Abstract
Objective/Hypothesis The aim of the study was to determine the impact of race on disease presentation and treatment of intracranial meningioma in the United States.
Study Design This study comprised of the analysis of a national population-based tumor registry.
Methods Analysis of the surveillance, epidemiology, and end results (SEER) database was performed, including all patients identified with a diagnosis of intracranial meningioma. Associations between race, disease presentation, treatment strategy, and overall survival were analyzed in a univariate and multivariable model.
Results A total of 65,973 patients with intracranial meningiomas were identified. Of these, 45,251 (68.6%) claimed white, 7,796 (12%) black, 7,154 (11%) Hispanic, 4,902 (7%) Asian, and 870 (1%) patients reported “other-unspecified” or “other-unknown.” The median annual incidence of disease was lowest among black (3.43 per 100,000 persons) and highest among white (9.52 per 100,000 persons) populations ( p < 0.001). Overall, Hispanic patients were diagnosed at the youngest age and white patients were diagnosed at the oldest age (mean of 59 vs. 66 years, respectively; p < 0.001). Compared with white populations, black, Hispanic, and Asian populations were more likely to present with larger tumors ( p < 0.001). After controlling for tumor size, age, and treatment center in a multivariable model, Hispanic patients were more likely to undergo surgery than white, black, and Asian populations. Black populations had the poorest disease specific and overall survival rates at 5 years following surgery compared with other groups.
Conclusion Racial differences among patients with intracranial meningioma exist within the United States. Understanding these differences are of vital importance toward identifying potential differences in the biological basis of disease or alternatively inequalities in healthcare delivery or access Further studies are required to determine which factors drive differences in tumor size, age, annual disease incidence, and overall survival between races.
Keywords: meningioma, radiosurgery, microsurgery, race, ethnicity, epidemiology
Introduction
Meningiomas represent the most common primary intracranial neoplasm and can arise anywhere along the skull-base or convexities thereby mandating a catalog of therapeutic considerations to individualize treatment. 1 The great majority of the intracranial meningioma literature has reported total population data, while very limited information exists regarding racial differences in disease epidemiology and management trends. 2 We sought to further investigate racial differences in intracranial meningiomas with regard to disease incidence, presentation, treatment, and outcome in the United States. Specifically, differences between white, black, Asian, and Hispanic populations are reported to provide greater detail regarding the largest minority populations in the United States.
Materials and Methods
Registry Data
The surveillance, epidemiology, and end results (SEER) database includes a set of 20 population-based tumor registries covering approximately 28% of the United States from 2004 to 2014. Cases of intracranial meningioma were included for analysis if their record was confirmed to contain one of the International Classification of Disease for Oncology (ICD-O) histology codes as well as one of the ICD-O topography codes. Topography codes included C70.0, cerebral meninges; C71.0, cerebrum; C71.1, frontal lobe; C71.2, temporal lobe; C71.3, parietal lobe; C71.4, occipital lobe; C71.5, ventricle, not otherwise specified (NOS); C71.56, cerebellum, NOS; C71.1, brain stem; C71.8, overlapping lesion of the brain; C71.9, brain, NOS; C72.2, olfactory nerve; C72.3, optic nerve; C72.5, cranial nerve, NOS; C75.1, pituitary gland; C75.2, craniopharyngeal duct; and C75.3, pineal gland. Tumor size was recorded and categorized as: 0 to < 1 cm, 1 to < 2 cm, 2 to < 3 cm, 3 to < 4 cm, 4 to < 5 cm, 5 to < 6 cm, ≥ 6 cm, and missing. Treatment was categorized based on SEER site specific surgery, radiotherapy, and radiation-surgery-sequence variables. Microsurgery was defined as gross or subtotal resection. Radiation alone was defined as patients without microsurgery who had a radiation code stating that treatment had been performed. Observation included all patients who did not undergo radiation therapy or microsurgery. Race groups analyzed included Asian, black, Hispanic, white, and others. Given limited population sizes for several groups, certain racial subpopulations were aggregated, including Chinese, Japanese, Korean, Vietnamese, Laotian, Kampuchean, Thai, Asian Indian or Pakistani, Pacific Islander, Asian NOS, and Oriental.
