Abstract
Context
We previously identified factors affecting thyroid status, including sex, age, and smoking.
Objective
In the current study, we increased the number of subjects examined and investigated the effects of these factors, particularly smoking and the thyroid peroxidase antibody (TPO-Ab), in Japanese patients with euthyroxinemia and serum free T4 levels within the normal range.
Participants
A total of 12,289 subjects who underwent health checkups were analyzed in a cross-sectional and longitudinal study.
Results
The mean age of subjects was 50 ± 10 years (age range: 21 to 88 years). Serum TSH levels and the prevalence of positivity for TPO-Ab increased with age in Japanese subjects with euthyroxinemia. Mean serum TSH levels were significantly lower in the smoking group than in the nonsmoking group except for women older than 50 years. Serum TSH levels were significantly higher in subjects with positivity for TPO-Ab than in those with negativity at all ages and in both sexes; however, smoking did not affect free T4 levels or positivity for TPO-Ab. Among men, the rate of smokers was significantly higher in patients with subclinical hyperthyroidism (25%) than in those with subclinical hypothyroidism (10%; P < 0.05). Furthermore, the results of the longitudinal study revealed a significant decrease in serum TSH levels 1 year after the start of smoking in men (P < 0.05).
Conclusion
Because smoking appeared to lower serum TSH levels in Japanese subjects with euthyroxinemia, their smoking status warrants careful consideration when evaluating subclinical thyroid function.
Keywords: smoking, serum TSH levels, Japanese
Recent studies showed that various factors, including age, sex, race, iodine intake, obesity, the thyroid peroxidase antibody (TPO-Ab), and/or smoking, influence thyroid status in men [1]. Regarding age and sex, our recent findings in a large number of patients, as well as those from several other studies, suggest that serum TSH levels increase with age in men and women [2–6]. This increase may be due to age-related alterations in the set point of serum TSH level against thyroid hormone level or reductions in the biological activity of serum TSH [5]. It may also be due to age-dependent increases in the prevalence of Hashimoto thyroiditis. In approximately 60% to 80% of patients with subclinical hypothyroidism (SCH), the disorder is associated with TPO-Ab, a marker of Hashimoto thyroiditis [7]. However, whether age-dependent increases are due to elevations in TPO-Ab levels in the iodine-rich country of Japan remains controversial.
Our previous findings indicated that smoking affects the prevalence of SCH [2]. Studies conducted in other countries also found that subnormal serum TSH concentrations were frequently observed in some healthy cigarette smokers [7–9]. Moreover, smoking has been reported to exert stimulatory as well as inhibitory effects on thyroid function and is a major risk factor for the development of thyroid disease. Graves disease, Graves ophthalmopathy, and thyroid hormone abnormalities have been linked to smoking [10, 11]. However, in Japan the effects of smoking on thyroid function, particularly in patients with euthyroxinemia, remain unknown.
In the current study, we increased the number of subjects analyzed and examined the effects of age, sex, TPO-Ab, and smoking status on thyroid function in subjects with serum free T4 level within the normal range. We concluded that smoking status should be considered when evaluating SCH and subclinical hyperthyroidism (SCT).
2. Subjects and Methods
A. Subjects
This was a cross-sectional, longitudinal follow-up study that included 15,660 Japanese subjects who underwent annual health checkups at Takasaki Hidaka Hospital between April 2003 and December 2015. Medical histories were reported by subjects via a self-administered questionnaire that included questions on health background and medication use as well as smoking habits. They were asked whether they currently do not smoke or are smokers. In the current study, a smoker was defined as a person who smoked at least one cigarette per day.
Among the 15,660 subjects, we excluded 3371 cases, including patients with any history of diseases such as Graves disease, liver cirrhosis, and renal failure; subjects currently taking medications such as levothyroxine, antithyroid drugs, insulin, and steroid hormones; and subjects with missing data.
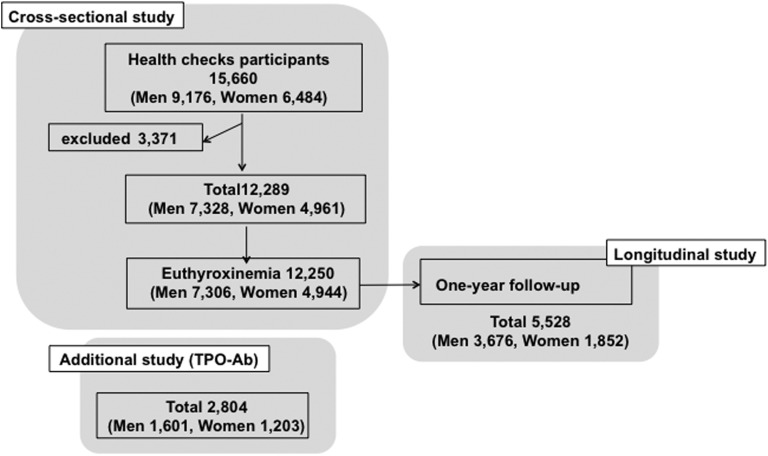
A total of 12,250 subjects had a free T4 level within the manufacturer’s normal reference range and were enrolled in the current study as subjects with euthyroxinemia. Furthermore, 2804 subjects who provided informed consent (i.e., 1601 men and 1203 women) underwent tests for TPO-Ab. A total of 5528 of 12,250 subjects with euthyroxinemia (i.e., 3676 men and 1852 women) revisited our hospital for annual health checkups and were followed up for 1 year (Fig. 1).
Figure 1.
Selection of subjects in this study. A total of 12,250 subjects had a free T4 level within the manufacturer’s normal reference range and were used in the current study as subjects with euthyroxinemia. Some subjects (1601 men and 1203 women) underwent tests for TPO-Ab. A total of 5528 of 12,250 subjects with euthyroxinemia, including 3676 men and 1852 women, revisited our hospital for annual health checkups and were followed up for 1 year
All subjects provided informed consent for this study, which was approved by the ethics committee of Hidaka Hospital.
