Abstract
Rezafungin acetate is a novel echinocandin in clinical development for prevention and treatment of invasive fungal infections. Rezafungin is differentiated by a pharmacokinetic/pharmacodynamic (PK/PD) profile that includes a long half‐life allowing once‐weekly administration, front‐loaded plasma drug exposures associated with antifungal efficacy, and penetration into deep‐seated infections, such as intra‐abdominal abscesses. In this series of in vivo studies, rezafungin demonstrated efficacy in the treatment of neutropenic mouse models of disseminated candidiasis, including infection caused by azole‐resistant Candida albicans, and aspergillosis. These results contribute to a growing body of evidence demonstrating the antifungal efficacy and potential utility of rezafungin in the treatment of invasive fungal infections.
Keywords: antifungal treatment, Aspergillus, azole resistance, Candida, CD101, echinocandin, in vivo efficacy, invasive aspergillosis, invasive candidiasis, rezafungin
Abbreviations
- AUC
area under the curve
- Cmax
peak drug concentration
- MEC
minimum effective concentration
- MIC
minimum inhibitory concentration
- PDA
potato dextrose agar
- SDA
sabouraud dextrose agar
1. INTRODUCTION
Invasive candidiasis and aspergillosis are serious opportunistic infections associated with significant morbidity and mortality.1, 2 Invasive candidiasis is a prevalent nosocomial infection, ranked fourth highest among bloodstream infections in the US alone.3 Invasive aspergillosis is relatively less common overall but highly prevalent among immunocompromised patients. With growing complexity in the treatment of underlying diseases and use of immunosuppressive therapies, there is increasing need for safe, efficacious antifungal treatment that can be safely coadministered in such patients at risk for invasive fungal infections.
Rezafungin is a novel echinocandin distinguished by its long‐acting pharmacokinetics (half‐life > 130 hours) and stability that support high plasma drug exposures and longer dosing intervals.4, 5, 6, 7, 8 In vitro, rezafungin demonstrates similar activity to that of current echinocandins against Candida and Aspergillus spp., as well as activity against resistant strains, including azole‐resistant Aspergillus spp. and subsets of echinocandin‐resistant Candida auris and Candida glabrata. 9, 10, 11, 12 Recent studies have evaluated pharmacokinetic/pharmacodynamic (PK/PD) factors relating to rezafungin efficacy.11, 13, 14, 15 The PK/PD index of AUC/MIC for rezafungin correlated well with efficacy and, compared with other echinocandins, rezafungin had lower PK/PD target exposures against Candida spp., including strains with resistance or reduced susceptibility to echinocandins.11 While Cmax also predicted rezafungin efficacy, AUC was selected as it is more reliably measured.16 Nevertheless, Cmax is an important index as shown in dose‐fractionation and PK/PD target attainment analyses that demonstrated how rezafungin activity is driven by both the extent and the shape of drug exposure. In effect, a single dose of rezafungin resulted in higher levels of drug exposure early in therapy and greater fungicidal activity compared with repeated daily dosing.14 Rezafungin also demonstrated extensive distribution to tissue and penetration at the site of infection, as compared with micafungin in an intra‐abdominal abscess candidiasis mouse model.15
A series of in vivo studies using a mouse model of disseminated candidiasis were conducted to further evaluate rezafungin efficacy: at extended intervals post‐infection, against azole‐resistant Candida albicans, and when antifungal treatment was delayed. Additionally, a neutropenic mouse model of disseminated aspergillosis was used to study the effect of a single rezafungin dose on survival, following a previous study in a similar mouse model that showed comparable survival rates among rezafungin‐ and amphotericin B‐treated mice following 5 days of treatment. Discussion of results from these in vivo studies will consider the relationship between PK‐PD determinants of antifungal efficacy, as observed for rezafungin.
Portions of these results were presented at ASM Microbe 2016 and Advances Against Aspergillosis 2016.
2. MATERIAL AND METHODS
2.1. Drug, chemical reagents, and other materials
Rezafungin (RZF; Cidara Therapeutics, Inc) and anidulafungin (ANF; Molcan, Toronto, Canada) were dissolved in 10% dimethyl sulfoxide (DMSO)/1% polysorbate (Tween) 20 in 0.9% saline. Fluconazole (FLU; Sigma‐Aldrich) was dissolved in water for injection. Amphotericin B (AmB; Sigma‐Aldrich) was dissolved in 0.9% saline. The dosing volume was 10 mL/kg for all groups.
