Highlights
-
•
Black peel pomegranate is a rare cultivar of pomegranate distinguished by a deep red color.
-
•
The peel extract of the pomegranate shows unique pharmaceutical properties such as antioxidant and antibacterial.
-
•
This extract synthesis silver nanoparticles in an ultra-fast pace without any heating or additional accelerators.
Keywords: Black peel pomegranate, Green synthesis, Selective cytotoxicity, Antioxidant agents, Anticancer agents
Abstract
"Black Peel Pomegranate" is a rare pomegranate cultivar that its specific features are still uncovered particularly in the bio-nano researches. The present study was organized to evaluate this pomegranate's potential in the biosynthesis of silver nanoparticles as well as bio-medical activities. According to the results, the pomegranate peel extract incredibly inhibited 100 % of DPPH free radicals (EC50 = 5 μg/mL). This extract also induced more than 70 % cell death in the treated breast tumor cell lines, BT-20 and MCF-7. Interestingly, the extract was capable of biosynthesis very stable and small (15.6 nm) silver nanoparticles at ambient temperature in an ultra-fast pace. Likewise, these nanoparticles inhibited 77 % of DPPH free radicals (EC50 = 9 μg/mL). Although this antioxidant capacity was lower than that of the extract, instead, the anticancer activity of the synthesized nanoparticles was significantly enhanced, so that they led to more than 81 % and 89 % cell death in the breast tumor cell lines BT-20 and MCF-7, respectively. Considerably, neither the extract nor the biosynthesized silver nanoparticles, showed significant toxicity against non-tumor cell lines (L-929) at the same concentrations. These features of the biosynthesized nanoparticles were quite outstanding in comparison with chemical/commercial ones. Overall, the present study introduces black peel pomegranate as a worthy bio-agent in the biosynthesis of silver nanoparticles with unique activities as well as a cancer treatment.
1. Introduction
Since ancient times, plant-derived compounds, the so-called phytocompounds, have been used to prevent or treat several diseases including cancer and infectious diseases [1]. However, investigations on pharmaceutical features of the plant-derived chemicals have become serious in the last few decades because of the prevalence of cancers and imperfections of current treatments. The potential application of phytocompounds against various cancers such as lung, breast, colon, etc. has been demonstrated in preclinical and clinical studies [1,2].
In this regard, pomegranate (Punica granatum L.) and its chemical components have also attracted much attention [3]. This delicious fruit possesses various pharmacological and toxicological properties including antioxidant [4], anti-inflammatory [5], anti-cancer, anti-angiogenesis and antibacterial activities [6,7]. These desirable bio-pharmaceutical activities are related to the abundance of polyphenols and flavonoids, anthocyanins, catechin, epicatechin, ellagic acid, and gallic acid in this fruit [3,7].
Nowadays most of the pomegranate cultivars have been studied and their bio-medical potentials are revealed [3]. However, some rare cultivars such as Black Peel Pomegranate has not received enough attention yet. As Fig. 1 shows, the dark purple-red color peel distinguishes this cultivar from the same cultivars. Based on the fact that flavonoids such as anthocyanins form red, blue, and purple plant pigments [8], it is thought that these compounds exist more in this kind of pomegranate. Therefore, it is highly likely that the black peel pomegranate has a higher therapeutic and nutritious potential. Although there is not any reliable report on the literature, it is expressed that this pomegranate has been traditionally used for treatment of several diseases such as diarrhea, jaundice and pertussis. Nonetheless, undiscovered features of this cultivar are abundant and need more expanded investigations.
Fig. 1.
Black peel pomegranates in nature. The unique kind of pomegranate's color is deep red in fact, while it seems black in a glans. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
In the present study, antioxidant and anticancer activities of black peel pomegranate extract (P. extract) have been investigated for the first time. Furthermore, the potential of the extract has been explored in the biosynthesis of silver nanoparticles (P. AgNPs), as both reducing and stabilizing agent. Synthesis of nanoparticles using biological agents is straightforward, inexpensive and eco-friendly [9,10]. In addition, during the stabilization process, the bioactive agents bind to the nanoparticles' surfaces, enhancing the biological activity of the nanoparticles [[11], [12], [13]]. Accordingly, an improvement in the properties of biosynthesized nanoparticles in comparison with the extract and commercial silver nanoparticles (CSNs) is expectable. This assumption has been examined by assessing the antioxidant and anticancer activity of biosynthesized silver nanoparticles.
2. Materials and methods
2.1. Materials
The Black peel pomegranate (Punica granatum) also called Black Leather pomegranate [14] was obtained as a gift from Medical Plant Garden of Isfahan University of Medical Sciences, Isfahan, Iran. All reagents used in this research were analytical grade and were used without further purification or treatment. High-purity chemical reagents, silver nitrate (99.98 %), ethanol (≥99.5 %), anhydrous dimethyl sulfoxide (DMSO) and Ascorbic acid were purchased from Merck, Germany. (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) and 1,1-diphenyl-2-picrylhydrazyl (DPPH), were also purchased from Sigma-Aldrich chemical company (Germany). Commercial silver nanoparticles (CSNs) (used as a control) were a product of US Research Nanomaterials Inc. (Houston, TX, USA). The Breast cancer cell lines (MCF-7 and BT-20) and the normal cell line (L-929) were purchased from Pasteur Institute, Tehran, Iran.
2.2. Preparation of P. extract
First, the medium fruits were washed with tap and deionized water and their black peel was carefully separated and chopped. Next, 30 g of the peel was mixed with 300 mL deionized water. The solution was then stirred up in a shaker incubator at 37 °C for 24 h. After that, the final mixture was refined using Whatman No. 1 filter papers and, finally, to make a powder form of the P. extract it was dried under lab conditions (27 °C – 30 °C).
