Abstract
Background:
Mescaline (3,4,5-trimethoxyphenethylamine), mainly found in the Peyote cactus (Lophophora williamsii), is one of the oldest known hallucinogenic agents that influence human and animal behavior, but its psychoactive mechanisms remain poorly understood.
Objectives:
This article aims to fully review pharmacokinetics and pharmacodynamics of mescaline, focusing on the in vivo and in vitro metabolic profile of the drug and its implications for the variability of response.
Methods:
Mescaline pharmacokinetic and pharmacodynamic aspects were searched in books and in PubMed (U.S. National Library of Medicine) without a limiting period. Biological effects of other compounds found in peyote were also reviewed.
Results:
Although its illicit administration is less common, in comparison with cocaine and Cannabis, it has been extensively described in adolescents and young adults, and licit consumption often occurs in religious and therapeutic rituals practiced by the Native American Church. Its pharmacodynamic mechanisms of action are primarily attributed to the interaction with the serotonergic 5-HT2A-C receptors, and therefore clinical effects are similar to those elicited by other psychoactive substances, such as lysergic acid diethylamide (LSD) and psilocybin, which include euphoria, hallucinations, depersonalization and psychoses. Moreover, as a phenethylamine derivative, signs and symptoms are consistent with a sympathomimetic effect. Mescaline is mainly metabolized into trimethoxyphenylacetic acid by oxidative deamination but several minor metabolites with possible clinical and forensic repercussions have also been reported.
Conclusion:
Most reports concerning mescaline were presented in a complete absence of exposure confirmation, since toxicological analysis is not widely available. Addiction and dependence are practically absent and it is clear that most intoxications appear to be mild and are unlikely to produce life-threatening symptoms, which favors the contemporary interest in the therapeutic potential of the drugs of the class.
Keywords: Mescaline, Peyote, Metabolism, Toxicity, Pharmacokinetics, Pharmacodynamics
1. INTRODUCTION
Hallucinogens, also known as psychedelics (“mind revealing”) or “psychotomimetics” (psychosis mimicking), were originally used in indigenous rituals, but their consumption is currently widespread [1]. At low concentrations, these compounds produce psychosis-like symptoms and can alter perception, feelings, thoughts and mood, without being addictive; a hallucination is an apparent sensory experience, i.e., something that the individual hears, sees, smells, feels, or tastes that does not really exist, and is commonly observed in people who suffer from mental disorders such as schizophrenia [2, 3]. Hallucinogens became very popular among the hippie culture in the 60s and 70s, but at that time legal concerns lead to their prohibition and the end of their research [1]. Recently, various hallucinogens have been proposed for the treatment of some pathologies, including alcoholism, depression and obsessive compulsive disorder [1, 2]. These agents all display a similar mechanism of action through the interaction with 5-hydroxytryptamine (5-HT; serotonin) receptors, particularly the 5-HT2A receptor which is associated with hallucinations [4]. A possible classification scheme groups hallucinogens as (i) serotonin-like, such as psilocybin, psilocin and lysergic acid diethylamide (LSD); and (ii) catecholamines (i.e., dopamine, noradrenaline and adrenaline)-like, such as mescaline [5].
Mescaline (3,4,5-trimethoxyphenethylamine) is a naturally-occurring alkaloid that has been used for millennia in religious rituals due to its psychedelic properties, and for medicinal purposes by the North American natives as far as 5700 years ago [6, 7]. Currently, mescaline continues to be legally used with apparent safety by the Native American Church during religious ceremonies, which are traditionally held at night and last for approximately 12 hours [7-9]. Nevertheless, both the cactus, and mainly mescaline, are being illegally consumed [10]. Mescaline was first isolated and identified in 1896 by the German chemist Arthur Heffter [11] and first synthesized in 1919 by Ernst Späth, who converted 3,4,5-trimethoxybenzoic acid into the respective aldehyde, subsequently reduced to mescaline [12].
Similar to several other hallucinogens, and following validation by preclinical research and several pilot clinical trials, mescaline has been claimed useful for the treatment of depression, anxiety, headache, obsessive compulsive disorder and addiction to certain substances, such as ethanol [13, 14]. Its use in alleviating ethanol withdrawal symptoms is practiced by the Native American Church, as the pleasant effects and sense of well-being provided by the consumption of mescaline may have led to successful stories on overcoming the symptoms of ethanol withdrawal and a lower prevalence of ethanol recidivism [15-17]. Its current status, as a controlled substance, limits the availability of the drug to researchers and by virtue of this, very few studies concerning the activity and potential therapeutic effects of mescaline in humans have been conducted since the early 1970s.
The aim of this manuscript is to review all the available data regarding mescaline pharmacokinetics and pharmacodynamics, focusing on major and minor metabolites and its pharmacological and toxicological relevance, as well as on peyote composition, that may help to explain the claimed therapeutic applications. This integrated overview will be useful to better understand clinical effects and variables that may influence both drug efficacy and toxicity, particularly at the molecular, cellular, and circuitry levels of the brain. Of note, the present review may also assist drug development processes and clinical and forensic interventions, where specific knowledge on pharmacokinetics and pharmacodynamic aspects are of utmost relevance.
