Abstract
Background The management of optic nerve sheath meningiomas (ONSMs) remains controversial. Surgical decompression through traditional resective techniques has been associated with significant morbidity. While radiation therapy, the current modality of choice is not exempt of risks.
Transnasal endoscopic optic nerve decompression (EOND) offers a direct route to the orbit, optic canal, and orbital apex, providing a minimally invasive alternative.
Objective The main objective of this article is to assess EOND as the initial management of symptomatic patients with primary ONSM.
Methods Patients with ONSMs without a history of radiotherapy who underwent EOND were retrospectively reviewed. Postoperative imaging, duration of follow-up, and visual outcomes at the last ophthalmology visit were assessed.
Results Four women (age range 25–63 years) with primary ONSMs that underwent EOND were identified. All patients displayed subjective and objective baseline signs of vision loss. Additionally, baseline proptosis, diplopia, optic nerve atrophy, and ocular pain were identified. In none of the cases, the optic nerve sheath was breached.
Following EOND, all patients deferred treatment with adjuvant radiotherapy. At a mean postoperative follow-up of 14 months, all patients were clinically stable without evidence of disease progression on imaging or physical examination. At last ophthalmologic evaluation, three out of four showed objective improvements from baseline visual acuity and visual field (remaining patient had baseline optic nerve atrophy).
Conclusion These results suggest that EOND could be a viable initial treatment modality of selected primary ONSM cases. Further studies are warranted to determine long-term efficacy and its role in a stepwise progression of management, preceding radiotherapy.
Keywords: orbital tumors, optic nerve sheath meningiomas, endoscopic endonasal approach, endoscopic optic nerve decompression
Introduction
Optic nerve sheath meningiomas (ONSM) are rare, representing less than 2% of all orbital tumors. However, with the advent of new imaging techniques, the frequency of its diagnosis has increased to become the most common neoplasia of the optic nerve sheath (ONS). 1 Arising from the arachnoid villi of the optic nerve, these tumors tend to grow progressively, manifesting with visual loss due to compression of the optic nerve or its vascularity. 2
Management of ONSM is controversial. In the past, patients were either observed or underwent surgery through transcranial approaches. However, suboptimal results with both treatment modalities catapulted radiotherapy (RT) into a primary role in the management of ONSM. Nowadays, the role of surgery remains undefined. The endoscopic approaches to the orbital apex and the optic canal have become an addition to the armamentarium of skull base surgeons, but the literature lacks an adequate evaluation of surgical outcomes.
This study assesses the outcomes of the largest reported series of patients with primary ONSM managed only with a bony decompression of the optic nerve. This study attempts to better define the benefits of this surgical approach.
Methodology
All patients who presented primary ONSM that had undergone an endoscopic endonasal optic decompression (without adjuvant radiation) at The James Cancer Hospital of the Wexner Medical Center at of The Ohio State University, from 2010 to 2016, were included in this retrospective study, from an IRB-approved database. Patients with secondary ONSM were excluded. Results from the first and last ophthalmologic evaluations were collected—these included visual acuity assessments with Snellen chart, and visual fields assessed with a 30–2 Humphrey Visual Field Analyzer testing (Carl Zeiss Meditec Inc., Dublin, California, United States). Follow-up period was calculated as time lapse between surgery and last reported visual acuity.
Surgery followed a standard unilateral technique by the same surgical team in all four patients. The following are the surgical steps during the procedure.
Outfracture of the inferior turbinate. Followed by right middle turbinectomy to improve access for instrumentation.
Anterior and posterior ethmoidectomy, with sphenoidotomies extended bilaterally to fully visualize anatomical landmarks, such as the bony protuberances of the cavernous internal carotid artery and the optic nerve at the canal with the respective opticocarotid recess.
Complete removal of the medial aspect of the optic canal with a high-speed drill under direct vision, exposing the periosteum from the orbital apex to the tuberculum sellae. The ONS was not incised in any of the cases.
Variation in the technique: In patients 3 and 4, the meningioma had an intraconal extension into the orbital apex, and the periorbita was incised. In patient 3, we extended the decompression to the superior orbital fissure and cavernous sinus.
As part of the literature review, PubMed was searched for the following terms: Optic nerve sheath meningioma, optic meningioma, intraorbital meningioma, optic canal decompression, optic nerve decompression
Results
Four patients harboring a primary ONSM were identified. All underwent an endoscopic endonasal optic canal decompression and endoscopic endonasal orbital decompression, as their primary treatment modality. All patients chose to defer adjuvant treatment in view of symptom improvement. At a mean follow-up of 14 months following surgery, all patients were clinically stable with no signs of disease progression.
Patient 1
A 32-year-old woman presented with a 4 months history of gradually decreasing OD vision, intermittent diplopia, and proptosis following her second childbirth. At evaluation, she had an oculus dexter (OD) visual acuity of 20/30, with inferonasal quadrant visual fields defect, horizontal diplopia, and mild proptosis. Fundoscopic examination revealed minimal optic disc edema (+1) and retinal deposits ( Supplementary Fig. S1 ). A diagnosis of primary ONSM was suggested by the magnetic resonance imaging (MRI) ( Fig. 1 ). She was brought to the operating room and an endoscopic endonasal approach and optic nerve and orbital decompression were performed with no complications as described in the methods section. During surgery, extreme flattening of the medial rectal muscle was observed. One month postoperatively she had an OD visual acuity of 20/20, but persisted with low-grade optic disc edema (+2). At 22 months follow-up, she has an OD visual acuity of 20/20, stable proptosis and diplopia at the extreme left and right gaze, but showed improvement of visual fields ( Fig. 2 ). No further progression of the disc edema or the lesion size has been observed.
