Abstract
Cordyceps militaris is a type of fungus consumed by people all over the world and renowned for their nutritional benefits and herbal formulas to promote health and longevity. In the present study investigation was carried out to explore the therapeutic properties and neuroprotective effect of the C. militaris on ischemic brain neuronal injury, impairment of memory and learning in experimental rats induced by a global cerebral ischemia-reperfusion injury in WISTAR rats. Vascular Dementia with transient global brain injuries induced by a four-vessel occlusion (4-VO) in WISTAR rats. Further, donepezil (5 mg/kg) and C. militaris was (100 and 300 mg/kg, p.o.) were orally administered for 7 days in 4-VO WISTAR rats. C. militaris has the ability to improve memory impairments due to global cerebral ischemia and scopolamine-induced memory deterioration. Our present findings suggest that C. militaris may be a potential candidate for the neuroprotection of hippocampus and the recovery of various vascular dementia or neuroinflammatory disorders.
Keywords: Cordyceps militaris; Global cerebral ischemia, learning; Memory
1. Introduction
Cerebral ischemia is a condition in which there is a insufficient blood flow to the brain cells to meet their metabolic demand. This is mainly due to cerebral hypoxia or deficient oxygen supply leads to the death of cerebral infarction or ischemic stroke in brain cells. This irreversible damage in brain and loss of functions of neuron was reported previously. Such metabolic impairments significantly cause temporary amnesia involving severe memory loss, involving attention deficits, permanently impaired learning and memory, dementias and disorders of judgment. Vascular Dementia is mainly caused by poor supply of blood to the brain cells, typically caused by a series of minor strokes, leading to worsening cognitive decline. Generally, coexisting with Alzheimer’s disease, mixed neurodegenerative and vascular dementia involved in age-related cognitive impairment (Costantino, 2013). In brain, damage is most frequent in the mammillothalamic region, medial temporal lobes and the cerebellum, especially in the hippocampus region (Petito et al., 1987). Animal models of global cerebral ischemia, CA1 layer of the hippocampus, highly vulnerable to ischemia show that CA1 layer is accompanied by deficits in conduct of memory and learning tasks. If any delay in restoration of blood supply, brain damage may be permanent. 4-VO model has numerous advantages, including a high incidence of predictable ischemic neuronal damage and ease of performing the experiments. This model has been widely applied to elucidate the effect of the properties of the therapeutic agents. During cerebral ischemia, pro-inflammatory cytokines was released by the activation of glial cells (Xiang et al., 2016). Microglia in the brain consists of 5% to 20% population of total glial cell (Filiano et al., 2015). Inflammation is important as a response necrotic cell followed by the generation of reactive oxygen species (ROS) and lead to activation of microglia. Inflammation generates secondary injury which exerts an impact on chronic and acute ischemic injury and recovery of the function of the brain. Once ischemia happens, microglia can become destructive and phagocytes as well (Perry et al., 2010). Phagocytosis of cellular debris and harmful substances along with the release of anti-inflammatory cytokines, and consequently reduce the noxious effects of inflammation and aid in tissue repair.
Cordyceps species are important natural products with a variety of biological functions. C. militaris, is a parasite on moth caterpillar larvae, is used widely in East Asia as a traditional medicine (Wasser, 2002) and many kinds of biological effects such as, hepatoprotective, nephroprotective, antioxidative, anti-inflammatory and antiapoptotic effects have been reported (Yamaguchi et al., 2000). It was previously reported that, supplementation of Cordyceps sp. in mice model reduced inflammatory markers in the brain, especially hippocampus (Tianzhu et al., 2014). C. militaris contains various bioactive compounds such as, cordycepic acid, polysaccharide, amino acids, cordycepin and trace elements (Fan and Lin, 2013). The methanolic extract of C. militaris fruiting body showed antifungal, antibacterial, antioxidant, and anticancer properties. Therefore, the present study was undertaken to investigate the neuroprotective properties of C. militaris in in vivo condition by performing behavior test and to assess memory function. In addition, we estimated cell death and performed an immunohistochemical analysis of OX-42 marker in the hippocampus of rats. Our data suggest that C. militaris is a novel therapeutic agent which may provide neuroprotection and antiinflammation in mouse model.