2.3. Biosynthesis of P. AgNPs
AgNPs were synthesized using the protocol that has been recently reported by our group [12] with some modification. Briefly, the P. extract powder was dissolved in deionized water and its pH was adjusted. Then, the silver nitrate solution was added to the extract solution and the flask containing the mixture was stirred at room temperature (22 °C – 25 °C) for 2 h. After that time, the mixture was centrifuged and the obtained pellet was washed twice with deionized water. Finally, the resulted precipitate was dried and stored for further characterization.
2.4. Characterizations
To assess the effect of time on P. AgNPs synthesis ultraviolet-visible (UV–Vis) spectrophotometer (JASCO V-670 UV-VIS-NIR; Tokyo, Japan) analysis was used within the wavelength range of 300–700 nm. Fourier transform infrared (FTIR) spectroscopy (JASCO Ltd., Tokyo, Japan) was employed to verify the possible effect of various phytochemicals present in the P. extract on the functionalization of the synthesized nanoparticles. Crystallographic properties of both P. extract and P. AgNPs were explored using X-ray powder diffraction (XRD) technique within 2θ = 10–80 using XRD instrument (D8 Advance, Bruker, Madison, WI, USA), having Cu Kα (λ = 1.540 Å) as a radiation source. The obtained pattern from the XRD was then analyzed using X'Pert High Score Plus software and the chemical composition, crystalline structure, and size of the compound were identified. Zeta potential (surface charge), hydrodynamic size (Z average) and polydispersity index (PDI) regarding P. AgNPs were analyzed using Horiba SZ-100 particle size analyzer (Kyoto, Japan). Investigating the size and morphology of the P. AgNPs as well as the presence/absence of any aggregation or agglomeration was conducted using the field emission scanning electron microscopy (FESEM) (MIRA3; TESCAN, Brno, Czech Republic) [12].
2.5. Antioxidant properties
The antioxidant activity of the P. extract and P. AgNPs in comparison to CNC and Ascorbic acid was assessed using the free radical DPPH scavenging assay as previously described by our group [12]. Briefly, the DPPH solution was distributed to each well of a 96-well plate and next, different concentrations of each sample were added to each well. Then, UV–vis absorbance of the samples was measured at 517 nm after 30 min as well as 1 -h incubation. Lastly, the percentage of free radical inhibition was calculated.
2.6. Anti-cancer properties
Conventional MTT reduction assay was employed to determine the cytotoxic and anticancer activity of P. extract, P. AgNPs and CSNs [15]. First, l-929, BT-20, and MCF-7 cells were seeded in 96-well plates and the plates were incubated for 24 h. After that, the previous medium was replaced with fresh medium along with different concentrations of the P. extract, P. AgNPs or CSNs and the plates were incubated at the same conditions for 24−48 hours. Next, the medium was replaced with a fresh one, immediately, the MTT solution was added to each well, and the plates were incubated for another 4 h. After that time, DMSO was added to each well and plates were incubated for 40 min in dark. Finally, the cell growth inhibition calculated using the absorbance intensity of each well at 492 nm.
2.7. Statistical analyses
The data were exposed to One-way Analysis of Variance (ANOVA) to determine the importance of individual differences at p < 0.05 level. Important means were compared by Duncan’s multiple range tests. All statistical analyses were performed using SPSS Version 16.
3. Results
Fig. 1 shows a black peel pomegranate in nature. Its unique color reflects the presence of unique pigments in the peel of the pomegranate. As mentioned earlier, anthocyanins are flavonoids that participate in the formation of plant pigments [16,17]. These compounds impart red, blue, and purple colors to flowers, leaves, fruits, and some vegetables [18,19]. The deep red color of pomegranate's peel very probably indicates that the total content of the flavonoids, especially anthocyanins, are significantly higher in the peel of black peel pomegranate than other common pomegranates.
3.1. Biosynthesis of P. AgNPs
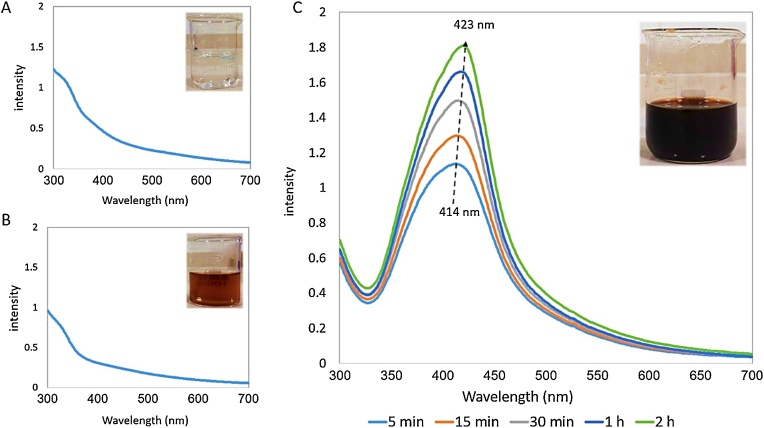
The changes in color, pH, and UV–Vis spectra were monitored during the biosynthesis process of P. AgNPs. The extract's color immediately changed from light brown to dark brown after addition of AgNO3 (Fig. 2, B and C). Also, a deep decrease from 8 to 3.2 was observed in the pH of the mixture during 2 first minutes.
Fig. 2.
