Abstract
Objective Posterior fossa meningiomas are surgically challenging tumors that are associated with high morbidity and mortality. We sought to investigate the anatomical distribution of clinically actionable mutations in posterior fossa meningioma to facilitate identifying patients amenable for systemic targeted therapy trials.
Methods Targeted sequencing of clinically targetable AKT1 , SMO , and PIK3CA mutations was performed in 61 posterior fossa meningioma using Illumina NextSeq 500 to a target depth of >500 × . Samples were further interrogated for 53 cancer-relevant RNA fusions by the Archer FusionPlex panel to detect gene rearrangements.
Results AKT 1 ( E17K ) mutations were detected in five cases (8.2%), four in the foramen magnum and one in the cerebellopontine angle. In contrast, none of the posterior fossa tumors harbored an SMO ( L412F ) or a PIK3CA ( E545K ) mutation. Notably, the majority of foramen magnum meningiomas (4/7, 57%) harbored an AKT1 mutation. In addition, common clinically targetable gene fusions were not detected in any of the cases.
Conclusion A large subset of foramen magnum meningiomas harbor AKT1 E17K mutations and are therefore potentially amenable to targeted medical therapy. Genotyping of foramen magnum meningiomas may enable more therapeutic alternatives and guide their treatment decision process.
Keywords: meningioma, skull base, targeted treatment, AKT1, genomics, fusions, oncology
Introduction
Meningiomas are the most common primary intracranial tumor, representing 36% of all primary central nervous system neoplasms. 1 Approximately 10% of all intracranial meningiomas arise in the posterior fossa. 2 Currently, surgical resection and radiation represent the most common treatment strategies. Due to the invasion of the skull base and the close vicinity to neurovascular structures, gross total resection is challenging and less achievable compared with supratentorial or convexity meningiomas. 3 4 5 6 At present, there is no effective chemotherapy to offer to patients with posterior fossa meningiomas in the setting of surgery or radiation failure.
Recent genomic studies in meningiomas have identified activating mutations in cancer driver genes that are potentially clinically actionable. 7 8 9 For instance, a subset of anterior skull base meningiomas was shown to harbor activating mutations in the AKT1 and PIK3CA genes, both members of the PI3K/AKT/mTOR pathway. 7 10 11 Notably, inhibitors targeting AKT1 and PIK3CA have shown promise in other malignancies. 12 13 14 Mutations in SMO , a sonic hedgehog (SHH) pathway gene, have also been previously reported in anterior skull base meningiomas; 7 8 15 SMO inhibitors are in clinical use in other cancers harboring alterations in the Hedgehog signaling pathway. 16 17 Consequently, targeted treatment in mutant meningiomas represent a promising strategy to decrease tumor burden prior to resection and potentially reduce postoperative morbidity. Furthermore, targeted treatment offers an alternative modality for patients with complex comorbidities deemed too high-risk for surgical intervention.
However, little is known about clinically actionable mutations in meningiomas originating in the posterior fossa. To build upon prior work, we sought to assess clinically actionable mutations in meningiomas arising in the posterior fossa to facilitate identifying amenable patients for ongoing targeted treatment trials.
Methods
Subject Cohort
The study was reviewed and approved by the human subjects' Institutional Review Boards of the Dana-Farber Cancer Institute and Massachusetts General Hospital and complied with the Health Insurance Portability and Accountability Act guidelines. We identified 93 patients who underwent surgical resection of posterior fossa meningiomas at our institution between 1995 and 2015. After excluding patients with neurofibromatosis type 2 and those who received cranial irradiation prior to developing a meningioma, 61 patients fulfilled the criteria and were included in our genomic analysis.
Targeted DNA Sequencing for AKT1 , SMO , and PIK3CA Hotspot Mutations
Representative formalin-fixed paraffin-embedded blocks were selected and reviewed by two board-certified pathologists (S. E. J., M. P. F.) to ensure histopathological diagnosis, grade, and tumor cell contents of each sample. DNA was extracted from FFPE specimens using the GeneRead DNA FFPE kit (Qiagen, Valencia, California, United States) and sheared using a Covaris E220 system (Covaris, Woburn, Massachusetts, United States). Targeted DNA sequencing was performed using a hybrid capture based sequencing platform targeting the genes of interest: AKT1 , SMO , and PIK3CA . Barcoded libraries were generated using the NEBNext Ultra II DNA Library Prep kit for Illumina (NEB, Ipswich, Massachusetts, United States). Target enrichment for the genes of interest within the barcoded libraries was performed using a customized version of SureSelect XT2 Hybridization capture library (Agilent, Santa Clara, California, United States) following the procedure for Capture library size < 3 Mb. Sequencing was performed on an Illumina NextSeq 500 to a target depth of >500× (Illumina, San Diego, California, United States).