2. METHODOLOGY
Search methodology was performed as described in previous publications on the metabolism and metabolomics of other drugs [5, 18-27]. Briefly, an English, Spanish, Portuguese and German extensive literature search was carried out in PubMed (U.S. National Library of Medicine) without a limiting period to identify relevant articles on pharmacokinetics and pharmacodynamics of mescaline, related known metabolizing enzymes and metabolites, and effects of mescaline and peyote. Electronic copies of the full papers were obtained from the retrieved journal articles, as well as books on peyote, mescaline and other hallucinogens, and then further reviewed to find additional publications related to human and non-human in vivo and in vitro studies.
3. DISTRIBUTION IN NATURE AND PLANT DESCRIPTION
Mescaline occurs naturally in some members of the Cactaceae plant family (Fig. 1), such as the North American peyote cactus (Lophophora williamsii), the South American San Pedro (alluding to St. Peter’s role as the gatekeeper to heaven) cactus (Echinopsis pachanoi syn. Trichocereus pachanoi), the Peruvian torch cactus (Echinopsis peruviana syn. Trichocereus peruvianus), the Bolivian torch cactus (Echinopsis lageniformis syn. Trichocereus bridgesii), and the Pereskia aculeata [28-35]. It is also found in small amounts in certain members of the Fabaceae (bean) family, including Acacia berlandieri [36]. The Trichocereus peruvianus and the Trichocereus bridgesii, both commonly known as wachuma, are used, though less frequently in comparison to Lophophora williamsii and Trichocereus pachanoi. Another cactus that contains mescaline, Pelecyphora aselliformis, is referred to as peyotillo by the Native Americans, or “little peyote”, as it is smaller than regular peyote cactus. Peyotillo does not contain as much mescaline as peyote, and Native Americans use it in folk medicine [37]. Lophophora williamsii is the most important representative; in English it is known as peyote (i.e., Spanish loanword), being mainly originated from the highlands of middle Mexico and southern Texas in North America. Neither mescaline should be confused with mescal (or mezcal in Spanish), the name of a tequila-like alcoholic beverage made from any type of agave plant (e.g., Agave angustifolia; (Fig. 2a), nor with the toxic hallucinogenic bean grown in Texas (i.e., the Sophora secundiflora or Dermatophyllum secundiflorum (Fig. 2b); erroneously called as “mescal bean” although it does not produce mescal) that contains cytisine, an alkaloid pharmacologically related to nicotine that binds with high affinity to the α4β2 subtype of the nicotinic acetylcholine receptor and is currently used in smoking cessation therapy [38-40]. Neither the liquor nor the bean contains the psychedelic chemical mescaline.
Fig. (1).

Cactaceae plant family containing mescaline. A and B - Lophophora williamsii; C and D - Echinopsis pachanoi; E - Echinopsis peruviana; F - Echinopsis lageniformis; G - Pereskia aculeate.
Fig. (2).

A - Agave angustifolia, the mescal plant; B - Dermatophyllum secundiflorum.
Lophophora spp. grow low to the ground and often form groups with numerous, crowded shoots. Peyote cacti are very slow growing, taking up to 30 years from the seedling to the blooming of flowers [3]. The blue-green, yellow-green, or sometimes reddish-green shoots, are mostly flattened spheres with sunken shoot tips without sharp spines. They can reach heights ranging from 2 to 7 cm and diameters of 4 to 12 cm, and may be found in clumps up to 1 m. Due to its size, peyote trafficking is not currently a worldwide significant problem. There are often significant, vertical ribs consisting of low and rounded or hump-like bumps/crowns. From the cusp areoles arises a tuft of soft, yellowish or whitish woolly hairs. Flowers are pink or white to slightly yellowish, sometimes reddish. They open during the day, varying from 1 to 2.4 cm long, and reach a diameter of 1 to 2.2 cm. The cactus produces flowers sporadically; these are followed by small edible pink fruit. The club-shaped to elongated fleshy fruits are bare and sort of colored. At maturity, they are brownish-white and dry. The fruits do not burst open on their own and they are between 1.5 and 2 cm long. They contain black, pear-shaped seeds that are 1 to 1.5 mm long and 1 mm wide. The seeds require hot and humid conditions to germinate. The top of the above-ground part of the cactus, the crown, consists of disc-shaped buttons. These should be cut above the roots, immediately below its base, leaving the subterranean portion of the plant along with a ring of green photosynthesizing area of the stem to regenerate new crowns/heads [41]. When done properly, the top of the root forms a callus and the root does not rot. When poor harvesting techniques are used, however, the entire plant dies. Due to the incorrect harvest technique (i.e., the cut of peyote too low since it can be very hard to distinguish where the shoot ends and the root begins) and the habitat destruction resulting from urban development, Lophophora williamsii populations have diminished, being endangered in large areas of South Texas, where “peyoteros” harvest the cactus for ceremonial purposes by the Native American Church. These heads are then dried to make disc-shaped buttons. The buttons are generally chewed, or boiled in water to produce a psychoactive tea. However, the taste of the cactus is bitter and most people are nauseated before they feel the onset of the psychoactive effects. Therefore, contemporary users often grind it into a powder and pour it into capsules to avoid the taste.
