Abstract
Experimental based evidence suggests that most of the medicinal plants possess wide-ranging pharmacological and biological activities that may possibly use in treatment of inflammation-related diseases. The current study was aimed to explore the acute toxicity, analgesic, sedative and antipyretic activities of Monotheca buxifolia and Bosea amherstiana in mices. In vivo experimental models were used in this study. Acute toxicity was evaluated for 24 h’ interval at concentration of 500, 1000, 1500 and 2000 mg/kg. The analgesic activity was estimated by acetic acid induced writhing test. White wood apparatus enclosed in stainless steel was used for sedative experiment and antipyretic activity was evaluated in brewer’s yeast induced hyperthermic at 50, 100 and 150 mg/kg i.p. Both plants were found safe at all tested doses. Monotheca buxifolia and Bosea amherstiana dose-dependently reduced abdominal constrictions in mice. Both plants exhibited significant (P < 0.0001) sedative effects in dose of 50, 150 and 150 mg/kg. Both plants markedly (P < 0.0001) reduced yeast induced hyperthermia. The inhibitions were dose-dependent and remained significant up to five hours of administration. These investigational results have linked a pharmacological indication for the traditional claim of the drugs to be used as an anti-inflammatory, analgesics and antipyretic agents.
Keywords: Monotheca buxifolia, Bosea amherstiana, Analgesic, Antipyretic, Sedative
1. Introduction
Many plants synthesized constituents that are beneficial for maintaining human health. The man was completely dependent on medicinal plants prior to synthetic drug availability for treating diseases (Singh et al., 2008). Natural medications produced from plants extracts are being progressively utilized for treating wide number of clinical diseases, however relatively little information about their mode of actions are available. The World Health Organization (WHO) encourages the addition of herbal medication in health cares because of the tremendous potentials they have. Natural products like herbs, vegetables, and fruits became more popular in recent era due to increasing interest, public awareness consumers and scientific community (Thaipong et al., 2006). Epidemiologically evidence has suggested that natural products constituents show many pharmacological activities, like antiviral and anti-inflammatory effects (Uddin et al., 2011, Rauf et al., 2012).
Inflammation is associated with protections of the body against infection, burns, allergens and toxic chemicals are considered as a basic physiological defense mechanism. If the inflammations are left uncontrolled, it will turn as an etiologic factor for many other chronic diseases (Kumar et al., 2015). It comprises a complex range of enzyme activation, intermediary proclamation, extravasation of the fluids, cell movement, tissues break down and its repairs (Vane and Botting, 1995). Previously, inflammation was considered as a sole disease caused by instabilities of body fluid, however, in the recent medical sciences, inflammation is considered as a vigorous process resultant from diseases or disturbance (Emamghoreishi et al., 2005). Currently, the inflammatory condition and related pain are succeeded by either narcotic, e.g., opioid or non-narcotics like salicylate and corticosteroid. Those synthetic medications are linked with some side effect (Gaddi et al., 2004).
Furthermore, these predictable medicines and drugs are either too expensive or toxic and not usually offered to the rural societies that constitute the key populace of the globe. So, the more effective and novel development of anti-inflammatory medications with a minimum side effect is essential. Plant origin medications have been using since ancient time with relatively minimum adverse effect. Therefore it is important to introduce novel therapeutic plant for cheaper drug development. Several herbal plants have been used to ameliorate the inflammation and related pain and fever with folk uses. Compounds that are isolated from plants can assist as a pattern for the production of new anti-inflammatory medications with higher therapeutic effects low toxicity level (Anilkumar, 2010). In developing countries, several medicinal plants have been used for the treatments of inflammation and pain. Monotheca buxifolia and Bosea amherstiana, are widely growing traditional plants in the northern hilly areas of Pakistan. These plants are used for wound healing and as antiseptic and for the gastrointestinal disorder treatment (Qureshi et al., 2007). The current study was conducted to determine the traditional use of Monotheca buxifolia and Bosea amherstiana as inflammation and related pain and fever.
2. Materials and methods
Diclofenac sodium was used as a standard drug. In writhing tests, the pain was inducted by acetic acid (Merck Germany). Control group was administrated with normal sterile saline water throughout the experiments while normal saline was used to prepare methanolic extracts. The reference drug diclofenac sodium was administrated as an analgesic drug by the intraperitoneal administration with a concentration of 10 mg/kg. Monotheca buxifolia (MB) and Bosea amherstiana (BA) at a dose of (50, 100 and 150 mg/kg), was dissolved in normal saline and intraperitoneal administrated. Diazepam was used as a depressant drug for sedative activity while paracetamol was used as a reference drug for anti-pyritic evaluation.