2.2. Test systems used
Minimal inhibitory concentration (MIC) and minimal effective concentration (MEC; echinocandins vs. molds only) values were performed in accordance with CLSI broth microdilution guidelines (M27‐Ed4 and M38‐Ed3, respectively). Both strains of C. albicans, R303 (RZF MIC, 0.125 μg/mL) and azole‐resistant R357 (RZF MIC, 0.125 μg/mL; FLU MIC, >64 μg/mL; AmB MIC, 0.5 μg/mL), are human bloodstream isolates and were obtained from Ricerca Biosciences, LLC (Concord, OH, USA). C. albicans was obtained from frozen working stock culture and thawed at room temperature. A 0.1 mL aliquot was transferred to a sabouraud dextrose agar (SDA) plate and incubated at 35‐37°C overnight. The culture was re‐suspended with 1 mL cold PBS and diluted with PBS (5 × 103 CFU/mL for R303; 5 × 105 CFU/mL for R357). Actual colony counts were determined by plating dilutions to SDA plates followed by 20‐24 hours incubation. Aspergillus fumigatus ATCC 13073 (RZF MEC, 0.008 μg/mL; ANF MEC, 0.008 μg/mL; AmB MIC, 2 μg/mL) was acquired from the American Type Culture Collection (Rockville, MD, USA). A. fumigatus growth was taken from 96‐hour potato dextrose agar (PDA) and re‐suspended in 0.1% Tween 80. The culture density was adjusted using optical density measurements to 1.5 × 105 CFU/mL in PBS. Actual colony counts were determined on PDA to confirm inoculation concentration.
In the invasive candidiasis model, 7‐week old, male ICR mice (Charles River Laboratories licensee, Taipei, Taiwan) (n = 5/group) weighing 22 ± 2 g were used. In the invasive aspergillosis model, female ICR mice (Charles River licensee, Taipei, Taiwan) (n = 10/group) weighing 22 ± 2 g were used. The sex of animals used in the experiments was selected to maintain the same sex used historically to validate each model, respectively. All studies were performed in accordance with the Guide for the Care and Use of Laboratory Animals: Eighth Edition, in an AAALAC‐accredited ABSL‐2 laboratory under the supervision of veterinarians. The animal care and use protocol was reviewed and approved by the IACUC at Eurofins Panlabs Taiwan, Ltd.
2.3. Experimental design
For all experiments in the invasive candidiasis model, mice were immunosuppressed using two intraperitoneal (IP) injections of cyclophosphamide17 with the first injection of 3 mg/mouse administered 4 days before C. albicans infection (Day –4) and the second injection of 2 mg/mouse 1 day before C. albicans infection (Day –1). For the extended‐interval dosing experiment only, persistent neutropenia was sustained for the duration of the study with cyclophosphamide 2 mg/mouse IP every 48 hours, on Days 1, 3, 5, and 7 post‐infection. On Day 0, mice were inoculated (0.2 mL/mouse intravenous [IV]) with C. albicans. Mice were sacrificed at various time points, and kidneys were harvested for fungal colony forming units per gram (CFU/g) and calculation of percentage decrease at the time of sacrifice.
2.3.1. Assessment of efficacy against Candida at increasing intervals
Rezafungin 1, 3, 10, or 30 mg/kg IP and FLU 20 mg/kg orally (PO) was administered 2 hours post‐infection (C. albicans R303; 1.41 × 103 CFU), and kidney CFU/g at 120 and 168 hours post‐infection in the rezafungin groups were compared with those of the FLU group at 24 hours and of the vehicle control group at 72 hours.
2.3.2. Assessment of efficacy against azole‐resistant Candida albicans (R357)
Rezafungin 3, 10, and 30 mg/kg IP, AmB 1 and 3 mg/kg IV, or FLU 20 mg/kg PO was administered 2 hours post‐infection (C. albicans ATCC R357; 1.41 × 105 CFU). Kidney fungal counts at 48 and 72 hours post‐infection in the treated and control groups were compared.
2.3.3. Assessment of efficacy against Candida with delayed treatment
Rezafungin 1, 3, 10, and 30 mg/kg IP or FLU 20 mg/kg PO was administered 24 hours post‐infection (C. albicans R303; 1.06 × 103 CFU). Kidney fungal counts at 96, 144, and 192 hours post‐infection in the rezafungin‐treated groups were compared with those of FLU‐treated mice at 48 hours and the vehicle control group at 72 hours.
For the experiment in the invasive aspergillosis model, mice were immunosuppressed using three IP injections of cyclophosphamide, with the first injection of 6 mg/mouse administered 3 days before A. fumigatus infection (Day −3) and the second and third injections of 2 mg/mouse 1 and 4 days after infection (Days + 1 and + 4), respectively. On Day 0, animals were inoculated (0.2 mL/mouse IV) with A. fumigatus (ATCC 13073), 2 × 104 CFU per mouse. Rezafungin was administered IP or IV as a one‐time 2 mg/kg dose 1 hour post‐infection. Rezafungin was also administered IP or IV with the same 2 mg/kg total dose fractionated as 0.2 mg/kg given twice daily for 5 days. AmB was administered IP as a one‐time 3 mg/kg dose or fractionated over 5 days as 0.3 mg/kg twice daily. Mortality was observed for 10 days.