2. Materials and methods
2.1. Materials
The chemicals, donepezil hydrochloride (±)-2-((1-benzylpiperidin-4-y) methyl)-5,6-dimethoxy-indan-1-one monohydrochloride: E2020) and scopolamine hydrobromide was purchased from Eisai Co., Ltd., Tokyo, Japan. It was dissolved in double distilled water and diluted with at desired concentrations. All other chemicals/materials were commercial grade with high purity.
2.2. Induction of ischemia
In the present study, transient forebrain ischemia was induced as suggested by Pulsinelli et al. (1982). In brief, under 1.5–2.0% isoflurane anesthesia (30%O2/70% N2O), vertebral arteries were electrocauterized carefully and further common carotid arteries were exposed. After 24 h, cerebral ischemia was induced in vertebral arteries and carotid arteries using aneurysm clips. After 10 min of this treatment, the aneurysm clips were carefully removed for reperfusion. Then samples were dissolved in double distilled water and orally administered twice at 100 mg/kg and 300 mg/kg for 0 and 90 min after reperfusion. The experimental rats in the vehicle-treated group were administered orally with double distilled water. Same surgical procedures were followed for Sham-operated animals and arteries were not occluded in this group. In another group (Ischemia + donepezil) administered 5 mg/kg donepezil per day. The dosing frequency and dosage of donepezil were selected as suggested previously by Kamat et al. (2010).
2.3. Brain tissue preparation
Brain tissue was prepared and was followed as per the previous publications (Pulsinelli and Brierley, 1979).
2.4. Cresyl violet staining
After 4 days of ischemia surgery, cresyl violet was used to stain cells (Burnett et al., 1987).
2.5. Immunohistochemistry
The brains tissue was sectioned using free floating method as described earlier (Briones et al., 2011). The primary antibody was applied against NeuN for overnight at 4 °C. Then secondary antibodies were rinsed and slide was prepared (Towfighi et al., 1997).
2.6. Microglia counts: Strain difference
CM exhibited more numbers activated microglia than that of control. The strain difference was evaluated in the histologically normal peri-infact region of the cortex surrounding the infarct.
2.7. Morris water maze experiment
Morris water maze experiment is widely used to explore the memory and spatial learning in animal models, including rats (Mao et al., 2018). The size of the water maze was 30 cm deep, 60 cm high, 136 cm diameter and circular in shape (Vorhees and Williams, 2006). The time it took the animal to find the platform represented the amount of learning and memory and was documented as described previously (Li et al., 2011).
2.8. Passive avoidance test
In the present study, passive avoidance test was carried out using the method suggested by Pitchaimani et al. (2012). Data represents mean ± SEM (n = 10).
2.9. Statistical analysis
One way ANOVA was used to test the significance of variation in the experiments. Two-way ANOVA was carried out to find the latency time in Morris water maze. The significance of variation was calculated at p < 0.05 level.
