Abstract
Tonic Activation of GluN2C/GluN2D-Containing NMDA Receptors by Ambient Glutamate Facilitates Cortical Interneuron Maturation
Hanson E, Armbruster M, Lau LA, Sommer ME, Klaft ZJ, Swanger SA, Traynelis SF, Moss SJ, Noubary F, Chadchankar J, Dulla CG. J Neurosci. 2019;39(19):3611-3626. doi:10.1523/JNEUROSCI.1392-18.2019. PMID: 30846615. Epub Mar 7, 2019.
Developing cortical GABAergic interneurons rely on genetic programs, neuronal activity, and environmental cues to construct inhibitory circuits during early postnatal development. Disruption of these events can cause long-term changes in cortical inhibition and may be involved in neurological disorders associated with inhibitory circuit dysfunction. We hypothesized that tonic glutamate signaling in the neonatal cortex contributes to, and is necessary for, the maturation of cortical interneurons. To test this hypothesis, we used mice of both sexes to quantify extracellular glutamate concentrations in the cortex during development, measure ambient glutamate-mediated activation of developing cortical interneurons, and manipulate tonic glutamate signaling using subtype-specific N-methyl-d-aspartic acid (NMDA) receptor antagonists in vitro and in vivo. We report that ambient glutamate levels are high (≈100 nm) in the neonatal cortex and decrease (to ≈50 nm) during the first weeks of life, coincident with increases in astrocytic glutamate uptake. Consistent with elevated ambient glutamate, putative parvalbumin-positive interneurons in the cortex (identified using G42: GAD1-eGFP reporter mice) exhibit a transient, tonic NMDA current at the end of the first postnatal week. GluN2C/GluN2D-containing NMDA receptors mediate the majority of this current and contribute to the resting membrane potential and intrinsic properties of developing putative parvalbumin interneurons. Pharmacological blockade of GluN2C/GluN2D-containing NMDA receptors in vivo during the period of tonic interneuron activation, but not later, leads to lasting decreases in interneuron morphological complexity and causes deficits in cortical inhibition later in life. These results demonstrate that dynamic ambient glutamate signaling contributes to cortical interneuron maturation via tonic activation of GluN2C/GluN2D-containing NMDA receptors. Significance statement: Inhibitory GABAergic interneurons make up 20% of cortical neurons and are critical to controlling cortical network activity. Dysfunction of cortical inhibition is associated with multiple neurological disorders, including epilepsy. Establishing inhibitory cortical networks requires in utero proliferation, differentiation, and migration of immature GABAergic interneurons and subsequent postnatal morphological maturation and circuit integration. Here, we demonstrate that ambient glutamate provides tonic activation of immature, putative parvalbumin-positive GABAergic interneurons in the neonatal cortex via high-affinity NMDA receptors. When this activation is blocked, GABAergic interneuron maturation is disrupted, and cortical networks exhibit lasting abnormal hyperexcitability. We conclude that temporally precise activation of developing cortical interneurons by ambient glutamate is critically important for establishing normal cortical inhibition.
Commentary
What exactly does a baby interneuron need to grow up and become a healthy, productive adult? Instructive advice from the environment? Interactions with other youngsters? Several pairs of good genes? The answer is very likely all of the above—and then some. Indeed, the processes that ensure the proper development of all neuron subtypes in the nervous system, as well as their connections with one another, are incredibly dynamic and complex.1,2
In 2015, Markram et al provided a blueprint outlining the end point of proper cortical development in the rodent.3 While the staggering complexity of cortical circuitry was well appreciated from many previous studies, the 2015 report encapsulated and reinforced this perspective with a set of experiments that drew from astonishing sample sizes (eg, intracellular recordings from >14 000 neurons in all 6 cortical layers). Within that study, we see again that while the cortex is predominantly composed of excitatory neurons, much of the neuronal diversity comes from a hodgepodge of many distinct inhibitory interneuron subtypes with exotic names such as Chandelier, Double Bouquet, and Martinotti.
