Abstract
Alliums and allied plant species are rich sources of sulfur compounds that have effects on vascular homeostasis and the control of metabolic systems linked to nutrient metabolism in mammals. In view of the multiple biological effects ascribed to these sulfur molecules, researchers are now using these compounds as inspiration for the synthesis and development of novel sulfur-based therapeutics. This research has led to the chemical synthesis and biological assessment of a diverse array of sulfur compounds representative of derivatives of S-alkenyl-l-cysteine sulfoxides, thiosulfinates, ajoene molecules, sulfides, and S-allylcysteine. Many of these synthetic derivatives have potent antimicrobial and anticancer properties when tested in preclinical models of disease. Therefore, the current review provides an overview of advances in the development and biological assessment of synthetic analogs of allium-derived sulfur compounds.
Keywords: allium, anticancer, sulfur compounds, gaseous mediators, hydrogen sulfide, garlic
1. Introduction
Approximately 700 plant species are known to contain S-alk(en)yl-l-cysteine sulfoxides (ACSOs), including many edibles like allium and brassica vegetables (Table 1). Popularity stems from their distinctive flavors, produced during the breakdown of each respective ACSOs [1,2]. As such, changes to the composition and quantities of each ACSO determines the odor, flavor variation, and biological activities ascribed to many plants containing these sulfur storage compounds [3]. At present, four major and two minor ACSOs exist, and these act as progenitor molecules in the production of other sulfur compounds like the lachrymatory factor, propanethial S-oxide and the antimicrobial compound allicin [3]. An additional 50+ sulfur compounds, including thiosulfinates, cysteine, and sulfides derivatives can also occur in allium tissue following preparation, and many of these compounds possess some level of biological activity in mammalian cells and tissue [4]. Indeed, researchers have shown crude allium-derived plant extracts as well as isolated sulfur compounds such as S-allylcysteine, diallyl disulfide, allicin, 1,2-vinyldithiin, 3-vinyldithiin, and ajoene can induce a range of physiological and biochemical changes in mammalian systems [5]. For example, several cellular targets are reported to be susceptible to sulfur compound treatment, including proinflammatory transcription factor nuclear factor-kappa beta (NF-κB) [6,7], various kinases, including p38 mitogen-activated protein kinase (p38 MAPK) [8], c-JunNH2-terminal kinase (JNK) [9], extracellular signal-regulated kinase (ERK) [10], and phosphoinositide 3-kinase-protein kinase B (PI-3K-Akt) [11]. Other cellular proteins, like nuclear factor erythroid 2-related factor 2 (Nrf-2) [12,13,14], p53 [15,16], AMP-activated protein kinase [17,18], proliferator-activated receptor γ [19], and NAD-dependent deacetylase sirtuin-1 (SIRT1) [20] are also targets of allium-derived sulfur species. Consequently, many sulfur molecules have been reported to have anticancer properties [21,22,23,24], are anti-inflammatory [7,25,26,27], act as cytoprotective agents [28,29], function as inhibitors of metastasis [30,31], and alter lipid metabolism [32,33]. Additionally, molecules like diallyl trisulfide (DATS) release hydrogen sulfide gas (H2S) in the presence of glutathione and cysteine [34]. The liberated H2S acts as a gaseous signaling molecule in mammalian cells with potential pharmacological effects in humans [35,36]. This property may contribute to the biological effects attributed to DATS and, more widely, other allium-derived sulfur species [37]. The identification of these molecular targets may explain why several ACSOs containing plant species, including garlic (Allium sativum) and onion (A. cepa) are common constituents of folklore remedies [38,39,40]. Moreover, several human population-based studies have shown that the consumption of diets rich in allium and brassica vegetables are associated with a reduced risk of developing several forms of cancers [41,42], type II diabetes [43], cardiovascular diseases [44,45], and changes in the gut microbiome [46].
Table 1.
Allium and brassica S-Alk(en)yl-l-cysteine sulfoxides (adapted from [2]).
| Chemical Name | Structure | Species |
|---|---|---|
| S-Methyl-l-Cysteine Sulfoxide |
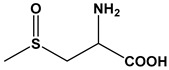
|
A. cepa
A. sativum A. chinensis Brassica Oleracea |
| S-Allyl-l-Cysteine Sulfoxide |
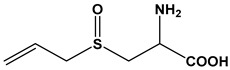
|
A. sativum
A. ursinium A. ampeloprasum |
| S-Propyl-l-Cysteine Sulfoxide |
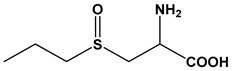
|
A. cepa
A. porrum A. fistulosum |
| S-Propenyl-l-Cysteine Sulfoxide |
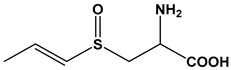
|
A. cepa
A. nutans A. schoenoprasum |
| S-Ethyl-l-Cysteine Sulfoxide |
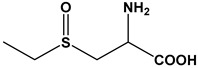
|
A. aflatunense
A. ampeloprasum A. victorialis |
| S-n-Butyl-l-Cysteine Sulfoxide |
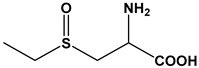
|
A. siculum |
In light of the potential roles of dietary sulfur compounds and their potential impact on health, it is timely to address some of the lesser-known aspects relating to research on allium-derived sulfur compounds, that of the biological screening of synthetic analogs of ACSOs and associated degradation products. Over the last decade, a number of researchers have synthesized a spectrum of synthetic sulfur compounds, representative of derivatives of thiosulfinates, cysteine analogs, ajoene-based compounds, and various sulfides. Sulfur-derived functional groups occur in a broad range of pharmaceuticals and natural products, and facilitated developments in modern drug design [47]. In the case of allium-based molecules, some are reportedly superior to their natural counterparts, and could be useful in the future development and design of new sulfur therapeutics, or as tools to explore sulfur metabolic networks in living organisms. Therefore, the current review describes recent advances in the biological assessment of some novel sulfur molecules that have been inspired by allium sulfur chemistry.
2. Allium Sulfur Storage Compounds and Generation of Biologically Active Compounds
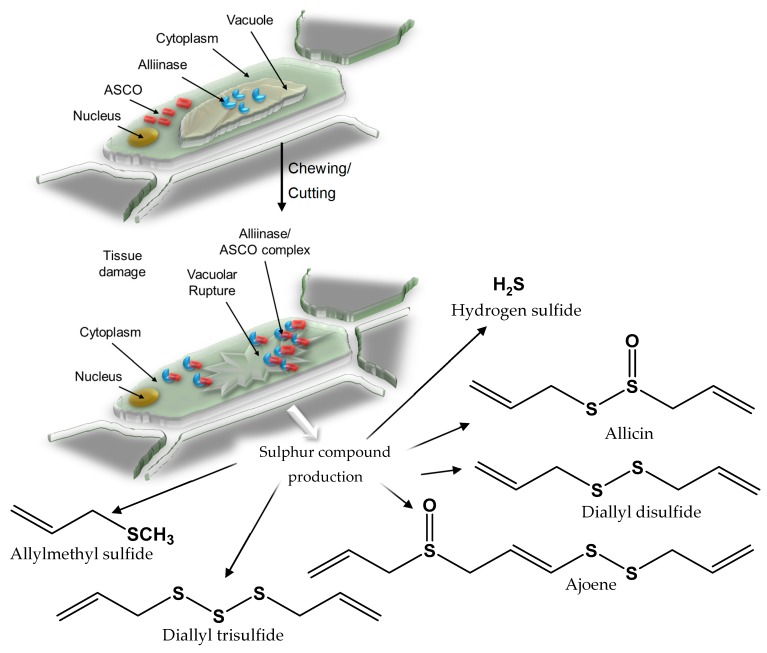
For the purposes of this review, only a brief overview of the types of sulfur compounds commonly encountered in allium and brassica plants is provided. We refer interested readers elsewhere for additional coverage of allium sulfur biochemistry [1,2,3,4,5]. In brief, the main parental storage compounds in alliums are (+)-S-allyl-l-cysteine sulfoxide (S-ACSO), commonly known as alliin, (+)-S-methyl-l-cysteine sulfoxide (methiin; MCSO), (+)-S-propyl-l-cysteine sulfoxide (propiin; PCSO), and (+)-S-trans-1-propenyl-l-cysteine sulfoxide or isoalliin (TPCSO) [1,2]. Isoalliin is the major sulfoxide present within intact onion tissue and is the source of the lachrymatory factor, thiopropanal-S-oxide. (+)-S-methyl-l-cysteine sulfoxide is by far the most ubiquitous, found in varying amounts in the intact tissue of A. sativum, A. cepa, A. porrum, and A. ursinum L., as well as in some brassica vegetables (Table 1). Enzymatic hydrolysis of ACSOs by the enzyme alliinase (EC 4.4.1.4) produces pyruvate, ammonia, and sulfenic acids [48]. Formed sulfenic acids further condense to form thiosulfinates, which in turn form a spectrum of additional sulfur compounds (Figure 1). These sulfur compounds are believed to be partly responsible for the reported health benefits attributed to the consumption of an allium- and brassica-rich diet [38,40,41,42,43,44,45,46]. Less widely reported are advances in the synthesis and biological assessment of allium-based sulfur analogs.
Figure 1.
Catabolism of S-alk(en)yl-l-cysteine sulfoxides (ASCOs) by alliinase enzyme generates a spectrum of individual sulfur compounds.
