Abstract
Objective This study was aimed to perform a systematic literature review by examining outcomes in patients with sporadic vestibular schwannoma (VS) undergoing ipsilateral cochlear implant (CI).
Data Sources PubMed-NCBI (National Center for Biotechnology Information) and Scopus databases were searched through October 2017.
Study Selection Studies reporting auditory outcomes for each patient when a CI was placed with an ipsilateral sporadic VS were included.
Main Outcome Measures Demographic variables, VS characteristics, preoperative hearing metrics, duration of deafness, CI type, approach to tumor resection, postoperative auditory outcomes, and postoperative tinnitus outcomes were reported for each eligible patient within studies. Each study was evaluated for quality and bias.
Results Fifteen studies and 45 patients met inclusion criteria. Mean speech discrimination score (SDS) improved from 30.0 to 56.4% after CI placement. The majority when reported had an improvement in tinnitus. Preoperative ipsilateral SDS was a negative predictor of postoperative SDS, while neither tumor resection status, tumor location, duration of deafness, ipsilateral pure tone average, nor timing of CI placement had a significant effect on patient outcome.
Conclusions Notwithstanding the challenges inherent with surveillance magnetic resonance imaging (MRI) in the setting of a cochlear implant magnet, select sporadic vestibular schwannoma patients can be considered for cochlear implantation.
Keywords: cochlear implant, vestibular schwannoma, acoustic neuroma, hearing outcomes, sporadic vestibular schwannoma
Introduction
A vestibular schwannoma (VS) is a benign tumor derived from Schwann cells of the vestibulocochlear nerve. It is the most common tumor of the cerebellopontine angle (CPA) with an incidence of approximately1 in 100,000 people. 1 The most common presenting symptom is unilateral hearing loss which is present in almost 80% of patients. 2 Ten years after presentation, less than one-quarter of patients who present with serviceable hearing maintain American Academy of Otolaryngology - Head and Beck Surgery class A or B 3 hearing regardless of intervention or lack thereof. 4
The generally accepted treatment options for VSs are observation with serial imaging, stereotactic radiotherapy, or surgery. 5 The priorities of treatment are: (1) preserving life, (2) preserving the facial nerve, (3) preserving hearing, and (4) minimizing other complications. 6 More recent trends in management favor less invasive treatment options. 7 However, the need for surgical treatment remains in many patients and inevitably, hearing preservation is sometimes not possible or unsuccessful.
The treatment algorithm shifts in a patient with neurofibromatosis type 2 (NF2) or a VS in an only hearing ear 5 as the patient may be faced with bilateral deafness. Auditory brainstem implants remain an option when the cochlear nerve requires sacrifice. When the cochlear nerve is intact, studies have shown a higher proportion of patients achieve functional open set speech recognition with CI compared with auditory brainstem implants. 8 9 10 11 12
Advancements in technology have improved outcomes in patients with cochlear implants (CI), leading to expanded indications in recent years. 13 In single-sided deafness, studies have shown that CIs are highly beneficial. 14 15 Härkönen et al 15 showed improvements in quality of life, spatial perception, sound localization, and speech intelligibility. The natural progression of this concept led to the consideration of placing a CI in the setting of a sporadic VS ipsilateral to the tumor regardless of the hearing status of the contralateral ear. CI could offer an appealing option for patients with poor hearing in a stable, nongrowing tumor. An implant could also be considered at tumor surgery when preoperative hearing is nonserviceable and/or hearing preservation surgery is not an option, and the cochlear nerve is preserved. These represent off-label uses of a CI.
To date, most studies reporting CI in VS involve NF2 patients. Far fewer studies have reported CIs in sporadic VS. Traditional view has held that in most sporadic VS, single-sided deafness is acceptable and concern for interference with magnetic resonance imaging (MRI) surveillance precluded placing a CI. However, we now understand that with few modifications MRI can still be effective with a CI in place. 16 17 The objective of this study is to perform a systematic review examining outcomes in patients with sporadic VS undergoing ipsilateral CI.
Methods
Eligibility Criteria
Inclusion was evaluated for each individual patient in all full text studies evaluated. Studies were included even if only a portion of the patients met inclusion criteria. For a study to be included, the data could not be aggregated; it had to be reported at a granular, patient level. The inclusion criteria for each patient were: (1) must have had a sporadic VS, (2) must have had a CI placed on the side of the VS, (3) must have had at least 6 months follow-up after their CI was placed, (4) CI auditory outcomes must have been reported, and (5) patients with NF2 were excluded. Of note, the VS need not have been resected for inclusion. We also used one patient from our personal experience which met set inclusion criteria.
Search Strategy
To identify relevant studies, searches were performed in PubMed-NCBI (National Center for Biotechnology Information) and Scopus by an academic librarian. The search strategies employed are included in the Appendix A . No restrictions based on year of publication were used. The studies could be in English or German.
Study Selection and Validation
Two reviewers independently screened each abstract and then evaluated the remaining full articles for eligibility. Discrepancies were resolved by a third reviewer.