Statistical Analysis
Univariate comparisons of demographic features, tumor characteristics, and treatment strategy were conducted using Chi-square tests and Student's t -tests, as appropriate. Multivariable logistic regression was performed to assess the independent impact of race on management modality while adjusting for tumor size, age, and treatment center. Overall survival was analyzed using the Kaplan–Meier method with the log-rank test for statistical significance between groups. Cox's proportional hazard regression analysis was performed to compare overall mortality between racial groups adjusting for age, tumor size, and treatment center differences. As SEER is a set of population-based registries, incidence may be calculated. The annual population denominators for the registries were taken from SEER-Stat and annual disease incidence was calculated. The Mayo Clinic Institutional Review Board (Mayo Clinic, Rochester, MN, U.S.A.) has deemed analyses of SEER data exempt from review. All analyses were performed using the SAS software package (version 9.4 for Windows; SAS Institute Inc., Cary, NC, U.S.A.), and p -values < 0.05 were considered statistically significant.
Results
In total, 65,973 patients with intracranial meningioma were identified over the time period of 2004 to 2014. The mean age at time of diagnosis was 65 years and 73% were women. Overall, 55% of patients received conservative management, 5% radiation alone, and 40% microsurgery alone. Of these, 45,251 (68.6%) claimed white, 7,796 (12%) black, 7,154 (11%) Hispanic, 4,902 (7%) Asian, and 870 (1%) patients reported other races. Comparison of the annual incidence of intracranial meningioma per 100,000 persons between race groups is described in Table 1 .
Table 1. Annual incidence of intracranial meningioma in the United States between race groups.
| n with Tumor | Incidence | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Y of surgery | White | Black | Hispanic | Asian or Pacific Islander | Other | Rate per 100,000 white | Rate per 100,000 Hispanic | Rate per 100,000 black | Rate per 100,000 Asian or Pacific Islander | Rate per 100,000 other |
| 2004 | 3,289 | 555 | 425 | 338 | 34 | 6.860 | 5.742 | 2.565 | 4.714 | 4.819 |
| 2005 | 3,568 | 556 | 525 | 376 | 54 | 7.471 | 5.751 | 3.083 | 5.101 | 7.644 |
| 2006 | 3,657 | 612 | 593 | 381 | 77 | 7.678 | 6.300 | 3.393 | 5.032 | 10.875 |
| 2007 | 3,866 | 674 | 601 | 431 | 67 | 8.130 | 6.846 | 3.349 | 5.553 | 9.438 |
| 2008 | 4,067 | 702 | 669 | 439 | 74 | 8.558 | 7.049 | 3.624 | 5.511 | 10.378 |
| 2009 | 4,376 | 747 | 697 | 461 | 86 | 9.215 | 7.419 | 3.674 | 5.653 | 11.995 |
| 2010 | 4,340 | 775 | 650 | 479 | 76 | 9.142 | 7.623 | 3.341 | 5.740 | 10.554 |
| 2011 | 4,432 | 760 | 708 | 462 | 93 | 9.336 | 7.402 | 3.567 | 5.401 | 12.837 |
| 2012 | 4,490 | 765 | 757 | 492 | 99 | 9.456 | 7.383 | 3.748 | 5.608 | 13.595 |
| 2013 | 4,618 | 834 | 752 | 517 | 97 | 9.728 | 7.979 | 3.662 | 5.738 | 13.245 |
| 2014 | 4,548 | 816 | 777 | 526 | 113 | 9.586 | 7.729 | 3.720 | 5.680 | 15.359 |
| Average per year | 9.516 | 7.020 | 3.430 | 5.430 | 10.976 | |||||
The median annual incidence was lowest among black (3.43 per 100,000 persons) and Asians (5.43 per 100,000 persons), intermediate for Hispanic populations (7.02 per 100,000 persons), and highest among whites (9.52 per 100,000 persons; p < 0.001). Incidence increased in each race over the evaluated time period. A comparison of baseline demographic features and tumor size between race groups is outlined in Table 2 . Overall, Hispanic patients were diagnosed at the youngest age, and white patients were diagnosed at the oldest age (mean of 59 vs. 66 years, respectively; p < 0.001). Compared with white populations, black, Hispanic, and Asian populations were more likely to present with larger tumors ( p < 0.001).