Fig. 1.

Preoperative magnetic resonance imaging (MRI) of patient 1. ( A ) T1 axial fat suppressed MRI with contrast, with lesion encircling the right optic nerve and showing enhancement tracking (“tram-track sign”). ( B ) Coronal view with “doughnut sign.”
Fig. 2.

Automated visual field of oculus dexter shows progressive improvement of inferior nasal arcuate visual fields defect ( A ) 2 months before surgery, ( B ) 12 months after surgery ( C ) and 15 months after surgery.
Patient 2
A 40-year-old woman presented with progressive oculus sinister (OS) vision loss over 4 months, worsening toward functional blindness, with intermittent ocular pain alternating with burning and tingling sensation. Examination revealed perception to hand movement only while fundoscopic examination showed severe pallor of the optic disc. A diagnosis of primary ONSM was suggested by imaging ( Fig. 3 ). An endoscopic endonasal approach and optic nerve and orbital decompression were performed with no complications, as described in the methods section. Following surgery, the patient's tingling and burning sensation resolved and her ocular pain progressively decreased in frequency and intensity, completely fading over a period of 2 months. One month following surgery, the patient described small visual field improvement, not reflected on the 30–2 HVF (Humphrey's Visual Field) and presented a transient improvement in OS visual acuity to 20/400. At 10 months of follow-up, there is no evidence of changes in the tumor size and after 20 months of follow-up, although her visual acuity regressed to baseline, she remains pain-free ( Fig. 4 ).
Fig. 3.
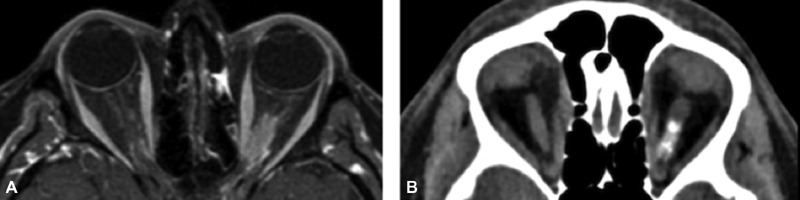
Preoperative imaging of patient 2. ( A ) T1 axial, fat suppressed, magnetic resonance imaging post-contrast with hyper-enhancing lesion encircling left optic nerve and extending to orbital apex. ( B ) Axial computed tomography without contrast showing calcifications in the left optic nerve.
Fig. 4.

Endoscopic endonasal decompression of left optic nerve in patient 2. ( A ) Sphenoidotomy. ( B ) Drilling the contour of the optic canal. (C) Endoscopic decompression of the optic nerve's circumference (270°). Abbreviations: OC, optic canal; P, periorbita; Pl, planum sphenoidale; S, sphenoid; Sep, septum; Sll, sella;; TS, tuberculum sellae..
Patient 3
A 63-year-old woman presented with a 4-month history of progressive diplopia, diminished perception of color brightness in OD, headaches, and eye pain. She was initially treated with oral corticosteroids resulting in improvement in the diplopia; however, it recurred after weaning. A second round of steroids failed to have any effect. Autoimmune and systemic inflammatory markers were negative. At evaluation, she had an OD visual acuity of 20/40 (deteriorated to 20/70 by the time of our evaluation), horizontal diplopia, complete cranial nerve VI (CN VI) palsy, a small central visual field defect, and an afferent pupillary defect, but no optic disc alterations at a fundoscopic examination. Imaging findings ( Fig. 5 ) were consistent with primary ONSM compressing the CN VI at the orbital apex, with a small intracanalicular component. She was brought to the operating room and an endoscopic endonasal approach with optic nerve and orbital decompression was performed with no complications as described in the methods section. For this patient, an extension of the decompression was performed to the superior orbital fissure. The medial wall of the orbit, optic canal, and superior orbital fissure were removed exposing the periosteum. The periorbit was incised to allow for further decompression of the orbital contents with orbital fat exposure.
Fig. 5.

Automated visual field of oculus sinister in patient 2 ( A ) One month before surgery and ( B ) one month after surgery, showing slight worsening of visual field defect; though patient expressed subjective clinical improvement.
One day after surgery, the eye pain had disappeared and the headaches resolved. The patient manifested better color perception and there was a partial improvement in the CN VI function, with the affected eye passing lateral to midline. The lesion remained stable at 4 months follow-up ( Fig. 6 ) while maintaining gradual improvement in visual fields ( Fig. 7 ). At 10 months postoperatively, the MRI showed stability of the tumor size ( Fig. 8 ) together with a visual acuity of 20/25–2. CN VI function improved progressively until only a subtle deficit remained. Although the diplopia improved, it never normalized, thereby requiring further surgery for esotropia, with an optimal outcome.
Fig. 6.

