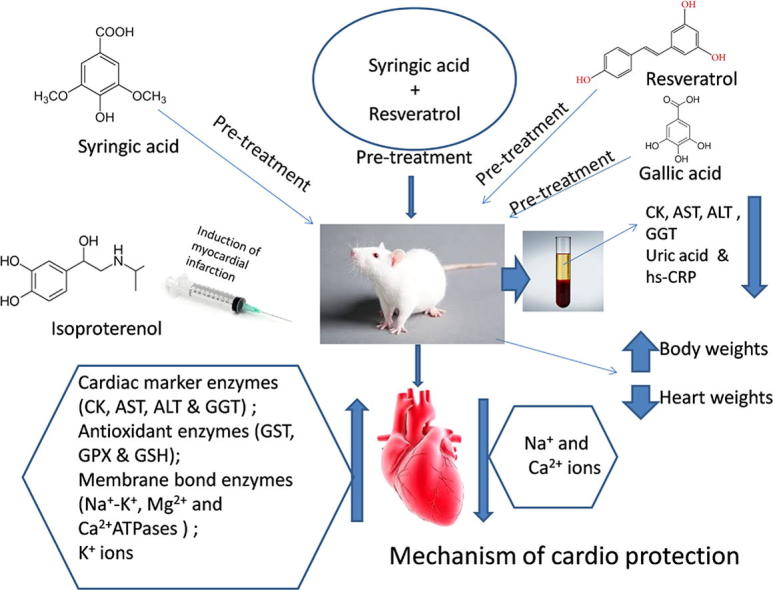
Graphical abstract
Keywords: Syringic acid, Resveratrol, Isoproterenol, Myocardial infarction
Abstract
Objective
To evaluate the cardio-protection of syringic acid (SA) in combination with resveratrol (RV) in isoproterenol (ISO) induced myocardial infarcted (MI) rats.
Methods
Groups of all rats were subjected oral pre-treatment at the beginning of the study with SA (50 mg/kg), RV (50 mg/kg) and combination (COMB) of SA (25 mg/kg) and RV (25 mg/kg) along with gallic acid (GA) (50 mg/kg) for 30 days. After sacrification, homogenate of heart tissue along with serum were utilized for further biochemical investigations. The effects on creatine kinase (CK), aspartate transaminase (AST), alanine transaminase (ALT) and gamma glutamyl transferase (GGT) were studied in serum and heart tissues. Glutathione-s-transferase (GST), glutathione peroxidase (GPX) and reduced glutathione (GSH), membrane bound enzymes and electrolytes were tested in heart tissues. Body weights and heart weights were also observed along with high sensitivity C-reactive protein (hs-CRP), uric acid and total protein content (TPC) in serum.
Results
CK, AST, ALT and GGT levels in serum were augmented significantly while these enzymes are decreased in cardiac tissue samples of ISO–treated rats. GST, GPX, GSH, Na+/K+, Mg2+, Ca2+ ATPases, K+ ions were significantly decreased while Na+ and Ca2+ ions were increased in the heart tissues of ISO-injected rats. Loss and gain of body and heart weights were noticed significantly in rats having ISO administration. ISO group showed significant increase in hs-CRP and Uric acid while significant decrease in TPC. All of actions of ISO were ameliorated by COMB.
Conclusions
COMB suppressed ISO induced MI in rats and exhibited cardio-protection.
1. Introduction
In both developed and developing countries, the main reason for deaths worldwide is nothing but Myocardial infarction (MI) or heart attack (Sharma et al., 2019). When blood supply to the heart is not in the balance with cardiac myocyte requirement, it will result in prolonged insufficient oxygen supply to cardiomyocytes and finally necrosis is seen in heart myocytes, a condition called as acute myocardial infarction (Boarescu et al., 2019).
Severe strain in the muscle cells and necrosis in myocardium are caused by isoproterenol (ISO), which is a β-adrenergic agonist. Overload of calcium (Ca2+), hypertension, and the condition of hypoxia, depletion of energy and enhanced formation of free radicals are caused by ISO (Do et al., 2015). Myocardial membrane is irreversibly damaged by lipid peroxidation and free radicals, which are influenced by ISO administration (Karthick and Stanely Mainzen Prince, 2006).
A phenolic chemical exists in the mycelium of shitake mushroom and the acai palm fruits, is syringic acid (SA) (Tanaka et al., 2017).
SA occurs in vegetables and fruits and shikimic acid pathway is key pathway through which SA synthesis occurs in plants. SA has wide-ranging beneficial applications in the avoidance of cardiovascular diseases CVDs, diabetes, cerebral ischemia, cancer as well as SA has anti-microbial, anti-oxidant, anti-endotoxic, anti-inflammatory, neuro and hepato-protective activities. SA obtains therapeutic activity due to containing methoxy groups at third and fifth carbons on aromatic ring. It additionally acts as scavenger of free radicals and reduces the markers of oxidative pressure. SA has beneficial effects on human health due to possessing strong anti-oxidant nature (Srinivasulu et al., 2018).
Prior proposals reveal that by various mechanisms, resveratrol (RV) acquired defensive properties against heart failure, oxidative pressure, inflammation (Das and Das, 2007) and also inhibits pathological hypertrophic signaling (Xi et al., 2009), improving Ca2+ handling (Dong et al., 2014), altering autophagy by means of diverse pathways inside the cell and diminishing apoptotic pathways (Kanamori et al., 2013).
In these days, scientific and public interest is using of phytochemicals in controlling human diseases (Srinivasulu et al., 2018). So we selected the combination (COMB) of phytochemicals such as SA and RV to test the prevention of myocardial damage.
2. Materials and methods
ISO, SA, RV, gallic acid (GA) and dimethyl sulfoxide (1% DMSO) were collected from Sigma Aldrich Company, USA and SDFCL, India respectively. Kits for Cardiac marker enzymes were purchased from Erba Company and Proton Biologicals India Pvt. Ltd. Kits were purchased from M/s Excel Diagnostics Pvt. Ltd., India for electrolytes. Erba Company kits were purchased for the assay of uric acid and high sensitivity C-reactive protein (hs-CRP). Other chemicals with Analytical grade were used in this research.