3. Results
3.1. Neuroprotection (cell density)
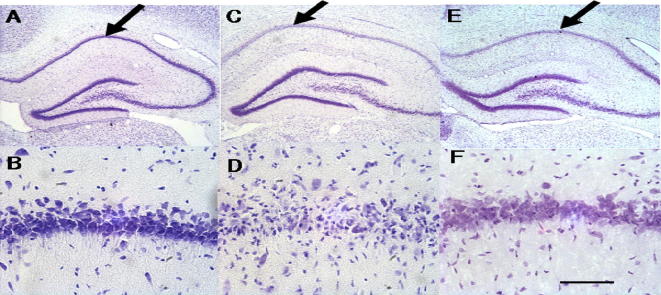
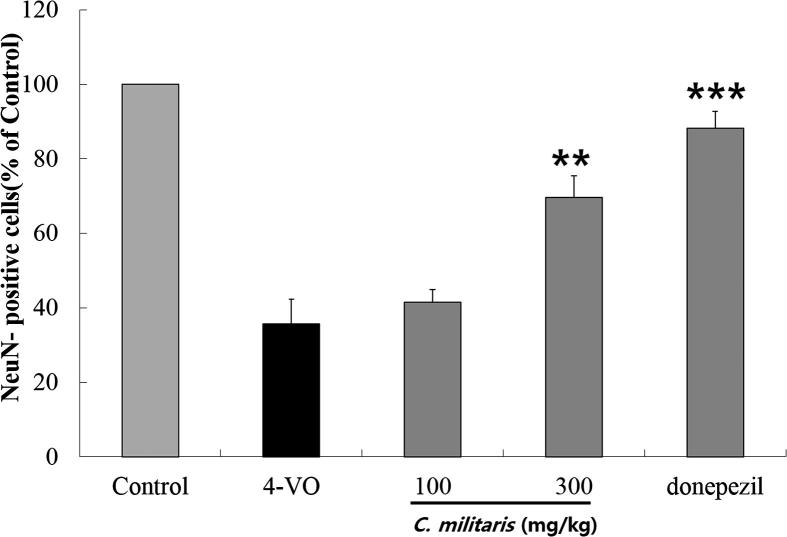
The images of rat hippocampal tissue which was stained with crezyl violet were described in Fig. 1. The surviving pyramidal neurons in the CA1 region of the hippocampal tissue were counted and the neuronal damage was clearly evaluated. Fig. 2 shows the neuronal cell density of hippocampal tissue of the experimental rats. In the present study, cell density was significantly reduced in the CA1 region in vehicle-treated group in the experimental animal and the cell density was 36.4 ± 9.1 cells/mm2. The sham-operated group consists of 280.7 ± 21.6 cells/mm2 in the CA1 region. Variable neurons have intact morphology and round in shape. However morphological changes were observed in the damaged neurons (Fig. 1B and D). In our study, C. militaris showed significant neuroprotective effect and increased cell density in brain tissue. A dose dependent inhibition against the cell density reduction was observed in our study and 78.4% inhibition was registered (p < 0.001) C. militaris in experimental rats at 300 mg/kg concentration. In the sham-operated group, a weak NeuN immunoreactivity was observed in all the group (Fig. 3). In the present study, NeuN-labeled cells was reduced significantly (35.7 ± 6.7 cells/mm2; p < 0.05) (Fig. 2). The number of NeuN-positive neurons increased by 52.7% in 300 mg/kg C. militaris treated (69.6 ± 5.9 cells/mm2) and by 81.7% increase was recorded in donepezil treated group (88.3 ± 4.5 cells/mm2) compared to 4-VO group (35.7 ± 6.7 cells/mm2).
Fig. 1.
Cresyl violet staining of the hippocampus in normal (A and B), negative control. (C and D, vehicle-treated), 300 mg/kg C. militaris – treated groups (E and F) one day after ischemia.
Fig. 2.
Number of surviving neurons in the hippocampal CA1 region after 10 min of ischemia followed by 7 days of reperfusion. The values are given as means ± SEM. ** p < 0.01, *** p < 0.001 for Group 4-VO Group.
Fig. 3.
Quantification of the number of NeuN levels in the CA1 region of vehicle, 300 mg/kg C. militaris treated ischemic groups. NeuN levels in ischemia C.militaris treated ischemic groups are lower than that in the corresponding vehicle-treated ischemic group, respectively (n = 7). The values are given as means ± SEM.** p < 0.01, ***p < 0.001 for 4-VO Group.
3.2. Microglia (OX-42)
In the present study, rat brain was sectioned and C. military significantly inhibited CA1 microglia activation (Fig. 4). In the experimental group animals, CA1 area of OX-42 showed significant morphological changes. In sham-operated group, no microglial cells were found (Fig. 4A and B). Activated microglia was evident in CA1 of hippocampus after seven days of global cerebral ischemia with the greatest density of cells being within the hippocampus (Fig. 4C, D). However, C. militaris reduced this activated microglia (Fig. 4E, F). Also, activated microglia had short projections, more distinct and densely stained cell bodies compared with resting microglia in 4-VO group.
Fig. 4.