3. New Sulfur Compounds Inspired by Allium Metabolites
In recent times, a number of researchers have focused their attention on the development of novel synthetic sulfur analogs of known allium-derived sulfur compounds. These molecules were conceptualized with the view to 1) produce more stable chemical entities with enhanced solubility; 2) develop molecules with specific chemical properties, like the ability to release H2S or nitric oxide (NO) gas; or 3) possess altered biological activities, such as enhanced anticancer activities, interesting antioxidant properties, or antimicrobial effects. At present, the chemical derivatives of alliin, allicin, S-allylcysteine, and garlic-derived sulfides have been reported in the literature. Of these, only a handful have been evaluated in preclinical models of disease and, as far as we are aware, none tested on humans (Table S1). Given the resurgent interest in sulfur biochemistry, it is reasonable to discuss advances in this area, as there is optimism that future research in this field will lead to the development of newer sulfur therapeutics.
3.1. S-Alk(en)yl-l-cysteine Sulfoxides and Thiosulfinate Analogs
To date, only a handful of studies have focused on the synthesis and evaluation of ASCO analogs. In 1985, the alliin molecule was isolated from the tissue of A. cepa, and found to inhibit platelet aggregation [49]. Further work led to the testing of several commercially available alliin derivatives, and to the identification of S-oxodiallyl disulfide that reportedly inhibits platelet aggregation at similar concentrations as alliin [50]. ASCO catabolism leads to the formation of thiosulfinates, and this chemical group has gained considerable interest over the years. Thiosulfinates react with thiol groups in cells, and this reaction mechanism is responsible for their antimicrobial and antifungal activities [2,51,52]. The electron-withdrawing effect of the oxygen atom generates an electrophilic sulfur center, which can react with thiolate ions of small-molecular-weight compounds or thiol-containing enzymes. Despite the potential of using the thiosulfinate allicin as an antimicrobial agent, it is unstable and readily decomposes or binds to cellular proteins [53,54]. Indeed, when allicin is maintained at 20 °C for 20 h, it decomposed to diallyl disulfide (DADS) (66%), diallyl sulfide (DAS) (14%), diallyl trisulfide (DATS) (9%), and sulfur dioxide (SO2) [55]. For this reason, the development of thiosulfinate-based therapeutics has been limited. Newer fluorinated and S-aryl alkylthiolsulfinate analogs appear to have greater chemical stability, and these molecules may be promising in the future developments of thiosulfinate-based therapeutics [56,57].
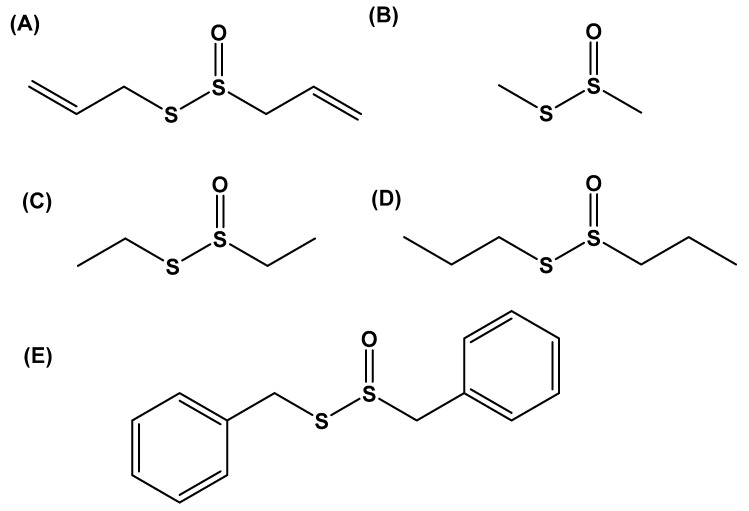
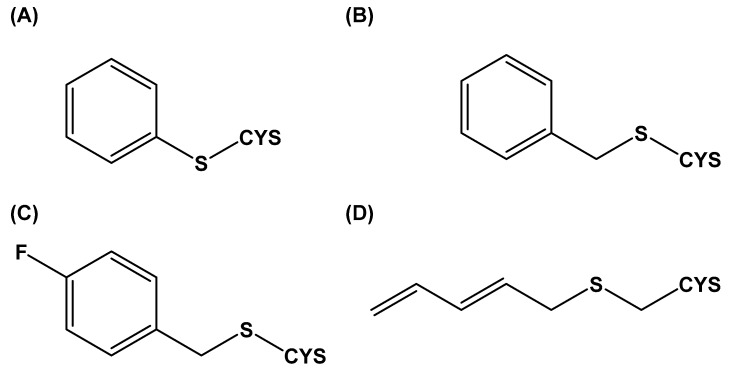
Recently, researchers have indeed raised the possibility of designing thiosulfinate-based prodrugs [58]. In this work, methionine- and cysteine-based compounds were tested. These molecules required catabolism by the methionine γ-lyase enzyme (MGL, EC 4.4.1.11) in order to generate reactive thiosulfinate intermediates. MGL can catalyze the γ- and β-elimination of methionine, and the cysteine analogs of l-methionine, l-methionine sulfoxide, S-ethyl-l-homocysteine, S-ethyl-l-homocysteine sulfoxide, O-acetyl-l-homoserine, S-ethyl-l-cysteine, S-ethyl-l-cysteine sulfoxide, and O-acetyl-l-serine and alliin. Of the tested compounds, l-methionine sulfoxide, alliin, S-ethyl-l-cysteine, and S-ethyl-l-homocysteine had bacteriostatic effects towards Citrobacter freundii and Staphylococcus aureus [58]. Similarly, Leontiev et al. focused on the synthesis of several antimicrobial analogs of allicin [59]; these compounds were representative of dimethyl-, diethyl-, diallyl-, allicin, dipropyl-, and dibenzyl- thiosulfinate derivatives (Figure 2A–E). In tests, MICs for the tested bacteria were in the range of 8–256 µg mL−1 and MBCs in the range of 16–256 µg mL−1 for various thiosulfinates. DMTS was more active against P. fluorescens than allicin was.
Figure 2.
Structures of novel antimicrobial and antifungal thiosulfinate derivatives of (A) allicin, (B) dimethylthiosulfinate, (C) diethylthiosulfinate, (D) dipropylthiosulfinate, and (E) dibenzylthiosulfinate (adapted from [61]).
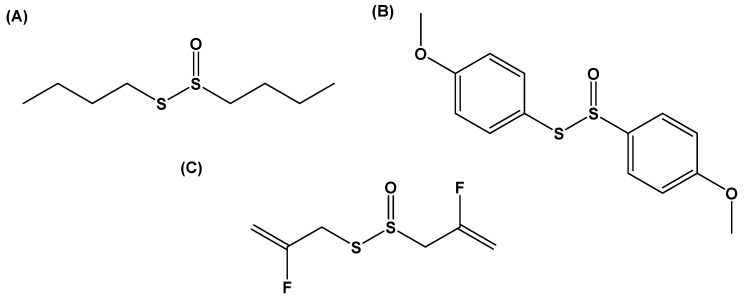
Other researchers have explored the use of thiosulfinates as novel anticancer agents. In the work of Roseblade et al., the synthesis and biological assessment of a series of 22 aromatic and aliphatic thiosulfinates were determined. These compounds were screened in an in vitro breast-cancer model using human adenocarcinoma breast-cancer cell lines MCF-7 and multidrug resistant (MDR) subline MCF-7/Dx [60]. Respective synthetic analogs S-butyl butane-1-sulfinothioate and S-4-methoxyphenyl-4-methoxybenzenesulfinothioate (Figure 3A,B) were identified as lead anticancer agents, having greater potency than that of allicin.
Figure 3.
Structures of novel synthetic sulfur compounds based on the naturally occurring thiosulfinate allicin. Several thiosulfinate derivatives have anticancer properties, like (A) S-butyl butane-1-sulfinothioate, (B) S-4-methoxyphenyl 4-methoxybenzenesulfinothioate [60], and (C) novel antithrombotic agent difluoroallicin [61].
Of the tested aromatic thiosulfinates, anticancer activity was correlated with the substituent attached to the sulfenyl sulfur moiety. Those molecules have an electron-rich 4-methoxybenzyl group and are anti-proliferative in mammalian cells. Further assessment of S-4-methoxyphenyl-4-methoxybenzenesulfinothioate showed this compound to inhibit cell proliferation by inducing G2/M phase cell-cycle arrest and apoptosis in drug-resistant cells. Finally, the synthesis, reactivity, and anti-thrombotic and anti-angiogenesis activity of a fluorinated allicin derivative have recently been tested [61]. Difluoroallicin (S-(2-fluoroallyl) 2-fluoroprop-2-ene-1-sulfinothioate; Figure 3C) dose-dependently inhibited angiogenesis and suppressed platelet aggregation [61].
3.2. Ajoene Derivatives
Ajoene (Figure 4A) is a common component of oil macerates of garlic and is present in aged crushed-garlic preparations [62]. The vinyl sulfur atom located within its disulfide backbone is electrophilic [63] and reacts with nucleophilic thiols groups. Indeed, past work has shown ajoene to react with cysteine residues present in and on proteins such as in glutathione reductase [64], trypanothione reductase [64], and human gastric lipase [65]. The ability of this molecule to react with thiol residues is partly responsible for its antimicrobial and anticancer properties [63,66,67]. Several ajoene-based analogs retain the central vinyl disulfide/sulfoxide core but have a variable terminal end group. Recent studies indicate that the antimicrobial properties of ajoene can be partly explained by its ability to disrupt quorum sensing (QS) in bacteria like in the pathogenic bacterium Pseudomonas aeruginosa via reducing the expression of key virulence genes [68]. This property suggests that ajoene and associated analogs could be useful antimicrobial agents. To date, one structure–activity relationship (SAR) screened 25 disulfide bond-containing ajoene analogs [69]. Synthesis started with the replacement of the allyl group with differing aliphatic and aromatic groups coupled with the retention of the benzothiazole disulfide moiety. This work lead to the discovery of a number of active antimicrobial agents, all of which required the presence of the benzothiazole group for activity. Para-chlorophenyl derivative 2-((4-chlorophenyl)disulfanyl)benzothiazole significantly reduced the levels of QS-regulated virulence factors like elastase, rhamnolipid, and pyocyanin and inhibited P. aeruginosa infection in a murine model of implant-associated infection [69].