Of the inhibitory neurons, the fairly common parvalbumin (PV)-containing basket cell has long been of keen interest for its capacity to strongly regulate the activity of its targets. Indeed, the neuron’s name was inspired by its characteristic, basket-like innervation of the cell body of targeted neurons, a location that can powerfully regulate the nearby site of action potential initiation.4–7 With this power, it is therefore not surprising to learn that PV interneurons are critical players in many brain functions, including in the generation of gamma oscillations associated with processing information in the brain.8–10
But what exactly does a growing PV interneuron need in order to successfully fulfill its important duties later in adulthood? Several studies show that activation of N-methyl-d-aspartate (NMDA) receptors by the excitatory neurotransmitter glutamate is important for interneuron development. Early postnatal NMDA receptor blockade results in malformed cortical layers,11 and young mice in which interneurons lack the NR1 subunit of the NMDA receptor exhibit abnormal cortical activity and schizophrenia-like behaviors later in adulthood.12 Thus, NMDA receptor activity early in life appears important for proper interneuron development. If true, then what are the conditions that give rise to NMDA receptor activation? A recent study by Hanson et al provides an answer to this important question.
In a series of neat experiments, Hanson et al demonstrate that inefficient removal of extracellular glutamate early in life, coupled with the timely expression of high-affinity NMDA receptors by PV interneurons, establishes a brief window of time during which glutamate provides PV interneurons with a tonic excitatory current that appears critical for proper development. Glutamate removal from the extracellular space is carried out by astrocytes that express excitatory amino acid transporters (EAATs), specialized proteins that efficiently take up the neurotransmitter.13 A previous report by the same group showed that astrocytic expression of EAATs in the cortex is generally low during the first postnatal week and ramps up during the second postnatal week.14 In their recent follow-up report, the authors show that such diminished EAAT expression results in higher concentrations of extracellular glutamate (also referred to as ambient glutamate) in the cortices of young mice.
The authors used two independent biosensing assays to conclude that young PV interneurons live in a soup containing concentrated glutamate. The first approach employed fluorescent, fluorescence resonance energy transfer (FRET)-based imaging techniques to ascertain glutamate levels, while the second approach included a series of electrophysiological recordings to approximate absolute concentrations of extracellular glutamate. Both approaches show that ambient glutamate is twice as concentrated in the cortex of a 3-day-old mouse relative to 14-day-old mouse. The authors specifically estimate that the ambient glutamate concentration is 100 nM in P3 mice but only 50 nM in P14 mice; intermediate glutamate concentrations were observed in intervening ages.
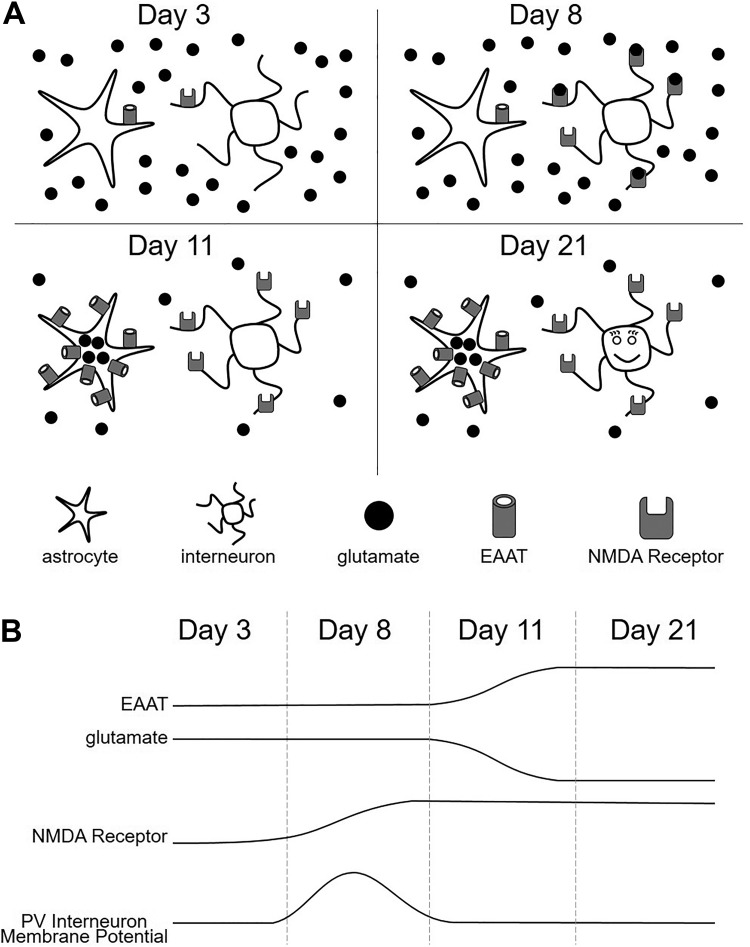