UV–Vis spectra of different solutions involved in the biosynthesis of the P. AgNPs process. (A) AgNO3 solution, (B) P. extract and (C) P. AgNPs colloid. Increasing the peak intensity with time, along with a clear red-shift indicates the synthesis of stable silver nanoparticles during the processing time. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
It is well known that the water suspension of AgNPs exhibits red-brown color, which is due to the excitation of surface plasmon resonances (SPR) of the metal nanoparticles [20,21]. Thus, these early observations demonstrate silver ions (Ag+) have been successfully reduced to silver nanoparticles through the P. extract. Although the exact mechanisms of reduction of Ag+ and formation of AgNPs have not been known yet, it is surely asserted that the major water-soluble phytoconstituents are involved in inter and intramolecular hydrogen bonding. It is very likely that these bioactive agents play the main role in both biosynthesis and stabilization of AgNPs through the formation of the intermediate Ag+-phytoconstituent complex [22,23]. The rapid pH decreasing may well is due to the participation of OH groups in the process of P. AgNPs synthesis. Moreover, the capability of doing such great performance at ambient temperatures without any additional heating is one of the most interesting features of the P. extract.
3.2. Characterizations of P. AgNPs
3.2.1. UV–vis spectroscopy
According to the results, AgNO3 and P. extract did not show any UV–vis absorbance peak in the range of 300–700 nm. While the intensity of the absorbance peaks of P. AgNPs colloid steadily increased during the time of the reaction up to 2 h. The changes were accompanied by a red-shift in λmax from 414 to 423 nm.
The increase associated with the red-shift in the UV–vis spectra of P. AgNPs is attributed to the initiation of the growth phase of nanocrystals resulting in the synthesis of more and larger particles without aggregation. It may well also happen because of the presence of extract constituents on the nanoparticles as capping agents [12]. It should be noted that these results are very similar to the reports in the literature [24,25]. In general, the dramatically rapid color change and the sharp absorbance intensity appeared in the first minutes of the process demonstrate the capability of P. extract in the ultra-fast synthesis of the nanoparticles. This property is one of the other advantages to the synthesis of nanoparticles using black peel pomegranate.
3.2.2. FTIR spectroscopy
Fig. 3 represents the FTIR spectra of the P. extract as well as its resultant P. AgNPs. The FTIR spectrum of the extract showed the major absorption bands at 3403 cm-1 and 1724 cm-1 which correspond to O—H stretching of the phenolic group and C–O of the carboxyl group, respectively. Further, the absorption bands at 1611 cm-1 and 1445 cm−1 were due to the presence of amine N—H and aromatic C C bond in the extract [22,26].
Fig. 3.
FTIR spectra of P. extract and P. AgNPs from 500 to 4000 cm−1. The resemblance between two spectra confirms the synthesis and functionalization of AgNPs carried out by the extract.
The likeness of the FTIR spectrum of the P. extract and P. AgNPs confirms that the phytoconstituents present in the extract have been efficiently involved in both synthesis and functionalization of AgNPs. Early researches have revealed the presence of polyphenols and flavonoids in the pomegranate peel extract [3,27].
3.2.3. XRD analysis
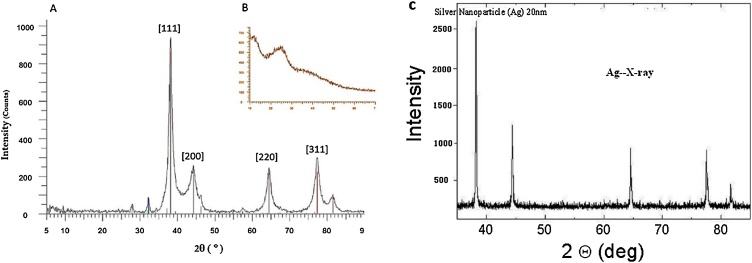
Analysis of the XRD spectra revealed the crystalline phase and the orientation of the synthesized nanoparticles. Based on the results, P. AgNPs' XRD spectrum (Fig. 4A) is homologous with the silver nanocrystal records (JCPDS NO 01-087- 0718). The spectrum shows peaks at 2θ = 38°, 44.56°, 64.5°, and 77°, corresponding to (111), (200), (220), and (311) planes of the face-centered cubic geometry of Ag nanocrystals. Whereas, the XRD spectrum of P. extract did not show any characteristic peak. Furthermore, Using the Scherrer equation [15], the size of P. AgNPs was calculated at about 10 nm (FWHM = 0.9 2θ).
Fig. 4.
X-ray powder diffraction spectra of (A) P. AgNPs and (B) P. extract and (C*) CNS. These distinct peaks correspond to the face-centered cubic geometry of the silver crystal. *The spectrum provided by the company that CSN was purchased from.
3.2.4. DLS analysis
The dynamic light scattering (DLS) technique was utilized to investigate the surface zeta potential, hydrodynamic size, and polydispersity index (PDI) of the samples. As Fig. 5A and B show, the average hydrodynamic size, PDI, and zeta potential for P. AgNPs are 31.6 nm, 0.32, and -39.6 mV (pH = 6.5, 25 °C), respectively.
Fig. 5.
Dynamic Light Scattering analysis of P. AgNPs indicating: (A) mean diameter and (B) zeta potential of the P. AgNPs. (C) Represents field emission scanning electron microscopy (FESEM) of P. extract. Note: part (D) is a TEM image of CSNs represented by the company that CSN was purchased from. Agglomeration of nanoparticles is clear in the slide.
PDI is dimensionless and scaled such that the PDI value “0″ represents the highest level of monodispersity whereas value “1″ represents a polydisperse distribution of particles [28]. Therefore, the PDI value of 0.32 obtained in this study is a good value and there is no doubt that the monodispersity enhances the subsequent functions of the nanoparticles. Zeta potential, also, is one of the other vital parameters to the stability of nanoparticles in an aqueous environment. It is generally argued that particles with zeta potentials greater than +30 mV and less than −30 mV are considered stable for colloidal dispersions in the absence of steric stabilization [29]. Consequently, the synthesized P. AgNPs with the zeta potential of −39.6 mV could be considered to be the adequately stable colloidal system. This character means P. extract sufficiently acts as a biological stabilizer.