Preoperative imaging of patient 3. ( A ) Axial view T1 MRI shows circumferential enhancement of posterior half of orbital portion (“tram-track sign”), more prominent laterally that seems to extend to anterior portion of optic canal. ( B ) Coronal Fat Suppressed with contrast showing “dough-nut sign.” ( C ) Axial head computed tomography demonstrating calcifications around the right optic nerve reinforcing the diagnosis of optic nerve sheath meningioma. ( D ) Endoscopic endonasal decompression of the circumference (270°) of the right optic nerve. Abbreviations: ICA, internal carotid artery; LOCR, lateral opticocarotid recess; ON, optic nerve; SLL, sella.
Fig. 7.

Automated visual field of oculus dexter shows improvement in visual fields in Patient 3: ( A ) One week before surgery and ( B ) two months after surgery.
Fig. 8.

At 4 months postoperative imaging of patient 3, demonstrating stability of tumor size. ( A ) T1 axial view magnetic resonance imaging (MRI) with contrast. ( B ) T1 coronal view MRI fat suppressed with contrast.
Patient 4
A 25-year-old woman presented with a 10-year history of migraine with visual aura, and transient episodes of vision loss who noticed OS proptosis associated with eye pain, epiphora, and blurry vision. Imaging showed an intraconal mass, consistent with ONSM ( Fig. 9 ). Her ophthalmologic evaluation revealed an OS visual acuity of 20/20–2, no changes in fundoscopic examination, and a slightly increased blind spot at HVF 24–2, although overall within normal limits. She was observed during a year, and re-evaluation showed progression with an OS visual acuity of 20/25 + 2. Due to function deterioration, she was referred to our department where she elected to undergo surgery. An endoscopic endonasal approach was performed with left side orbit and optic canal decompression. In her case, the periorbita was incised to further decompress the orbital contents with orbital fat exposure. After the surgical procedure patient expressed immediate vision improvement and at 3-month follow-up, almost total resolution of the proptosis is observed, with an OS visual acuity of 20/20 and an improvement in her visual field ( Fig. 10 ).
Fig. 9.

( A ) Preoperative axial orbit fat-suppressed three-dimensional T1-weighted magnetic resonance imaging (MRI) stealth of patient 4, showing circumferential encasement of posterior orbital segment of the optic nerve. The tumor extends into the canalicular segment, without extraconal extension. (“tram-track sign”). ( B ) axial orbit fat-suppressed turbo spin echo MRI showing postoperative changes.
Fig. 10.

Automated visual field of OD shows progressive improvement in visual field defect ( A ) 1 month before surgery, ( B ) 3 months after surgery.
Discussion
Once considered rare tumors, ONSMs have been increasingly diagnosed as a result of advancements in imaging. Despite representing fewer than 2% of all orbital tumors, they remain the most common neoplasia to originate in the ONS, and the second most common to affect the ON, 2 after gliomas. 3 4 Most commonly seen in middle-aged women 5 6 (average 40.8 years), 2 they have also been encountered in young women 7 appearing to be associated with pregnancy, 5 8 possibly due to hormone-driven growth. 9 10 Primary ONSMs arise from the “cap” cells of the arachnoid villi in the orbit, and less commonly in the intracanalicular segments of the ONS. Conversely, secondary ONSMs are more common, originating in extraorbital tissues and extending into the subdural space of ONS, through the optic canal or the superior orbital fissure. As it can be challenging to clearly determine a primary origin, further categories in their classification have been proposed, such as probable primary ONSM and probable secondary ONSM, 11 based upon their intraorbital or extraorbital predominance.
Patients usually present with slow, gradual vision loss, ∼1 to 5 years before a diagnosis is made. 1 Blurry vision is most commonly reported (77–80% 4 12 ), though 45% have a visual acuity of 20/40 or better at presentation. 2 Other signs and symptoms include visual field defects (especially in peripheral fields), transient vision loss (15–23%), proptosis (9–60%), diplopia (9–39%), headaches and orbital pain (7–9%), 6 12 color vision disturbance, and afferent pupillary defects. 13 Although ONSMs have been associated with the Hoyt-Spencer triad of visual loss, optic atrophy (pallor), and optociliary shunt vessels, 14 it is rare to find these three features together. 2 At fundoscopy, it is common to find chronic disc swelling and choroidal folds. 15 However, the fundoscopy may be normal in some tumors of the orbital apex and optic canal 16 17 that present rapidly progressive symptoms 16 18 (causing a great number of misdiagnoses, including optic neuritis, 17 19 20 ischemic optic neuropathy, and optic disc vasculitis) 21 and also in few cases of chronic compressive optic neuropathy. 22
Currently, ONSM is diagnosed with a combination of clinical suspicion and imaging. 15 Computed tomography (CT) imaging shows thickening of the ONS and may show calcifications in 20 to 50%, 23 as encountered in two of our patients. However, the preferred diagnostic test is MRI with gadolinium-enhanced T1-weighted sequences with fat suppression, 1 24 which shows a contrast-enhancing peripheral lesion separated from the nonenhancing ON, observed on axial and coronal views as the “tram-track” and “doughnut” signs, respectively.