All methods were performed in accordance with relevant guidelines and regulations, including ethical guidelines for Medical and Health Research Involving Human Subjects presented by the Ministry of Health, Labor and Welfare in Japan. This study was approved by the Ethics Committee on Human Research of Hidaka Hospital (approval number 3: Hidaka Hospital Human Genome Ethics Committee.). According to the ethical guidelines for Medical and Health Research Involving Human Subjects, in this research design, written informed consent is not necessarily required. Therefore, we widely disclosed the outline of our study and provided opportunities for disagreement. Since TPO-Ab measurement was not included in the health checkup, studies on underwent test for TPO-Ab were also approved by the Ethics Committee on Human Research of Hidaka Hospital (approval number 89: Hidaka Hospital Human Genome Ethics Committee), and each subject provided written informed consent.
B. Blood Test for Serum Thyroid Hormone and TSH Levels
Blood samples were collected from all subjects between 8:00 and 9:00 am after they had fasted for at least 11 hours. Plasma TSH and free T4 levels were measured using the following kits: TSH: Chemilumi ACS II, CLEIA (Siemens Healthcare Diagnostics, Inc., Shinagawa-ku, Tokyo, Japan) and free T4: Chemilumi E-FT4, CLEIA (Siemens Healthcare Diagnostics, Inc.). The manufacturers’ reference ranges were 0.4 to 4.0 mU/L and 0.8 to 1.9 ng/dL for serum TSH and free T4, respectively. Anti‒TPO-Ab was measured using a Serodia-AMC kit (FIJIREBIO INC., Tokyo, Japan).
C. Definitions of Thyroid Dysfunction
SCH was defined as a serum free T4 level within the reference range but a serum TSH level >4.0 mU/L. SCT was defined as a normal free T4 level but a TSH level <0.4 mU/L. Overt hyperthyroidism was defined as a free T4 level >1.9 ng/dL with a TSH level <0.4 mU/L, and overt hypothyroidism as a free T4 level <0.8 ng/dL with a TSH level >4.0 mU/L.
D. Statistical Analyses
All results were expressed as median or mean ± SD for continuous variables and as absolute numbers and relative percentages for categorical variables. Group comparisons were performed by ANOVA and the Student t test for normally distributed data or the Wilcoxon rank-sum test for nonnormally distributed data for continuous variables. The χ2 test was used for categorical variables. A multiple comparison was performed with the Dunnett test. A paired t test was performed to compare two observation variables. Multiple regression analyses were performed to examine the relationships between smoking and nonsmoking and also TPO-Ab positivity and negativity. A multivariate logistic regression analysis compared the percentage of smokers among subjects with SCT and SCH. All tests for significance and the resulting P values were two-sided, with a level of significance of 5%. Statistical analyses were performed using JMP 9.0.2 (SAS Institute Inc., Cary, NC). The estimated sample size was based on the prevalence of smokers among subjects grouped by various TSH levels, with a two-sided type 1 error of <5% and a power of 80%.
3. Results
A. Effects of Age and Sex on Baseline Serum TSH and Free T4 Levels and TPO-Ab Positivity in 12,250 Subjects With Euthyroxinemia
Because we previously reported the effects of age and sex on the prevalence of SCH, we herein increased the number of subjects analyzed and reexamined the effects of these factors on the presence of TPO-Ab in addition to obtaining more detailed information on serum TSH and free T4 levels. Table 1 shows serum TSH and free T4 levels in each decade of life in 12,250 euthyroid participants. The total number of subjects with euthyroxinemia was 12,250, with 7306 (60%) being male. The mean age (± SD) of subjects was 50 ± 10 years among men (from 21 to 88 years old) and 49 ± 10 years among women (from 21 to 88 years old).
Table 1.
Baseline Serum TSH and Free T4 Levels in Each Decade of Life in 12,250 Subjects With Euthyroxinemia
| TSH (mU/L) | FreeT4 (ng/mL) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Age Group (y) | n | Median | 2.5th | 97.5th | Mean | Median | 2.5 th | 97.5th | Mean |
| Men | |||||||||
| 21–30 | 83 | 1.5 | 0.4 | 4.3 | 1.7 ± 1.0 | 1.4 | 1.0 | 1.8 | 1.4 ± 0.2 |
| 31–40 | 1267 | 1.3 | 0.4 | 3.7 | 1.5 ± 0.8 | 1.3 | 1.1 | 1.7 | 1.3 ± 0.2 |
| 41–50 | 2464 | 1.4 | 0.5 | 4.0 | 1.6 ± 1.1 | 1.3 | 1.0 | 1.6 | 1.3 ± 0.2 |
| 51–60 | 2495 | 1.5 | 0.5 | 4.7 | 1.7 ± 1.3 | 1.3 | 1.0 | 1.6 | 1.3 ± 0.2 |
| 61–70 | 863 | 1.6 | 0.5 | 6.0 | 2.1 ± 1.7 | 1.2 | 1.0 | 1.6 | 1.3 ± 0.2 |
| 71– | 134 | 2.0 | 0.4 | 6.5 | 2.5 ± 1.5 | 1.2 | 1.0 | 1.6 | 1.2 ± 0.2 |
| Women | |||||||||
| 21–30 | 91 | 1.6 | 0.2 | 5.7 | 1.6 ± 1.4 | 1.2 | 0.9 | 1.6 | 1.2 ± 0.2 |
| 31–40 | 1059 | 1.5 | 0.4 | 4.8 | 1.8 ± 2.0 | 1.2 | 0.9 | 1.5 | 1.2 ± 0.2 |
| 41–50 | 1699 | 1.6 | 0.5 | 4.9 | 1.8 ± 1.3 | 1.2 | 0.9 | 1.5 | 1.2 ± 0.1 |
| 51–60 | 1490 | 1.8 | 0.5 | 5.4 | 2.0 ± 1.4 | 1.2 | 0.9 | 1.5 | 1.2 ± 0.2 |
| 61–70 | 516 | 2.0 | 0.7 | 7.6 | 2.6 ± 3.1 | 1.2 | 0.9 | 1.6 | 1.2 ± 0.2 |
| 71– | 89 | 1.8 | 0.6 | 7.9 | 2.4 ± 1.8 | 1.2 | 0.9 | 1.5 | 1.2 ± 0.2 |
2.5th and 97.5th indicate 2.5th and 97.5th percentiles, and n indicates number.