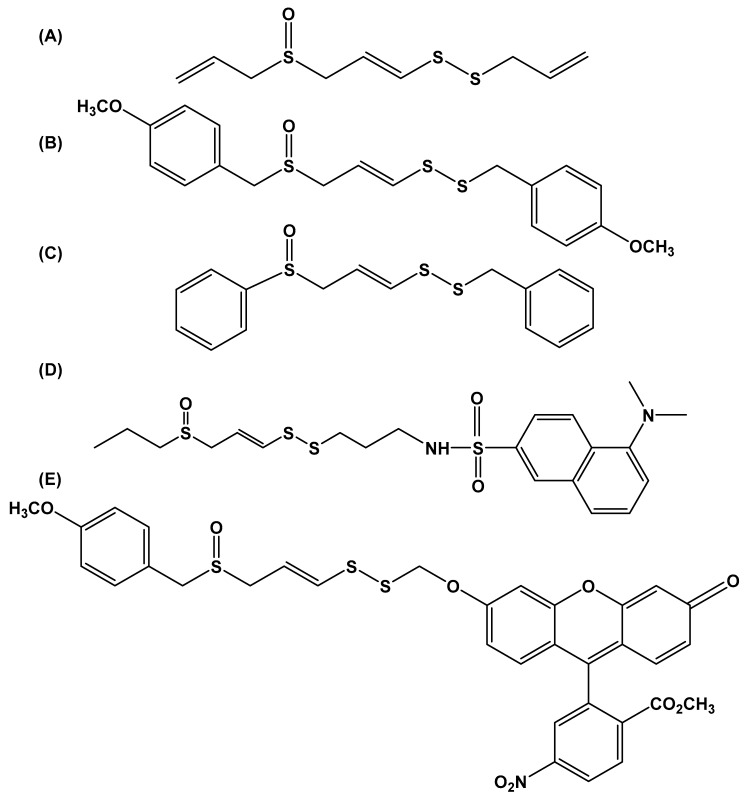
Figure 4.
(A) Ajoene has inspired derivatives synthesis like (B) (E,Z)-1,8-(Bis-para-methoxyphenyl)-2,3,7-trithia-octa-4-ene [73], and (C) SPA3015 [74], molecules with anti-proliferative effects in cancer cells. Other studies have produced (D) a labelled dansyl and (E) fluorescein ajoene derivatives to assist in the identification of molecular targets prone to ajoene modification in mammalian cells [75,76].
Some ajoene derivatives act as anticancer agents [70]. As shown in Table S1, a number of ajoene derivatives containing the central vinyl disulfide/sulfoxide core are cytotoxic to cancer cells. The para-methoxybenzyl (bisPMB) molecule (Figure 4B) and associated derivatives are anti-proliferative when tested in transformed CT-1 fibroblast cells. These same compounds also inhibited cancer-cell proliferation when tested on cultured prostrate, breast, cervical, and esophageal cancer cells [71,72]. Interestingly, analogs lacking the disulfide bond were inactive, indicating that the vinyl sulfur atom is important for biological activity. Mechanistic work indicates that bisPMB induces alternate splicing of transcription factor XBP-1 and alters the expression of ER stress proteins GRP78 and CHOP/GADD153, CHOP expression being central to the cytotoxicity of this molecule [73]. Other research has led to the identification and characterization of another ajoene derivative, SPA3015 (Figure 4C). SPA3015 reduces cell viability in P-gp-overexpressing MDR cancer cells, and suppresses NF-κB signaling. The inhibition of NF-κB decreases expression of anti-apoptotic proteins, the cellular inhibitor of apoptosis protein-1 (CIAP1), the cellular inhibitor of apoptosis protein-1 (CIAP2), the X-linked inhibitor of apoptosis protein (XIAP), and B-cell lymphoma-extra large protein (Bcl-XL), [74]. Of equal importance are efforts to identify cellular targets modified by ajoene. Kaschula and colleagues reported on the total synthesis of two fluorescently tagged ajoene probes. These probes allowing researchers to track the movement and localization of ajoene in cancer cells. Both a dansyl-tagged ajoene (DP; Figure 4D) and a fluorescein-tagged ajoene (FOX; Figure 4E) were used to study the localizes of ajoene in mammalian breast-cancer cells [75]. Dansyl ajoene, S-thiolates cellular proteins, and the use of this compound reportedly allowed for the identification of vimentin as a cellular target prone to ajoene attack [76].
3.3. Cysteine Analogs
Almost 20 years ago, Pinto and colleagues described the cytotoxic properties of a series of synthetic S-cysteinyl compounds that resemble garlic constituents (Figure 5A–D). In this work, the anti-proliferative effects of these compounds on human prostate carcinoma (LNCaP) cells were reported [77].
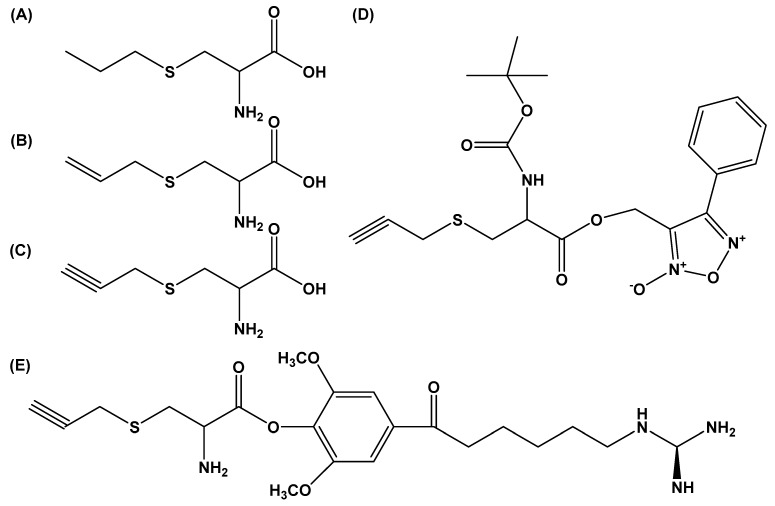
Figure 5.
Synthetic thioallyl derivatives described in the work of Pinto [77]. (A) Phenyl-S-cysteine, (B) benzyl-S-cysteine, (C) para-fluorobenzyl-S-cysteine, and (D) penta-1,3-dienyl-S-cysteine.
In the last decade, one of the most widely characterized cysteine analogs is S-propylargyl cysteine (SPRC; Figure 6) identified after an initial screening of several analogs of S-allylcysteine [78,79].
Figure 6.
(A) S-propylcysteine, (B) S-allylcysteine, (C) S-propargylcysteine, three widely characterized cysteine analogs. (D) Dual H2S and NO hybrid molecule ZYZ803. (E) Leonurine–SPRC conjugate, 3,5-dimethoxy-4-(2-amino-3-prop-2-ynylsulfanyl-propionyl)-benzoic acid 4-guanidino-butyl ester. This molecule has cardioprotective effect against hypoxia-induced neonatal rat ventricular myocyte damage [80].
The pharmacological effects of SPRC are associated with its cardioprotective and proangiogenic properties as determined in several ischemic heart models. Studies show that SPRC is neuroprotective when tested in a model of Alzheimer’s disease [81,82], has anticancer effects [83,84], and is anti-inflammatory [85]. In the cardiovascular system SPRC reduces infarct size, improves cardiac function, and mitigates the production and damaging effects of oxidative stress. These effects are associated with the induction of antioxidant defenses in cardiac tissue, including catalase and superoxide dismutase, and changes in the circulatory levels of H2S gas via the upregulation of H2S biosynthetic enzyme cystathionine-gamma-lyase (CSE), [82]. Additional research has confirmed the cardioprotective effects of SPRC in animal models of heart failure [86], myocardial infarction [87], doxorubicin-induced cardiotoxicity [88], hypoxic stress [89], and glucose-mediated damage [90]. When tested on primary human umbilical vein endothelial cells, SPRC was proangiogenic via the activation of STAT3 signaling [91]. The anti-inflammatory effects of SPRC were linked to the inhibition of TNF-α-induced ROS production and JNK1/2/NF-κB activation in cells, and reduced the expression of adhesion proteins ICAM and VCAM in endothelial cells. In animal models, SPRC reduced inflammation in models of acute pancreatitis in mice [92], alleviated inflammation anemia [93], and had anti-inflammatory effects in a rodent model of rheumatoid arthritis by modulating Nrf2-ARE signaling [94]. Moreover, in heart failure, SPRC preserves mitochondrial dysfunction through S-sulfhydration of Ca2+/calmodulin-dependent protein kinase II (CAMKII) [95]. The same group recently developed a novel SPRC conjugate, designated ZYZ-803, that functions as a combined H2S–NO-releasing molecule [96] (Figure 6D). ZYZ-803 contains the H2S-releasing moiety of S-propargylcysteine (SPRC) combined with the NO-releasing group of furoxan. Characterization of this ‘gas’ donor revealed that ZYZ-803 can time- and dose-dependently relax the sustained contraction induced by phenylephrine in rat aortic rings, and has 1.5- to 100-fold greater efficacy than that of furoxan or SPRC alone. Additional studies indicated that ZYZ-803 can stimulate endothelial cell angiogenesis [97] via induction of STAT3 signaling and the activation of CAMKII [98]. ZYZ-803 can improve left ventricular remodeling and preserve left ventricular function in isoprenaline-induced heart failure in mice [99]. These protective effects corresponded with the upregulation of CSE, an enzyme important for H2S generation, and the enzyme endothelial NO synthase (eNOS) needed for NO production in cardiac tissue. Various endogenous antioxidant proteins, like glutathione peroxidase (GPx) and heme oxygenase 1 (HO-1), also showed increased expression in tissue. Lastly, ZYZ-803 treatment in animals mitigates endoplasmic reticulum stress-related necroptosis following acute myocardial infarction [100].