Table 2. Univariate Comparisons of baseline demographic features and tumor size between racial groups with intracranial meningioma.
| Overall ( n = 65,973) | Non-Hispanic white ( n = 45,251) | Non-Hispanic black ( n = 77,96) | Hispanic ( n = 7,154) | Non-Hispanic Asian or Pacific Islander ( n = 4,902) |
Other/unknown ( n = 870) | p -Value | |
|---|---|---|---|---|---|---|---|
| Age (y) | < 0.0001 | ||||||
| Mean (SD) | 64.2 (16.1) | 65.8 (15.8) | 61.5 (15.8) | 59.0 (16.6) | 63.2 (15.9) | 57.9 (15.0) | |
| Median | 65 | 67 | 62 | 59 | 64 | 58 | |
| Age group (y) | < 0.0001 | ||||||
| 0–49 (%) | 13,832 (21.0) | 8,254 (18.2) | 1,958 (25.1) | 2,280 (31.9) | 1,075 (21.9) | 265 (30.5) | |
| 50–59 (%) | 11,254 (17.1) | 7,251 (16.0) | 1,598 (20.5) | 1,345 (18.8) | 856 (1,7.5) | 204 (23.4) | |
| 60+ (%) | 40,887 (62.0) | 29,746 (65.7) | 4,240 (54.4) | 3,529 (49.3) | 2,971 (60.6) | 401 (46.1) | |
| Sex | 0.0001 | ||||||
| Male (%) | 17,527 (26.6) | 12,239 (27.0) | 2,068 (26.5) | 1,802 (25.2) | 1,209 (24.7) | 209 (24.0) | |
| Female (%) | 48,446 (73.4) | 33,012 (73.0) | 5,728 (73.5) | 5,352 (74.8) | 3,693 (75.3) | 661 (76.0) | |
| Tumor size group | < 0.0001 | ||||||
| 1–< 2 cm (%) | 16,290 (24.7) | 11,842 (26.2) | 1,688 (21.7) | 1,503 (21.0) | 1,035 (21.1) | 222 (25.5) | |
| 2–< 3 cm (%) | 11,514 (17.5) | 7,921 (17.5) | 1,435 (18.4) | 1,238 (17.3) | 787 (16.1) | 133 (15.3) | |
| 3–< 4 cm (%) | 7,601 (11.5) | 5,026 (11.1) | 989 (12.7) | 917 (12.8) | 574 (11.7) | 95 (10.9) | |
| 4–< 5 cm (%) | 5,436 (8.2) | 3,507 (7.8) | 679 (8.7) | 669 (9.4) | 512 (10.4) | 69 (7.9) | |
| 5–< 6 cm (%) | 3,628 (5.5) | 2,274 (5.0) | 487 (6.2) | 461 (6.4) | 355 (7.2) | 51 (5.9) | |
| > 6 cm (%) | 3,833 (5.8) | 2,438 (5.4) | 495 (6.3) | 467 (6.5) | 393 (8.0) | 40 (4.6) | |
| Missing (%) | 12,420 (18.8) | 8,363 (18.5) | 1,505 (19.3) | 1,433 (20.0) | 924 (18.8) | 195 (22.4) |
With regard to management, 55% of patients underwent observation, 5% underwent radiation treatment, and 40% underwent microsurgery across the entire cohort ( Table 3 ). Treatment over time was evaluated and trends toward decreased surgery (48–36%) and radiation (6–4%) were noted with increase in observation (46–60%) over time ( p < 0.0001). When comparing differences between racial groups, Hispanic patients most frequently underwent microsurgery whereas Asian populations were most likely to undergo radiation treatment. Observation was most common among white patients when compared with other groups. After controlling for tumor size, age, and treatment center in a multivariable model, the Hispanic patient population was more likely to undergo microsurgery compared with Non-Hispanic white patients ( Table 4 ). When comparing race groups and treatment modalities, black populations showed decreased overall survival. Similarly, on subgroup analysis, overall mortality following surgery was higher in the black population (hazard ratio [HR]: 1.35) compared with the white population on univariate analysis and when accounting for baseline differences in age, tumor size, and treatment center in a multivariate model ( Figs. 1 and 2 ). It is important to note that 20% of the information on tumor size was missing in the SEER data, which could affect interpretation of results.