Table 1 shows the means, medians, and 95th percentiles of TSH and free T4 levels in each decade of life among subjects. Mean and median serum TSH levels increased with age in men and women. Serum TSH levels were higher in women in most age groups, except for those older than 71 years. The 97.5th percentiles of serum TSH levels also significantly increased with age, from 3.7 mU/L in 31- to 40-year-old men to 6.5 mU/L in men older than 71 years and from 4.8 mU/L in 31- to 40-year-old women to 7.9 mU/L in women older than 71 years.
Serum free T4 levels in men progressively decreased with age. The mean free T4 level was 1.4 ± 0.2 ng/dL (mean ± SD) among men aged 20 to 29 years and 1.2 ± 0.2 ng/dL among those older than 70 years. However, no significant changes were observed in women, with 1.2 ± 0.2 ng/dL among those aged 20 to 29 years and among those older than 70 years. Serum free T4 levels were significantly lower in women than in men at most ages, as reported previously [2]
Table 2 shows the serum TSH and free T4 levels in each decade of life in 2279 euthyroid subjects who underwent tests for TPO-Ab. Among subjects with euthyroxinemia, the percentage of TPO-Ab positivity also increased with age and was significantly higher in women than in men in most age groups (7.8% in 31- to 40-year-old women and 12.1% in 61- to 70-year-old women; 2.8% in 31- to 40-year-old men and 8.1% in 61- to 70-year-old men).
Table 2.
Baseline Serum TPO Antibody Level in Each Decade of Life in 2797 Subjects With Euthyroxinemia
| Age Group (y) | n | TSH (mU/L) | TPO-Ab | ||
|---|---|---|---|---|---|
| Median | 2.5th | 97.5th | (%) | ||
| Men | |||||
| 21–30 | 6 | 1.3 | 0.5 | 2.2 | N/A |
| 31–40 | 212 | 1.2 | 0.3 | 2.9 | 2.8 |
| 41–50 | 501 | 1.2 | 0.4 | 3.3 | 4.2 |
| 51–60 | 564 | 1.4 | 0.4 | 4.3 | 5.1 |
| 61–70 | 259 | 1.4 | 0.6 | 4.8 | 8.1 |
| 71– | 56 | 2.1 | 0.7 | 6.4 | N/A |
| Women | |||||
| 21–30 | 8 | 1.3 | 0.7 | 2.6 | N/A |
| 31–40 | 258 | 1.3 | 0.3 | 3.9 | 7.8 |
| 41–50 | 407 | 1.3 | 0.4 | 4.0 | 7.6 |
| 51–60 | 342 | 1.5 | 0.5 | 5.1 | 11.7 |
| 61–70 | 149 | 1.6 | 0.6 | 5.8 | 12.1 |
| 71– | 35 | 1.8 | 0.3 | 4.0 | 11.4 |
2.5th and 97.5th indicate 2.5th and 97.5th percentiles, and n indicates number.
Abbreviation: N/A, not available.
B. Overall Thyroid Status in Japanese Subjects
On the basis of the results on baseline thyroid function shown in Tables 1 and 2, the thyroid status of 12,289 subjects in this study are shown in Table 3. The overall prevalence of normal serum TSH and free T4 levels was 6966 (95.1%) in men and 4610 (92.9%) in women. The prevalence of SCH was 278 (3.8%) in men and 291 (5.9%) in women, and that of SCT was 62 (0.8%) in men and 44 (1.0%) in women.
Table 3.
Thyroid Status in All 12,289 Subjects
| Men | Women | |
|---|---|---|
| Number of subjects (%) | 7319 (100) | 4958 (100) |
| Overt hyperthyroidism | 10 (1.3) | 10 (0.2) |
| Subclinical hyperthyroidism | 62 (0.8) | 44 (1.0) |
| Normal thyroid function | 6966 (95.1) | 4609 (92.9) |
| Subclinical hypothyroidism | 278 (3.8) | 291 (5.9) |
| Overt hypothyroidism | 3 (0.1) | 4 (0.1) |
C. Effects of Smoking on Serum TSH and Free T4 Levels in Subjects With Euthyroxinemia
We examined the effects of smoking on serum TSH and free T4 levels in 12,250 subjects with euthyroxinemia. Table 4 shows the means, medians, and 95th percentiles of TSH and free T4 levels in each decade of life with or without smoking.
Table 4.