3.4. Sulfide Species
Sulfides are common in many allium preparations, and compounds such as DADS, DATS, and allyl methyl trisulfide (AMS) can occur in appreciable amounts in oils produced during steam distillation. Garlic essential oils contain high amount of sulfur compounds like diallyl trisulfide (37.3–45.9%), diallyl disulfide (17.5–35.6%), and methyl allyl trisulfide (7.7–10.4%) [101,102] (Figure 7A). Sadly, like other allium-derived sulfur compounds, many sulfides are chemically reactive and prone to decomposition, and this limits their use as viable therapeutic agents. Attention has been focused on the screening of sulfide analogs as anti-proliferative agents in cancer cells [103], as inhibitors of HMG–CoA reductase, which is a key enzyme in lipid metabolism [104,105], and as antimicrobial agents [106], (Figure 7B). Research by Saini and colleagues identified bis[3-(3-fluorophenyl) prop-2-ene]disulphide from an initial assessment of several synthetic DADs analogs. Bis[3-(3-fluorophenyl) prop-2-ene]disulfide induces ROS generation, loss in mitochondrial membrane potential, and changes in the expression of Bcl-2/Bax ratio, leading to the induction of apoptosis in human pancreatic cancer MIA PaCa-2 cells [107]. This compound caused G2/M phase cell-cycle arrest, and the upregulation of p-Chk1 and of inactive phosphorylated Cdc25C protein. Similarly, other synthetic sulfide derivatives appear to be anti-proliferative in cancer cells, suggesting some commonality in their anti-proliferative effects. This property appears to correspond with the induction of ROS production in cancer cells [108,109] and the S-thiolation of intracellular proteins [110]. Other pronounced biological effects of synthetic sulfide derivatives include neuroprotection by bis[2-(3,4-dimethoxy phenyl) ethenyl] dithioperoxy anhydride and bis[2-(3-methoxy,4-hydroxy phenyl) ethenyl] dithioperoxy anhydride derivatives (Figure 7C,D) [111,112], and cardioprotective properties [113]. In recent times, interest in the development of H2S-releasing therapeutics has increased. As mentioned, compounds like DATS can generate H2S gas, and this property is partly responsible for the biological effects attributed to dietary foods containing this class of molecule [37,114,115,116]. H2S is the third gaseous signaling molecule to be characterized, and it has a range of important biochemical and physiological roles in mammals [35,117]. With this in mind, several research groups have focused efforts on the development of synthesis of H2S-releasing sulfides, particularly polysulfide species [118]. This work afforded the synthesis of tetrasulfide species in high yields with little need for extensive purification. Several bis(aryl) tetrasulfides and bis(alkyl) tetrasulfides, including N-acetylcysteine-derived tetrasulfides, were produced. These approaches provide a convenient route for the production of several H2S-releasing molecules. Similar efforts have led to the production of synthetic benzyl polysulfides (ranging from monosulfide to tetrasulfide derivatives) that can release H2S in the presence of thiols like cysteine and glutathione (Figure 7E,F). Both benzyl trisulfide and benzyl tetrasulfide also suppress cell proliferation in bEnd.3 cells [119].
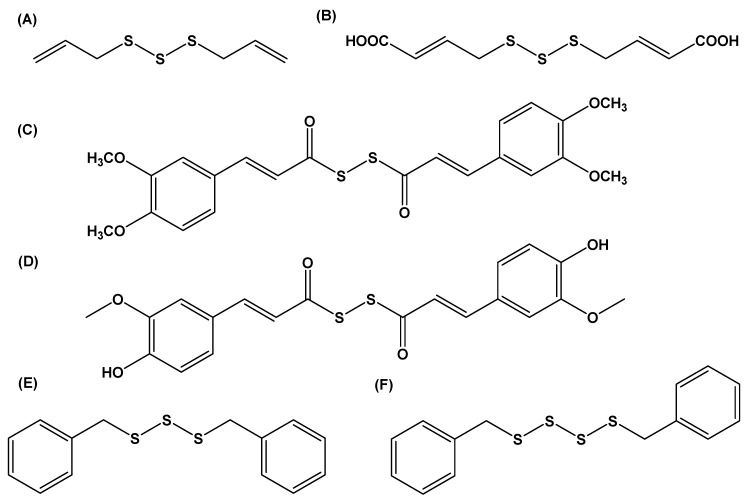
Figure 7.
In recent years, interest in (A) DATS led to the design and synthesis of several novel sulfide derivatives including (B) novel antibacterial agent (2E,2E)-4, 4-trisulfanediylbis(but-2-enoic acid), and DADS derivatives, (C) bis[2-(3,4-dimethoxy phenyl) ethenyl] dithioperoxy anhydride and (D) bis[2-(3-methoxy,4-hydroxy phenyl) ethenyl] dithioperoxy anhydride. These two molecules are templates for developing new multifunctional therapeutics for Alzheimer’s disease treatment [114]. Each compound inhibits Aβ1-42-induced neuronal cell death in human neuroblastoma SH-SY5Y cells. Novel H2S-releasing benzyl polysulfides (E) benzyl trisulfide and (F) benzyl tetrasulfide.
4. Conclusions
Over the years, researchers have been fascinated with the isolation of functional components present in the tissue of allium plants, and this has led to the characterization of a whole spectrum of biologically active sulfur compounds. Less widely reported is the growing body of work focused on the development of novel therapeutic agents based on naturally occurring sulfur species found in alliums. Currently, this drive has focused on the identification and optimization of synthetic routes in the production of derivatives of ajoene, S-allylcysteine, and various sulfide species. This research has led to the production of several new antimicrobial and anticancer agents that are currently evaluated in preclinical cell-culture and animal models. In the future, we anticipate that this field of research will drive developments in the production of newer sulfur therapeutics.
Supplementary Materials
Supplementary materials are available online at https://www.mdpi.com/1420-3049/24/21/4006/s1.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- 1.Block E. The Organosulfur Chemistry of the Genus Allium—Implications for the Organic Chemistry of Sulfur. Angew. Chem. Int. Ed. Engl. 1992;31:1135–1178. doi: 10.1002/anie.199211351. [DOI] [Google Scholar]
- 2.Rose P., Whiteman M., Moore P.K., Zhu Y.Z. Bioactive S-alk(en)yl cysteine sulfoxide metabolites in the genus Allium: The chemistry of potential therapeutic agents. Nat. Prod. Rep. 2005;22:351–368. doi: 10.1039/b417639c. [DOI] [PubMed] [Google Scholar]
- 3.Jones M.G., Hughes J., Tregova A., Milne J., Tomsett A.B., Collin H.A. Biosynthesis of the flavour precursors of onion and garlic. J. Exp. Bot. 2004;55:1903–1918. doi: 10.1093/jxb/erh138. [DOI] [PubMed] [Google Scholar]
- 4.Yoshimoto N., Saito K. S-Alk(en)ylcysteine sulfoxides in the genus Allium: Proposed biosynthesis, chemical conversion, and bioactivities. J. Exp. Bot. 2019;70:4123–4137. doi: 10.1093/jxb/erz243. [DOI] [PubMed] [Google Scholar]
- 5.Jacob C. A scent of therapy: Pharmacological implications of natural products containing redox-active sulfur atoms. Nat. Prod. Rep. 2006;23:851–863. doi: 10.1039/b609523m. [DOI] [PubMed] [Google Scholar]
- 6.Bauer D., Redmon N., Mazzio E., Taka E., Reuben J.S., Day A., Sadrud-Din S., Flores-Rozas H., Soliman K.F., Darling-Reed S. Diallyl disulfide inhibits TNFα induced CCL2 release through MAPK/ERK and NF-Kappa-B signaling. Cytokine. 2015;75:117–126. doi: 10.1016/j.cyto.2014.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lee H.H., Jeong J.W., Hong S.H., Park C., Kim B.W., Choi Y.H. Diallyl Trisulfide Suppresses the Production of Lipopolysaccharide-induced Inflammatory Mediators in BV2 Microglia by Decreasing the NF-κB Pathway Activity Associated With Toll-like Receptor 4 and CXCL12/CXCR4 Pathway Blockade. J. Cancer Prev. 2018;23:134–140. doi: 10.15430/JCP.2018.23.3.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang Y., Sun Z., Chen S., Jiao Y., Bai C. ROS-mediated activation of JNK/p38 contributes partially to the pro-apoptotic effect of ajoene on cells of lung adenocarcinoma. Tumour. Biol. 2016;37:3727–3738. doi: 10.1007/s13277-015-4181-9. [DOI] [PubMed] [Google Scholar]
- 9.Jiang X., Zhu X., Huang W., Xu H., Zhao Z., Li S., Li S., Cai J., Cao J. Garlic-derived organosulfur compound exerts antitumor efficacy via activation of MAPK pathway and modulation of cytokines in SGC-7901 tumor-bearing mice. Int. Immunopharmacol. 2016;48:135–145. doi: 10.1016/j.intimp.2017.05.004. [DOI] [PubMed] [Google Scholar]