4. PEYOTE COMPOSITION
Peyote contains a large spectrum of phenylethylamine (or phenethylamine or β-phenylethylamine) alkaloids, the principal being mescaline for which the content of Lophophora williamsii is about 0.4% fresh (undried) and 3-6% dried [28, 42]. Besides mescaline, dozens of different alkaloids have already been identified in this cactus (Fig. 3) [43-46]. This “cocktail of compounds” may enhance mescaline effects, although some of these, when taken alone, are devoid of pharmacological activity. Some are solely present in a few parts of the plant, such as hordenine, an antibiotic found only in the roots, while others, such as lophophorine, can be detected both in the roots and buds of the cactus [43]. Mescaline is mostly concentrated in cactus buds (i.e., the photosynthetic portion of the stem above ground), being also detected in small concentrations in non-chlorophyllous stem and roots [47]. Tyrosine and phenylalanine serve as the metabolic precursors to the biosynthesis of mescaline.
Fig. (3).
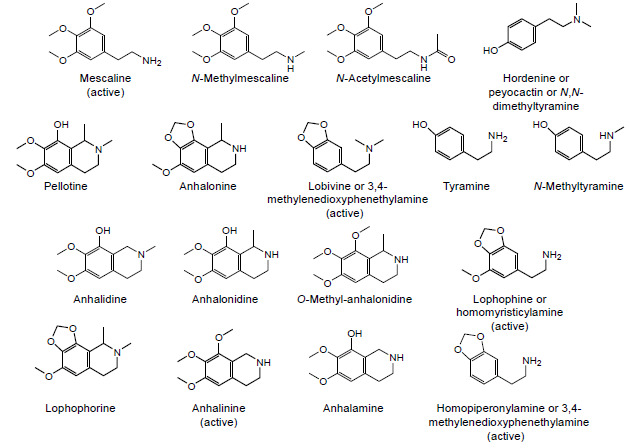
Main alkaloids found in the peyote cactus.
Besides mescaline, hordenine also has forensic relevance, namely as a qualitative and quantitative marker for beer consumption since it is formed during malting of barley grains in beer brewing [48]. Hordenine is a N,N-dimethyl derivative of the well-known biogenic amine tyramine, from which it is biosynthetically derived and with which it shares some pharmacological properties. The sympathomimetic effect of hordenine is attributed to its structural similarity to neurotransmitters, including dopamine and adrenaline; it leads to hypertension (in cats) and increases respiratory and cardiac frequencies after application of 2 mg per kilogram of body weight [49].
About a century ago, pellotine was marketed as a sedative/hypnotic by Boehringer & Sohn in Germany, but it was then discontinued after the advent of barbiturates. Worth to note that pellotine is the second most abundant alkaloid in Lophophora williamsii, but it is by far the most abundant alkaloid in the other Lophophora spp., accounting for 70-90% of its total alkaloid content. In those species, mescaline is present only in trace concentrations, not necessarily high enough to produce pharmacological effects following ingestion of the cactus.
Anhalinine is another stimulant alkaloid that can be isolated from Lophophora williamsii [50]. While also being active, lophophine (3-methoxy-4,5-methylenedioxyphene- thylamine), homopiperonylamine and lobivine are minor components of both peyote and San Pedro cacti [51]. In contrast to the three methoxy groups present in the molecule of mescaline, an interesting chemical feature of the tetrahydroisoquinoline alkaloids (such as pellotine, anhalonidine, lophophorine and anhalonine) is the existence of a methylenedioxy substituent on the aromatic ring, as occurs in 3,4-methylenedioxymethamphetamine (MDMA or “ecstasy”). The preponderant role played by the methylenedioxy moiety in the toxicity of this amphetamine designer drug is widely acknowledged [52]; similar toxicological mechanisms might therefore be hypothesized for these peyote constituents.
5. PHARMACODYNAMICS
The 5-HT2 receptor subfamily consists of the 5-HT2A, 5-HT2B, and 5-HT2C receptors, exhibiting considerable homology: 46-50% in their overall amino acid sequence and more than 70% within the transmembrane domains. They all mediate excitatory neurotransmission and are coupled to the Gq (Gαq or Gq/11) family of G-proteins that activate phospholipase C, leading to hydrolysis of phosphatidylinositol 4,5-bisphosphate (PIP2) into soluble inositol 1,4,5-triphosphate (IP3), therefore increasing its cytosolic levels (Fig. 4). IP3 then diffuses through the cytosol to bind to IP3 receptors, particularly to calcium channels in the endoplasmic reticulum (ER), increasing cytosolic Ca2+ levels [53]. 5-HT2 receptors exhibit distinct expression profiles [54] with distribution in the cortex, locus coeruleus, basal ganglia, hippocampus, platelets and vascular smooth muscle [55]. The hallucinogenic effects of mescaline result from the interference with the neuronal serotoninergic mechanisms, as an agonist; although it exhibits affinity to 5-HT1A receptor, its action is primarily at 5-HT2 level, with affinity to 5-HT2A and 5-HT2B receptors being relatively low compared to 5-HT2C receptor, for which it is full agonist [56, 57].