Data Abstraction
Information was extracted at two levels, a study level and a patient level. Information extracted from each study included author, year of publication, article language, number of patients, whether it was retrospective or prospective, the study's level of evidence was based on the Oxford Centre for Evidence Based Medicine, 2011 criteria. 18 Information extracted from individual patients, when available, included gender, age, laterality, tumor location, tumor size, preoperative hearing metrics, duration of deafness before CI, CI type, whether VS was resected, approach to tumor resection, timing of CI placement compared with resection, complications, postoperative auditory outcomes, and postoperative tinnitus outcomes. The data were entered into an electronic research database (REDCap hosted at Loyola University Medical Center). 19
Assessment of Quality and Bias of Individual Studies
The National Institutes of Health's (NIH) Quality Assessment of Case Series Studies 20 was used to evaluate quality and bias of individual studies. Fig. 1 shows the criteria used in this assessment.
Fig. 1.

Criteria for the National Institutes of Health's quality assessment of case series studies. 18
Statistical Methods
Descriptive statistics were used to summarize patient characteristics and outcomes of interest. Associations between predictors and hearing function were tested using regression analysis on the pooled sample. Between-study variation was accounted for by incorporating fixed study effects. Cases with missing data were excluded from the analyses. All analyses were performed using SAS Version 9.4 (Cary, NC).
Results
Study Selection
A total of 477 studies were identified from the PubMed-NCBI and Scopus searches. After duplicates were removed, 304 abstracts were screened. After implementation of our selection criteria, 284 studies were excluded based on their abstracts. The remaining 20 full articles were reviewed, and 16 articles fulfilled all criteria for inclusion. The reasons for exclusion of studies are listed in Fig. 2 . One patient was included from our own experience.
Fig. 2.
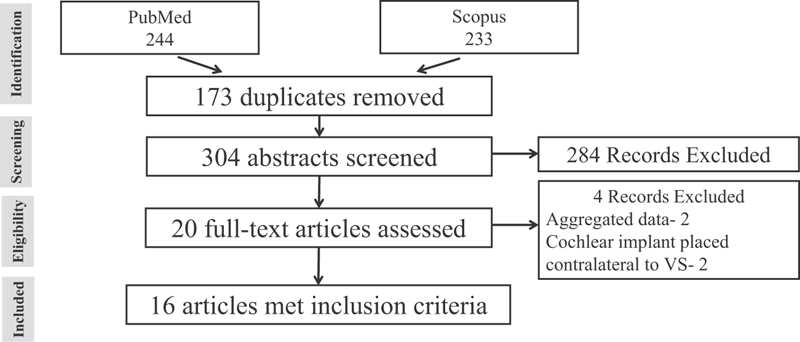
Study selection process and reasons for exclusion. VS, vestibular schwannoma.
Study Characteristics
The studies that met inclusion criteria were published between 1995 and 2017. Table 1 shows studies' reported outcome measures. The assessment of bias and quality for each study is shown in Table 2 .
Table 1. Study characteristics.
| Ref. | First Author | PY | Lang | Pts. in Study | Pts. Used | Reason for pt. removal | Outcomes Measures |
|---|---|---|---|---|---|---|---|
| 37 | Aschendorff | 2017 | E | 8 | 5 | CI not placed | SDS |
| 38 | Bohr | 2017 | G | 6 | 5 | NF2 | PAT, SDS |
| 39 | DeHart | 2017 | E | 1 | 1 | PAT, AzBio, CNC | |
| 40 | Plontke | 2017 | E | 12 | 5 | CI not placed | SDS |
| 41 | Carlson | 2016 | E | 10 | 3 | NF2 | PAT, AzBio, CNC |
| 42 | Dagna | 2016 | E | 1 | 1 | PTA, HINT, THI | |
| 21 | Hassepass | 2016 | E | 11 | 3 | CI not placed or no CI outcomes | HINT, localization test, tinnitus VAS, subjective survey |
| 43 | Huo | 2016 | E | 3 | 2 | C/l CI placement | PAT, SDS mono/di/sentence, THI, telephone use |
| 44 | Kim | 2016 | E | 2 | 2 | PAT, HINT | |
| 45 | Schipper | 2017 | G | 10 | 10 | SDS | |
| 46 | Schutt | 2014 | E | 1 | 1 | PAT, SDS | |
| 47 | Di Lella | 2013 | E | 10 | 3 | C/l CI placement | Disyllabic SDS, sentence recognition |
| 48 | Helbig | 2009 | E | 1 | 1 | SDS | |
| 49 | Zanetti | 2008 | E | 1 | 1 | SDS | |
| 50 | Arriaga | 1995 | E | 1 | 1 | PAT |
Abbreviations: AzBio, Arizona biomedical institute sentence test; CI, cochlear implant; CNC, Maryland consonant-vowel nucleus-consonant test; E, English; G, German; HINT, hearing in noise test; Lang., language; NF2, neurofibromatosis type 2; PAT, postimplant audiometry threshold; Pt, patients; Pts, patients; C/I, contralateral; Ref, reference number; SDS, speech discrimination score; THI, tinnitus handicap index; VAS, visual analog scale.
