Highlights
-
•
In this study, two immunogenic antigens based on recombinant PspA proteins were immunized mice.
-
•
The protective effects of developed anti-PspA antibodies in mice in intranasal and intraperitoneal challenges were proved.
-
•
Based on the obtained results, immunization with the B-regions of PspA antigens are crucial in protection of challenged mice with S. pneumoniae strains.
Keywords: Streptococcus pneumoniae, Pneumococcal surface protein A (PspA), Immune protection
Abstract
Streptococcus pneumoniae is a major pathogen in human respiratory tract which causes significant morbidity and mortality across from the world. Currently available vaccines are not completely effective and cannot cover all pathogenic strains so there is an important need to develop an alternative cost-effective vaccine, based on conserved protein antigens. Pneumococcal surface protein A (PspA) is one of interesting candidates for development of a serotype-independent vaccine against pneumococcal infections. PspA is grouped into two major families with five clades, and broad-reacting PspA-based vaccines should contain at least one functional fragment from each of the two families.
In this study, we developed two immunogenic antigens based on recombinant PspA proteins that including the different antigenic regions of PspA from both two families. The cross-reactivity of antibodies elicited against two PspA proteins PspAB1-5 and PspA4ABC and their role in complement deposition with three strains of pneumococci were tested. The protective effects of developed anti-PspA antibodies in mice in intranasal and intraperitoneal challenges were evaluated using a strain from clade 2. Sera from immunized mice with PspAB1-5 in comparison with PspA4ABC was able to deposit more C3 complement component on surface of pneumococci bearing diverse PspA from both families 1 and 2, and immunized mice with the PspAB1-5 showed a higher protection than PspA4ABC in pneumococcal challenges.
The obtained results from this study indicate that a PspA-based antigen composed of B region from all clades in addition to conserved domains, can provide a significant protection against multiple strains of S. pneumoniae and may overcome the limitation of polysaccharide vaccines.
1. Introduction
Streptococcus pneumoniae is the major pathogen that causes acute bacterial infections such as meningitis, sepsis and pneumonia in human [1]. The capsular polysaccharides are considered the primary basis for the pathogenicity of this organism [2,3] and there are more than 90 serotypes of S. pneumoniae according to distinct polysaccharide capsules [4]. Current pneumococcal polysaccharide vaccines are composed of capsular polysaccharides from the most prevalent serotypes of pneumococcus [3]. The limited vaccine coverage, replacement by non-vaccine serotypes [3] and non-encapsulated S. pneumoniae (NESp) which have been isolated from patients with invasive and non-invasive pneumococcal disease [5,6] and increasing antibiotic resistance [7] are some serious threats in the near future; Therefore, the search for new candidates for a vaccine that elicit protection against a broader range of pneumococcal strains is necessary [[8], [9], [10]]. Pneumococcal surface protein A (PspA) is a very promising candidate for novel vaccine development against pneumococcal infections [11]. PspA have been found in all the clinical isolates [[12], [13], [14]]. This antigen prevents complement deposition on the surface of the bacterium [[15], [16], [17]]. Several studies have shown that active or passive immunization by recombinant PspAs can protect animal models from pneumococcal lethal challenge [[18], [19], [20]]. In addition, PspA has been administered to human adults in early clinical trials [21,22]. PspA is composed of five domains:1- a signal peptide, 2- an α-helical highly charged (N-terminal) domain, 3- a proline-rich region domain, 4- a choline-binding domain, and 5- a short hydrophobic tail (C-terminal) [23]. The N-terminal region of PspA is surface accessible and has an α-helical coiled-coil structure with protection-eliciting epitopes, and it has been divided into three regions, A, B and C [[24], [25], [26]]. Most 100 amino acids from the C-terminal of the α-helical region exhibit serological variability, known as the B window or clade-defining region (CDR). The sequence similarity in the B window of diverse pneumococcus strains was the basis for the classification of PspA into three families and six clades [27]. Clades 1 and 2 belong to family 1, clades 3 to 5 belong to family 2, and family 3 is comprised of clade 6. Families 1 and 2 are present in the at least 98% of clinical isolates [14,28,29]. Previous studies have shown that the similarity degree among the amino acid sequences within the B regions determines the level of cross-reactivity among different PspA fragments. So higher degree of cross-reactivity among PspA fragments within the same clade is expected [30]; Furthermore, different studies have shown that some antibodies against N-terminal domain of PspA were reactive against the B region [27] and the immunization of mice with B region induced high antibody levels against the whole N-terminal domain of a homologous PspA fragments [20]. Since some researches have demonstrated that the immunity elicited by family 1 or family 2 was clade dependent [25,27,31], it is suggested that high antigenic fragments of all clades, that have the greatest impact on cross-reactivity, should be included in a chimeric PspA-based vaccine. Also, the proline-rich region and the A region domain contain more conserved epitopes across the PspA with the effect on cross-reactivity [27,32]. In this context, Zhenyu Piao et al. generated three recombinant PspA proteins consist of N-terminal and proline rich region from two PspA families. They examined the reactivity and protective effect of antisera raised in mice immunized with these PspA fusion protein with five different PspA clades. They reported PspA3+2 vaccine has an advantage over the PspA2+4 or PspA2+5 vaccine candidate in terms of a broad range of cross-reactivity with clinical isolates and cross-protection [33].