3.2.5. FE-SEM analysis
Fig. 5C shows the results of the FE-SEM analysis of P. AgNPs. Based on the results, the nanoparticles are spherical in shape, with an average size of 15.6 nm (SD = 3). Furthermore, due to the high electrostatic repulsive forces between the particles, confirmed by their zeta potential, no distinct aggregation and agglomeration of the nanoparticles are observed.
Herein, there is a slight difference between data obtained by XRD, DLS and microscopic (FESEM) examination regarding particle size. The differences are acceptable because the sample preparation process and nature of the techniques are different in these analyses. Furthermore, the DLS method measures all particles in the sample analyzed, while a limited number of particles can be assessed by microscopic techniques [12]. It is worth mentioning that the good monodispersity observed by the microscopic studies in the particle size distribution, confirming the DLS data and the PDI value of 0.32.
3.3. Antioxidant properties assessment
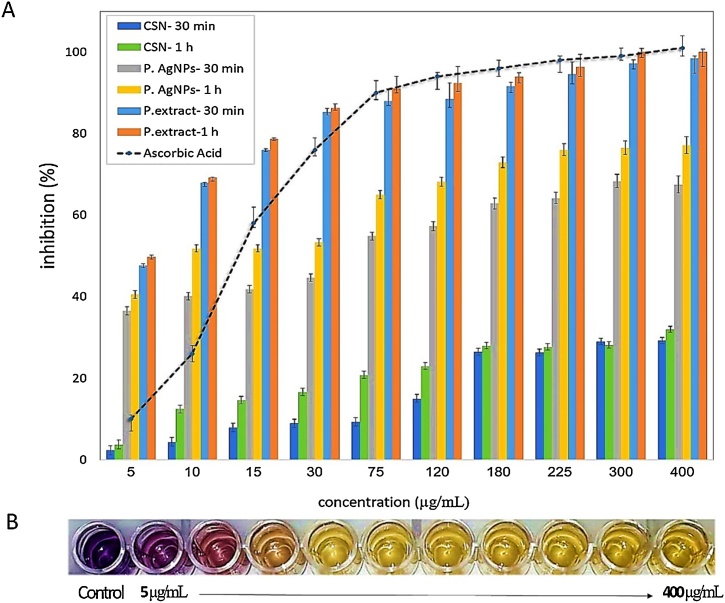
The results of DPPH-free radical eliminating (Fig. 6) revealed both P. AgNPs and P. extract are highly capable of scavenging elevated concentrations of the free radicals, within 77 % (EC50 = 9 μg/mL), and 100 % (EC50 = 5 μg/mL) of DPPH radical inhibition, respectively. Meanwhile, Ascorbic Acid (as positive control) showed an antioxidant activity equivalent of P. extract at the high concentrations. Though, the activity of P. extract at low concentrations was higher than that of Ascorbic acid. On the other hand, CSNs exhibited less free-radical scavenging ability, within the maximum scavenging yield of 32 % (EC50 not determined). Remarkably, the activity of P. extract and Ascorbic acid depends only on their concentrations, but in the case of P. AgNPs, it seems to be dependent on the exposure time of the nanoparticles as well. As Fig. 6 shows, the EC50 of P. AgNPs decreased from 65 to 9 μg/mL after just 30 min. This observation could be attributed to the controlled antioxidant activity of P. AgNPs. In other words, these nanoparticles scavenge free radicals slowly and continuously.
Fig. 6.
(A) Antioxidant behavior of P. extract, P. AgNPs, CSNthe and Ascorbic acid in different concentrations (5–400 μg/mL) and time (30 min and 1 h), Ascorbic acid activity presented after 1 h. (B) The alterations of DPPH color after exposing to different concentrations of P. extract.
DPPH assay method is based on the measurement of the reducing ability of antioxidants toward DPPH radical [30,31]. DPPH has a maximum UV–Vis absorption within the range of 515–520 nm. This organic nitrogen radical reacts with hydrogen/electron donor compounds and after reduction, the radical solution becomes discolored or yellow and its absorbance decreases. Results are normally expressed using the EC50 value, defined as the concentration of antioxidant that causes 50 % of DPPH radical inhibition [32,33].
Previous studies have revealed that antioxidant activity has a high correlation with the total phenolic composition of food materials [31,32]. A study conducted by Li et al., reported the contents of total phenolics and flavonoids are higher in the peel extract than pulp and seed of pomegranate and the peel extract has the highest antioxidant activity [34,35]. Azarpazhooh et al. have also recently reported an EC50 equivalent to 560 μg/mL for microencapsulated peel extract using a DPPH assay [36]. In another study, the ethanolic extract of a pomegranate peel has shown the highest DPPH radical scavenging ability at 250 μg/mL concentration (EC50 approximately 90 μg/mL) [37]. These reported EC50 values are more than the results obtained in the present study, indicating that the black peel pomegranate extract is more potent in terms of free-radical scavenging compared to the previously reported findings. The elevated antioxidant activity of black peel pomegranate could be attributed to the large amount of flavonoids such as anthocyanin and ellagic acid in the pomegranate's peel [14,16].
It is proposed that the lower antioxidant property of P. AgNPs is due to the neutralization of some functional groups of P. extract during the reduction of Ag+ to AgNPs and their stabilization. The low antioxidant activity of CSNs also could be due to their agglomeration during the time. What justifies more potent antioxidant activity of P. extract than Ascorbic acid is highly likely that the extract is a compound of various bioactive agents, while Ascorbic acid is a solitary agent.
