Abstract
Lymphatic vessels mediate fluid flows that affect antigen distribution and delivery, lymph node stromal remodeling, and cell-cell interactions, to thus regulate immune activation. Here we review the functional role of lymphatic transport and lymph node biomechanics in immunity. We present experimental tools that enable quantitative analysis of lymphatic transport and lymph node dynamics in vitro and in vivo. Finally, we discuss the current understanding for how changes in lymphatic transport and lymph node biomechanics contribute to pathogenesis of conditions including cancer, aging, neurodegeneration, and infection.
Subject Areas: Lymphology, Biophysics, Biomechanics, Biological Sciences Tools
Graphical Abstract
Lymphology; Biophysics; Biomechanics; Biological Sciences Tools
Introduction
The lymphatic system is composed of a hierarchy of vessels that facilitate directional lymph transport from peripheral tissues to secondary lymphoid organs (lymph nodes, LNs) where adaptive immune responses are initiated. As such, lymphatic vessels are appreciated as necessary physical conduits that facilitate immune surveillance, but an understanding of how changes in lymphatic transport, the movement of lymph, cells, and antigen through this system, is actively regulated during inflammatory processes and how these changes impact immunity is only beginning to emerge. Dissecting the mechanisms by which lymphatic vessels impact immune responses through changes in fluid flows, macromolecule distribution, leukocyte trafficking, and LN organization requires rigorous, quantitative approaches both in vivo and in vitro paired with a deep understanding of relevant disease processes and models. This review aims to integrate concepts and experimental approaches across the fields of immunology, lymphatic physiology, and bioengineering to showcase new approaches to understanding disease. Herein, we will highlight the role of lymphatic transport in immune physiology, describe bioengineering and biophysical tools that enable the interrogation of these effects on lymphatic physiology and immunity in vitro and in vivo, and detail the current understandings of how lymphatic transport is altered in disease.
Lymphatic Transport in Immunity
Lymph Formation and Antigen Transport
Lymph is formed in the periphery, where vascular transudate is transported through the interstitium at rates of 0.1–1.0 μm/s, and subsequently enters initial lymphatic capillaries through characteristic gaps between lymphatic endothelial cells (LECs) (Wiig and Swartz, 2012, Huxley and Scallan, 2011). Interstitial fluid flows and intralymphatic lymph propulsion are mediated by intrinsic (phasic smooth muscle contraction) collecting lymphatic pumping and extrinsic (e.g., respiration) physiological forces (Dixon, 2010) that drive net directional fluid flow within lymphatic vessels, maintain a pressure gradient from the periphery on into the vessel, and ultimately transport lymph and its contents to LNs (Figure 1). Lymph itself is composed of soluble proteins, pre-processed antigens, and metabolites (Zawieja, 2009) as well as larger particulates, exosomes, and protein complexes (~30 nm), which are most efficiently transported by lymphatic vessels (Reddy et al., 2007) and accumulate in LNs (Rohner and Thomas, 2016, Rohner and Thomas, 2017). Importantly, the size exclusion of peripheral lymphatic vessels is conserved in tumors (Rohner and Thomas, 2016) but may change under various inflammatory contexts (Loo et al., 2017, Zhang et al., 2018) where remodeling of endothelial junctions limits passive, paracellular transport. As such, the information delivered to LNs by draining lymphatic vessels may be context dependent, and further exploration is needed to discern the significance of these changes.
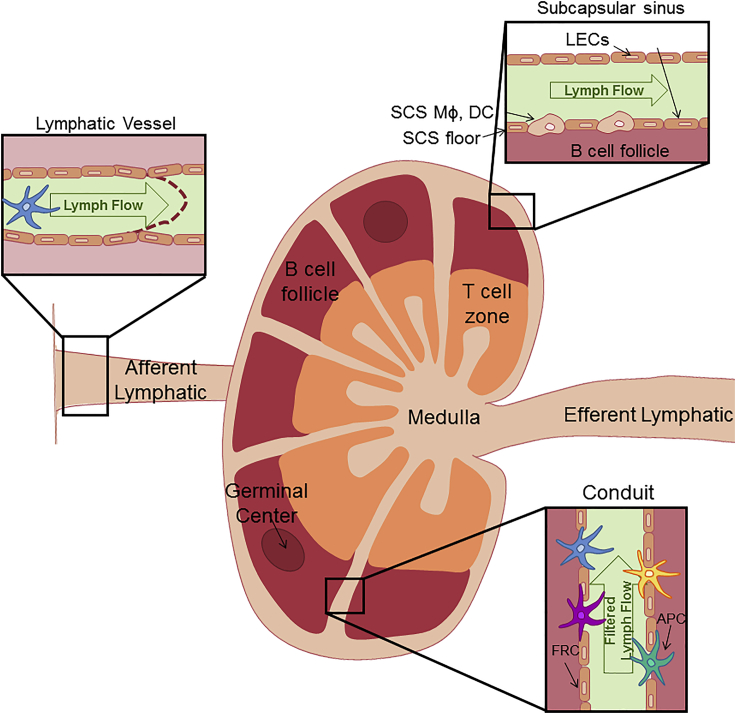
Figure 1.
Lymph Node Structure
The typical structure of a lymph node, demonstrating normal partitioning and structures, including the T cell zone, medulla, B cell follicle and germinal center, subcapsular sinus, conduits, and afferent and efferent lymphatics, is represented, along with improved visualization of regions of fluid flows, including the flow of lymph through afferent lymphatic vessels and the subcapsular sinus, and the flow of filtered lymph through the conduit system.
Afferent lymph enters the LN (Figure 1) where cells and antigen are compartmentalized to coordinate and synchronize immune responses. Two layers of LECs facilitate lymph flow through the subcapsular sinus (SCS) and around the LN cortex (Figure 1). These specialized LECs support resident subcapsular sinus macrophages (Mondor et al., 2016, Junt et al., 2007, Phan et al., 2007) and dendritic cells (DCs) (Gerner et al., 2015) that regulate antigen, viral, and bacterial access to B cell follicles for the induction of humoral immunity (Junt et al., 2007, Kastenmuller et al., 2012). Lymph flows either around the SCS or through the LN parenchyma via a network of reticular collagen conduits ensheathed by fibroblastic reticular cells (FRCs) (Heath et al., 2004, Sixt et al., 2005, Junt et al., 2007, Roozendaal et al., 2009) (Figure 1). Large particulates (>70 kDa), including intact antibodies and pathogens, are unable to access reticular conduits and are restricted to the SCS, whereas soluble antigens move freely into the paracortical network for sampling by resident DCs (Gretz et al., 2000, Sixt et al., 2005, Roozendaal et al., 2009, Reynoso et al., 2019). Specialized diaphragms in LECs that line the SCS floor actively filter lymph-borne macromolecules and determine the size exclusion properties of the conduits (Rantakari et al., 2015). Although this model of physical filtration has held up over multiple studies, the size exclusion properties of the LN were recently challenged by descriptions of rapid transfer of intact lymph-borne antibodies to LN stroma following subcutaneous injection. Antibody transport was isotype dependent and mediated by receptor-independent vesicular transcytosis in LECs (Kahari et al., 2019). Furthermore, intradermal injection of viruses resulted in rapid uptake of infectious particles in the LN paracortex, including the large enveloped DNA Vaccinia virus (Reynoso et al., 2019). We note, however, that both observations may depend on the biomechanics of injection. Interestingly, Vaccinia virus applied by scarification, which is not associated with high interstitial fluid pressures, does not infect draining LNs (Loo et al., 2017). The interstitial pressures and shear flows associated with injection may increase passive lymphatic uptake and active mechanisms of transcytosis to alter LN delivery (Triacca et al., 2017). Thus, given the sensitivity of the lymphatic network to changes in interstitial fluid pressures, studies of lymph transport to LNs should consider the biophysics of subcutaneous or intradermal injection as it will impact the distribution of lymph-borne particulates and may fail, in some cases, to model endogenous physiology.
Even in the presence of rapid, soluble antigen transport, active delivery of antigen by migratory, professional antigen-presenting cells (APCs) is critical to the generation of adaptive immune responses (Allan et al., 2006). Lymphatic vessels are necessary for DC migration to LNs (Thomas et al., 2012, Loo et al., 2017, Platt et al., 2013), actively facilitate DC homing and transendothelial migration (Jackson, 2014, Miteva et al., 2010, Russo et al., 2016, Teijeira et al., 2017), and shape chemokine gradients to permit migration into the LN paracortex (Ulvmar et al., 2014), making them critical features of an efficient adaptive immune response. Importantly, temporal control over the kinetics of specialized migratory DC subsets from peripheral tissue to LNs coordinates sequential interactions with naive CD4+ and CD8+ T cells driving efficient effector responses (Hor et al., 2015, Kissenpfennig et al., 2005). Migratory DCs can also deliver antigen to resident DCs (Gurevich et al., 2017, Allan et al., 2006). It is within the LN paracortex, and specifically along the reticular conduit network, that mature resident and migratory DCs interact with naive T cells in a chemokine receptor 7 (CCR7)-dependent manner (Braun et al., 2011, Schumann et al., 2010, Gunn et al., 1998). Importantly, lymph flow itself appears to partially maintain paracortical structure and chemokine expression (Tomei et al., 2009) and, along with lymph-borne migratory DCs, supports high endothelial venules (HEVs) (Webster et al., 2006, Chyou et al., 2008) that facilitate naive lymphocyte entry into LNs (Kumar et al., 2010, Kumar et al., 2012). Following activation and expansion, T cells exit LNs following sphingosine 1-phosphate gradients that are generated and maintained by efferent lymphatic vessels (Pham et al., 2010, Ito et al., 2014, Mendoza et al., 2017) leading to eventual recirculation in blood and homing to sites of inflammation. It is therefore the movement and filtration of lymph through LNs that determines both cellular localization and antigen distribution to enable rapid development of immune responses following peripheral challenge (Thomas et al., 2016).
Fluid Flows, Morphogen Gradients, and Cell Migration
In addition to the role lymph plays in moving immunological information (e.g., solutes, chemokines, cytokines, cells) from peripheral tissues to lymphoid organs, fluid flow itself provides critical organizational cues that influence morphogen asymmetry and directional behavior. Within 3D tissues, fluid flows bias macromolecular distribution, enhance ligand expression, align extracellular matrix (ECM) components, and subsequently shape active mechanisms of cell migration (Schineis et al., 2019, Platt et al., 2013). In mice lacking dermal lymphatic vessels and afferent lymphatic transport to LNs, skin-draining LNs exhibit significantly reduced cellularity and altered stromal morphology (Thomas et al., 2012). Fluid flows across endothelial monolayers (Miteva et al., 2010) and through FRC networks (Tomei et al., 2009) enhance expression of homeostatic and inflammatory chemokines that direct leukocyte localization and migration patterns. Within the interstitial matrix, fluid flow aligns matrix components (Wiig and Swartz, 2012, Freund et al., 2012, Ranft et al., 2012) that along with bound chemokines provide haptotactic cues and organize leukocyte localization within tissue (Lucas and Tamburini, 2019, Schumann et al., 2010) and activate proteases that release matrix bound morphogens to drive local signaling behavior (Fleury et al., 2006). Interestingly, both mathematical modeling and in vitro experimentation indicate that even low levels of convective flow are sufficient to bias chemokine distribution, creating directional paracellular gradients that inform trafficking behavior (Shields et al., 2007, Jafarnejad et al., 2017). For cells simultaneously both producing chemokines and expressing the matched receptor, autologous chemokine gradients are generated by fluid flows that direct trafficking behavior toward draining lymphatic vessels and may be implicated in metastatic seeding of regional LNs (Shields et al., 2007). Different cell types, however, may integrate fluid flow information differently. In vitro data indicate that macrophages migrate in opposition to fluid flows, a behavior, that if true in vivo, may explain their localization to sites of high interstitial fluid pressure such as tumors (Li et al., 2018). Interestingly, fluid flows may affect leukocyte phenotype, with increased flows enhancing transforming growth factor β, arginase, and scavenger receptor expression in 3D in vitro macrophage cultures (Li et al., 2018) and enhancing proliferation and drug sensitivity in engineered models of diffuse large B cell lymphoma (Apoorva et al., 2018). How fluid flows, overlapping morphogen gradients, and the biochemical makeup of the tissue itself integrate to determine complex cellular behaviors in vivo remains to be carefully dissected. Importantly, rates of interstitial fluid flow and subsequent directional cell migration depend upon downstream lymphatic vessel function (Thomas et al., 2012, Platt et al., 2013). Thus, the functional status of the lymphatic vessel hierarchy impacts both upstream lymphatic homing and tissue organization as well as downstream leukocyte positioning and activation. The extent to which fluid flows contribute immune responses independently or rather in collaboration with inflammatory mediators remains to be carefully dissected.