Some studies have demonstrated that the antiserum to PspA clade 4 have the broadest cross-reactivity among all clades of PspAs [27,34].
In the present study, we evaluated the protection conferred against pneumococcal infections by immunization of mice with two PspA recombinant antigens. One of these molecules is consisted of the B regions from clades one to five and the other one is composed of complete ABC domain from clade 4 of strain EF5668. We also analyzed the ability of generated antibodies against these two recombinant fragments to bind and increase complement deposition on bacteria with PspAs from both families. The in vivo function of these immunogenic antigens was evaluated through measurement of nasopharyngeal colonization and protection of mice against intranasal and intraperitoneal challenge by strain ATCC-6305 (clade 2) of pneumococci.
2. Materials and methods
2.1. Construction and cloning of PspAB1-5 and PspA4ABC coding sequences
The constructs of fusion proteins were designed including B region fragments of PspA from clades 1 to 5 (PspAB1-5) and A, B, and C region fragments of PspA clade 4 (PspA4ABC). DNA fragments encoding portions of the N-terminal regions (A, B and C regions, ABC) of strain EF5668 (family 2, clade 4) [GenBank: U89711.1], and coding sequences of B regions from clades 1 to 5, strain St 435/96 (clade 1) [GenBank: AY082387.1], strain RX1 (clade 2) [GenBank: M74122], strain EF3296 (clade 3) [GenBank: AF071816.1], strain EF5668 (clade 4) [GenBank: U89711.1], and strain ATCC6303 (clade5) [GenBank: AF071820.1], were synthesized (Genecust, Luxembourg) in a single coding sequence (B1-5).
The PspA4ABC and PspAB1-5 genes were cloned into pET15b expression vector (Merck-Millipore, Germany), and the insertion was confirmed by colony PCR and enzymatic digestion methods.
2.2. PspA expression and purification
E. coli BL21 (DE3) competent cells (Invitrogen, USA) were transformed with pET15b vectors containing the recombinant PspA coding sequences. Expression induction of proteins were induced in the mid-log-phase cultures by 0.75 mmol/L IPTG (Applichem, Germany). The recombinant proteins with His-tag were purified from lysate of cultured cells by affinity chromatography using HisPur Ni-NTA Resin (Thermo Fisher Scientific, USA) as described before [35]. The purified recombinant proteins were evaluated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), western blotting using anti-His-tag monoclonal antibody (Qiagen, Germany) and ELISA using mouse anti-Pneumococcal polyclonal antibody against three pneumococci bacteria strains (ATCC 6305, 49619, and 700678) (Pasteur Institute of Iran, Iran). Filtration with Amicon Ultra-0.5 mL Centrifugal Filter (Merck Millipore, Germany) were performed according to the manufacturer’s instruction and concentrated proteins were stored at −20 °C.
2.3. Animal immunization and antibody development against the recombinant PspA fragments
Five 6–8 weeks old female BALB/c mice (Pasture Institute of Iran, Iran) were immunized intraperitoneally with 10 μg of PspAB1-5 or PspA4ABC in PBS solution plus 50 μl of Alum adjuvant (Pasture Institute of Iran, Iran) in final volume of 200 μl per mouse. The prime immunization followed by three boosts of 5 μg of the recombinant proteins along with Alum adjuvant at two-week intervals. The adjuvant (50 μl) with PBS solution in a total volume of 200 μl was used as negative control.
All mice were housed in same-sex groups of 5 in 11 × 7×5-inch cages. Mice were maintained by using standard husbandry practices, with cages changed weekly. Food and tap water were available. Blood samples were collected from mice 7 days after each immunization and before prime immunization (non-immunized serum) and the sera were stored at −20 °C for further evaluation. All animal handling and experimental procedures were in accordance with the regulations and ethical considerations of Laboratory Animal Care and Use Committees at Tehran University of Medical Sciences and Pasteur Institute of Iran.
2.4. Determination of anti-PspA antibodies by ELISA
The collected sera were assayed for specific raised antibody responses. Briefly, a 96-well plate (Nunc, Denmark) was coated with 50 μl recombinant PspA (PspA4ABC and PspAB1-5) at a concentration of 5 μg/ml in PBS and incubated overnight at 4 °C. The plate was washed with PBS containing 0.05% Tween 20 (PBST) and blocked with PBS containing 2% bovine serum albumin (BSA, Sigma, Germany) at 37 °C for 1.5 h. Following washing with PBST, the plate was incubated with 1:1000 sera from immunized mice at room temperature for 1 h. The plate was washed with PBST and incubated with 1:2000 goat anti-mouse HRP conjugate (Sigma, Germany) followed by a 1h-incubation at room temperature. The color was developed by adding 3,3,5,5 –tetra methyl benzidine substrate (TMB, Sigma, Germany). After color development (10 min), the reaction was stopped by 0.5 N H2SO4 and absorbance was measured at 450 nm. The serum of non-immunized mice was used as negative control. Each assay was repeated in three different runs (experimental triplicates).