2.1. Animals
Male albino Wistar rats weighing nearly 100–120 g were acclimatized seven days of period to the animal house environment. Proper management was taken on animals by stabilizing the temperature at 25 °C and dark and light cycles at 12/12 h along with sufficient ventilation. Sufficient pellet diet and water were provided to all rats. Permission was granted to the university (Biochemistry, Sri Krishnadevaraya University, Anantapur, India) for conducting researches on animals from Committee for the Purpose of Control and Supervision of Experiments on animals (CPCSEA) (Regd. 1889/GO/Re/S/16/CPCSEA), and the research was approved by the IAEC protocol No. SKU/Biochem/08/2017.
2.2. Experimental protocol
Totally 42 rats were equally categorized into seven groups having 6 rats in each group:
Group 1: Control: received DMSO orally for duration of 30 days.
Group 2: ISO [50 mg/kg bw]: received ISO sub-cutaneously on 29th and 30th days with a 24 time gap hours.
Group 3: SA [50 mg/kg bw]: received SA orally for duration of 30 days.
Group 4: GA+I [50 mg/kg bw]: received GA orally for duration of 30 days and on 29th and 30th days (with a gap of 24 h), ISO was administered sub-cutaneously.
Group 5: SA+I [50 mg/kg bw]: received SA orally for duration of 30 days and on 29th and 30th days (with a gap of 24 h), ISO was administered sub-cutaneously.
Group 6: RV+I [50 mg/kg bw]: received RV orally for duration of 30 days and on 29th and 30th days (with a gap of 24 h), ISO was administered sub-cutaneously.
Group 7: COMB+I [SA (25 mg/kg bw) and RV (25 mg/kg bw)]: received combination of SA and RV orally for duration of 30 days and on 29th and 30th days (with a gap of 24 h), ISO was administered sub-cutaneously.
SA and RV were dissolved in DMSO while GA and ISO were dissolved in water. For the oral treatment, utilized device was intragastric tube.
2.3. Collection of samples
Without delay after the handling of rats with second dose of ISO, sodium pentobarbital (35 mg/kg I.P.) was injected by intraperitoneal (I.P.) route to anesthetize all animals and rats were subjected to sacrifice by cervical decapitation. Hearts and blood were collected right away. Collected blood was used for the serum isolation and hearts were rinsed with ice-cold physiological saline and homogenized, centrifuged to collect the supernatant for different biochemical examinations. At −80 °C, all tissue and serum samples were preserved.
2.4. Biochemical measurements
Gamma glutamyl transferase (GGT) and total protein content (TPC) were examined carefully according to the methods of Young and Stirling Donald, 1995, Lowry et al., 1951 respectively. The protocols followed for observation of activities of glutathione-s-transferase (GST), glutathione peroxidase (GPX) and reduced glutathione (GSH) were Habig et al., 1974, Routruck et al., 1973, and Ellman (1959) respectively. Bonting, 1970, Ohnishi et al., 1982, and Hjertén and Pan (1983) procedures were selected to check the activities of sodium-potassium (Na+/K+), magnesium (Mg2+) and Ca2+ adenosine tri phsophatases (ATPases) respectively. Diagnostic kits were helped in the assessment of creatine kinase (CK), aspartate transaminase (AST), alanine transaminase (ALT), Na+, K+, Ca2+, uric aicd and hs-CRP.
2.5. Statistical study
To perform the statistical study, results were examined by subjecting to Duncan’s multiple range (DMR) test by considering p value at p < 0.05. The outcome data was communicated as means ± SD.
3. Results
3.1. Impacts of COMB on cardiac marker enzymes
COMB showed impact on markers of heat injury in heart tissues and serum samples (Table 1). When p value is less than 0.05, CK, AST, ALT and GGT revealed a significant raise and drop in serum and heart respectively in ISO rats, compared to untreated rats. COMB pre-treatment to ISO rats significantly (p < 0.05) reversed the situation by decreasing and increasing these cardiac marker enzymes respectively in serum and heart, while compared to ISO alone administered rats. Both SA and control group of rats demonstrated insignificant (p < 0.05) difference.
Table 1.
Effect of combination (COMB) of syringic acid (SA) and resveratrol (RV) on creatine kinase (CK), alanine transaminase (ALT), aspartate transaminase (AST) and gamma glutamyl transferase (GGT) in serum and heart tissues of normal and isoproterenol (ISO) treated groups.