2.1. Plant materials and extract preparation
Aerial parts of Monotheca buxifolia (MB) and Bosea amherstiana (BA) were collected from, Dir Lower, KPK, Pakistan, in March 2014 and September 2014 respectively. The plants samples were authenticated by Dr. Jehandar Shah, Taxonomist; Ex-Vice Chancellor Shaheed Benazir University Shiringal, Pakistan.
The well-established protocol was followed to get a methanolic extract from the powder plant material (Ibrar and Muhammad, 2011, Khan et al., 2012). Extracts were concentrated under vacuum at 40 °C after filtration. The methanolic extract was screened for in vivo pharmacological assays using albino mice.
2.2. Animals
Albino rat (180–220 g) and mice (18–22 g) of either sex were attained from animal house, Animal Sciences Department, Quaid-e-Azam University, Pakistan. Standard laboratory protocols were provided to animals (25 °C light and dark cycle, i.e. 12/12 h) with standard quality water and food.
2.3. Acute-toxicity assay
The clinical trials for the determination of acute toxicity of both plants were done to evaluate the possibility of any type of acute toxicities. Mices of either sex were assessed by oral administering various dose (500, 1000, 1500 and 2000 mg/kg), whereas distilled water was provided to control group (1 mL/kg). Gross effects of first 4 h in all groups were observed then 24 h mortality was observed (Muhammad et al., 2013).
2.4. Acetic acid induced writhing’s test
The analgesic evaluation of methanolic extracts of MB and BA were estimated by acetic acid induced writhing’s test (Koster, 1959). Albino mice of each sex were randomly divided into four groups comprising of six in each. Normal saline at concentration of 10 mL/Kg was injected to Group I as control, diclofenac sodium as a standard drug was injected to Group II at concentration of 10 mg/kg, while different concentrations of methanolic extracts (50, 100 and 150 mg/kg), through i,p route were injected respectively. Acetic acid (1%) was injected after 30 min after the above treatment. After five minutes the abdominal writhes were counted for the periods of ten minutes.
2.5. Sedative effect
White wood apparatus enclosed in stainless steel was used in this experiment, divided by black lines in different squares. Mices of either sex were used for this activity. Mices were familiarized under red light prior to experiment for one hour in lab condition with feed supply. The animals used for experiment were treated with a reference drug diazepam (0.5 mg/kg i.p.) normal saline (control), methanolic extracts (50, 100 and 150 mg/kg i.p). Each mouse was putted in the center of wood box after 30 min of the treatment and counted down the lines crossed by each animal (Muhammad et al., 2013).
2.6. Antipyretic activity
Antipyretic activity against yeast induced pyrexia of methanolic extracts of Monotheca buxifolia and Bosea amherstiana in mices were evaluated according to the previously described procedure with slight modification (Metowogo et al., 2008). Animals were kept fasting for overnight and were divided into five groups consisting of 6 mices in each group. By subcutaneous pyrexia was induced at concentration of 2 mL/100 g of 20% aqueous suspensions of brewer in yeasts. Group I with normal saline (2 mL/kg) kept as a control treatment. Group 2, 3 and 4 were administrated with methanolic extracts (50, 100 and 150 mg/kg p.o.) respectively, while group 5 was served with paracetamol (Standard drug) 100 mg/kg p.o. The rectal temperature was recorded after 24 h of inducting of pyrexia. The animals were treated according to protocols with plants extracts, standard and control. The rectal temperature was measured at the interval of 30, 90 and 150 min after drug and samples administrations.
2.7. Statistical analysis
The data generated was statistically analyzed by computer software program JMP. SAS (Version 7.0. SAS, USA), followed by Student’s t-test.
3. Results
3.1. Acute toxicity
Both plants were found safe for all tested dose. All treated animals were found normal during the first four hours and after 24 h’ assessment.