An in-house custom bioinformatics pipeline was used for the quality assessment and variant calling, and filtering and annotation. Briefly, raw FASTQ files were mapped to the human genome (hg19) with bwa-mem (v0.7.12), 18 and reads with MAPQ ≥ 30 were retained. Mapped reads were deduplicated using sambamba (v0.6.6) 19 and samtools (v1.5) 20 and filtered for high-quality alignments with a custom Python script. Filtered mapped reads intersecting with targeted regions were selected using bedtools (v2.26). SNVs were called using Freebayes (v0.9.21–19-gc003c1e) and filtered using the following parameters: variant allele frequency ≥ 3.25%, total depth ≥ 100, and alternate allele depth ≥ 10. Insertions and deletions were called using Pindel (v0.2.4) 21 and filtered using the following parameters: variant allele frequency ≥ 7.3%, total depth ≥ 100, and alternate allele depth ≥ 10. Variant annotation was performed using VEP based on RefSeq annotations (v89.7). 22 Finally, for our analysis, we considered the known hotspot mutations AKT1 ( E17K ), SMO ( L412F and W535L ), and PIK3CA ( E545K ) as clinically actionable. 19 20 21 22 23
Archer FusionPlex
Samples were interrogated for fusions by the Archer FusionPlex Solid Tumor (AK0034; Table 1 ) kit. This technology uses an anchored multiplex polymerase chain reaction (AMP) technique that detects gene rearrangements in a fusion partner agnostic manner. 24 FASTQ data analysis, including fusion calling, was performed by ArcherDx Analysis software v5.0.6 using default parameters. 24
Table 1. Fusion genes assessed by the Archer FusionPlex SolidTumor kit.
| AKT3 | EWSR1 | NOTCH1/2 | RAF1 |
| ALK | FGFR1/2/3 | NRG1 | RELA |
| ARHGAP26 | FGR | NTRK1/2/3 | RET |
| AXL | INSR | NUMBL | ROS1 |
| BRAF | MAML2 | NUTM1 | RSPO2/3 |
| BRD3/4 | MAST1/2 | PDGFRA/B | TERT |
| EGFR | MET | PIK3CA | TFE3 |
| ERG | MSMB | PKN1 | TFEB |
| ESR1 | MUSK | PPARG | THADA |
| ETV1/4/5/6 | MYB | PRKCA/B | TMPRSS2 |
Statistical Analysis
The Mann–Whitney U test and Fisher exact test were used to test for the association of clinical variables and AKT1/SMO/PIK3CA alterations. Value of p < 0.05 was considered as statistically significant. The Kaplan–Meier technique was used to estimate progression-free survival (PFS) and overall survival (OS). Significant differences were analyzed by the log-rank test using the statistical software SPSS (IBM Analytics). PFS was calculated from the day of surgery until magnetic resonance imaging (MRI)-confirmed tumor progression or end of follow-up. OS was defined as the interval from the day of first surgery until death or the end of follow-up.
Results
Patient Cohort
The study cohort consisted of 48 females and 13 males who underwent surgical excision of posterior fossa meningiomas, with an average age at surgery of 57.5 ± 11.5 years (range: 34.7–85 years). By histology, 51 meningiomas were World Health Organization (WHO) grade I and 10 were WHO grade II. Meningothelial histology was the most common histological subtype (44.2%). Cerebellopontine meningiomas accounted for the majority of cases (52.4%) followed by petroclival meningiomas (14.7%). Cerebellar dysfunction and cranial neuropathies were the most common neurologic presenting symptoms. Gross total resection was achieved in 59% of all cases ( Table 2 ).