2.5. Cross-reactivity of developed anti-PspA antibodies
ELISA was used for analyzing cross-reactivity of anti-PspA antibodies. A 96-well plate was coated with the whole cell of three strains of pneumococcus. Strains ATCC 6305 (clade 2), ATCC 49619 (clade 1) and 700678 (clade 5) were grown to late log phase in Todd-Hewitt broth (THY) medium supplemented with 0.5% yeast extract, harvested by centrifugation and killed by the addition of 70% ethanol. Then 100 μL of each suspension were used to coat each well, corresponding to ∼107 cells well−1. The plate was incubated overnight at 4 °C. On the next day, wells were washed one time with PBST containing 0.1% Tween 20 and blocked with 10% fat-free skimmed milk (Sigma, Germany) in PBST. Following three times wash with PBST, the plate was then incubated with 1:1000 dilutions of individual sera from mice immunized with PspA4ABC or PspAB1-5 (collected 1 week after the last immunization) in PBST for 1 h at 37 °C. The wells were washed and incubated with goat anti-mouse HRP conjugat (1:2000) in PBST for 1 h at 37 °C. Following three more washes, antibodies were detected by adding TMB substrate. After color development (10 min), the reaction was stopped and absorbance was measured at 450 nm. Differences between groups were analyzed by ANOVA test. A p-value <0.05 was considered statistically significant. The serum of non-immunized mice was used as negative control. Each assay was repeated in three different runs (experimental triplicates).
2.6. Complement deposition assay
Coating and blocking of pneumococci in 96 well plate was performed as described above. Then, the bacteria were washed three times with PBST and were incubated with anti-PspAs sera at a final dilution of 1:1000 in PBS for 1.5 h at 37 °C. Following washing three times, fresh serum from a BALB/c mouse was added as complement source. After a 1h-incubation, the plate was washed with PBST and incubated with 1:1000 dilution of anti-C3 antibody (Cedarlane, Canada) for 1 h at 37 °C. After three more washes, goat anti-mouse HRP-conjugate was added and the next steps were performed as similar as described above. Differences between groups were analyzed by ANOVA test. P-value <0.05 was considered statistically significant. Each assay was repeated in three different runs (experimental triplicates).
2.7. Colonization test and animal challenge
Fifteen days after the last immunization, immunized mice with PspAB1-5 and PspA4ABC recombinant proteins, were challenged intra-nasally with fresh cultured strain ATCC 6305 of pneumococci. Approximately 106 bacteria were inoculated in 20 μL normal saline into nostrils of mice previously anesthetized through i.p route with 200 μL of a 0.2% xilazine and 1.0% ketamine. The nasopharyngeal colonization with S. pneumoniae were examined 10 days after the challenge, the nasal cavity of each mouse was washed by 100 μL of sterile normal saline. Then the nasal wash was spread on the blood agar plates (containing 5% defibrinated sheep) and incubated overnight at 37 °C. The colonies on the blood agar plates were tested using optochin (Rosco; Denmark) susceptibility testing (Kellogg et al. 2001) to detect S. pneumoniae colonies. At ≥ 1 optochin positive CFU/50 μl of nasal wash was detected, the mouse was considered to be nasally colonized. To evaluate protection against intraperitoneal challenge by pneumococci, the mice were challenged intraperitoneally with 104 CFU of ATCC6305 prepared as previously described [36,37], in sterile animal care cages in a laminar air flow (LAF) cabinet. Mice survival was investigated every 24 h for the next 21 days.
