Abstract
Cancer is one of the most impacting life-threatening disease for the human populace. Hence, over the years we have seen a consistent interest to study and investigate new treatments to cure and prevent this disease. Medicinal plants have played a progressive part in treatment since many years. In this research study, we have explored the cytotoxicity effect of purified bioactive compound isolated from Stevia rebaudiana leaves and the key mechanism responsible for apoptosis in human breast cancer cells. The anticancer properties of Stevia rebaudiana leaves has been suggested in earlier literature. Hence, the aim of this study was to investigate the cytotoxicity of purified stevioside in human breast cancer cell lines MDA-MB-231 and SKBR3. Results showed that purified stevioside inhibited the growth of cancerous cell lines. The IC50 obtained after treatment with stevioside on cancer cells MDA-MB-231 and SKBR3 are 55 µM and 66 µM respectively. This shows purified stevioside is capable of inducing apoptosis indicating its promising anticancer activity. However, so far chemosensitization effects of stevioside on breast cancer have not been fully explained by other studies. Hence, additionally, this study also evaluates the chemosensitization potential of stevioside in combination with 5-FU. This research study shows the importance of Stevia rebaudiana as a good source of bioactive compounds with high anti-cancer property.
Keywords: Cancer, Cytotoxicity effects, Stevia rebaudiana, Apoptosis, MDA-MB-231, SKBR3
1. Introduction
In 2011, Cancer emerged as the ‘top killer’. There are some studies which indicate that the number of cancer cases and deaths will be doubled by 2030. Among the various cancers known to mankind, Breast cancer is the most prevalent cancer that affects many women across the world. These cases are particularly on rise in some Asian countries like India, Japan. In India, breast cancer leads to death of about 50,000 women every year (Kumar et al., 2013). In western countries, the number of breast cancer cases has been stable or has declined in the recent past. Yet, the disease is a key contributor to cancer-related deaths and incidents of the disease in younger women worldwide, 25 years to 35 years, and deaths (Youlden et al., 2014) have also raised the concerns over the disease highlighting the urgent need to invest in studies in breast cancer preventive and cure strategies.
The current methods of cancer treatments, chemo and radiotherapies, though are popular in this disease space, have many immeasurable side effects, making it imperative to explore options that come with minimal side effects. The major challenges thrown by the chemotherapy are the extreme side effects and chemoresistance which occur due to upregulation of the survival signals. Chemosensitizers are used to increase the efficacy of conventional chemotherapy by downregulating survival signals (Eldahshan., 2017).Curcumin, genistein, green tea polyphenols, resveratrol, ursolic acid are some of the natural compounds that can be used in sensitizing tumor cells to chemotherapeutic mediums by hindering pathways that lead resistance of treatment. In a recent study, it has been shown that resveratrol sensitizes TRAIL by inhibiting p53 gene and it also potentiates cisplatin when given in combination in lung cancer (Rasheduzzaman et al., 2018, Hu et al., 2016). Plants are the immense source of bioactive compound and can have anti-cancer and sensitization potential (Svejda et al., 2010, Shanmugam et al., 2012).
A number of studies have shown that plant derived compound and marine extracts are beneficial in the treatment of cancer (Ho and Pan, 2013, Noolu et al., 2013, Ouhtit et al., 2013, Srivastava et al., 2014). This suggests that some easily available food ingredients and herbs might have anti-cancer and anti-tumor properties. Therefore, in this study, we have investigated the efficacy of purified component (bioactive compound), Stevioside to increase the impact of conventional drug available for treatment of cancer on breast cancer cell lines MDA-MB-231 and SKBR3.
Stevia rebaudiana Bertoni, also known as Sweet-Leaf, belongs to the plant family-Asteraceae and is an herbaceous perennial shrub. Several past studies reveal that it has good anticancer properties. Stevioside, a major component of Stevia rebaudiana leaf is used as a sweetener in many parts of the world as they are non-caloric and sweeter then sucrose (Hai et al., 2017). Few studies have suggested that stevioside may also have therapeutic effect as they possess immunomodulatory, anti-inflammatory, anti-hypertensive and antitumor properties.