Table 2. Patient demographics and clinical characteristics.
| All patients, N = 61 | AKT1 -mutant, N = 5 | Wild-type, N = 56 | |
|---|---|---|---|
| Age at surgery (years) | 57.5 (36–85.2) | 60.6 (42.1–73.8) | 58.2 (36–85.2) |
| Sex | |||
| Female | 48 | 5 | 43 |
| Male | 13 | 0 | 13 |
| Presenting symptoms | |||
| Headache | 17 | 0 | 17 |
| Altered mental status | 4 | 0 | 4 |
| Cerebellar dysfunction | 26 | 1 | 25 |
| Cranial nerve neuropathy | 21 | 1 | 20 |
| Hearing loss | 16 | 0 | 16 |
| Asymptomatic | 2 | 0 | 2 |
| WHO grade | |||
| I | 51 | 4 | 47 |
| II | 10 | 1 | 9 |
| Tumor location | |||
| Cerebellopontine angle | 32 | 1 | 31 |
| Petroclival | 9 | 0 | 9 |
| Tentorium | 8 | 0 | 8 |
| Foramen magnum | 7 | 4 | 3 |
| Jugular foramen | 3 | 0 | 3 |
| Clivus | 2 | 0 | 2 |
| Histological subtype | |||
| Meningothelial | 27 | 3 | 24 |
| Transitional | 10 | 0 | 10 |
| Atypical | 10 | 1 | 9 |
| Psammomatous | 4 | 1 | 3 |
| Secretory | 9 | 0 | 9 |
| Focal rhabdoid | 1 | 0 | 1 |
| Treatment | |||
| Gross total resection | 36 | 2 | 34 |
| Postoperative radiation | 11 | 0 | 0 |
| Chemotherapy | 1 | 0 | 1 |
| Postoperative progression/recurrence | 11 | 1 | 10 |
Abbreviation: WHO, World Health Organization.
AKT1 , SMO , and PIK3CA Mutations and Anatomical Location
In total, five posterior fossa meningiomas (8.2%) harbored an AKT1 c.49G > A ( E17K ) mutation, whereas none of the tumors had detectable SMO or PIK3CA hotspot mutations. All patients with an AKT1- mutant meningioma were female, and four out of the five meningiomas harboring an AKT1 mutation were located in the foramen magnum, representing 57% of all foramen magnum meningiomas (4/7) in our series. The tumors were classified as anterior ( n = 3) or lateral ( n = 3) intradural foramen magnum meningiomas, as depicted on preoperative magnetic resonance (MR) images ( Fig. 1 ). Most meningiomas harboring an AKT1 mutation had a meningothelial WHO grade I ( n = 3) as a histological subtype, whereas the remaining two cases had either a psammomatous WHO grade I or an atypical meningioma WHO grade II ( Table 2 ).
Fig. 1.
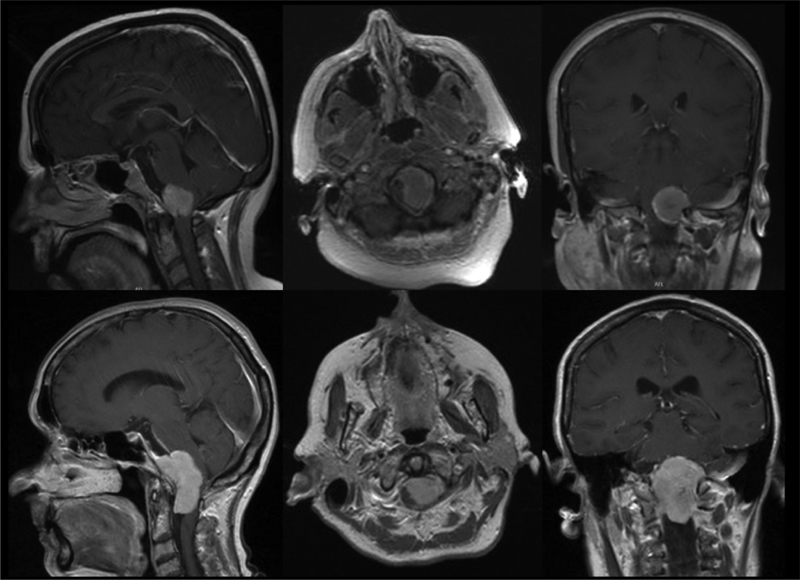
Representative magnetic resonance (MR) images of two patients with AKT1 -mutant foramen magnum meningiomas.
Gene Rearrangements Are Not Common in Posterior Fossa Meningiomas
Fusion oncogenes have been described in several human cancers including meningiomas 26 27 and have been successfully targeted in several tumor types. 28 To assess whether clinically targetable gene fusions were present in posterior fossa meningiomas, we performed targeted RNA fusion gene analysis in all samples using Archer FusionPlex and did not detect any relevant RNA fusion transcripts ( Table 1 ).