LN Expansion and Contraction
During peripheral tissue inflammation, lymphocyte proliferation is accompanied by a transient reduction in lymphocyte exit leading to dramatic LN expansion. What can be a 10- to 20-fold increase in leukocyte number drives expansion-induced strain that must be supported by synchronized stromal remodeling. During development, fluid flows mediated by perinodal lymphatic vessel maturation cooperate with lymphotoxin beta signaling to amplify stromal CXCL13 and facilitate LN development (Bovay et al., 2018). These observations raise the interesting possibility that altered or disrupted fluid flows in the adult might contribute to LN expansion and contraction through similar mechanisms. What we know is that during peripheral tissue inflammation, all major stromal cell types within the LN exhibit dynamic transcriptional profile changes and undergo a proliferative expansion followed by contraction (Malhotra et al., 2012, Gregory et al., 2017).
Importantly, these cumulative stromal changes (Gregory et al., 2017) impact the mechanical properties, e.g., stiffness (Rohner et al., 2015), of the LN. Changes in LN mechanics and the underlying microarchitecture allow for the dramatic changes in volume that are associated with inflammation and may play important roles in tuning the kinetics and overall extent of nodal immune expansion. A significant player in the regulation of LN elasticity are FRCs, specialized myofibroblasts that exert contractile forces on their matrix environment. FRCs depend on the surface glycoprotein podoplanin to maintain their contractile phenotype. During inflammation, migratory DCs infiltrating the LN parenchyma engage podoplanin through C-type lectin-like 2 (CLEC-2) and attenuate FRC contraction leading to reticular network relaxation. FRC relaxation increased LN volume and thereby supported vaccine-induced lymphocyte expansion (Astarita et al., 2015, Acton et al., 2014). Similarly, human follicular DCs exhibit contractile behavior and may modulate elasticity within B cell follicles as a function of their regional cytokine environment (Munoz-Fernandez et al., 2014). Thus, stromal remodeling drives significant changes in LN microarchitecture, fluid flows, permeability, and biomechanics that are necessary to regulate the extent and duration of adaptive immune responses. How the physical changes observed in inflamed LNs integrate with the biochemical signals classically associated with immunity remains to be fully dissected. Efforts in this area may generate novel insights into how lymphatic structures support immune health and, importantly, how loss of structural dynamics may drive disease.
Experimental Systems and Techniques to Quantify and Model Lymphatic Transport
Based on the developing understanding that lymphatic vessels and the fluid flows associated with their transport function affect immune responses, numerous methods to dissect their regulation and mechanistic role in health and disease have been developed. We describe here current bioengineering and biophysical approaches to evaluate the role physiological forces and transport exerted on and by the lymphatic system play in influencing the immune system (Figure 2). The discussion here is limited to experimental methodologies, noting that numerous important contributions to this field have been made using computational approaches, and the reader is referred to excellent reviews on this topic (Mirsky et al., 2011, Novkovic et al., 2018).
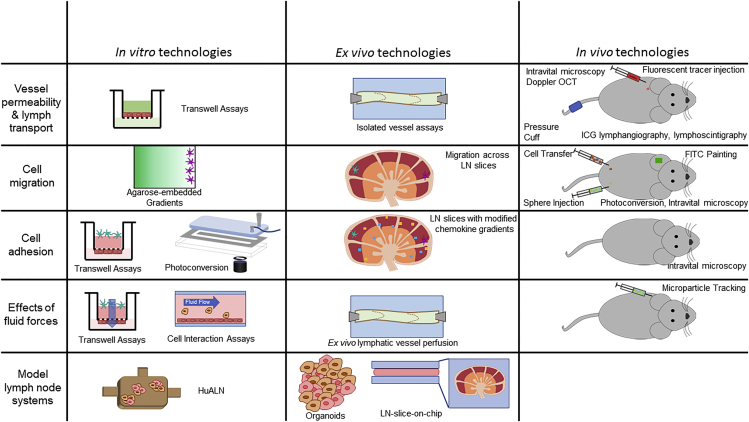
Figure 2.
Technologies to Study Properties of Lymphatics
Example technologies of use to assess vessel permeability and lymph transport, cell migration, cell adhesion, effects of fluid forces, and model lymph node systems, divided into in vitro, ex vivo, and in vivo methodologies. Optical coherence tomorgraphy (OCT); Indocyanine Green (ICG).
Lymphatic Vessel Fluid Flows and Biomechanics
The advancement of lymphatic mechanobiology and system modeling has been historically limited by a lack of quantitation of lymph flows in experimental model systems typically used for immunology studies. The last few decades have seen an emergence of advanced techniques to tackle this problem, improving the field's understanding of flow microenvironments experienced within the lymphatic system of rodents, which are the most commonly used immunobiology models with a wide availability of transgenics, along with improvements in clinical imaging modalities. A few relatively recent advances are described.
Indocyanine green lymphangiography is often regarded as the most effective lymphatic imaging technique (Mihara et al., 2012). Indocyanine green dye is injected intradermally into the skin, followed by near-infrared fluorescent imaging (Unno et al., 2007) (Figure 2). In clinical settings, this has allowed for diagnoses of lymphatic malformations (Seki et al., 2019) and lymphedema even before clinical presentation (Akita et al., 2016). The alternative, lymphoscintigraphy, involves subcutaneous injection of radiotracers, which enter the lymphatic system and can be detected with a gamma camera (Threefoot et al., 1963, Yoshida et al., 2016). This system can be used to diagnose lymphatic disorders with only small amounts of radiotracers and can be used in any patient (even those in critical condition), although it suffers compared with lymphangiography from low spatial resolution, exposure of patients to radiation, and the need for specialized, expensive equipment. Neither technique directly quantifies flow, but rather provide an indirect measure of flow through quantitation of tracer transport.
To enable the more direct measurement of lymphatic flows in vivo, microparticles tagged with near-infrared dyes have been applied to facilitate intravital imaging of lymphatic vessels (Figure 2). In such approaches, ultrahigh-speed imaging is used to measure the velocities of flowing beads whose movement can be traced by fluorescence or phase contrast. From such measurements, fluid shear stress levels within collecting lymphatic vessels in vivo were measured for the first time (Dixon et al., 2005, Dixon et al., 2006). Doppler optical coherence tomography, which uses measurements of frequency shifts based on movement of scattering material, has also be applied in a similar manner (Blatter et al., 2016). Using these techniques, velocities of pulsatile lymphatic flow have been shown to range from −1 to 7 mm/s, which can be used to estimate shear stress levels exerted on LECs.
Lymph transport is an active process requiring the integration of intrinsic and extrinsic pumping processes that generate net unidirectional fluid flow from peripheral tissues to LNs, whose regulatory mechanisms are still being established (Gashev and Zawieja, 2010). Intrinsic pumping, which consists of the rapid and phasic contractions of collecting vessels, is modulated by local physical factors including changes in pressure, stretch, flow, and shear, whereas extrinsic pumping is driven by physiological processes such as blood vessel pulsations, muscle contraction, and breathing movements (Gashev and Zawieja, 2010). To non-invasively quantify the minimum pumping pressure required to drive lymph flow, near-infrared tracers are injected distal to the vessel of interest and pressure subsequently applied to the limb or tail of an animal using a cuff to occlude the lymphatic vessel (Figure 2). This stops flow, the cessation of which is visualized by fluorescence imaging of tracer movement, and pressure is then gradually released (Nelson et al., 2014). Restoration of lymph transport as pressure is decreased is monitored via fluorescence to quantify qualitative attributes of lymphatic function, including effective pumping pressure and vessel emptying rate along with pumping parameters (e.g., amplitude, frequency, systolic time, and diastolic time) (Nelson et al., 2014).
Despite numerous advancements in quantitation of in vivo lymphatic flow, evaluating effects of fluid flow on vessel function, which is shear stress regulated (Kornuta et al., 2015), remains challenging. One technique that was developed to overcome this limitation is an ex vivo lymphatic perfusion system, which consists of saline-bathed, isolated lymphatic vessels (Kornuta and Dixon, 2014) (Figure 2). Vessels are cannulated and perfused with fluid to evaluate the impact of shear stresses on lymphatic vessel pumping, an approach that demonstrated that increased flow impairs lymphatic vessel contraction (Gashev et al., 2002), (Gashev et al., Kornuta and Dixon, Kornuta et al.). Each of these techniques provides new insights into understanding lymphatic vessel flows, with high applicability for further evaluation of lymphatic pumping responses.
The Biological Effects of Fluid Flow
As discussed, LECs and the LN stroma are highly mechanosensitive. For example, shear forces that result from fluid flows regulate collecting lymphatic vessel transport (Kornuta et al., 2015, Gashev et al., 2002, Gashev et al., 2004) and organization of the LN stroma (Mebius et al., 1991). Interrogating the biological consequences of physiological force resulting from interstitial, transmural, and luminal flows provides important insights into lymphatic tissue development and physiology (Sabine et al., 2012, Sabine et al., 2015, Sweet et al., 2015). Furthermore, macrophages (Wang et al., 2016) as well as B (Shaheen et al., 2017) and T cells (Nataraj et al., 2018, O'connor et al., 2012, Lambert et al., 2017) differentially sense and respond to matrix microenvironment topography and stiffness, respectively, indicating the need to build models that simultaneously evaluate multiple biophysical cues. In vitro systems that provide substantial advantages with respect to throughput, cost, and parameterization are accelerating progress in these areas.
Transwell inserts are used to explore the effects of transmural flow on LECs in vitro and assess multiple functional outcomes including transendothelial migration and monolayer permeability (Figure 2). A monolayer of LECs plated on the underside of the transwell permit application of directional transmural flows in a physiologic apical to luminal orientation. ECM with or without embedded cells provides resistance to achieve physiologically relevant rates of fluid flow through the engineered matrix and across the LEC monolayer. Using this system, increasing transmural flow upregulates migration cues and adhesion molecules, including CCL21 and E-selectin (Miteva et al., 2010), which are necessary for leukocyte transmigration, and thereby suggests a functional role for transmural flows in the regulation of LN homing.
Interstitial flow effects on LNs have also been investigated using an in vitro device consisting of cells cultured in a perfused 3D collagen and Matrigel matrix (Tomei et al., 2009) (Figure 2). The system was used to show that FRCs remodel in a shear-dependent manner, recapitulating LN responses to diminished or absent flow seen in vivo (Mebius et al., 1991, Tomei et al., 2009, Thomas et al., 2012). Flow changes, common in inflammation, may thus direct LN cellular organization that is important in regulating adaptive immune responses. In addition to effects on the stroma, lymph transport and associated fluid flows also likely influence leukocyte interactions within the LN. Recent work evaluated the influence of force on DC-T cell interactions. Moura Rosa et al. developed a microfluidic chip where T cells were flowed across a monolayer of DCs and imaged by confocal microscopy (Moura Rosa et al., 2016) (Figure 2). By controlling rates of shear stress (0.1–100 dyn/cm2) and coincident antigen presentation they quantified the force threshold for sustained T cell/DC interactions.
Lymphatic Vessel and LN Permeability and Molecular Transport
In addition to regulating fluid adsorption, lymphatic capillaries facilitate intercellular signaling between peripheral tissues and draining LNs through solute transport. Solute transport by LECs occurs both passively and actively, wherein molecules may pass in a paracellular fashion through intercellular junctions or be transported across the LEC cell body via active uptake or transcytosis pathways (Rantakari et al., 2015, Kahari et al., 2019). The relative contribution of paracellular and transcellular transport can vary with disease state and local inflammation (Zolla et al., 2015, Gousopoulos et al., 2016) and thereby substantially effect overall permeability of the lymphatic endothelium and influence signaling within draining LNs (Kuan et al., 2015, Loo et al., 2017, Srinivasan et al., 2016).
In vitro systems are often used to directly evaluate molecular transport across endothelial monolayers at steady state and in the presence of inflammatory stimuli. For example, effective permeability can be determined using Transwell systems that allow sampling of media on either side of a LEC monolayer to quantitatively monitor changes over time of fluorescent tracer content (Triacca et al., 2017) (Figure 2). Organotypic microfluidic platforms are used to explore complex tissue architecture and incorporate intercellular interactions and cross talk. For example, Sato et al. engineered a microfluidic chip containing both LEC- and blood endothelial cell-lined channels on either side of a model tissue interstitium to simultaneously quantify permeability across both blood and lymphatic capillaries (Sato et al., 2015). This system can be used to account for both the lymphatic and blood components of clearance from the interstitium as it occurs in vivo and how these pathways are differentially regulated in response to stimuli.