In this study, work was carried out to check the cytotoxic activity of purified stevioside isolated from Stevia rebaudiana leaves and its inhibition effect against the growth of breast cancer cells MDA-MB-231 and SKBR3. Further, investigation was also done to study the ability of purified stevioside to potentiate the chemo-sensitivity of breast cancer cells to conventional drug 5-FU.
2. Materials and methods
2.1. Cell lines and cell culture
The cancer cell lines MDA-MB-231, SKBR3 were procured from National Centre for Cell Science, Pune. These cell lines were cultured in Dulbecco's Modified Eagle's Medium (DMEM) supplemented with Fetal bovine serum (FBS) and penicillin at 37 °C in 5% CO2 which were obtained from Sigma Biotech. Antibodies against Caspase 3, Caspase 9, Bax, Bcl-2 and β-Actin were purchased from Cell Signalling Technology Inc. (Beverly, MA, USA). 5-Fluorouracil (5-FU) drug were purchased from sigma. 3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide (MTT), 4′,6-diamidino-2-phenylindole dihydrochloride (DAPI) and Annexin V apoptosis detection kit were obtained from Sigma Biotech. The enhanced chemiluminescence (ECL) kit was bought from Amersham Life Science. Stevioside was isolated, purified and characterized from S. rebaudiana leaves earlier as per protocol.
2.2. Cell treatment and MTT assay
The impact of purified stevioside on the viability of cells was identified by MTT assay. Drug treatment was performed after 24 h of seeding. Treatment was done in two phases i.e., the cells undergoing combination studies were pre-treated with 10 µM of stevioside followed by 8 h incubation. After 8 h of incubation, all the cells including those undergoing combination studies were treated with 10 µM purified stevioside, 25 µM 5-FU and the combination of both- 10 µM stevioside and 10 µM 5-FU. Incubation time was determined based on the trial. The viability of the cells was then detected by performing MTT assay. For this, 20 μL of MTT solution were loaded in each well, and the mixture was incubated for 4 h at 37 °C. After that, the MTT solution was separated and 120 μL of dimethyl sulfoxide was mixed to the wells (Paul et al., 2012). The absorbance was then measured by utilizing a microplate reader at a wavelength of 540 nm.
2.3. Flowcytometry
The extent of apoptosis was assessed by flow cytometry using Annexin V-FITC. After post treatment with combination, breast carcinoma cells were washed with Phosphate Buffer Saline. The cells were then incubated with Annexin V-FITC and PI for 10 min in the dark. Flow cytometer Accuri C6 was used to detect to cells (Paul et al., 2012).
2.4. Western blot
Protease inhibitor and lysis buffer (1:1000) were utilized to separate the proteins from the cells. Proteins were loaded on a 10% SDS PAGE gel. After running SDS-PAGE electrophoresis, the obtained bands was transferred on polyvinylidenedifluoride (PVDF) membranes. After that, blocking was done in 5% non-fat milk for an hour at 28 °C. This was incubated with different primary antibodies overnight at 4 °C. After that, incubation was done with relevant secondary antibodies for an hour at room temperature. ECL assay kit was used to visualize the protein bands. In the treated cells, β-actin was used as internal reference for protein expression (Hai et al., 2017).
2.5. DNA fragmentation assay
Treatment was done with different concentration of purified stevioside and 5-FU followed by incubation of cells for a day. Trypsinization technique was used to collect the cells after 24 h. Equal proportion of phenol:chloroform:isoamyl alcohol was used to purify DNA. Precipitation of DNA was done with two-thirds volume of cold isopropanol followed by the centrifugation. 70% ethanol was used to wash the pellet. After washing, pellet was resuspended in deionized water. This was followed by the analysis of DNA by 1.5% agarose gel electrophoresis (Ho and Pan, 2013).