Fig. (4).
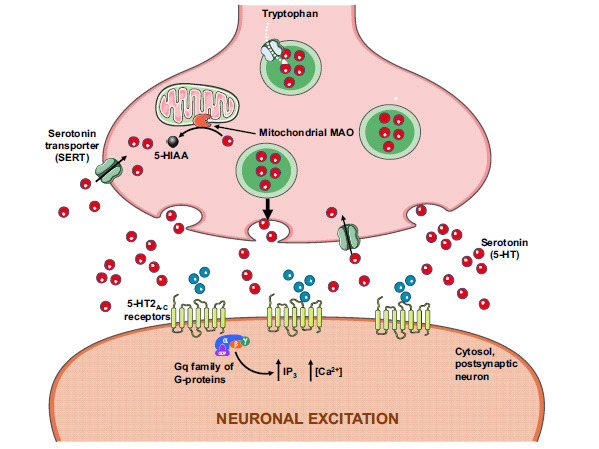
Mescaline pharmacodynamics at the serotonergic terminal is mediated through 5-HT2A-C receptors. 5-HIAA, 5-hydroxyindoleacetic acid; MAO, monoamine oxidase; IP3, inositol 1,4,5-triphosphate.
Freedman et al. [58] reported that low doses of mescaline decreased brain levels of 5-hydroxyindoleacetic acid (5-HIAA), the major metabolite of serotonin, while at high doses increased brain 5-HIAA. Consistent with this high dose effect, Tilson and Sparber [59] reported that mescaline increased the release and/or re-uptake of serotonin. Since mescaline possesses a phenylethylamine moiety, and thus is a structural analog of amphetamine, a prototypic dopamine-releasing agent, dopaminergic activity was also documented, but it is probably a modest influence [60]. In accordance, there is no evidence to support addiction and dependence to mescaline [43]. Cross tolerance of mescaline with other serotonergic drugs such as LSD and psilocybin has been described in humans and other animals [61]; mescaline tolerance develops after a few days of consumption but sensitivity is restored after 3-4 days of drug abstinence [43, 61].
Although it has an action similar to other traditional hallucinogens, mescaline is the least potent drug of the group, but its effects may last longer than 10-12 hours (e.g., less than an hour for N,N-dimethyltryptamine or DMT) [2]. Several pharmacological differences between these drugs are outlined in the Table 1.
Table 1. Pharmacological differences between mescaline, lysergic acid diethylamide (LSD) and psilocybin. (Adapted from [3]).
| - | MESCALINE | LSD | PSILOCYBIN |
|---|---|---|---|
| Average dose | 20-500 mg | 0.05-0.2 mg | 20-40 mg |
| Potency in comparison to mescaline | - | 2,000× stronger | 20× stronger |
| Time to onset of effects | 1-3 hours | 30-40 minutes | 20-30 minutes |
| Duration of effects | >10-12 hours | 8-12 hours | 4 hours |
| Occurrence of flashbacks | Rare | Common | Rare |
6. ABSORPTION, DISTRIBUTION AND EXCRETION
Mescaline is mainly administered per os, but can also be smoked and insufflated. Tablets containing mescaline are typically ingested, or more commonly cactus buttons are chewed or used to prepare infusions (i.e., peyote cactus tea) [10, 43]. Through this route, doses usually ranging 200-400 mg of mescaline sulfate or 178-356 mg of mescaline hydrochloride are administered, being the average amount contained in 3 to 6 cactus buds or roughly 10 to 20 g of dried peyote [2, 43]. Nevertheless, the concentrations of the compounds rely heavily on the species, geoclimatic and development conditions, cactus age, the harvested part, amongst other factors, making it difficult to accurately estimate doses without previous extraction of mescaline [62]. Interestingly, aiming to enhance the psychedelic effects of peyote, the participants of religious ceremonies usually fast to increase gastrointestinal absorption of the drug.
Mescaline is rapidly absorbed from the gastrointestinal tract [43, 63]. A great percentage of the dose of mescaline is distributed to the kidneys and liver, and combined with hepatic proteins, delaying its concentration in blood, increasing its half-life, and delaying the occurrence of effects [43, 64]. Indeed, several studies reported the detection of larger amounts of mescaline in the liver and in the kidney than in the brain and blood [65]. Mescaline has a low lipid solubility and therefore low ability to cross the blood brain barrier, higher doses being required to produce similar effects to those caused by other hallucinogens [66]. Accordingly, LSD is approximately 2,000 times more potent than mescaline in producing an altered state of consciousness [67]. Usually, the effects appear within 30 minutes per os, the psychedelic peak effect occurs after 2 hours and disappears after 10-12 hours [43, 68, 69]. The peak of the effects does not co-occur with the mescaline concentration peak in the brain, suggesting that mescaline undergoes bioactivation in order to produce the maximum effect. Previously, it was shown that the administration of chlorpromazine (15 mg/kg) to mice 30 minutes prior to or 45 minutes after a tracer dose of mescaline, caused marked retention of the hallucinogen in the brain and other tissues examined [70]. The effect was speculated to be due to the blockade of removal of mescaline from various tissues, since the initial entry of the hallucinogen remained unaffected [70].