Although in vitro studies provide important insights in well-controlled conditions, they do not fully recapitulate in vivo 3D tissues. Tools for ex vivo and in vivo investigations of vessel permeability can instead quantitate transport in a physiological context. In one such ex vivo approach, lymphatic vessels are excised, cannulated, perfused with fluorophores of defined molecular weights, and fluorescence flux across the vessel wall calculated (Ono et al., 2005) (Figure 2). Molecular mechanisms that regulate permeability in intact vessels and lymphatic muscle responses and effects of pathology or drugs on lymphatic vessel function have been revealed using this technique (Ono et al., 2005, Scallan et al., 2013, Davis et al., 2012). To assess in vivo lymphatic vessel permeability, lymphatic vessels are cannulated and perfused with fluorescent tracers in situ at different pressure differentials whereby flux is assessed by fluorescent imaging (Scallan and Huxley, 2010) (Figure 2). This system was used to show that increasing intraluminal pressure decreases spontaneous lymphatic vessel contraction and permeability. The permeability of naive and inflamed (tumor-draining) FRC-lined LN conduits (Riedel et al., 2016) has been assessed using confocal microscopy on excised samples and that of LN blood vessels assessed by intravital multiphoton microscopy (Gerner et al., 2015, Kastenmuller et al., 2012, Meijer et al., 2017).
Numerous techniques are implemented in pre-clinical disease models for the experimental assessment of lymphatic transport of solutes and molecular or particulate cargos. For example, fluorescently tagged tracers (typically dextrans) can be used to quantitate lymphatic drainage patterns in vivo (Gretz et al., 2000, Angeli et al., 2006, Thomas et al., 2012) (Figure 2). In such studies, tracers of known hydrodynamic radii are tagged with fluorescent molecules and injected into the interstitium. LNs, along with systemic tissues, are assessed for bulk fluorescence (Rohner and Thomas, 2016), imaged in whole or tissue sections using confocal microscopy (Gretz et al., 2000), or analyzed flow cytometrically to discern patterns of cellular uptake (Thomas et al., 2012). This improves understanding of the in vivo barriers to passive lymphatic drainage of differently sized molecules and has been used to better understand the structure of the LN (Gretz et al., 2000) and impacts of cancer progression on transport (Rohner and Thomas, 2016). Fluorescein isothiocyanate (FITC) painting can also be used to measure lymphatic solute transport in vivo (Figure 2). In this approach, FITC is painted onto the skin of an animal, and the FITC content is analyzed in tissues on a bulk basis to assess total transport (Thomas et al., 2012), within cells using flow cytometry to delineate cell subtype-specific uptake of FITC (Thomas et al., 2012), and via imaging of LN slices (Angeli et al., 2006). Transport of particulate cargos, such as drug delivery vehicles or cell-derived extracellular vesicles, via lymphatic trafficking cells can be assessed quantitatively by injecting larger (>100 nm), fluorescently tagged tracers (polymer particles, for example) into peripheral tissues and quantifying fluorescence in LNs draining the site of injection (dLN) on a bulk (Rohner and Thomas, 2016, Rohner and Thomas, 2017) or individual cell (Thomas et al., 2012) basis using total fluorescence of tissue homogenates or flow cytometry, respectively.
Cellular Migration
One of the most important roles of the lymphatic system is coordinating the transport of immune cells to facilitate cell-cell signaling and cross talk. Several approaches to assess these processes and how they are affected by disease have thus been developed.
How immune cells sense and respond to chemokine cues to invade lymphatic vessels as well as LNs is of major interest to determine how lymphatic tissues orchestrate immune responses. To directly measure chemotaxis in 3D, an agarose-based microfluidic device was developed by Haessler et al. wherein cell migration can be queried in the presence of rapidly formed, stable chemokine gradients (Haessler et al., 2009, Haessler et al., 2011) (Figure 2). In so doing, physiological chemokine gradients could be recapitulated and their effects on cells assessed in a high-throughput and tunable manner. Such a system was used to investigate DC responses to gradients of soluble versus matrix-bound CCR7 ligands and has high applicability to other chemotaxis programs that orchestrate cell homing to and within lymphoid tissues. Further investigations into DC responses to CCR7 ligands were also performed by allowing DCs to migrate across live LN slices or ligand-coated carbon fibers (Schumann et al., 2010) (Figure 2). These studies improve understandings of the effects of immobilization of CCR7 ligands in a physiological setting. This provides a method to investigate cell responses to the different structures present in lymphoid tissues, although physiological flows were not recapitulated in either system.
Although limited to date to the study of cancer metastasis, invasion into lymphatic vessels and flow effects on this process have been modeled in vitro using Transwell systems (Pisano et al., 2015) (Figure 2). In this model, the insert containing cells cultured in a collagen/Matrigel ECM solution is placed in a cell culture insert, which fits into a custom-fabricated microfluidic chamber. The chamber is designed to allow culture media to flow both through and across multiple cell-containing gels, enabling high-throughput evaluation of cell invasion under biologically relevant shear and transmural flows.
Effects of luminal flow fields on cell migration have been widely explored, although this work has been primarily confined to the study of cells circulating through the blood vasculature. Nevertheless, these fluidic systems are highly relevant to modeling myeloid cell and lymphocyte homing to LNs via HEVs as well as DC and lymphocyte migration to LNs through afferent lymphatic vessels. As examples of the former, microfluidic chips with tightly controlled patterning of adhesion molecules have been implemented to evaluate bond strength and cooperative effects of receptor-ligand interactions on cell adhesion in the context of hemodynamic forces using videomicroscopy (Tong et al., 2012, Edwards and Thomas, 2017) (Figure 2). A velocimetric photoconversion technique (Edwards et al., 2018) was also recently described, for use in conjunction with microfluidic devices, that model circulating cell adhesion in the context of fluid flow. In this approach, cells expressing a photoconvertible fluorophore, which irreversibly alters its spectral properties after light exposure, are perfused through an engineered microfluidic system illuminated with photoconverting light and fabricated to present vascular endothelium-expressed adhesion receptors (Figure 2). Cells are thus exposed to activating light for times proportional to their transit time through a confined area of illumination. As a result, the fluorescence of each cell shifts in proportion to its adhesive behavior. When used in conjunction with multi-color flow cytometry, features of interacting versus non-interacting cells can be explored in high content and throughput. Both techniques could putatively be used to model and analyze lymphocyte recruitment to LNs through HEVs that exhibit dynamic morphologies to adapt to inflammation state.
Transit of migratory immune cells to LNs and vice versa is controlled by regulatory cues from the lymphatic system at multiple stages. Accordingly, numerous tools have been generated that permit in vivo examination of the adhesion and migration of immune cells within lymphatic capillaries. First, animal models are used to image immune cell-LEC interactions in vivo. In these experiments, fluorescently tagged immune cells are transferred into animals bearing fluorescent LECs and migration is imaged using multiphoton microscopy (Russo et al., 2016) (Figure 2). Such systems show that LECs generate CCL21 gradients under flow conditions, which direct DC migration (Russo et al., 2016), and that T cells interact with lymphatic capillaries via intercellular adhesion molecule 1 (ICAM-1)/lymphocyte function-associated antigen 1 (LFA-1) interactions, allowing them to enter the lymphatic collectors where they passively flow into LNs (Teijeira et al., 2017). Cells can also be loaded with latex beads or simply tagged fluorescently and adoptively transferred into the periphery (Angeli et al., 2006). The location and movement of these adoptively transferred cells can then be tracked via analysis of beads, fluorescence, or congenic labels (e.g., Thy1.1 or CD45.1) at downstream sites (Figure 2). This allows for closer tracking of specific cell types but requires adoptive transfer of cells, which may change immune responses. Finally, photoconversion represents an exciting technique to also assess cellular trafficking in vivo without the requirement for endogenous cell transfer. Regional application of light in an intact transgenic animal enables confined photoconversion and transient tracking of leukocytes as they migrate within and out of the tissue of interest (Tomura et al., 2008) (Figure 2). In this way, photoconversion can assess patterns of dermal DC and Langerhans cell migration from the skin to LNs (Tomura et al., 2014), assess leukocyte trafficking within the gut (Morton et al., 2014), and investigate movement of T cells out of tumors (Steele et al., 2019, Torcellan et al., 2017), among other diverse applications. These techniques can be widely used to understand how barriers and mechanisms of transport impact cell trafficking from the periphery to LNs.
Engineered In Vitro LN Models
LNs are highly complex multicellular tissues whose microarchitecture tunes disparate immune activity. Delineating the mechanisms of dysregulated tissue organization and function associated with disease is thus of high interest for development and testing of new immunotherapeutic approaches, modeling disease mechanisms, and therapeutic resistance. Furthermore, in vitro and ex vivo technologies offer the promise of personalized and precision medicine (Wang et al., 2015). Accordingly, there has been a groundswell of interest in recent years in in vitro LN models (Wang et al., 2015, Shanti et al., 2018).
To study DC-T cell interactions, an LN-on-a-chip was developed whereby a central and outer reservoir containing DCs and T cells, respectively, are cultured to promote self-assembly of tissue-mimicking structures (Giese et al., 2006) (Figure 2). The subsequent assembly of lymphoid tissue-like structures facilitates DC-mediated early activation of T cells within this artificial system. Expanding on this early-generation LN-on-a-chip, the human artificial LN, containing expanded bioreactors for higher-throughput studies, was developed (Giese et al., 2010) (Figure 2). Immunomodulatory agents (Hepatitis A vaccine and cytomegalovirus) were introduced into the system as model antigens, and cytokine profiles assessed. These profiles mirrored those of human patients, demonstrating the applicability of this system for predicting immunological responses. This system has since been commercialized to test the effects of pharmaceutical drugs on immune cell responses.
A next-generation technology termed an “LN slice-on-a-chip” (Ross et al., 2017) utilizes sliced LNs from patients or animals embedded in agarose gel within microfluidic devices (Figure 2). Ports positioned on the underside of the tissue slice are used to introduce stimuli with high spatiotemporal control. Using this device, B cells were shown to exhibit increased glucose-conjugate uptake compared with T cells (Ross et al., 2017). In an alternative approach using a similar LN slice-on-a-chip system, a small microneedle is used to inject fluorescent tracer into live tissue samples and the sample is imaged via live integrated optical imaging to analyze tracer diffusion and the functional consequence of altered cytokine environments (Ross and Pompano, 2018).
In addition to LN-on-chip technologies, cells can be directed into developing 3D structures, termed organoids, which better mimic the physiological status compared with typical 2D culture (Figure 2). An LN B cell follicle organoid that is highly tunable and representative of in vivo follicles was generated by Purwada et al. (Purwada et al., 2015, Purwada and Singh, 2017). In this system, a scaffold of gelatin and silicate particles houses B cells co-incubated with CD40L-expressing and B cell activating factor-producing stromal cells. Co-culture resulted in spontaneous generation of viable follicles capable of functional B cell antibody class switching. Utilizing air-liquid interface technology, organoids can also be generated to model tumor immune microenvironments using both patient- and animal-derived samples (Neal et al., 2018). In such approaches, immune cells from tumor samples are maintained at similar ratios and activation states in the organoids compared with patient samples, resulting in tumor-infiltrating lymphocyte responses to immune checkpoint blockade, which mimic the therapeutic responses seen in vivo, suggesting that such in vitro model systems could be used to personalize immunotherapy.
Lymphatic Transport and LN Biomechanics in Disease
Evidence continues to emerge in support of the relevance of lymphatic vessel biology and lymphatic transport in disease pathogenesis. Environmental (Kajiya et al., 2007) and pathogen exposure (Fonseca et al., 2015, Jones et al., 2018, Loo et al., 2017), aging (Da Mesquita et al., 2018, Ecker et al., 2019, Gasheva et al., 2007, Nagai et al., 2011, Zolla et al., 2015), and chronic (Schwager and Detmar, 2019) pathologies are all associated with direct effects on lymphatic transport that likely influence regional fluid homeostasis, tissue immunity, and fibrosis (Figure 3). The integration of the tools described earlier with controlled disease models will be necessary to mechanistically dissect the multifactorial impact of lymphatic vessel biology on disease and reveal new opportunities for therapeutic immunomodulation.