- 10.Xiao X., Chen B., Liu X., Liu P., Zheng G., Ye F., Tang H., Xie X. Diallyl disulfide suppresses SRC/Ras/ERK signaling-mediated proliferation and metastasis in human breast cancer by up-regulating miR-34a. PLoS ONE. 2014;9:e112720. doi: 10.1371/journal.pone.0112720. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 11.Yu L., Di W., Dong X., Li Z., Xue X., Zhang J., Wang Q., Xiao X., Han J., Yang Y., et al. Diallyl trisulfide exerts cardioprotection against myocardial ischemia-reperfusion injury in diabetic state, role of AMPK-mediated AKT/GSK-3β/HIF-1α activation. Oncotarget. 2017;8:74791–74805. doi: 10.18632/oncotarget.20422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Munday R., Munday J.S., Munday C.M. Comparative effects of mono-, di-, tri-, and tetrasulfides derived from plants of the Allium family: redox cycling in vitro and hemolytic activity and Phase 2 enzyme induction in vivo. Free Radic. Biol. Med. 2003;34:1200–1211. doi: 10.1016/S0891-5849(03)00144-8. [DOI] [PubMed] [Google Scholar]
- 13.Kay H.Y., Won Yang J., Kim T.H., Lee D.Y., Kang B., Ryu J.H., Jeon R., Kim S.G. Ajoene, a stable garlic by-product, has an antioxidant effect through Nrf2-mediated glutamate-cysteine ligase induction in HepG2 cells and primary hepatocytes. J. Nutr. 2010;140:1211–1219. doi: 10.3945/jn.110.121277. [DOI] [PubMed] [Google Scholar]
- 14.Zhang M., Pan H., Xu Y., Wang X., Qiu Z., Jiang L. Allicin Decreases Lipopolysaccharide-Induced Oxidative Stress and Inflammation in Human Umbilical Vein Endothelial Cells through Suppression of Mitochondrial Dysfunction and Activation of Nrf2. Cell. Physiol. Biochem. 2017;41:2255–2267. doi: 10.1159/000475640. [DOI] [PubMed] [Google Scholar]
- 15.Bottone F.G., Baek S.J., Nixon J.B., Eling T.E. Diallyl disulfide (DADS) induces the antitumorigenic NSAID-activated gene (NAG-1) by a p53-dependent mechanism in human colorectal HCT 116 cells. J. Nutr. 2002;132:773–778. doi: 10.1093/jn/132.4.773. [DOI] [PubMed] [Google Scholar]
- 16.Arora A., Siddiqui I.A., Shukla Y. Modulation of p53 in 7,12-dimethylbenz[a]anthracene-induced skin tumors by diallyl sulfide in Swiss albino mice. Mol. Cancer Ther. 2004;3:1459–1466. [PubMed] [Google Scholar]
- 17.Han C.Y., Ki S.H., Kim Y.W., Noh K., Lee D.Y., Kang B., Ryu J.H., Jeon R., Kim E.H., Hwang S.J., et al. Ajoene, a stable garlic by-product, inhibits high fat diet-induced hepatic steatosis and oxidative injury through LKB1-dependent AMPK activation. Antioxid. Redox Signal. 2011;14:187–202. doi: 10.1089/ars.2010.3190. [DOI] [PubMed] [Google Scholar]
- 18.Lee M.S., Kim I.H., Kim C.T., Kim Y. Reduction of body weight by dietary garlic is associated with an increase in uncoupling protein mRNA expression and activation of AMP-activated protein kinase in diet-induced obese mice. J. Nutr. 2011;141:1947–1953. doi: 10.3945/jn.111.146050. [DOI] [PubMed] [Google Scholar]
- 19.Shi L., Lin Q., Li X., Nie Y., Sun S., Deng X., Wang L., Lu J., Tang Y., Luo F. Alliin, a garlic organosulfur compound, ameliorates gut inflammation through MAPK-NF-κB/AP-1/STAT-1 inactivation and PPAR-γ activation. Mol. Nutr. Food Res. 2017;61 doi: 10.1002/mnfr.201601013. [DOI] [PubMed] [Google Scholar]
- 20.Kim H.K. Protective Effect of Garlic on Cellular Senescence in UVB-Exposed HaCaT Human Keratinocytes. Nutrients. 2016;8:464. doi: 10.3390/nu8080464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu Y., Zhao Y., Wang Y., Zhu P., Wei Z., Wang S., Tao L., Liu Z., Wu H., Sheng X., et al. Suppressive role of diallyl trisulfide in the activated platelet-mediated hematogenous metastasis of MDA-MB-231 human breast cancer cells. Int. J. Mol. Med. 2017;39:1516–1524. doi: 10.3892/ijmm.2017.2953. [DOI] [PubMed] [Google Scholar]
- 22.Wang K., Wang Y., Qi Q., Zhang F., Zhang Y., Zhu X., Liu G., Luan Y., Zhao Z., Cai J., et al. Inhibitory effects of S-allylmercaptocysteine against benzo(a)pyrene-induced precancerous carcinogenesis in human lung cells. Int. Immunopharmacol. 2016;34:37–43. doi: 10.1016/j.intimp.2016.02.017. [DOI] [PubMed] [Google Scholar]
- 23.Li S., Yang G., Zhu X., Cheng L., Sun Y., Zhao Z. Combination of rapamycin and garlic-derived S-allylmercaptocysteine induces colon cancer cell apoptosis and suppresses tumor growth in xenograft nude mice through autophagy/p62/Nrf2 pathway. Oncol. Rep. 2017;38:1637–1644. doi: 10.3892/or.2017.5849. [DOI] [PubMed] [Google Scholar]
- 24.Arulselvan P., Wen C.C., Lan C.W., Chen Y.H., Wei W.C., Yang N.S. Dietary administration of scallion extract effectively inhibits colorectal tumor growth: cellular and molecular mechanisms in mice. PLoS ONE. 2012;7:e44658. doi: 10.1371/journal.pone.0044658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Keophiphath M., Priem F., Jacquemond-Collet I., Clément K., Lacasa D. 1,2-Vinyldithiin from garlic inhibits differentiation and inflammation of human preadipocytes. J. Nutr. 2009;139:2055–2060. doi: 10.3945/jn.109.105452. [DOI] [PubMed] [Google Scholar]
- 26.Yang G., Li S., Li B., Cheng L., Jiang P., Tian Z., Sun S. Protective Effects of Garlic-Derived S-Allylmercaptocysteine on IL-1β-Stimulated Chondrocytes by Regulation of MMPs/TIMP-1 Ratio and Type II Collagen Expression via Suppression of NF-κB Pathway. Biomed. Res. Int. 2017:8686207. doi: 10.1155/2017/8686207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lee H.H., Han M.H., Hwang H.J., Kim G.Y., Moon S.K., Hyun J.W., Kim W.J., Choi Y.H. Diallyl trisulfide exerts anti-inflammatory effects in lipopolysaccharide-stimulated RAW 264.7 macrophages by suppressing the Toll-like receptor 4/nuclear factor-κB pathway. Int. J. Mol. Med. 2015;35:487–495. doi: 10.3892/ijmm.2014.2036. [DOI] [PubMed] [Google Scholar]
- 28.Chen C., Pung D., Leong V., Hebbar V., Shen G., Nair S., Li W., Kong A.N. Induction of detoxifying enzymes by garlic organosulfur compounds through transcription factor Nrf2: Effect of chemical structure and stress signals. Free Radic. Biol. Med. 2004;37:1578–1590. doi: 10.1016/j.freeradbiomed.2004.07.021. [DOI] [PubMed] [Google Scholar]
- 29.Miltonprabu S., Sumedha N.C., Senthilraja P. Diallyl trisulfide, a garlic polysulfide protects against As-induced renal oxidative nephrotoxicity, apoptosis and inflammation in rats by activating the Nrf2/ARE signaling pathway. Int. Immunopharmacol. 2017;50:107–120. doi: 10.1016/j.intimp.2017.06.011. [DOI] [PubMed] [Google Scholar]
- 30.Howard E.W., Ling M.T., Chua C.W., Cheung H.W., Wang X., Wong Y.C. Garlic-derived S-allylmercaptocysteine is a novel in vivo antimetastatic agent for androgen-independent prostate cancer. Clin. Cancer Res. 2007;13:1847–1856. doi: 10.1158/1078-0432.CCR-06-2074. [DOI] [PubMed] [Google Scholar]
- 31.Lui Y., Zhu P., Wang Y., Wei Z., Tao L., Zhu Z., Sheng X., Wang S., Ruan J., Liu Z., et al. Antimetastatic Therapies of the Polysulfide Diallyl Trisulfide against Triple-Negative Breast Cancer (TNBC) via Suppressing MMP2/9 by Blocking NF-κB and ERK/MAPK Signaling Pathways. PLoS ONE. 2015;10:e0123781. doi: 10.1371/journal.pone.0123781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ragavan G., Muralidaran Y., Sridharan B., Nachiappa Ganesh R., Viswanathan P. Evaluation of garlic oil in nano-emulsified form: Optimization and its efficacy in high-fat diet induced dyslipidemia in Wistar rats. Food Chem. Toxicol. 2017;105:203–213. doi: 10.1016/j.fct.2017.04.019. [DOI] [PubMed] [Google Scholar]
- 33.González-Peña D., Checa A., de Ancos B., Wheelock C.E., Sánchez-Moreno C. New insights into the effects of onion consumption on lipid mediators using a diet-induced model of hypercholesterolemia. Redox Biol. 2017;11:205–212. doi: 10.1016/j.redox.2016.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Benavides G.A., Squadrito G.L., Mills R.W., Patel H.D., Isbell T.S., Patel R.P., Darley-Usmar V.M., Doeller J.E., Kraus D.W. Hydrogen sulfide mediates the vasoactivity of garlic. Proc. Natl. Acad. Sci. USA. 2007;104:17977–17982. doi: 10.1073/pnas.0705710104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wallace J.L., Wang R. Hydrogen sulfide-based therapeutics: Exploiting a unique but ubiquitous gasotransmitter. Nat. Rev. Drug Discov. 2015;14:329–345. doi: 10.1038/nrd4433. [DOI] [PubMed] [Google Scholar]