Representative photomicrographs of OX-42 immunostaining in the hippocampus CA1 subregion after 10 min of ischemia. (A, B) Normal control CA1 area contained weakly OX-42-positive cells. After 10 min of ischemia, these cells became strongly immunoreactive at one day, (C, D) The immunoreactivity peaked at 7 days, (E, F) OX-42-positive cells were also significantly increased in ischemic animals with C. militaris deficiency in comparison to the sham groups.(D) The cells at 7 days after ischemia transformation to the reactive form. (F) The positive cells at 7 days showed rod-like nuclei counterstained by DAB. Scale bar = 100 μm. Ox-42.
3.3. Behavior test
Morris water maze test was carried out to evaluate the behavior changes in rats. This test revealed that 4-VO group showed prolonged escape latency than sham operation group. Whereas, C. militaris shortened the path length and the result was statistically significant (***p < 0.001); and swimming speed was not affected. In the probe trial, the swimming times within the target quadrant in the C. militaris (100, 300 mg/kg) experimental group were higher than those in the vehicle-treated control rats (*P < 0.05; Fig. 5). The present finding suggested that 4-VO administered experimental rats treated with C. militaris performed well compare with the sham-operated rats. However, the 4-VO experimental animals treated with vehicle performed very poorly to reach this task. In day 4, the C. militaris and donepezil- treated experimental group showed the shorter mean latency (12.2 ± 0.7 s, 10.6 ± 1.6 s), however the vehicle-treated experimental animals required greater time to locate the pedestal (29.7 ± 7.6 s). The experimental rats spent time within the 10 cm diameter circle where the pedestal had been located during acquisition training is represented (Fig. 5B). In the present study, significant difference among the groups such 4-VO showed less time on target (1.3 ± 0.1 s) than that of sham-groups (3.1 ± 1.2 s) or C. militaris - treated experimental animals (2.3 ± 0.4 s). In our study, probe testing on day 5 of experiments showed a pattern of greater time spent the rats in the quadrant where the pedestal was earlier located by the C. militaris - treated animal groups. C. militaris administered rats at 100 and 300 mg/kg prolonged the shortened swimming distance in the provided platform quadrant and significantly increased the frequency of the rats crossing the target quadrant with 4-VO group. The present finding revealed that donepezil and C. militaris significantly improve the spatial memory ability of 4-VO group.
Fig. 5.
Effects of C. militaris training trials (A) and in the probe trial (B) of the Morris water maze task in global cerebra ischemia –induced memory deficits rats.
In the present study, latency times were varied significantly among the experimental groups in acquisition trials. C. militaris (100, 200, 300 mg/kg) and donepezil (5 mg/kg) administered in scopolamine-induced amnesia in rats showed a significant reduction in transfer latency and a significant increase in escape latency. All the treatment groups are compared with scopolamine-induced amnesia, normal control group. Fig. 6. The escape latency time was 24.27 ± 2.84 s in the sham group, 10.74 ± 0.86 sec in the scopolamine-induced group, 11.89 ± 1.13 s in the scopolamin- induced group and 100 mg/kg C. militaris- treated group, 12.29 ± 1.47 s in the scopolamin- induced group and 200 mg/kg C. militaris- treated group, 21.47 ± 3.72 s in the scopolamin- induced group and 300 mg/kg C. militaris- treated group, 22.09 ± 4.33 s in the scopolamin- induced group and 5 mg/kg donepezil – treated rats. These findings showed that short-term memory was severely affected by scopolamine, and the C. militaris treatment alleviated the short-term memory impairment. The shorter step-through latencies induced by scopolamine were decreased by C. militaris (100, 200, 300 mg/kg) and the variation was statistically significant (P < 0.05).
Fig. 6.
Effects of C. militaris with scopolamine in the passive avoidance task.