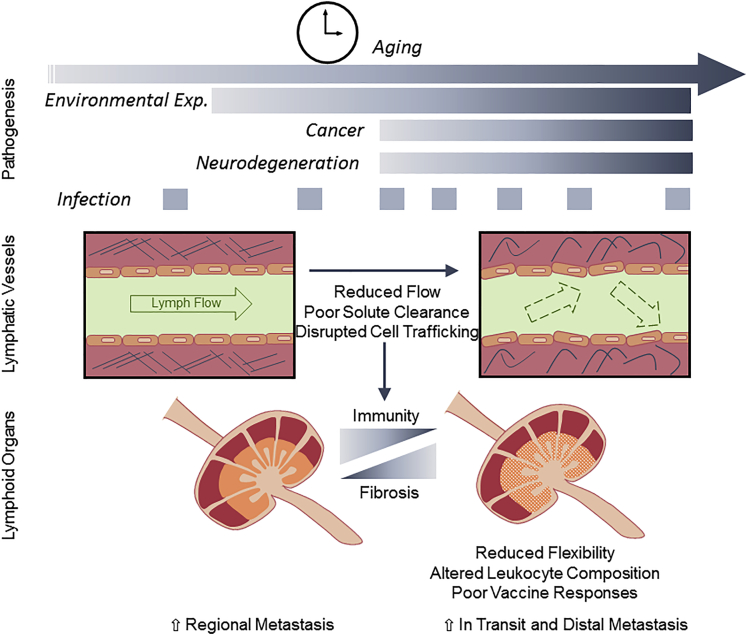
Figure 3.
Progressive Lymphatic Dysfunction Is Associated with Disease
Age, environmental, and infection-related progressive lymphatic dysfunction may in part underscore pathophysiology associated with cancer, neurodegeneration, and other disease states. Changes in lymphatic transport affect peripheral tissue fluid dynamics, cell migration patterns, and solute clearance and lymph node (LN) fibrosis that drive tissue accumulation of inflammatory mediators and disrupt spatiotemporal control over protective immune responses.
Cancer
The contribution of lymphatic vessels and their transport function in cancer progression has long been attributed to their role in regional dissemination of metastatic tumor cells (Stacker et al., 2014). LN metastasis is associated with poor outcomes across a variety of solid tumor types. Mechanistic studies indicate that interstitial fluid flows and lymphatic vessel-secreted chemokine gradients directly facilitate directional homing (Issa et al., 2009, Shields et al., 2007), transmigration (Miteva et al., 2010, Pisano et al., 2015, Triacca et al., 2017), and eventual seeding of tumor cells in sentinel LNs (Commerford et al., 2018, Ogawa et al., 2014, Olmeda et al., 2017). In addition to their contribution to metastasis, progressive lymphatic vessel dysfunction in tumors (Karnezis et al., 2012) and regional fibrosis significantly alter solute efflux out of tumors. This raises interstitial fluid pressures that disrupt drug and nutrient delivery (Leu et al., 2000, Stylianopoulos et al., 2013). Thus, remodeling of lymphatic vessel transport in tumor microenvironments impacts the biophysical, biochemical, and metabolic tumor context.
The implications of lymphangiogenesis and lymphatic transport on anti-tumor immunity are of increasing interest to the field. Overexpression of vascular endothelial growth factor (VEGF)C and subsequent tumor-associated lymphangiogenesis drives intratumoral inflammation that at baseline favors immune suppression (Lund et al., 2012) but is more readily activated by immunotherapy (Fankhauser et al., 2017) indicating enhanced tumor/host communication. Consistently, complete loss of dermal lymphatic vessels dampens host tumor recognition leading to immunologically cold tumor microenvironments (Lund et al., 2016). These studies argue that the extent to which lymphatic vessels are engaged with a developing tumor is a significant control point for host immune responses.
In addition to evidence for the role these vessels play as active conduits to draining LNs, there is evidence that, at least in skin, tumor-associated lymphatic vessels may directly inhibit effector lymphocyte accumulation and function through multiple mechanisms. Tumor-associated and LN LECs scavenge and cross-present tumor antigen leading to the dysfunctional activation of naive CD8+ T cells (Hirosue et al., 2014, Lund et al., 2012). Furthermore, recent data indicate that peripheral LECs in melanoma and non-malignant skin activate expression of the T cell inhibitory ligand programmed death ligand 1 (PD-L1) in an interferon ɣ-dependent manner (Lane et al., 2018). Loss of interferon ɣ receptor specifically in lymphatic vessels interrupted cross talk with infiltrating cytotoxic T cells and improved CD8+ T cell-mediated tumor killing in murine melanomas. This suggests direct lymphatic vessel-mediated control of effector immunity within peripheral tissues. Lymphatic vessels, and their transport function, are consequently emerging as active, relevant, and targetable players in anti-tumor immune responses and immunotherapy (Lund, 2016).
Aging
Peripheral lymphatic vessels exhibit progressive dysfunction with age, associated with intrinsic loss of endothelial and mural cell responsiveness, lymphatic muscle cell atrophy, destruction of elastic matrix, and reductions in both the number of lymphatic vessels and number of connections made in existing lymphatic networks (Gashev and Zawieja, 2010). Reduced lymphatic pumping (contraction amplitude and frequency) is specifically mediated by age-related disturbances in nitric oxide-dependent regulatory pathways and limits lymph propulsion to draining LNs (Gasheva et al., 2007, Nagai et al., 2011). Age-related destabilization of lymphatic structures is also associated with changes in the endothelial glycocalyx in collecting vessels (Zolla et al., 2015) and an aging ECM (Ecker et al., 2019), where the resulting hyperpermeability allows both pathogen (Zolla et al., 2015) and tumor cell escape (Ecker et al., 2019).
Meningeal Lymphatic Vessels and Neurodegeneration
Of increasing interest is lymphatic drainage in the central nervous system and its functional relevance in the pathobiology of aging and neurodegeneration. Meningeal lymphatic vessels appear to represent a major outflow route for small and large molecules administered by intraventricular infusion (Aspelund et al., 2015, Ma et al., 2017, Louveau et al., 2015); however, the exact anatomy of lymphatic vessel-mediated drainage to cervical LNs remains controversial. Although initial descriptions focused on dorsal vessels (Louveau et al., 2015, Aspelund et al., 2015), their uptake of fluorescent tracers may be limited (Ma et al., 2017) and more recent work implicates basal lymphatic vessels (at lateral or basal parts of the skull) as dominant outflow pathways for cerebrospinal fluid drainage (Ahn et al., 2019). Lymphatic vessels are additionally implicated in cerebrospinal fluid outflow from the sacral spine (Ma et al., 2019). Importantly, age-related changes in meningeal lymphatic transport may have significant implications for disease (Da Mesquita et al., 2018, Ma et al., 2017, Ahn et al., 2019). Recent data indicate that disrupted lymphatic transport accelerates the accumulation of amyloid beta (Aβ) and exacerbates disease in murine models of Alzheimer’s disease (Da Mesquita et al., 2018). Although mouse studies indicate lymphatic vessels may be a critical route for Aβ efflux, careful histological analysis failed to identify specific Aβ staining either within or around human dural lymphatic vessels (Goodman et al., 2018) as has been described for blood vessels. Whether this suggests that Aβ is not cleared by lymphatic vessels in humans, or that it may simply be harder to detect than for blood vessels, remains to be carefully considered. Still, the presence of functional lymphatic vessels draining the central nervous system argues for a more careful investigation of the role of lymphatic drainage in neurological disease, inflammation, and immune privilege.
Environment and Infection
In addition to the progressive process of aging, environmental factors such as sun exposure (Kajiya et al., 2007) and infection (Fonseca et al., 2015, Jones et al., 2018, Loo et al., 2017) drive persistent and progressive remodeling of lymphatic vessel density and function. Although acute cutaneous infection transiently reduces lymph transport to prevent viral dissemination (Loo et al., 2017), persistent local inflammation drives lymphatic vessel maladaptation, impairs lymphatic contraction, and promotes regional immunosuppression (Liao et al., 2011). Direct pathogen-related lymphatic vessel dysfunction is observed following methicillin-resistant Staphylococcus aureus infection that induces death in lymphatic muscle cells and impaired pumping (Jones et al., 2018). Importantly, even following pathogen clearance, chronic loss of mesenteric lymphatic transport perturbs regional immunity and cellular trafficking through altered exchange with regional adipose depots (Fonseca et al., 2015, Kuan et al., 2015). Thus, the history of pathogen and environmental exposure within a given tissue may determine future immune responsiveness and immune-related pathologies owing to long-lived alterations in lymphatic structures.
Lymphatic and LN Fibrosis
Fibrosis within the lymphatic system, both vessels and LNs, may significantly contribute to altered fluid homeostasis and immune surveillance. Ectopic recruitment of smooth muscle cells to lymphatic capillaries driven by platelet-derived growth factor B signaling in LECs disrupts lymphatic function in the context of disease. Accumulation of smooth muscle cells, fibroblasts, and fibrogenic molecules to the perilymphatic space causes capillary fibrosis, disrupts fluid transport, and contributes to the pathogenesis of pulmonary fibrosis (Meinecke et al., 2012, Wang et al., 2017). How tissue type and disease context may differentially predispose lymphatic vessels to fibrotic reactions remains to be carefully dissected. Interestingly, in the context of melanoma, lymphatic transport is transiently increased (Harrell et al., 2007) and then progressively reduced (Rohner and Thomas, 2016). These changes in transport are likely regulated by multiple processes including increasing interstitial fluid pressures and biomechanical stresses.
Importantly, these age and disease-associated changes in lymphatic transport profoundly impact the structure and function of downstream LNs such that LN fibrosis is a common feature of pathophysiology. Tumor-dLNs exhibit FRC activation (Riedel et al., 2016), ECM remodeling, and progressive stiffening with high interstitial pressures (Rohner et al., 2015). Since immune activation is dependent on the coordinated recruitment and interaction of leukocyte subsets within the dynamic 3D LN structure, reduction in reticular conduit flexibility may limit these responses. As an example, persistent Leishmania mexicana infection impairs LN expansion inhibiting new leukocyte recruitment and limiting local immune reactivity and parasite control (Hsu and Scott, 2007). Furthermore, repetitive ischemic injuries to the kidney induce dLN disorganization, fibrosis, and FRC disorganization and death (Maarouf et al., 2018), and as injuries occur more frequently, LN remodeling impairs immune responses and exacerbates disease. Patients with HIV exhibit extensive LN fibrosis owing to progressive replacement of FRCs with collagen driven by transforming growth factor β-expressing CD8+ T cells (Huang et al., 2018), and the condition is associated with impaired CD4+ T cell survival (Sanchez et al., 2015, Zeng et al., 2012). Consistent with these observed changes, individual pathogen exposure history and associated LN fibrosis may determine subsequent response to challenge and explain differential vaccine responses in endemic regions (Kityo et al., 2018). Thus, both passive and active remodeling of the lymphatic system, from its fluid and cellular transport properties to LN architecture and function, stand to significantly alter local immune reactivity in persistent and progressive ways.
Conclusions and Future Directions
Lymphatic transport is a critical organizing force for regional immune responses. Both actively regulated in the acute setting and progressively remodeled in the context of disease, lymphatic transport coordinates the delivery of soluble and cell-associated immunological signals to LNs. LNs further depend on fluid flows to maintain structure and function. Future studies that interrogate mechanisms of tissue immunity and tolerance should integrate an understanding of lymphatic transport and its biochemical and biophysical effects within complex 3D tissues. This will allow for exploration into how information delivery to LNs contributes to underlying immune responsiveness or deficiency. These mechanistic studies will define a new paradigm to support efforts to directly modulate transport as a novel immunological control point and further provide rationale for immunotherapy strategies that exploit lymphatic transport.
Acknowledgments
This work was supported by US National Institutes of Health grants R01CA207619 (S.N.T.), U01CA214354 (S.N.T.), T32GM008433 (M.J.O.), R21CA202849 (S.N.T.), R01CA238163 (A.W.L.), and P30-CA069533 (OHSU Knight Cancer Institute, A.W.L.) and grants from Winship Cancer Institute of Emory University (S.N.T.), Department of Defense Peer Reviewed Cancer Research Program (W81XH-15-1-0348, A.W.L.), American Cancer Society (RSG-18-169-01-LIB, A.W.L.), and Cancer Research Institute (Lloyd J. Old STAR, A.W.L.).
Contributor Information
Amanda W. Lund, Email: lunda@ohsu.edu.
Susan N. Thomas, Email: susan.thomas@gatech.edu.