Progression and Survival Data
Progressive disease was reported in 11 cases, whereas four patients died without evidence for recurrence or progression during follow-up. Only one patient with an AKT1 -mutant meningioma developed a recurrent tumor. Both the median PFS and OS were not reached in the AKT1 -mutant and wild-type cases ( Fig. 2 ). Given the low number of survival events, the study was underpowered to make any significant conclusions about differences in the survival outcome between AKT1 -mutant and wild-type meningioma patients.
Fig. 2.
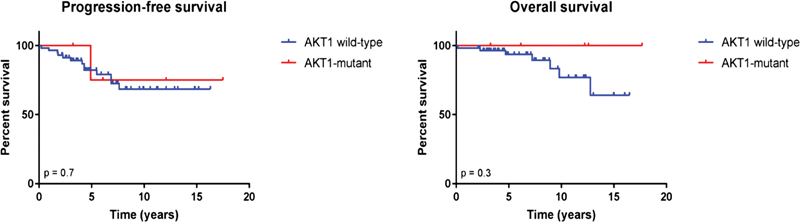
Kaplan–Meier estimates of progression-free and overall survival in fossa posterior meningioma patients in relation to AKT1 mutation status.
Discussion
We performed targeted sequencing of AKT1 , SMO , and PIK3CA hotspot mutations, as well as a targeted RNA fusion gene analysis, in a large cohort of meningiomas located in the posterior fossa to identify targetable genomic alterations.
Among all posterior fossa meningiomas, our study demonstrates that foramen magnum was a common anatomical site with frequent AKT1 mutations, making a subset of these tumors candidates for targeted therapy. Our data expand on prior studies that reported on small numbers of posterior fossa meningiomas with AKT1 mutations. 8 11 Foramen magnum represents the beginning of the spinal dura, which is continuous with the cranial dura mater. Taking a previous report on recurrent AKT1 mutations in spinal meningiomas into consideration, 29 the high rate of foramen magnum meningiomas harboring AKT1 mutations may be suggestive of a tumor originating in the spinal dura. However, given the small sample cohort of our study, further work is needed to confirm this finding in larger cohorts.
Foramen magnum meningiomas account for 1 to 3% of all meningiomas and 7% of all posterior fossa meningiomas. 30 31 The surgical resection of these lesions is particularly challenging and is often associated with a high postoperative morbidity and mortality. 3 25 31 32 Given these surgical challenges, enrolling affected patients into the ongoing multicenter phase II study that will investigate the efficacy of afuresertib ( AKT1 inhibitor, ClinicalTrials.gov NCT02523014) has the potential to shift management paradigms for these tumors. Such an approach requires screening of foramen magnum meningiomas for AKT1 E17K mutations.
In contrast to AKT1 mutations, SMO or PIK3CA mutations were absent in the posterior fossa, suggesting that these mutations are exclusive to meningiomas originating in the anterior skull base, such as in the olfactory groove or tuberculum sellae, as previously reported. 7 8 15 However, our study was designed to detect AKT1/SMO / PIK3CA alterations . Therefore, it cannot be excluded that other genomic alterations drive growth in posterior fossa meningiomas.
Finally, while novel fusions were recently discovered in a subset of meningiomas, 26 27 33 we did not detect any clinically actionable gene fusions in the meningiomas in our study. This may be explained by the fact that the majority of the cases in our cohort have a low-grade histology, whereas gene fusions, such as those involving TERT (telomerase reverse transcriptase), have been described in high-grade meningiomas. 27 33 Furthermore, while NF2 gene rearrangements have been reported in patients who received cranial irradiation in childhood, this group of patients was excluded in our study.
In summary, a large subset of foramen magnum meningiomas harbor AKT1 E17K mutations and is therefore potentially amenable to targeted therapy. We suggest that genotyping of foramen magnum meningiomas can facilitate therapeutic alternatives and guide in their treatment decision process. Further studies are needed to investigate the impact of actionable mutations on survival and identify meningioma patients who may derive the greatest possible benefit from targeted therapeutic strategies.
Funding Statement
Funding This work is supported by U.S. NIH 1R21NS099844 (to D. P. Cahill and P. K. Brastianos), the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation) Projektnummer, 401837860 (to Dr. T. Juratli), the Damon Runyon Award (to P. K. Brastianos), Brain Science Foundation (to P. K. Brastianos), and the American Brain Tumor Association (to P. K. Brastianos).