Table 2. Assessment of quality and individual bias for individual studies based on the Oxford Centre for Evidence Based Medicine (OCEBM) 16 2011 criteria and the standardized risk assessment of individual studies based on the National Institute of Health (NIH) quality assessment tool for case series studies 18 .
| Ref. | First Author | PY | P/R | OCEBM | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 37 | Aschendorff | 2017 | R | 4 | Y | Y | Y | Y | Y | Y | Y | NA | Y |
| 38 | Bohr | 2017 | R | 4 | Y | Y | Y | Y | Y | Y | Y | Y | Y |
| 39 | DeHart | 2017 | R | 4 | Y | Y | NA | Y | Y | Y | Y | NA | Y |
| 40 | Plontke | 2017 | R | 4 | Y | Y | Y | Y | Y | Y | Y | NA | Y |
| 41 | Carlson | 2016 | P | 4 | Y | Y | Y | Y | Y | Y | Y | Y | Y |
| 42 | Dagna | 2016 | R | 4 | Y | Y | NA | Y | Y | Y | Y | NA | Y |
| 21 | Hassepass | 2016 | R | 4 | Y | Y | Y | Y | Y | Y | Y | NA | Y |
| 43 | Huo | 2016 | R | 4 | Y | Y | Y | Y | Y | Y | Y | NA | Y |
| 44 | Kim | 2016 | R | 4 | Y | Y | Y | Y | Y | Y | Y | NA | Y |
| 45 | Schipper | 2017 | R | 4 | Y | Y | Y | Y | Y | Y | Y | N | Y |
| 46 | Schutt | 2014 | R | 4 | Y | Y | NA | Y | Y | Y | Y | NA | Y |
| 47 | Di Lella | 2013 | R | 4 | Y | Y | Y | Y | Y | Y | Y | NA | Y |
| 48 | Helbig | 2009 | R | 4 | Y | Y | NA | Y | Y | Y | Y | NA | Y |
| 49 | Zanetti | 2008 | R | 4 | Y | Y | NA | Y | Y | Y | Y | NA | Y |
| 50 | Arriaga | 1995 | R | 4 | Y | Y | NA | Y | Y | Y | Y | NA | Y |
Abbreviations: N, no; NA, not applicable; P, prospective; PY, publication year; R, retrospective; Ref., reference number; Y, yes.
Note : Numbers are based on questions from Fig. 1 .
Patient Characteristics
Patients of all ages were included in the study. The mean age was 53 years old, with a range of 22 to 83 years old. There was a male predominance, with males making up 61.5% of patients.
Preoperative characteristics and hearing results are listed in Tables 3 and 4 . The tumor was located on the right more often than the left (61.5%). The internal auditory canal was the most common location of VS (57.1%) and the vestibular structures were the second most common at 28.6%. A single tumor may involve more than one location. Tumors involved more than one site in 23.8% of cases. Patients presented with an average of 22.5 months of deafness. The mean preoperative ipsilateral pure tone average (PTA) was 85 dB and speech discrimination score (SDS) was 30.0%. A CI was placed at the time of surgical resection of the tumor in 48.7% of patients. Patients were followed for an average of 20 months after CI placement. Several different approaches to resect tumors were performed. The most common approach was translabyrinthine (61.5%). Multiple approaches were used in 7.7% of cases. Most patients receiving a CI had tumors surgically resected (86.7%). Promontory stimulation was reported and positive in 11 patients before implantation.
Table 3. Preoperative characteristics.
| Characteristic | n | Result |
|---|---|---|
| Side: Right | 39 | |
| Right | 24 (61.5%) | |
| Left | 15 (38.5%) | |
| Tumor location | 42 | |
| CPA | 5 (11.9%) | |
| IAC | 24 (57.1%) | |
| Intracochlear | 11 (26.2%) | |
| Vestibular structures | 12 (28.6%) | |
| Two locations | 10 (23.8%) | |
| Approach for resection | 39 | |
| Translabyrinthine | 24 (61.5%) | |
| Retrosigmoid | 2 (5.1%) | |
| Labyrinthectomy | 8 (20.5%) | |
| Cochleoectomy | 8 (20.5%) | |
| Retrolabyrinthine | 1 (2.6%) | |
| Combined approaches | 3 (7.7%) | |
| CI placed concurrent with resection | 39 | 19 (48.7%) |
| Tumor resected | 45 | 39 (86.7%) |
Abbreviations: CI, cochlear implant; CPA, cerebellopontine angle; IAC, internal auditory canal; n , number of patients where data reported.
Table 4. Preoperative hearing results.
| Hearing Results | n | Mean +/− SD |
|---|---|---|
| Duration of deafness | 18 | 89.8 +/− 132.9 mo |
| Follow-up time | 41 | 20.2 +/− 15.3 mo |
| Ipsilateral PTA preoperative | 20 | 79.8 +/− 32.7 dB |
| Ipsilateral SDS preoperative | 29 | 30.0 +/− 40.0% |
Abbreviations: dB, decibels; n , number of patients; PTA, pure tone average; SD, standard deviation; SDS, speech discrimination score.
Auditory Outcomes
Postoperative hearing outcomes are described in Table 5 . Average SDS was 56.4% with a standard deviation of 27.6 ( Fig. 3 ). AzBio testing (Arizona biomedical institute sentence test) showed an average of 75%. The average postimplant audiometry threshold was 28.8 dB.