Effects of Smoking on Serum TSH and Free T4 Levels in 12,250 Subjects With Euthyroxinemia
| Age Group (y) | TSH (mU/L) | ||||||
|---|---|---|---|---|---|---|---|
| Nonsmoker | Smoker | Multivariatea (P Value) | |||||
| n | Median (2.5th, 97.5th) | Mean | n | Median (2.5th, 97.5th) | Mean | ||
| Men | |||||||
| 21–30 | 50 | 1.6 (0.2, 6.0) | 1.8 ± 1.1 | 33 | 1.1 (0.4, 3.7) | 1.4 ± 0.9 | <0.05 |
| 31–40 | 794 | 1.4 (0.5, 3.9) | 1.6 ± 1.0 | 473 | 1.2 (0.4, 2.9) | 1.3 ± 0.6 | <0.01 |
| 41–50 | 1616 | 1.5 (0.5, 4.5) | 1.7 ± 1.2 | 848 | 1.2 (0.5, 3.2) | 1.3 ± 0.7 | <0.01 |
| 51–60 | 1746 | 1.5 (0.5, 5.0) | 1.9 ± 1.2 | 749 | 1.3 (0.4, 4.1) | 1.5 ± 1.2 | <0.01 |
| 61–70 | 671 | 1.8 (0.6, 6.2) | 2.2 ± 1.8 | 192 | 1.4 (0.5, 4.3) | 1.6 ± 0.9 | <0.01 |
| 71– | 120 | 2.1 (0.4, 6.6) | 2.5 ± 1.6 | 14 | 1.8 (0.6, 3.7) | 1.9 ± 0.9 | 0.15 |
| Women | |||||||
| 21–30 | 75 | 1.6 (0.1, 5.9) | 2.0 ± 1.4 | 16 | 1.6 (0.5, 5.8) | 1.9 ± 1.3 | 0.97 |
| 31–40 | 924 | 1.6 (0.4, 4.9) | 1.9 ± 2.1 | 135 | 1.3 (0.5, 4.1) | 1.5 ± 0.9 | <0.01 |
| 41–50 | 1541 | 1.6 (0.5, 4.9) | 1.9 ± 1.3 | 158 | 1.4 (0.3, 4.2) | 1.6 ± 1.0 | <0.01 |
| 51–60 | 1355 | 1.8 (0.5, 5.4) | 2.1 ± 1.5 | 135 | 1.5 (0.5, 5.8) | 1.8 ± 1.5 | 0.30 |
| 61–70 | 491 | 2.0 (0.7, 8.1) | 2.6 ± 3.1 | 25 | 1.6 (0.4, 5.2) | 2.1 ± 1.4 | 0.18 |
| 71– | 86 | 2.1 (0.6, 8.0) | 2.4 ± 1.8 | 3 | N/A | N/A | N/A |
| Age Group (y) | FreeT4 (ng/mL) | ||||||
| Nonsmoker | Smoker | Multivariatea (P Value) | |||||
| n | Median (2.5th, 97.5th) | Mean | n | Median (2.5th, 97.5th) | Mean | ||
| Men | |||||||
| 21–30 | 50 | 1.4 (1.1, 2.0) | 1.4 ± 0.2 | 33 | 1.3 (1.0, 1.8) | 1.3 ± 0.2 | <0.05 |
| 31–40 | 794 | 1.3 (1.1, 1.6) | 1.3 ± 0.2 | 473 | 1.3 (1.0, 1.7) | 1.3 ± 0.2 | 0.06 |
| 41–50 | 1616 | 1.3 (1.0, 1.6) | 1.3 ± 0.2 | 848 | 1.3 (1.0, 1.6) | 1.3 ± 0.2 | 0.45 |
| 51–60 | 1746 | 1.3 (1.0, 1.6) | 1.3 ± 0.2 | 749 | 1.3 (1.0, 1.7) | 1.3 ± 0.2 | 0.38 |
| 61–70 | 671 | 1.2 (0.9, 1.6) | 1.3 ± 0.2 | 192 | 1.2 (0.9, 1.6) | 1.3 ± 0.2 | 0.38 |
| 71– | 120 | 1.3 (1.0, 1.6) | 1.3 ± 0.2 | 14 | 1.3 (1.0, 1.8) | 1.3 ± 0.2 | 0.48 |
| Women | |||||||
| 21–30 | 75 | 1.2 (0.9, 1.5) | 1.2 ± 0.2 | 16 | 1.2 (1.0, 1.5) | 1.2 ± 0.2 | 0.69 |
| 31–40 | 924 | 1.2 (0.9, 1.5) | 1.2 ± 0.1 | 135 | 1.2 (0.9, 1.6) | 1.2 ± 0.2 | 0.90 |
| 41–50 | 1541 | 1.2 (0.9, 1.5) | 1.2 ± 0.1 | 158 | 1.2 (0.9, 1.6) | 1.2 ± 0.2 | 0.30 |
| 51–60 | 1355 | 1.2 (0.9, 1.5) | 1.2 ± 0.2 | 135 | 1.2 (1.0, 1.5) | 1.2 ± 0.1 | 0.71 |
| 61–70 | 491 | 1.2 (1.0, 1.5) | 1.2 ± 0.2 | 25 | 1.2 (1.0, 1.5) | 1.3 ± 0.2 | 0.12 |
| 71– | 86 | 1.2 (0.9, 1.5) | 1.2 ± 0.2 | 3 | N/A | N/A | N/A |
Abbreviation: N/A, not available.
Adjusted for age.
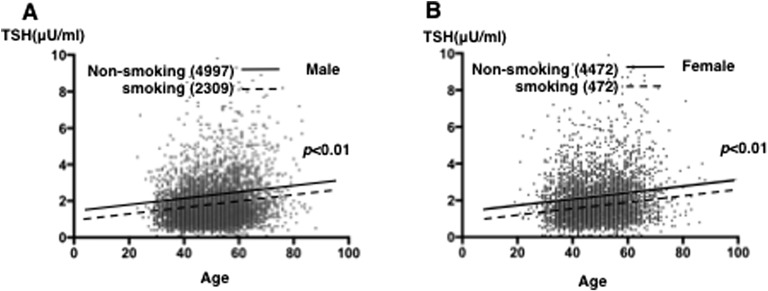
Mean and median serum TSH levels increased with age in smokers and nonsmokers; however, they were significantly lower in smokers than in nonsmokers among men and women in most age groups. Among 31- to 40-year-old men, the median 97.5th percentiles of TSH levels were 1.2 and 2.9 mU/L in smokers and 1.4 and 3.9 mU/L in nonsmokers (P < 0.01); among 61- to 70-year-old men, the median 97.5th percentiles of TSH levels were 1.4 and 4.3 mU/L in smokers and 1.8 and 6.2 mU/L in nonsmokers (P < 0.01). The multiple regression analysis revealed a difference in serum TSH levels at all ages in men and women, as shown in Fig. 2A and 2B.
Figure 2.
Effects of smoking on serum TSH and free T4 levels in subjects with euthyroxinemia. The relationship between age and serum TSH level in nonsmokers (solid line: 4997 men and 4472 women) and smokers (dotted line: 2309 men and 472 women) among (A) men and (B) women is shown. The line represents the linear regression fit to the data points calculated using JMP software (SAS Institute Inc.).
However, smoking had a negligible effect on serum TSH levels in women older than 50 years: among 31- to 40-year-old women, the levels were 1.3 mU/L in smokers and 1.6 mU/L in nonsmokers (P < 0.01); among 51- to 60-year-old women, the levels were 1.5 mU/L and 1.8 mU/L, respectively (P = 0.3). Furthermore, consistent with our previous findings, the current study confirmed that serum free T4 levels in men progressively decreased with age, whereas no significant change was observed in women (Tables 1 and 2) [2]. Smoking did not affect the relationship between age and serum free T4 levels in men or women, except for men in their 20s (Table 4).