Table 3. Univariate comparisons of treatment strategies between racial groups with intracranial meningioma.
| Overall | Non-Hispanic white | Non-Hispanic black | Hispanic | Non-Hispanic Asian or Pacific Islander | Other/unknown | p -Value | |
|---|---|---|---|---|---|---|---|
| n | 65,973 | 45,251 | 7,796 | 7,154 | 4,902 | 870 | |
| Type of treatment | < 0.0001 | ||||||
| No surgery or radiation (%) | 36,206 (54.9) | 25,820 (57.1) | 4,294 (55.1) | 3,240 (45.3) | 2,396 (48.9) | 456 (52.4) | |
| Radiation only (%) | 3,484 (5.3) | 2,352 (5.2) | 391 (5.0) | 389 (5.4) | 288 (5.9) | 64 (7.4) | |
| Surgery (%) | 26,283 (39.8) | 17,079 (37.7) | 3,111 (39.9) | 3,525 (49.3) | 2,218 (45.2) | 350 (40.2) | |
| Radiation after surgery (also included in surgery category) (%) | 2,352 (3.6) | 1,427 (3.2) | 308 (4.0) | 356 (5.0) | 237 (4.8) | 24 (2.8) |
Table 4. Probability of receiving primary surgery between racial groups, after controlling for age, tumor size, and treatment center.
| Variable | OR | Lower CI | Upper CI | p -Value |
|---|---|---|---|---|
| Asian vs. white | 1.035 | 0.957 | 1.118 | 0.392 |
| Black vs. white | 0.806 | 0.757 | 0.859 | < 0.001 |
| Hispanic vs. white | 1.112 | 1.042 | 1.187 | < 0.0001 |
| Other vs. white | 0.794 | 0.667 | 0.945 | 0.009 |
Abbreviations: CI, confidence interval; OR, odds ratio.
Fig. 1.
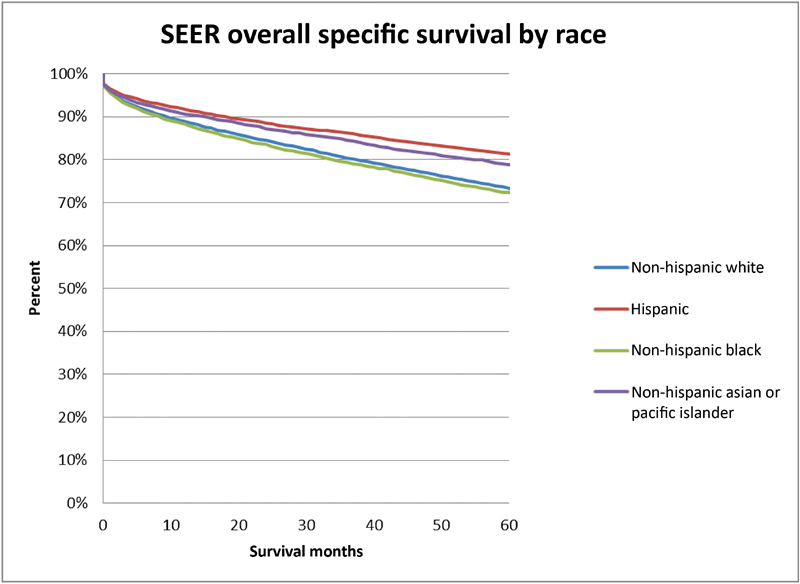
| Kaplan–Meier analysis comparing overall survival between racial groups with intracranial meningioma.
Fig. 2.