Table 5. Postoperative auditory outcomes.
| Auditory Outcome | n | Mean +/− SD |
|---|---|---|
| SDS | 30 | 56.4 +/− 27.6% |
| PAT | 11 | 28.8 +/- 8.3 dB |
| AzBio | 5 | 75.0 +/− 14.3% |
Abbreviations: AzBio, Arizona biomedical institute sentence test; n , number of patients; PAT, postimplant audiometry threshold; SD, standard deviation; SDS, speech discrimination score.
Fig. 3.
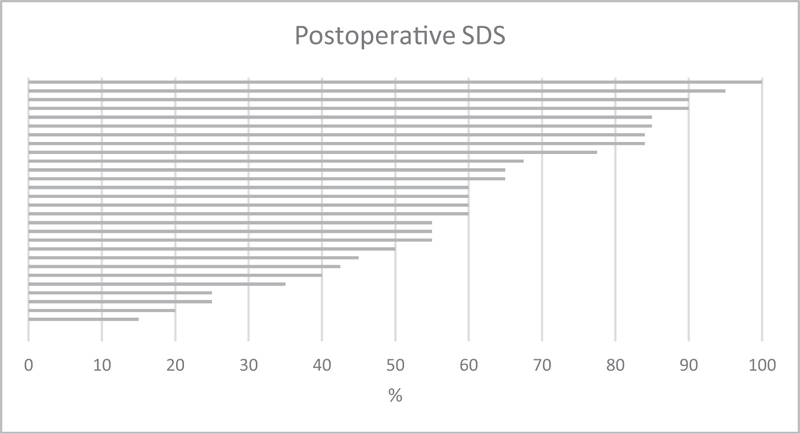
Postoperative speech discrimination score for each patient. SDS, speech discrimination score.
Auditory Outcome Predictors
Univariable regression analysis found that an increase in preoperative ipsilateral SDS predicted a lower SDS. Table 6 shows that neither tumor resection status, tumor location, duration of deafness, ipsilateral PTA, nor timing of CI placement had a significant effect on patients' outcome.
Table 6. Outcome predictors.
| Predictor | n | EC | SE | p -Value |
|---|---|---|---|---|
| Tumor resected: yes vs. no | 30 | −18.46 | 20.66 | 0.380 |
| Tumor location | 30 | |||
| Intracochlear vs. IAC | 6.71 | 17.94 | 0.712 | |
| Vestibular vs IAC | 7.75 | 17.58 | 0.663 | |
| Multiple locations vs IAC | −4.28 | 20.49 | 0.836 | |
| Duration of deafness, mo | 10 | 0.13 | 0.12 | 0.303 |
| Ipsilateral PTA | 8 | 0.27 | 0.60 | 0.668 |
| Ipsilateral SDS | 20 | −0.44 | 0.21 | 0.049 |
| Timing of CI | 28 | 15.84 | 10.88 | 0.158 |
Abbreviations: CI, cochlear implant; EC, estimated coefficient; IAC, internal auditory canal; n , number of patients; PTA, pure tone average; SDS, speech discrimination score; SE, standard error.
Tinnitus
Tinnitus was reported in seven patients, five among them completed a standardized questionnaire. In all, except in one patient, tinnitus improvement was noted. The patient with worsening of tinnitus postoperatively was the only patient whose tumor was not resected.
Discussion
Most patients with sporadic VS will experience a decline of hearing in the involved ear whether undergoing observation, radiation treatment, or surgery. Current standard treatment options for this hearing loss including bone anchored hearing aids or contralateral routing of signal (CROS) hearing aids offering contralateral signal routing but do not provide auditory input into the involved ear. 21 There is a relative abundance of research on hearing restoration using CI in patients with NF2 8 9 10 11 12 22 23 24 25 26 but thus far there is limited data available in CI with sporadic VS. This is the first systematic literature review focusing on outcomes in patients with sporadic VS and CI in the affected ear.
Hearing outcomes were evaluated by combining all cases reported in the literature. We found that mean SDS improved from 30.0 to 56.4%. Additionally, studies have shown that functional hearing can be achieved even if natural hearing must be sacrificed 27 which has precedence in CI after labyrinthectomy in Meniere's disease. 28 This study suggests that despite the presence of tumor and/or potential intraoperative injury, the number of surviving axons in the cochlear nerve is usually sufficient for CI rehabilitation. Our analysis showed that a lower preoperative SDS predicted an increased SDS following CI placement. This is a somewhat surprising finding and the clinical relevance is unclear.
At this time, there is insufficient directly comparable data in the literature to compare CI outcomes when a VS is the cause of deafness compared with other causes of deafness. In comparison to hearing sparing approaches, such as middle fossa or retrosigmoid, the outcomes with CI in this study compare favorably. Wilkinson et al's 29 large study showed mean PTA and SDS was 64.4 dB and 60.8% for middle fossa and 81.3 dB and 46.3% for retrosigmoid approaches, respectively. Dead ears resulted in 29.0% of patients with middle fossa and 40.7% of retrosigmoid approaches. In our study, mean postimplant audiometry threshold was 28.8 dB and SDS was 56.4%. While native hearing and digital hearing are inherently different, the outcomes expected from a CI in the setting of sporadic VS could be considered comparable to what is expected with a hearing sparing approach. It is important to note that there is inherent variability of these audiometric tests across centers, so these values must be taken as indicative only.