E. Effects of Smoking on TPO-Ab
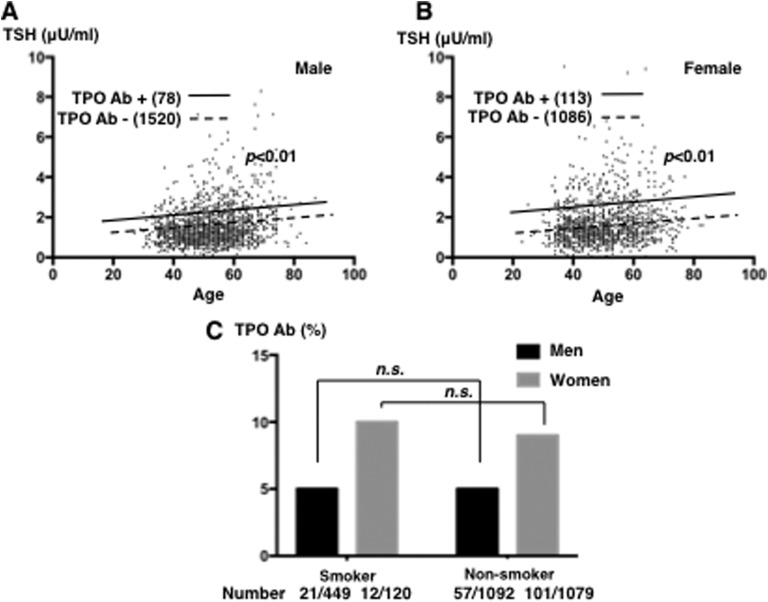
We evaluated the effects of smoking on the percentage of positivity for serum TPO-Ab. We examined the effects of the presence of serum TPO-Ab on age- and sex-dependent changes in serum TSH levels. As shown in Fig. 3A and 3B, serum TSH levels were significantly higher with TPO-Ab positivity in all decades of life among men and women (adjusted for age: P < 0.01 and P < 0.01, respectively). However, no significant differences were observed in the percentages of men and women with TPO-Ab positivity with or without smoking (the percentage of men with TPO-Ab positivity was 5% in both smokers and nonsmokers; the percentages in women were 10% and 9%, respectively (Fig. 3C).
Figure 3.
Effects of TPO-Ab on serum TSH and free T4 levels in subjects with euthyroxinemia. The relationship between age and serum TSH level in TPO-Ab‒positive (solid line: 78 men and 113 women) and TPO-Ab‒negative (dotted line: 1520 men and 1086 women) (A) men and (B) women among subjects with euthyroxinemia is shown. (C) The percentages of TPO-Ab‒positive smokers (21 of 449 men and 12 of 120 women) and nonsmokers (57 of 1092 men and 101 of 1079 women) are depicted. n.s., not significant.
F. Percentage of Smokers for Each Quartile of Serum TSH and Free T4 Levels and the Prevalence of SCH and SCT
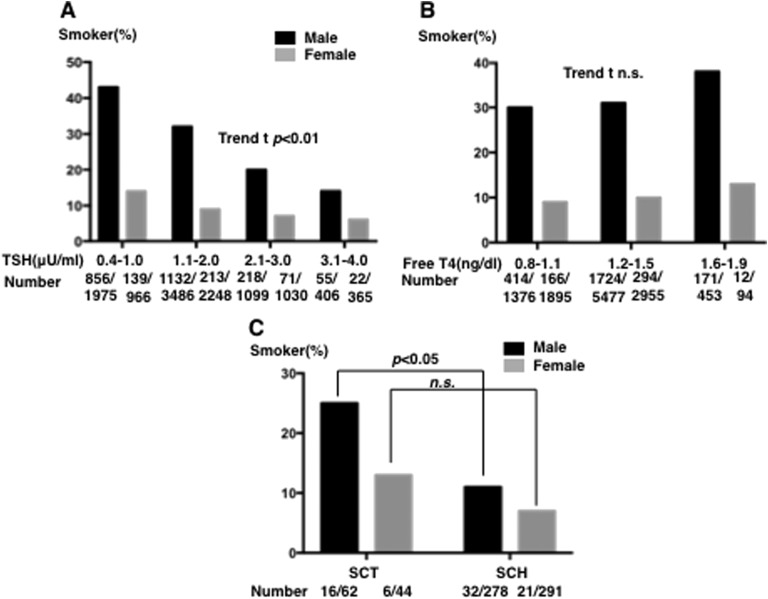
We investigated the relationships between sex, smoking for each quartile of TSH values, and free T4 level. As expected, when we examined serum TSH levels, which were within the normal range, the percentage of smokers was lower at higher serum TSH levels: 43% of smokers in the group with serum TSH levels between 0.4 and 1.0 mU/L and 15% in the group with serum TSH levels between 3.1 and 4.0 mU/L in men; t trend test (P < 0.01); 14% and 5%, respectively, in women (P < 0.01) (Fig. 4A).
Figure 4.
Effects of smoking on serum TSH and free T4 levels in subjects with euthyroxinemia. (A) Graph shows the percentage of smokers in each quartile of serum TSH groups within the normal TSH range (among men: 856 of 1975 in the group with serum TSH levels between 0.4 and 1.0 mU/L, 856 of 3488 with levels between 1.1 and 2.0 mU/L, 1132 of 1099 with levels between 2.1 and 3.0 mU/L, and 55 of 406 with levels between 3.1 and 4.0 mU/L; in women, the percentages were 139 of 966, 213 of 2249, 71 of 1030, and 22 of 365, respectively; t-trend: P < 0.01 and P < 0.01). (B) Graph shows the percentage of smokers in each quartile of serum free T4 groups within the normal free T4 range (in men: 414 of 1376 in the group with serum free T4 levels between 0.8 and 1.1 ng/dL, 1727 of 5477 between 1.2 and 1.5 ng/dL, and 171 of 453 between 1.6 and 1.9 ng/dL; in women, the percentages were 166 of 1896, 294 of 2955, and 12 of 94, respectively; t-trend: n.s. and n.s., respectively). (C) Graph comparing the prevalence of smokers among men with SCT and those with SCH (16 of 62 and 32 of 278, respectively) and among women with SCT and SCH (6 of 44 and 21 of 291, respectively). n.s., not significant.