2.4. Compliance with design and statistical analysis requirements
Group comparisons for each infection model were made between groups of equal size (n = 5, invasive candidiasis model; n = 10, invasive aspergillosis model). Randomization and blinding were not part of the study design of these in vivo experiments, and bias due to their absence was considered to be minimal.
2.5. Data analysis and statistical procedures
For the mouse model of disseminated candidiasis, fungal counts in kidneys were calculated and the decrease percentage was calculated by the following formula: Decrease (%) = [(CFU/g of vehicle – CFU/g of treatment)/(CFU/g of vehicle)] x 100%. A 99% decrease in the fungal counts or more (≥99%), or a 2‐log reduction in counts, compared to the vehicle control group indicates significant activity. One‐way ANOVA followed by Dunnett's test was also applied in the assessment of efficacy against azole‐resistant Candida albicans (R357) to assess statistical significance. For the mouse model of disseminated aspergillosis, an increase of 50 percent or more (≥50%) in the survival rate, compared to the vehicle control group, indicated significant antifungal activity.
3. RESULTS
3.1. In vivo efficacy: mouse model of invasive candidiasis
3.1.1. Prolonged efficacy against azole‐susceptible Candida albicans
Rezafungin demonstrated significant efficacy at up to 168 hours post‐infection (data not shown). Kidney fungal counts were reduced to near or below the limit of detection (LOD; 1.34 log CFU/mL) at 168 hours post‐infection in the rezafungin 1 mg/kg and 3 mg/kg groups. All rezafungin‐treated groups demonstrated significant antifungal efficacy at the longest post‐infection periods evaluated (120 and 168 hours post‐infection). Fluconazole, at the same dose used in historical validation of this infection model (20 mg/kg), elicited a significant reduction in colony counts at 24 hours compared with vehicle controls, thus demonstrating its appropriateness as the positive control in the current experiments.
3.1.2. Efficacy against azole‐resistant Candida albicans (R357)
Treatment with rezafungin at all tested doses resulted in ≥ 99% reduction of kidney fungal counts at 48 and 72 hours after infection with azole‐resistant C. albicans. Similar results in percentage change in kidney fungal counts were observed at 72 hours in the AmB 3 mg/kg group, and reductions in all rezafungin and AmB groups were significant compared with vehicle (P > .05). Fluconazole 20 mg/kg reductions in fungal counts at 48 and 72 hours post‐infection reached 51% and 84%, respectively. Figure 1 shows for each group the change in log10 fungal counts from 2 hours to 48 and 72 hours post‐infection.
Figure 1.
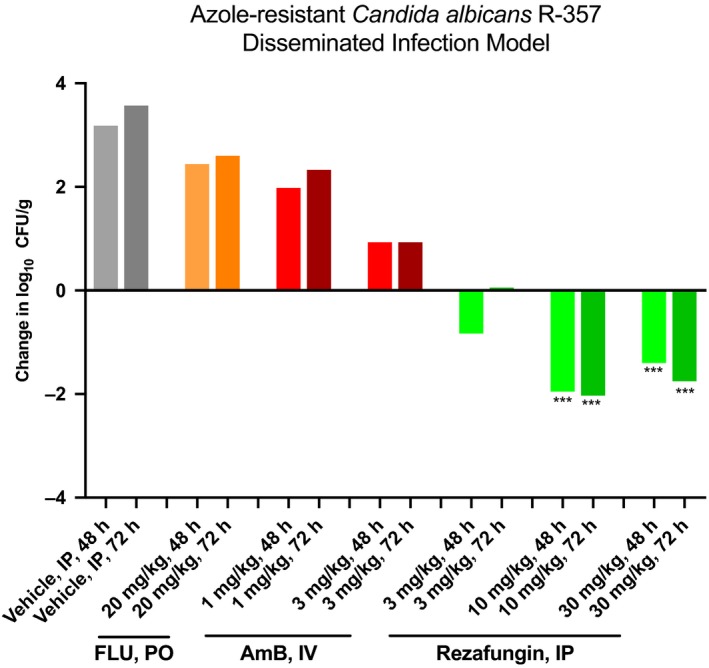
Efficacy against azole‐resistant Candida: change in log counts in kidneys of fluconazole (FLU)‐, amphotericin B (AmB)‐, and rezafungin‐treated mice at 48 and 72 h post‐infection in an azole‐resistant Candida albicans (R357) disseminated infection model. IP, intraperitoneal. *** P < .001 (reduction)
3.1.3. Efficacy with delayed treatment
In mice treated with rezafungin 24 hours post‐infection, significant antifungal effects (≥99%, 2‐log reduction in CFU/g) were observed at 48, 96, 144, and 192 hours post‐infection (up to 168 hours following treatment) at all rezafungin doses tested. Kidney fungal counts in mice treated with rezafungin were reduced to near or under the limit of detection (LOD = 1.28 log CFU/mL) at 144 hours post‐infection in the 3 and 30 mg/kg group and at 192 hours post‐infection in the 3, 10, and 30 mg/kg groups (Figure 2).