4. Discussion
This is the first report on the neuroprotective and therapeutic effect of Cordyceps militaris in mice model. In this study, C. militaris reduced neuronal cell death, spatial memory loss on Morris water maze after 4-VO in rats after seven days of treatment. Analyses of hippocampal region and neuron count in the CA1 region of hippocampus gave neuroprotection of C. militaris after 4-VO treatment. Cerebral ischemia in animals causes apoptosis, chromatin condensation, DNA fragmentation, cell shrinkage and membrane damage (Sekerdag et al., 2018). Apoptosis is the major pathway of cell death after cerebral ischemia. The 4-VO technique was effective to study neuronal damage in the hippocampus and was reported previously by various research groups. In our study, cell density was reduced significantly in the CA1 region in vehicle-treated group compare with sham-operated group after seven days of treatment in the experimental rats. This result clearly suggests that C. militaris has significant neuroprotective effects caused by cerebral ischemia.
The observed neuroprotective effect in C. militaris is good agreement with earlier studies (Hwang et al., 2008). In rats, the mycelial extract of Cordyceps ophioglossoides prevented decreased β-amyloid peptide-induced memory deficits and neuronal death. In another study, Cordyceps sinensis extract showed antioxidant properties and severely inhibited lipid peroxidation (Kreutzberg, 1996). It was also reported that the majority of neurons are positive to NeuN, hence NeuN immunoreactivity was frequently used as a marker to differentiate neurons in tissue sections and to evaluate the neuron/glia ratio in brain regions (Herculano-Houzel and Lent, 2005). NeuN is expressed in the nucleus and cytoplasm of mature neurons as a transcription factor (Mullen et al., 1992). There is a complicated factor including microglia, neurophils, monocytes, astrocytes and neurons in brain inflammation (Jeong et al., 2013). It was previously reported that the activated microglia was caused by 4-VO in brain and cause neuronal cell death in CA1 region. Cytokines, especially IL-1 beta and TNF alpha and adhesion molecules were involved in the maintenance and propagation of CNS inflammatory response. In central nervous system, microglia acts as an important form of active immune defense and the activated microglia secret various cytotoxic substances. Mabuchi et al. (2000) reported the involvement of microglia in the development of the ischemic area in experimental rats. The results of this study, observed effect are related to anti-inflammatory activity as indicated by a reduction in microglia activation using immunohisto chemical techniques (Sulkowski et al., 2002).
In the present study, the marker, OX-42 was observed in the vehicle-treated animals and this was considerably reduced in C. militaris treated group. Also, C. militaris significantly reduced the activity of microglia. This finding was consistent with previous studies showing reduction in glial activation and protection against ischemic brain damage by targeting inflammatory response (Yrjänheikki et al., 1998).
4-VO group treated with C. militaris showed reductions in the ischemia-induced damage measured in the hippocampus region, and these rats showed passive avoidance and water maze performances. C. militaris has potential pharmaceutical properties and could be the alternatives in health management to treat neurological disorders. Because of high cost to develop novel synthetic drugs, more attention has been paid in recent years on natural products. These natural sources proved to be more effective and safe with little or no side effects. Cordyceps militaris could effectively be used as a functional food (Yue et al., 2008). In the present study increase of OX-42 and immunoreactivity were observed in vehicle-treated group however, decreased effect was observed in C. militaris treated group. C. militaris extract significantly reduced the expressions of microglia because of the presence of bioactive compounds.
5. Conclusion
In conclusion, the present finding shows that C. militaris has various biological properties. The bioactivity of the fungus protected delayed neuronal death in the CA1 region of the hippocampus in rats. The sample involved in inhibition of OX-42 expression because of its anti-inflammatory properties and improve memory impairments due to global cerebral ischemia and scopolamine-induced memory deterioration. Our present findings suggest that C. militaris may be a potential therapeutic candidate for the neuroprotection of hippocampus and the recovery of function in the hippocampus in patients with global ischemia, such as various neuroinflammatory disorders or vascular dementia.
Conflict of interest
None of the authors declared conflict of interest
Footnotes
Peer review under responsibility of King Saud University.
Contributor Information
Young Ock Kim, Email: kyo9128abcd@gmail.com.
Gasem Mohammad Abu-Taweel, Email: gmabutaweel@iau.edu.sa.
Gi-Ho Sung, Email: sung97330@gmail.com.