It is not required to obtain a diagnostic biopsy. The small but potentially disabling risk of optic nerve damage outweighs potential benefits, unless an atypical response to treatment forces the reconsideration of the diagnosis. In addition, obtaining enough tissue from the posterior optic nerve 25 26 and analyzing the samples can be challenging. 27 Somatostatin receptor scintigraphy and positron emission tomography/CT and single-photon emission computed tomography/CT with somatostatin receptor ligands have shown promise in aiding the diagnosis, planning radiotherapeutic protocols and evaluating treatment responses. 28 29 30
In the absence of an ideal management algorithm, observing these patients is still a viable option, especially in adults with preserved and stable vision. 31 The slow-growth nature of some of these tumors may allow observation, until clinical symptoms or tumor growth progress is noted. Initially, Dutton 2 reported 84% of progressive visual loss in observed patients, but other follow-up studies have shown that observation alone could offer long-term stability of good visual acuity and even allow spontaneous improvement. 12 32 33 On the other hand, observation alone of symptomatic patients could preclude visual improvement. A visual acuity of 20/40 has been proposed as a threshold to initiate treatment. 34
Although it was initially believed that ONSMs were resistant to RT, 35 nowadays it is the preferred treatment modality, superseding surgery. Turbin et al 36 in a multi-institutional study reported that fractionated RT results in superior preservation of visual acuity and fewer complications, than those observed with surgery alone or surgery plus RT, although with a complication rate of 33%. Advances such as conformal fractionated stereotactic radiotherapy (FSRT), intensity modulated RT, and 3D-conformal RT have increased long-term tumor control rates to almost 100% with at least 80% vision preservation or improvement, 37 though not without associated treatment morbidity. 38 39 Stereotactic radiosurgery remains a growing field with various satisfactory results reported with frameless using CyberKnife. 37 Altogether, radiation therapy must be carefully considered in asymptomatic patients to avoid a treatment-related vision loss and should not be favored in the presence of rapid symptoms of tumor compression, as less than 10% of ONSM regress in size after treatment. 40
The surgical management of ONSMs is determined by the treatment needs. Partial resections may be performed to obtain surgical specimens in patients unresponsive to RT. 41 42 Surgery can also be considered in the presence of ONSMs with intracranial components (to prevent contralateral extension), 43 44 to debulk tumors occasioning a disfiguring proptosis, in patients with nonfunctional vision, and as an adjuvant to RT 41 to expedite improvement in severe acute visual deterioration or prominent disc edema. Notably, patients with previous surgery show a lower rate of visual improvement following FSRT. 45
Resection alone with curative intent is challenging. ONSMs arise between the optic nerve and its extradural-derived blood supply, engaging pial blood vessels in a way that tumor removal risks the reduction in the optic nerve vascularity. 46 An endoscopic endonasal resection of an intracanalicular primary ONSM 47 was reported, along with normalization of visual deficits over a 10 months period. Still, it has long been considered that total resection is seldom compatible with vision preservation. 2 27 36 Successful outcomes are mostly associated with exophytic tumors without nerve invasion. 47 48 The surgical complexities of the area could account for the high reported rate of local recurrences 2 along with seeding into orbital tissues, 49 during procedures such as needle biopsies and optic nerve sheath decompression (ONSD). Radiation therapy is thought to diminish the risk of seeding after transgression of the ONS. 25 41
ONSD, also called ONS fenestration, involves the opening of slits or a dural window to reduce subarachnoid compartment pressure. This procedure, similar in technique to a biopsy, also allows resecting tumor components that extrude through the created window. Saeed et al 12 observed that 8 out of 10 patients had significantly worse vision following ONSD, either immediately or up to 2 years after surgery. On the other hand, visual acuity recovery has been reported after biopsies and partial resections. 36 41 49 50 Most of these cases additionally underwent radiation therapy, but still pressure reduction could be partly responsible for the improvement.
Compression of the optic nerve leads to pial vessel injury and ischemia, with diminished axonal transport and demyelination. The bony walls of the optic canal represent an outward force over the growing tumor that could create a pressure disequilibrium that displaces the lamina cribosa, 51 while compressing the ganglion cells axons of the optic nerve. Decompressing the optic apparatus decreases these extrinsic forces, and it is thought to reverse some of the consequences of compressive injury. 52 But its isolated beneficial effect remains poorly addressed in the literature, as it has been mainly conducted as a preparatory step to more invasive procedures.
The introduction and increasing applicability of endoscopic endonasal techniques to access lesions in the optic canal and orbit 51 53 54 55 have progressively displaced well-established transcranial approaches. These techniques allow resection and decompression of benign and malignant pathologies of the medial optic apparatus with an increased exposure, 56 reduced postoperative pain, manipulation of the brain, 57 58 optic nerve and extraocular muscles, 59 and avoidance of skin incisions. Pressure reduction obtained through an endoscopic optic canal decompression could be even greater if associated with an endoscopic endonasal orbital decompression, 60 though there is scarce literature to support it, especially for other tumors than skull base meningiomas. Approximations can be made between ONSM and skull base meningiomas that invade the optic canal, but the latter usually do not adhere to the ONS. 61
In our series, all patients were symptomatic; therefore, observation was not considered as an option. After an endoscopic endonasal optic canal and orbital decompression, all patients in this series refused radiation therapy or any other surgical procedure, either because of symptoms improvement or personal preference. All of them were advised that at any sign of deterioration of their vision or tumor progression confirmed on imaging, radiation would be the next step in their treatments.