3. Results
3.1. PspA expression and purification
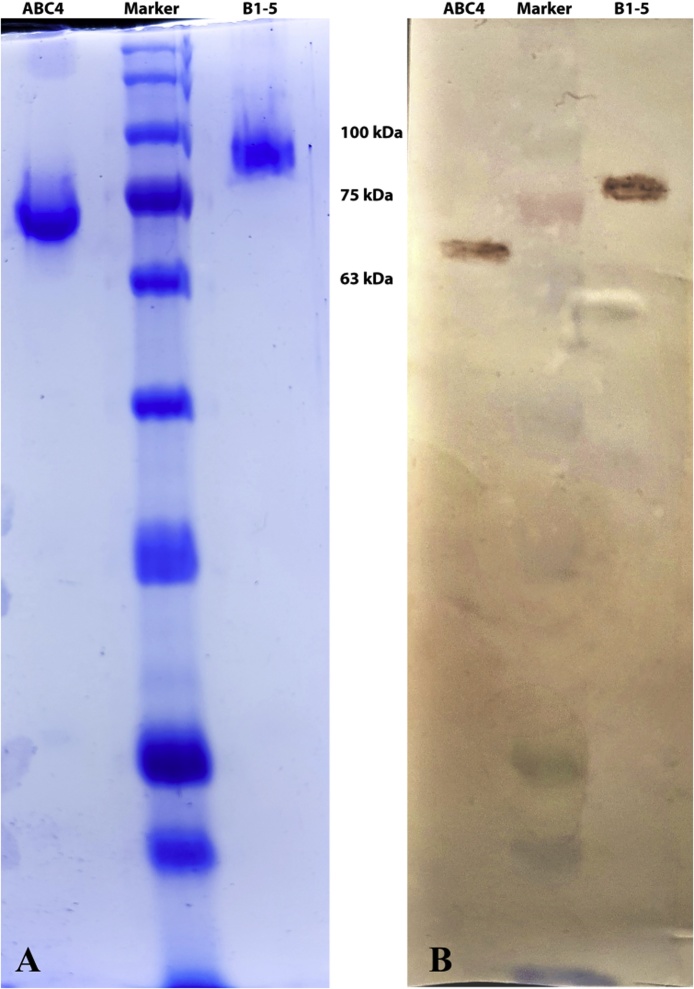
PspA4ABC fragment and fusion protein containing B region from clades 1 to 5 were expressed in fusion with a C-terminus His-tag in transformed competent E. coli strain and purified through Ni-NTA affinity chromatography to ensure the high purity because high purity is necessary for the immunization experiments. The purified recombinant proteins were electrophoresed on SDS–PAGE gels, followed by Coomassie blue staining and western blotting using anti-His tag antibody (Fig. 1), and ELISA using mouse anti-pneumococcal polyclonal sera (data not shown). The apparent sizes of the fragments on SDS-PAGE was larger than the predicted masses (49 kDa for PspA4ABC and 63 kDa for PspAB1-5), an effect that has been formerly reported by others [31,38].
Fig. 1.
Characterization of purified recombinant PspA fragments by SDS-PAGE (A) and western blotting (B). From left to right: lane 1: PspA4ABC; lane 2: protein marker; line 3: PspAB1-5.
3.2. Anti-PspA antibodies binding to the pneumococcal surface
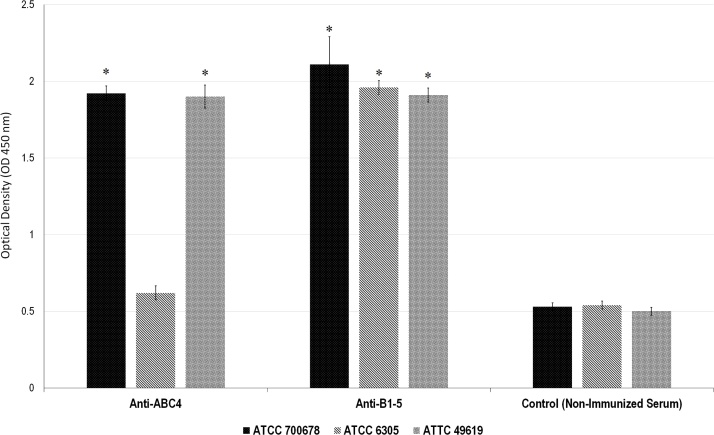
BALB/c mice were immunized with four doses of PspA4ABC and PspAB1-5 in alum at 14-day intervals. Two weeks after the last immunization, sera were collected and tested to measure the antibody titers and for the ability to bind to the intact pneumococci surface bearing PspAs of clades 1, 2 and 5. The results are shown in Fig. 2. Anti-PspAB1-5 antibodies showed a strong binding to 6305 strains and anti-PspA4ABC was not reactive with the 6305 strain. Results showed that although both PspA fragments were able to recognize pneumococcus strains from clade 1 and 5, the presence of one fragment from each clade in the PspAB1-5 hybrid seems to be important to extend the reactivity of sera to all three PspA clades.
Fig. 2.
Binding of anti-PspA fragments antibodies to the pneumococcal surface with 3 different PspA clades. Sera from immunized mice with recombinant PspA4ABC and PspAB1-5 were analysed by ELISA against pneumococcus strains with PspA fragments from clade 1 (ATCC 49619), clade 2 (ATCC 6305) and clade 5 (ATCC 700678). Non-immunized mouse serum was used as negative control.