Figure 2.
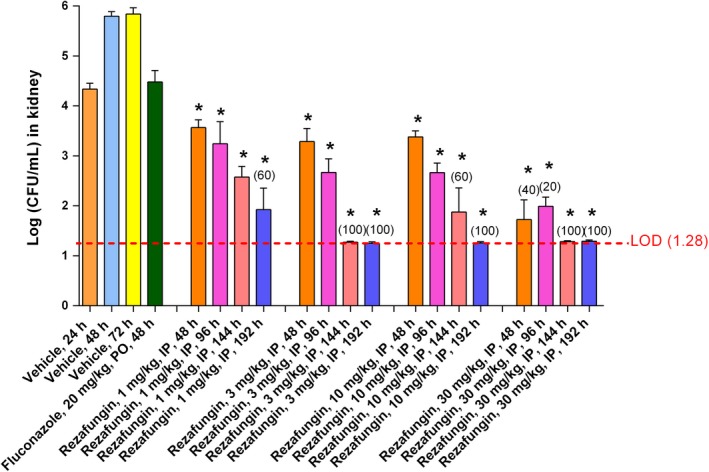
Efficacy with delayed treatment against Candida: change in log fungal counts in kidneys of fluconazole‐ and rezafungin‐treated mice up to 192 h post‐infection (168 h post‐treatment) in a Candida albicans (R303) disseminated infection model. * indicates ≥ 2‐log reduction in the kidney counts of the treatment groups compared to the vehicle group. The limit of detection (LOD) of fungal counts is 1.28 (dashed line). The percentage of animals with counts below the LOD is in parentheses (% clearance) above the data bar
3.2. In vivo efficacy: mouse model of disseminated Aspergillosis
In mice infected with A. fumigatus ATCC 13073 and treated with a one‐time dose of rezafungin 2 mg/kg, the 10‐day survival rates were significantly higher (100%) than in the vehicle group (20%). Survival rates following a one‐time dose of rezafungin 2 mg/kg (either as a single dose or fractionated dose given twice daily for 5 days), administered IV or IP, were comparable to those of AmB given 3 mg/kg (Figure 3).
Figure 3.
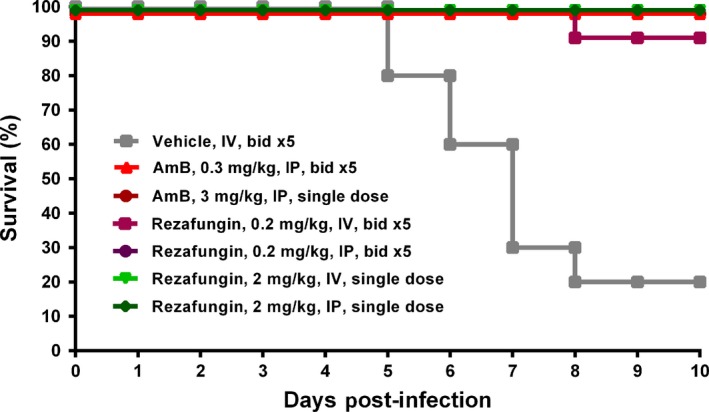
Survival rates in an Aspergillus fumigatus disseminated infection model following a single dose (2 mg/kg, single dose) or fractionated doses (0.2 mg/kg twice daily for 5 days [bid x 5]) of rezafungin or amphotericin B (AmB, 3 mg/kg, single dose, or 0.3 mg/kg, bid x 5)
4. DISCUSSION
Rezafungin is a novel echinocandin that was designed for greater stability and for pharmacokinetics that would enable more flexible dosing regimens than those of existing echinocandins, while retaining the general activity and safety of the class.18 Truly, since its discovery, extensive research has demonstrated the enhanced stability and safety, in vitro activity, and distinctive pharmacokinetics of rezafungin.6, 7, 8, 19, 20, 21 Studies also have evaluated the efficacy of rezafungin in vivo and with respect to pharmacometrics– ie, the pharmacokinetic and pharmacodynamic relationships of exposure and response that predict efficacy.13 The current series of experiments expand on previous studies (Table 1) and, to the extent that in vivo findings may translate to clinical outcomes, provide greater insight on the potential of rezafungin in the treatment of invasive fungal infections.