3. Results
3.1. Stevioside induced cytotoxicity on various breast cancer cells
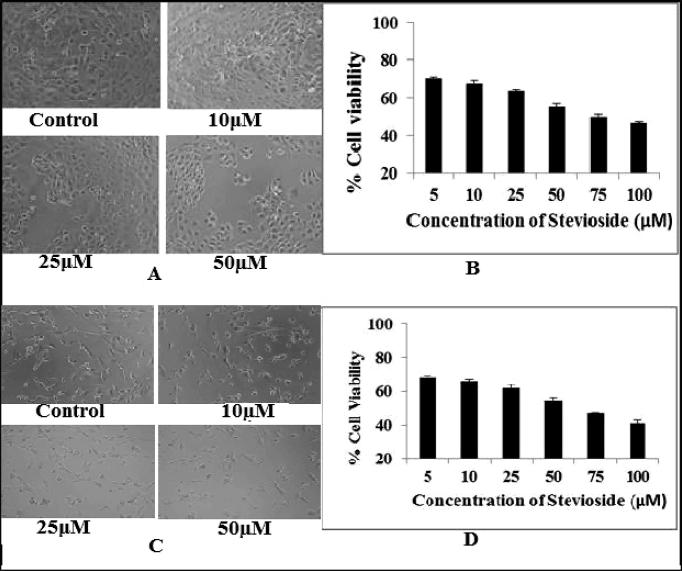
MTT Assay was performed to observe the anticancer effect of stevioside on the breast cancer cells. 96-well plate was used to seed 5000 cells. This was followed by incubation for 24 h. Cell lines were then treated with different concentrations of purified stevioside (5, 10, 25, 50, 75, 100 μM) for 24 h and 48 h. 20 µL of MTT solution was mixed to each well followed by incubation for 4 h at 37 °C. This was followed by measuring Absorbance at 540 nm wavelength and relative cell viability was then calculated as shown in Fig. 1.
Fig. 1.
(A) Represents treated SKBR3 with different concentration of Stevioside. (B) Represents relative cell viability on SKBR3 cell line treated with different concentrations of stevioside. (C) Represents treated MDA-MB-231 with different concentration of Stevioside. (D) Represents relative cell viability on MDA-MB-231 cell line with different concentration of Stevioside. MTT assay was used to determine the cell viability. All experiments were done in triplets to confirm reproducibility. P < 0.05 compared to the control group.
3.2. Stevioside sensitizes MDA-MB-231 and SKBR3 to 5-FU confirmed by cytotoxicity
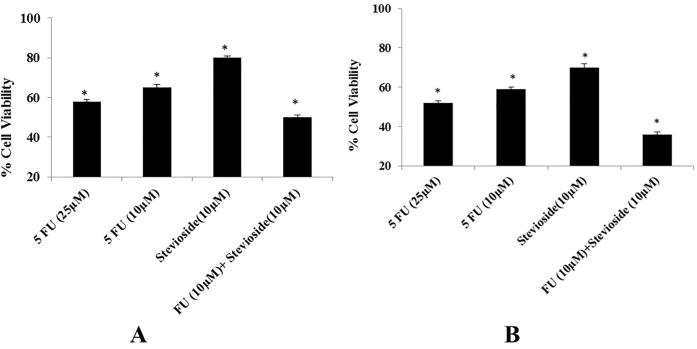
The cell Cytotoxicity shown with varied results like, MDA-MB-231 showed the highest cytotoxicity when treated with the combination of purified stevioside and 5-FU. MDA-MB-231 cells when treated with 5-FU (25 µM), the cell viability was 52%, when treated with 5-FU (10 µM) cell viability was found to be 59%, when treated with purified stevioside (10 µM) alone, it has been observed that cell viability was 70%. Whereas the combination of purified stevioside (10 µM) and 5-FU (10 µM) treated cells exhibited a relative viability of 36%. In case of SKBR3 cell line, when treated with 5-FU alone 25 µM and 10 µM, the cell viabilities were 58% and 65% respectively and when treated with purified stevioside 10 µM, viability was 80% and when treated with combination of both the observation of viability decreased to 40% as clearly shown in Fig. 2. On the view of relative cell viability calculations, MDA-MB-231 cells treated with the combination of purified stevioside 10 µM and 5-FU 10 µM, showed considerable increase in cytotoxicity of about 64% which was found to be 16% higher than the cytotoxicity obtained by 25 µM 5-FU alone and 4% higher than the additive effect of purified stevioside 10 µM and 5-FU 10 µM in SKBR3. This inveterate that the purified stevioside have potentiate the effect of 5-FU more in MDA-MB-231 as compared to SKBR3.