References
- Acton S.E., Farrugia A.J., Astarita J.L., Mourao-Sa D., Jenkins R.P., Nye E., Hooper S., Van Blijswijk J., Rogers N.C., Snelgrove K.J. Dendritic cells control fibroblastic reticular network tension and lymph node expansion. Nature. 2014;514:498–502. doi: 10.1038/nature13814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahn J.H., Cho H., Kim J.-H., Kim S.H., Ham J.-S., Park I., Suh S.H., Hong S.P., Song J.-H., Hong Y.-K. Meningeal lymphatic vessels at the skull base drain cerebrospinal fluid. Nature. 2019;572:62–66. doi: 10.1038/s41586-019-1419-5. [DOI] [PubMed] [Google Scholar]
- Akita S., Nakamura R., Yamamoto N., Tokumoto H., Ishigaki T., Yamaji Y., Sasahara Y., Kubota Y., Mitsukawa N., Satoh K. Early detection of lymphatic disorder and treatment for lymphedema following breast cancer. Plast. Reconstr. Surg. 2016;138:192e–202e. doi: 10.1097/PRS.0000000000002337. [DOI] [PubMed] [Google Scholar]
- Allan R.S., Waithman J., Bedoui S., Jones C.M., Villadangos J.A., Zhan Y., Lew A.M., Shortman K., Heath W.R., Carbone F.R. Migratory dendritic cells transfer antigen to a lymph node-resident dendritic cell population for efficient CTL priming. Immunity. 2006;25:153–162. doi: 10.1016/j.immuni.2006.04.017. [DOI] [PubMed] [Google Scholar]
- Angeli V., Ginhoux F., Llodra J., Quemeneur L., Frenette P.S., Skobe M., Jessberger R., Merad M., Randolph G.J. B cell-driven lymphangiogenesis in inflamed lymph nodes enhances dendritic cell mobilization. Immunity. 2006;24:203–215. doi: 10.1016/j.immuni.2006.01.003. [DOI] [PubMed] [Google Scholar]
- Apoorva F., Loiben A.M., Shah S.B., Purwada A., Fontan L., Goldstein R., Kirby B.J., Melnick A.M., Cosgrove B.D., Singh A. How biophysical forces regulate human B cell lymphomas. Cell Rep. 2018;23:499–511. doi: 10.1016/j.celrep.2018.03.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aspelund A., Antila S., Proulx S.T., Karlsen T.V., Karaman S., Detmar M., Wiig H., Alitalo K. A dural lymphatic vascular system that drains brain interstitial fluid and macromolecules. J. Exp. Med. 2015;212:991–999. doi: 10.1084/jem.20142290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Astarita J.L., Cremasco V., Fu J., Darnell M.C., Peck J.R., Nieves-Bonilla J.M., Song K., Kondo Y., Woodruff M.C., Gogineni A. The CLEC-2-podoplanin axis controls the contractility of fibroblastic reticular cells and lymph node microarchitecture. Nat. Immunol. 2015;16:75–84. doi: 10.1038/ni.3035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blatter C., Meijer E.F.J., Nam A.S., Jones D., Bouma B.E., Padera T.P., Vakoc B.J. In vivo label-free measurement of lymph flow velocity and volumetric flow rates using Doppler optical coherence tomography. Sci. Rep. 2016;6:29035. doi: 10.1038/srep29035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bovay E., Sabine A., Prat-Luri B., Kim S., Son K., Willrodt A.-H., Olsson C., Halin C., Kiefer F., Betsholtz C. Multiple roles of lymphatic vessels in peripheral lymph node development. J. Exp. Med. 2018;215:2760–2777. doi: 10.1084/jem.20180217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun A., Worbs T., Moschovakis G.L., Halle S., Hoffmann K., Bolter J., Munk A., Forster R. Afferent lymph-derived T cells and DCs use different chemokine receptor CCR7-dependent routes for entry into the lymph node and intranodal migration. Nat. Immunol. 2011;12:879–887. doi: 10.1038/ni.2085. [DOI] [PubMed] [Google Scholar]
- Chyou S., Ekland E.H., Carpenter A.C., Tzeng T.-C.J., Tian S., Michaud M., Madri J.A., Lu T.T. Fibroblast-type reticular stromal cells regulate the lymph node vasculature. J. Immunol. 2008;181:3887–3896. doi: 10.4049/jimmunol.181.6.3887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Commerford C.D., Dieterich L.C., He Y., Hell T., Montoya-Zegarra J.A., Noerrelykke S.F., Russo E., Rocken M., Detmar M. Mechanisms of tumor-induced lymphovascular niche formation in draining lymph nodes. Cell Rep. 2018;25:3554–3563.e4. doi: 10.1016/j.celrep.2018.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Da Mesquita S., Louveau A., Vaccari A., Smirnov I., Cornelison R.C., Kingsmore K.M., Contarino C., Onengut-Gumuscu S., Farber E., Raper D. Functional aspects of meningeal lymphatics in ageing and Alzheimer's disease. Nature. 2018;560:185–191. doi: 10.1038/s41586-018-0368-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis M.J., Scallan J.P., Wolpers J.H., Muthuchamy M., Gashev A., Zaweija D.C. Intrinsic increase in lymphangion muscle contractility in response to elevated afterload. Am. J. Physiol. Heart Circ. Physiol. 2012;303:H795–H808. doi: 10.1152/ajpheart.01097.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon J.B. Lymphatic lipid transport: sewer or subway? Trends Endocrinol. Metab. 2010;21:480–487. doi: 10.1016/j.tem.2010.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon J.B., Greiner S.T., Gashev A., Cote G.L., Moore J.E., Zawieja D.C. Lymph flow, shear stress, and lymphocyte velocity in rat mesenteric prenodal lymphatics. Microcirculation. 2006;13:597–610. doi: 10.1080/10739680600893909. [DOI] [PubMed] [Google Scholar]
- Dixon J.B., Zaweija D.C., Gashev A., Cote G.L. Measuring microlymphatic flow using fast video microscopy. J. Biomed. Opt. 2005;10:064016. doi: 10.1117/1.2135791. [DOI] [PubMed] [Google Scholar]
- Ecker B.L., Kaur A., Douglass S.M., Webster M.R., Almeida F.V., Marino G.E., Sinnamon A.J., Neuwirth M.G., Alicea G.M., Ndoye A. Age-related changes in HAPLN1 increase lymphatic permeability and affect routes of melanoma metastasis. Cancer Discov. 2019;9:82–95. doi: 10.1158/2159-8290.CD-18-0168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards E.E., Birmingham K.G., O'melia M.J., Oh J., Thomas S.N. Fluorometric quantification of single-cell velocities to investigate cancer metastasis. Cell Syst. 2018;7:496–509. doi: 10.1016/j.cels.2018.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards E.E., Thomas S.N. P-Selectin and ICAM-1 synergy in mediating THP-1 monocyte adhesion in hemodynamic flow is length dependent. Integr. Biol. 2017;9:313–327. doi: 10.1039/c7ib00020k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fankhauser M., Broggi M.A.S., Potin L., Bordry N., Jeanbart L., Lund A.W., Da Costa E., Hauert S., Rincon-Restrepo M., Tremblay C. Tumor lymphangiogenesis promotes T cell infiltration and potentiates immunotherapy in melanoma. Sci. Transl Med. 2017;9:eaal4712. doi: 10.1126/scitranslmed.aal4712. [DOI] [PubMed] [Google Scholar]
- Fleury M.E., Boardman K.C., Swartz M.A. Autologous morphogen gradients by subtle interstitial flow and matrix interactions. Biophysical J. 2006;91:113–121. doi: 10.1529/biophysj.105.080192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fonseca D.M., Hand T.W., Han S.J., Gerner M.Y., Glatman Zaretsky A., Byrd A.L., Harrison O.J., Ortiz A.M., Quinones M., Trinchieri G. Microbiota-dependent sequelae of acute infection compromise tissue-specific immunity. Cell. 2015;163:354–366. doi: 10.1016/j.cell.2015.08.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freund J.B., Goetz J.G., Hill K.L., Vermot J. Fluid flows and forces in development: functions, features and biophysical principles. Development. 2012;139:1229–1245. doi: 10.1242/dev.073593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gashev A., Davis M.J., Delp M.D., Zawieja D.C. Regional variations of contractile activity in isolated rat lymphatics. Microcirculation. 2004;11:477–492. doi: 10.1080/10739680490476033. [DOI] [PubMed] [Google Scholar]
- Gashev A., Davis M.J., Zaweija D.C. Inhibition of the active lymph pump by flow in rat mesenteric lymphatics and thoracic duct. J. Physiol. 2002;540:1023–1037. doi: 10.1113/jphysiol.2001.016642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gashev A.A., Zawieja D.C. Hydrodynamic regulation of lymphatic transport and the impact of aging. Pathophysiology. 2010;17:277–287. doi: 10.1016/j.pathophys.2009.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasheva O.Y., Knippa K., Nepiushchikh Z.V., Muthuchamy M., Gashev A.A. Age-related alterations of active pumping mechanisms in rat thoracic duct. Microcirculation. 2007;14:827–839. doi: 10.1080/10739680701444065. [DOI] [PubMed] [Google Scholar]
- Gerner M.Y., Torabi-Parizi P., Germain R.N. Strategically localized dendritic cells promote rapid T cell responses to lymph-borne particulate antigens. Immunity. 2015;42:172–185. doi: 10.1016/j.immuni.2014.12.024. [DOI] [PubMed] [Google Scholar]
- Giese C., Demmler C.D., Ammer R., Hartmann S., Lubitz A., Miller L., Muller R., Marx U. A human lymph node in vitro - challenges and progress. Artif. Organs. 2006;30:803–808. doi: 10.1111/j.1525-1594.2006.00303.x. [DOI] [PubMed] [Google Scholar]
- Giese C., Lubitz A., Demmler C.D., Reuschel J., Bergner K., Marx U. Immunological substance testing on human lymphatic micro-organoids in vitro. J. Biotechnol. 2010;148:38–45. doi: 10.1016/j.jbiotec.2010.03.001. [DOI] [PubMed] [Google Scholar]
- Goodman J.R., Adham Z.O., Woltjer R.L., Lund A.W., Iliff J.J. Characterization of dural sinus-associated lymphatic vasculature in human Alzheimer's dementia subjects. Brain Behav. Immun. 2018;73:34–40. doi: 10.1016/j.bbi.2018.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gousopoulos E., Proulx S.T., Scholl J., Uecker M., Detmar M. Prominent lymphatic vessel hyperplasia with progressive dysfunction and distinct immune cell infiltration in lymphedema. Am. J. Pathol. 2016;186:2193–2203. doi: 10.1016/j.ajpath.2016.04.006. [DOI] [PubMed] [Google Scholar]
- Gregory J.L., Walter A., Alexandre Y.O., Hor J.L., Liu R., Ma J.Z., Devi S., Tokuda N., Owada Y., Mackay L.K. Infection programs sustained lymphoid stromal cell responses and shapes lymph node remodeling upon secondary challenge. Cell Rep. 2017;18:406–418. doi: 10.1016/j.celrep.2016.12.038. [DOI] [PubMed] [Google Scholar]
- Gretz J.E., Norbury C.C., Anderson A.O., Proudfoot A.E.I., Shaw S. Lymph-borne chemokines and other low molecular weight molecules reach high endothelial venules via specialized conduits while a functional barrier limits access to the lymphocyte microenvironments in lymph node cortex. J. Exp. Med. 2000;192:1425–1440. doi: 10.1084/jem.192.10.1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunn M.D., Tangemann K., Tam C., Cyster J.G., Rosen S.D., Wiliams L.T. A chemokine expressed in lymphoid high endothelial venules promotes the adhesion and chemotaxis of naive T lymphocytes. Proc. Natl. Acad. Sci. U S A. 1998;95:258–263. doi: 10.1073/pnas.95.1.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurevich I., Feferman T., Milo I., Tal O., Golani O., Drexler I., Shakhar G. Active dissemination of cellular antigens by DCs facilitates CD8+ T-cell priming in lymph nodes. Eur. J. Immunol. 2017;47:1802–1818. doi: 10.1002/eji.201747042. [DOI] [PubMed] [Google Scholar]
- Haessler U., Kalinin Y., Swartz M.A., Wu M. An agarose-based microfluidic platform with a gradient buffer for 3D chemotaxis studies. Biomed. Microdevices. 2009;11:827–835. doi: 10.1007/s10544-009-9299-3. [DOI] [PubMed] [Google Scholar]