- 36.Wallace J.L., Nagy P., Feener T.D., Allain T., Ditrói T., Vaughan D.J., Muscara M.N., de Nucci G., Buret A.G. A proof-of-concept, Phase 2 clinical trial of the gastrointestinal safety of a hydrogen sulfide-releasing anti-inflammatory drug. Br. J. Pharmacol. 2019 doi: 10.1111/bph.14641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rose P., Moore P.K., Zhu Y.Z. Garlic and Gaseous Mediators. Trends Pharmacol. Sci. 2018;39:624–634. doi: 10.1016/j.tips.2018.03.009. [DOI] [PubMed] [Google Scholar]
- 38.Kahn G. History of garlic. In: Koch H.P., Lawson L.D., editors. Garlic: The Science and Therapeutic Application of Allium sativum L and Related Species. Williams & Wilkins; New York, NY, USA: 1996. pp. 25–36. [Google Scholar]
- 39.Moyers S. Garlic in Health, History and World Cuisine. Suncoast Press; St Petersburg, FL, USA: 1996. p. 1036. [Google Scholar]
- 40.Petrovska B.B., Cekovska S. Extracts from the history and medical properties of garlic. Pharmacogn. Rev. 2010;4:106–110. doi: 10.4103/0973-7847.65321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nicastro H.L., Ross S.A., Milner J.A. Garlic and onions: Their cancer prevention properties. Cancer Prev. Res. 2015;8:181–189. doi: 10.1158/1940-6207.CAPR-14-0172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Van Poppel G., Verhoeven D.T., Verhagen H., Goldbohm R.A. Brassica vegetables and cancer prevention. Epidemiology and mechanisms. Adv. Exp. Med. Biol. 1999;472:159–168. doi: 10.1007/978-1-4757-3230-6_14. [DOI] [PubMed] [Google Scholar]
- 43.Bahadoran Z., Mirmiran P., Momenan A.A., Azizi F. Allium vegetable intakes and the incidence of cardiovascular disease, hypertension, chronic kidney disease, and type 2 diabetes in adults: a longitudinal follow-up study. J. Hypertens. 2017;35:1909–1916. doi: 10.1097/HJH.0000000000001356. [DOI] [PubMed] [Google Scholar]
- 44.Xiong X.J., Wang P.Q., Li S.J., Li X.K., Zhang Y.Q., Wang J. Garlic for hypertension: A systematic review and meta-analysis of randomized controlled trials. Phytomedicine. 2016;22:352–361. doi: 10.1016/j.phymed.2014.12.013. [DOI] [PubMed] [Google Scholar]
- 45.Blekkenhorst L.C., Bondonno C.P., Lewis J.R., Devine A., Zhu K., Lim W.H., Woodman R.J., Beilin L.J., Prince R.L., Hodgson J.M. Cruciferous and Allium Vegetable Intakes are Inversely Associated With 15-Year Atherosclerotic Vascular Disease Deaths in Older Adult Women. J. Am. Heart Assoc. 2016;6:e006558. doi: 10.1161/JAHA.117.006558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ried K., Travica N., Sali A. The Effect of Kyolic Aged Garlic Extract on Gut Microbiota, Inflammation, and Cardiovascular Markers in Hypertensives: The GarGIC Trial. Front. Nutr. 2018;11:122. doi: 10.3389/fnut.2018.00122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Feng M., Tang B., Liang S.H., Jiang X. Sulfur Containing Scaffolds in Drugs: Synthesis and Application in Medicinal Chemistry. Curr. Top. Med. Chem. 2016;16:1200–1216. doi: 10.2174/1568026615666150915111741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ovesná J., Mitrová K., Kučera L. Garlic (A. sativum L.) alliinase gene family polymorphism reflects bolting types and cysteine sulphoxides content. BMC Genet. 2015;16 doi: 10.1186/s12863-015-0214-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Liakopoulou-Kyriakides M., Sinakos Z., Kyriakidis D.A. Identification of alliin, a constituent of Allium cepa with an inhibitory effect on platelet aggregation. Phytochemistry. 1985;24:600–601. doi: 10.1016/S0031-9422(00)80777-3. [DOI] [Google Scholar]
- 50.Liakopoulou-Kyriakides M. Relation between the structure of alliin analogues and their inhibitory effect on platelet aggregation. Phytochemistry. 1985;24:1593–1594. doi: 10.1016/S0031-9422(00)81070-5. [DOI] [Google Scholar]
- 51.Müller A., Eller J., Albrecht F., Prochnow P., Kuhlmann K., Bandow J.E., Slusarenko A.J., Leichert L.I. Allicin Induces Thiol Stress in Bacteria through S-Allylmercapto Modification of Protein Cysteines. J. Biol. Chem. 2016;291:11477–114790. doi: 10.1074/jbc.M115.702308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hunter R., Caira M., Stellenboom N. Thiolsulfinate allicin from garlic: Inspiration for a new antimicrobial agent. Ann. N. Y. Acad. Sci. 2005;1056:234–241. doi: 10.1196/annals.1352.011. [DOI] [PubMed] [Google Scholar]
- 53.Brodnitz M.H., Pascale J.V., Van Derslice L. Flavor components of garlic extract. J. Agric. Food Chem. 1971;192:273–275. doi: 10.1021/jf60174a007. [DOI] [Google Scholar]
- 54.Lawson L.D., Hughes B.G. Characterization of the formation of allicin and other thiosulfinates from garlic. Planta Med. 1992;58:345–350. doi: 10.1055/s-2006-961482. [DOI] [PubMed] [Google Scholar]
- 55.Lawson L.D., Gardner C.D. Composition, stability, and bioavailability of garlic products used in a clinical trial. J. Agric. Food Chem. 2005;53:6254–6261. doi: 10.1021/jf050536+. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Brace N.O. Oxidation chemistry of perfluoroalkyl-segmented thiols, disulfides, thiosulfinates, and thiosulfonates. The role of the perfluoroalkyl group in searchingout new chemistry. J. Fluorine Chem. 2000;105:11–23. doi: 10.1016/S0022-1139(00)00261-X. [DOI] [Google Scholar]
- 57.Stellenboom N., Hunter R., Caira M.R., Bourne S.A., Cele K., Qwebani T., le Roex T. Synthesis and inclusion of S-aryl alkylthiosulfinates as stable allicin mimics. ARKIVOC. 2007;9:53–63. [Google Scholar]
- 58.Anufrieva N.V., Morozova E.A., Kulikova V.V., Bazhulina N.P., Manukhov I.V., Degtev D.I., Gnuchikh E.Y., Rodionov A.N., Zavilgelsky G.B., Demidkina T.V. Sulfoxides, Analogues of L-Methionine and L-Cysteine As Pro-Drugs against Gram-Positive and Gram-Negative Bacteria. Acta Nat. 2015;7:128–135. doi: 10.32607/20758251-2015-7-4-128-135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Leontiev R., Hohaus N., Jacob C., Gruhlke M.C.H., Slusarenko A.J. A comparison of the antibacterial and antifungal activities of thiosulfinate analogues of allicin. Sci. Rep. 2018;30:6763. doi: 10.1038/s41598-018-25154-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Roseblade A., Ung A., Bebawy M. Synthesis and in vitro biological evaluation of thiosulfinate derivatives for the treatment of human multidrug-resistant breast cancer. Acta Pharmacol. Sin. 2017;38:1353–1368. doi: 10.1038/aps.2016.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Block E., Bechand B., Gundala S., Vattekkatte A., Wang K., Mousa S.S., Godugu K., Yalcin M., Mousa S.A. Fluorinated Analogs of Organosulfur Compounds from Garlic (Allium sativum): Synthesis, Chemistry and Anti-Angiogenesis and Antithrombotic Studies. Molecules. 2017;22:2081. doi: 10.3390/molecules22122081. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 62.Yoo M., Lee S., Kim S., Shin D. Optimizing conditions for E-and Z-ajoene formation from garlic juice using response surface methodology. Food Sci. Nutr. 2014;2:605–611. doi: 10.1002/fsn3.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kaschula C.H., Hunter R., Parker M.I. Garlic-derived anticancer agents: Structure and biological activity of ajoene. Biofactors. 2010;36:78–85. doi: 10.1002/biof.76. [DOI] [PubMed] [Google Scholar]
- 64.Gallwitz H., Bonse S., Martinez-Cruz A., Schlichting I., Schumacher K., Krauth-Siegel R.L. Ajoene is an inhibitor and subversive substrate of human glutathione reductase and Trypanosoma cruzi trypanothione reductase: Crystallographic, kinetic, and spectroscopic studies. J. Med. Chem. 1999;42:364–372. doi: 10.1021/jm980471k. [DOI] [PubMed] [Google Scholar]
- 65.Gargouri Y., Moreau H., Jain M.K., de Haas G.H., Verger R. Ajoene prevents fat digestion by human gastric lipase in vitro. Biochim. Biophys. Acta. 1989;1006:137–139. doi: 10.1016/0005-2760(89)90335-4. [DOI] [PubMed] [Google Scholar]