Table 1.
Summary of rezafungin in vivo efficacy
| Reference | Objective | Preclinical model/ Pathogen | Materials and methods | Results and conclusions |
|---|---|---|---|---|
| Ong et al6 | To evaluate RZF efficacy in preclinical models of systemic infection | Neutropenic mouse/ disseminated Candida albicans (R303) |
‐‐‐
|
Significant efficacy (≥99%, 2‐log reduction in CFU/g) with RZF 0.6 and 0.8 mg/kg at 24, 48, and 72 h and with ANF 0.6 mg/kg at 24 and 48 h One dose of RZF demonstrated potent antifungal efficacy in a neutropenic mouse model of C. albicans infection, up to 72 h after a single dose |
| Neutropenic mouse/ disseminated Aspergillus fumigatus (ATCC 13073) |
‐‐‐
|
Significant increase in 10‐day survival rate compared with vehicle (P < .05) with all RZF groups (0.2, 1, and 5 mg/kg) and with ANF 1 and 5 mg/kg at 24 and 48 h RZF administered BID for 5 days demonstrated potent antifungal efficacy in a neutropenic mouse model of A. fumigatus infection |
||
| Lakota et al14 | To evaluate the effects of front‐loaded dosing regimens on RZF efficacy | Neutropenic mouse/ disseminated C. albicans (R303) |
RZF total doses (0.7, 2, and 7 mg/kg) administered on 3 dosing schedules: single dose, twice weekly, and daily (eg, RZF 2 mg/kg total was evaluated as a single administration of 2 mg/kg, as 1 mg/kg given twice weekly, and as 0.29 mg/kg given daily for 7 days). (MIC, 0.125 μg/mL) By IP injection starting 24 h post‐infection |
A higher degree of fungal killing was achieved when RZF 2 mg/kg (total) was front‐loaded ‐ ie, delivered entirely in one dose versus divided into daily or twice weekly doses. There was a > 2 log10 CFU reduction from baseline at 168 h, whereas twice‐weekly and daily regimens resulted in net stasis or log CFU similar to no‐treatment controls. RZF PK/PD produces beneficial effects on efficacy due to front‐loaded dosing and the associated exposure shape of RZF (ie, high drug exposures achieved early in the course of therapy) |
| Zhao et al15 | To evaluate the effects of tissue drug exposure on RZF efficacy | Mouse/ intraabdominal C. albicans (SC5314) |
|
RZF demonstrated extensive tissue distribution and rapid penetration into abscesses. At 24 h after a single dose, the mean drug concentration within lesions was ~ 4‐fold higher for RZF than for MCF at the same dosage, indicating superior lesion penetration by RZF. Four of 5 mouse livers were sterilized by RZF 20 mg/kg, and liver infection resolved in one of the 5 mice. No liver sterilization was observed in MCF‐treated mice. RZF demonstrated higher tissue exposure and lesion penetration compared with MCF. |
| Hager et al 33 | To evaluate the efficacy of RZF in treatment of disseminated infection caused by Candida auris | Immunosuppressed mouse/ disseminated C. auris (MRL35368) |
|
Mice treated with RZF had significantly lower average log10 CFU compared with AMB‐ and vehicle‐treated mice on all days when kidneys were harvested and compared with the MCF‐treated group on Day 10. RZF demonstrated in vivo efficacy against C. auris. |
Abbreviations: AMB, amphotericin B; ANF, anidulafungin; CFU, colony‐forming units; IP, intraperitoneal; IV, intravenous; MCF, micafungin; MEC, minimum effective concentration; MIC, minimum inhibitory concentration; RZF, rezafungin.