3.4. Anti-cancer properties assessment
Two different breast cancer cell lines (BT-20 and MCF-7) and a normal cell line (L-929) were used in order to assess the cytotoxicity of these agents using the MTT assay. Results expressed as cell death percentage and IC50 value, a concentration that a cytotoxic agent induces 50 percent inhibition of cell viability [38].
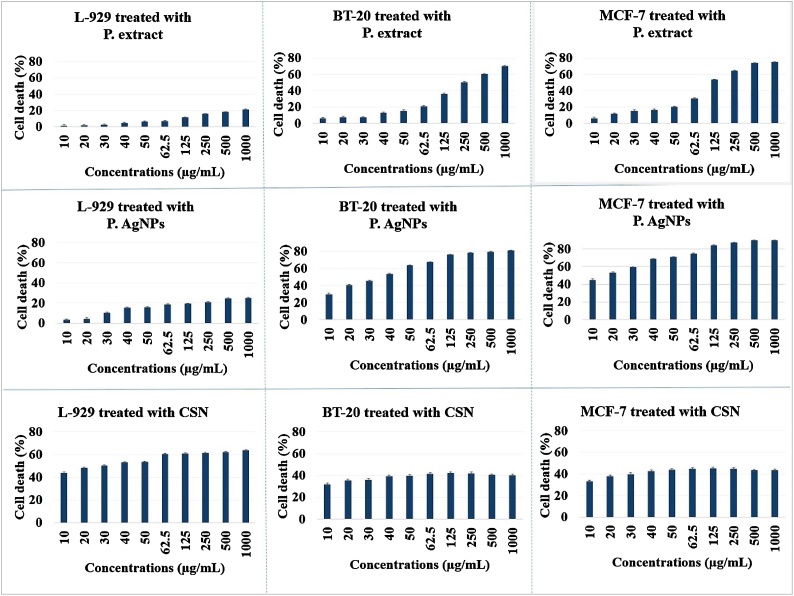
The cytotoxicity assessment of different concentrations of P. extract and P. AgNPs on MCF-7 and BT-20 revealed that either the extract or nanoparticles are very toxic against these cancerous cell lines. Moreover, P. AgNPs clearly show more cytotoxicity. Interestingly, neither P. extract nor P. AgNPs did show significant toxicity against the normal cell line at the same concentrations. In contrast, the CSNs not only exhibited low toxicity toward tumor cell lines but showed also significant toxicity against the normal cells (Fig. 7).
Fig. 7.
Cytotoxicity evaluation of P. extract, P. AgNPs and CSNs on l-929, BT-20, and MCF-7 cell lines.
A more detailed look at Table 1 shows, after 48 -hs of exposure, the maximum cell death (MCD) detected for the P. AgNPs reached 89.6 % against MCF-7 cell lines. However, CSNs resulted in approximately only 43 % cell death against the cancerous cells, while they induced more than 63 % cell death in the normal cell line. This is when the P. extract showed just over 75 % toxicity against the breast tumor cells. As can be seen in Fig. 7, the cytotoxicity of P. extract and P. AgNPs are all concentration-dependent. Moreover, the cytotoxicity of P. AgNPs remarkably increases during 48 h, while that of CSNs is not striking, arguably the nanoparticles agglomerate in the high concentrations and over time. The present report is in good agreement with the data in the literature, which report the concentration-dependent toxicity of nanoparticles, particularly at lower levels of concentration [39,40].
Table 1.
Maximum cell death (MCD) and IC50 values of P. extract, P. AgNPs and CSNs against l-929 (as a normal cell line) and BT-20 and MCF-7 (breast cancer cell lines).
| L-929 |
BT-20 |
MCF-7 |
||||
|---|---|---|---|---|---|---|
| MCD (%) | IC50 (μg/mL) | MCD (%) | IC50 (μg/mL) | MCD (%) | IC50 (μg/mL) | |
| P. extract | 21.2 | ND* | 70.2 | 250 | 75.2 | 120 |
| P. AgNPs | 25.1 | ND | 81.2 | 37 | 89.6 | 17 |
| CSN | 63.7 | 30 | 42.4 | ND | 45.3 | ND |
Not determined.
It is well known that radical scavenging is correlated with growth inhibition of cancerous cells [31,41]. Hence, it is expectable that the P. extract and P. AgNPs with the unique antioxidant property would show considerable anticancer activity. Till now, several studies have proven that polyphenols and flavonoids are toxic against cancerous cells and are harmless toward normal cells [35,42]. Likewise, It has been reported that pomegranate fruit extract can decrease proliferation, invasion, and motility in aggressive breast cancer phenotypes with suppressed NF-κB gene expression and a decrease in RhoC and RhoA protein expression [[43], [44], [45]]. Also, dominant compounds in the extract Ellagic acid, for example, [14,46], are well known for their antiproliferative and antioxidant properties in cancer cell lines and animal models.
Given that this study is the first investigation on the cytotoxicity of black peel pomegranate extract and there are insufficient data on the phytochemistry of the cultivar, an exact mechanism for its anticancer properties remain to be unclear and needs further evaluation. Nonetheless, the level of selective cytotoxicity and the smart discrimination between normal and cancerous cells presented by the extract is highly considerable. Moreover, compared to published reports, the extract and resultant AgNPs have a low IC50 which confirms their higher anticancer activity than other similar bio-agents.
In the end, these nanoparticles have been covered by the extract compounds, in addition, they have a small size, making them capable of easily enter the cells, interact with cell constituents, and eventually disturb the cellular functions [47]. So, the higher anticancer activity of the P. AgNPs than P. extract could be attributed to an additive effect of AgNPs and their covering polyphenols [12,48]. It is also very possible that abnormal metabolism and high proliferation rate [49] of cancerous cells makes them more vulnerable toward these anticancer agents [12].