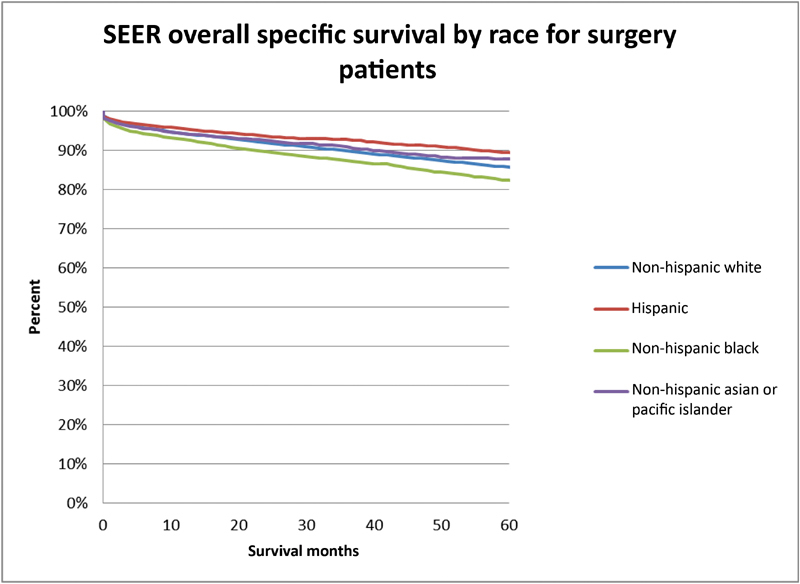
Kaplan–Meier analysis comparing overall survival between racial groups that underwent intracranial meningioma microsurgical resection.
Discussion
In recent years, race, ethnicity, and socioeconomic-related differences in medical care have received increasing attention. The influence of these factors on healthcare access, delivery, and outcomes are pervasive and far-reaching. To date, few studies have evaluated the impact of economic, social, cultural, and health system access on disease presentation and treatment outcomes of nonmalignant tumors, such as intracranial meningioma.
There have been several environmental risk factors associated with intracranial meningioma. Ionizing radiation has been linked to meningioma development. 3 Meningioma risk might be lower in those with a history of allergy, although this association may be limited to eczema alone. 4 There is no clear evidence of ethnic variation in eczema incidence. Initial work indicates that meningioma risk might be higher in those who have used hormone replacement therapy (HRT). 5 There is some evidence that white women are more likely to use HRT than other groups. 6 Multiparity may also be a risk factor for meningioma. 5
Several studies have evaluated potential genetic associations with meningioma development. The Interphone study identified 12 single nucleotide polymorphisms associated with development of meningioma, 7 with a later meta-analysis revealing that Caucasians have a significantly higher risk (odds ratio [OR] = 1.31, 95% confidence interval [CI] 1.05–1.63, p = 0.02) of meningioma if they carry the computed tomography [CT] genotype of the methylenetetrahydrofolate reductase gene. 8 Ibebuike et al sought to identify a genetic cause to explain a disproportionately high incidence of meningiomas in the Sotho ethnic nationality in their analysis of intracranial meningiomas at two South African hospitals; however, the overall relationship between ethnicity and genetic risk factors was indeterminate. 9 Kshettry et al suggested that black and Asian Pacific Islander (API) races present with a higher proportion of aggressive meningiomas, defined as both World Health Organization Grade II and III status. 10 Turner syndrome and neurofibromatoses type 2 are associated with a higher incidence of meningioma. 11 However, there is no evidence of race predilection for these conditions.