- 66.Naganawa R., Iwata N., Ishikawa K., Fukuda H., Fujino T., Suzuki A. Inhibition of microbial growth by ajoene, a sulfur-containing compound derived from garlic. Appl. Environ. Microbiol. 1996;62:4238–4242. doi: 10.1128/aem.62.11.4238-4242.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Agarwal K.C. Therapeutic actions of garlic constituents. Med. Res. Rev. 1996;16:111–124. doi: 10.1002/(SICI)1098-1128(199601)16:1<111::AID-MED4>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 68.Jakobsen T.H., van Gennip M., Phipps R.K., Shanmugham M.S., Christensen L.D., Alhede M., Skindersoe M.E., Rasmussen T.B., Friedrich K., Uthe F., et al. Ajoene, a sulfur-rich molecule from garlic, inhibits genes controlled by quorum sensing. Antimicrob. Agents Chemother. 2012;56:2314–2325. doi: 10.1128/AAC.05919-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Fong J., Yuan M., Jakobsen T.H., Mortensen K.T., Delos Santos M.M., Chua S.L., Yang L., Tan C.H., Nielsen T.E., Givskov M. Disulfide Bond-Containing Ajoene Analogues As Novel Quorum Sensing Inhibitors of Pseudomonas aeruginosa. J. Med. Chem. 2017;60:215–222. doi: 10.1021/acs.jmedchem.6b01025. [DOI] [PubMed] [Google Scholar]
- 70.Hunter R., Kaschula C.H., Parker I.M., Caira M.R., Richards P., Travis S., Taute F., Qwebani T. Substituted ajoenes as novel anti-cancer agents. Bioorg. Med. Chem. Lett. 2008;18:5277–5279. doi: 10.1016/j.bmcl.2008.08.056. [DOI] [PubMed] [Google Scholar]
- 71.Kaschula C.H., Hunter R., Hassan H.T., Stellenboom N., Cotton J., Zhai X.Q., Parker M.I. Anti-proliferation activity of synthetic ajoene analogues on cancer cell-lines. Anticancer Agents Med. Chem. 2011;11:260–266. doi: 10.2174/187152011795347450. [DOI] [PubMed] [Google Scholar]
- 72.Kaschula C.H., Hunter R., Stellenboom N., Caira M.R., Winks S., Ogunleye T., Richards P., Cotton J., Zilbeyaz K., Wang Y., et al. Structure-activity studies on the anti-proliferation activity of ajoene analogues in WHCO1 oesophageal cancer cells. Eur. J. Med. Chem. 2012;50:236–254. doi: 10.1016/j.ejmech.2012.01.058. [DOI] [PubMed] [Google Scholar]
- 73.Siyo V., Schäfer G., Hunter R., Grafov A., Grafova I., Nieger M., Katz A.A., Parker M.I., Kaschula C.H. The cytotoxicity of the ajoene analogue BisPMB in WHCO1 oesophageal cancer cells is mediated by CHOP/GADD153. Molecules. 2017;22:892. doi: 10.3390/molecules22060892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hwang J.W., Cho H., Lee J.Y., Jeon Y., Kim S.N., Lee S.J., Bae G.U., Yoon S., Jeon R., Kim Y.K. The synthetic ajoene analog SPA3015 induces apoptotic cell death through crosstalk between NF-κB and PPARγ in multidrug-resistant cancer cells. Food Chem. Toxicol. 2016;96:35–42. doi: 10.1016/j.fct.2016.07.020. [DOI] [PubMed] [Google Scholar]
- 75.Kaschula C.H., Hunter R., Cotton J., Tuveri R., Ngarande E., Dzobo K., Schäfer G., Siyo V., Lang D., Kusza D.A., et al. The garlic compound ajoene targets protein folding in the endoplasmic reticulum of cancer cells. Mol. Carcinog. 2016;55:1213–1228. doi: 10.1002/mc.22364. [DOI] [PubMed] [Google Scholar]
- 76.Kaschula C.H., Tuveri R., Ngarande E., Dzobo K., Barnett C., Kusza D.A., Graham L.M., Katz A.A., Rafudeen M.S., Parker M.I., et al. The garlic compound ajoene covalently binds vimentin, disrupts the vimentin network and exerts anti-metastatic activity in cancer cells. BMC Cancer. 2019;19:1248. doi: 10.1186/s12885-019-5388-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Pinto J.T., Qiao C., Xing J., Rivlin R.S., Protomastro M.L., Weissler M.L., Tao Y., Thaler H., Heston W.D. Effects of garlic thioallyl derivatives on growth, glutathione concentration, and polyamine formation of human prostate carcinoma cells in culture. Am. J. Clin. Nutr. 1997;66:398–405. doi: 10.1093/ajcn/66.2.398. [DOI] [PubMed] [Google Scholar]
- 78.Wang Q., Liu H.R., Mu Q., Rose P., Zhu Y.Z. S-propargyl-cysteine protects both adult rat hearts and neonatal cardiomyocytes from ischemia/hypoxia injury: the contribution of the hydrogen sulfide-mediated pathway. J. Cardiovasc. Pharmacol. 2009;54:139–146. doi: 10.1097/FJC.0b013e3181ac8e12. [DOI] [PubMed] [Google Scholar]
- 79.Wang Q., Wang X.L., Liu H.R., Rose P., Zhu Y.Z. Protective effects of cysteine analogues on acute myocardial ischemia: novel modulators of endogenous H(2)S production. Antioxid. Redox Signal. 2010;12:1155–1165. doi: 10.1089/ars.2009.2947. [DOI] [PubMed] [Google Scholar]
- 80.Liu C., Gu X., Zhu Y.Z. Synthesis and biological evaluation of novel leonurine-SPRC conjugate as cardioprotective agents. Bioorg. Med. Chem. Lett. 2010;20:6942–6946. doi: 10.1016/j.bmcl.2010.09.135. [DOI] [PubMed] [Google Scholar]
- 81.Gong Q.H., Wang Q., Pan L.L., Liu X.H., Xin H., Zhu Y.Z. S-propargyl-cysteine, a novel hydrogen sulfide-modulated agent, attenuates lipopolysaccharide-induced spatial learning and memory impairment: involvement of TNF signaling and NF-κB pathway in rats. Brain Behav. Immun. 2011;25:110–119. doi: 10.1016/j.bbi.2010.09.001. [DOI] [PubMed] [Google Scholar]
- 82.Gong Q.H., Pan L.L., Liu X.H., Wang Q., Huang H., Zhu Y.Z. S-propargyl-cysteine (ZYZ-802), a sulphur-containing amino acid, attenuates beta-amyloid-induced cognitive deficits and pro-inflammatory response: Involvement of ERK1/2 and NF-κB pathway in rats. Amino Acids. 2011;40:601–610. doi: 10.1007/s00726-010-0685-1. [DOI] [PubMed] [Google Scholar]
- 83.Ma K., Liu Y., Zhu Q., Liu C.H., Duan J.L., Tan B.K., Zhu Y.Z. H2S donor, S-propargyl-cysteine, increases CSE in SGC-7901 and cancer-induced mice: evidence for a novel anti-cancer effect of endogenous H2S? PLoS ONE. 2011;6:e20525. doi: 10.1371/journal.pone.0020525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Wang W., Cheng J., Zhu Y. The JNK Signaling Pathway Is a Novel Molecular Target for S-Propargyl-L-Cysteine, a Naturally-Occurring Garlic Derivatives: Link to Its Anticancer Activity in Pancreatic Cancer In Vitro and In Vivo. Curr. Cancer Drug Targets. 2015;15:613–623. doi: 10.2174/1568009615666150602143943. [DOI] [PubMed] [Google Scholar]
- 85.Pan L.L., Liu X.H., Gong Q.H., Zhu Y.Z. S-Propargyl-cysteine (SPRC) attenuated lipopolysaccharide-induced inflammatory response in H9c2 cells involved in a hydrogen sulfide-dependent mechanism. Amino Acids. 2011;41:205–215. doi: 10.1007/s00726-011-0834-1. [DOI] [PubMed] [Google Scholar]
- 86.Huang C., Kan J., Liu X., Ma F., Tran B.H., Zou Y., Wang S., Zhu Y.Z. Cardioprotective effects of a novel hydrogen sulfide agent-controlled release formulation of S-propargyl-cysteine on heart failure rats and molecular mechanisms. PLoS ONE. 2013;8:e69205. doi: 10.1371/journal.pone.0069205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Tran B.H., Huang C., Zhang Q., Liu X., Lin S., Liu H., Wang S., Zhu Y.Z. Cardioprotective effects and pharmacokinetic properties of a controlled release formulation of a novel hydrogen sulfide donor in rats with acute myocardial infarction. Biosci. Rep. 2015;35:e00216. doi: 10.1042/BSR20140185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Wu J., Guo W., Lin S.Z., Wang Z.J., Kan J.T., Chen S.Y., Zhu Y.Z. Gp130-mediated STAT3 activation by S-propargyl-cysteine, an endogenous hydrogen sulfide initiator, prevents doxorubicin-induced cardiotoxicity. Cell Death Dis. 2016;7:e2339. doi: 10.1038/cddis.2016.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Liang Y.H., Shen Y.Q., Guo W., Zhu Y.Z. SPRC protects hypoxia and re-oxygenation injury by improving rat cardiac contractile function and intracellular calcium handling. Nitric Oxide. 2014;41:113–119. doi: 10.1016/j.niox.2014.05.010. [DOI] [PubMed] [Google Scholar]
- 90.Yang H., Mao Y., Tan B., Luo S., Zhu Y. The protective effects of endogenous hydrogen sulfide modulator, S-propargyl-cysteine, on high glucose-induced apoptosis in cardiomyocytes: A novel mechanism mediated by the activation of Nrf2. Eur. J. Pharmacol. 2015;761:135–143. doi: 10.1016/j.ejphar.2015.05.001. [DOI] [PubMed] [Google Scholar]