| Groups | CK (IU/L) |
ALT (IU/L) |
AST (IU/L) |
GGT (U/L) |
||||
|---|---|---|---|---|---|---|---|---|
| Serum | Heart | Serum | Heart | Serum | Heart | Serum | Heart | |
| Control | 561.1 ± 11.7b | 139.6 ± 6.5a* | 43.8 ± 2.6c | 38.5 ± 6.8a* | 189.0 ± 6.9c | 12.16 ± 2.3a2 | 7.35 ± 0.05e | 5.71 ± 0.37a |
| ISO | 1268.5 ± 335.4a | 74.0 ± 5.2c | 95.1 ± 2.0a | 22.9 ± 2.2c | 394.8 ± 5.3a | 3.96 ± 0.3b | 12.43 ± 0.09a | 2.15 ± 0.32f |
| SA | 558.3 ± 10.7b | 139.6 ± 8.3a | 43.6 ± 2.6c | 38.5 ± 1.7a# | 188.8 ± 3.2c | 12.25 ± 1.7a1 | 7.32 ± 0.03e | 5.83 ± 0.18a |
| GA+I | 582.3 ± 9.8b | 120.0 ± 2.8b | 73.1 ± 1.9b | 26.0 ± 1.2b | 273.3 ± 3.4b | 6.82 ± 1.1a | 9.39 ± 0.09b | 2.68 ± 0.22e |
| SA+I | 578.8 ± 12.9b | 126.0 ± 2.7b | 47.0 ± 1.4c* | 29.0 ± 3.0b | 213.3 ± 14.0c# | 7.36 ± 2.2a | 8.30 ± 0.19c | 3.24 ± 0.08d |
| RV+I | 575.8 ± 12.9b | 132.8 ± 3.4a | 46.3 ± 2.0c | 33.5 ± 2.3a | 211.0 ± 6.6c* | 9.00 ± 1.2a* | 7.67 ± 0.16d | 3.64 ± 0.21c |
| COMB+I | 563.0 ± 31.5b | 134.1 ± 1.8a | 45.6 ± 2.0c | 35.0 ± 2.6a | 203.0 ± 31.6c | 10.36 ± 0.9a# | 7.39 ± 0.05e | 4.15 ± 0.09b |
Results are mean ± SD values with unusual lower case letters (a–f) significantly differ from one another [p < 0.05, Duncan’s multiple range test (DMRT)]. CK-heart-a* – Control group is significantly different with RV+I group, ALT-serum-c* – SA+I group is significantly different with Control and SA groups, ALT-heart-a* – Control group is significantly different with RV+I group, a# – SA group is significantly different with RV+I group, AST-serum-c# – SA+I group is significantly different with control and SA groups, c* – RV+I group is significantly different with control and SA groups.
3.2. Effect of COMB on anti-oxidants
COMB shows effect on anti-oxidants in heart (Table 2). ISO administration brought down the levels of the GST, GPX and GSH significantly (p < 0.05) in ISO group of rats, compared to control rats. Our surprising results showed clearly that COMB intake to ISO injected rats significantly (p < 0.05) brought up these anti-oxidant levels, when compared with ISO alone injected rats. Change of Significance (p < 0.05) was not established in between control and SA group rats.
Table 2.
Effect of combination (COMB) of syringic acid (SA) and resveratrol (RV) on glutathione-s-transferase (GST), glutathione peroxidise (GPX) and reduced glutathione (GSH) heart tissues of normal and isoproterenol (ISO) treated groups.
| Groups | GST (µM/min/mg protein) | GPX (µg GSH/min/mg protein) | GSH (µg GSH/mg protein) |
|---|---|---|---|
| Control | 15.41 ± 0.38a | 49.20 ± 0.22a | 26.13 ± 1.47a |
| ISO | 10.06 ± 0.22e | 32.85 ± 0.44e | 15.12 ± 0.66f |
| SA | 15.40 ± 0.22a | 49.23 ± 0.34a | 26.14 ± 0.21a |
| GA+I | 11.95 ± 0.30d | 36.36 ± 0.34d | 18.76 ± 0.37e |
| SA+I | 12.16 ± 0.23d | 38.68 ± 0.41c | 19.85 ± 0.16d |
| RV+I | 12.51 ± 0.14c | 38.86 ± 0.46c | 21.43 ± 0.23c |
| COMB+I | 13.92 ± 0.18b | 43.49 ± 0.25b | 23.41 ± 0.36b |
Results are mean ± SD values with unusual lower case letters (a–f) significantly differ from one another [p < 0.05, Duncan’s multiple range test (DMRT)].
3.3. Effect of COMB on membrane bound enzymes
Table 3 indicates the impact of COMB and membrane bound enzymes in heart. The activities of Na+-K+, Mg2+ and Ca2+ ATPases were demonstrated a significance (p < 0.05) declinement in ISO challenged rats, while comparing with normal group. Upon comparing with ISO-operated rats, COMB intake orally to ISO operated animals, displayed enhancement in these membrane bound enzyme activities at the significance of p < 0.05. Pre-treatment for 30 days with SA indicated that no significant (p < 0.05) difference with untreated rats.
Table 3.
Effect of combination (COMB) of syringic acid (SA) and resveratrol (RV) on sodium-potassium (Na+/K+), magnesium (Mg2+) and calcium (Ca2+) adenosine tri phosphatases (ATPases) in the heart tissues of normal and isoproterenol (ISO) treated groups.
| Groups | Na+/K+-ATPase (µM of phosphorous liberated/mg protein) | Mg2+-ATPase (µM of phosphorous liberated/mg protein) | Ca2+-ATPase (µM of phosphorous liberated/mg protein) |
|---|---|---|---|
| Control | 3.47 ± 0.03a | 5.37 ± 0.04a | 2.57 ± 0.10a |
| ISO | 1.68 ± 0.07f | 1.64 ± 0.05f | 0.74 ± 0.09f |
| SA | 3.50 ± 0.10a | 5.47 ± 0.04a | 2.98 ± 0.05a |
| GA+I | 1.95 ± 0.09e | 2.29 ± 2.29e | 1.62 ± 0.09e |
| SA+I | 2.28 ± 0.07d | 2.75 ± 0.04d | 1.96 ± 0.04d |
| RV+I | 2.69 ± 0.13c | 3.41 ± 0.15c | 2.20 ± 0.05c |
| COMB+I | 3.06 ± 0.09b | 4.60 ± 0.11b | 2.38 ± 0.13b |
Results are mean ± SD values with unusual lower case letters (a–f) significantly differ from one another [p < 0.05, Duncan’s multiple range test (DMRT)].