Regarding serum free T4 levels, although higher levels were observed in smokers, no significant differences were noted in serum free T4 levels between smokers and nonsmokers in men and women (Fig. 4B).
We also investigated the percentage of smokers in patients with SCT and SCH. As shown in Fig. 4C, the multivariate logistic regression analysis showed the percentage of smokers in men was significantly higher among subjects with SCT than among those with SCH (the percentage of smokers was 25% with SCT and 10% with SCH; adjusted for age, P < 0.01), but not in women (SCT vs SCT, 12% vs 6%, respectively; not significant).
G. Longitudinal Study on the Effects of Smoking On-Off for 1 Year
In a longitudinal study, we examined the effects of smoking on serum TSH levels for 1 year. We defined stopped smoking as subjects who answered “smoking” in the first examination but “nonsmoking” in the next examination after 1 year and started smoking as subjects who answered “nonsmoking” in the first examination and “smoking” in the next examination after 1 year.
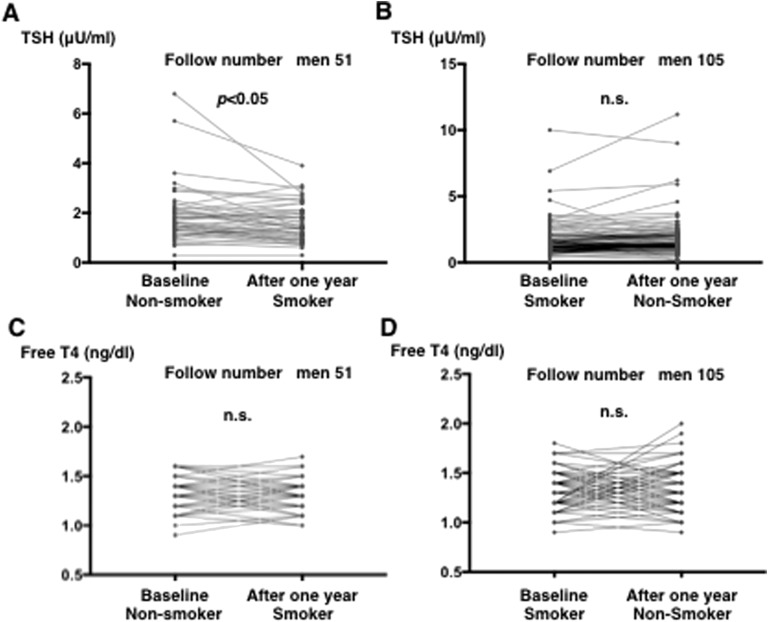
Among men, we analyzed 51 subjects who started smoking and 107 who stopped. As shown in Fig. 5A, serum TSH levels significantly decreased after 1 year of smoking (adjusted for age, P < 0.05). In contrast, no significant change was observed in serum TSH levels in men who stopped smoking for 1 year (Fig. 5B).
Figure 5.
Changes in serum TSH and free T4 levels in 1 y in subjects with euthyroxinemia who (A and C) started and (B and D) stopped smoking. In those who started to smoke in 1 y (51 men), serum TSH levels significantly decreased (adjusted for age, paired t; P < 0.05). (A and B) However, no significant change was observed in serum TSH levels after 1 y of smoking cessation (105). (C and D) No significant changes were noted in serum free T4 levels 1 y after starting or stopping smoking. n.s., not significant.
Moreover, no significant changes were noted in serum free T4 levels after starting or stopping smoking for 1 year (Fig. 5C and 5D).
3. Discussion
The effects of age, race, and sex on thyroid function have been extensively examined in iodine-deficient countries such as the United States (NHANES III, a population-based study) [3, 12, 13]. In the current study, the relationships between a number of factors (including age, sex, TPO-Ab, and smoking) and serum TSH levels in Japanese subjects with euthyroxinemia were examined. The results demonstrated that although serum TSH levels increased with age in men and women, they were higher in women than in men in all age groups. In addition, we found that smoking had significant effects on serum TSH levels in Japanese euthyroid subjects.
A similar increase in serum TSH level with age was previously reported in several countries, whereas SCH is more common in iodine-sufficient countries [7, 14] and occurs in 4% to 20% of the adult population. In the NHANES III study conducted in the United States, among a population negative for TPO-Ab, the 97.5th percentile was 3.56 mIU/L for the 20- to 29-year-old group, and it progressively increased to 7.49 mU/L in the group older than 80 years [3]. In another Japanese study, Takeda et al. [15] reported that among subjects who tested negative for thyroid antibodies, the 97.5th percentile of serum TSH level was 4.16 mU/L in those in their 20s and 30s, and it increased to 5.37 mU/L in subjects older than 70 years. However, it is important to note that both studies investigated men and women together, without considering sex-based differences.
In the current study, we analyzed men and women separately. Although we found that both sexes showed a significant increase in serum TSH levels with age, clear sex-based differences were noted: The 97.5th percentile of serum TSH levels was 3.8 mU/L in men aged 31 to 40 years and 8.6 mU/L in men older than 70 years, and it was 4.5 mU/L in women aged 31 to 40 years and 10.0 mU/L in women older than 70 years. Furthermore, it was clear that serum TSH levels were significantly higher in women than in men at all ages. Free T4 levels decreased with age in men, but not in women. Therefore, age-dependent increases in serum TSH levels may be due to age-dependent decreases in serum free T4 levels in men. However, it remains unclear why women did not show any changes in free T4 levels.