Access to healthcare and specifically advanced neurodiagnostic imaging, likely plays a significant role in the epidemiology of intracranial meningioma and stage at diagnosis. Elective diagnostic imaging has been shown to be more accessible to patients with higher socioeconomic backgrounds and professional work experience. One commonly held belief is that the increasing incidence in meningiomas correlates with the availability of magnetic resonance imaging (MRI) scanners and receiving MRI's for other problems. Certainly the number of head MRI in the U.S. performed has increased, and since meningioma is the most common intracranial tumor, we would expect to find more with time. The availability of MRI scanners may be vulnerable to racial disparity; however, it also may be the case that certain reasons to perform a head MRI may be more common amongst different ethnic groups. 12 For instance, Nicholson et al described lower levels of headache utilization, diagnosis, and treatment African Americans in comparison to Caucasians. 13 This finding may lead to an identification bias, as Caucasians may be more likely to undergo further workup for headaches and subsequently result in identifying incidental meningiomas. Additionally, it has been reported that whites have a higher incidence of multiple sclerosis which may results in increased incidental meningiomas identified due to the associate MRI workup. 13
Due to the indolent nature of meningioma growth in most cases and insidious progression of symptoms, the threshold for imaging workup in patients with superior health insurance coverage may be lower than in patients with substandard insurance coverage and higher out-of-pocket costs. As a possible downstream effect, Inskip et al described a positive association between household income and the odds of being diagnosed with meningioma. 14 Dolecek et al reported that black race was associated with poorer survival outcomes for meningiomas, whereas for most other CNS tumors, blacks had lower incidence and little differences in survival compared with other races. 15 This finding is similar to reports on outcomes of high-grade gliomas from Robertson et al 16 and Barnholtz-Sloan et al 17 A possible explanation is that the more sudden and severe symptomatology from an aggressive glioma, for example, is more likely to drive a diagnostic workup regardless of insurance status.
In 2010, Mukherjee et al (2010) performed a retrospective analysis of 76,436 adult patients and found that African Americans had disproportionately worse access to high-quality neuro-oncology care compared with whites. 18 A 2013 study of 2,321 Medicaid patients found significant disparities in the care of African American patients after brain tumor surgery. 19 Additionally, Nuño et al sampled a nationwide inpatient population and similarly found that black and Hispanic patients had more severe disease at presentation. 20 In the present study, black, Hispanic, and Asian populations were more likely to present with larger tumors than their white counterparts. Furthermore, on average white patients presented at a later age and had a higher incidence of disease compared with other races. These findings infer that inequalities in healthcare access and delivery drive underdiagnoses and diagnostic delays within many minority populations.
Epidemiological analyses of other cancers, including breast, skin, and lung cancer, have demonstrated wide differences in diagnosis between racial groups, often with black patients being diagnosed at a lower rate than white patients. 21 22 23 24 25 For example, a study published by Bach et al investigated racial differences in treatment strategies for nonsmall cell lung cancer and found that the rate of surgical treatment for resectable disease was 64% in African Americans versus 77% in whites; as a result, the 5-year survival rates were lower in African American than white patients at 26 versus 34%, respectively ( p < 0.001). 25 When analyzing factors predicting deep brain stimulation (DBS) use in 2,408,302 patients with Parkinson's disease, Chan et al found that although African American patients are more often discharged from hospitals with conditions predicting need for DBS use, these patients received disproportionately fewer DBS procedures compared with their non-African American counterparts. 26
In many cases, these differences are also associated with disparities in outcomes, including higher mortality and higher likelihood of adverse events in non-whites. Previous research on patients undergoing craniotomy for brain tumors has similarly demonstrated worse outcomes and survival among racial minorities. 27 Specifically, a study by Curry et al (2010) showed that black patients undergoing craniotomy were more likely to present with more severe disease to lower-volume hospitals, and subsequently died at a higher rate, and were more likely to have an adverse discharge disposition. 27 McClelland III et al found an increased risk of mortality following vestibular schwannoma surgical resection in African Americans compared with Caucasians, with an OR of greater than eight, based on data from the Nationwide Inpatient Sample (Healthcare Cost and Utilization Project) from 1994 to 2003. 28 Similarly, Mukherjee et al found that African Americans with meningiomas had higher odds of developing a 30-day complication ( p < 0.05) and were significantly more likely to have longer length of stay ( p < 0.05) and greater total charges ( p < 0.001) relative to Caucasians. 19 Corroborating these findings, the current study identified poorer overall survival following microsurgery among black and Hispanic populations compared with Asian, other, and white patients. It is important to note that 870 patients in the study identified as “other,” which may result in some minor skewing of the data.
Conclusion
Issues of quality and equity in healthcare are complex. The current study highlights racial disparity in the management of intracranial meningioma. Evaluation of equity in healthcare may help alleviate disparities and improve care for all patients with intracranial meningioma. The strength of the study is highlighted by its inclusion and analysis of over 63,700 patients. Inherent to use of a generic national tumor registry, limitations include absence of data pertaining to socioeconomic variables, patient comorbidities, as well as disease-specific pre- and posttreatment morbidity and mortality.