4. Conclusion
Herein, we investigated the unique biological features of a rare cultivar of pomegranate, Black Peel Pomegranate, for the first time. The deep red color of the black peel pomegranate distinguished it from common pomegranates and granting specific antioxidant and anticancer activities. Interestingly, the extract demonstrated smart cytotoxicity against cells; so that they were toxic only against tumor cell lines. In addition, the extract showed an outstanding capability of the biosynthesis of silver nanoparticles since it could reduce silver ions to very small and stable spherical silver nanoparticles in an ultra-fast pace pattern without any heating or additional accelerators. It is worth mentioning that the resultant nanoparticles possess an elevated biological activity in comparison to the extract and even commercial nanoparticles as consequences of the synergy between pomegranate-derived bioactive agents and silver nanoparticles.
Declaration of Competing Interest
None.
Contributor Information
Sadegh Khorrami, Email: s.khorrami.992@gmail.com.
Ali Zarrabi, Email: alizarrabi@sabanciuniv.edu.
References
- 1.Aqil F., Munagala R., Agrawal A.K., Gupta R. New Look to Phytomedicine. Elsevier; 2019. Anticancer phytocompounds: experimental and clinical updates; pp. 237–272. [DOI] [Google Scholar]
- 2.Zhang Y.-J., Gan R.-Y., Li S., Zhou Y., Li A.-N., Xu D.-P., Li H.-B. Antioxidant phytochemicals for the prevention and treatment of chronic diseases. Molecules. 2015;20:21138–21156. doi: 10.3390/molecules201219753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Monica R., Loizzo Francesca Aiello, Tenuta Maria C., Leporini Mariarosaria, Tiziana Falco R.T. Nonvitamin and Nonmineral Nutritional Supplements. Elsevier Inc.; 2019. Pomegranate (punica granatum L.) pp. 467–472. [DOI] [Google Scholar]
- 4.Kotamballi N., Murthy Chidambara, Guddadarangavvahally K., Jayaprakasha, R.P.S Studies on antioxidant activity of pomegranate (Punica granatum) peel extract using in vivo models. J. Agric. 2002;50:4791–4795. doi: 10.1021/jf0255735. [DOI] [PubMed] [Google Scholar]
- 5.Larrosa M., González-Sarrías A., Yáñez-Gascón M.J., Selma M.V., Azorín-Ortuño M., Toti S., Tomás-Barberán F., Dolara P., Espín J.C. Anti-inflammatory properties of a pomegranate extract and its metabolite urolithin-A in a colitis rat model and the effect of colon inflammation on phenolic metabolism. J. Nutr. Biochem. 2010;21:717–725. doi: 10.1016/j.jnutbio.2009.04.012. [DOI] [PubMed] [Google Scholar]
- 6.Kanatt S.R., Chander R., Sharma A. Antioxidant and antimicrobial activity of pomegranate peel extract improves the shelf life of chicken products. Int. J. Food Sci. Technol. 2010;45:216–222. doi: 10.1111/j.1365-2621.2009.02124.x. [DOI] [Google Scholar]
- 7.Olaniyi A., Fawole N.P.M., U.L.O Antibacterial, antioxidant and tyrosinase-inhibition activities of pomegranate fruit peel methanolic extract. BMC Complement. Altern. Med. 2012;12:1–11. doi: 10.1186/1472-6882-12-200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mazza G. CRC press; 2018. Anthocyanins in Fruits, Vegetables, and Grains. [DOI] [Google Scholar]
- 9.Chauhan M., Sharma B., Kumar R., Chaudhary G.R., Hassan A.A., Kumar S. Green synthesis of CuO nanomaterials and their proficient use for organic waste removal and antimicrobial application. Environ. Res. 2019;168:85–95. doi: 10.1016/j.envres.2018.09.024. [DOI] [PubMed] [Google Scholar]
- 10.Gautam P.K., Singh A., Misra K., Sahoo A.K., Samanta S.K. Synthesis and applications of biogenic nanomaterials in drinking and wastewater treatment. J. Environ. Manage. 2019;231:734–748. doi: 10.1016/j.jenvman.2018.10.104. [DOI] [PubMed] [Google Scholar]
- 11.Fahimirad S., Ajalloueian F., Ghorbanpour M. Synthesis and therapeutic potential of silver nanomaterials derived from plant extracts. Ecotoxicol. Environ. Saf. 2019;168:260–278. doi: 10.1016/j.ecoenv.2018.10.017. [DOI] [PubMed] [Google Scholar]
- 12.Khorrami S., Zarrabi A., Khaleghi M., Danaei M., Mozafari M. Selective cytotoxicity of green synthesized silver nanoparticles against the MCF-7 tumor cell line and their enhanced antioxidant and antimicrobial properties. Int. J. Nanomed. 2018;13:8013–8024. doi: 10.2147/IJN.S189295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Khorrami S., Jafari Najafabadi F., Zarepour A., Zarrabi A. Is Astragalus gossypinus honey a natural antibacterial and cytotoxic agent? An investigation on A. Gossypinus honey biological activity and its green synthesized silver nanoparticles. Bionanoscience. 2019:1–8. doi: 10.1007/s12668-019-00646-8. [DOI] [Google Scholar]
- 14.Mousavinejad G., Emam-Djomeh Z., Rezaei K., Khodaparast M.H.H. Identification and quantification of phenolic compounds and their effects on antioxidant activity in pomegranate juices of eight Iranian cultivars. Food Chem. 2009;115:1274–1278. doi: 10.1016/j.foodchem.2009.01.044. [DOI] [Google Scholar]