The small sample size of this current review makes difficult to comment on CI in sporadic VS outcomes regarding tinnitus and impact of duration of deafness. According to Bell et al, 30 preoperative tinnitus persists in 83% of patients following VS resection. Improvement in tinnitus in six of seven patients in this study suggests CIs may improve tinnitus prognosis. We also found no significant difference in hearing outcomes based on the duration of deafness prior to CI placement. Lazard et al 31 showed that in postlingually deafened adults, CI speech performance decreased by 0.23% per year of preimplantation moderate hearing loss. However, once again, because of our small sample size, we believe these questions deserve further study.
As discussed in the introduction, the benefits to CI placement in single-sided deafness are well established. 14 15 Sanna et al 27 prospectively evaluated CI placement simultaneous with translabyrinthine tumor resection with intact contralateral hearing with good results. The data gathered from this study was insufficient to evaluate the effect of an intact contralateral ear on CI outcomes with VS. As long as there is sufficient cochlear nerve function for a CI to function normally, it is reasonable to expect similar outcomes in these patients as others with single-sided deafness. It is especially important to make sure these patients are motivated to learn how to use their CI effectively.
There are several unique challenges to using CIs with sporadic VS. First, the use of MRI in a patient status post CI is possible with appropriate precautions; however, imaging artifact along the posterior fossa with a CI magnet in place is inevitable and may limit the ability to closely follow a VS. 16 17 The patient must also be comfortable in accepting the challenges of undergoing MRI if deemed necessary. For these reasons, the practitioner should choose to implant sporadic VS patients with tumor deemed low risk for requiring future intervention and counsel the patient appropriately. Second, obtaining insurance approval for a single-sided deafness CI can be difficult in many cases.
There are several potential contexts where CIs could be implemented in the treatment of hearing loss in sporadic VS patient. In all scenarios the cochlear nerve must be intact, that is, tumors undergoing observation, radiation treatment, or resection with preserved cochlear nerve. The first scenario would involve placing a CI in a patient undergoing observation or radiation treatment. As hearing declines, the practitioner offers CI after having observed a small stable tumor with serial MRI and deeming the patient low risk for future intervention.
A second scenario would involve patients under consideration for hearing ablative translabyrinthine tumor surgery. Admittedly, many of these patients will have tumors of size and position such that the cochlear nerve cannot be preserved at time of surgery. However, in selected patients, a plan for possible CI placement with translabyrinthine approach when the cochlear nerve can be functionally preserved may be an appealing option. Implantation concurrent with tumor surgery should be considered because of the potential for cochlear ossification following labyrinthectomy, although this is controversial. 32
A final application would be in the uncommon case of an intracochlear or intravestibular VS. Eighteen of the patients in this study had intracochlear or intravestibular involvement and CI hearing outcomes were reasonably good with or without resecting the tumor. CI is especially appealing in isolated intravestibular tumors where the cochlear nerve is uninvolved. Cochlear implant placement following a transmastoid labyrinthectomy for intractable Meniere's disease is an analogous established practice. 28
There are several ways that an intact cochlear neural pathway could be verified before placement of a CI. A positive promontory stimulation test has been shown to be predictive of positive CI outcomes at 100 and 200 Hz, 33 but since the patient must be awake it could not be used at the time of resection for a potential simultaneous CI placement. Neural response telemetry could be used to confirm CI function; however, the absence of a response does not necessarily indicate lack of stimulation/transmission through the cochlear nerve, 34 and since committing to CI would be necessary to perform neural response telemetry (NRT), a poor test result is unlikely to change management. If the auditory brainstem response (ABR) is monitored in a hearing sparing approach for resection, a delay in latency and reduction in amplitude in wave V can predict cochlear nerve damage and portends a poor CI functional outcome. 35 This could be taken into account if a delayed CI placement is being considered with a poor hearing outcome following resection. An electrical ABR can be used in a translabyrinthine approach because of the accessibility of the cochlea and it has been shown to correlate with CI outcomes. 36 This represents an excellent option for simultaneous VS resection with CI placement.
Cochlear implantation is not meant for all patients with sporadic VS. In selected patients with a functionally preserved cochlear nerve where the practitioner and patient are comfortable with the challenges inherent to MRI surveillance, a CI can improve auditory function.
Conclusion
CI in sporadic VS can be considered in a subset of patients where the cochlear nerve is intact. This study demonstrates that selected patients with sporadic VS can benefit from ipsilateral CI placement, notwithstanding the challenges inherent with surveillance MRI in the setting of a CI magnet.
Acknowledgments
Special thanks to Ayrin Molefe, PhD, from the Loyola University Clinical Research Office–Division of Biostatistics for her statistical analysis and Jeanne Sadlik, MLS, from the Loyola University Chicago Health Sciences Library for performing the literature search.
Footnotes
Conflicts of Interests There was no funding or conflicts of interest to disclose for this study.
Appendix A.