3.3. Complement deposition in the presence of anti-PspA antibodies
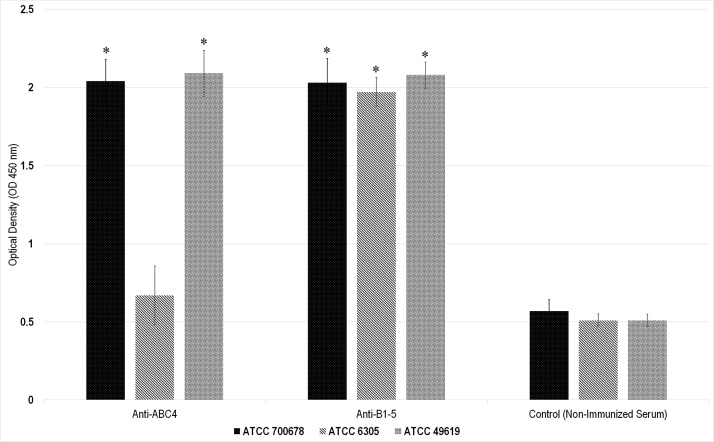
We also determined the ability of the elicited anti-PspA antibodies to rise complement deposition on the pneumococcal surface. Pneumococci were incubated with anti-PspA4ABC, anti-PspAB1-5 and 10% fresh-frozen normal mouse serum as the complement source. The samples were washed and incubated with anti-C3 antibodies. The C3 deposition on the bacteria was determined by ELISA (Fig. 3). Antibodies induced against PspA4ABC increased the amount of C3 deposited onto the pneumococcal strains bearing PspA fragments from clades 1 and 5, while showed lower C3 deposition on the strains expressing clade 2 (p-value = 0.052). In addition, the immune sera to the PspAB1-5 fusion proteins caused higher levels of C3 deposition on all three strains that was correlated with the presence of B regions in the hybrid and the sequence identity with PspA present on the bacterium (p-value = 0.014).
Fig. 3.
Complement deposition assay on 3 pneumococcal strains with anti-PspA fragments antibodies. Bacteria containing PspAs of clade 1 (ATCC49619), clade 2 (ATCC6305), and clade 5 (700678) were incubated with normal mouse serum and sera of immunized mice with PspA4ABC and PspAB1-5. The obtained OD of complement deposited bacteria in ELISA is represented for each group. Non-immunized serum was used as negative control.
3.4. Colonization test and animal challenge
Immunized mice were challenged by intraperitoneal injection of 104 CFU with S. pneumoniae strain ATCC6305 (PspA clade 2, serotype 5) 15 days after last immunization. Animals were then monitored for 3 weeks. The survival rate in PspAB1-5 mice group was greater than PspA4ABC group. Two mice of PspA4ABC group and all five mice of PspAB1-5 group survived while all mice in control group died (Table1).
Table 1.
Animal challenge study of immunized and non-immunized mice. Survival of immunized mice with PspA fragments after a challenge with pneumococcal strain containing PspA from clade 2 was investigated. Survival was inspected 21 days after the challenge.
| Mice group | No. of challenged mice (Survived/Total) |
P value |
|---|---|---|
| PspA4ABC | 2/5 | 0.155 |
| PspAB1-5 | 5/5 | 0.001 |
| Alum (negative control) | 0/5 | --- |
There was no alteration in consumption of food and water in the mice after vaccination, and no weight loss was observed. After the challenge, a reduction in the mobility and water and food consumption was observed in the eventually dead mice.
Colony formation of S. pneumoniae in the nasopharynx of mice that were immunized with recombinant PspAB1-5 protein did not observed (0 of 5 mice). In the group that immunized with recombinant PspA4ABC protein, 2 of the 5 mice showed nasopharyngeal colonization and in the negative control group, 4 of the 5 mice showed nasopharyngeal colonization.
4. Discussion
PspA antigen is a promising vaccine candidate with protein nature against pneumococcal infections to overcome the limitations of polysaccharide-based vaccines [32]. PspA shows considerable antigenic diversity among clinical isolates and it is divided into three families with six clades. For developing a cost-effective and widely available PspA-based vaccines, it is suggested that a combination of PspA antigens from different PspA families is more appropriate.
In this study, we produced two PspA fragments, PspA4ABC and PspAB1-5 recombinant antigens. We compared the binding ability of antibodies to these recombinant proteins in cross-reactivity with the strain of both families. Because complement components deposition on the surface of pneumococcus is facilitated by the anti-PspA antibodies [28], we also investigated whether antibodies against PspA fragments could enhance C3 complement deposition on the cell surface of pneumococci. In addition to cross-reactivity, the ability of anti-PspA antibodies to increase complement deposition depends on the PspA family in the bacterium. On the basis of obtained results, the PspAB1-5 antigen could better enhance both cross-reactivity and complement deposition on pneumococcal strains from both families (Fig. 2, Fig. 3). The mouse challenge experiment showed that the vaccination with PspAB1-5, which also contains B region of clade 2, could protect mice from infection by clade 2 strains.
One limitation of this study was the evaluation of protection against only one strain of pneumococci (ATCC 6305, clade 2, capsular serotype 5). Since there is no correlation between capsular serotype and the expression of a specific PspA clade, the protection effect of immunization with this recombinant PspA protein should be evaluated against pneumococcal strains with other capsular serotype that expressing different PspA clades.
Although, some researches have indicated that PspA fragment from clade 4, especially those include all cell-surface domain, can induce high cross-reactive antibodies, our results and the conclusions from other researcher [31,32] have shown that anti-sera against PspA from clade 4 is often family-dependent and sometimes restricted to the same clade.