- Haessler U., Pisano M., Wu M., Swartz M.A. Dendritic cell chemotaxis in 3D under defined chemokine gradients reveals differential response to ligands CCL21 and CCL19. Proc. Natl. Acad. Sci. U S A. 2011;108:5614–5619. doi: 10.1073/pnas.1014920108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrell M.I., Iritani B.M., Ruddell A. Tumor-induced sentinel lymph node lymphangiogenesis and increased lymph flow precede melanoma metastasis. Am. J. Pathol. 2007;170:774–786. doi: 10.2353/ajpath.2007.060761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heath W.R., Belz G.T., Behrens G.M.N., Smith C.M., Forehan S.P., Parish I.A., Davey G.M., Wilson N.S., Carbone F.R., Villadangos J.A. Cross-presentation, dendritic cell subsets, and the generation of immunity to cellular antigens. Immunol. Rev. 2004;199:9–26. doi: 10.1111/j.0105-2896.2004.00142.x. [DOI] [PubMed] [Google Scholar]
- Hirosue S., Vokali E., Raghavan V.R., Rincon-Restrepo M., Lund A.W., Corthesy-Henrioud P., Capotosti F., Halin Winter C., Hugues S., Swartz M.A. Steady-state antigen scavenging, cross-presentation, and CD8+ T cell priming: a new role for lymphatic endothelial cells. J. Immunol. 2014;192:5002–5011. doi: 10.4049/jimmunol.1302492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hor J.L., Whitney P.G., Zaid A., Brooks A.G., Heath W.R., Mueller S.N. Spatiotemporally distinct interactions with dendritic cell subsets facilitates CD4+ and CD8+ T cell activation to localized viral infection. Immunity. 2015;43:554–565. doi: 10.1016/j.immuni.2015.07.020. [DOI] [PubMed] [Google Scholar]
- Hsu A.C., Scott P. Leishmania mexicana infection induces impaired lymph node expansion and Th1 cell differentiation despite normal T cell proliferation. J. Immunol. 2007;179:8200–8207. doi: 10.4049/jimmunol.179.12.8200. [DOI] [PubMed] [Google Scholar]
- Huang L., Deng J., Xu W., Wang H., Shi L., Wu F., Wu D., Nei W., Zhao M., Mao P., Zhou X. CD8+ T cells with high TGFbeta1 expression cause lymph node fibrosis following HIV infection. Mol. Med. Rep. 2018;18:77–86. doi: 10.3892/mmr.2018.8964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huxley V.H., Scallan J.P. Lymphatic fluid: exchange mechanisms and regulation. J. Physiol. 2011;589:2935–2943. doi: 10.1113/jphysiol.2011.208298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Issa A., Le T.X., Shoushtari A.N., Shields J.D., Swartz M.A. Vascular endothelial growth factor-C and C-C chemokine receptor 7 in tumor cell-lymphatic cross-talk promote invasive phenotype. Cancer Res. 2009;69:349–357. doi: 10.1158/0008-5472.CAN-08-1875. [DOI] [PubMed] [Google Scholar]
- Ito K., Morimoto J., Kihara A., Matsui Y., Kurotaki D., Kanayama M., Simmons S., Ishii M., Sheppard D., Takaoka A., Uede T. Integrin a9 on lymphatic endothelial cells regulates lymphocyte egress. Proc. Natl. Acad. Sci. U S A. 2014;111:3080–3085. doi: 10.1073/pnas.1311022111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson D.G. Lymphatic regulation of cellular trafficking. J. Clin. Cell Immunol. 2014;5:258. doi: 10.4172/2155-9899.1000258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jafarnejad M., Zawieja D.C., Brook B.S., Nibbs R.J.B., Moore J. A novel computational model predicts key regulators of chemokine gradient formation in lymph nodes and site-specific roles for CCL19 and ACKR4. J. Immunol. 2017;199:2291–2304. doi: 10.4049/jimmunol.1700377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones D., Meijer E.F.J., Blatter C., Liao S., Pereira E.R., Bouta E.M., Jung K., Chin S.M., Huang P., Munn L.L. Methicillin-resistant Staphylococcus aureus causes sustained collecting lymphatic vessel dysfunction. Sci. Transl Med. 2018;10:eaam7964. doi: 10.1126/scitranslmed.aam7964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Junt T., Moseman E.A., Iannacone M., Massberg S., Lang P.A., Boes M., Fink K., Henrickson S.E., Shayakhmetov D.M., Di Paolo N.C. Subcapsular sinus macrophages in lymph nodes clear lymph-borne viruses and present them to antiviral B cells. Nature. 2007;450:110–114. doi: 10.1038/nature06287. [DOI] [PubMed] [Google Scholar]
- Kahari L., Fair-Makela R., Auvinen K., Rantakari P., Jalkanen S., Ivaska J., Salmi M. Transcytosis route mediates rapid delivery of intact antibodies to draining lymph nodes. J. Clin. Invest. 2019;129:3086–3102. doi: 10.1172/JCI125740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kajiya K., Kunstfeld R., Detmar M., Chung J.H. Reduction of lymphatic vessels in photodamaged human skin. J. Dermatol. Sci. 2007;47:241–243. doi: 10.1016/j.jdermsci.2007.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karnezis T., Shayan R., Caesar C., Roufail S., Harris N.C., Ardipradja K., Zhang Y.F., Williams S.P., Farnsworth R.H., Chai M.G. VEGF-D promotes tumor metastasis by regulating prostaglandins produced by the collecting lymphatic endothelium. Cancer Cell. 2012;21:181–195. doi: 10.1016/j.ccr.2011.12.026. [DOI] [PubMed] [Google Scholar]
- Kastenmuller W., Torabi-Parizi P., Subramanian N., Lammermann T., Germain R.N. A spatially-organized multicellular innate immune response in lymph nodes limits systemic pathogen spread. Cell. 2012;150:1235–1248. doi: 10.1016/j.cell.2012.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kissenpfennig A., Henri S., Dubois B., Laplace-Builhe C., Perrin P., Romani N., Tripp C.H., Douillard P., Leserman L., Kaiserlian D. Dynamics and function of Langerhans cells in vivo: dermal dendritic cells colonize lymph node areas distinct from slower migrating Langerhans cells. Immunity. 2005;22:643–654. doi: 10.1016/j.immuni.2005.04.004. [DOI] [PubMed] [Google Scholar]
- Kityo C., Makamdop K.N., Rothenberger M., Chipman J.G., Hoskuldsson T., Beilman G.J., Grzywacz B., Mugyenyi P., Ssali F., Akondy R.S. Lymphoid tissue fibrosis is associated with impaired vaccine responses. J. Clin. Invest. 2018;128:2763–2773. doi: 10.1172/JCI97377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornuta J.A., Dixon J.B. Ex vivo lymphatic perfusion system for independently controlling pressure gradient and transmural pressure in isolated vessels. Ann. Biomed. Eng. 2014;42:1691–1704. doi: 10.1007/s10439-014-1024-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornuta J.A., Nepiyushchikh Z., Gasheva O.Y., Mukherjee A., Zaweija D.C., Dixon J.B. Effects of dynamic shear and transmural pressure on wall shear stress sensitivity in collecting lymphatic vessels. Am. J. Physiol. Heart Circ. Physiol. 2015;309:R1122–R1134. doi: 10.1152/ajpregu.00342.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuan E.L., Ivanov S., Bridenbaugh E.A., Victora G., Wang W., Childs E.W., Platt A.M., Jakubzick C.V., Mason R.J., Gashev A.A. Collecting lymphatic vessel permeability facilitates adipose tissue inflammation and distribution of antigen to lymph node-homing adipose tissue dendritic cells. J. Immunol. 2015;194:5200–5210. doi: 10.4049/jimmunol.1500221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar V., Chyou S., Stein J.V., Lu T.T. Optical projection tomography reveals dynamics of HEV growth after immunization with protein plus CFA and features shared with HEVs in acute autoinflammatory lymphadenopathy. Front. Immunol. 2012;3:282. doi: 10.3389/fimmu.2012.00282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar V., Scandella E., Danuser R., Onder L., Nitschke M., Fukui Y., Halin C., Ludewig B., Stein J.V. Global lymphoid tissue remodeling during a viral infection is orchestrated by a B cell-lymphotoxin-dependent pathway. Blood. 2010;115:4725–4733. doi: 10.1182/blood-2009-10-250118. [DOI] [PubMed] [Google Scholar]
- Lambert L.H., Goebrecht G.K.E., De Leo S.E., O'connor R.S., Nunez-Cruz S., Li T.-D., Yuan J., Milone M.C., Kam L.C. Improving T cell expansion with a soft touch. Nano Lett. 2017;17:821–826. doi: 10.1021/acs.nanolett.6b04071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane R.S., Femel J., Breazeale A.P., Loo C.P., Thibault G., Kaempf A., Mori M., Tsujikawa T., Chang Y.H., Lund A.W. IFNgamma-activated dermal lymphatic vessels inhibit cytotoxic T cells in melanoma and inflamed skin. J. Exp. Med. 2018;215:3057–3074. doi: 10.1084/jem.20180654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leu A.J., Berk D.A., Lymboussaki A., Alitalo K., Jain R.K. Absence of functional lymphatics within a murine sarcoma: a molecular and functional evaluation. Cancer Res. 2000;60:4324–4327. [PubMed] [Google Scholar]
- Li R., Serrano J.C., Xing H., Lee T.A., Azizgolshani H., Zaman M., Kamm R.D. Interstitial flow promotes macrophage polarization toward an M2 phenotype. Mol. Biol. Cell. 2018;29:1927–1940. doi: 10.1091/mbc.E18-03-0164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao S., Cheng G., Conner D.A., Huang Y., Kucherlapati R.S., Munn L.L., Ruddle N.H., Jain R.K., Fukumura D., Padera T.P. Impaired lymphatic contraction associated with immunosuppression. Proc. Natl. Acad. Sci. U S A. 2011;108:18784–18789. doi: 10.1073/pnas.1116152108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loo C.P., Nelson N.A., Lane R.S., Booth J.L., Loprinzi Hardin S.C., Thomas A., Slifka M.K., Nolz J.C., Lund A.W. Lymphatic vessels balance viral dissemination and immune activation following cutaneous viral infection. Cell Rep. 2017;20:3176–3187. doi: 10.1016/j.celrep.2017.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louveau A., Smirnov I., Keyes T.J., Eccles J.D., Rouhani S.J., Peske J.D., Derecki N.C., Castle D., Mandell J.W., Lee K.S. Structural and functional features of central nervous system lymphatic vessels. Nature. 2015;523:337–341. doi: 10.1038/nature14432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucas E.D., Tamburini B.A.J. Lymph node lymphatic endothelial cell expansion and contraction and the programming of the immune response. Front. Immunol. 2019;10:36. doi: 10.3389/fimmu.2019.00036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lund A.W. Rethinking lymphatic vessels and antitumor immunity. Trends Cancer. 2016;2:548–551. doi: 10.1016/j.trecan.2016.09.005. [DOI] [PubMed] [Google Scholar]
- Lund A.W., Duraes F.V., Hirosue S., Raghavan V.R., Nembrini C., Thomas S.N., Issa A., Hugues S., Swartz M.A. VEGF-C promotes immune tolerance in B16 melanomas and cross-presentation of tumor antigen by lymph node lymphatics. Cell Rep. 2012;1:191–199. doi: 10.1016/j.celrep.2012.01.005. [DOI] [PubMed] [Google Scholar]