Search strategies
1. PubMed-NCBI: “Neuroma, Acoustic” or vestibular schwannoma or acoustic neuroma and “Cochlear Implantation” or “Cochlear Implants” or cochlear implant.
2. Scopus: (TITLE-ABSTRACT-KEY (acoustic neuroma or vestibular schwannoma) and TITLE-ABSTRACT-KEY (cochlear implantation or cochlear implants or cochlear implant))
References
- 1.Propp J M, McCarthy B J, Davis F G, Preston-Martin S. Descriptive epidemiology of vestibular schwannomas. Neuro-oncol. 2006;8(01):1–11. doi: 10.1215/S1522851704001097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Foley R W, Shirazi S, Maweni R M et al. Signs and symptoms of acoustic neuroma at initial presentation: an exploratory analysis. Cureus. 2017;9(11):e1846. doi: 10.7759/cureus.1846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Committee on Hearing and Equilibrium guidelines for the evaluation of hearing preservation in acoustic neuroma (vestibular schwannoma). American Academy of Otolaryngology-Head and Neck Surgery Foundation, INC. Otolaryngol Head Neck Surg. 1995;113(03):179–180. doi: 10.1016/S0194-5998(95)70101-X. [DOI] [PubMed] [Google Scholar]
- 4.Carlson M L, Link M J, Wanna G B, Driscoll C LW. Management of sporadic vestibular schwannoma. Otolaryngol Clin North Am. 2015;48(03):407–422. doi: 10.1016/j.otc.2015.02.003. [DOI] [PubMed] [Google Scholar]
- 5.Doherty J K, Friedman R A. Controversies in building a management algorithm for vestibular schwannomas. Curr Opin Otolaryngol Head Neck Surg. 2006;14(05):305–313. doi: 10.1097/01.moo.0000244186.72645.d4. [DOI] [PubMed] [Google Scholar]
- 6.Rutherford S A, King A T. Vestibular schwannoma management: what is the ‘best’ option? Br J Neurosurg. 2005;19(04):309–316. doi: 10.1080/02688690500305399. [DOI] [PubMed] [Google Scholar]
- 7.Carlson M L, Habermann E B, Wagie A E et al. The changing landscape of vestibular schwannoma management in the United States–a shift toward conservatism. Otolaryngol Head Neck Surg. 2015;153(03):440–446. doi: 10.1177/0194599815590105. [DOI] [PubMed] [Google Scholar]
- 8.Colletti L, Shannon R, Colletti V. Auditory brainstem implants for neurofibromatosis type 2. Curr Opin Otolaryngol Head Neck Surg. 2012;20(05):353–357. doi: 10.1097/MOO.0b013e328357613d. [DOI] [PubMed] [Google Scholar]
- 9.Lloyd S K, Glynn F J, Rutherford S A et al. Ipsilateral cochlear implantation after cochlear nerve preserving vestibular schwannoma surgery in patients with neurofibromatosis type 2. Otol Neurotol. 2014;35(01):43–51. doi: 10.1097/MAO.0000000000000185. [DOI] [PubMed] [Google Scholar]
- 10.Roehm P C, Mallen-St Clair J, Jethanamest D et al. Auditory rehabilitation of patients with neurofibromatosis type 2 by using cochlear implants. J Neurosurg. 2011;115(04):827–834. doi: 10.3171/2011.5.JNS101929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Carlson M L, Breen J T, Driscoll C L et al. Cochlear implantation in patients with neurofibromatosis type 2: variables affecting auditory performance. Otol Neurotol. 2012;33(05):853–862. doi: 10.1097/MAO.0b013e318254fba5. [DOI] [PubMed] [Google Scholar]
- 12.Behr R, Colletti V, Matthies C et al. New outcomes with auditory brainstem implants in NF2 patients. Otol Neurotol. 2014;35(10):1844–1851. doi: 10.1097/MAO.0000000000000584. [DOI] [PubMed] [Google Scholar]
- 13.Carlson M L, Sladen D P, Gurgel R K, Tombers N M, Lohse C M, Driscoll C L. Survey of the American Neurotology Society on cochlear implantation: part 1, candidacy assessment and expanding indications. Otol Neurotol. 2018;39(01):e12–e19. doi: 10.1097/MAO.0000000000001632. [DOI] [PubMed] [Google Scholar]
- 14.Vlastarakos P V, Nazos K, Tavoulari E F, Nikolopoulos T P. Cochlear implantation for single-sided deafness: the outcomes. An evidence-based approach. Eur Arch Otorhinolaryngol. 2014;271(08):2119–2126. doi: 10.1007/s00405-013-2746-z. [DOI] [PubMed] [Google Scholar]
- 15.Härkönen K, Kivekäs I, Rautiainen M, Kotti V, Sivonen V, Vasama J P. Single-sided deafness: the effect of cochlear implantation on quality of life, quality of hearing, and working performance. ORL J Otorhinolaryngol Relat Spec. 2015;77(06):339–345. doi: 10.1159/000439176. [DOI] [PubMed] [Google Scholar]
- 16.Carlson M L, Neff B A, Link M J et al. Magnetic resonance imaging with cochlear implant magnet in place: safety and imaging quality. Otol Neurotol. 2015;36(06):965–971. doi: 10.1097/MAO.0000000000000666. [DOI] [PubMed] [Google Scholar]