3.4. Effect of COMB on electrolytes
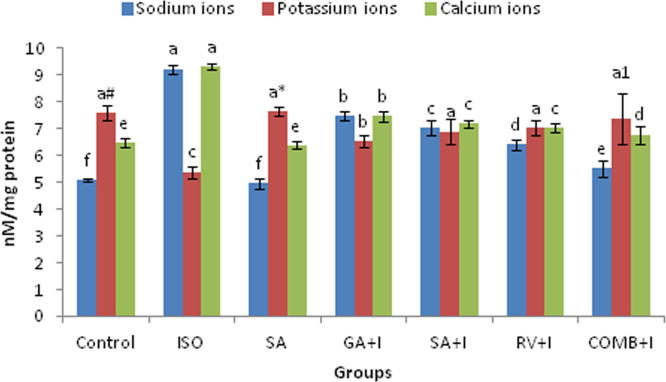
Fig. 1 indicates the impact of COMB on the levels of electrolytes in heart. An elevation of Na+, Ca2+ and depression of K+ ion proportions were detected at the significance of p < 0.05 in the group of ISO challenged animals than in rats of group 1. 30 days oral dispension of COMB to ISO injected rats, leads to depression of Na+, Ca2+ and elevation of K+ ion levels significantly (p < 0.05), after comparing with ISO group. SA alone therapy revealed no significant (p < 0.05) change with untreated rats.
Fig. 1.
Effect of combination (COMB) of syringic acid (SA) and resveratrol (RV) on sodium (Na+), potassium (K+) and calcium (Ca2+) ions in the heart tissues of control and isoproterenol (ISO) treated rats. Values are mean ± SD (n = 6 rats). Values that do not share a common superscript (a–f) differ significantly from each other (p < 0.05, Duncan’s multiple range test). a# – C group is significantly different with SA+I and RV+I groups, a* – SA group is significantly different with SA+I and RV+I groups while a1 – COMB+I group is significantly different with SA+I group.
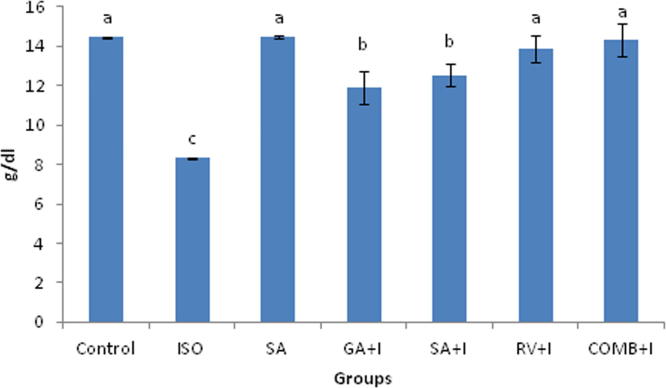
3.5. Effects of COMB on body weights and heart weights
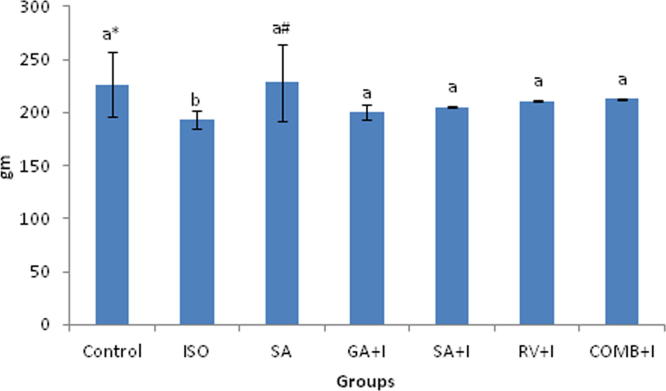
COMB shows impact on body weights as well as heart weights (Fig. 2a, Fig. 2b). At the significance value of p < 0.05, body and heart weights were indicated their decrease and increase respectively in ISO induced rats, upon comparing with rats belonging to normal group. Than in ISO injected rats, COMB pre-treatment clearly manifest a significant (p < 0.05) consequence by increasing and decreasing body weights and heart weights respectively. Control and SA groups clearly point out no significant (p < 0.05) changes.
Fig. 2a.
Effect of combination (COMB) of syringic acid (SA) and resveratrol (RV) on body weights of control (C) and isoproterenol (ISO) treated rats. Values are mean ± SD (n = 6 rats). Values that do not share a common superscript (a and b) differ significantly from each other (p < 0.05, Duncan’s multiple range test). a* – control group is significantly different with GA+I group, while a# – SA group is significantly different with GA+I group.
Fig. 2b.
Effect of combination (COMB) of syringic acid (SA) and resveratrol (RV) on heart weights of control and isoproterenol (ISO) treated rats. Values are mean ± SD (n = 6 rats). Values that do not share a common superscript (a–d) differ significantly from each other (p < 0.05, Duncan’s multiple range test).
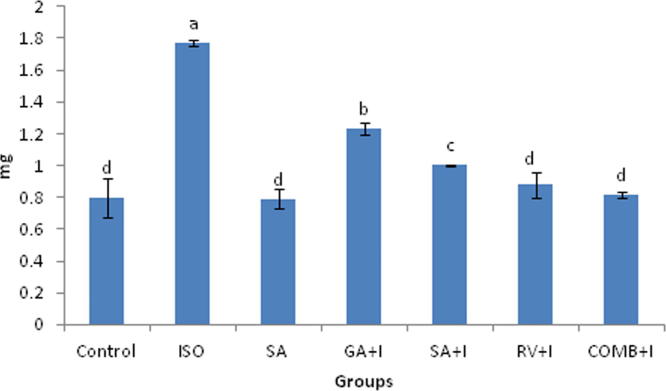
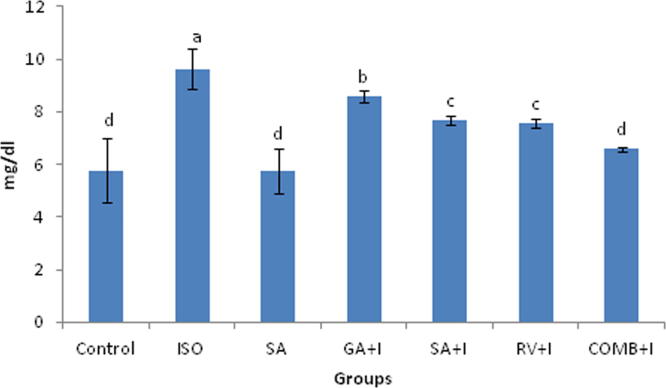
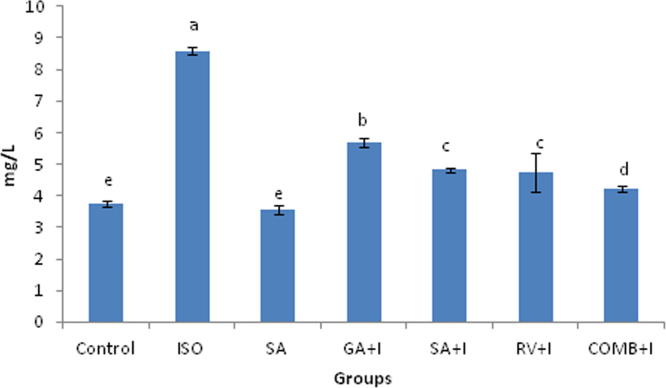
3.6. Effects of COMB on hs-CRP and uric acid
Uric acid along with was affected by COMB (Fig. 3, Fig. 4). Serum collected from the rats with ISO administration exhibited a raise in uric acid and hs-CRP proportions significantly (p < 0.05), upon comparing with rats of untreated group. There was significant (p < 0.05) drop of proportions of inflammatory markers (uric acid and hs-CRP) in rats, which were pre-treated with COMB orally for 30 days duration, in contrasting with ISO administered rats. No altered significance (p < 0.05) was noticed between groups of SA and control animals.