Conflict of Interest Dr. Cahill report honoraria and travel expenses from Merck. He also reports being a consultant to Lilly outside the submitted work. Dr. Silverman reports being a stockholder for Incyte.
Note
Portions of this work were presented at the North American Skull Base Society Annual Meeting, New Orleans, Louisiana, United States, March 4, 2017.
These authors contributed equally to this work.
References
- 1.Ostrom Q T, Gittleman H, Liao Pet al. CBTRUS Statistical Report: primary brain and other central nervous system tumors diagnosed in the United States in 2010-2014 Neuro-oncol 201719(5, suppl_5):v1–v88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Castellano F, Ruggiero G. Meningiomas of the posterior fossa. Acta Radiol Suppl. 1953;104:1–177. [PubMed] [Google Scholar]
- 3.Meling T R, Da Broi M, Scheie D, Helseth E.Meningiomas: skull base versus non-skull baseNeurosurg Rev 2018 (e-pub ahead of print). doi:10.1007/s10143-018-0976-7 [DOI] [PubMed]
- 4.Voß K M, Spille D C, Sauerland C et al. The Simpson grading in meningioma surgery: does the tumor location influence the prognostic value? J Neurooncol. 2017;133(03):641–651. doi: 10.1007/s11060-017-2481-1. [DOI] [PubMed] [Google Scholar]
- 5.Nanda A, Vannemreddy P. Recurrence and outcome in skull base meningiomas: do they differ from other intracranial meningiomas? Skull Base. 2008;18(04):243–252. doi: 10.1055/s-2007-1016956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Javalkar V, Banerjee A D, Nanda A. Posterior cranial fossa meningiomas. J Neurol Surg B Skull Base. 2012;73(01):1–10. doi: 10.1055/s-0032-1304835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brastianos P K, Horowitz P M, Santagata S et al. Genomic sequencing of meningiomas identifies oncogenic SMO and AKT1 mutations . Nat Genet. 2013;45(03):285–289. doi: 10.1038/ng.2526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Clark V E, Erson-Omay E Z, Serin Aet al. Genomic analysis of non-NF2 meningiomas reveals mutations in TRAF7 , KLF4 , AKT1 , and SMO Science 2013339(6123):1077–1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sahm F, Schrimpf D, Stichel D et al. DNA methylation-based classification and grading system for meningioma: a multicentre, retrospective analysis. Lancet Oncol. 2017;18(05):682–694. doi: 10.1016/S1470-2045(17)30155-9. [DOI] [PubMed] [Google Scholar]
- 10.Abedalthagafi M, Bi W L, Aizer A A et al. Oncogenic PI3K mutations are as common as AKT1 and SMO mutations in meningioma . Neuro-oncol. 2016;18(05):649–655. doi: 10.1093/neuonc/nov316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yesilöz Ü, Kirches E, Hartmann C et al. Frequent AKT1E17K mutations in skull base meningiomas are associated with mTOR and ERK1/2 activation and reduced time to tumor recurrence . Neuro-oncol. 2017;19(08):1088–1096. doi: 10.1093/neuonc/nox018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hyman D M, Smyth L M, Donoghue M TA et al. AKT inhibition in solid tumors with AKT1 mutations . J Clin Oncol. 2017;35(20):2251–2259. doi: 10.1200/JCO.2017.73.0143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Janku F, Wheler J J, Westin S N et al. PI3K/AKT/mTOR inhibitors in patients with breast and gynecologic malignancies harboring PIK3CA mutations . J Clin Oncol. 2012;30(08):777–782. doi: 10.1200/JCO.2011.36.1196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Janku F, Wheler J J, Naing A et al. PIK3CA mutation H1047R is associated with response to PI3K/AKT/mTOR signaling pathway inhibitors in early-phase clinical trials . Cancer Res. 2013;73(01):276–284. doi: 10.1158/0008-5472.CAN-12-1726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Strickland M R, Gill C M, Nayyar N et al. Targeted sequencing of SMO and AKT1 in anterior skull base meningiomas . J Neurosurg. 2017;127(02):438–444. doi: 10.3171/2016.8.JNS161076. [DOI] [PubMed] [Google Scholar]
