Park J., Park S., Kim S.H. Selective detection of crystalline cellulose in plant cell with sum-frequency-generation (SFG) vibration spectroscopy. Biomacromolecules. 2011;12(7):2434–2439. doi: 10.1021/bm200518n. [DOI] [PubMed] [Google Scholar]
- 2.Machado Cristina. 2010. Situación de los Biocombustibles de 2da y 3era Generación en América Latina y Caribe: 1-99. En Biocombustibles de 2da y 3era Generación – OLADE/IICA. [Google Scholar]
- 3.Bhat M., Bhat S. Cellulose degrading enzymes and their potential industrial applications. Biotechnol. Adv. 1997;15(3/4):583–620. doi: 10.1016/s0734-9750(97)00006-2. [DOI] [PubMed] [Google Scholar]
- 4.Filson P.B., Dawson B.E. Sono-chemical preparation of cellulose nanocrystals from lignocellulose derived materials. Bioresour. Technol. 2009;100:2259–2264. doi: 10.1016/j.biortech.2008.09.062. [DOI] [PubMed] [Google Scholar]
- 5.SIAP . 2018. Servicio de Informacion Agroalimentaria y Pesquera.https://www.gob.mx/siap Retrieved May 7, 2018, from. [Google Scholar]
- 6.SAGARPA . 2016. Secretaria de Agricultura, Ganaderia, Desarrollo rural, Pesca y Alimentacion.https://www.gob.mx/sagarpa Retrieved March 13, 2016, from. [Google Scholar]
- 7.Santos T.D.N., Dutra E.D., Gomes do Prado A., Leite F.C.B., de Souza R.D.F.R., dos Santos D.C., de Abreu C.A.M., Simões D.A., de Morais M.A., Jr, Menezes R.S.C. Potential for biofuels from the biomass of prickly pear cladodes: challenges for bioetanol and biogas production in dry areas. Biomass Bioenergy. 2016;85:215–222. [Google Scholar]
- 8.Berumen L., Paez J., Soto N.O., Murillo M., Herrera E., Muro A. Chemical composition, in vitro gas production and energetic value of prickly pear fermented with and without Kluyveromyces marxianus. J. Biosci. Biotechnol. 2015;4(3):359–364. [Google Scholar]
- 9.Owen N.A., Griffiths H. Marginal land bioethanol yield potential of four crassulacean acid metabolism candidates (Agave fourcroydes, Agave salmiana, Agave tequilana and Opuntia ficus-indica) in Australia. GCB Bioenergy. 2014;6(6):687–703. [Google Scholar]
- 10.Kuloyo O., du Preez J., García M., Kilian S., Steyn L., Görgens J. Opuntia ficus-indica cladodes as feedstock for ethanol production by Kluyveromyces marxianus and Saccharomyces cerevisiae. World J. Microbiol. Biotechnol. 2014;30:3173–3183. doi: 10.1007/s11274-014-1745-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fucikovski L. Proceedings of the Third Annual Texas Prickly Pear Council; Texas, USA; 1992. [Google Scholar]
- 12.Lachance M.A., Starmer W.T., Phaff H.J. Identification of yeasts found in decaying cactus tissue. Can. J. Microbiol. 1988;34(9):1025–1036. [Google Scholar]
- 13.Vavaro L., Granata G., Balestra G. Severe Erwinia-Caused damage of Opuntia ficus-indica in Italy. J. Phyropathol. 1993;138(4):325–330. [Google Scholar]
- 14.Bernabé L. Universidad Nacional de Tumbes; Perú, Tesis de maestría: 2016. Identificación de bacterias cultivadas y no cultivadas asociadas a las rizósfera de Opuntia ficus-indica (L.) mill (Cactaceae) en ecosistemas áridos; pp. 29–30. [Google Scholar]
- 15.Atlas R. 3rd ed. CRC press; Boca Raton, USA: 2004. Handbook of Microbiological Media. [Google Scholar]
- 16.Miller G.L. Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal. Chem. 1959;31(3):426–428. [Google Scholar]
- 17.Bohringer P., Jacob L. The determination of alcohol using chromic acid. Zeitschr. Flussinges Abstracts. 1964;31:223. [Google Scholar]
- 18.Ghose T. Measurement of cellulase activities. Int. Union Pure Appl. Chem. 1987;59(2):257–268. [Google Scholar]
- 19.Vegasa E., Nieves B. Acientobacter spp.: Aspectos microbiológicos, clínicos y epidemiológicos. Revista de la Sociedad Venezolana de Microbiología. 2005;25(2) [Google Scholar]
- 20.Ekperigin M.M. Preliminary studies of cellulase production by Acinetobacter anitratus and Branhamella sp. Afr. J. Biotechnol. 2007;6(1):28–33. [Google Scholar]
- 21.Deka D., Bhargavi P., Sharma A., Goyal D., Jawed M., Goyal A. Enhancemen to cellulase activity from a new strain of Bacillus subtilis by medium optimization and analysis with various cellulosic substrates. Enzyme Res. 2011:51656. doi: 10.4061/2011/151656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Irfan M., Safdar A., Syed Q., Nadeem M. Isolation and screening of cellulolytic bacteria from soil and optimization of cellulase production and activity. Turk. J. Biochem. 2012;37:287–293. [Google Scholar]
- 23.Alcarraz M., Flores A., Godoy Producción de celulasas por inmovilización celular para el tratamiento de efluentes industriales lignocelulósicos. Revista del Instituto de Investigación de la Facultad de Ingeniería Geológica, Minera, Metalúrgica y Geográfica. 2010;13:97–102. [Google Scholar]
- 24.Poszytek K., Ciezkowska M., Sklodowska A., Drewniak L. Microbial consortium with high cellulolytic activity (MCHCA) for enhanced biogas production. Front. Microbiol. 2016;7(324):1–11. doi: 10.3389/fmicb.2016.00324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Raimondi S., Zanni E., Amaretti A., Palleschi C., Uccellettii D., Rossi M. Thermal adaptability of Kluyveromyces marxianus in recombinant protein production. Microb. Cell Fact. 2013;12(34):2–7. doi: 10.1186/1475-2859-12-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.López-Domínguez C., Wu D., Ramírez-Sucre M., Rodríguez-Buenfil I. Determinación de la actividad hidrolítica de bacterias silvestres empleando harina de cladodios de Opunta ficus-indica como sustrato. Memories of the Food and Biotechnology International Congress; Pag. 115. Amalgama Arte Editorial S.A. de C.V. México, D.F; 2016. [Google Scholar]
- 27.Yanase S., Husunuma T., Yamada T., Tanaka T., Ogino C., Fukuda H., Kondo A. Direct ethanol production from cellulosic materials at high temperature using the thermotolerant yeast Kluyveromyces marxianus displaying cellulolytic enzymes. Appl. Microbiol. Biotechnol. 2010;88:381–388. doi: 10.1007/s00253-010-2784-z. [DOI] [PubMed] [Google Scholar]
- 28.Alencar B.R.A., Dutra E.D., Sampaio E.V.S.B., Menezes R.S.C., de Morais M.A., Jr Enzymatic hydrolysis of cactus pear varieties with high solids loading for bioetanol production. Bioresour. Technol. 2018;250:273–280. doi: 10.1016/j.biortech.2017.11.042. [DOI] [PubMed] [Google Scholar]