This group is to our knowledge, the largest single-institution series of patients with primary ONSM managed with endoscopic endonasal optic nerve decompression (EOND). The improvement observed in our patients ranges from modest to significant, and was not restricted to medially located lesions. Visual improvement seems to be common in patients with a rapid visual decline 42 and less preoperative deficit, 62 as seen in patients 1 and 3. While patient 2 presented no enduring visual improvement, as expected in patients with preoperative disc pallor (a known negative prognostic factor), 63 the procedure was done to alleviate pain. Still, impressive visual improvement has been reported following surgery of some patients with signs of chronic optic nerve compression. 41 64 On the other hand, patient 1 presented with symptoms following her second childbirth, but the presence of optic disc changes and the behavior of these tumors suggest that a long-standing asymptomatic ONSM underwent accelerated growth during pregnancy. Based on the experience with this cohort, and a review of the outcomes yielded by current treatment options, the surgical decompressive techniques could be a feasible alternative in the management of symptomatic patients with a suspected primary ONSM.
Berhouma was the first to describe the benefits of endoscopic optic canal decompression and orbital apex decompression in ONS 65 66 in three patients that also received RT. Despite differences in management, their results were similar to ours, with two out of three patients obtaining visual acuity improvement at 6 months follow-up, and papillary atrophy present in a patient without visual improvement. While it is usually expected for surgery of primary ONSM to include an incision on the ONS (especially in the context of a sudden visual deterioration), our results suggest that it is not always necessary to obtain clinical improvement. A less dogmatic approach could be proposed, where the avoidance of opening the dural sheath allows to reserve it as a secondary management option. It is important to consider the added benefit of avoiding the risk of ophthalmic artery lesions, cerebrospinal fluid leaks, and seeding.
Although we are aware that surgery is usually not a definitive solution, and radiation therapy is regarded as a superior option, in some selected cases, a transient improvement may be enough to sustain the patient through a period of accelerated tumor growth. Turbin et al 36 reported that only 4 out of 13 observed patients showed progressive growth of the tumor, in a median time of 10.8 years.
Larger studies with longer follow-up are needed to further support this proposal. But EOND could offer the benefit of early symptoms relief while allowing a stepwise progression, before proceeding unto more aggressive treatment choices (such as ONSD and radiation therapy). Still, it is always imperative to keep a strict ophthalmologic and imaging surveillance, allowing a timely detection of tumor growth or clinical progression.
Conflict of Interest None declared.
Note
This work was presented as a poster at NASBS, Coronado 2018.
Supplementary Material
References
- 1.Shapey J, Sabin H I, Danesh-Meyer H V, Kaye A H. Diagnosis and management of optic nerve sheath meningiomas. J Clin Neurosci. 2013;20(08):1045–1056. doi: 10.1016/j.jocn.2013.03.008. [DOI] [PubMed] [Google Scholar]
- 2.Dutton J J. Optic nerve sheath meningiomas. Surv Ophthalmol. 1992;37(03):167–183. doi: 10.1016/0039-6257(92)90135-g. [DOI] [PubMed] [Google Scholar]
- 3.Rush J A, Younge B R, Campbell R J, MacCarty C S. Optic glioma. Long-term follow-up of 85 histopathologically verified cases. Ophthalmology. 1982;89(11):1213–1219. [PubMed] [Google Scholar]
- 4.Chutorian A M, Schwartz J F, Evans R A, Carter S. Optic gliomas in children. Neurology. 1964;14:83–95. doi: 10.1212/wnl.14.2.83. [DOI] [PubMed] [Google Scholar]
- 5.Wright J E.Primary optic nerve meningiomas: clinical presentation and management Trans Sect Ophthalmol Am Acad Ophthalmol Otolaryngol 197783(4 Pt 1):617–625. [PubMed] [Google Scholar]
- 6.Sibony P A, Krauss H R, Kennerdell J S, Maroon J C, Slamovits T L. Optic nerve sheath meningiomas. Clinical manifestations. Ophthalmology. 1984;91(11):1313–1326. doi: 10.1016/s0161-6420(84)34148-3. [DOI] [PubMed] [Google Scholar]
- 7.Wright J E, Call N B, Liaricos S. Primary optic nerve meningioma. Br J Ophthalmol. 1980;64(08):553–558. doi: 10.1136/bjo.64.8.553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Eddleman C S, Liu J K. Optic nerve sheath meningioma: current diagnosis and treatment. Neurosurg Focus. 2007;23(05):E4. doi: 10.3171/FOC-07/11/E4. [DOI] [PubMed] [Google Scholar]