Significant in vivo efficacy (≥99%, 2‐log reduction in CFU/g) was previously observed following rezafungin treatment at up to 72 hours post‐infection in a neutropenic mouse model of disseminated candidiasis.6, 22 The present experiment using a similar infection model showed that one dose of rezafungin was efficacious at up to 192 hours (8 days) post‐infection, with reductions in kidney fungal colony counts to levels at or below the limit of detection in groups treated with rezafungin. The long duration of rezafungin efficacy is consistent with its pharmacokinetics which, in multiple animal species (mice, rats, dogs, and nonhuman primates),7 were consistent and linear across species in terms of low clearance, long half‐life, and dose‐dependent plasma exposure. More recently, clinical translation of the long half‐life of rezafungin and correspondingly prolonged efficacy was demonstrated in STRIVE, the Phase 2 study of rezafungin administered once weekly in patients with invasive candidiasis and/or candidemia, which met both its primary safety and efficacy endpoints.23, 24
The experiment of rezafungin treatment 24 hours after infection also builds upon previous evaluations of antifungal efficacy, as well as of drug exposure as a determinant of efficacy.11 The efficacy of rezafungin despite later treatment initiation may be attributable in part to the front‐loaded pattern of rezafungin drug exposure.13, 14 Briefly, the concentration‐dependent fungicidal action and high plasma drug exposure of rezafungin lend themselves to rapid, extensive killing early in the course of treatment when fungal burden is greatest. The benefit to efficacy from front‐loaded patterns of exposure has been substantiated with both single and intermittent doses of rezafungin14 compared with once‐daily administration of currently approved echinocandins.25 In target attainment analyses based on Phase 1 data, single and once‐weekly doses of rezafungin achieved high (≥90% and 100%, respectively) probabilities of target attainment against contemporary strains of C. albicans and Candida glabrata.16 The PK/PD profile of rezafungin, namely its high plasma drug exposure and wide safety margin, may also be relevant to preventing resistance, as postulated by the mutant selection window hypothesis and mutant prevention concentration (MPC; the minimal concentration of a drug that inhibits development of mutant subpopulations) determined for rezafungin.15 While prevention of resistance remains hypothetical, PK/PD determinants of efficacy clearly favor the ability to readily achieve and safely maintain high levels of drug in plasma.
The in vivo efficacy of rezafungin reported herein against azole‐resistant C. albicans (R357) expands the database on rezafungin against less susceptible and resistant Candida spp.,11, 12, 15 including the inherently multidrug‐resistant and difficult‐to‐treat Candida auris (Table 1).9, 10, 26, 27, 28, 29 Similarly, the in vivo model of invasive aspergillosis, in which treatment with rezafungin 2 mg/kg (human equivalent dose in mouse, 10‐30 mg/kg) and AmB 3 mg/kg administered as a single dose or twice‐daily fractionated doses were associated with similar survival rates in mice infected with A. fumigatus (ATCC 13073), contributes to a growing body of research on rezafungin against Aspergillus spp.30, 31 Further in vivo evaluation of rezafungin against azole‐resistant Aspergillus spp. as well as the potential benefits of extended‐interval dosing32 would be of interest, particularly when considering situations involving azole resistance or intolerability.
Certain methodological details in the invasive candidiasis experiments may warrant explanation. In the assessments of efficacy at increasing intervals post‐infection and when treatment was delayed, rezafungin effects at 168 hours and 192 hours, respectively, were compared with those of fluconazole and vehicle controls at earlier timepoints (at 24 and 48 hours, respectively, for fluconazole and at 72 hours for vehicle controls). Fluconazole use, as a positive control to demonstrate the success of the infection model (ie, that infection would respond to active treatment), was maintained from historical validation of the model, and animals in the vehicle control group were moribund by 72 hours and were sacrificed for ethical reasons. Secondly, the use of different routes of administration (PO and IV for treatment, and IP for control) may limit but do not entirely preclude comparisons, based on the assumption that responses in vehicle‐treated animals would be similar to those of untreated control animals.
In this series of in vivo studies, rezafungin demonstrated efficacy in a variety of situations that expand on previous preclinical evaluations and may translate to potential clinical scenarios, such as treatment of resistant strains of Candida and Aspergillus and when treatment is not immediately initiated. These findings further substantiate the in vivo efficacy of rezafungin and support its ongoing clinical development in the prevention and treatment of invasive fungal infections.
DISCLOSURES
None declared.
AUTHORS CONTRIBUTIONS
LM and KYL are employees of Eurofins Panlabs. VO is an employee and stockholder of Cidara Therapeutics, Inc. All authors made substantial contributions to the design of the work and/or to the acquisition of data, their analysis, and their interpretation. All authors were involved in the drafting and/or critical review of the manuscript, and all authors approved of the final version.
ACKNOWLEDGMENTS
These studies and medical writing assistance for the manuscript (provided by T. Chung, Scribant Medical) was funded by Cidara Therapeutics, Inc. The study sponsor was involved in the study design and the decision to submit the manuscript for publication; the study sponsor was not involved in the collection, analysis, or interpretation of data.
Miesel L, Lin K‐Y, Ong V. Rezafungin treatment in mouse models of invasive candidiasis and aspergillosis: Insights on the PK/PD pharmacometrics of rezafungin efficacy. Pharmacol Res Perspect. 2019;00:e00546 10.1002/prp2.546
DATA AVAILABILITY STATEMENT
Data may be available from the corresponding author upon reasonable request.