- 17.Edmonson H A, Carlson M L, Patton A C, Watson R E. MR imaging and cochlear implants with retained internal magnets: reducing artifacts near highly inhomogeneous magnetic fields. Radiographics. 2018;38(01):94–106. doi: 10.1148/rg.2018170135. [DOI] [PubMed] [Google Scholar]
- 18.Oxford centre for evidence-based medicine–levels of evidence (March 2009). Available from:https://www.cebm.net/2009/06/oxford-centre-evidence-based-medicine-levels-evidence-march-2009/. Accessed March 23, 2018
- 19.Harris P A, Taylor R, Thielke R, Payne J, Gonzalez N, Conde J G. Research electronic data capture (REDCap)–a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(03):377–381. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Study quality assessment tools: National Institutes of Health. Available from:https://www.nhlbi.nih.gov/health-pro/guidelines/in-develop/cardiovascular-risk-reduction/tools/case_series. Accessed March 23, 2018
- 21.Hassepass F, Arndt S, Aschendorff A, Laszig R, Wesarg T. Cochlear implantation for hearing rehabilitation in single-sided deafness after translabyrinthine vestibular schwannoma surgery. Eur Arch Otorhinolaryngol. 2016;273(09):2373–2383. doi: 10.1007/s00405-015-3801-8. [DOI] [PubMed] [Google Scholar]
- 22.Hoa M, Slattery W H., IIINeurofibromatosis 2 Otolaryngol Clin North Am 20124502315–332., viii [DOI] [PubMed] [Google Scholar]
- 23.North H J, Mawman D, O'Driscoll M et al. Outcomes of cochlear implantation in patients with neurofibromatosis type 2. Cochlear Implants Int. 2016;17(04):172–177. doi: 10.1080/14670100.2016.1197587. [DOI] [PubMed] [Google Scholar]
- 24.Trotter M I, Briggs R J. Cochlear implantation in neurofibromatosis type 2 after radiation therapy. Otol Neurotol. 2010;31(02):216–219. doi: 10.1097/MAO.0b013e3181c348e7. [DOI] [PubMed] [Google Scholar]
- 25.Lassaletta L, Aristegui M, Medina M et al. Ipsilateral cochlear implantation in patients with sporadic vestibular schwannoma in the only or best hearing ear and in patients with NF2. Eur Arch Otorhinolaryngol. 2016;273(01):27–35. doi: 10.1007/s00405-014-3450-3. [DOI] [PubMed] [Google Scholar]
- 26.Neff B A, Wiet R M, Lasak J M et al. Cochlear implantation in the neurofibromatosis type 2 patient: long-term follow-up. Laryngoscope. 2007;117(06):1069–1072. doi: 10.1097/MLG.0b013e31804b1ae7. [DOI] [PubMed] [Google Scholar]
- 27.Sanna M, Medina M D, Macak A, Rossi G, Sozzi V, Prasad S C. Vestibular schwannoma resection with ipsilateral simultaneous cochlear implantation in patients with normal contralateral hearing. Audiol Neurotol. 2016;21(05):286–295. doi: 10.1159/000448583. [DOI] [PubMed] [Google Scholar]
- 28.Doobe G, Ernst A, Ramalingam R, Mittmann P, Todt I. Simultaneous labyrinthectomy and cochlear implantation for patients with single-sided Ménière's disease and profound sensorineural hearing loss. BioMed Res Int. 2015;2015:457318. doi: 10.1155/2015/457318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wilkinson E P, Roberts D S, Cassis A, Schwartz M S. Hearing outcomes after middle fossa or retrosigmoid craniotomy for vestibular schwannoma tumors. J Neurol Surg B Skull Base. 2016;77(04):333–340. doi: 10.1055/s-0035-1571166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bell J R, Anderson-Kim S J, Low C, Leonetti J P. The persistence of tinnitus after acoustic neuroma surgery. Otolaryngol Head Neck Surg. 2016;155(02):317–323. doi: 10.1177/0194599816642427. [DOI] [PubMed] [Google Scholar]
- 31.Lazard D S, Vincent C, Venail F et al. Pre-, per- and postoperative factors affecting performance of postlinguistically deaf adults using cochlear implants: a new conceptual model over time. PLoS One. 2012;7(11):e48739. doi: 10.1371/journal.pone.0048739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rodgers B, Stucken E, Metrailer A, Sargent E. Factors influencing cochlear patency after translabyrinthine surgery. Otolaryngol Head Neck Surg. 2017;157(02):269–272. doi: 10.1177/0194599817703072. [DOI] [PubMed] [Google Scholar]
- 33.Alfelasi M, Piron J P, Mathiolon C et al. The transtympanic promontory stimulation test in patients with auditory deprivation: correlations with electrical dynamics of cochlear implant and speech perception. Eur Arch Otorhinolaryngol. 2013;270(06):1809–1815. doi: 10.1007/s00405-012-2125-1. [DOI] [PubMed] [Google Scholar]