3.2. Acetic acid induced test/analgesic activity
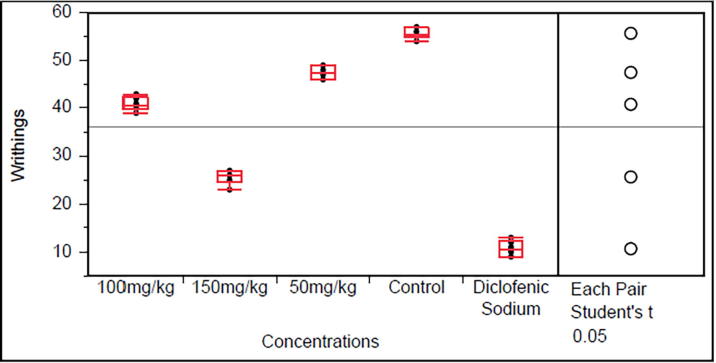
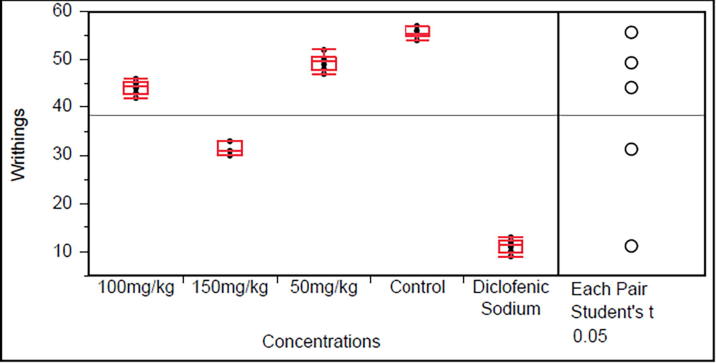
Monotheca buxifolia and Bosea amherstiana (50, 100 and 150 mg/kg) dose-dependently reduced abdominal constrictions in mice. The reduction was significantly (P < 0.0001) when compared with control (Fig. 1, Fig. 2). The effect of the extracts was comparable to that of diclofenac sodium (10 mg/kg) the standard drug.
Fig. 1.
Effect of Monotheca buxifolia 50, 100 and 150 mg/kg in acetic acid induced test (Data are reported as mean ± S.E.M. for group of six animals. The data was analyzed by ANOVA followed by Student’s t test with significance value P > 0.0001).
Fig. 2.
Effect of Bosea amherstiana50, 100 and 150 mg/kg in acetic acid induced test (Data are reported as mean ± S.E.M. for group of six animals. The data was analyzed by ANOVA followed by Student’s t test with significance value P > 0.0001).
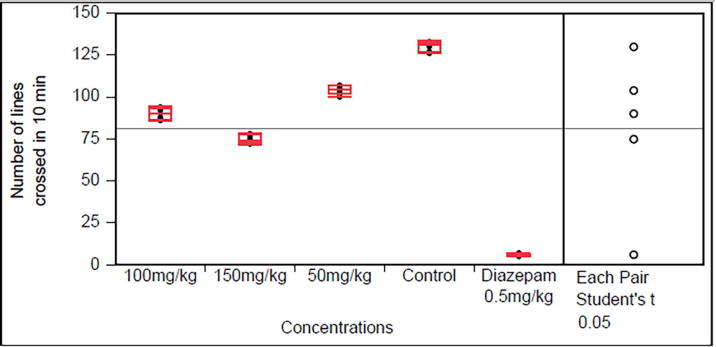
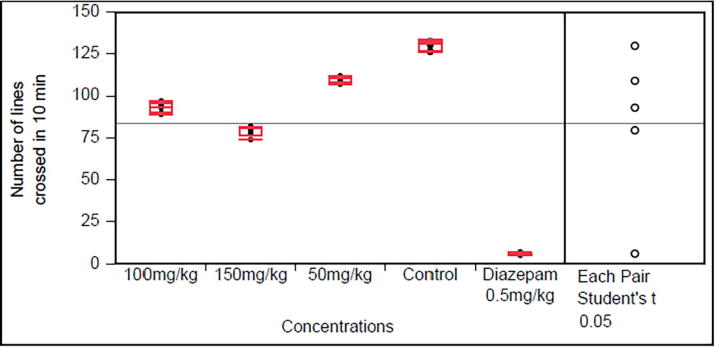
3.3. Sedative effect
The sedative effect, the open field test of MB and BA are given in Fig. 3, Fig. 4. Diazepam the standard drug used exhibit maximum sedative effect which was significantly (p < 0.0001) compared with the control group. Both plants exhibit (p < 0.0001) sedative effects in dose of 50, 150 and 150 mg/kg, however, these should be considered mild to moderate sedative as the sedation induced was for less standard drug diazepam.