- 9.Maxwell M, Galanopoulos T, Neville-Golden J, Antoniades H N. Expression of androgen and progesterone receptors in primary human meningiomas. J Neurosurg. 1993;78(03):456–462. doi: 10.3171/jns.1993.78.3.0456. [DOI] [PubMed] [Google Scholar]
- 10.Wan W L, Geller J L, Feldon S E, Sadun A A. Visual loss caused by rapidly progressive intracranial meningiomas during pregnancy. Ophthalmology. 1990;97(01):18–21. doi: 10.1016/s0161-6420(90)32634-9. [DOI] [PubMed] [Google Scholar]
- 11.Bosch M M, Wichmann W W, Boltshauser E, Landau K. Optic nerve sheath meningiomas in patients with neurofibromatosis type 2. Arch Ophthalmol. 2006;124(03):379–385. doi: 10.1001/archopht.124.3.379. [DOI] [PubMed] [Google Scholar]
- 12.Saeed P, Rootman J, Nugent R A, White V A, Mackenzie I R, Koornneef L. Optic nerve sheath meningiomas. Ophthalmology. 2003;110(10):2019–2030. doi: 10.1016/S0161-6420(03)00787-5. [DOI] [PubMed] [Google Scholar]
- 13.Charpentier P, Mouriaux F. Total recovery of optic nerve sheath meningioma. BMJ Case Rep. 2016;2016:2016. doi: 10.1136/bcr-2016-215532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Frisèn L, Royt W F, Tengroth B M. Optociliary veins, disc pallor and visual loss. A triad of signs indicating spheno-orbital meningioma. Acta Ophthalmol (Copenh) 1973;51(02):241–249. doi: 10.1111/j.1755-3768.1973.tb03801.x. [DOI] [PubMed] [Google Scholar]
- 15.Turbin R E, Pokorny K. Diagnosis and treatment of orbital optic nerve sheath meningioma. Cancer Contr. 2004;11(05):334–341. doi: 10.1177/107327480401100508. [DOI] [PubMed] [Google Scholar]
- 16.Misra S, Misra N, Gogri P, Mehta R. A rare case of bilateral optic nerve sheath meningioma. Indian J Ophthalmol. 2014;62(06):728–730. doi: 10.4103/0301-4738.136238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Alroughani R, Behbehani R. Optic nerve sheath meningioma masquerading as optic neuritis. Case Rep Neurol Med. 2016;2016:5.419432E6. doi: 10.1155/2016/5419432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jackson A, Patankar T, Laitt R D. Intracanalicular optic nerve meningioma: a serious diagnostic pitfall. AJNR Am J Neuroradiol. 2003;24(06):1167–1170. [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang T L, Shao S F, Zhang T et al. Idiopathic inflammation of optic nerve simulating optic nerve sheath meningioma: CT demonstration. J Comput Assist Tomogr. 1987;11(02):360–361. doi: 10.1097/00004728-198703000-00037. [DOI] [PubMed] [Google Scholar]
- 20.Sawaya R A, Sidani C, Farah N, Hourani-Risk R. Presumed bilateral optic nerve sheath meningiomas presenting as optic neuritis. J Neuroophthalmol. 2008;28(01):55–57. doi: 10.1097/WNO.0b013e3181674331. [DOI] [PubMed] [Google Scholar]
- 21.Mao J F, Xia X B, Tang X B, Zhang X Y, Wen D. Analyses on the misdiagnoses of 25 patients with unilateral optic nerve sheath meningioma. Int J Ophthalmol. 2016;9(09):1315–1319. doi: 10.18240/ijo.2016.09.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jiang L, Shi J, Liu W, Kang J, Wang N.[Clinical feature of chronic compressive optic neuropathy without optic atrophy][Article in Chinese]Zhonghua Yan Ke Za Zhi 20145012889–893. [PubMed] [Google Scholar]
- 23.Miller N R. Primary tumours of the optic nerve and its sheath. Eye (Lond) 2004;18(11):1026–1037. doi: 10.1038/sj.eye.6701592. [DOI] [PubMed] [Google Scholar]
- 24.Mafee M F, Goodwin J, Dorodi S.Optic nerve sheath meningiomas. Role of MR imaging Radiol Clin North Am 1999370137–58., ix [DOI] [PubMed] [Google Scholar]
- 25.Gündüz K, Catak E, Erden E. Optic nerve biopsy via a medial transconjunctival orbitotomy approach in the diagnosis of optic nerve and sheath tumors. Orbit. 2010;29(04):190–193. doi: 10.3109/01676831003664368. [DOI] [PubMed] [Google Scholar]
- 26.Khong J J, McNab A A. Medial transconjunctival intrinsic optic nerve biopsy: surgical technique and indications. Orbit. 2012;31(04):227–232. doi: 10.3109/01676830.2012.669010. [DOI] [PubMed] [Google Scholar]
- 27.Kim J W, Rizzo J F, Lessell S. Controversies in the management of optic nerve sheath meningiomas. Int Ophthalmol Clin. 2005;45(04):15–23. doi: 10.1097/01.iio.0000176367.16758.f4. [DOI] [PubMed] [Google Scholar]
- 28.Saeed P, Tanck M W, Freling N, Baldeschi L, Mourits M P, Bennink R J. Somatostatin receptor scintigraphy for optic nerve sheath meningiomas. Ophthalmology. 2009;116(08):1581–1586. doi: 10.1016/j.ophtha.2009.02.004. [DOI] [PubMed] [Google Scholar]