- Lund A.W., Wagner M., Fankhauser M., Steinskog E.S., Broggi M.A., Spranger S., Gajewski T.F., Alitalo K., Eikesdal H.P., Wiig H., Swartz M.A. Lymphatic vessels regulate immune microenvironments in human and murine melanoma. J. Clin. Invest. 2016;126:3389–3402. doi: 10.1172/JCI79434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Q., Decker Y., Muller A., Ineichen B.V., Proulx S.T. Clearance of cerebrospinal fluid from the sacral spine through lymphatic vessels. J. Exp. Med. 2019;216:2492–2502. doi: 10.1084/jem.20190351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Q., Ineichen B.V., Detmar M., Proulx S.T. Outflow of cerebrospinal fluid is predominantly through lymphatic vessels and is reduced in aged mice. Nat. Commun. 2017;8:1434. doi: 10.1038/s41467-017-01484-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maarouf O.H., Uehara M., Kasinath V., Solhjou Z., Banouni N., Bahmani B., Jiang L., Yilmam O.A., Guleria I., Lovitch S.B. Repetitive ischemic injuries to the kidneys result in lymph node fibrosis and impaired healing. JCI Insight. 2018;3:120546. doi: 10.1172/jci.insight.120546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malhotra D., Fletcher A.L., Astarita J.L., Lukacs-Kornek V., Tayalia P., Gonzalez S.F., Elpek K.G., Chang S.K., Knoblich K., Hemler M.E., Consortium, I. G. P Transcriptional profiling of stroma from inflamed and resting lymph nodes defines immunological hallmarks. Nat. Immunol. 2012;13:499–510. doi: 10.1038/ni.2262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mebius R.E., Streeter P.R., Breve J., Duijvestijn A.M., Kraal G. The influence of afferent lymphatic vessel interruption on vascular addressin expression. J. Cell Biol. 1991;115:85–95. doi: 10.1083/jcb.115.1.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meijer E.F.J.M., Blatter C., Chen I.X., Bouta E., Jones D., Pereira E.R., Jung K., Vakoc B.J., Baish J.W., Padera T.P. Lymph node effective vascular permeability and chemotherapy uptake. Microcirculation. 2017;24 doi: 10.1111/micc.12381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meinecke A.-K., Nagy N., Lago G.D.A., Kirmse S., Klose R., Schrodter K., Zimmermann A., Helfrich I., Rundqvist H., Theegarten D. Aberrant mural cell recruitment to lymphatic vessels and impaired lymphatic drainage in a murine model of pulmonary fibrosis. Blood. 2012;119:5931–5942. doi: 10.1182/blood-2011-12-396895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendoza A., Fang V., Chen C., Serasinghe M., Verma A., Muller J., Chaluvadi V.S., Dustin M.L., Hla T., Elemento O. Lymphatic endothelial S1P promotes mitochondrial function and survival in naive T cells. Nature. 2017;546:158–161. doi: 10.1038/nature22352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mihara M., Hara H., Araki J., Kikuchi K., Narushima M., Yamamoto T., Iida T., Yoshimatsu H., Murai N., Mitsui K. Indocyanine green (ICG) lymphangiography is superior to lymphoscintigraphy for diagnostic imaging of early lymphedema of the upper limbs. PLoS One. 2012;7:e38182. doi: 10.1371/journal.pone.0038182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirsky H.P., Miller M.J., Linderman J.J., Kirschner D.E. Systems biology approaches for understanding cellular mechanisms of immunity in lymph nodes during infection. J. Theor. Biol. 2011;287:160–170. doi: 10.1016/j.jtbi.2011.06.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miteva D.O., Rutkowski J.M., Dixon J.B., Kilarski W., Shields J., Swartz M.A. Transmural flow modulates cell and fluid transport functions of lymphatic endothelium. Circ. Res. 2010;106:920–931. doi: 10.1161/CIRCRESAHA.109.207274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mondor I., Jorquera A., Sene C., Adriouch S., Adams R.H., Zhou B., Wienert S., Klauschen F., Bajenoff M. Clonal proliferation and stochastic pruning orchestrate lymph node vasculature remodeling. Immunity. 2016;45:877–888. doi: 10.1016/j.immuni.2016.09.017. [DOI] [PubMed] [Google Scholar]
- Morton A.M., Sefik E., Upadhyay R., Weissleder R., Benoist C., Mathis D. Endoscopic photoconversion reveals unexpectedly broad leukocyte trafficking to and from the gut. Proc. Natl. Acad. Sci. U S A. 2014;111:6696–6701. doi: 10.1073/pnas.1405634111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moura Rosa P., Gopalakrishnan N., Ibrahim H., Haug M., Halaas O. The intercell dynamics of T cells and dendritic cells in a lymph node-on-a-chip flow device. Lab Chip. 2016;16:3728–3740. doi: 10.1039/c6lc00702c. [DOI] [PubMed] [Google Scholar]
- Munoz-Fernandez R., Prados A., Tirado-Gonzalez I., Martin F., Abadia A.C., Olivares E.G. Contractile activity of human follicular dendritic cells. Immunol. Cell Biol. 2014;92:851–859. doi: 10.1038/icb.2014.61. [DOI] [PubMed] [Google Scholar]
- Nagai T., Bridenbaugh E.A., Gashev A.A. Aging-associated alterations in contractility of rat mesenteric lymphatic vessels. Microcirculation. 2011;18:463–473. doi: 10.1111/j.1549-8719.2011.00107.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nataraj N.M., Dang A.P., Kam L.C., Lee J.H. Ex vivo induction of regulatory T cells from conventional CD4(+) T cells is sensitive to substrate rigidity. J. Biomed. Mater. Res. A. 2018;106:3001–3008. doi: 10.1002/jbm.a.36489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neal J.T., Li X., Zhu J., Giangarra V., Grzeskowiak C.L., Ju J., Liu I.H., Chiou S.-H., Salahudeen A.A., Smith A.R. Organoid modeling of the tumor immune microenvironment. Cell. 2018;175:1972–1988. doi: 10.1016/j.cell.2018.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson T.S., Akin R.E., Weiler M.J., Kassis T., Kornuta J.A., Dixon J.B. Minimally invasive method for determining the effective lymphatic pumping pressure in rats using near-infrared imaging. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2014;306:R281–R290. doi: 10.1152/ajpregu.00369.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novkovic M., Onder L., Cheng H.-W., Bocharov G., Ludewig B. Integrative computational modeling of the lymph node stromal cell landscape. Front. Immunol. 2018;9:2428. doi: 10.3389/fimmu.2018.02428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'connor R.S., Hao X., Shen K., Bashour K., Akimova T., Hancock W.W., Kam L.C., Milone M.C. Substrate rigidity regulates human T cell activation and proliferation. J. Immunol. 2012;189:1330–1339. doi: 10.4049/jimmunol.1102757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa F., Amano H., Eshima K., Ito Y., Matsui Y., Hosono K., Kitasato H., Iyoda A., Iwabuchi K., Kumagai Y. Prostanoid induces premetastatic niche in regional lymph nodes. J. Clin. Invest. 2014;124:4882–4894. doi: 10.1172/JCI73530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olmeda D., Cerezo-Wallis D., Riveiro-Falkenbach E., Pennacchi P.C., Contreras-Alcalde M., Ibarz N., Cifdaloz M., Catena X., Calvo T.G., Canon E. Whole-body imaging of lymphovascular niches identifies pre-metastatic roles of midkine. Nature. 2017;546:676–680. doi: 10.1038/nature22977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ono N., Mizuno R., Ohhashi T. Effective permeability of hydrophilic substances through walls of lymph vessels: roles of endothelial barrier. Am. J. Physiol. Heart Circ. Physiol. 2005;289:H1676–H1682. doi: 10.1152/ajpheart.01084.2004. [DOI] [PubMed] [Google Scholar]
- Pham T.H.M., Baluk P., Xu Y., Grigorova I.L., Bankovich A.J., Pappu R., Coughlin S.R., Mcdonald D.M., Schwab S.R., Cyster J.G. Lymphatic endothelial cell sphingosine kinase activity is required for lymphocyte egress and lymphatic patterning. J. Exp. Med. 2010;207:17–27. doi: 10.1084/jem.20091619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phan T.G., Grigorova I.L., Okada T., Cyster J.G. Subcapsular encounter and complement-dependent transport of immune complexes by lymph node B cells. Nat. Immunol. 2007;8:992–1000. doi: 10.1038/ni1494. [DOI] [PubMed] [Google Scholar]
- Pisano M., Triacca V., Barbee K.A., Swartz M.A. An in vitro model of the tumor-lymphatic microenvironment with simultaneous transendothelial and luminal flows reveals mechanisms of flow enhanced invasion. Integr. Biol. 2015;7:525–533. doi: 10.1039/c5ib00085h. [DOI] [PubMed] [Google Scholar]
- Platt A.M., Rutkowski J.M., Martel C., Kuan E.K., Ivanov S., Swartz M.A., Randolph G.J. Normal dendritic cell mobilization to lymph nodes under conditions of severe lymphatic hypoplasia. J. Immunol. 2013;190:4608–4620. doi: 10.4049/jimmunol.1202600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purwada A., Jaiswal M.K., Ahn H., Nojima T., Kitamura D., Gaharwar A.K., Cerchietti L., Singh A. Ex vivo engineered immune organoids for controlled germinal center reactions. Biomaterials. 2015;63:24–34. doi: 10.1016/j.biomaterials.2015.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purwada A., Singh A. Immuno-engineered organoids for regulating the kinetics of B-cell development and antibody production. Nat. Protoc. 2017;12:168–182. doi: 10.1038/nprot.2016.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranft J., Prost J., Julicher F., Joanny J.F. Tissue dynamics with permeation. Eur. Phys. J. E Soft Matter. 2012;35:46. doi: 10.1140/epje/i2012-12046-5. [DOI] [PubMed] [Google Scholar]
- Rantakari P., Auvinen K., Jappinen N., Kapraali M., Valtonen J., Karikoski M., Gerke H., Iftakhar-E-Khuda I., Keuschnigg J., Umemoto E. The endothelial protein PLVAP in lymphatics controls the entry of lymphocytes and antigens into lymph nodes. Nat. Immunol. 2015;16:386–396. doi: 10.1038/ni.3101. [DOI] [PubMed] [Google Scholar]
- Reddy S.T., Van Der Vlies A.J., Simeoni E., Angeli V., Randolph G.J., O'neil C.P., Lee L.K., Swartz M.A., Hubbell J.A. Exploiting lymphatic transport and complement activation in nanoparticle vaccines. Nat. Biotechnol. 2007;25:1159–1164. doi: 10.1038/nbt1332. [DOI] [PubMed] [Google Scholar]
- Reynoso G.V., Weisberg A.S., Shannon J.P., Mcmanus D.T., Shores L., Americo J.L., Stan R.V., Yewdell J.W., Hickman H.D. Lymph node conduits transport virions for rapid T cell activation. Nat. Immunol. 2019;20:602–612. doi: 10.1038/s41590-019-0342-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riedel A., Shorthouse D., Haas L., Hall B.A., Shields J. Tumor-induced stromal reprogramming drives lymph node transformation. Nat. Immunol. 2016;17:1118–1127. doi: 10.1038/ni.3492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohner N.A., Mcclain J., Tuell S.L., Warner A., Smith B., Yun Y., Mohan A., Sushnitha M., Thomas S.N. Lymph node biophysical remodeling is associated with melanoma lymphatic drainage. FASEB J. 2015;29:4512–4522. doi: 10.1096/fj.15-274761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohner N.A., Thomas S.N. Melanoma growth effects on molecular clearance from tumors and biodistribution into systemic tissues versus draining lymph nodes. J. Control Release. 2016;223:99–108. doi: 10.1016/j.jconrel.2015.12.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohner N.A., Thomas S.N. Flexible macromolecule versus rigid particle retention in the injected skin and accumulation in draining lymph nodes are differentially influenced by hydrodynamic size. ACS Biomater. Sci. Eng. 2017;3:153–159. doi: 10.1021/acsbiomaterials.6b00438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roozendaal R., Mempel T.R., Pitcher L.A., Gonzalez S.F., Verschoor A., Mebius R.E., Von Andrian U.H., Carroll M.C. Conduits mediate transport of low-molecular-weight Antigen to lymph node follicles. Immunity. 2009;30:264–276. doi: 10.1016/j.immuni.2008.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross A.E., Belanger M.C., Woodroof J.F., Pompano R.R. Spatially resolved microfluidic stimulation of lymphoid tissue ex vivo. Analyst. 2017;142:649–659. doi: 10.1039/c6an02042a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross A.E., Pompano R.R. Diffusion of cytokines in live lymph node tissue using microfluidic integrated optical imaging. Anal. Chim. Acta. 2018;1000:205–213. doi: 10.1016/j.aca.2017.11.048. [DOI] [PubMed] [Google Scholar]
- Russo E., Teijeira A., Vaahtomeri K., Willrodt A.-H., Bloch J.S., Nitschke M., Santambrogio L., Kerjaschki D., Sixt M., Halin C. Intralymphatic CCL21 promotes tissue egress of dendritic cells through afferent lymphatic vessels. Cell Rep. 2016;14:1723–1734. doi: 10.1016/j.celrep.2016.01.048. [DOI] [PubMed] [Google Scholar]