- 15.Khorrami S., Abdollahi Z., Eshaghi G., Khosravi A., Bidram E., Zarrabi A. An improved method for fabrication of Ag-GO nanocomposite with controlled anti-cancer and anti-bacterial behavior; a comparative study. Sci. Rep. 2019;9:9167. doi: 10.1038/s41598-019-45332-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Norhaslinda R., Noratiqah J.M., Amin B.A., Mohd R., Khalili A., Norhaslinda R., Noratiqah J.M. Quantitative and optimization of anthocyanin extracted from pomegranate (Punica granatum) extract by high-performance liquid chromatography (HPLC) Pharmacogn. Prelim. Phytochem. Investig. Jatrophae Curcatis Semen. 2018;10:650–653. doi: 10.5530/pj.2018.4.107. [DOI] [Google Scholar]
- 17.Solovchenko A., Yahia E.M., Chen C. Postharvest Physiology and Biochemistry of Fruits and Vegetables. Elsevier; 2019. Pigments; pp. 225–252. [DOI] [Google Scholar]
- 18.Burton-Freeman B., Sandhu A., Edirisinghe I. Nutraceuticals. Elsevier; 2016. Anthocyanins; pp. 489–500. [DOI] [Google Scholar]
- 19.Zhao X., Yuan Z., Yin Y., Feng L. Patterns of pigment changes in pomegranate (punica granatum l.) peel during fruit ripening. Acta Hortic. 2015;1089:83–89. doi: 10.17660/ActaHortic.2015.1089.9. [DOI] [Google Scholar]
- 20.Khaleghi M., Khorrami S., Ravan H. Identification of Bacillus thuringiensis bacterial strain isolated from the mine soil as a robust agent in the biosynthesis of silver nanoparticles with strong antibacterial and anti-biofilm activities. Biocatal. Agric. Biotechnol. 2019;18 doi: 10.1016/j.bcab.2019.101047. [DOI] [Google Scholar]
- 21.Nakuleshwar Dut Jasuja, Gupta Deepak Kumar, Mohtashim Reza S.C.J. Green Synthesis of AgNPs Stabilized with biowaste and their antimicrobial activities. Braz. J. Microbiol. 2014;1332:1325–1332. doi: 10.1590/s1517-83822014000400024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Edison T.J.I., Sethuraman M.G. Biogenic robust synthesis of silver nanoparticles using Punica granatum peel and its application as a green catalyst for the reduction of an anthropogenic pollutant 4-nitrophenol. Spectrochim. Acta - Part A Mol. Biomol. Spectrosc. 2013;104:262–264. doi: 10.1016/j.saa.2012.11.084. [DOI] [PubMed] [Google Scholar]
- 23.Kumar I., Mondal M., Sakthivel N. Elsevier; 2019. Green Synthesis of Phytogenic Nanoparticles, in: Green Synthesis, Characterization and Applications of Nanoparticles; pp. 37–73. [DOI] [Google Scholar]
- 24.Ahmed S., Kaur G., Sharma P., Singh S., Ikram S. Evaluation of antioxidant, antibacterial and anticancer (lung cancer cell line A549) activity of Punica granatum mediated silver nanoparticles. Toxicol. Res. (Camb). 2018;7:923–930. doi: 10.1039/C8TX00103K. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Allafchian A.R., Mirahmadi-Zare S.Z., Jalali S.A.H., Hashemi S.S., Vahabi M.R. Green synthesis of silver nanoparticles using phlomis leaf extract and investigation of their antibacterial activity. J. Nanostruct. Chem. 2016;6:129–135. doi: 10.1007/s40097-016-0187-0. [DOI] [Google Scholar]
- 26.Bulut E., Ozacar M. Rapid, facile synthesis of silver nanostructure using hydrolyzable tannin. Ind. Eng. Chem. Res. 2009;48:5686–5690. doi: 10.1021/ie801779f. [DOI] [Google Scholar]
- 27.Nisha M.H., Tamileswari R., Jesurani S.S., Kanagesan S., Hashim M., Alexander S.C.P. Green synthesis of silver nanoparticles from pomegranate (Punicagranatum) leaves and analysis of anti-bacterial activity. Int. J. Adv. Technol. Eng. Sci. 2015;4:1–8. [Google Scholar]
- 28.Danaei M., Dehghankhold M., Ataei S., Hasanzadeh Davarani F., Javanmard R., Dokhani A., Khorasani S., Mozafari M.R. Impact of particle size and polydispersity index on the clinical applications of lipidic nanocarrier systems. Pharmaceutics. 2018;10:1–17. doi: 10.3390/pharmaceutics10020057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nayak D., Ashe S., Rauta P.R., Kumari M., Nayak B. Bark extract mediated green synthesis of silver nanoparticles: evaluation of antimicrobial activity and antiproliferative response against osteosarcoma. Mater. Sci. Eng. C. 2016;58:44–52. doi: 10.1016/j.msec.2015.08.022. [DOI] [PubMed] [Google Scholar]
- 30.Chen F., Huang G. Antioxidant activity of polysaccharides from different sources of ginseng. Int. J. Biol. Macromol. 2019;125:906–908. doi: 10.1016/j.ijbiomac.2018.12.134. [DOI] [PubMed] [Google Scholar]
- 31.Dabbagh A., Res B.M.C., Dabbagh B., Al Elhaty I.A., Elhaw M., Murali C., Mansoori A.Al. Antioxidant and anticancer activities of chamomile (Matricaria recutita L.) BMC Res. Notes. 2019;12:1–8. doi: 10.1186/s13104-018-3960-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ersus S., Yurdagel U. Microencapsulation of anthocyanin pigments of black carrot (Daucus carota L.) by spray drier. J. Food Eng. 2007;80:805–812. doi: 10.1016/j.jfoodeng.2006.07.009. [DOI] [Google Scholar]