- 29.Gehler B, Paulsen F, Oksüz M O et al. [68Ga]-DOTATOC-PET/CT for meningioma IMRT treatment planning. Radiat Oncol. 2009;4:56. doi: 10.1186/1748-717X-4-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Klingenstein A, Haug A R, Miller C, Hintschich C. Ga-68-DOTA-TATE PET/CT for discrimination of tumors of the optic pathway. Orbit. 2015;34(01):16–22. doi: 10.3109/01676830.2014.959185. [DOI] [PubMed] [Google Scholar]
- 31.Berman D, Miller N R. New concepts in the management of optic nerve sheath meningiomas. Ann Acad Med Singapore. 2006;35(03):168–174. [PubMed] [Google Scholar]
- 32.Egan R A, Lessell S. A contribution to the natural history of optic nerve sheath meningiomas. Arch Ophthalmol. 2002;120(11):1505–1508. doi: 10.1001/archopht.120.11.1505. [DOI] [PubMed] [Google Scholar]
- 33.Schick U, Jung C, Hassler W E. Primary optic nerve sheath meningiomas: a follow-up study. Cent Eur Neurosurg. 2010;71(03):126–133. doi: 10.1055/s-0029-1246136. [DOI] [PubMed] [Google Scholar]
- 34.Kennerdell J S, Maroon J C, Malton M, Warren F A. The management of optic nerve sheath meningiomas. Am J Ophthalmol. 1988;106(04):450–457. doi: 10.1016/0002-9394(88)90882-3. [DOI] [PubMed] [Google Scholar]
- 35.Dyke C G, Davidoff L M. Philadelphia: Lea & Febiger; 1942. Roentgen Treatment of Diseases of the Nervous System. 3rd edition; p. 113. [Google Scholar]
- 36.Turbin R E, Thompson C R, Kennerdell J S, Cockerham K P, Kupersmith M J.A long-term visual outcome comparison in patients with optic nerve sheath meningioma managed with observation, surgery, radiotherapy, or surgery and radiotherapy Ophthalmology 200210905890–899., discussion 899–900 [DOI] [PubMed] [Google Scholar]
- 37.Bloch O, Sun M, Kaur G, Barani I J, Parsa A T. Fractionated radiotherapy for optic nerve sheath meningiomas. J Clin Neurosci. 2012;19(09):1210–1215. doi: 10.1016/j.jocn.2012.02.010. [DOI] [PubMed] [Google Scholar]
- 38.Subramanian P S, Bressler N M, Miller N R. Radiation retinopathy after fractionated stereotactic radiotherapy for optic nerve sheath meningioma. Ophthalmology. 2004;111(03):565–567. doi: 10.1016/j.ophtha.2003.06.025. [DOI] [PubMed] [Google Scholar]
- 39.Hamilton S N, Nichol A, Truong P et al. Visual outcomes and local control after fractionated stereotactic radiotherapy for optic nerve sheath meningioma. Ophthal Plast Reconstr Surg. 2018;34(03):217–221. doi: 10.1097/IOP.0000000000000914. [DOI] [PubMed] [Google Scholar]
- 40.Metellus P, Kapoor S, Kharkar S et al. Fractionated conformal radiotherapy for management of optic nerve sheath meningiomas: long-term outcomes of tumor control and visual function at a single institution. Int J Radiat Oncol Biol Phys. 2011;80(01):185–192. doi: 10.1016/j.ijrobp.2010.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Turbin R E, Wladis E J, Frohman L P, Langer P D, Kennerdell J S. Role for surgery as adjuvant therapy in optic nerve sheath meningioma. Ophthal Plast Reconstr Surg. 2006;22(04):278–282. doi: 10.1097/01.iop.0000225420.06323.76. [DOI] [PubMed] [Google Scholar]
- 42.Roser F, Nakamura M, Martini-Thomas R, Samii M, Tatagiba M. The role of surgery in meningiomas involving the optic nerve sheath. Clin Neurol Neurosurg. 2006;108(05):470–476. doi: 10.1016/j.clineuro.2005.08.001. [DOI] [PubMed] [Google Scholar]
- 43.Schick U, Dott U, Hassler W. Surgical management of meningiomas involving the optic nerve sheath. J Neurosurg. 2004;101(06):951–959. doi: 10.3171/jns.2004.101.6.0951. [DOI] [PubMed] [Google Scholar]
- 44.Norris J H, Norris J S, Akinwunmi J, Malhotra R. Optic canal decompression with dural sheath release; a combined orbito-cranial approach to preserving sight from tumours invading the optic canal. Orbit. 2012;31(01):34–43. doi: 10.3109/01676830.2011.605500. [DOI] [PubMed] [Google Scholar]
- 45.Adeberg S, Welzel T, Rieken S, Debus J, Combs S E. Prior surgical intervention and tumor size impact clinical outcome after precision radiotherapy for the treatment of optic nerve sheath meningiomas (ONSM) Radiat Oncol. 2011;6:117. doi: 10.1186/1748-717X-6-117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Miller N R. New concepts in the diagnosis and management of optic nerve sheath meningioma. J Neuroophthalmol. 2006;26(03):200–208. doi: 10.1097/01.wno.0000235569.19131.ac. [DOI] [PubMed] [Google Scholar]
- 47.Hunt P J, DeMonte F, Tang R A, Su S Y, Raza S M. Surgical resection of an optic nerve sheath meningioma: relevance of endoscopic endonasal approaches to the optic canal. J Neurol Surg Rep. 2017;78(02):e81–e85. doi: 10.1055/s-0037-1600897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mark L E, Kennerdell J S, Maroon J C, Rosenbaum A E, Heinz R, Johnson B L. Microsurgical removal of a primary intraorbital meningioma. Am J Ophthalmol. 1978;86(05):704–709. doi: 10.1016/0002-9394(78)90194-0. [DOI] [PubMed] [Google Scholar]