- 34.Cosetti M K, Shapiro W H, Green J E et al. Intraoperative neural response telemetry as a predictor of performance. Otol Neurotol. 2010;31(07):1095–1099. doi: 10.1097/MAO.0b013e3181ec1b8c. [DOI] [PubMed] [Google Scholar]
- 35.Lundin K, Stillesjö F, Rask-Andersen H. Prognostic value of electrically evoked auditory brainstem responses in cochlear implantation. Cochlear Implants Int. 2015;16(05):254–261. doi: 10.1179/1754762815Y.0000000005. [DOI] [PubMed] [Google Scholar]
- 36.Lassaletta L, Polak M, Huesers J et al. Usefulness of electrical auditory brainstem responses to assess the functionality of the cochlear nerve using an intracochlear test electrode. Otol Neurotol. 2017;38(10):e413–e420. doi: 10.1097/MAO.0000000000001584. [DOI] [PubMed] [Google Scholar]
- 37.Aschendorff A, Arndt S, Laszig R, Wesarg T, Hassepaß F, Beck R. Treatment and auditory rehabilitation of intralabyrinthine schwannoma by means of cochlear implants : English version. HNO. 2017;65 01:46–51. doi: 10.1007/s00106-016-0217-8. [DOI] [PubMed] [Google Scholar]
- 38.Bohr C, Müller S, Hornung J, Hoppe U, Iro H. [Hearing restoration with cochlear implants after translabyrinthine vestibular schwannoma resection] HNO. 2017;65(09):758–765. doi: 10.1007/s00106-017-0404-2. [DOI] [PubMed] [Google Scholar]
- 39.DeHart A N, Broaddus W C, Coelho D H. Translabyrinthine vestibular schwannoma resection with simultaneous cochlear implantation. Cochlear Implants Int. 2017;18(05):278–284. doi: 10.1080/14670100.2017.1337665. [DOI] [PubMed] [Google Scholar]
- 40.Plontke S K, Rahne T, Pfister M et al. Intralabyrinthine schwannomas : surgical management and hearing rehabilitation with cochlear implants. HNO. 2017;65 02:136–148. doi: 10.1007/s00106-017-0364-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Carlson M L, Neff B A, Sladen D P, Link M J, Driscoll C L. Cochlear implantation in patients with intracochlear and intralabyrinthine schwannomas. Otol Neurotol. 2016;37(06):647–653. doi: 10.1097/MAO.0000000000001016. [DOI] [PubMed] [Google Scholar]
- 42.Dagna F, Murri A, Albera R, Cuda D. [Cochlear implantation in delayed sudden hearing loss after conservative vestibular schwannoma surgery] Acta Otorhinolaryngol Ital. 2016;36(05):428–430. doi: 10.14639/0392-100X-561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Huo Z, Zhang Z, Huang Q et al. Hearing restoration for adults with vestibular schwannoma in the only hearing ear: ipsilateral or contralateral cochlear implantation? ORL J Otorhinolaryngol Relat Spec. 2016;78(05):281–288. doi: 10.1159/000451003. [DOI] [PubMed] [Google Scholar]
- 44.Kim J W, Han J H, Kim J W, Moon I S. Simultaneous translabyrinthine tumor removal and cochlear implantation in vestibular schwannoma patients. Yonsei Med J. 2016;57(06):1535–1539. doi: 10.3349/ymj.2016.57.6.1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schipper J, Kristin J, Volpert S, Klenzner T. [Hearing rehabilitation with the cochlea implant following translabyrinthine CPA tumor removal? ] Laryngorhinootologie. 2017;96(12):836–843. doi: 10.1055/s-0043-119546. [DOI] [PubMed] [Google Scholar]
- 46.Schutt C A, Kveton J F. Cochlear implantation after resection of an intralabyrinthine schwannoma. Am J Otolaryngol. 2014;35(02):257–260. doi: 10.1016/j.amjoto.2013.11.002. [DOI] [PubMed] [Google Scholar]
- 47.Di Lella F, Merkus P, Di Trapani G, Taibah A, Guida M, Sanna M. Vestibular schwannoma in the only hearing ear: role of cochlear implants. Ann Otol Rhinol Laryngol. 2013;122(02):91–99. doi: 10.1177/000348941312200204. [DOI] [PubMed] [Google Scholar]
- 48.Helbig S, Rader T, Bahmer A, Baumann U. A case of bilateral cochlear implantation in single-sided untreated acoustic neurinoma. Acta Otolaryngol. 2009;129(06):694–696. doi: 10.1080/00016480802527545. [DOI] [PubMed] [Google Scholar]
- 49.Zanetti D, Campovecchi C B, Pasini S, Nassif N. Simultaneous translabyrinthine removal of acoustic neuroma and cochlear implantation. Auris Nasus Larynx. 2008;35(04):562–568. doi: 10.1016/j.anl.2007.11.011. [DOI] [PubMed] [Google Scholar]
- 50.Arriaga M A, Marks S. Simultaneous cochlear implantation and acoustic neuroma resection: imaging considerations, technique, and functional outcome. Otolaryngol Head Neck Surg. 1995;112(02):325–328. doi: 10.1016/s0194-5998(95)70257-1. [DOI] [PubMed] [Google Scholar]