- 33.Zheng Chen, Riccardo Bertin G.F. EC50 estimation of antioxidant activity in DPPH assay using several statistical programs. Food Chem. 2013;138:414–420. doi: 10.1016/j.foodchem.2012.11.001. [DOI] [PubMed] [Google Scholar]
- 34.Guo C., Yang J., Wei J., Li Y., Xu J., Jiang Y. Antioxidant activities of peel, pulp and seed fractions of common fruits as determined by FRAP assay. Nutr. Res. 2003;23:1719–1726. doi: 10.1016/j.nutres.2003.08.005. [DOI] [Google Scholar]
- 35.Li Y., Guo C., Yang J., Wei J., Xu J., Cheng S. Evaluation of antioxidant properties of pomegranate peel extract in comparison with pomegranate pulp extract. Food Chem. 2006;96:254–260. doi: 10.1016/j.foodchem.2005.02.033. [DOI] [Google Scholar]
- 36.Azarpazhooh E., Sharayei P., Zomorodi S., Ramaswamy H.S. Physicochemical and phytochemical characterization and storage stability of freeze-dried encapsulated pomegranate peel anthocyanin and in vitro evaluation of its antioxidant activity. Food Bioprocess Technol. 2018;12:199–210. doi: 10.1007/s11947-018-2195-1. [DOI] [Google Scholar]
- 37.Gaikwad M., Kamble S., Kshirsagar R., Kulkarni A. Phytochemical analysis and anti-oxidant activity of Punica granatum L. Int. J. Curr. Res. Life Sci. 2018;07:2327–2330. [Google Scholar]
- 38.Mohammadizadeh F., Falahati-pour S.K., Rezaei A., Mohamadi M., Hajizadeh M.R., Mirzaei M.R., Khoshdel A., Fahmidehkar M.A., Mahmoodi M. The cytotoxicity effects of a novel Cu complex on MCF-7 human breast cancerous cells. BioMetals. 2018;31:233–242. doi: 10.1007/s10534-018-0079-5. [DOI] [PubMed] [Google Scholar]
- 39.Devanesan S., AlSalhi M.S., Balaji R.V., Ranjitsingh A.J.A., Ahamed A., Alfuraydi A.A., AlQahtani F.Y., Aleanizy F.S., Othman A.H. Antimicrobial and cytotoxicity effects of synthesized silver nanoparticles from Punica granatum peel extract. Nanoscale Res. Lett. 2018;13:315. doi: 10.1186/s11671-018-2731-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kaur P., Thakur R., Malwal H., Manuja A., Chaudhury A. Biosynthesis of biocompatible and recyclable silver/iron and gold/iron core-shell nanoparticles for water purification technology. Biocatal. Agric. Biotechnol. 2018;14:189–197. [Google Scholar]
- 41.Grigalius I., Petrikaite V. Relationship between antioxidant and anticancer activity of Trihydroxyflavones. Molecules. 2017;22:2169. doi: 10.3390/molecules22122169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dai J., Mumper R.J. Plant phenolics: extraction, analysis and their antioxidant and anticancer properties. Molecules. 2010;15:7313–7352. doi: 10.3390/molecules15107313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Khan G.N., Gorin M.A., Rosenthal D., Pan Q., Bao L.W., Wu Z.F., Newman R.A., Pawlus A.D., Yang P., Lansky E.P. Pomegranate fruit extract impairs invasion and motility in human breast cancer. Integr. Cancer Ther. 2009;8:242–253. doi: 10.1177/1534735409341405. [DOI] [PubMed] [Google Scholar]
- 44.N Syed D., Chamcheu J.-C., M Adhami V., Mukhtar H. Pomegranate extracts and cancer prevention: molecular and cellular activities. Anticancer Agents Med. Chem. 2013;13:1149–1161. doi: 10.2174/1871520611313080003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Naghma Khan, Syed Deeba N., Pal Harish Chandra, Hasan Mukhtar F.A. Pomegranate fruit extract inhibits UVB‐induced inflammation and proliferation by modulating NF‐κB and MAPK signaling pathways in mouse skin. Photochem. Photobiol. 2011:1–9. doi: 10.1111/j.1751-1097.2011.01063.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Negi P.S., Jayaprakasha G.K., Jena B.S. Antioxidant and antimutagenic activities of pomegranate peel extracts. Food Chem. 2003;80:393–397. doi: 10.1016/S0308-8146(02)00279-0. [DOI] [Google Scholar]
- 47.Park E.J., Yi J., Kim Y., Choi K., Park K. Silver nanoparticles induce cytotoxicity by a Trojan-horse type mechanism. Toxicol. In Vitro. 2010;24:872–878. doi: 10.1016/j.tiv.2009.12.001. [DOI] [PubMed] [Google Scholar]
- 48.Gudkov S.V., Guryev E.L., Gapeyev A.B., Sharapov M.G., Bunkin N.F., Shkirin A.V., Zabelina T.S., Glinushkin A.P., Sevost M.A., Belosludtsev K.N., Chernikov A.V., Bruskov V.I., Zvyagin A.V. Unmodified hydrated C 60 fullerene molecules exhibit antioxidant properties, prevent damage to DNA and proteins induced by reactive oxygen species and protect mice against injuries caused by radiation-induced oxidative stress. Nanomed. Nanotechnol., Biol. Med. 2019;15:37–46. doi: 10.1016/j.nano.2018.09.001. [DOI] [PubMed] [Google Scholar]
- 49.Cairns R.A., Harris I.S., Mak T.W. Regulation of cancer cell metabolism. Nat. Rev. Cancer. 2011;11:85–95. doi: 10.1038/nrc2981. [DOI] [PubMed] [Google Scholar]