Centro de Biotecnologia et al. [27] constructed a PspA hybrid comprised of complete N-terminal region of clade 1 plus only B region of clade 4 (PspA1ABC-4B). Their results showed that this hybrid could extend the recognition capacity of antibodies raised against only PspA1ABC, and PspA1ABC-4B could show stronger reaction with not only PspA clade 1 but also with PspA from clade 4- and 5-bearing pneumococcal isolates; Nevertheless, Eliane N. Miyaji et al. [30] showed that immunization of mice with DNA vaccine encoding PspA1ABC-4B could not induce significant protection against infection with strain expressing clade 4. Nevertheless, this difference in results could be due to antigen delivery method or the differences in amino acid sequences and structure of PspA fragments. In addition, the induction of antibody titers by DNA vaccination was lower than the induction of antibody titers by protein immunization. These results can prove that including B region from each clade, which be expressed as a hybrid protein, can induce effective antibodies against all clade from both families. It is suggested that the selected B region for including in a PspA-based vaccine, be less divergent among B region sequences of PspA antigens in that clade.
In general, our results indicated that although the PspAB1-5 antigen did not have the A and C conserved regions but it, indeed, was able to increase complement deposition, cross-reactivity, and potential protection against Pneumococcal infections. Therefore, these results suggest that the B region contains the key antigenic epitopes in immunization against S. pneumoniae.
Taken together, our results suggested that for construction of a PspA-based anti-pneumococcal vaccine candidate, the B region from all clade should be included. It also proposed that including the more conserved domain such as A and C region to this hybrid, can be helpful to extend the cross-protection of the vaccine, because this region also play a significant role in inducing of cross-reactive antibody production.
However, it needs further studies to evaluate the protective ability of PspAB1-5 hybrid against different strains of pneumococci from all clades in addition of isolated strains from infected patients.
Declaration of Competing Interest
There is no conflict of interest to be declared.
Acknowledgment
This work was supported by Pasteur Institute of Iran and Tehran University of Medical Sciences.
Contributor Information
Babak Negahdari, Email: b-negahdari@sina.tums.ac.ir.
Mahdi Habibi-Anbouhi, Email: habibi_m@pasteur.ac.ir.
References
- 1.Moffitt K., Malley R. Rationale and prospects for novel pneumococcal vaccines. Hum. Vaccin. Immunother. 2016;12:383–392. doi: 10.1080/21645515.2015.1087625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jedrzejas M.J. Pneumococcal virulence factors: structure and function. Microbiol. Mol. Biol. Rev. 2001;65:187–207. doi: 10.1128/MMBR.65.2.187-207.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pichichero M.E., Khan M.N., Xu Q. Next generation protein based Streptococcus pneumoniae vaccines. Hum. Vaccin. Immunother. 2016;12:194–205. doi: 10.1080/21645515.2015.1052198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lin J., Kaltoft M.S., Brandao A.P., Echaniz-Aviles G., Brandileone M.C.C., Hollingshead S.K., Benjamin W.H., Nahm M.H. Validation of a multiplex pneumococcal serotyping assay with clinical samples. J. Clin. Microbiol. 2006;44:383–388. doi: 10.1128/JCM.44.2.383-388.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Keller L.E., Robinson D.A., McDaniel L.S. Nonencapsulated Streptococcus pneumoniae: emergence and pathogenesis. MBio. 2016;7 doi: 10.1128/mBio.01792-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Langereis J.D., de Jonge M.I. Non-encapsulated Streptococcus pneumoniae, vaccination as a measure to interfere with horizontal gene transfer. Virulence. 2017;8:637–639. doi: 10.1080/21505594.2017.1309492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hsu H.E., Shutt K.A., Moore M.R., Beall B.W., Bennett N.M., Craig A.S., Farley M.M., Jorgensen J.H., Lexau C.A., Petit S. Effect of pneumococcal conjugate vaccine on pneumococcal meningitis. N. Engl. J. Med. 2009;360:244–256. doi: 10.1056/NEJMoa0800836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Grabenstein J., Klugman K. A century of pneumococcal vaccination research in humans. Clin. Microbiol. Infect. 2012;18:15–24. doi: 10.1111/j.1469-0691.2012.03943.x. [DOI] [PubMed] [Google Scholar]
- 9.Mann B., Thornton J., Heath R., Wade K.R., Tweten R.K., Gao G., El Kasmi K., Jordan J.B., Mitrea D.M., Kriwacki R. Broadly protective protein-based pneumococcal vaccine composed of pneumolysin toxoid–CbpA peptide recombinant fusion protein. J. Infect. Dis. 2013;209:1116–1125. doi: 10.1093/infdis/jit502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mohammadzadeh M., Pourakbari B., Doosti A., Mahmoudi S., Habibi-Anbouhi M., Mamishi S. Construction and evaluation of a whole-cell pneumococcal vaccine candidate. J. Appl. Microbiol. 2018 doi: 10.1111/jam.14079. [DOI] [PubMed] [Google Scholar]