Showing that the cortex of a young mouse has high concentrations of ambient glutamate is important but possibly irrelevant if receptors are not available to bind the neurotransmitter. Thus, Hanson et al further show that expression of GluN2D, the subunit that confers NMDA receptors with a high affinity for glutamate, is elevated in PV interneurons of a 7-day-old mouse cortex relative to a 3-day-old mouse cortex. Critically, this upregulation of GluN2D in the interneurons occurs when EAAT expression is still relatively low and, therefore, ambient glutamate concentrations are relatively high. This developmental window of upregulated NMDA expression and elevated glutamate concentration is brief, occurring between 7 and 9 days of age and provides PV interneurons with a positive conductance that significantly depolarizes their resting membrane potential (Figure 1). However, this conductance is transient, subsiding during the second postnatal week when EAAT expression is upregulated and glutamate is efficiently removed from the extracellular space.
Figure 1.
Staggered expression of N-methyl-d-aspartic acid (NMDA) receptors and excitatory amino acid transporters (EAATs) establishes a brief window during development characterized by elevated ambient glutamate concentrations and depolarization of parvalbumin (PV) interneurons. (A) Shortly after birth, PV interneuron expression of GluN2C/D subunit-containing NMDA receptors and astrocytic expression of EAATs is low. The low EAAT expression results in elevated ambient glutamate concentrations (eg, day 3). Soon thereafter, NMDA receptor expression by PV interneurons is upregulated, and ambient glutamate promotes their activation (eg, day 8). Later, as EAAT expression is upregulated, ambient glutamate concentrations decrease, resulting in weaker NMDA receptor activation (eg, day 11). The transient depolarization provided by high ambient glutamate concentration occurs during days 7 to 9 and appears critical for the development of a healthy adult PV interneuron (eg, day 21). (B) Graphical representation of the developmental profiles of EAAT expression (top), ambient glutamate (middle, top), NMDA receptor expression (middle, bottom), and PV interneuron membrane potential (bottom).
Finally, what is the significance of a small, depolarizing tonic current observed in PV interneurons for just a few days during development? Hanson et al address this question by pharmacologically blocking GluN2D-containing NMDA receptors in vivo from postnatal days 7 to 9. The long-term consequences of this acute manipulation are impressive. The density of anatomically defined inhibitory synapses is reduced in adult mice following early postnatal exposure to the NMDA receptor blocker as is the frequency of observed inhibitory postsynaptic currents measured with electrophysiological techniques. Consistent with these observations, the authors also show that adult PV interneurons treated with the NMDA receptor blocker at an early age exhibit reduced morphological complexity. None of these abnormalities were observed in vehicle-treated mice or in mice treated with NMDA receptor blockers between postnatal days 11 and 13. Collectively, these results strongly support the conclusion that early, albeit brief activation of NMDA receptors expressed by PV interneurons during development is necessary for proper morphology and function in adulthood. The consequences of denying PV interneurons activation are deleterious, as the cortices of adult mice treated with the NMDA receptor blocker early in life are hyperexcitable and prone to produce seizure-like events.