Fig. 3.
Sedative activity of Monotheca buxifolia (Data are reported as mean ± S.E.M. for group of six animals. The data was analyzed by ANOVA followed by Student’s t test with significance value P > 0.0001).
Fig. 4.
Sedative activity of Bosea amherstiana (Data are reported as mean ± S.E.M. for group of six animals. The data was analyzed by ANOVA followed by Student’s t test with significance value P > 0.0001).
3.4. Antipyretic test
Both plants markedly (P < 0.0001) reduced yeast induced hyperthermia. The dose-dependent inhibitions remained significant up to five hours (Table 1). The maximum effect against pyrexia was observed at Monotheca buxifolia 150 mg/kg and Bosea amherstiana 150 mg/kg, while, the antipyretic effect of paracetamol was 37.67 ± 0.44. The pyrexia inhibition temperature is shown in Table 1.
Table 1.
Antipyretic activity of Monotheca buxifolia (MB) and Bosea amherstiana (BA).
| Treatment | Dose | Normal (A) | 24 h (B) | 1 h (C1) | 2 h (C1) | 3 h (C1) | 4 h (C1) | 5 h (C1) |
|---|---|---|---|---|---|---|---|---|
| N. Saline | 10 mL/kg | 36.67 ± 0.44 | 39 ± 0.67 | 38 ± 0.67 | 38.67 ± 0.67 | 39.33 ± 0.67 | 38.33 ± 0.44 | 38 ± 0.67 |
| Paracetamol | 100 mg/kg | 36.67 ± 0.44 | 38.67 ± 0.44 | 37.67 ± 0.44 | 37.33 ± 0.44 | 37.67 ± 0.56 | 37.67 ± 0.67 | 37.67 ± 0.44 |
| MB | 50 mg/kg | 36.33 ± 0.44 | 39.33 ± 0.44 | 38.67 ± 0.44 | 38.67 ± 0.44 | 38.67 ± 0.67 | 38.67 ± 0.44 | 38.67 ± 0.44 |
| MB | 100 mg/kg | 36 ± 0.67 | 39 ± 0.67 | 38.67 ± 0.44 | 38.33 ± 0.44 | 38 ± 0.67 | 38.33 ± 0.44 | 38 ± 00 |
| MB | 150 mg/kg | 36 ± 0.67 | 38.67 ± 0.44 | 37.67 ± 0.44 | 37.67 ± 0.44 | 38.67 ± 0.44 | 37.67 ± 0.67 | 37.67 ± 0.89 |
| BA | 50 mg/kg | 36.67 ± 0.44 | 38.33 ± 0.44 | 38 ± 0.67 | 38.33 ± 0.44 | 38.33 ± 0.44 | 39 ± 0.67 | 38.67 ± 0.44 |
| BA | 100 mg/kg | 36.67 ± 0.44 | 38.67 ± 0.44 | 38.33 ± 0.44 | 39 ± 0.67 | 38.33 ± 0.44 | 38.67 ± 0.44 | 38.67 ± 0.44 |
| BA | 150 mg/kg | 37.33 ± 044 | 38.33 ± 0.44 | 38.33 ± 0.44 | 38.33 ± 0.44 | 38.33 ± 0.44 | 38.33 ± 0.44 | 38.33 ± 0.44 |
Data are reported as mean ± S.E.M. for group of six animals. The data was analyzed by ANOVA followed by Student’s t test with significance value P > 0.0001.