- 11.Greene C.J., Marks L.R., Hu J.C., Reddinger R., Mandell L., Roche-Hakansson H., King-Lyons N.D., Connell T.D., Hakansson A.P. Novel strategy to protect against influenza virus-induced pneumococcal disease without interfering with commensal colonization. Infect. Immun. 2016;84:1693–1703. doi: 10.1128/IAI.01478-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Converso T., Goulart C., Rodriguez D., Darrieux M., Leite L. Rational selection of broadly cross-reactive family 2 PspA molecules for inclusion in chimeric pneumococcal vaccines. Microb. Pathog. 2017 doi: 10.1016/j.micpath.2017.06.004. [DOI] [PubMed] [Google Scholar]
- 13.Daniels C.C., Briles T.C., Mirza S., Håkansson A.P., Briles D.E. Capsule does not block antibody binding to PspA, a surface virulence protein of Streptococcus pneumoniae. Microb. Pathog. 2006;40:228–233. doi: 10.1016/j.micpath.2006.01.007. [DOI] [PubMed] [Google Scholar]
- 14.Hollingshead S.K., Baril L., Ferro S., King J., Coan P., Briles D.E., Group P.P.E.S. Pneumococcal surface protein A (PspA) family distribution among clinical isolates from adults over 50 years of age collected in seven countries. J. Med. Microbiol. 2006;55:215–221. doi: 10.1099/jmm.0.46268-0. [DOI] [PubMed] [Google Scholar]
- 15.Li J., Glover D.T., Szalai A.J., Hollingshead S.K., Briles D.E. PspA and PspC minimize immune adherence and transfer of pneumococci from erythrocytes to macrophages through their effects on complement activation. Infect. Immun. 2007;75:5877–5885. doi: 10.1128/IAI.00839-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mirza S., Benjamin W.H., Coan P.A., Hwang S.-A., Winslett A.-K., Yother J., Hollingshead S.K., Fujihashi K., Briles D.E. The effects of differences in pspA alleles and capsular types on the resistance of Streptococcus pneumoniae to killing by apolactoferrin. Microb. Pathog. 2016;99:209–219. doi: 10.1016/j.micpath.2016.08.029. [DOI] [PubMed] [Google Scholar]
- 17.Mukerji R., Mirza S., Roche A.M., Widener R.W., Croney C.M., Rhee D.-K., Weiser J.N., Szalai A.J., Briles D.E. Pneumococcal surface protein A inhibits complement deposition on the pneumococcal surface by competing with the binding of C-reactive protein to cell-surface phosphocholine. J. Immunol. 2012;189:5327–5335. doi: 10.4049/jimmunol.1201967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Daniels C.C., Coan P., King J., Hale J., Benton K.A., Briles D.E., Hollingshead S.K. The proline-rich region of pneumococcal surface proteins A and C contains surface-accessible epitopes common to all pneumococci and elicits antibody-mediated protection against sepsis. Infect. Immun. 2010;78:2163–2172. doi: 10.1128/IAI.01199-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kono M., Hotomi M., Hollingshead S.K., Briles D.E., Yamanaka N. Maternal immunization with pneumococcal surface protein A protects against pneumococcal infections among derived offspring. PLoS One. 2011;6 doi: 10.1371/journal.pone.0027102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Roche H., Håkansson A., Hollingshead S.K., Briles D.E. Regions of PspA/EF3296 best able to elicit protection against Streptococcus pneumoniae in a murine infection model. Infect. Immun. 2003;71:1033–1041. doi: 10.1128/IAI.71.3.1033-1041.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Briles D.E., Hollingshead S.K., King J., Swift A., Braun P.A., Park M.K., Ferguson L.M., Nahm M.H., Nabors G.S. Immunization of humans with recombinant pneumococcal surface protein A (rPspA) elicits antibodies that passively protect mice from fatal infection with Streptococcus pneumoniae bearing heterologous PspA. J. Infect. Dis. 2000;182:1694–1701. doi: 10.1086/317602. [DOI] [PubMed] [Google Scholar]
- 22.Nabors G.S., Braun P.A., Herrmann D.J., Heise M.L., Pyle D.J., Gravenstein S., Schilling M., Ferguson L.M., Hollingshead S.K., Briles D.E. Immunization of healthy adults with a single recombinant pneumococcal surface protein A (PspA) variant stimulates broadly cross-reactive antibodies to heterologous PspA molecules. Vaccine. 2000;18:1743–1754. doi: 10.1016/s0264-410x(99)00530-7. [DOI] [PubMed] [Google Scholar]
- 23.Hollingshead S.K., Becker R., Briles D.E. Diversity of PspA: mosaic genes and evidence for past recombination in Streptococcus pneumoniae. Infect. Immun. 2000;68:5889–5900. doi: 10.1128/iai.68.10.5889-5900.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Daniels C.C., Rogers P.D., Shelton C.M. A review of pneumococcal vaccines: current polysaccharide vaccine recommendations and future protein antigens. J. Pediatr. Pharmacol. Ther. 2016;21:27–35. doi: 10.5863/1551-6776-21.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Goulart C., Darrieux M., Rodriguez D., Pimenta F.C., Brandileone M.C.C., de Andrade A.L.S., Leite L.C. Selection of family 1 PspA molecules capable of inducing broad-ranging cross-reactivity by complement deposition and opsonophagocytosis by murine peritoneal cells. Vaccine. 2011;29:1634–1642. doi: 10.1016/j.vaccine.2010.12.074. [DOI] [PubMed] [Google Scholar]