- Sabine A., Agalarov Y., Maby-El Hajjami H., Jaquet M., Hagerling R., Pollmann C., Bebber D., Pfenniger A., Miura N., Dormond O. Mechanotransduction, PROX1, and FOXC2 cooperate to control Connexin37 and calcineurin during lymphatic-valve formation. Dev. Cell. 2012;22:430–445. doi: 10.1016/j.devcel.2011.12.020. [DOI] [PubMed] [Google Scholar]
- Sabine A., Bovay E., Demir C.S., Kimura W., Jaquet M., Agalarov Y., Zangger N., Scallan J.P., Graber W., Gulpinar E. FOXC2 and fluid shear stress stabilize postnatal lymphatic vasculature. J. Clin. Invest. 2015;125:3861–3877. doi: 10.1172/JCI80454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez J.L., Hunt P.W., Reilly C.S., Hatano H., Beilman G.J., Khoruts A., Jasurda J.S., Somsouk M., Thorkelson A., Russ S. Lymphoid fibrosis occurs in long-term nonprogressors and persists with antiretroviral therapy but may be reversible with curative interventions. J. Infect. Dis. 2015;211:1068–1075. doi: 10.1093/infdis/jiu586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato M., Sasaki N., Ato M., Hirakawa S., Sato K., Sato K. Microcirculation-on-a-chip: a microfluidic platform for assaying blood- and lymphatic-vessel permeability. PLoS One. 2015;10:e0137301. doi: 10.1371/journal.pone.0137301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scallan J.P., Davis M.J., Huxley V.H. Permeability and contractile responses of collecting lymphatic vessels elicited by atrial and brain natriuretic peptides. J. Physiol. 2013;591:5071–5081. doi: 10.1113/jphysiol.2013.260042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scallan J.P., Huxley V.H. In vivo determination of collecting lymphatic vessel permeability to albumin: a role for lymphatics in exchange. J. Physiol. 2010;588:243–254. doi: 10.1113/jphysiol.2009.179622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schineis P., Runge P., Halin C. Cellular traffic through afferent lymphatic vessels. Vasc. Pharmacol. 2019;112:31–41. doi: 10.1016/j.vph.2018.08.001. [DOI] [PubMed] [Google Scholar]
- Schumann K., Lammermann T., Bruckner M., Legler D.F., Polleux J., Spatz J.P., Schuler G., Forster R., Lutz M.B., Sorokin L., Sixt M. Immobilized chemokine fields and soluble chemokine gradients cooperatively shape migration patterns of dendritic cells. Immunity. 2010;32:703–713. doi: 10.1016/j.immuni.2010.04.017. [DOI] [PubMed] [Google Scholar]
- Schwager S., Detmar M. Inflammation and lymphatic function. Front. Immunol. 2019;10:308. doi: 10.3389/fimmu.2019.00308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seki Y., Kajikawa A., Yamamoto T., Takeuchi T., Terashima T., Kurogi N. Real-time indocyanine green videolymphography navigation for lymphaticovenular anastomosis. Plast. Reconstr. Surg. Glob. Open. 2019;7:e2253. doi: 10.1097/GOX.0000000000002253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaheen S., Wan Z., Li Z., Chau A., Li X., Czhang S., Liu Y., Yi J., Zeng Y., Wang J. Substrate stiffness governs the initiation of B cell activation by the concerted signaling of PKCbeta and focal adhesion kinase. Elife. 2017;6:e23060. doi: 10.7554/eLife.23060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shanti A., Teo J., Stefanini C. In Vitro immune organs-on-chip for drug development: a review. Pharmaceutics. 2018;10:278. doi: 10.3390/pharmaceutics10040278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shields J.D., Fleury M.E., Yong C., Tomei A.A., Randolph G.J., Swartz M.A. Autologous chemotaxis as a mechanism of tumor cell homing to lymphatics via interstitial flow and autocrine CCR7 signaling. Cancer Cell. 2007;11:526–538. doi: 10.1016/j.ccr.2007.04.020. [DOI] [PubMed] [Google Scholar]
- Sixt M., Kanazawa N., Selg M., Samson T., Roos G., Reinhardt D.P., Pabst R., Lutz M.B., Sorokin L. The conduit system transports soluble antigens from the afferent lymph to resident dendritic cells in the T cell area of the lymph node. Immunity. 2005;22:19–29. doi: 10.1016/j.immuni.2004.11.013. [DOI] [PubMed] [Google Scholar]
- Srinivasan S., Vannberg F., Dixon J.B. Lymphatic transport of exosomes as a rapid route of information dissemination to the lymph node. Sci. Rep. 2016;6:24436. doi: 10.1038/srep24436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stacker S.A., Williams S.P., Karnezis T., Shayan R., Fox S.B., Achen M.G. Lymphangiogenesis and lymphatic vessel remodelling in cancer. Nat. Rev. Cancer. 2014;14:159–172. doi: 10.1038/nrc3677. [DOI] [PubMed] [Google Scholar]
- Steele M.M., Churchill M.J., Breazeale A.P., Lane R.S., Nelson N.A., Lund A.W. Quantifying leukocyte egress via lymphatic vessels from murine skin and tumors. J. Vis. Exp. 2019;143:e57804. doi: 10.3791/58704. [DOI] [PubMed] [Google Scholar]
- Stylianopoulos T., Martin J.D., Snuderl M., Mpekris F., Jain S.R., Jain R.K. Coevolution of solid stress and interstitial fluid pressure in tumors during progression: implications for vascular collapse. Cancer Res. 2013;73:3833–3841. doi: 10.1158/0008-5472.CAN-12-4521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweet D.T., Jimenez J.M., Chang J., Hess P.R., Mericko-Ishizuka P., Fu J., Xia L., Davies P.F., Kahn M.L. Lymph flow regulates collecting lymphatic vessel maturation in vivo. J. Clin. Invest. 2015;125:2995–3007. doi: 10.1172/JCI79386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teijeira A., Hunter M.C., Russo E., Proulx S.T., Frei T., Debes G.F., Coles M., Melero I., Detmar M., Rouzaut A., Halin C. T cell migration from inflamed skin to draining lymph nodes requires intralymphatic crawling supported by ICAM-1/LFA-1 interactions. Cell Rep. 2017;18:857–865. doi: 10.1016/j.celrep.2016.12.078. [DOI] [PubMed] [Google Scholar]
- Thomas S.N., Rohner N.A., Edwards E.E. Implications of lymphatic transport to lymph nodes in immunity and immunotherapy. Annu. Rev. Biomed. Eng. 2016;18:207–233. doi: 10.1146/annurev-bioeng-101515-014413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas S.N., Rutkowski J.M., Pasquier M., Kuan E.L., Alitalo K., Randolph G.J., Swartz M.A. Impaired humoral immunity and tolerance in K14-VEGFR-3-ig mice that lack dermal lymphatic drainage. J. Immunol. 2012;189:2181–2190. doi: 10.4049/jimmunol.1103545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Threefoot S.A., Kent W.T., Hatchett B.F. Lymphaticovenous and lymphaticolymphatic communications demonstrated by plastic corrosion models of rats and by postmortem lymphangiography in man. J. Lab Clin. Med. 1963;61:9–22. [PubMed] [Google Scholar]
- Tomei A.A., Siegert S., Britschgi M.R., Luther S.A., Swartz M.A. Fluid flow regulates stromal cell organization and CCL21 expression in a tissue-engineered lymph node microenvironment. J. Immunol. 2009;183:4273–4283. doi: 10.4049/jimmunol.0900835. [DOI] [PubMed] [Google Scholar]
- Tomura M., Hata A., Matsuoka S., Shand F.H., Nakanishi Y., Ikebuchi R., Ueha S., Tsutsui H., Inaba K., Matsushima K. Tracking and quantification of dendritic cell migration and antigen trafficking between the skin and lymph nodes. Sci. Rep. 2014;4:6030. doi: 10.1038/srep06030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomura M., Yoshida N., Tanaka J., Karasawa S., Miwa Y., Miyawaki A., Kanagawa O. Monitoring cellular movement in vivo with photoconvertible fluorescence protein “Kaede” transgenic mice. Proc. Natl. Acad. Sci. U S A. 2008;105:10871–10876. doi: 10.1073/pnas.0802278105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong Z., Cheung L.S.-L., Stebe K.J., Konstantopoulos K. Selectin-mediated adhesion in shear flow using micropatterned substrates: multiple-bond interactions govern the critical length for cell binding. Integr. Biol. 2012;4:847–856. doi: 10.1039/c2ib20036h. [DOI] [PubMed] [Google Scholar]
- Torcellan T., Hampton H.R., Bailey J., Tomura M., Brink R., Chtanova T. In vivo photolabeling of tumor-infiltrating cells reveals highly regulated egress of T-cell subsets from tumors. Proc. Natl. Acad. Sci. U S A. 2017;114:5677–5682. doi: 10.1073/pnas.1618446114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Triacca V., Guc E., Kilarski W.W., Pisano M., Swartz M.A. Transcellular pathways in lymphatic endothelial cells regulate changes in solute transport by fluid stress. Circ. Res. 2017;120:1440–1452. doi: 10.1161/CIRCRESAHA.116.309828. [DOI] [PubMed] [Google Scholar]
- Ulvmar M.H., Werth K., Braun A., Kelay P., Hub E., Eller K., Chan L., Lucas B., Novitzky-Basso I., Nakamura K. The atypical chemokine receptor CCRL1 shapes functional CCL21 gradients in lymph nodes. Nat. Immunol. 2014;15:623–630. doi: 10.1038/ni.2889. [DOI] [PubMed] [Google Scholar]
- Unno N., Inuzuka K., Suzuki M., Yamamoto N., Sagara D., Nishiyama M., Konno H. Preliminary experience with a novel fluorescence lymphography using indocyanine green in patients with secondary lymphedema. J. Vasc. Surg. 2007;45:1016–1021. doi: 10.1016/j.jvs.2007.01.023. [DOI] [PubMed] [Google Scholar]
- Wang T., Luu T.U., Chen A., Khine M., Liu W.F. Topographical modulation of macrophage phenotype by shrink-film multi-scale wrinkles. Biomater. Sci. 2016;4:948–952. doi: 10.1039/c6bm00224b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., Jin Y., Mae M.A., Zhang Y., Ortsater H., Betsholtz C., Makinen T., Jakobsson L. Smooth muscle cell recruitment to lymphatic vessels requires PDGFB and impacts vessel size but not identity. Development. 2017;144:3590–3601. doi: 10.1242/dev.147967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z., Samanipour R., Koo K.-I., Kim K. Organ-on-a-Chip platforms for drug delivery and cell characterization: a review. Sensor Mat. 2015;27:487–506. [Google Scholar]
- Webster B., Ekland E.H., Agle L.M., Chyou S., Ruggieri R., Lu T.T. Regulation of lymph node vascular growth by dendritic cells. J. Exp. Med. 2006;203:1903–1913. doi: 10.1084/jem.20052272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiig H., Swartz M.A. Interstitial fluid and lymph formation and transport: physiological regulation and roles in inflammation and cancer. Physiol. Rev. 2012;92:1005–1060. doi: 10.1152/physrev.00037.2011. [DOI] [PubMed] [Google Scholar]
- Yoshida R.Y., Kariya S., Ha-Kawa S., Tanigawa N. Lymphoscintigraphy for imaging of the lymphatic flow disorders. Tech. Vasc. Interv. Radiol. 2016;19:273–276. doi: 10.1053/j.tvir.2016.10.009. [DOI] [PubMed] [Google Scholar]
- Zawieja D.C. Contractile physiology of lymphatics. Lymphatic Res. Biol. 2009;7:87–96. doi: 10.1089/lrb.2009.0007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng M., Paiardini M., Engram J.C., Beilman G.J., Chipman J.G., Schacker T.W., Silvestri G., Haase A.T. Critical role of CD4 T cells in maintaining lymphoid tissue structure for immune cell homeostasis and reconstitution. Blood. 2012;120:1856–1867. doi: 10.1182/blood-2012-03-418624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang F., Zarkada G., Han J., Li J., Dubrac A., Ola R., Genet G., Boye K., Michon P., Kunzel S.E. Lacteal junction zippering protects against diet-induced obesity. Science. 2018;361:599–603. doi: 10.1126/science.aap9331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zolla V., Nizamutdinova I.T., Scharf B., Clement C.C., Maejima D., Akl T., Nagai T., Luciani P., Leroux J.C., Halin C. Aging-related anatomical and biochemical changes in lymphatic collectors impair lymph transport, fluid homeostasis, and pathogen clearance. Aging Cell. 2015;14:582–594. doi: 10.1111/acel.12330. [DOI] [PMC free article] [PubMed] [Google Scholar]