- 16.Sekulic A, Migden M R, Oro A E et al. Efficacy and safety of vismodegib in advanced basal-cell carcinoma. N Engl J Med. 2012;366(23):2171–2179. doi: 10.1056/NEJMoa1113713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tang J Y, Mackay-Wiggan J M, Aszterbaum M et al. Inhibiting the hedgehog pathway in patients with the basal-cell nevus syndrome. N Engl J Med. 2012;366(23):2180–2188. doi: 10.1056/NEJMoa1113538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li H, Durbin R. Fast and accurate short read alignment with Burrows-Wheeler transform. Bioinformatics. 2009;25(14):1754–1760. doi: 10.1093/bioinformatics/btp324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tarasov A, Vilella A J, Cuppen E, Nijman I J, Prins P. Sambamba: fast processing of NGS alignment formats. Bioinformatics. 2015;31(12):2032–2034. doi: 10.1093/bioinformatics/btv098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li H, Handsaker B, Wysoker A et al. The Sequence Alignment/Map format and SAMtools. Bioinformatics. 2009;25(16):2078–2079. doi: 10.1093/bioinformatics/btp352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ye K, Schulz M H, Long Q, Apweiler R, Ning Z. Pindel: a pattern growth approach to detect break points of large deletions and medium sized insertions from paired-end short reads. Bioinformatics. 2009;25(21):2865–2871. doi: 10.1093/bioinformatics/btp394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McLaren W, Gil L, Hunt S E et al. The Ensembl Variant Effect Predictor. Genome Biol. 2016;17(01):122. doi: 10.1186/s13059-016-0974-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pietrantonio F, Di Nicolantonio F, Schrock A Bet al. ALK , ROS1 , and NTRK rearrangements in metastatic colorectal cancer J Natl Cancer Inst 2017;109(12) [DOI] [PubMed]
- 24.Zheng Z, Liebers M, Zhelyazkova B et al. Anchored multiplex PCR for targeted next-generation sequencing. Nat Med. 2014;20(12):1479–1484. doi: 10.1038/nm.3729. [DOI] [PubMed] [Google Scholar]
- 25.Bruneau M, George B.Foramen magnum meningiomas: detailed surgical approaches and technical aspects at Lariboisière Hospital and review of the literature Neurosurg Rev 2008310119–32., discussion 32–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Agnihotri S, Suppiah S, Tonge P D et al. Therapeutic radiation for childhood cancer drives structural aberrations of NF2 in meningiomas . Nat Commun. 2017;8(01):186. doi: 10.1038/s41467-017-00174-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Juratli T A, Thiede C, Koerner M VA et al. Intratumoral heterogeneity and TERT promoter mutations in progressive/higher-grade meningiomas . Oncotarget. 2017;8(65):109228–109237. doi: 10.18632/oncotarget.22650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Alvarez-Breckenridge C, Miller J J, Nayyar N et al. Clinical and radiographic response following targeting of BCAN-NTRK1 fusion in glioneuronal tumor . NPJ Precis Oncol. 2017;1(01):5. doi: 10.1038/s41698-017-0009-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sahm F, Bissel J, Koelsche C et al. AKT1E17K mutations cluster with meningothelial and transitional meningiomas and can be detected by SFRP1 immunohistochemistry . Acta Neuropathol. 2013;126(05):757–762. doi: 10.1007/s00401-013-1187-5. [DOI] [PubMed] [Google Scholar]
- 30.Rhoton A L., Jr Meningiomas of the cerebellopontine angle and foramen magnum. Neurosurg Clin N Am. 1994;5(02):349–377. [PubMed] [Google Scholar]
- 31.Borba L A, de Oliveira J G, Giudicissi-Filho M, Colli B O.Surgical management of foramen magnum meningiomas Neurosurg Rev 2009320149–58., discussion 59–60 [DOI] [PubMed] [Google Scholar]
- 32.Leon-Ariza D S, Campero A, Romero Chaparro R J, Prada D G, Vargas Grau G, Rhoton A L., Jr Key aspects in foramen magnum meningiomas: from old neuroanatomical conceptions to current far lateral neurosurgical intervention. World Neurosurg. 2017;106:477–483. doi: 10.1016/j.wneu.2017.07.029. [DOI] [PubMed] [Google Scholar]
- 33.Juratli T A, McCabe D, Nayyar N et al. DMD genomic deletions characterize a subset of progressive/higher-grade meningiomas with poor outcome. Acta Neuropathol. 2018;136(05):779–792. doi: 10.1007/s00401-018-1899-7. [DOI] [PubMed] [Google Scholar]