References
- Briones T.L., Rogozinska M., Woods J. Modulation of ischemia-induced NMDAR1 activation by environmental enrichment decreases oxidative damage. J. Neurotrauma. 2011;28:2485–2492. doi: 10.1089/neu.2011.1842. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Burnett R.A., Brown I.L., Findlay J. Cresyl fast violet staining method for Campylobacter like organisms. J. Clin. Pathol. 1987;40(3):353. doi: 10.1136/jcp.40.3.353-b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costantino L. Costantino The pathobiology of vascular dementia 2013. Neuron. 2013;80:844–866. doi: 10.1016/j.neuron.2013.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sekerdag Emine, Solaroglu Ihsan, Gursoy-Ozdemir Yasemin. Cell death mechanisms in stroke and novel molecular and cellular treatment options. Curr. Neuropharmacol. 2018 doi: 10.2174/1570159X16666180302115544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan H.T., Lin H.S. Advances on Cordyceps militaris constituents and pharmacological effect. China J. Chinese Mater. Med. 2013;38:2549–2551. [PubMed] [Google Scholar]
- Filiano A.J., Gadani S.P., Kipnis J. Interactions of innate and adaptive immunity in brain development and function. Brain Research. 2015;1617:18–27. doi: 10.1016/j.brainres.2014.07.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herculano-Houzel S., Lent R. Isotropic fractionator: A simple, rapid method for the quantification of total cell and neuron numbers in the brain. J. Neurosci. 2005;25(10):2518–2521. doi: 10.1523/JNEUROSCI.4526-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeong Hey-Kyeong, Ji Kyungmin, Min Kyungjin, Joe Eun-Hye. Brain inflammation and microglia: Facts and misconceptions. Exp. Neurobiol. 2013;22(2):59–67. doi: 10.5607/en.2013.22.2.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang I.K., Lim S.S., Yoo K.Y., Lee Y.S., Kim H.G., Kang I.J., Kwon H.J., Park J., Choi S.Y., Won M.H. A phytochemically characterizedextract of Cordyceps militaris and cordycepin protect hippocampal neurons from ischemic injury in gerbils. Planta Med. 2008;74(2):114–119. doi: 10.1055/s-2008-1034277. [DOI] [PubMed] [Google Scholar]
- Yrjänheikki Ju.ha., Keinänen Riitta, Hökfelt Milla PellikkaTomas, Koistinaho Jari. Tetracyclines inhibit microglial activation and are neuroprotective in global brain ischemia. Proc. Natl. Acad. Sci. USA. 1998;95(26):15769–15774. doi: 10.1073/pnas.95.26.15769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamat P.K., Tota S., Saxena G., Shukla R., Nath C. Okadaic acid (ICV) induced memory impairment in rats: a suitable experimental model to test anti-dementia activity. Brain Res. 2010;1309:66–74. doi: 10.1016/j.brainres.2009.10.064. [DOI] [PubMed] [Google Scholar]
- Kreutzberg G.W. Microglia: a sensor for pathologicalevents in the CNS. Trends Neurosci. 1996;19(8):312–318. doi: 10.1016/0166-2236(96)10049-7. [DOI] [PubMed] [Google Scholar]
- Li L.-X., Cheng Y.-F., Lin H.-B., Wang C., Xu J.-P., Zhang H.-T. Prevention of cerebral ischemia-induced memory deficits by inhibition of phosphodiesterase-4 in rats. Metab. Brain Dis. 2011;26(1):37–47. doi: 10.1007/s11011-011-9235-0. [DOI] [PubMed] [Google Scholar]
- Mao Li-Min, Faris Hunter J., Wang John Q. Muscarinic acetylcholine receptors inhibit fyn activity in the rat striatum in vivo. J. Mol. Neurosci. 2018:1–10. doi: 10.1007/s12031-018-1053-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mabuchi T., Kitagawa K., Ohtsuki T., Kuwabara K., Yagita Y., Yanagihara T., Hori M., Matsumoto M. Contribution of microglia/macrophages to expansion of infarction and response of oligodendrocytes after focal cerebral ischemia in rats. Stroke. 2000;31(7):1735–1743. doi: 10.1161/01.str.31.7.1735. [DOI] [PubMed] [Google Scholar]