Because we found age-dependent decreases in free T4 levels in men, we investigated changes in TPO-Ab with aging. We found clear age-dependent increases in patients with TPO-Ab positivity. Similar increases were reported by Kasagi et al. [16], who showed that positivity for TPO-Ab increased with age: in men, 6.2% for those aged 40 to 50 years, 6.7% for those aged 50 to 60 years, and 11.7% in those older than 60 years vs in women, 13% for those aged 40 to 50 years, 16.2% for those aged 50 to 60 years, 14.9% for those older than 60 years. In the United States, NHANES III demonstrated that after adjustments for other characteristics, the odds of thyroid autoantibody positivity were higher with each year increase in age [OR (95% CI): 1.019 (1.017, 1.025)] and higher in females than in males [OR (95% CI): 2.0 (1.7, 2.3)] [12]. Therefore, the significant decrease observed in free T4 levels and increase in serum TSH levels may be due to a greater prevalence of Hashimoto thyroiditis.
In the current investigation, we found that smoking appeared to reduce serum TSH levels in a cross-sectional and longitudinal study over 1 year. This finding provided details on the relationship between smoking and serum TSH levels in an iodine-rich country, although these effects of smoking have been reported in an iodine-deficient country. The NHANES III survey revealed that the TSH distribution in active smokers shifted toward levels that were lower than those of nonsmokers [12]. This survey showed that smokers had TSH levels that were significantly lower than 4.5 mU/mL, with an OR (95% CI) of 0.6 (0.5, 0.8) after adjustments for age, sex, race-ethnicity, and iodine status [12]. The fifth Tromsø study in Norway also reported lower TSH levels in smokers than in nonsmokers (in males, 1.63 ± 0.88 mU/L vs 1.95 ± 1.04 mU/L; in females, 1.55 ± 0.86 mU/L vs 1.86 ± 1.01 mU/L, respectively) [17]. Our results also indicated the effects of smoking on lowering serum TSH levels, except in elderly women, for whom the effects may be due to menopause, differences in race, and/or insufficient sample size.
The mechanisms by which smoking lowers serum TSH levels remain unknown. There are several mechanisms by which smoking affects thyroid hormone levels. In the fifth Tromsø study, serum free T4 and free T3 levels were significantly higher in smokers than in nonsmokers [14.0 ± 2.2 pmol/L vs 13.4 ± 2.4 pmol/L for free T4 (P < 0.05) and 3.89 ± 0.79 pmol/L vs 3.72 ± 0.67 pmol/L for free T3 (P < 0.01); males and females analyzed together] [17]. The reduction in TSH levels may be secondary to the increase in serum free T3 and free T4 levels because a short exposure to cigarette smoke for just 1 hour increased serum freeT3 and freeT4 levels; however, this was not followed by a significant decrease in serum TSH levels (10). Therefore, smoking may increase thyroid hormone synthesis and release via TSH-independent pathways.
We also examined herein the effects of smoking on TPO-Ab but found no significant changes. NHANES III reported that smokers had 43% lower odds of having thyroid autoantibodies than nonsmokers [OR (95% CI): 0.57 (0.48, 0.67)] [12]. This discrepancy may be due to iodine intake, racial differences, or smaller sample sizes. Activation of the sympathetic nervous system may be involved via its innervation of the thyroid gland [10]. Furthermore, tobacco smoke contains toxins such as thiocyanate and 2,3-hydroxypyridine. Thiocyanate may be a goitrogen [11, 18]. Thiocyanate, which has a half-life of more than 6 days, inhibits iodide transport and organification as well as increasing the efflux of iodide from the gland. In the presence of an iodine deficiency, thiocyanate causes goiter. On the other hand, 2,3-hydroxypyridine inhibits thyroxine deiodination by limiting iodothyronine deiodinase activity [11, 19]. This effect may temporarily elevate serum thyroxine levels as a result of its deiodinase-altering activity before decreasing these levels [11, 20]. However, we did not observe any changes in free T4 levels between nonsmokers and smokers or in the longitudinal study for 1 year of smoking, even with significant decreases in serum TSH levels. Therefore, the mechanisms responsible for smoking-induced reductions in serum TSH levels remain controversial.
The results of the present and previous studies suggest that smoking influences the prevalence of subclinical thyroid diseases (such as SCH and SCT), as they are diagnosed on the basis of blood TSH levels. In our study, the incidence of SCH was lower in smokers than in nonsmokers, whereas the incidence of SCT was higher in the former. In the HUNT STUDY, the incidences of overt and subclinical hypothyroidisms in smokers were both reduced by half [21]. Furthermore, smoking was recently reported to directly influence thyroid function and gland volume even in healthy individuals. As for the relationship between smoking and autoimmune thyroid diseases, it has been suggested that smokers have a lower risk of Hashimoto disease and a higher risk of Graves disease. Their risks of thyroid carcinoma and nontoxic multinodular goiter are lower and higher, respectively [10]
The present results and previous findings collectively indicate that sex differences and smoking status should be considered when evaluating SCH and SCT in Japan.
A. Limitations
Several limitations of this study need to be considered when interpreting the results. Unlike a population-based cohort study, there was bias in the backgrounds of subjects in terms of economic status, as only 17% of subjects received financial support from mutual aid associations. We obtained information regarding smoking status by self-questionnaires only and did not evaluate the amount of smoking; therefore, we did not investigate the effects of heavy smoking on TSH levels. These results may not be extrapolated to countries with current or recent lower iodine intakes, where the increase in TSH level related to aging is less obvious.
Acknowledgments
We thank Dr. Santosh Sapkota for his assistance with the writing of this manuscript in English.
Glossary
Abbreviations:
- NHANES
National Health and Nutrition Examination Survey
- SCH
subclinical hypothyroidism
- SCT
subclinical hyperthyroidism
- TPO-Ab
thyroid peroxidase antibody
Additional Information
Disclosure Summary: The authors have nothing to disclose.
Data Availability: All data generated or analyzed during this study are included in this published article or in the data repositories listed in References.