- 91.Kan J., Guo W., Huang C., Bao G., Zhu Y., Zhu Y.Z. S-propargyl-cysteine, a novel water-soluble modulator of endogenous hydrogen sulfide, promotes angiogenesis through activation of signal transducer and activator of transcription 3. Antioxid. Redox Signal. 2014;20:2303–2316. doi: 10.1089/ars.2013.5449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Sidhapuriwala J.N., Hegde A., Ang A.D., Zhu Y.Z. Bhatia M. Effects of S-propargyl-cysteine (SPRC) in caerulein-induced acute pancreatitis in mice. PLoS ONE. 2012;7:e32574. doi: 10.1371/journal.pone.0032574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Wang M., Tang W., Xin H., Zhu Y.Z. S-Propargyl-Cysteine, a Novel Hydrogen Sulfide Donor, Inhibits Inflammatory Hepcidin and Relieves Anemia of Inflammation by Inhibiting IL-6/STAT3 Pathway. PLoS ONE. 2016;11:e0163289. doi: 10.1371/journal.pone.0163289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Wu W.J., Jia W.W., Liu X.H., Pan L.L., Zhang Q.Y., Yang D., Shen X.Y., Liu L., Zhu Y.Z. S-propargyl-cysteine attenuates inflammatory response in rheumatoid arthritis by modulating the Nrf2-ARE signaling pathway. Redox Biol. 2016;10:157–167. doi: 10.1016/j.redox.2016.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Wu D., Hu Q., Tan B., Rose P., Zhu D., Zhu Y.Z. Amelioration of mitochondrial dysfunction in heart failure through S-sulfhydration of Ca2+/calmodulin-dependent protein kinase II. Redox Biol. 2018;19:250–262. doi: 10.1016/j.redox.2018.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Wu D., Hu Q., Ma F., Zhu Y.Z. Vasorelaxant Effect of a New Hydrogen Sulfide-Nitric Oxide Conjugated Donor in Isolated Rat Aortic Rings through cGMP Pathway. Oxid. Med. Cell Longev. 2016:7075682. doi: 10.1155/2016/7075682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Hu Q., Wu D., Ma F., Yang S., Tan B., Xin H., Gu X., Chen X., Chen S., Mao Y., et al. Novel Angiogenic Activity and Molecular Mechanisms of ZYZ-803, a Slow-Releasing Hydrogen Sulfide-Nitric Oxide Hybrid Molecule. Antioxid. Redox Signal. 2016;25:498–514. doi: 10.1089/ars.2015.6607. [DOI] [PubMed] [Google Scholar]
- 98.Xiong Y., Chang L.L., Tran B., Dai T., Zhong R., Mao Y.C., Zhu Y.Z. ZYZ-803, a novel hydrogen sulfide-nitric oxide conjugated donor, promotes angiogenesis via cross-talk between STAT3 and CaMKII. Acta Pharmacol. Sin. 2019 doi: 10.1038/s41401-019-0255-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Wu D., Hu Q., Xiong Y., Zhu D., Mao Y., Zhu Y.Z. Novel H2S-NO hybrid molecule (ZYZ-803) promoted synergistic effects against heart failure. Redox Biol. 2018;15:243–252. doi: 10.1016/j.redox.2017.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Chang L., Wang Z., Ma F., Tran B., Zhong R., Xiong Y., Dai T., Wu J., Xin X., Guo W., et al. ZYZ-803 mitigates endoplasmic reticulum stress-related necroptosis after acute myocardial infarction through downregulating the RIP3-CaMKII signaling pathway. Oxid. Med. Cell. Longev. 2019:6173685. doi: 10.1155/2019/6173685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Dziri S., Casabianca H., Hanchi B., Hosni K. Composition of garlic essential oil (Allium sativum L.) as influenced by drying method. J. Essent. Oil Res. 2014;26:91–96. doi: 10.1080/10412905.2013.868329. [DOI] [Google Scholar]
- 102.Satyal P., Craft J.D., Dosoky N.S., Setzer W.N. The Chemical Compositions of the Volatile Oils of Garlic (Allium sativum) and Wild Garlic (Allium vineale) Foods. 2017;6:63. doi: 10.3390/foods6080063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Rai S.K., Sharma M., Tiwari M. Synthesis, DNA binding, and cytotoxic evaluation of new analogs of diallyldisulfide, an active principle of garlic. Bioorg. Med. Chem. 2008;16:7302–7310. doi: 10.1016/j.bmc.2008.06.029. [DOI] [PubMed] [Google Scholar]
- 104.Rai S.K., Sharma M., Tiwari M. Inhibitory effect of novel diallyldisulfide analogs on HMG-CoA reductase expression in hypercholesterolemic rats: CREB as a potential upstream target. Life Sci. 2009;85:211–219. doi: 10.1016/j.lfs.2009.05.020. [DOI] [PubMed] [Google Scholar]
- 105.Sharma M., Tiwari M., Chandra R. Bis[3-(4’-substituted phenyl)prop-2-ene]disulfides as a new class of antihyperlipidemic compounds. Bioorg. Med. Chem. Lett. 2004;14:5347–5350. doi: 10.1016/j.bmcl.2004.08.015. [DOI] [PubMed] [Google Scholar]
- 106.Wu T., Huang Y., Chen Y., Zhang M. Antibacterial Effect of (2E,2E)-4,4-Trisulfanediylbis(but-2-enoic acid) against Staphylococcus aureus. PLoS ONE. 2018;13:e0197348. doi: 10.1371/journal.pone.0197348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Saini V., Manral A., Arora R., Meena P., Gusain S., Saluja D., Tiwari M. Novel synthetic analogs of diallyl disulfide triggers cell cycle arrest and apoptosis via ROS generation in MIA PaCa-2 cells. Pharmacol. Rep. 2017;69:813–821. doi: 10.1016/j.pharep.2017.03.006. [DOI] [PubMed] [Google Scholar]
- 108.Bhattacherjee D., Basu C., Bhardwaj Q., Mal S., Sahu S., Sur R., Bhabak K.P. Design, Synthesis and Anti-Cancer Activities of Benzyl Analogues of Garlic-Derived Diallyl Disulfide (DADS) and the Corresponding Diselenides. Chem. Select. 2017;2:7399–7406. doi: 10.1002/slct.201700499. [DOI] [Google Scholar]
- 109.Xiaoyan H.E. Effect of the Molecular Properties of Diallyl Trisulfide Analogues on ROS Production. Lat. Am. J. Pharm. 2015;34:1766–1771. [Google Scholar]
- 110.Smith M., Hunter R., Stellenboom N., Kusza D.A., Parker M.I., Hammouda A.N., Jackson G., Kaschula C.H. The cytotoxicity of garlic-related disulphides and thiosulfonates in WHCO1 oesophageal cancer cells is dependent on S-thiolation and not production of ROS. Biochim. Biophys. Acta. 2016;1860:1439–1449. doi: 10.1016/j.bbagen.2016.03.032. [DOI] [PubMed] [Google Scholar]
- 111.Manral A., Saini V., Meena P., Tiwari M. Multifunctional novel Diallyl disulfide (DADS) derivatives with β-amyloid-reducing, cholinergic, antioxidant and metal chelating properties for the treatment of Alzheimer’s disease. Bioorg. Med. Chem. 2015;23:6389–6403. doi: 10.1016/j.bmc.2015.08.024. [DOI] [PubMed] [Google Scholar]
- 112.Manral A., Meena P., Saini V., Siraj F., Shalini S., Tiwari M. DADS Analogues Ameliorated the Cognitive Impairments of Alzheimer-Like Rat Model Induced by Scopolamine. Neurotox Res. 2016;30:407–426. doi: 10.1007/s12640-016-9625-5. [DOI] [PubMed] [Google Scholar]
- 113.Sharma D.K., Manral A., Saini V., Singh A., Srinivasan B.P., Tiwari M. Novel diallyldisulfide analogs ameliorate cardiovascular remodeling in rats with L-NAME-induced hypertension. Eur. J. Pharmacol. 2012;691:198–208. doi: 10.1016/j.ejphar.2012.07.022. [DOI] [PubMed] [Google Scholar]
- 114.Liang D., Wu H., Wong M.W., Huang D. Diallyl Trisulfide Is a Fast H2S Donor, but Diallyl Disulfide Is a Slow One: The Reaction Pathways and Intermediates of Glutathione with Polysulfides. Org. Lett. 2015;17:4196–4199. doi: 10.1021/acs.orglett.5b01962. [DOI] [PubMed] [Google Scholar]
- 115.Tocmo R., Wu Y., Liang D., Fogliano V., Huang D. Boiling enriches the linear polysulfides and the hydrogen sulfide-releasing activity of garlic. Food Chem. 2017;221:1867–1873. doi: 10.1016/j.foodchem.2016.10.076. [DOI] [PubMed] [Google Scholar]
- 116.Tocmo R., Liang D., Lin Y., Huang D. Chemical and biochemical mechanisms underlying the cardioprotective roles of dietary organopolysulfides. Front Nutr. 2015;2:1. doi: 10.3389/fnut.2015.00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Rose P., Moore P.K., Zhu Y.Z. H2S biosynthesis and catabolism: new insights from molecular studies. Cell. Mol. Life. Sci. 2017;74:1391–1412. doi: 10.1007/s00018-016-2406-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Cerda M.M., Hammers M.D., Earp M.S., Zakharov L.N., Pluth M.D. Applications of Synthetic Organic Tetrasulfides as H2S Donors. Org. Lett. 2017;19:2314–2317. doi: 10.1021/acs.orglett.7b00858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Bolton S.G., Cerda M.M., Gilbert A.K., Pluth M.D. Effects of sulfane sulfur content in benzyl polysulfides on thiol-triggered H2S release and cell proliferation. Free Radic. Biol. Med. 2019;131:393–398. doi: 10.1016/j.freeradbiomed.2018.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.