- 26.McDaniel L.S., Ralph B.A., McDaniel D.O., Briles D.E. Localization of protection-eliciting epitopes on PspA of Streptococcus pneumoniae between amino acid residues 192 and 260. Microb. Pathog. 1994;17:323–337. doi: 10.1006/mpat.1994.1078. [DOI] [PubMed] [Google Scholar]
- 27.Darrieux M., Moreno A.T., Ferreira D.M., Pimenta F.C., de Andrade A.L.S., Lopes A.P., Leite L.C., Miyaji E.N. Recognition of pneumococcal isolates by antisera raised against PspA fragments from different clades. J. Med. Microbiol. 2008;57:273–278. doi: 10.1099/jmm.0.47661-0. [DOI] [PubMed] [Google Scholar]
- 28.Ren B., Szalai A.J., Hollingshead S.K., Briles D.E. Effects of PspA and antibodies to PspA on activation and deposition of complement on the pneumococcal surface. Infect. Immun. 2004;72:114–122. doi: 10.1128/IAI.72.1.114-122.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Xin W., Li Y., Mo H., Roland K.L., Curtiss R. PspA family fusion proteins delivered by attenuated Salmonella enterica serovar Typhimurium extend and enhance protection against Streptococcus pneumoniae. Infect. Immun. 2009;77:4518–4528. doi: 10.1128/IAI.00486-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Miyaji E.N., Ferreira D.M., Lopes A.P., Brandileone M.C.C., Dias W.O., Leite L.C. Analysis of serum cross-reactivity and cross-protection elicited by immunization with DNA vaccines against Streptococcus pneumoniae expressing PspA fragments from different clades. Infect. Immun. 2002;70:5086–5090. doi: 10.1128/IAI.70.9.5086-5090.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Darrieux M., Miyaji E., Ferreira D., Lopes L., Lopes A., Ren B., Briles D., Hollingshead S., Leite L. Fusion proteins containing family 1 and family 2 PspA fragments elicit protection against Streptococcus pneumoniae that correlates with antibody-mediated enhancement of complement deposition. Infect. Immun. 2007;75:5930–5938. doi: 10.1128/IAI.00940-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kristian S.A., Ota T., Bubeck S.S., Cho R., Groff B.C., Kubota T., Destito G., Laudenslager J., Koriazova L., Tahara T. Generation and improvement of effector function of a novel broadly reactive and protective monoclonal antibody against pneumococcal surface protein A of Streptococcus pneumoniae. PLoS One. 2016;11 doi: 10.1371/journal.pone.0154616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Piao Z., Akeda Y., Takeuchi D., Ishii K.J., Ubukata K., Briles D.E., Tomono K., Oishi K. Protective properties of a fusion pneumococcal surface protein A (PspA) vaccine against pneumococcal challenge by five different PspA clades in mice. Vaccine. 2014;32:5607–5613. doi: 10.1016/j.vaccine.2014.07.108. [DOI] [PubMed] [Google Scholar]
- 34.Moreno A.T., Oliveira M.L.S., Ferreira D.M., Ho P.L., Darrieux M., Leite L.C., Ferreira J.M., Pimenta F.C., Andrade A.L.S., Miyaji E.N. Immunization of mice with single PspA fragments induces antibodies capable of mediating complement deposition on different pneumococcal strains and cross-protection. Clin. Vaccine Immunol. 2010;17:439–446. doi: 10.1128/CVI.00430-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yousefi-Rad N., Shokrgozar M.A., Behdani M., Moradi-Kalbolandi S., Motamedi-Rad M., Habibi-Anbouhi M. Antigenic assessment of a recombinant human CD90 protein expressed in prokaryotic expression system. Protein Expr. Purif. 2015;116:139–143. doi: 10.1016/j.pep.2015.08.017. [DOI] [PubMed] [Google Scholar]
- 36.Knapp S., Hareng L., Rijneveld A.W., Bresser P., van der Zee J.S., Florquin S., Hartung T., van der Poll T. Activation of neutrophils and inhibition of the proinflammatory cytokine response by endogenous granulocyte colony-stimulating factor in murine pneumococcal pneumonia. J. Infect. Dis. 2004;189:1506–1515. doi: 10.1086/382962. [DOI] [PubMed] [Google Scholar]
- 37.Vorderstrasse B.A., Lawrence B.P. Protection against lethal challenge with Streptococcus pneumoniae is conferred by aryl hydrocarbon receptor activation but is not associated with an enhanced inflammatory response. Infect. Immun. 2006;74:5679–5686. doi: 10.1128/IAI.00837-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yother J., Handsome G., Briles D. Truncated forms of PspA that are secreted from Streptococcus pneumoniae and their use in functional studies and cloning of the pspA gene. J. Bacteriol. 1992;174:610–618. doi: 10.1128/jb.174.2.610-618.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]