The half-life of ingested mescaline in humans is about 6 hours [68] and in the mouse brain was found to be approximately 1 hour [71]. Charalampous et al. [68] reported that, following an oral administration of mescaline to humans, 81.4% is eliminated unchanged in urine within the first hour, and 13.2% of the dose is excreted as 3,4,5-trimethoxypheny- lacetic acid (TMPA), with increasing elimination of this metabolite over the course of time. Accordingly, 87% of TMPA was excreted within the first 24 hours and 96% within 48 hours [68]. Other studies also demonstrated that mescaline is mainly excreted in the urine, mostly in the unchanged form (28-58%) and the remaining as TMPA [64, 72, 73]. Another study demonstrated that the percentage of mescaline eliminated unchanged in the urine of rats and mice was 18.4% and 79.4%, respectively [43]. Other minor metabolites have been identified in human urine, such as N-acetyl-3,4-dimethoxy-5-hydroxyphenylethylamine, 3,4,5-trimethoxybenzoic acid, 3,4-dimethoxy-5-hydroxyphenethylamine, and 3,4-dihydroxy-5-methoxyphenacetylglutamine [66]. According to Shah and Himwich [74], 24 hours after an intraperitoneal administration of mescaline-8-C14 to mice, approximately 61-68% of the dose was detected in urine, of which 31% was in the form of TMPA.
7. METABOLISM
The major and minor metabolic routes of mescaline are presented in (Fig. 5). In rabbit, mescaline metabolism occurs mainly in the liver by the action of an amine oxidase. While showing a significantly lower expression of amine oxidase, the lung also contributes for the clearance of mescaline, due to a larger blood flow, in comparison with the liver [75]. Mescaline undergoes detoxification mainly by oxidative deamination into an intermediate and unstable aldehyde, 3,4,5-trimethoxyphenylacetaldehyde, that is rapidly oxidized to the inactive TMPA or reduced to the inactive 3,4,5-trimethoxyphenylethanol [68, 71, 76, 77]. The fact that the peak of mescaline effects does not coincide with its peak concentration in brain, provided evidence on the contribution of its metabolites for hallucinogenic effects. In agreement, a study with rats treated with calcium carbimide (i.e., an aldehyde dehydrogenase inhibitor) showed that the metabolism into acid and alcohol follows the oxidation of mescaline to the aldehyde, whose concentration increased by metabolic inhibition, thus implicating this metabolite in the effects of the drug [43, 77]. The enzyme responsible for the deamination of mescaline to the aldehyde derivative is still a controversial issue among the scientific community. This reaction may be carried out by a monoamine oxidase (MAO) or a diamine oxidase (DAO). Studies with mice have shown that this route is inhibited by TPN, nicotinamide, iproniazid, semicarbazid, and other inhibitor compounds of mono or diamine oxidase [43, 78]. However, some authors discredit the role of these enzymes, as there are studies where their inhibition showed little relevance in the alteration of the metabolic profile, suggesting the existence of a mescaline oxidase [76, 79].
Fig. (5).
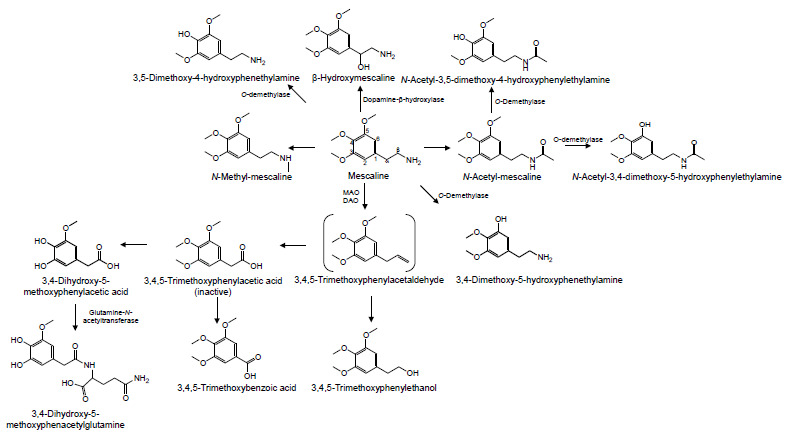
Major and minor metabolic routes of mescaline. MAO, monoamine oxidase; DAO, diamine oxidase.