REFERENCES
- 1. Kontoyiannis DP, Marr KA, Park BJ, et al. Prospective surveillance for invasive fungal infections in hematopoietic stem cell transplant recipients, 2001–2006: overview of the Transplant‐Associated Infection Surveillance Network (TRANSNET) Database. Clinical Infect Dis. 2010;50:1091‐1100. [DOI] [PubMed] [Google Scholar]
- 2. Pappas PG, Alexander BD, Andes DR, et al. Invasive fungal infections among organ transplant recipients: results of the Transplant‐Associated Infection Surveillance Network (TRANSNET). Clin Infect Dis. 2010;50:1101‐1111. [DOI] [PubMed] [Google Scholar]
- 3. Sanguinetti M, Posteraro B, Lass‐Florl C. Antifungal drug resistance among Candida species: mechanisms and clinical impact. Mycoses. 2015;58(Suppl 2):2‐13. [DOI] [PubMed] [Google Scholar]
- 4. Krishnan BR, James KD, Polowy K, et al. CD101, a novel echinocandin with exceptional stability properties and enhanced aqueous solubility. J Antibiot (Tokyo). 2017;70:130‐135. [DOI] [PubMed] [Google Scholar]
- 5. Lakota EA, Bader JC, Thye D, et al. PK‐PD target attainment analyses to support the selection of extended interval CD101 dosing regimens. Open Forum Infect Dis. 2016;3(suppl 1): 10.1093/ofid/ofw172.1542 [DOI] [Google Scholar]
- 6. Ong V, Hough G, Schlosser M, et al. Preclinical evaluation of the stability, safety, and efficacy of CD101, a novel echinocandin. Antimicrob Agents Chemother. 2016;60:6872‐6879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ong V, James KD, Smith S, Krishnan BR. Pharmacokinetics of the novel echinocandin CD101 in multiple animal species. Antimicrob Agents Chemother. 2017;61:e01626-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Sandison T, Ong V, Lee J, Thye D. Safety and pharmacokinetics of CD101 IV, a novel echinocandin, in healthy adults. Antimicrob Agents Chemother. 2017;61:pii: e01627‐01616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Berkow EL, Lockhart SR. Activity of CD101, a long‐acting echinocandin, against clinical isolates of Candida auris . Diagn Microbiol Infect Dis. 2018;90:196‐197. [DOI] [PubMed] [Google Scholar]
- 10. Lepak A, Zhao M, Andes D. Pharmacodynamic evaluation of rezafungin (CD101) against Candida auris in the neutropenic mouse invasive candidiasis model. Antimicrob Agents Chemother. 2018a;62:e01572 10.1128/AAC.01572-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lepak AJ, Zhao M, VanScoy B, Ambrose PG, Andes DR. Pharmacodynamics of a long‐acting echinocandin, CD101, in a neutropenic invasive‐candidiasis murine model using an extended‐interval dosing design. Antimicrob Agents Chemother. 2018b;62:e02154‐17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Pfaller MA, Messer SA, Rhomberg PR, Jones RN, Castanheira M. Activity of a long‐acting echinocandin, CD101, determined using CLSI and EUCAST reference methods, against Candida and Aspergillus spp., including echinocandin‐ and azole‐resistant isolates. J Antimicrob Chemother. 2016;71:2868‐2873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bader J, Bhavnani S, Andes D, Ambrose P. We can do better: a fresh look at echinocandin dosing. J Antimicrob Chemother. 2018a;73:i44‐i50. [DOI] [PubMed] [Google Scholar]
- 14. Lakota E, Bader J, Ong V, et al. Pharmacological basis of CD101 efficacy: exposure shape matters. Antimicrob Agents Chemother. 2017;61:e00758‐00717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Zhao Y, Prideaux B, Nagasaki Y, et al. Unraveling drug penetration of echinocandin antifungals at the site of infection in an intra‐abdominal abscess model. Antimicrob Agents Chemother. 2017;61:e01009‐01017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Bader JC, Lakota EA, Flanagan S, et al. Overcoming the resistance hurdle: PK‐PD target attainment analyses of rezafungin (CD101) for Candida albicans and Candida glabrata . Antimicrob Agents Chemother. 2018b;62: pii: e02614-17. 10.1128/AAC.02614-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Zuluaga AF, Salazar BE, Rodriguez CA, Zapata AX, Agudelo M, Vesga O. Neutropenia induced in outbred mice by a simplified low‐dose cyclophosphamide regimen: characterization and applicability to diverse experimental models of infectious diseases. BMC Infect Dis. 2006;6:55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. James KD, Laudeman CP, Malkar NB, Krishnan R, Polowy K. Structure‐Activity relationships of a series of echinocandins and the discovery of CD101, a highly stable and soluble echinocandin with distinctive pharmacokinetic properties. Antimicrob Agents Chemother. 