- 49.Meeker A R, Ko M W, Carruth B P, Strumpf K B, Bersani T A. Diagnosis of optic nerve sheath meningioma during optic nerve sheath decompression. Orbit. 2017;36(01):35–38. doi: 10.1080/01676830.2017.1279648. [DOI] [PubMed] [Google Scholar]
- 50.Levin M H, Ney J J, Venneti S et al. Optic nerve biopsy in the management of progressive optic neuropathy. J Neuroophthalmol. 2012;32(04):313–320. doi: 10.1097/WNO.0b013e31825be81e. [DOI] [PubMed] [Google Scholar]
- 51.Zhao J W, Wang D S, Chang Q L et al. [Theoretic explanation about visual disorder induced by fibrous dysplasia of skull: pressure disequilibrium between lamina cribrosa] Zhonghua Yi Xue Za Zhi. 2010;90(19):1317–1321. [PubMed] [Google Scholar]
- 52.Sergott R C, Savino P J, Bosley T M. Optic nerve sheath decompression: a clinical review and proposed pathophysiologic mechanism. Aust N Z J Ophthalmol. 1990;18(04):365–373. doi: 10.1111/j.1442-9071.1990.tb01819.x. [DOI] [PubMed] [Google Scholar]
- 53.Luxenberger W, Stammberger H, Jebeles J A, Walch C. Endoscopic optic nerve decompression: the Graz experience. Laryngoscope. 1998;108(06):873–882. doi: 10.1097/00005537-199806000-00016. [DOI] [PubMed] [Google Scholar]
- 54.Jho H D. Endoscopic endonasal approach to the optic nerve: a technical note. Minim Invasive Neurosurg. 2001;44(04):190–193. doi: 10.1055/s-2001-19927. [DOI] [PubMed] [Google Scholar]
- 55.Lund V J, Larkin G, Fells P, Adams G. Orbital decompression for thyroid eye disease: a comparison of external and endoscopic techniques. J Laryngol Otol. 1997;111(11):1051–1055. doi: 10.1017/s0022215100139313. [DOI] [PubMed] [Google Scholar]
- 56.Jacquesson T, Abouaf L, Berhouma M, Jouanneau E. How I do it: the endoscopic endonasal optic nerve and orbital apex decompression. Acta Neurochir (Wien) 2014;156(10):1891–1896. doi: 10.1007/s00701-014-2199-1. [DOI] [PubMed] [Google Scholar]
- 57.de Lara D, Ditzel Filho L F, Prevedello D Met al. Endonasal endoscopic approaches to the paramedian skull base World Neurosurg 201482(6, Suppl):S121–S129. [DOI] [PubMed] [Google Scholar]
- 58.Stokken J, Gumber D, Antisdel J, Sindwani R. Endoscopic surgery of the orbital apex: Outcomes and emerging techniques. Laryngoscope. 2016;126(01):20–24. doi: 10.1002/lary.25539. [DOI] [PubMed] [Google Scholar]
- 59.Murchison A P, Rosen M R, Evans J J, Bilyk J R. Endoscopic approach to the orbital apex and periorbital skull base. Laryngoscope. 2011;121(03):463–467. doi: 10.1002/lary.21357. [DOI] [PubMed] [Google Scholar]
- 60.McKinney K A, Snyderman C H, Carrau R L et al. Seeing the light: endoscopic endonasal intraconal orbital tumor surgery. Otolaryngol Head Neck Surg. 2010;143(05):699–701. doi: 10.1016/j.otohns.2010.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Pletcher S D, Sindwani R, Metson R.Endoscopic orbital and optic nerve decompression Otolaryngol Clin North Am 20063905943–958., vi vi [DOI] [PubMed] [Google Scholar]
- 62.Sade B, Lee J H.High incidence of optic canal involvement in tuberculum sellae meningiomas: rationale for aggressive skull base approach Surg Neurol 20097202118–123., discussion 123 [DOI] [PubMed] [Google Scholar]
- 63.Bulters D O, Shenouda E, Evans B T, Mathad N, Lang D A. Visual recovery following optic nerve decompression for chronic compressive neuropathy. Acta Neurochir (Wien) 2009;151(04):325–334. doi: 10.1007/s00701-009-0192-x. [DOI] [PubMed] [Google Scholar]
- 64.Carlson A P, Stippler M, Myers O. Predictive factors for vision recovery after optic nerve decompression for chronic compressive neuropathy: systematic review and meta-analysis. J Neurol Surg B Skull Base. 2013;74(01):20–38. doi: 10.1055/s-0032-1329624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Tamura R, Takahashi S, Horikoshi T, Yoshida K. Improvement of long-term blindness caused by compression from inner-third sphenoid wing meningioma after optic canal decompression: an extremely rare case report. Surg Neurol Int. 2016;7:67. doi: 10.4103/2152-7806.184579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Berhouma M, Jacquesson T, Abouaf L, Vighetto A, Jouanneau E. Endoscopic endonasal optic nerve and orbital apex decompression for nontraumatic optic neuropathy: surgical nuances and review of the literature. Neurosurg Focus. 2014;37(04):E19. doi: 10.3171/2014.7.FOCUS14303. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