TMPA is afterward metabolized to 3,4-dihydroxy-5-methoxyphenylacetic acid or 3,4,5-trimethoxybenzoic acid (3,4,5-TMBA). The first metabolite is formed by TMPA demethylation, being further combined with glutamine, by glutamine N-acyltransferase, to be eliminated as 3,4-dihydroxy-5-methoxyphenacetylglutamine, which has already been identified in human urine [43, 69]. This reaction is similar to the degradation of amphetamines in benzoic acid, which is later combined with glycine to be eliminated [80, 81]. The TMPA conversion to 3,4,5-TMBA has already been clarified in vivo, in mouse brain and liver [71]; and in vitro, in mouse brain, hepatic, cardiac and kidney homogenates [82]. The highest concentrations of 3,4,5-TMBA, both in vivo and in vitro, were detected in the brain, in comparison to other studied tissues [71, 82]. This can be explained by the location of the enzyme that catalyzes the 3,4,5-TMBA formation, which seems to mainly occur in the nuclear and microsomal fractions of this organ; the reaction depends on the presence of oxygen and NAD(P)H, and is inhibited by SKF 525-A. It seems that MAO and DAO inhibitors do not have any effect on the formation of this metabolite [82].
N-acetylation of mescaline seems to be an important metabolic route, at least in the brain. Indeed, following an administration of [14C]mescaline to rats, Musacchio and Goldstein [83] verified the formation of N-acetylmescaline and its O-demethylated metabolites N-acetyl-3,5-dimethoxy-4-hydroxy- phenylethylamine and N-acetyl-3,4-dimethoxy-5-hydroxy- phenylethylamine. These N-acetylated derivatives represented about 30% of the total amount eliminated through urine. Seiler and Demisch [71] have also observed similar results with mice. Upon treatment with iproniazid (i.e., an inhibitor of deamination metabolism), the excretion of N-acetylate metabolites increased, reinforcing the importance of this metabolic route to the metabolism of mescaline [83]. The N-acetylation has also been observed in humans by Charalampous et al. [68], who measured N-acetylated metabolites in urine. In spite of being a minor route, experiments in humans with labeled mescaline revealed that the radioactivity of the cerebrospinal fluid increased for up to 5 hours, and although unchanged mescaline still made up about half of the counts, N-acetylmescaline and N-acetyl-3,4-dimethoxy-5-hydroxyphen- ethylamine were the most abundant metabolites [68]. Studies carried out in the brain with deamination inhibitors showed no significant changes in the metabolites formed, leading authors to believe that the N-acetylation is the main central nervous system route of detoxification of mescaline. In contrast, deamination appears to be relevant in the liver, suggesting that different organs metabolize mescaline through different routes [74].
Minor routes of mescaline metabolism include its demethylation, through the O-demethylase, to 3,5-dimethoxy-4-hydroxyphenethylamine and 3,4-dimethoxy-5-hydroxy- phenethylamine with production of formaldehyde. Indeed, the incubation of mescaline with microsomal enzymes obtained from rabbit liver led to the formation of these metabolites [72, 84], and Friedhoff and Hollister [85] also identified the demethylated metabolites in human urine. Mescaline demethylation does not seem to be dependent on metabolism by CYP2D6 [86]. The N-methylation of mescaline has also been observed in rabbit lung, but the amount of N-methyl-mescaline was not properly representative and its formation in vivo has not been described yet [76].
The minor metabolite 3,4-dihydroxy-5-methoxyphene- thylamine is methylated to 3,5-dimethoxy-4-hydroxyphene- thylamine by catecholamine O-methyl transferase (COMT) [72], and a very small amount of 3,4,5-trimethoxybenzoic acid was later identified as an additional urinary metabolite [87].
Similar to some biogenic amines, it has been suggested that mescaline could be bioactivated by dopamine-β-hydroxylase, leading to the formation of β-hydroxymescaline [88]. Nevertheless, the formation of this metabolite has not yet been detected in animal or human models [76].
Phase II metabolism, leading to conjugates of the ring-hydroxylated metabolites, seems to be present but is also relatively unimportant [68, 69].
8. SIGNS AND SYMPTOMS OF EXPOSURE
Despite displaying different chemical properties, all hallucinogens generally produce similar psychological effects, but mescaline and peyote have some distinctive properties [89]. Shortly after administration, hallucinations occur as classical intensifications of visual stimulus of object forms, and touch and sounds hypersensitivity with distorted pitch [61]. Prominence of light and color is distinctive, appearing brilliant and intense. Typically, hallucinations may persist longer than 10-12 hours. Subjective effects may include altered thinking processes, an altered sense of time and self-awareness, and closed- and open-eyes visual phenomena [73]. Time is often perceived as passing more slowly and the sense of smell is enhanced. As with LSD, synesthesia can occur and is especially intensified by music [43, 73, 90, 91]. An unusual but unique characteristic of mescaline use is the “geometrization” of three-dimensional objects. The object can appear flattened and distorted, similar to the presentation of a Cubist painting [92]. The user often feels like “an alien in entirely new surroundings, and may feel as if he or she is floating or weighted down by some strange gravitational force” [3]. It is common for the individual to believe that is communicating with God or other deities, and is able to transcend the limits of earth, time and space to another world. It is for this reason that mescaline is often used during religious ceremonies, particularly by Native Americans, and that peyote is called the “divine” or “sacred” cactus [3].