2017;61:e01541-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Pfaller MA, Messer SA, Rhomberg PR, Castanheira M. Activity of a long‐acting echinocandin (CD101) and seven comparator antifungal agents tested against a global collection of contemporary invasive fungal isolates in the SENTRY 2014 antifungal surveillance program. Antimicrob Agents Chemother 2017a;61:e02045–02016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Pfaller MA, Messer SA, Rhomberg PR, Castanheira M. CD101, a long‐acting echinocandin, and comparator antifungal agents tested against a global collection of invasive fungal isolates in the SENTRY 2015 Antifungal Surveillance Program. Int J Antimicrob Agents. 2017b;50:352–358. [DOI] [PubMed] [Google Scholar]
- 21. Wiederhold NP, Tran BH, Locke JB, Daruwala P, Bartizal K. Rezafungin (CD101) demonstrates potent in vitro activity against Aspergillus, including azole‐resistant A. fumigatus isolates and cryptic species. J Antimicrob Chemother. 2018b;73(11):3063‐3067. [DOI] [PubMed] [Google Scholar]
- 22. Zhao Y, Perez WB, Jimenez‐Ortigosa C, et al. CD101: a novel long‐acting echinocandin. Cell Microbiol. 2016;18:1308‐1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Thompson GR, Honore PM, Horcajada JP, et al. Rezafungin clinical safety and efficacy in the treatment of candidemia and/or invasive candidiasis: combined results from the STRIVE Phase 2 trial Parts A and B. Nice, France: Presented at Trends in Medical Mycology;2019. [Google Scholar]
- 24. Thompson GR, Vazquez J, Soriano A, et al. Clinical efficacy and safety of rezafungin (CD101): results from STRIVE, a randomized, double‐blind, multicenter, phase 2 study in the treatment of candidemia and/or invasive candidiasis. Atlanta, GA: Presented at ASM Microbe;2018. [Google Scholar]
- 25. Bader JC, Lakota EA, Bhavnani SM, Ambrose PG. Emerging Candida glabrata resistance and echinocandin dosing: A Call to Arms!. Open Forum Infectious Diseases. 2016;3:1973‐1973. [Google Scholar]
- 26. Arendrup MC, Jørgensen KM, Chowdhary A, Meis JF. EUCAST susceptibility testing of rezafungin (CD101): activity against Candida auris. Madrid, Spain: Presented at European Congress of Clinical Microbiology and Infectious Diseases;2018. [Google Scholar]
- 27. Calvo B, Melo AS, Perozo‐Mena A, et al. First report of Candida auris in America: Clinical and microbiological aspects of 18 episodes of candidemia. J Infect. 2016;73:369‐374. [DOI] [PubMed] [Google Scholar]
- 28. Lockhart SR, Etienne KA, Vallabhaneni S, et al. Simultaneous emergence of multidrug‐resistant Candida auris on 3 continents confirmed by whole‐genome sequencing and epidemiological analyses. Clin Infect Dis. 2017;64:134‐140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Vallabhaneni S, Kallen A, Tsay S, et al. Investigation of the first seven reported cases of Candida auris, a globally emerging invasive, multidrug‐resistant fungus ‐ United States, May 2013‐August 2016. MMWR Morb Mortal Wkly Rep. 2016;65:1234‐1237. [DOI] [PubMed] [Google Scholar]
- 30. Wiederhold N, Najvar LK, Jaramillo R, Olivo M, Catano G, Patterson TF. Rezafungin is efficacious against invasive aspergillosis caused by azole-resistant Aspergillus fumigatus harboring the TR34/L98H mutation. San Francisco, CA: Presented at ASM Microbe;2019a. [Google Scholar]
- 31. Wiederhold NP, Locke JB, Daruwala P, Bartizal K. Rezafungin (CD101) demonstrates potent in vitro activity against Aspergillus, including azole‐resistant Aspergillus fumigatus isolates and cryptic species. J Antimicrob Chemother. 2018a;73(11):3063–3067. [DOI] [PubMed] [Google Scholar]
- 32. Wiederhold NP, Najvar LK, Jaramillo R, et al. Extended‐interval dosing of rezafungin against azole‐resistant Aspergillus fumigatus . Antimicrob Agents Chemother. 2019b;63:e01165‐19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Hager CL, Larkin EL, Long LA, Ghannoum MA. Evaluation of the efficacy of rezafungin, a novel echinocandin, in the treatment of disseminated Candida auris infection using an immunocompromised mouse model. J Antimicrob Chemother. 2018;73:2085–2088. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data may be available from the corresponding author upon reasonable request.