- Mullen R.J., Buck C.R., Smith A.M., Neu N. A neuronal specific nuclear protein in vertebrates. Development. 1992;116:201–211. doi: 10.1242/dev.116.1.201. [DOI] [PubMed] [Google Scholar]
- Perry V.H., Nicoll J.A., Holmes C. Microglia in neurodegenerative disease. Nat. Rev. Neurol. 2010;6:193–201. doi: 10.1038/nrneurol.2010.17. [DOI] [PubMed] [Google Scholar]
- Petito C.K., Feldmann E., Pulsinelli W.A., Plum F. Delay edhippocampal damage in humans following cardiorespiratory arrest. Neurology. 1987 Aug;37(8):1281–1286. doi: 10.1212/wnl.37.8.1281. [DOI] [PubMed] [Google Scholar]
- Pitchaimani V., Arumugam S., Thandavarayan R.A., Thiyagarajan M.K., Aiyalu R., Sreedhar R. Nootropic activity of acetaminophen against colchicine induced cognitive impairment in rats. J. Clin. Biochem. Nutr. 2012;50:241–244. doi: 10.3164/jcbn.11-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pulsinelli W.A., Brierley J.B., Plum F. Temporal profile of neuronal damage in a model of transient forebrain ischemia. Ann. Neurol. 1982;11(5):491–498. doi: 10.1002/ana.410110509. [DOI] [PubMed] [Google Scholar]
- Pulsinelli, W.A., Brierley, J.B., 1979. A new model of bilateral hemispheric ischemia in the unanesthetized rat Stroke. 10 (3), 267–272. [DOI] [PubMed]
- Sulkowski G., Bubko I., Struzyńska L., Januszewski S., Walski M., Rafałowska U. Astrocytic response in the rodent model of global cerebral ischemia and during reperfusion. Exp. Toxicol. Pathol. 2002;54(1):31–38. doi: 10.1078/0940-2993-00229. [DOI] [PubMed] [Google Scholar]
- Tianzhu Z., Shihai Y., Juan D. Antidepressant-like effects of cordycepin in a mice model of chronic unpredictable mild stress. Evid. Based Complement Alternat. Med. 2014 doi: 10.1155/2014/438506. Epub 2014 Dec 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towfighi J., Mauger D., Vannucci R.C., Vannucci S.J. Influence of age on the cerebral lesions in an immature rat model of cerebral hypoxia-ischemia: a light microscopic study. Brain Res. Dev. Brain Res. 1997;100:149–160. doi: 10.1016/s0165-3806(97)00036-9. [DOI] [PubMed] [Google Scholar]
- Vorhees C.V., Williams M.T. Morris water maze: procedures for assessing spatial and related forms of learning and memory. Nat. Protoc. 2006;1(2):848–858. doi: 10.1038/nprot.2006.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wasser S.P. Medicinal mushrooms as a source of antitumor and immunomodulating polysaccharides. Appl. Microbiol. Biotechnol. 2002;60(3):258–274. doi: 10.1007/s00253-002-1076-7. [DOI] [PubMed] [Google Scholar]
- Xiang Y., Zhao H., Wang J., Zhang L., Liu A., Chen Y. Inflammatory mechanisms involved in brain injury following cardiac arrest and cardiopulmonary resuscitation. Biomed. Rep. 2016;5:11–17. doi: 10.3892/br.2016.677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi Y., Kagota S., Nakamura K., Shinozuka K., Kunitomo M. Antioxidant activity of the extracts from fruiting bodies of cultured Cordyceps sinensis. Phytother. Res. 2000;14:647–649. doi: 10.1002/1099-1573(200012)14:8<647::aid-ptr670>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- Yue G.-G., Lau C.-B., Fung K.-P., Leung P.-C., Ko W.-H. Effects of cordyceps sinensis, cordyceps militaris and their isolated compounds on ion transport in calu-3 human airway epithelial cells. J. Ethnopharmacol. 2008;117:92–101. doi: 10.1016/j.jep.2008.01.030. [DOI] [PubMed] [Google Scholar]