Fig. 2.
(A) and (B) represent relative cell viability on cell line treated SKBR3 and MDA-MB-231 respectively with 25 µM, 10 µM 5-FU alone, 10 µM stevioside alone and in combination 5-FU 10 µM & 10 µM stevioside. Experiments were done in triplets to confirm reproducibility. * P < 0.05 compared to the control group.
3.3. Sensitization effect of stevioside on breast cancer cell line to 5-FU as assessed by apoptosis and cell cycle distribution in MDA-MB-231 cell line
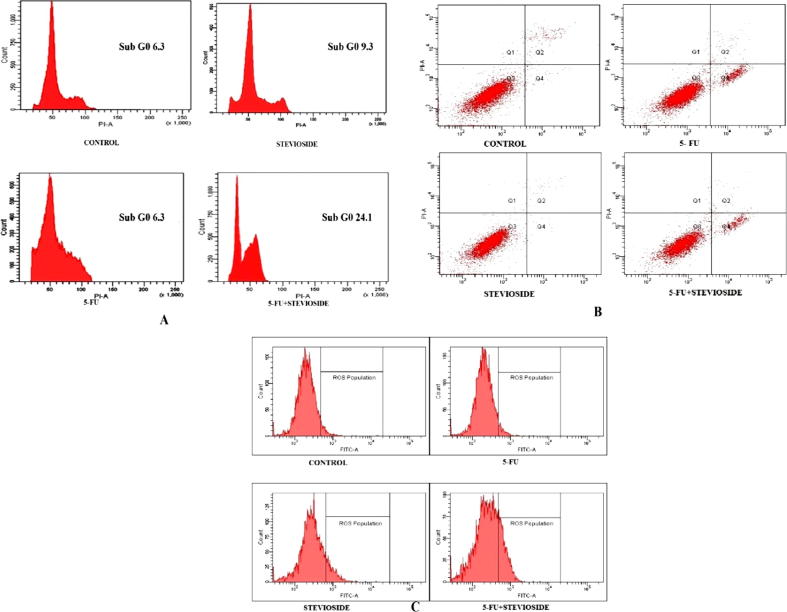
To further enumerate the apoptosis triggered on selected cell line i.e., MDA-MB-231, flow cytometry was used to analyse the percentages of apoptotic cells. After treating the cells with different concentrations of purified stevioside, standard and in combination for 24 h, the number of early and late apoptotic cells in G1, S, G2/M and sub G0 phases are found to be 61.0, 21.2, 11.5 and 6.3 respectively. In purified stevioside 10 µM alone treated cells, the percentage of cells in G1, S, G2/M and sub G0 phases are found to be 54.7, 20.3, 15.7 and 9.3 respectively. In 5-FU 10 µM alone treated cells, the percentage of cells in G1, S, G2/M and sub G0 phases were found to be 52.9, 31.3 9.5 and 6.3 respectively. In 5-FU 10 µM and purified stevioside 10 µM combination treated cells, the percentage of cells in G1, S, G2/M and sub G0 phases are found 45.7, 22.3, 7.9 and 24.1 respectively shown in Fig. 3A. The percentage of apoptosis occurred is represented by the percentage of cells in sub G0 phase. The cell of control showed 6.3% while purified stevioside alone showed 9.3% and 5-FU showed 6.3% of cells in sub G0 phase. For the cells exposed with combination of 5-FU and purified stevioside 10 µM each, the cells percentage in sub G0 phase was found to be 24.1% which is 17.7% more compared to 5-FU alone treated cells. This clearly showed that the combination is able to increase the rate of apoptosis in cells. Annexin staining reveals that combination treatment together induces apoptosis but not necrosis as demonstrated in Fig. 3B. All these results demonstrated that purified stevioside together with 5-FU could trigger early and late apoptotic events in cancer cells. We further investigated ROS level as many studies reveal ROS to be apoptosis inducer. Combination treatment also increased ROS level significantly in MDA-MB-231. DCFDA staining showed that it increased the ROS levels from an average of 5.0% to 23% within 24 h in MDA-MB-231 as compared in alone treatment shown in Fig. 3C.