References and Notes
- 1. Lago-Sampedro AM, Gutiérrez-Repiso C, Valdés S, Maldonado C, Colomo N, Almaraz MC, Rubio-Martín E, Morcillo S, Esteva I, Ruiz de Adana MS, Perez-Valero V, Soriguer F, Rojo-Martínez G, García-Fuentes E. Changes in thyroid function with age: results from the Pizarra population-based longitudinal study. Int J Clin Pract. 2015;69(5):577–587. [DOI] [PubMed] [Google Scholar]
- 2. Nakajima Y, Yamada M, Akuzawa M, Ishii S, Masamura Y, Satoh T, Hashimoto K, Negishi M, Shimomura Y, Kobayashi I, Andou Y, Mori M. Subclinical hypothyroidism and indices for metabolic syndrome in Japanese women: one-year follow-up study. J Clin Endocrinol Metab. 2013;98(8):3280–3287. [DOI] [PubMed] [Google Scholar]
- 3. Surks MI, Hollowell JG. Age-specific distribution of serum thyrotropin and antithyroid antibodies in the US population: implications for the prevalence of subclinical hypothyroidism. J Clin Endocrinol Metab. 2007;92(12):4575–4582. [DOI] [PubMed] [Google Scholar]
- 4. Canaris GJ, Manowitz NR, Mayor G, Ridgway EC. The Colorado thyroid disease prevalence study. Arch Intern Med. 2000;160(4):526–534. [DOI] [PubMed] [Google Scholar]
- 5. Bremner AP, Feddema P, Leedman PJ, Brown SJ, Beilby JP, Lim EM, Wilson SG, O’Leary PC, Walsh JP. Age-related changes in thyroid function: a longitudinal study of a community-based cohort. J Clin Endocrinol Metab. 2012;97(5):1554–1562. [DOI] [PubMed] [Google Scholar]
- 6. Boekholdt SM, Titan SM, Wiersinga WM, Chatterjee K, Basart DC, Luben R, Wareham NJ, Khaw KT. Initial thyroid status and cardiovascular risk factors: the EPIC-Norfolk prospective population study. Clin Endocrinol (Oxf). 2010;72(3):404–410. [DOI] [PubMed] [Google Scholar]
- 7. Cooper DS, Biondi B. Subclinical thyroid disease. Lancet. 2012;379(9821):1142–1154. [DOI] [PubMed] [Google Scholar]
- 8. Biondi B, Palmieri EA, Klain M, Schlumberger M, Filetti S, Lombardi G. Subclinical hyperthyroidism: clinical features and treatment options. Eur J Endocrinol. 2005;152(1):1–9. [DOI] [PubMed] [Google Scholar]
- 9. Biondi B, Cooper DS. The clinical significance of subclinical thyroid dysfunction. Endocr Rev. 2008;29(1):76–131. [DOI] [PubMed] [Google Scholar]
- 10. Wiersinga WM. Smoking and thyroid. Clin Endocrinol (Oxf). 2013;79(2):145–151. [DOI] [PubMed] [Google Scholar]
- 11. Kapoor D, Jones TH. Smoking and hormones in health and endocrine disorders. Eur J Endocrinol. 2005;152(4):491–499. [DOI] [PubMed] [Google Scholar]
- 12. Belin RM, Astor BC, Powe NR, Ladenson PW. Smoke exposure is associated with a lower prevalence of serum thyroid autoantibodies and thyrotropin concentration elevation and a higher prevalence of mild thyrotropin concentration suppression in the third National Health and Nutrition Examination Survey (NHANES III). J Clin Endocrinol Metab. 2004;89(12):6077–6086. [DOI] [PubMed] [Google Scholar]
- 13. Hollowell JG, Staehling NW, Flanders WD, Hannon WH, Gunter EW, Spencer CA, Braverman LE. Serum TSH, T4, and thyroid antibodies in the United States population (1988 to 1994): National Health and Nutrition Examination Survey (NHANES III). J Clin Endocrinol Metab. 2002;87(2):489–499. [DOI] [PubMed] [Google Scholar]
- 14. Teng W, Shan Z, Teng X, Guan H, Li Y, Teng D, Jin Y, Yu X, Fan C, Chong W, Yang F, Dai H, Yu Y, Li J, Chen Y, Zhao D, Shi X, Hu F, Mao J, Gu X, Yang R, Tong Y, Wang W, Gao T, Li C. Effect of iodine intake on thyroid diseases in China. N Engl J Med. 2006;354(26):2783–2793. [DOI] [PubMed] [Google Scholar]
- 15. Takeda K, Mishiba M, Sugiura H, Nakajima A, Kohama M, Hiramatsu S. Evaluated reference intervals for serum free thyroxine and thyrotropin using the conventional outliner rejection test without regard to presence of thyroid antibodies and prevalence of thyroid dysfunction in Japanese subjects. Endocr J. 2009;56(9):1059–1066. [DOI] [PubMed] [Google Scholar]
- 16. Kasagi K, Takahashi N, Inoue G, Honda T, Kawachi Y, Izumi Y. Thyroid function in Japanese adults as assessed by a general health checkup system in relation with thyroid-related antibodies and other clinical parameters. Thyroid. 2009;19(9):937–944. [DOI] [PubMed] [Google Scholar]
- 17. Jorde R, Sundsfjord J. Serum TSH levels in smokers and non-smokers: the 5th Tromsø study. Exp Clin Endocrinol Diabetes. 2006;114(7):343–347. [DOI] [PubMed] [Google Scholar]
- 18. Fukayama H, Nasu M, Murakami S, Sugawara M. Examination of antithyroid effects of smoking products in cultured thyroid follicles: only thiocyanate is a potent antithyroid agent. Acta Endocrinol (Copenh). 1992;127(6):520–525. [DOI] [PubMed] [Google Scholar]
- 19. Sugawara M, Park DL, Hershman JM. Antithyroid effect of 2,3-dihydroxypyridine in vivo and in vitro. Proc Soc Exp Biol Med. 1982;170(4):431–435. [DOI] [PubMed] [Google Scholar]
- 20. Fisher CL, Mannino DM, Herman WH, Frumkin H. Cigarette smoking and thyroid hormone levels in males. Int J Epidemiol. 1997;26(5):972–977. [DOI] [PubMed] [Google Scholar]
- 21. Åsvold BO, Bjøro T, Nilsen TI, Vatten LJ. Tobacco smoking and thyroid function: a population-based study. Arch Intern Med. 2007;167(13):1428–1432. [DOI] [PubMed] [Google Scholar]