Funding Statement
Funding This study involved no internal/external funding.
Footnotes
Conflict of Interest The authors declare no conflicts of interest regarding publication of this paper.
References
- 1.Agarwal V, McCutcheon B A, Hughes J D et al. Trends in management of intracranial meningiomas: analysis of 49,921 cases from modern cohort. World Neurosurg. 2017;106:145–151. doi: 10.1016/j.wneu.2017.06.127. [DOI] [PubMed] [Google Scholar]
- 2.Curry W T, Jr., Barker F G., II Racial, ethnic and socioeconomic disparities in the treatment of brain tumors. J Neurooncol. 2009;93(01):25–39. doi: 10.1007/s11060-009-9840-5. [DOI] [PubMed] [Google Scholar]
- 3.Taylor A J, Little M P, Winter D L et al. Population-based risks of CNS tumors in survivors of childhood cancer: the British Childhood Cancer Survivor Study. J Clin Oncol. 2010;28(36):5287–5293. doi: 10.1200/JCO.2009.27.0090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang M, Chen C, Qu J et al. Inverse association between eczema and meningioma: a meta-analysis. Cancer Causes Control. 2011;22(10):1355–1363. doi: 10.1007/s10552-011-9808-6. [DOI] [PubMed] [Google Scholar]
- 5.Qi Z Y, Shao C, Huang Y L, Hui G Z, Zhou Y X, Wang Z. Reproductive and exogenous hormone factors in relation to risk of meningioma in women: a meta-analysis. PLoS One. 2013;8(12):e83261. doi: 10.1371/journal.pone.0083261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Harris T J, Cook D G, Wicks P D, Cappuccio F P.Ethnic differences in use of hormone replacement therapy: community based survey BMJ 1999319(7210):610–611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bethke L, Murray A, Webb E et al. Comprehensive analysis of DNA repair gene variants and risk of meningioma. J Natl Cancer Inst. 2008;100(04):270–276. doi: 10.1093/jnci/djn004. [DOI] [PubMed] [Google Scholar]
- 8.Ding H, Liu W, Yu X, Wang L, Shao L, Yi W. Risk association of meningiomas with MTHFR C677T and GSTs polymorphisms: a meta-analysis. Int J Clin Exp Med. 2014;7(11):3904–3914. [PMC free article] [PubMed] [Google Scholar]
- 9.Ibebuike K, Ouma J. Demographic profile of patients diagnosed with intracranial meningiomas in two academic hospitals in Johannesburg, South Africa: a 12-month prospective study. Afr Health Sci. 2014;14(04):939–945. doi: 10.4314/ahs.v14i4.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kshettry V R, Ostrom Q T, Kruchko C, Al-Mefty O, Barnett G H, Barnholtz-Sloan J S. Descriptive epidemiology of World Health Organization grades II and III intracranial meningiomas in the United States. Neuro-oncol. 2015;17(08):1166–1173. doi: 10.1093/neuonc/nov069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Maile E J, Barnes I, Finlayson A E, Sayeed S, Ali R. Nervous system and intracranial tumour incidence by Ethnicity in England, 2001-2007: a descriptive epidemiological study. PLoS One. 2016;11(05):e0154347. doi: 10.1371/journal.pone.0154347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Amezcua L, Rivas E, Joseph S, Zhang J, Liu L.Multiple sclerosis mortality by race/ethnicity, age, sex, and time period in the United States, 1999-2015 Neuroepidemiology 201850(1, 2):35–40. [DOI] [PubMed] [Google Scholar]
- 13.Nicholson R A, Rooney M, Vo K, O'Laughlin E, Gordon M. Migraine care among different ethnicities: do disparities exist? Headache. 2006;46(05):754–765. doi: 10.1111/j.1526-4610.2006.00453.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Inskip P D, Tarone R E, Hatch E E et al. Sociodemographic indicators and risk of brain tumours. Int J Epidemiol. 2003;32(02):225–233. doi: 10.1093/ije/dyg051. [DOI] [PubMed] [Google Scholar]