Fig. 3.
Effect of combination (COMB) of syringic acid (SA) and resveratrol (RV) on uric acid in the serum of control and isoproterenol (ISO) treated rats. Values are mean ± SD (n = 6 rats). Values that do not share a common superscript (a–d) differ significantly from each other (p < 0.05, Duncan’s multiple range test).
Fig. 4.
Effect of combination (COMB) of syringic acid (SA) and resveratrol (RV) on high sensitivity C-reactive protein (hs-CRP) of control and isoproterenol (ISO) treated rats. Values are mean ± SD (n = 6 rats). Values that do not share a common superscript (a–e) differ significantly from each other (p < 0.05, Duncan’s multiple range test).
3.7. Effect of COMB on TPC
Fig. 5 illustrates the effect of COMB on TPC in serum. Depleted levels TPC were significantly (p < 0.05) detected surprisingly in ISO animals than untreated rats. Upon pre-treating ISO rats with COMB, surprising significant (p < 0.05) effect was seen in TPC enrichment than ISO alone injected rats. SA alone treated rats were found to exhibit no significant (p < 0.05) difference with control rats.
Fig. 5.
Effect of combination (COMB) of syringic acid (SA) and resveratrol (RV) on total protein content in the serum of control and isoproterenol (ISO) treated rats. Values are mean ± SD (n = 6 rats). Values that do not share a common superscript (a–c) differ significantly from each other (p < 0.05, Duncan’s multiple range test).
4. Discussion
CK, AST, ALT and GGT in serum and heart tissues of ISO operated rats were increased and decreased respectively. Myocardial film integrity and permeability were altered by ISO administration (Thippeswamy et al., 2009), which leads to the leakage of cardiac marker enzymes into serum from heart, thereby increase and decrease of these enzyme levels in serum and heart respectively were observed. Rats of COMB group interestingly showed a noticeable drop and raise in these marker enzymes of cardiac injury in serum and heart respectively. This result was occurred due to COMB may acting as protecting agent against myocardial membrane by increasing the antioxidants in myocardium and thereby reducing the free radicals attack on myocardial membrane, which leads to the prevention of leakage of cardiac marker enzymes into the serum from heart. Previous reports (Shen et al., 2019, Raish et al., 2019, Prasad et al., 2017) supported our outcome.
Declined activities of above mentioned anti-oxidants were found in ISO operated rats. This outcome was due to the inactivation or decrease of anti-oxidants by the enhanced levels of hydrogen peroxide and superoxide (Karthikeyan et al., 2007). These anti-oxidants were markedly increased in ISO treated rats when pre-treated with COMB, which exposing the free radicals scavenging property by having hydroxyl groups. Past outcomes (Mangalanathan et al., 2019, Mounir et al., 2019) are in line with our report.
By comparing with normal rats, depressed activities of above mentioned membrane bound enzymes were clearly noticed in rats, those were injected with ISO. Oxidation of “SH” groups leads to changes in these enzyme conformations. The process of peroxidation in lipids of membrane could diminish these enzymes (Kako et al., 1988). Even minute conformational alterations in membrane bound enzymes may influence the cardiac capacities. Outcome with COMB pre-treatment showed a clear indication that COMB enhanced these enzyme activities by stabilizing membranes. Our results are upheld by the past report (Hamsika et al., 2018).
Rats, those were handled with ISO showed depressed proportions of K+ ions and elevated Na+ and Ca+2 ion proportions in heart. Myocardial cytoplasm contains high concentrations of K+ ions, which are maintained by Na+/K+ ATPase, which was in turn affected by catecholamine, insulin, aldosterone and hyperkalemia (Clausen, 1981). Due to decreased functionality of Na+/K+ ATPase, levels of K+ ions were depressed and levels of Na+ ions increased (Rajadurai and Prince, 2007). Due to increased activity of adenylate cyclase enzyme, levels of cyclic adenosine mono phosphate were elevated, which leads to the phoshporylation reactions at many sites on C-terminal sites. This may be the cause for opening of Ca2+ ion channels (Varadi et al., 1995), which in turn leads to the higher levels of Ca2+ ions in myocardial cells due to Ca2+ influx. Pre-treatment with COMB depressed Na+ and Ca2+ ion levels and elevated K+ ion amounts in all ISO challenged rats, demonstrating the antioxidant assets of COMB in opposition to oxidation of SH groups on ATPase enzymes and preventive action of COMB against lipid peroxidation, which imply the membrane stabilization character of COMB. Our results are linear with the previous report (Althaf Hussain et al., 2018).
Chief cause for decrease in body weights is less food intake by ISO rats (Patel et al., 2010). COMB showed increased food intake activity by increasing body weights. Increased heart weights were detected in ISO group. Because edematous fluid compartments of intramuscular spaces are accumulated with fluid and fibers of myocardium are subjected to necrosis in the hearts ISO treated rats (Patel et al., 2010). COMB proves it’s amelioration of cardiac necrosis by decreasing heart weights. Prior report is in line with our outcome (Hamsika et al., 2018).
Upon comparing with control rats, raise in the hs-CRP levels were noticed in ISO challenged rats. The raised hs-CRP levels may be the cause of ISO alone injection to rats, leads to the formation of inflammatory cytokines, which results in the cardiotoxic effects (Garg and Khanna, 2014). Serum shows enhancement in the hs-CRP levels against acute infections, conditions of inflammation (Kamath et al., 2015). Increased risk for CVD will be detected with the help of C-reactive protein, which is a stable inflammation marker (Euteneuer et al., 2012). COMB pre-treatment in rats with ISO induction resulted in reduced levels of hs-CRP, which may reveals COMB as an anti-inflammatory agent. Our result is in concord with earlier report (Zaafan et al., 2013).
On comparison with control rats, ISO treated rats exhibited the serum upgraded uric acid levels. MI improvement is seen in the condition of higher levels of uric acid, a hazardous marker of MI (Niskanen et al., 2004). In tissues with low oxygen levels, adenosine tri phosphate consumption occurs, causing accumulation of hypoxanthine. At the point when tissues are exasperates, SH groups undergo oxidation, leads to the creation of xanthine oxidase from xanthine dehydrogenase, which in turn form superoxide, uric acid and xanthine (McCord, 1988). This could be one reason for the upgraded uric acid levels in ISO injected rats. COMB therapy for 30 days degraded the serum uric acid levels. This might be due to anti-inflammatory nature of COMB. Our consequences are in according with previous report (Nahar et al., 2018). Hence amplified free radical creation by ISO administration (Padmanabhan et al., 2008), ISO challenged rats exhibited a drastic depleted content of total protein in the serum. 30 days treatment with COMB enriched the content of total protein in the serum. It reveals the potency of COMB to scavenge free radicals. Our outcome is in accordance with past report (Nagasaraswathi et al., 2013).
5. Conclusion
Amelioration of cardiac markers, anti-oxidant and membrane bound enzymes, electrolytes, inflammatory markers and TPC by 30 days intake of COMB. Thus our examination reveals the cardio-protective potency of COMB in ISO induced oxidative stress in rats.
Declaration of Competing Interest
The authors declared that there is no conflict of interest.
Acknowledgements
Appreciations of all authors for giving funding through research group number (RG-1438-058) by Deanship of Scientific Research at King Saud University, Saudi Arabia.
Footnotes
Peer review under responsibility of King Saud University.
References
- Althaf Hussain S., Kareem M.A., Rasool S.N., Al Omar S.Y., Saleh A., Al-Fwuaires M.A., Daddam J.R., Devi K.L. Trace element determination and cardioprotection of terminalia pallida fruit ethanolic extract in isoproterenol induced myocardial infarcted rats by ICP-MS. Biol. Trace Elem. Res. 2018;181:112–121. doi: 10.1007/s12011-017-1037-8. [DOI] [PubMed] [Google Scholar]
- Boarescu P.-M., Chirilă I., Bulboacă A.E., Bocșan I.C., Pop R.M., Gheban D., Bolboacă S.D. Effects of curcumin nanoparticles in isoproterenol-induced myocardial infarction. Oxid. Med. Cell. Longev. 2019 doi: 10.1155/2019/7847142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonting S.L. Membrane and ion transport. In: Bilter E.E., editor. presence of Enzyme Systems in Mammalian Tissues. Wiley Interscience; London: 1970. pp. 257–263. [Google Scholar]
- Clausen T. Physiology of Non-Excitable Cells: Proceedings of the 28th International Congress of Physiological Sciences. 1981. The hormonal regulation of active electrogenic Na+-K+- transport in skeletal muscle; pp. 209–220. [Google Scholar]
- Das S., Das D.K. Anti-inflammatory responses of resveratrol. Inflamm. Allergy Drug Targ. 2007;6:168–173. doi: 10.2174/187152807781696464. [DOI] [PubMed] [Google Scholar]
- Do R., Stitziel N.O., Won H.H., Jørgensen A.B., Duga S., Merlini P.A., Kiezun A., Farrall M., Goel A., Zuk O., Guella I., Asselta R., Lange L.A., Peloso G.M., Auer P.L., Girelli D., Martinelli N., Farlow D.N., DePristo M.A., Roberts R., Stewart A.F.R., Saleheen D., Danesh J., Epstein S.E., Sivapalaratnam S., Hovingh G.K., Kastelein J.J., Samani N.J., Schunkert H., Erdmann J., Shah S.H., Kraus W.E., Davies R., Nikpay M., Johansen C.T., Wang J., Hegele R.A., Hechter E., Marz W., Kleber M.E., Huang J., Johnson A.D., Li M., Burke G.L., Gross M., Liu Y., Assimes T.L., Heiss G., Lange E.M., Folsom A.R., Taylor H.A., Olivieri O., Hamsten A., Clarke R., Reilly D.F., Yin W., Rivas M.A., Donnelly P., Rossouw J.E., Psaty B.M., Herrington D.M., Wilson J.G., Rich S.S., Bamshad M.J., Tracy R.P., Cupples L.A., Rader D.J., Reilly M.P., Spertus J.A., Cresci S., Hartiala J., Tang W.H.W., Hazen S.L., Allayee H., Reiner A.P., Carlson C.S., Kooperberg C., Jackson R.D., Boerwinkle E., Lander E.S., Schwartz S.M., Siscovick D.S., McPherson R., Tybjaerg-Hansen A., Abecasis G.R., Watkins H., Nickerson D.A., Ardissino D., Sunyaev S.R., O’Donnell C.J., Altshuler D., Gabriel S., Kathiresan S. Exome sequencing identifies rare LDLR and APOA5 alleles conferring risk for myocardial infarction. Nature. 2015;518:102–106. doi: 10.1038/nature13917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong Q., Wu Z., Li X., Yan J., Zhao L., Yang C., Lu J., Deng J., Chen M. Resveratrol ameliorates cardiac dysfunction induced by pressure overload in rats via structural protection and modulation of Ca2+ cycling proteins. J. Transl. Med. 2014;12:323. doi: 10.1186/s12967-014-0323-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellman G.L. Tissue sulfhydryl groups. Arch. Biochem. Biophys. 1959;82:70–77. doi: 10.1016/0003-9861(59)90090-6. [DOI] [PubMed] [Google Scholar]
- Euteneuer F., Mills P.J., Rief W., Ziegler M.G., Dimsdale J.E. Association of in vivo β-adrenergic receptor sensitivity with inflammatory markers in healthy subjects. Psychosom. Med. 2012;74:271–277. doi: 10.1097/PSY.0b013e318245d762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garg M., Khanna D. Exploration of pharmacological interventions to prevent isoproterenol-induced myocardial infarction in experimental models. Ther. Adv. Cardiovasc. Dis. 2014;8:155–169. doi: 10.1177/1753944714531638. [DOI] [PubMed] [Google Scholar]
- Habig W.H., Pabst M.J., Jakoby W.B. Glutathione S transferases. The first enzymatic step in mercapturic acid formation. J. Biol. Chem. 1974;249:7130–7139. [PubMed] [Google Scholar]
- Hamsika C., Sakeena Q., Ramakrishnan G., Sugumar M., Sathyanarayan B., Setty M.V., Tousif A.H., Chethan N., Abid B., Bipul R., Chidambaram S.B. Protective role of heartogen against myocardial infarction in rats. Int. J. Nutr. Pharmacol. Neurol. Dis. 2018;8:92–100. [Google Scholar]
- Hjertén S., Pan H. Purification and characterization of two forms of a low-affinity Ca2+-ATPase from erythrocyte membranes. Biochim. Biophys. Acta. 1983;728:281–288. doi: 10.1016/0005-2736(83)90480-7. [DOI] [PubMed] [Google Scholar]
- Kako K., Kato M., Matsuoka T., Mustapha A. Depression of membrane-bound Na+-K+-ATPase activity induced by free radicals and by ischemia of kidney. Am. J. Physiol. 1988;254:C330–C337. doi: 10.1152/ajpcell.1988.254.2.C330. [DOI] [PubMed] [Google Scholar]
- Kamath D.Y., Xavier D., Sigamani A., Pais P. High sensitivity C-reactive protein (hsCRP) & cardiovascular disease: an Indian perspective. Indian J. Med. Res. 2015;142:261–268. doi: 10.4103/0971-5916.166582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanamori H., Takemura G., Goto K., Tsujimoto A., Ogino A., Takeyama T., Kawaguchi T., Watanabe T., Morishita K., Kawasaki M., Mikami A., Fujiwara T., Fujiwara H., Seishima M., Minatoguchi S. Resveratrol reverses remodeling in hearts with large, old myocardial infarctions through enhanced autophagy-activating AMP kinase pathway. Am. J. Pathol. 2013;182:701–713. doi: 10.1016/j.ajpath.2012.11.009. [DOI] [PubMed] [Google Scholar]
- Karthick M., Stanely Mainzen Prince, P. Preventive effect of rutin, a bioflavonoid, on lipid peroxides and antioxidants in isoproterenol-induced myocardial infarction in rats. J. Pharm. Pharmacol. 2006;58:701–707. doi: 10.1211/jpp.58.5.0016. [DOI] [PubMed] [Google Scholar]
- Karthikeyan K., Sarala Bai B.R., Niranjali Devaraj S. Grape seed proanthocyanidins ameliorates isoproterenol-induced myocardial injury in rats by stabilizing mitochondrial and lysosomal enzymes: an in vivo study. Life Sci. 2007;81:1615–1621. doi: 10.1016/j.lfs.2007.09.033. [DOI] [PubMed] [Google Scholar]
- Lowry O.H., Rosebrough N.J., Farr A.L., Randall R.J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- Mangalanathan M., Devendhiran T., Uthamaramasamy S., Kumarasamy K., Mohanraj K., Devendhiran K., Ilavarasan L., Lin M.-C. Efficacy of Zanthoxylum armatum fruit on isoproterenol induced myocardial infarction in rats. South Asian J. Eng. Technol. 2019;8:4–11. [Google Scholar]
- McCord J.M. Free radicals and myocardial ischemia: overview and outlook. Free Radic. Biol. Med. 1988;4:9–14. doi: 10.1016/0891-5849(88)90005-6. [DOI] [PubMed] [Google Scholar]
- Mounir A.M., El A.N., Azeem A.M.A. Evaluating the Efficiency of gamma Irradiated Frankincense against Isoprenaline Induced Myocardial Infarction in Rats. Pakis. J. Zool. 2019;51:219–226. [Google Scholar]
- Nagasaraswathi M., Rafi Khan P., Aleemuddin M.A., Gopi K., Sravani K. Effect of indigofera tinctoria linn against isoproterenol induced myocardial infarction on albino wistar rats. J. Curr. Chem. Pharm. Sci. 2013;3:222–230. [Google Scholar]
- Nahar K., Kabir F., Islam P., Rahman M.M., Al Mamun M.A., Faruk M., Subhan N., Rahman G.M.S., Reza H.M., Alam M.A. Cardioprotective effect of Amaranthus tricolor extract in isoprenaline induced myocardial damage in ovariectomized rats. Biomed. Pharmacother. 2018;103:1154–1162. doi: 10.1016/j.biopha.2018.04.151. [DOI] [PubMed] [Google Scholar]
- Niskanen L.K., Laaksonen D.E., Nyyssönen K., Alfthan G., Lakka H.-M., Lakka T.A., Salonen J.T. Uric acid level as a risk factor for cardiovascular and all-cause mortality in middle-aged men: a prospective cohort study. Arch. Intern. Med. 2004;164:1546–1551. doi: 10.1001/archinte.164.14.1546. [DOI] [PubMed] [Google Scholar]
- Ohnishi T., Suzuki T., Suzuki Y., Ozawa K. A comparative study of plasma membrane Mg2+-ATPase activities in normal, regenerating and malignant cells. Biochim. Biophys. Acta. 1982;684:67–74. doi: 10.1016/0005-2736(82)90050-5. [DOI] [PubMed] [Google Scholar]
- Padmanabhan M., Rajadurai M., Prince P.S.M. Preventive effect of S-allylcysteine on membrane-bound enzymes and glycoproteins in normal and isoproterenol-induced cardiac toxicity in male Wistar rats. Basic Clin. Pharmacol. Toxicol. 2008;103:507–513. doi: 10.1111/j.1742-7843.2008.00244.x. [DOI] [PubMed] [Google Scholar]
- Patel V., Upaganlawar A., Zalawadia R., Balaraman R. Cardioprotective effect of melatonin against isoproterenol induced myocardial infarction in rats: a biochemical, electrocardiographic and histoarchitectural evaluation. Eur. J. Pharmacol. 2010;644:160–168. doi: 10.1016/j.ejphar.2010.06.065. [DOI] [PubMed] [Google Scholar]
- Prasad E.M., Mopuri R., Islam M.S., Kodidhela L.D. Cardioprotective effect of Vitex negundo on isoproterenol-induced myocardial necrosis in wistar rats: a dual approach study. Biomed. Pharmacother. 2017;85:601–610. doi: 10.1016/j.biopha.2016.11.069. [DOI] [PubMed] [Google Scholar]
- Raish M., Ahmad A., Ansari M.A., Alkharfy K.M., Ahad A., Khan A., Ali N., Ganaie M.A., Hamidaddin M.A.A. Beetroot juice alleviates isoproterenol-induced myocardial damage by reducing oxidative stress, inflammation, and apoptosis in rats. 3 Biotech. 2019;9(4):147. doi: 10.1007/s13205-019-1677-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajadurai M., Prince P.S.M. Preventive effect of naringin on isoproterenol-induced cardiotoxicity in Wistar rats: an in vivo and in vitro study. Toxicology. 2007;232:216–225. doi: 10.1016/j.tox.2007.01.006. [DOI] [PubMed] [Google Scholar]
- Rotruck J.T., Pope A.L., Ganther H.E., Swanson A.B., Hafeman D.G., Hoekstra W. Selenium: biochemical role as a component of glutathione peroxidase. Science. 1973;179:588–590. doi: 10.1126/science.179.4073.588. [DOI] [PubMed] [Google Scholar]
- Sharma E., Kushwah A.S., Singh T. Evaluation of the cardioprotective role of 7-hydroxy-4-methylcoumarin on isoproterenol induced myocardial infarction in rats. MOJ Drug Design Dev. Theapy. 2019;3:12–17. [Google Scholar]
- Shen Z., Geng Q., Huang H., Yao H., Du T., Chen L., Wu Z., Miao X., Shi P. Antioxidative and cardioprotective effects of schisandra chinensis bee pollen extract on isoprenaline-induced myocardial infarction in rats. Molecules. 2019;24:1090. doi: 10.3390/molecules24061090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srinivasulu C., Ramgopal M., Ramanjaneyulu G., Anuradha C.M., Suresh Kumar C. Syringic acid (SA) – a review of its occurrence, biosynthesis, pharmacological and industrial importance. Biomed. Pharmacother. 2018;108:547–557. doi: 10.1016/j.biopha.2018.09.069. [DOI] [PubMed] [Google Scholar]
- Tanaka T., Kawaguchi N., Zaima N., Moriyama T., Fukuta Y., Shirasaka N. Antiosteoporotic activity of a syringic acid diet in ovariectomized mice. J. Nat. Med. 2017;71:632–641. doi: 10.1007/s11418-017-1105-6. [DOI] [PubMed] [Google Scholar]
- Thippeswamy B.S., Thakker S.P., Tubachi S., Kalyani G.A., Netra M.K., Patil U., Desai S., Gavimath C.C., Veerapur V.P., Nagar S.R., Nagar S.R., Lingayat K., Campus J., Bagh U., Nagar S.R. Cardioprotective effect of cucumis trigonus roxb on isoproterenol-induced myocardial infarction in rat. Am. J. Pharmacol. Toxicol. 2009;4:29–37. [Google Scholar]
- Varadi G., Mori Y., Mikala G., Schwartz A. Molecular determinants of Ca2+ channel function and drug action. Trends Pharmacol. Sci. 1995;16:43–49. doi: 10.1016/s0165-6147(00)88977-4. [DOI] [PubMed] [Google Scholar]
- Xi J., Wang H., Mueller R.A., Norfleet E.A., Xu Z. Mechanism for resveratrol-induced cardioprotection against reperfusion injury involves glycogen synthase kinase 3beta and mitochondrial permeability transition pore. Eur. J. Pharmacol. 2009;604:111–116. doi: 10.1016/j.ejphar.2008.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young, Donald Stirling. fourth ed. AACC Press; Washington D.C: 1995. Effects of Drugs on Clinical Laboratory Tests. [Google Scholar]
- Zaafan M.A., Zaki H.F., El-Brairy A.I., Kenawy S.A. Protective effects of atorvastatin and quercetin on isoprenaline-induced myocardial infarction in rats. Bull. Fac. Pharm. Cairo Univ. 2013;51:35–41. [Google Scholar]