In sum, how the brain assembles itself during development is clearly a question of great importance. Such assembly seems to involve improbable complexity, and faulty assembly can have devastating consequences. By showing that healthy brain function in an adult depends on early expression of high-affinity glutamate receptors by PV inhibitory neurons, coupled with slightly delayed astrocyte-mediated glutamate clearance, Hanson et al now provide an important contribution to our understanding of this assembly process.
By Mark Beenhakker
Footnotes
ORCID iD: Mark Beenhakker  https://orcid.org/0000-0002-4541-0201
https://orcid.org/0000-0002-4541-0201
References
- 1. Hu JS, Vogt D, Sandberg M, Rubenstein JL. Cortical interneuron development: a tale of time and space. Development. 2017;144(21):3867–3878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Lui JH, Hansen DV, Kriegstein AR. Development and evolution of the human Neocortex. Cell. 2011;146(1):18–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Markram H, Muller E, Ramaswamy S, et al. Reconstruction and simulation of neocortical Microcircuitry. Cell. 2015;163(2):456–492. [DOI] [PubMed] [Google Scholar]
- 4. Veres JM, Nagy GA, Hájos N. Perisomatic GABAergic synapses of basket cells effectively control principal neuron activity in amygdala networks. Huguenard J, editor. eLife. 2017;6:e20721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Marin-Padilla M. Origin of the pericellular baskets of the pyramidal cells of the human motor cortex: a golgi study. Brain Res. 1969;14(3):633–646. [DOI] [PubMed] [Google Scholar]
- 6. Somogyi P, Kisvárday ZF, Martin KA, Whitteridge D. Synaptic connections of morphologically identified and physiologically characterized large basket cells in the striate cortex of cat. Neuroscience. 1983;10(2):261–294. [DOI] [PubMed] [Google Scholar]
- 7. Cajal SRY. Histologie du Systdme Nerveux de l’Homme et des Vertebres. Vol. 2 Paris: A. Maloine; 1909. –2011. [Google Scholar]
- 8. Cardin JA, Carlén M, Meletis K, et al. Driving fast-spiking cells induces gamma rhythm and controls sensory responses. Nature. 2009;459(7247):663–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ferguson BR, Gao WJ. PV Interneurons: Critical Regulators of E/I Balance for prefrontal cortex-dependent behavior and psychiatric disorders. Front Neural Circuits [Internet]. 2018;12 https://www.frontiersin.org/articles/10.3389/fncir.2018.00037/full#h8. Accessed July 8, 2019. [DOI] [PMC free article] [PubMed]
- 10. Jadi MP, Behrens MM, Sejnowski TJ. Abnormal Gamma Oscillations in N-Methyl-D-Aspartate Receptor Hypofunction Models of Schizophrenia. Biol Psychiatry. 2016;79(9):716–726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Reiprich P, Kilb W, Luhmann HJ. Neonatal nmda receptor blockade disturbs neuronal migration in rat somatosensory cortex in Vivo. Cereb Cortex. 2005;15(3):349–358. [DOI] [PubMed] [Google Scholar]
- 12. Belforte JE, Zsiros V, Sklar ER, et al. Postnatal NMDA receptor ablation in corticolimbic interneurons confers schizophrenia-like phenotypes. Nat Neurosci. 2010;13(1):76–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Danbolt NC. Glutamate uptake. Prog Neurobiol. 2001;65(1):1–105. [DOI] [PubMed] [Google Scholar]
- 14. Hanson E, Armbruster M, Cantu D, et al. Astrocytic glutamate uptake is slow and does not limit neuronal NMDA receptor activation in the neonatal neocortex. Glia. 2015;63(10):1784–1796. [DOI] [PMC free article] [PubMed] [Google Scholar]