- 15.Dolecek T A, Dressler E V, Thakkar J P, Liu M, Al-Qaisi A, Villano J L. Epidemiology of meningiomas post-public law 107-206: the benign brain tumor cancer registries amendment act. Cancer. 2015;121(14):2400–2410. doi: 10.1002/cncr.29379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Robertson J T, Gunter B C, Somes G W. Racial differences in the incidence of gliomas: a retrospective study from Memphis, Tennessee. Br J Neurosurg. 2002;16(06):562–566. [PubMed] [Google Scholar]
- 17.Barnholtz-Sloan J S, Sloan A E, Schwartz A G. Racial differences in survival after diagnosis with primary malignant brain tumor. Cancer. 2003;98(03):603–609. doi: 10.1002/cncr.11534. [DOI] [PubMed] [Google Scholar]
- 18.Mukherjee D, Zaidi H A, Kosztowski T et al. Disparities in access to neuro-oncologic care in the United States. Arch Surg. 2010;145(03):247–253. doi: 10.1001/archsurg.2009.288. [DOI] [PubMed] [Google Scholar]
- 19.Mukherjee D, Patil C G, Todnem N et al. Racial disparities in Medicaid patients after brain tumor surgery. J Clin Neurosci. 2013;20(01):57–61. doi: 10.1016/j.jocn.2012.05.014. [DOI] [PubMed] [Google Scholar]
- 20.Nuño M, Mukherjee D, Elramsisy A et al. Racial and gender disparities and the role of primary tumor type on inpatient outcomes following craniotomy for brain metastases. Ann Surg Oncol. 2012;19(08):2657–2663. doi: 10.1245/s10434-012-2353-z. [DOI] [PubMed] [Google Scholar]
- 21.Ward E, Jemal A, Cokkinides V et al. Cancer disparities by race/ethnicity and socioeconomic status. CA Cancer J Clin. 2004;54(02):78–93. doi: 10.3322/canjclin.54.2.78. [DOI] [PubMed] [Google Scholar]
- 22.Tammemagi C M, Nerenz D, Neslund-Dudas C, Feldkamp C, Nathanson D. Comorbidity and survival disparities among black and white patients with breast cancer. JAMA. 2005;294(14):1765–1772. doi: 10.1001/jama.294.14.1765. [DOI] [PubMed] [Google Scholar]
- 23.Shin J Y, Truong M T. Racial disparities in laryngeal cancer treatment and outcome: A population-based analysis of 24,069 patients. Laryngoscope. 2015;125(07):1667–1674. doi: 10.1002/lary.25212. [DOI] [PubMed] [Google Scholar]
- 24.Molina M A, Cheung M C, Perez E A et al. African American and poor patients have a dramatically worse prognosis for head and neck cancer: an examination of 20,915 patients. Cancer. 2008;113(10):2797–2806. doi: 10.1002/cncr.23889. [DOI] [PubMed] [Google Scholar]
- 25.Bach P B, Cramer L D, Warren J L, Begg C B. Racial differences in the treatment of early-stage lung cancer. N Engl J Med. 1999;341(16):1198–1205. doi: 10.1056/NEJM199910143411606. [DOI] [PubMed] [Google Scholar]
- 26.Chan A K, McGovern R A, Brown L T et al. Disparities in access to deep brain stimulation surgery for Parkinson disease: interaction between African American race and Medicaid use. JAMA Neurol. 2014;71(03):291–299. doi: 10.1001/jamaneurol.2013.5798. [DOI] [PubMed] [Google Scholar]
- 27.Curry W T, Jr., Carter B S, Barker F G., IIRacial, ethnic, and socioeconomic disparities in patient outcomes after craniotomy for tumor in adult patients in the United States, 1988-2004 Neurosurgery 20106603427–437., discussion 437–438 [DOI] [PubMed] [Google Scholar]
- 28.McClelland S, III, Guo H, Okuyemi K S. Morbidity and mortality following acoustic neuroma excision in the United States: analysis of racial disparities during a decade in the radiosurgery era. Neuro-oncol. 2011;13(11):1252–1259. doi: 10.1093/neuonc/nor118. [DOI] [PMC free article] [PubMed] [Google Scholar]