Symptoms of mescaline poisoning are consistent with a sympathomimetic toxidrome, namely hyperreflexia, tachycardia, agitation, muscle stiffness, ataxia, seizures, mydriasis, sialorrhea, hyperthermia and paresthesia [10, 61, 93]. The methoxy side chains are likely responsible for the hallucinogenic effects of mescaline and are found in similar compounds that are known hallucinogens, including the “designer” street drug 2,5-dimethoxy-4-methylphenylisopropylamine (also known as STP or DOM) [94]. Occasionally, compensatory bradycardia may also occur [2, 10, 43, 90]. Nausea, emesis and anorexia have been inconsistently reported after peyote ingestion [10]. Although not entirely clarified, it is likely due to the very bitter taste of the plant [95] rather than to the effects of the active substance, mescaline. In a study with volunteers who were administered with synthetic mescaline, vomiting was not observed in any participants [89]. Several approaches have been described for reducing emesis, such as mixing the plant material with fruit juices or gelatin, or pulverizing the buttons and placing the powder into gelatin capsules [96].
There are only scarce data regarding the prevalence of sequels caused by the chronic consumption of mescaline, but a state of prolonged psychosis similar to schizophrenia has been reported [43]. Other studies performed in subsets of Navajo Native Americans with distinct consumption habits, revealed no evidence of long-term psychological or cognitive problems related to peyote use amongst the Native American Church ceremonies [7]. Compared to the group whose individuals reported minimal substance use, the group composed by members who regularly ingested peyote showed no significant deficits on the Rand Mental Health Inventory or any neuropsychological measures. Authors recognized, however, that these findings may not generalize to illicit hallucinogen users [7]. Moreover, peyote use in religious context does not appear to be associated with hallucinogen persisting perception disorder (i.e., “flashbacks”), dependence or addition [97]. Although evidence indicates that mescaline crosses placental barrier in monkeys, fetal adverse outcomes were not reported for peyote use during pregnancy and childbirth [98].
Table 2. Median lethal doses (LD50) observed for mescaline, when administered to animals.
| - | Species, Strain or Other Taxonomic Group | Administration Route a | LD50b | References |
|---|---|---|---|---|
| Rodent | Mouse Swiss Webster | i.p. | 212 | [90] |
| Mouse | i.p. | 315 | [43] | |
| per os | 880 | |||
| s.c. | 534 | |||
| i.v. | 157 | |||
| Sprague Dawley Rat | i.p. | 132 | [90] | |
| Rat | i.p. | 330-410 | [43] | |
| i.v. | 157 | |||
| s.c. | 534 | |||
| i.m. | 330 | |||
| Guinea pig | i.p. | 328 | [90] | |
| s.c. | 500 | [43] | ||
| Dog | Mongrel dog | i.v. | 54 | [90] |
| Rhesus macaque | Macaca mulatta | i.v. | 130 | [90] |
a i.p. – Intraperitoneal; s.c. – Subcutaneous; i.v. –Intravenous; i.m. –Intramuscular.
b Median lethal dose (LD50) is the amount of mescaline that kills fifty percent of the tested population and is expressed in milligrams of the hydrochloride salt per kilogram of bodyweight (mg/kg).
9. INTOXICATION CASES
There are some reports of intoxications caused by hallucinogens requiring emergency management, including fatalities, but the information available on these cases is very limited [99]. Generally, the duration of the symptoms lasts less than 24 hours. Typically, most patients treated in healthcare facilities only require sedation and supportive measures. If a patient arrives at the emergency room with a suspected hallucinogen intoxication, multidrug screening tests should be done in order to assess the possibility of concomitant intake of other substances rather than detecting hallucinogens, since they are rapidly eliminated from the body [100]. Symptomatic treatment of intoxications is carried out with chlorpromazine, trioxanize and pipradol [43, 101, 102]. Also, the intravenous administration of sodium succinate has been shown to be very effective in attenuating the psychotic effects of mescaline, but effects may relapse within few hours after the administration of the antidote, albeit with less intensity [102]. The symptoms of mescaline appear to be exacerbated by previous administration of reserpine [43]. The median lethal doses (LD50) observed for mescaline in studies in several species are displayed in Table 2. The dog seems to be the most sensitive species and in humans the lowest toxic dose has been extrapolated from data obtained in laboratory animals at 2500 μg/kg (i.m.) with reported effects of euphoria, distorted perceptions and hallucinations. Considering the human dose range reported above, it would be very difficult to consume enough mescaline to cause death. Serious effects from peyote ingestion have been described only rarely and include Mallory-Weiss lacerations from severe vomiting [103] and botulism from ingestion of improperly stored peyote buttons [104].