4. Discussion
The present study indicates that Monotheca buxifolia and Bosea amherstiana has noticeable anti-inflammatory, analgesic and antipyretic effects with a rational safety profile. The anti-inflammatory protocols are a well-established and used for assessing therapeutic agents for their analgesic effect. The reduction in abdominal constrictions was significantly when compared with control. The induced pain due to liberating endogenous constituents as well as another pain mediator such as arachidonic acid via cyclooxygenase, and biosynthesis of prostaglandin (Khan et al., 2010). This pain pattern is generally used for the evaluation of peripheral analgesic assay due to its response and sensitivity to the containing compounds at dose with no effect in other procedures approaches. The locally peritoneal receptors might be the main cause of abdominal writhing (Mbiantcha et al., 2011). The sensation of pain is caused by phospholipid in tissue via cyclooxygenase (COX), and producing prostaglandins specifically PGF2α and PGE2, in peritoneal fluids the level of increase may occur in lipoxygenase production (Khan et al., 2010). The pain and inflammations may cause due to prostaglandins and lipoxygenase products by rising capillary penetrability. The writhing inhibiting substances have the analgesic effect preferable by inhibiting synthesis of prostaglandin, a fringe mechanism of inhibiting pain (Duarte et al., 1988). Concerning the outcomes of our plants extract in acetic acid induced abdominal constrictions evaluation, a significance inhibition of writhing’s reflux was noted. These results strongly endorse that both plants have peripheral analgesic effects and their way of act may be facilitated through inhibitions of locally peritoneal receptor which may be the contribution in inhibition of cyclooxygenase potentials.
Diazepam belongs to the group benzodiazepine a depressant of nervous system and is used for sleeping disorders. The binding site of benzodiazepine is GABA receptor type ionosphere complex. It reduce the activity, balance the excitement and calm down the recipient. The drug diazepam used as a reference reduced the onset and enhance the interval of barbiturate induced sleep and lower down exploratory activity owning potential as sedative (Hossain et al., 2016a). Pyrexia induced by Brewer’s yeast is recommended as an appropriate test for the screening of medicinal plants as well as drugs formed synthetically for their antipyretic effects (Khan et al., 2009, Devi et al., 2003). Yeast-induced pyrexia is also known as pathogenic fever (Hossain et al., 2016b, Hossain et al., 2017). The intraperitoneal administration of Monotheca buxifolia and Bosea amherstiana significantly attenuating rectal temperature of yeast induced mice.
5. Conclusion
These investigational results have linked a pharmacological indication for the traditional claim of the drugs to be used as an analgesic, anti-inflammatory, and antipyretic agent. Supplementary studies on the probable mechanism of actions and isolation of active compounds responsible for such activities are presently under progress in our laboratory.
Acknowledgments
We are thankful to Department of Animal Sciences, Faculty of Biological Sciences, Quaid-i-Azam University Islamabad for experimental laboratory facilitation.
Footnotes
Peer review under responsibility of King Saud University
References
- Anilkumar M. 10. Ethnomedicinal plants as anti-inflammatory and analgesic agents. Ethnomed.: Source Complementary Therap. 2010:267–293. [Google Scholar]
- Devi B.P., Boominathan R., Mandal S.C. Evaluation of antipyretic potential of Cleome viscosa Linn. (Capparidaceae) extract in rats. J. Ethnopharmacol. 2003;87:11–13. doi: 10.1016/s0378-8741(03)00099-0. [DOI] [PubMed] [Google Scholar]
- Duarte I., Nakamura M., Ferreira S. Participation of the sympathetic system in acetic acid-induced writhing in mice. Braz. J. Med. Biol. Res.= Revista brasileira de pesquisas medicas e biologicas. 1988;21:341–343. [PubMed] [Google Scholar]
- Emamghoreishi M., Khasaki M., Aazam M.F. Coriandrum sativum: evaluation of its anxiolytic effect in the elevated plus-maze. J. Ethnopharmacol. 2005;96:365–370. doi: 10.1016/j.jep.2004.06.022. [DOI] [PubMed] [Google Scholar]
- Gaddi A., Cicero A.F., Pedro E.J. Clinical perspectives of anti-inflammatory therapy in the elderly: the lipoxigenase (LOX)/cycloxigenase (COX) inhibition concept. Arch. Gerontol. Geriatr. 2004;38:201–212. doi: 10.1016/j.archger.2003.10.001. [DOI] [PubMed] [Google Scholar]
- Hossain M., Islam M.M., Azad M.A.K., AL Faruq A. In-vivo investigation of analgesic, ANTIPYRETIC anti-diarrheal and anxiolytic activity of blumea densiflora DC. Eur. J. Pharm. Med. Res. 2017;3:50–55. [Google Scholar]
- Hossain M.F., Talukder B., Rana M.N., Tasnim R., Nipun T.S., Uddin S.N., Hossen S.M. In vivo sedative activity of methanolic extract of Stericulia villosa Roxb. leaves. BMC Complementary Altern. Med. 2016;16:398. doi: 10.1186/s12906-016-1374-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hossain M.S., Akter S., Das A., Sarwar M.S. CNS depressant, antidiarrheal and antipyretic activities of ethanolic leaf extract of Phyllanthus acidus L. on Swiss Albino Mice. British J. Pharm. Res. 2016;10:1. [Google Scholar]
- Ibrar M., Muhammad N. Evaluation of Zanthoxylum armatum DC for in-vitro and in-vivo pharmacological screening. African J. Pharm. Pharmacol. 2011;5:1718–1723. [Google Scholar]
- Khan H., Saeed M., Khan M.A., Dar A., Khan I. The antinociceptive activity of Polygonatum verticillatum rhizomes in pain models. J. Ethnopharmacol. 2010;127:521–527. doi: 10.1016/j.jep.2009.10.003. [DOI] [PubMed] [Google Scholar]
- Khan H., Saeed M., Khan M.A., Khan I., Ahmad M., Muhammad N., Khan A. Antimalarial and free radical scavenging activities of rhizomes of Polygonatumverticillatum supported by isolated metabolites. Med. Chem. Res. 2012;21:1278–1282. [Google Scholar]
- Khan I., Nisar M., Ebad F., Nadeem S., Saeed M., Khan H., Khuda F., Karim N., Ahmad Z. Anti-inflammatory activities of Sieboldogenin from Smilax china Linn.: experimental and computational studies. J. Ethnopharmacol. 2009;121:175–177. doi: 10.1016/j.jep.2008.10.009. [DOI] [PubMed] [Google Scholar]
- Koster R. Acetic acid for analgesic screening. Fed. Proc. 1959:412. [Google Scholar]
- Kumar, V., Abbas, A.K., Aster, J.C., Perkins, J.A., 2015. Robbins and Cotran pathologic basis of disease.
- Mbiantcha M., Kamanyi A., Teponno R., Tapondjou A., Watcho P., Nguelefack T. Analgesic and anti-inflammatory properties of extracts from the bulbils of Dioscorea bulbifera L. var sativa (Dioscoreaceae) in mice and rats. Evidence-Based Complementary Altern. Med. 2011 doi: 10.1155/2011/912935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metowogo K., Agbonon A., Eklu-Gadegbeku K., Aklikokou A., Gbeassor M. Anti-ulcer and anti-inflammatory effects of hydroalcohol extract of Aloe buettneri A. Berger (Lilliaceae) Trop. J. Pharm. Res. 2008;7:907–912. [Google Scholar]
- Muhammad N., Saeed M., Khan H., Haq I. Evaluation of n-hexane extract of Viola betonicifolia for its neuropharmacological properties. J. Nat. Med. 2013;67:1–8. doi: 10.1007/s11418-012-0636-0. [DOI] [PubMed] [Google Scholar]
- Qureshi R.A., Ghufran M.A., Gilani S.A., Sultana K., Ashraf M. Ethnobotanical studies of selected medicinal plants of sudhan gali and ganga chotti hills, district bagh, azad kashmir. Pak. J. Bot. 2007;39:2275–2283. [Google Scholar]
- Rauf A., Muhammad N., Khan A., Uddin N., Atif M. Antibacterial and phytotoxic profile of selected Pakistani medicinal plants. World Appl. Sci. J. 2012;20:540–544. [Google Scholar]
- Singh A., Malhotra S., Subban R. Anti-inflammatory and analgesic agents from Indian medicinal plants. Int. J. Integr. Biol. 2008;3:57–72. [Google Scholar]
- Thaipong K., Boonprakob U., Crosby K., Cisneros-Zevallos L., Byrne D.H. Comparison of ABTS, DPPH, FRAP, and ORAC assays for estimating antioxidant activity from guava fruit extracts. J. Food Compos. Anal. 2006;19:669–675. [Google Scholar]
- Uddin G., Rauf A., Siddiqui B.S., Shah S.Q. Preliminary comparative phytochemical screening of Diospyros lotus Stewart. Middle-East J. Sci. Res. 2011;10:78–81. [Google Scholar]
- Vane J., Botting R. New insights into the mode of action of anti-inflammatory drugs. Inflamm. Res. 1995;44:1–10. doi: 10.1007/BF01630479. [DOI] [PubMed] [Google Scholar]