Fig. 3.
(A) Alone and combination treatment of 5-FU and stevioside on cell. Cell lines were seeded after 24 h of drug treatment and stained with propidium iodide and then assayed by flowcytometry. Histograms show the percentages of cells in G1, S, G2/M and sub-G0 phases of the cell cycle. (B) Experiment was conducted to check whether the purified stevioside and 5-FU together are inducing apoptosis or necrosis. Annexin staining showed that the stevioside induced only apoptosis and not necrosis with 24 h treatment and potentiate 5-FU when given in combination. (C) Ros level significantly increased with combination treatment. All experiment was conducted in triplets to confirm reproducibility.
3.4. Sensitization effect assessed by cleavage of Caspase3/Caspase9 and Bax/Bcl-2 ratio
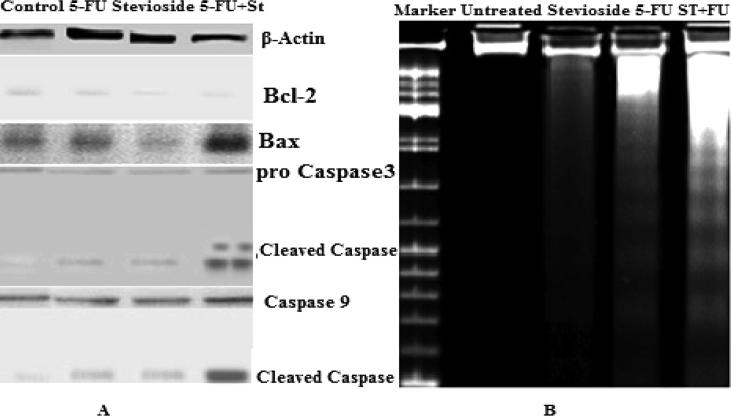
To confirm the apoptotic effect of purified stevioside on breast carcinoma cell, caspase 9 and caspase 3 activities were assessed as indicators of apoptosis. After treatment, cleavage in caspase 3 caspase 9 activities was observed in combined treated cancer cells. β Actin was utilized as an internal control reference. As shown in Fig. 4A. our experimental result indicates that in control, there were no bands whereas in purified stevioside treated cells, thin bands were found indicating less cleavage. Cleavage of caspase 3 and caspase 9 is visible in 5-FU treated cells but in combination treated cells, thick cleavage band of caspase 9 and caspase 3 has been observed as compared with alone treated cells which designate the additive effect of purified stevioside. The apoptosis is also associated with upregulation of pro-apoptotic proteins and with the retardation of anti-apoptotic proteins (Konopleva et al., 2002). Bcl-2 and Bax are important proteins that take part in apoptosis. Investigational outcome showed that combination treatment of 5-FU along with the stevioside increased the ratio of Bax to Bcl-2 in MDA-MB-231 thereby triggering apoptosis as compared to alone treatment of 5-FU or purified compound.
Fig. 4.
(A) Western blots demonstrated that purified stevioside enhanced the activity of 5-FU by inducing caspase cleavage in MDA-MB-231 cells and also it increases bax/Bcl-2 ratio. Cell lysates were prepared after treating MDA-MB-231 cells with combination and alone treatment after 48 h and examined by anti-caspase antibodies. (B) DNA fragmentation was visualized on agarose gel. All experiments were done in triplet to confirm the reproducibility.
3.5. Stevioside sensitizes MDA-MB-231 examined through DNA fragmentation
DNA fragmentation study is the most important part to utilize for the location of apoptosis. DNA fracture investigation of treated cells demonstrated a laddering design, cells untreated has no bands, cells treated alone with purified stevioside have very less bands as shown below, while for cells treated with purified stevioside and 5-FU, fragmentation is more which is normal for apoptosis, showing internucleosomal DNA degradation shown in the Fig. 4B.
4. Discussion
Search for new substances with pharmaceutical benefits have been carried out since many years. Amid the most recent twenty years, there has been a recovery of enthusiasm to explore natural products, as they make up half of the medications utilized clinically in developed countries, of which 25% come from higher plants. In recent years, scientists have been focusing on dietary phytochemicals after revelation about their action in treating various cancers (Tan et al., 2011). Studies from across the world have found that phytochemicals present in medicinal plants inhibit cell proliferation and induce apoptosis and thereby retarding the growth of various cancer cells. The polyphenolic compounds found in plants also demonstrate several pharmacological properties such as antioxidant and anti-carcinogenic activities (Wahle et al., 2010, Yoshida et al., 2010, Arul and Subramanian, 2013). Several studies conducted on both cell lines and animal tissues emphasized that the natural plant extract, with multiple bioactive molecules, has several advantages compared to single therapeutics (Carvalho et al., 2010, Ovadje et al., 2014). Few studies conducted on cell line demonstrated the anti-cancer property of Stevioside. It induced antitumor activity in colon cancer (Hai et al., 2017). Study also suggested that it also induces apoptosis through ROS regulation in MCF 7 breast cancer cell line (Paul et al., 2012). The study following to the present work with purified Stevioside isolated from Stevia rebaudiana leaves affirmed the anticancer activity and sensitization effect on breast cancer cell line MDA-MB-231 and SKBR3. Cell viability assays were conducted on the MDA-MB-231 and SKBR3 cancer cell lines to assess the relative toxicity of the purified stevioside. The toxicity of stevioside was found active and higher against MDA-MB-231 followed by SKBR3, with cell viability decreasing with increasing drug concentration. This propensity is upheld by past investigations showing a connection between tests with high antioxidant activity and anti-cancer properties (Dai and Mumper, 2010, Wani et al., 2013). We further investigated caspase 3 caspase 9 and Bax/Bcl-2 ratio as they play crucial role in apoptosis. Treatment with 5-FU along with stevioside on caspase 3 and caspase 9 shows activated caspases which underlying the mechanism of apoptosis and we also found Bax is upregulated and Bcl-2 is down regulated as investigated by Western blot (Ouyang et al., 2012, Guerra-Vladusic et al., 1999). To facilitate bits of knowledge into the method of cell death caused by Stevioside, its impact on the DNA fragmentation which is the most important part for the induction of apoptosis, was examined. DNA fracture investigation of purified stevioside treated cells along with 5-FU demonstrated a laddering design, which is normal for apoptosis, showing internucleosomal DNA degradation.
5. Conclusion
In conclusion, the study has demonstrated that stevioside compound isolated from S. rebaudiana leaves exerts an anti-tumor activity on breast cancer cell lines by restraining cell proliferation on MDA-MB-231 and SKBR3. In addition, the result also demonstrated that purified stevioside, in vitro, can amplify the chemosensitivity of breast cancer cells to 5-FU treatments. However, further investigations are needed to study this phenomenon and its effects in detail.
Conflict of interest
Authors declare that there is no conflict of interest.
Acknowledgement
The authors are thankful to Department of Bioengineering, Birla Institute of Technology, Mesra Ranchi – Jharkhand, Central Drug Research Institute, Lucknow – Uttar Pradesh and Synteny Life Sciences Hyderabad – Telangana to provide facilitation for the research work.