CONCLUSION & FUTURE PERSPECTIVES
The hallucinogens psilocybin, LSD and mescaline were extensively used in psychiatry before they were placed in Schedule I of the UN Convention on Drugs in 1967 due to recreational misuse. Currently, in addition to psychoactive use, some Native American tribes use peyote in the belief that it may have curative properties in toothache, pain in childbirth, fever, breast pain, skin diseases, rheumatism, alcoholism and other drug addictions, diabetes, colds, blindness [91] and for “strength in walking” [95]. Peyote extracts also demonstrated antibiotic properties against various strains of the bacteria Staphylococcus aureus probably due to the presence of hordenine [105]. Lumholtz [106] described its use in the treatment of snakebites, burns, wounds and rheumatism. During the last few years, as ancient tradition seems to suggest, the interest in the beneficial therapeutic applications of hallucinogens have resurged [5, 107], renovating the attention paid to the class [108]. In spite of the increasing number of relevant recent publications, most of the existing information is yet ancient and limited, urging systematic studies on this topic, both in vitro and in vivo.
This article attempted to fully review pharmacokinetics and pharmacodynamics of mescaline, focusing in its potential therapeutic application, as hallucinogens appear to present favorable toxicological profiles for this purpose. Mescaline acts similarly to other psychedelic agents; it binds to and activates mainly the serotonin 5-HT2c receptor with a high affinity [56, 57]. In comparison with LSD, binding affinities to the 5-HT2A receptor were lower for all of the tryptamines, including psilocin and DMT, mescaline being the least potent psychedelic [109]. Investigators also observed inhibition of serotonin (SERT), noradrenaline (NAT) or dopamine (DAT) transporters for all tested substances, with the exception of mescaline and LSD [109]. Although only LSD showed affinity to dopaminergic D1-3 receptors [109], other studies demonstrated that the characteristic behavioral effects of mescaline in cats were nearly completely blocked by pretreatment with low doses of either serotonin (methysergide) or dopamine (haloperidol) specific antagonists [60]. Most peyote intoxications appear to be mild in nature and are unlikely to produce life-threatening symptoms. Therefore, it is clear that mescaline distinctive behavioral profile suggests a complex mechanism of action that is still not fully understood.
Much work is needed to address variations in mescaline content within species and within individual cactus, and the influence of soil, season, and even hour of sampling [107]. Moreover, besides mescaline, other alkaloids have also been reported in Lophophora williamsii. Among them, lophophine is also psychoactive in man [51, 110]; the activity following its ingestion was described as “a peaceful elevation of mood, the generation of an euphoric state, and the enhancement of visual perception especially in the color sense”. Lophophine is also closely related to 3-methoxy-4,5-methylenedioxy- amphetamine, which is a potent psychotomimetic agent [51, 110]. Moreover, it is important to remember that abuse of peyote cactus may escape detection during routine drug testing, making clinical and forensic diagnosis difficult. Likewise, intoxication reports are usually described in a complete absence of exposure confirmation, as assays for measurement of plasma mescaline levels are not widely available [10].
Finally, while hallucinogens are widely used, both by drug abusers and by members of traditional cultures for religious or healing purposes, the long-term residual psychological and cognitive effects of these drugs remain poorly understood. Renewed interest in the possible therapeutic applications of psychedelic drugs may offer further insights for the development of more potent analogues.
Acknowledgements
Declared none.
Consent for Publication
Not applicable.
Funding
This work was supported by grants from CESPU (TramTap-CESPU-2016, Chronic-TramTap_CESPU_2017 and TraTapMDMA-CESPU-2018) and Norte Portugal Regional Operational Program (NORTE 2020), under the PORTUGAL 2020 Partnership Agreement, through the European Regional Development Fund (ERDF).
Conflict of Interest
The authors declare no conflict of interest, financial or otherwise.
REFERENCES
- 1.Bogenschutz M.P., Johnson M.W. Classic hallucinogens in the treatment of addictions. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2016;64:250–258. doi: 10.1016/j.pnpbp.2015.03.002. [DOI] [PubMed] [Google Scholar]
- 2.Nichols D.E. Hallucinogens. Pharmacol. Ther. 2004;101:131–181. doi: 10.1016/j.pharmthera.2003.11.002. [DOI] [PubMed] [Google Scholar]
- 3.Olive M.F., Triggle D.J. Drugs the straight facts: Peyote and mescaline. New York: Chelsea House; 2007. [Google Scholar]
- 4.Lopez-Gimenez J.F., Gonzalez-Maeso J. Hallucinogens and Serotonin 5-HT2A Receptor-Mediated Signaling Pathways. Curr. Top. Behav. Neurosci. 2018;36:45–73. doi: 10.1007/7854_2017_478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dinis-Oliveira R.J. Metabolism of psilocybin and psilocin: Clinical and forensic toxicological relevance. Drug Metab. Rev. 2017;49:84–91. doi: 10.1080/03602532.2016.1278228. [DOI] [PubMed] [Google Scholar]
- 6.El-Seedi H.R., De Smet P.A., Beck O., Possnert G., Bruhn J.G. Prehistoric peyote use: Alkaloid analysis and radiocarbon dating of archaeological specimens of Lophophora from Texas. J. