Introduction
Drug-induced hypersensitivity syndrome (DIHS), also known as drug reaction with eosinophilia and systemic symptoms (DRESS), is a severe reaction to medications that presents with rash, fever, and lymphadenopathy. Patients typically have eosinophilia and end-organ damage, most commonly to the kidneys or liver. If the heart is involved, either hypersensitivity myocarditis or acute necrotizing eosinophilic myocarditis (ANEM) can occur; the former is often reversible, and the latter is usually fatal.1 DIHS can persist for months after drug withdrawal, and different organ systems can be affected at different times. Symptoms can persist for a year or more.1 DIHS is treated with high doses of corticosteroids, which may be administered for months.
DIHS is a type IV hypersensitivity reaction resulting in a T helper cell 2 (Th2)–predominant immune response with recruitment and activation of eosinophils. Interleukin 5 (IL-5), an eosinophil activator, and eosinophil chemokines, C-C motif chemokine ligand (CCL)17 and CCL22, are involved in DIHS and other eosinophilic disorders.2, 3, 4, 5, 6 In DIHS, other cytokines including IL-6, IL-10, and IL-13 are also thought to play a role in pathogenesis.7, 8 IL-5 blockers can be used to treat some eosinophilic disorders but these agents do not block these other pathogenic cytokines. However, all of these cytokines rely on the Janus kinase–signal transducer and activator of transcription (JAK-STAT) pathway and can be simultaneously targeted using JAK inhibitors.
It is not known whether molecularly targeted small molecule inhibitors are effective or work rapidly enough to treat severe drug reactions such as DIHS. Here we report 2 consecutive patients with severe DIHS with myocardial involvement treated with the JAK inhibitor tofacitinib.
Patient 1
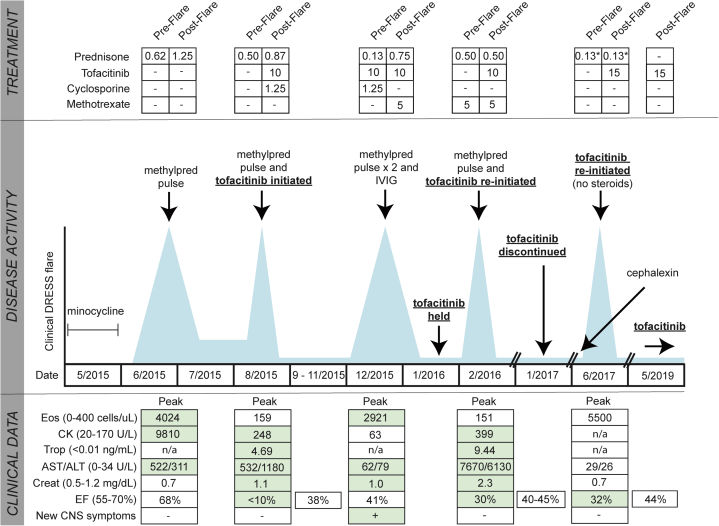
A 37-year-old woman developed an exanthematous rash, facial edema, fever, and lymphadenopathy 3 weeks after starting minocycline. She was found to have elevated transaminases and eosinophilia (1035 cells/μL). Her RegiSCAR9 score was 4 and DIHS was diagnosed (Fig 1; see Supplement). She was started on prednisone 80 mg once daily but had worsening eosinophilia (4024 cells/μL), increasing transaminase elevation, and creatine kinase elevation. Magnetic resonance imaging of multiple anatomic areas showed rhabdomyolysis, which can be observed in DIHS.10 Pulse methylprednisolone led to improvement, and the patient was transitioned to a prednisone taper. One month later, taking prednisone 40 mg daily, she became dyspneic and was found to have troponin elevation and biventricular heart failure with left ventricular ejection fraction (LVEF) less than 10%, consistent with ANEM (see Supplement). She was treated with an intra-aortic balloon pump, vasopressors, and pulse methylprednisolone. Her LVEF recovered, and she was transitioned to a prednisone taper plus cyclosporine. Given the life-threatening nature of her disease, similarities between DIHS and hypereosinophilic syndrome, and our experience treating hypereosinophilic syndrome with tofacitinib,11 tofacitinib 5 mg twice daily was also initiated.
Fig 1.
Summary of clinical course in patient 1. Treatments before and after clinical DIHS flare are listed in the top panel. Doses of prednisone and cyclosporine are shown as milligram per kilogram per day. Doses of tofacitinib are milligrams per day and methotrexate milligrams per week. Methylprednisolone was given at 1 g daily for 3-5 days. Intravenous immunoglobulin (IVIG) was given at 2 g/kg divided over 5 days. Middle panel shows DIHS activity/disease flares (blue spikes), but are not to scale. Lower panel shows the most relevant clinical data during DIHS flares, including ejection fraction (EF) during and after recovery from the flare, when possible. Green highlight signifies an abnormal value. Asterisk signifies every other day. ALT, alanine aminotransferase; AST, aspartate aminotransferase; CK, creatine kinase; CNS, central nervous system; Creat, creatinine; Eos, eosinophils; n/a, data not available; Trop, troponin. Additional details for this case can be found in the Supplement.
While tapering prednisone (to 10 mg daily), diplopia, flaccid bilateral lower extremity weakness, and urinary retention developed, and DIHS flare involving the central nervous system was diagnosed. Other causes for her neurologic symptoms were evaluated and ruled out (Fig 1; see Supplement). She was treated with pulse methylprednisolone and intravenous immunoglobulin. She was discharged on prednisone, methotrexate, and tofacitinib, 5 mg twice daily.
She was stable for 4 weeks until tofacitinib was discontinued due to insurance issues. Nine days later, while taking prednisone, 40 mg daily, and methotrexate, she was admitted in cardiogenic shock with recurrent ANEM requiring intra-aortic balloon pump, vasopressors, and pulse methylprednisolone. Tofacitinib, 5 mg twice daily, was restarted. She had significant recovery of her LVEF and was discharged 2.5 weeks later on a prednisone taper, methotrexate, and tofacitinib, 5 mg twice daily.
She was stable for 10 months, and so tofacitinib was discontinued; at that time she was also taking prednisone, 10 mg every other day. Five months later she presented with fever and malaise (see Supplement). Laboratory evaluation showed peripheral eosinophilia (5,500 cells/μL). A relapse of DIHS (RegiSCAR score 6) was diagnosed, and she was treated with tofacitinib, 5 mg twice daily, monotherapy. Her symptoms resolved immediately, but because her peripheral eosinophil count was still 5,000 cells/μL after 3 days, the dose of tofacitinib was increased to 10 mg in the morning and 5 mg at night. By day 6 her eosinophil count normalized (600 cells/μL). She has continued on tofacitinib 15 mg daily for 23 months. Her cardiovascular function has nearly normalized, with recent LVEF of 44% and no evidence of heart failure.
Patient 2
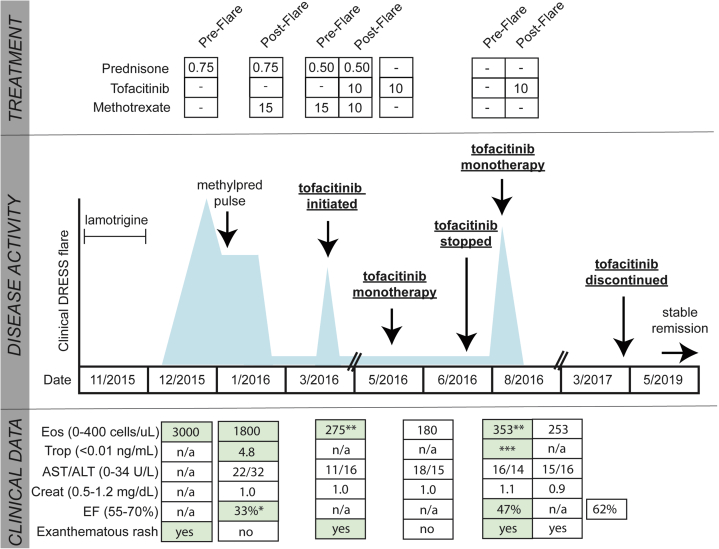
A 31-year-old man was started on lamotrigine and 5 weeks later developed an exanthematous rash, facial swelling, fever, and lymphadenopathy. He was found to have peripheral eosinophilia (3000 cells/μL). RegiSCAR score was 5, consistent with DIHS. After a 3-week prednisone taper (starting at 60 mg daily), his peripheral eosinophil count continued to be elevated (approximately 1800 cells/μL on prednisone, 40 mg daily), and he developed new chest pain (Fig 2). Troponin was elevated, and echocardiogram and cardiac magnetic resonance imaging showed LVEF of 33%, regional wall motion abnormalities, and a delayed enhancement pattern consistent with myocarditis. DIHS-associated hypersensitivity myocarditis was diagnosed, and he was treated with pulse methylprednisolone, resulting in resolution of eosinophilia and clinical improvement. He was started on methotrexate and a prednisone taper.
Fig 2.
Summary of clinical course in patient 2. Treatments before and after clinical DIHS flare are listed in the top panel. Doses of prednisone are shown as milligram per kilogram per day. Doses of tofacitinib are milligrams per day and methotrexate milligrams per week. Methylprednisolone was given at 1 g daily for 3 days. Middle panel shows DIHS activity/disease flares (blue spikes), but are not to scale. Lower panel shows the most relevant clinical data during DIHS flares, including ejection fraction (EF) during and after recovery from the flare, when possible. Green highlight signifies an abnormal value. Single asterisk signifies associated with wall motion abnormalities, double asterisk signifies trending upwards, and triple asterisk signifies associated with chest pain. ALT, alanine aminotransferase; AST, aspartate aminotransferase; CK, creatine kinase; CNS, central nervous system; Creat, creatinine; Eos, eosinophils; n/a, data not available; Trop, troponin.
Six weeks later, taking methotrexate and prednisone, 20 mg twice daily, the exanthematous eruption recurred. Tofacitinib, 5 mg twice daily, was started, and the dose of methotrexate was decreased. Over the next 8 weeks, prednisone was tapered and discontinued, together with methotrexate. He continued on tofacitinib monotherapy for 4 weeks without evidence of DIHS activity. Tofacitinib was discontinued, and 1 week later the rash and chest pain recurred (RegiSCAR score 3). Tofacitinib was restarted and the symptoms and rash remitted. Tofacitinib was continued for 5 months, at which time repeat imaging showed interval improvement in the delayed enhancement pattern and LVEF. Tofacitinib was continued for an additional 2 months and then discontinued without recurrence of disease.
Cytokine analysis in peripheral blood of patient 1
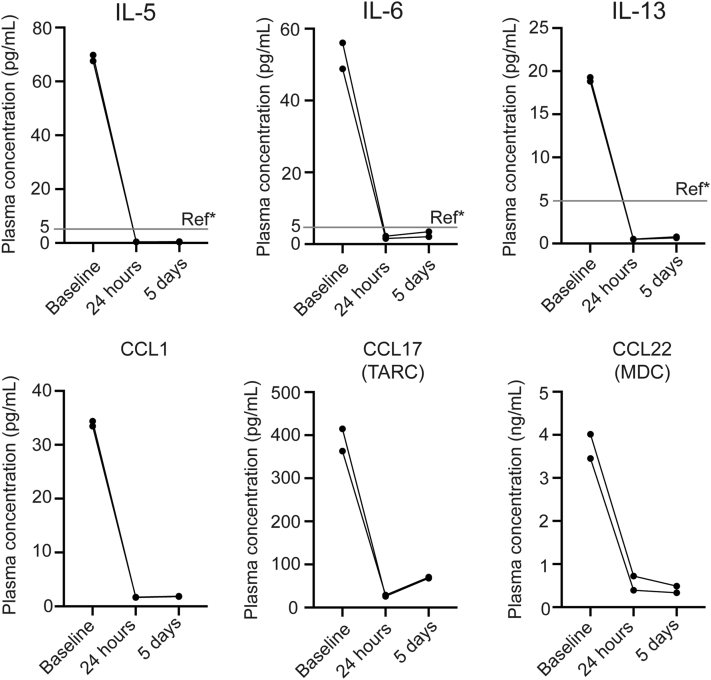
In patient 1, plasma was acquired during a DIHS flare (Fig 1, June 2017), just before reinitiation of tofacitinib monotherapy and then again after 24 hours and 5 days of treatment (see Supplement). IL-5, IL-6, and IL-13 were markedly elevated before initiation of tofacitinib, and within 24 hours of treatment, levels of these cytokines normalized (Fig 3). Other eosinophil chemokines including CCL1, CCL17, and CCL22 also normalized within 24 hours of tofacitinib (Fig 3). IL-10, IL-12, and C-X-C motif chemokine ligand 10 were also highly elevated and rapidly improved on therapy (not shown).
Fig 3.
Cytokine measurements in patient 1. In patient 1, plasma was collected during a DIHS flare (Fig 1, June 2017) just prior to (baseline) reinitiation of tofacitinib and then again after 24 hours and 5 days of therapy. Each measurement was performed in duplicate. This analysis was not performed in patient 2. Measurement of cytokines described in the Supplement. *Ref indicates references ranges from ARUP laboratories.
Discussion
DIHS may have a protracted course and be lethal. The mainstay of treatment, corticosteroids, is sometimes insufficient. There are no guidelines for management of DIHS, and which steroid-sparing agents are preferable in patients with severe disease is not clear.12 JAK-STAT–dependent cytokines such as IL-5, IL-6, IL-10, and IL-13 play a role in DIHS pathogenesis and signal via the JAK-STAT pathway. We describe 2 cases of DIHS with cardiac involvement that responded to the JAK1/3 inhibitor, tofacitinib. Although the clinical courses in both cases are complicated, DIHS flares in both patients, including ANEM (which has a mortality rate >50%13), appeared to respond well to tofacitinib treatment. In patient 1, a reduction in plasma levels of pathogenic cytokines such as IL-5 and IL-6 and chemokines such as CCL17 and CCL22 were observed within 24 hours of tofacitinib monotherapy. The rapid clinical and molecular responses, including normalization of organ function and eosinophil levels and reduction in levels of IL-5 and other pathogenic cytokines/chemokines in our patients, suggest that ;JAK inhibition should be further explored in the treatment of drug hypersensitivity reactions.
Footnotes
Funding sources: Supported by a gift (to Dr King) from the Ranjini and Ajay Poddar Resource Fund for Dermatologic Diseases Research. Dr Damsky is supported by the Dermatology Foundation. Dr Meyer was supported by the Fogarty International Center/National Institutes of Health (K01TW008764-02).
Conflicts of Interest: Dr Damsky has research funding from Pfizer and is a consultant for Eli Lilly. Dr Vesely's spouse works at Regeneron. Dr Choi has served on advisory boards for Merck, Pfizer, and EMD Serono. This article was prepared while Dr Meyer was employed by Yale University. The opinions expressed in this article are Dr Meyer's own and do not reflect the views of the US Army Medical Research and Material Command, the Department of the Army, the Department of Defense, or the United States Government. Dr King an investigator for Concert Pharmaceuticals Inc, Eli Lilly and Company, and Pfizer Inc and is a consultant to and/or has served on advisory boards for Aclaris Therapeutics, Arena Pharmaceuticals, Concert Pharmaceuticals Inc, Dermavant Sciences, Eli Lilly and Company, and Pfizer Inc; he is on speakers bureaus for Pfizer Inc, Regeneron, and Sanofi Genzyme. The rest of the authors have no conflicts to disclose.
Supplemental methods
RegiSCAR scoring criteria for DRESS/DIHS
The RegiSCAR score is calculated using clinical and laboratory parameters and is used for establishing the diagnosis of drug rash with eosinophilia and systemic symptoms (DRESS)/drug-induced hypersensitivity syndrome (DIHS). The complete scoring rubric is available elsewhere.S1 Table I shows RegiSCAR scores and their associated diagnoses. Adapted from Kardaun et al (2007).S1
Table I.
RegiSCAR scores and their associated diagnoses
| Score | Diagnosis |
|---|---|
| −4 to 2 | Not DRESS |
| 2 to 3 | Possible DRESS |
| 4 to 5 | Probable DRESS |
| 5 to 9 | “Definite” DRESS |
Cytokine analysis
Ten milliliters of blood was collected into ethylenediamine tetraacetic acid (EDTA)-coated tubes prior to reinitiation of tofacitinib and then again 24 hours and 5 days after reinitiating tofacitinib. Plasma was prepared using density gradient centrifugation (Lymphoprep, Stem Cell Technologies, Cambridge, MA) according to the manufacturer's instructions and stored at −80°C. Cytokine analysis was performed by Eve Technologies in Ontario, Canada. The Human Cytokine/Chemokine 65-Plex Panel was run in the plasma samples. The Panel includes: epidermal growth factor, Eotaxin, fibroblast frowth factor-2, Flt-3 ligand, Fractalkine, granulocyte colony-stimulating factor, granulocyte-macrophage colony-stimulating factor, GRO, interferon (IFN)-α2, IFN-γ, interleukin (IL)-10, IL-12 (p40), IL-12 (p70), IL-13, IL-15, IL-17A, IL-18, IL-1ra, IL-1α, IL-1β, IL-2, IL-3, IL-4, IL-5, IL-6, IL-7, IL-8, IL-9, IP-10, MCP-1, MCP-3, MDC (CCL22), MIP-1α, MIP-1β, platelet-derived growth factor (PDGF)-AA, PDGF-AB/BB, RANTES, transforming growth factor (TGF)-α, tumor necrosis factor (TNF)-α, TNF-β, vascular endothelial growth factor, sCD40L, Eotaxin-2, MCP-2, BCA-1, MCP-4, I-309, IL-16, TARC, 6CKine, Eotaxin-3, LIF, TPO, SCF, TSLP, IL-33, IL-20, IL-21, IL-23, TRAIL, CTACK, SDF-1a+B, ENA-78, MIP-1d, IL-28A.
Supplemental information regarding patient 1
Clinical presentation
A previously healthy 37-year-old woman had an exanthematous rash, fever, arthralgias and myalgias 3 weeks after starting minocycline for acne vulgaris. She was found to have facial edema, submandibular lymphadenopathy, elevated hepatic transaminases (aspartate aminotransferase [AST], 61 U/L; alanine aminotransferase [ALT], 108 U/L) (Ref: 10-30 U/L and 6-29 U/L, respectively) and peripheral blood eosinophilia (1,035/μL3). Atypical lymphocytes were not evaluated at initial presentation. Creatinine was normal. A diagnosis of DIHS was made, and she was started on 80 mg prednisone (1 mg/kg) daily. One week later, despite prednisone, severe muscle weakness developed, and she had worsening transaminase elevation (AST, 447 U/L; ALT, 269 U/L) and a markedly elevated creatine kinase (CK) of 9,810 U/L (Ref: 24-170 U/L). Echocardiogram was unremarkable and left ventricular ejection fraction (LVEF) was 68%. Magnetic resonance imaging of the hips found intramuscular edema and decreased T2 signal concerning for necrosis. A diagnosis of DIHS-associated rhabdomyolysisS2 was established. Pulse methylprednisolone (240 mg daily for 3 days) was administered with good effect and serum enzyme normalization. She was transitioned to prednisone (tapering from 100 mg daily). Other rheumatologic disorders were excluded using standard serum markers.
One month later, taking prednisone, 40 mg daily, she had dyspnea on minimal exertion. Laboratory evaluation found severe hyponatremia (118 mmol/L, Ref: 135-146 mmol/L) with elevated transaminases (AST, 234 U/L; ALT, 642 U/L) and a troponin T level of 4.69 ng/mL (Ref: <0.01). Creatinine levels were normal. There was no rash or fever. The RegiSCAR score at this time was 4 consistent with probable DIHS. A diagnosis of DIHS flare was made given the clinical context.
Diagnosis of acute necrotizing eosinophilic myocarditis in the setting of DIHS
An electrocardiogram showed a new right bundle branch block and ST segment changes and an echocardiogram (ECHO) showed biventricular heart failure with LVEF less than 10%. Two forms of DIHS/DRESS myocarditis have been reported: hypersensitivity myocarditis and acute necrotizing eosinophilic myocarditis. They have very different prognosis.S3 Hypersensitivity myocarditis is less severe and is characterized by variable cardiac enzyme elevation and systolic dysfunction as determined by ECHO. Electrocardiogram is either normal or shows nonspecific changes. On the other hand, acute necrotizing eosinophilic myocarditis (ANEM) is distinguished by biventricular heart failure on ECHO and has a mortality rate of greater than 50%, with median survival of 3 to 4 days.S4 Because of the severity of heart failure including biventricular failure, ANEM was diagnosed. Endomyocardial biopsy was performed at a later admission for a subsequent disease flare (below) and showed myocyte hypertrophy with edema and focal lymphocytic infiltrate. Although these histopathologic findings are not diagnostic of ANEM, the biopsy was performed while the patient was on high-dose corticosteroids and tofacitinib, and histopathologic involvement by ANEM is known to be patchy, meaning myocardial biopsy has a relatively low sensitivity for establishing the diagnosis of ANEM.S5 Cytomegalovirus, adenovirus, simian virus 40 and Congo red stains on the myocardial biopsy were negative. An extensive workup for other causes of myocarditis was negative.
For the ANEM, she required intra-aortic balloon pump (IABP) and vasopressor support and was treated with methylprednisolone, 1000 mg daily for 5 days. Captopril, 25 mg daily, amiodarone, 400 mg daily, and spironolactone, 25 mg daily, were started. She was transitioned to prednisone, 60 mg twice daily, plus cyclosporine, 50 mg twice daily. Tofacitinib, 5 mg twice daily, was also added given the severity of her DIHS. Ten days after admission, mechanical support and vasopressors were discontinued, and her LVEF was 40%.
One month later, while taking prednisone, 10 mg daily, cyclosporine, 50 mg twice daily, and tofacitinib, 5 mg twice daily, she was re-admitted to the hospital for difficulty ambulating. She had a symmetric 4/5 proximal lower extremity weakness, hyperreflexia, and urinary retention. Cardiac function was stable at this time. There was no rash or fever. Her lower extremity weakness progressed over the next 5 days and she had bilateral foot drop, areflexia, and was unable to ambulate with 0 to 1/5 weakness in the bilateral lower extremities with relative sparing of plantarflexion and inversion (3/5).
Diagnosis of Neurologic Involvement of DIHS
Magnetic resonance imaging of the brain and spine demonstrated (1) T2 hyperintensity of the left brachial points and the central grey matter of the spinal cord from the cervical spine to the conus medullaris; (2) diffuse leptomeningeal enhancement of the skull base, spinal cord, and cauda equina; and (3) a well-circumscribed enhancing lesion in the T10 vertebral body. Cerebrospinal fluid (CSF) analysis was notable for elevated white blood cell count of 67 cells per μL (98% lymphocytes, 2% monocytes), mild decrease in glucose 65 mg/dL (Ref: 45-80 mg/dL), and increased protein (105 mg/dL, Ref: <50 mg/dL). CSF cultures were negative. Flow cytometry and B- and T-cell gene rearrangement polymerase chain reaction (PCR) of the CSF was assessed twice and was unremarkable. Paraneoplastic autoantibody panel of the CSF was negative. CSF PCR for herpes simplex virus, varicella zoster virus, enterovirus and human herpes virus 6 (HHV) was negative. PCR for Epstein-Barr virus was positive.
Plasma Vitamin B12 level was 1028 pg/mL (Ref: 180-914 pg/mL). Serum antibodies were negative for HIV, Venereal Disease Research Laboratory, Strongyloides, Cystercosis, Trichinella, Trypanosoma cruzi, and Coccioides. Urine Histoplasma antigen was negative. Poliovirus PCR was negative. Quantiferon Gold testing was negative. Antinuclear cytoplasmic antibodies and anti–double-stranded DNA antibodies were negative as was neuromyolitis optica antibody. Serum paraneoplastic antibody panel was also negative.
Viral reactivation is at times reported in DIHS/DRESS,S6 albeit with unclear significance. Evaluation for cytomegalovirus, herpes simplex virus, HHV6, and HHV7 in the serum were negative. PCR for Epstein-Barr virus was positive in the serum, but the viral load was less than 500 copies/mL. The presentation was felt to be consistent with neurologic involvement of her DIHS, particularly as all other testing to explain the longitudinally extensive transverse myelitis and leptomeningeal inflammation was negative. Neurologic involvement in DRESS is uncommon but well described and most commonly manifests as meningitis and encephalitis.S7 Myelitis, although rare, has also been reported in DRESS.S8
The patient was treated for central nervous system involvement of her DIHS with daily pulse methylprednisolone (1 g/d for 5 days) plus intravenous immunoglobulin for 5 days. Cyclosporine was discontinued, but tofacitinib was continued. Repeat imaging found interval improvement in the leptomenigeal enhancement. Her urinary retention improved, but her flaccid areflexic paraplegia did not. Electromyography found acute axonal motor neuropathy in a radicular pattern.
Ten days later diplopia developed, and she was found to have mild bilateral sixth nerve palsies. Repeat imaging found an interval worsening of the leptomeningeal enhancement, and she received another 5-day course of solumedrol (1 g/d), and prednisone was increased to 60 mg daily. During the remainder of her 5-week hospitalization, her diplopia improved, but her lower extremity strength did not. Her cardiac function remained stable, and she was discharged on prednisone, 60 mg daily, tofacitinib, 5 mg twice daily, and methotrexate, 5 mg weekly.
During this hospitalization, an incidental apical, cavitary lung lesion was noted on chest computed tomography scan. A biopsy of the lesion found organizing pneumonitis with eosinophils. Cultures from this lesion, including for acid-fast bacilli, were all negative. A broad infectious workup was negative.
The patient's condition remained stable for 4 weeks until tofacitinib was discontinued because of lack of insurance coverage. Nine days after discontinuing tofacitinib, while taking prednisone, 40 mg daily, and methotrexate, 5 mg weekly, she was admitted to the hospital again with cardiogenic shock. She had evidence of myocardial necrosis with a troponin T of 9.44 ng/mL, elevated transaminases (AST, 6130 U/L and ALT, 7670 U/L), LVEF 30%, and hypotension requiring intra-aortic balloon pump and inotropic support. She was treated with pulse methylprednisolone (1 g/d for 3 days), and tofacitinib, 5 mg twice daily was restarted. The endomyocardial biopsy described above was performed during this admission. Three days later, the patient experienced significant recovery of her LVEF to 40%-45%. She was discharged on a prednisone taper, tofacitinib, 5 mg twice daily, and methotrexate, 5 mg once weekly.
The patient's condition was stable, without evidence of heart failure or worsening neurologic disease, over the next 10 months, so it was decided to discontinue tofacitinib; at that time, she was taking prednisone, 10 mg every other day, and tofacitinib, 5 mg twice daily (the methotrexate had been discontinued 5 months previously). Five months later, she presented with fever and malaise. This occurred 3 weeks after taking cephalexin for automated implantable cardioverter defibrillator placement. DIHS recurrence with structurally unrelated culprit drugs has been reported.S9 She was febrile and had return of her morbilliform eruption and lymphadenopathy. Laboratory evaluation found return of peripheral eosinophilia to 5500 cells/μL. Hepatic transaminases and creatinine levels were at baseline. Her RegiSCAR score was 6, consistent with definite DIHS, according to the scoring criteria. Recurrent DIHS was diagnosed, which was treated with tofacitinib monotherapy (5 mg twice daily). She improved clinically, but because her peripheral eosinophil count was still 5000 cells/μL after 3 days of therapy, the tofacitinib was increased to 10 mg in the morning and 5 mg at night, and on day 6 her eosinophil count was normal at 600 cells/μL. Plasma samples for cytokine analysis were obtained during this admission (before reinitiation of tofacitinib and 24 hours and 5 days later). She has continued on tofacitinib, 15 mg daily, for 23 months. Her cardiovascular function has nearly normalized, with last LVEF of 45% and N-terminal pro B-type natriuretic peptide levels of 400 pg/mL (peak of 4567 pg/mL, Ref: <300 pg/mL).
Repeated imaging of the neuroaxis has not found any new lesions, but her flaccid areflexic paraplegia remains only minimally improved. She remains wheelchair bound although can now independently transfer and walk short distances with Candian crutches.
Evaluation for Hypereosinophilic Syndrome
Given the protracted nature of her presentation, an extensive evaluation for hypereosinophilic syndrome was undertaken. Her serum tryptase was variably elevated, the maximum was 18.8 μg/L (Ref: <11μg/L) and vitamin B12 level was 1028 pg/mL (Ref: 180-914 pg/mL) but was at other times normal. IgE levels were variably elevated with a maximum of 1051 kU/L (Ref: <115 kU/L) but were at other times normal. Serum protein electrophoresis and serum free light chains were unremarkable, and immunofixation electrophoresis was negative. Flow cytometry of the blood was unremarkable. Fluorescence in situ hybridization on peripheral blood mononuclear cells (PBMCs) was negative for pathogenic alterations in PDGFRA, PDGFRB, FGFR1, D8Z2, ABL1, BCR, MYH11, and CBFB. PCR of PBMCs was negative tor JAK2 V617F and KIT D816V. A bone marrow biopsy found normal tri-lineage hematopoiesis with 19% eosinophils; flow cytometry was unremarkable.
Further evaluation of her PBMCs with a clinical panel evaluating 26 genes for AML/MDS driver mutations was performed (ASXL1, CBL, CEBPA, CSF3R, DNMT3A, ETV6, EZH2, FLT3, HRAS, IDH1, IDH2, JAK2, KIT, KRAS, MLL, MPL, NPM1, NRAS, PHF6, RUNX1, SF3B1, SRSF2, TET2, TP53, WT1). This analysis found a heterozygous missense ASXL1 mutation 1945G>A resulting in Gly652Ser. This mutation was interpreted as being of unknown/unlikely significance in the panel. Further, this mutation is listed as a benign SNP in the 1000 genomes project (Variation ID: 133592). This mutation has never been reported in hematologic malignancy. High throughput T-cell receptor sequencing was also performed on PBMCs (clonoSEQ from Adaptive Technologies) and found polyclonally, ruling out lymphocytic hypereosinophilic syndrome. Last, exome sequencing of the patient's PBMCs (performed by the Choi laboratory, author JC) failed to reveal any pathologic mutations (data not shown).
References
- 1.Husain Z., Reddy B.Y., Schwartz R.A. DRESS syndrome: Part I. Clinical perspectives. J Am Acad Dermatol. 2013;68:693.e1–693.e14. doi: 10.1016/j.jaad.2013.01.033. quiz 706-8. [DOI] [PubMed] [Google Scholar]
- 2.Choquet-Kastylevsky G., Intrator L., Chenal C., Bocquet H., Revuz J., Roujeau J.C. Increased levels of interleukin 5 are associated with the generation of eosinophilia in drug-induced hypersensitivity syndrome. Br J Dermatol. 1998;139:1026–1032. doi: 10.1046/j.1365-2133.1998.02559.x. [DOI] [PubMed] [Google Scholar]
- 3.Tsai Y.G., Liou J.H., Hung S.I. Increased type 2 innate lymphoid cells in patients with drug reaction with eosinophilia and systemic symptoms syndrome. J Invest Dermatol. 2019;139:1722–1731. doi: 10.1016/j.jid.2018.10.048. [DOI] [PubMed] [Google Scholar]
- 4.Watanabe H. Recent Advances in drug-induced hypersensitivity syndrome/drug reaction with eosinophilia and systemic symptoms. J Immunol Res. 2018;2018:5163129. doi: 10.1155/2018/5163129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yi S., Zhai J., Niu R. Eosinophil recruitment is dynamically regulated by interplay among lung dendritic cell subsets after allergen challenge. Nat Commun. 2018;9:3879. doi: 10.1038/s41467-018-06316-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Karlsson A.K., Walles K., Bladh H., Connolly S., Skrinjar M., Rosendahl A. Small molecule antagonists of CCR8 inhibit eosinophil and T cell migration. Biochem Biophys Res Commun. 2011;407:764–771. doi: 10.1016/j.bbrc.2011.03.097. [DOI] [PubMed] [Google Scholar]
- 7.Shiohara T., Mizukawa Y., Aoyama Y. Monitoring the acute response in severe hypersensitivity reactions to drugs. Curr Opin Allergy Clin Immunol. 2015;15:294–299. doi: 10.1097/ACI.0000000000000180. [DOI] [PubMed] [Google Scholar]
- 8.Teraki Y., Fukuda T. Skin-homing IL-13-producing T cells expand in the circulation of patients with drug rash with eosinophilia and systemic symptoms. Dermatology. 2017;233:242–249. doi: 10.1159/000475546. [DOI] [PubMed] [Google Scholar]
- 9.Kardaun S.H., Sidoroff A., Valeyrie-Allanore L. Variability in the clinical pattern of cutaneous side-effects of drugs with systemic symptoms: does a DRESS syndrome really exist? Br J Dermatol. 2007;156:609–611. doi: 10.1111/j.1365-2133.2006.07704.x. [DOI] [PubMed] [Google Scholar]
- 10.Engel J.N., Mellul V.G., Goodman D.B. Phenytoin hypersensitivity: a case of severe acute rhabdomyolysis. Am J Med. 1986;81:928–930. doi: 10.1016/0002-9343(86)90371-2. [DOI] [PubMed] [Google Scholar]
- 11.King B., Lee A.I., Choi J. Treatment of hypereosinophilic syndrome with cutaneous involvement with the JAK inhibitors tofacitinib and ruxolitinib. J Invest Dermatol. 2017;137:951–954. doi: 10.1016/j.jid.2016.10.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Husain Z., Reddy B.Y., Schwartz R.A. DRESS syndrome: Part II. Management and therapeutics. J Am Acad Dermatol. 2013;68:709.e1–709.e9. doi: 10.1016/j.jaad.2013.01.032. quiz 18-20. [DOI] [PubMed] [Google Scholar]
- 13.Sabatine M.S., Poh K.K., Mega J.L., Shepard J.A., Stone J.R., Frosch M.P. Case records of the Massachusetts General Hospital. Case 36-2007. A 31-year-old woman with rash, fever, and hypotension. N Engl J Med. 2007;357:2167–2178. doi: 10.1056/NEJMcpc079030. [DOI] [PubMed] [Google Scholar]
Supplemental References
- Kardaun S.H., Sidoroff A., Valeyrie-Allanore L. Variability in the clinical pattern of cutaneous side-effects of drugs with systemic symptoms: does a DRESS syndrome really exist? Br J Dermatol. 2007;156:609–611. doi: 10.1111/j.1365-2133.2006.07704.x. [DOI] [PubMed] [Google Scholar]
- Engel J.N., Mellul V.G., Goodman D.B. Phenytoin hypersensitivity: a case of severe acute rhabdomyolysis. Am J Med. 1986;81:928–930. doi: 10.1016/0002-9343(86)90371-2. [DOI] [PubMed] [Google Scholar]
- Bourgeois G.P., Cafardi J.A., Groysman V., Hughey L.C. A review of DRESS-associated myocarditis. J Am Acad Dermatol. 2012;66:e229–e236. doi: 10.1016/j.jaad.2010.11.057. [DOI] [PubMed] [Google Scholar]
- Sabatine M.S., Poh K.K., Mega J.L., Shepard J.A., Stone J.R., Frosch M.P. Case records of the Massachusetts General Hospital. Case 36-2007. A 31-year-old woman with rash, fever, and hypotension. N Engl J Med. 2007;357:2167–2178. doi: 10.1056/NEJMcpc079030. [DOI] [PubMed] [Google Scholar]
- Burke A.P., Saenger J., Mullick F., Virmani R. Hypersensitivity myocarditis. Arch Pathol Lab Med. 1991;115:764–769. [PubMed] [Google Scholar]
- Cho Y.T., Yang C.W., Chu C.Y. Drug reaction with eosinophilia and systemic symptoms (dress): an interplay among drugs, viruses, and immune system. Int J Mol Sci. 2017;18 doi: 10.3390/ijms18061243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Husain Z., Reddy B.Y., Schwartz R.A. DRESS syndrome: Part I. Clinical perspectives. J Am Acad Dermatol. 2013;68:693.e1–693.e14. doi: 10.1016/j.jaad.2013.01.033. quiz 706-8. [DOI] [PubMed] [Google Scholar]
- Scheibe F., Metz I., Radbruch H. Drug reaction with eosinophilia and systemic symptoms after daclizumab therapy in MS. Neurol Neuroimmunol Neuroinflamm. 2018;5:e479. doi: 10.1212/NXI.0000000000000479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picard D., Vellar M., Janela B., Roussel A., Joly P., Musette P. Recurrence of drug-induced reactions in DRESS patients. J Eur Acad Dermatol Venereol. 2015;29:801–804. doi: 10.1111/jdv.12419. [DOI] [PubMed] [Google Scholar]