Footnotes
Peer review under responsibility of King Saud University.
References
- Arul D., Subramanian P. Inhibitory effect of naringenin (citrus flavonone) on N-nitrosodiethylamine induced hepatocarcinogenesis in rats. Biochem. Biophys. Res. Commun. 2013;434(2):203–209. doi: 10.1016/j.bbrc.2013.03.039. [DOI] [PubMed] [Google Scholar]
- Carvalho M., Ferreira P.J., Mendes V.S., Silva R., Pereira J.A., Jerónimo C., Silva B.M. Human cancer cell antiproliferative and antioxidant activities of Juglansregia L. Food Chem. Toxicol. 2010;48(1):441–447. doi: 10.1016/j.fct.2009.10.043. [DOI] [PubMed] [Google Scholar]
- Dai J., Mumper R.J. Plant phenolics: extraction, analysis and their antioxidant and anticancer properties. Molecules. 2010;15(10):7313–7352. doi: 10.3390/molecules15107313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eldahshan O.A. Chemosensitization of tumors by natural compounds. Int. J. Pharm. Chin. Med. 2017;1(2) [Google Scholar]
- Guerra-Vladusic F.K., Scott G., Weaver V., Vladusic E.A., Tsai M.S., Benz C.C., Lupu R. Constitutive expression of Heregulin induces apoptosis in an erbB-2 overexpressing breast cancer cell line SKBr-3. Int. J. Oncol. 1999;15(5):883–975. doi: 10.3892/ijo.15.5.883. [DOI] [PubMed] [Google Scholar]
- Hai P.R., Xiao Y.Y., Hai Y.Y., Hai F.X. Stevioside induced cytotoxicity in colon cancer cells via reactive oxygen species and mitogen-activated protein kinase signaling pathways-mediated apoptosis. Oncol. Lett. 2017;13:2337–2343. doi: 10.3892/ol.2017.5744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho Y.S., Pan M.H. Nutrition, Functional and Sensory Properties of Foods. 2013. Tea extracts confer its antiproliferating effects through inhibition of nicotine-and estrogen-induced 9-nicotinic acetylcholine receptor upregulation in human breast cancer cells; pp. 256–268. [Google Scholar]
- Hu S., Li X., Xu R., Ye L., Kong H., Zeng X., Wang H., Xie W. The synergistic effect of resveratrol in combination with cisplatin on apoptosis via modulating auto phagy in A549 cells. Acta Biochim. Biophys. Sin. 2016;48(6):528–535. doi: 10.1093/abbs/gmw026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konopleva M., Konoplev S., Hu W., Zaritskey A.Y., Afanasiev B.V., Andreeff M. Stromal cells prevent apoptosis of AML cells by up-regulation of anti-apoptotic proteins. Leukemia. 2002;16(9):1713–1724. doi: 10.1038/sj.leu.2402608. [DOI] [PubMed] [Google Scholar]
- Kumar P., Bolshette N.B., Jamdade V.S., Mundhe N.A., Thakur K.K., Saikia K.K., Lahkar M. Breast cancer status in India: an overview. Biomed. Prevent. Nutr. 2013;3(2):177–183. [Google Scholar]
- Noolu B., Ajumeera R., Chauhan A., Nagalla B., Manchala R., Ismail A. Murraya koenigii leaf extract inhibits proteasome activity and induces cell death in breast cancer cells. BMC Complem. Altern. Med. 2013;13(1):7. doi: 10.1186/1472-6882-13-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouhtit A., Gaur R.L., Abdraboh M., Ireland S.K., Rao P.N., Raj S.G., Hollenbach A. Simultaneous inhibition of cell-cycle, proliferation, survival, metastatic pathways and induction of apoptosis in breast cancer cells by a phytochemical super-cocktail: genes that underpin its mode of action. J. Cancer. 2013;4(9):703–715. doi: 10.7150/jca.7235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouyang L., Shi Z., Zhao S., Wang F.T., Zhou T.T., Liu B., Bao J.K. Programmed cell death pathways in cancer: a review of apoptosis, autophagy and programmed necrosis. Cell Prolif. 2012;45(6):487–498. doi: 10.1111/j.1365-2184.2012.00845.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ovadje P., Ma D., Tremblay P., Roma A., Steckle M., Guerrero J.A., Pandey S. Evaluation of the efficacy & biochemical mechanism of cell death induction by piper longum extract selectively in in-vitro and in-vivo models of human cancer cells. PloS One. 2014;9(11) doi: 10.1371/journal.pone.0113250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul S., Sengupta S., Bandoupadhaya T.K., Bhattacharya A. Stevioside induced ROS-mediated apoptosis through mitochondrial pathway in human breast cancer cell line MCF-7. Nutr. Cancer. 2012;64(7):1087–1094. doi: 10.1080/01635581.2012.712735. [DOI] [PubMed] [Google Scholar]
- Rasheduzzaman M., Jeong J.K., Park S.Y. Resveratrol sensitizes lung cancer cell to TRAIL by p53 independent and suppression of Akt/NF-kB signaling. Life Sci. 2018;208:208–220. doi: 10.1016/j.lfs.2018.07.035. [DOI] [PubMed] [Google Scholar]
- Shanmugam S., Rajendran K., Suresh K. Traditional uses of medicinal plants among the rural people in Sivagangai district of Tamil Nadu, Southern India. Asian Pac. J. Trop. Biomed. 2012;2(1):S429–S434. [Google Scholar]
- Svejda B., Aguiriano-Moser V., Sturm S., Höger H., Ingolic E., Siegl V., Pfragner R. Anticancer activity of novel plant extracts from Trailliaedoxa gracilis (WW Smith & Forrest) in human carcinoid KRJ-I cells. Anticancer Res. 2010;30(1):55–64. [PubMed] [Google Scholar]
- Srivastava M., Hegde M., Chiruvella K.K., Koroth J., Bhattacharya S., Choudhary B., Raghavan S.C. Sapodilla plum (Achrassapota) induces apoptosis in cancer cell lines and inhibits tumor progression in mice. Sci. Rep. 2014;4:6147. doi: 10.1038/srep06147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan A.C., Konczak I., Sze D.M.Y., Ramzan I. Molecular pathways for cancer chemoprevention by dietary phytochemicals. Nutr. Cancer. 2011;63(4):495–505. doi: 10.1080/01635581.2011.538953. [DOI] [PubMed] [Google Scholar]
- Wahle K.W., Brown I., Rotondo D., Heys S.D. Bio-Farms for Nutraceuticals. 2010. Plant phenolics in the prevention and treatment of cancer; pp. 36–51. [DOI] [PubMed] [Google Scholar]
- Wani B.A., Ramamoorthy D., Rather M.A., Arumugam N., Qazi A.K., Majeed R., Hamid A., Ganie S.A., Gania B.A., Anand R., Gupta A.P. Induction of apoptosis in human pancreatic MiaPaCa-2 cells through the loss of mitochondrial membrane potential (ΔΨm) by Gentianakurroo root extract and LC-ESI-MS analysis of its principal constituents. Phytomedicine. 2013;20(8–9):723–733. doi: 10.1016/j.phymed.2013.01.011. [DOI] [PubMed] [Google Scholar]
- Yoshida H., Takamura N., Shuto T., Ogata K., Tokunaga J., Kawai K., Kai H. The citrus flavonoids hesperetin and naringenin block the lipolytic actions of TNF-α in mouse adipocytes. Biochem. Biophys. Res. Commun. 2010;394(3):728–732. doi: 10.1016/j.bbrc.2010.03.060. [DOI] [PubMed] [Google Scholar]
- Youlden D.R., Cramb S.M., Yip C.H., Baade P.D. Incidence and mortality of female breast cancer in the Asia-Pacific region. Cancer Biol. Med. 2014;11(2):101–115. doi: 10.7497/j.issn.2095-3941.2014.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]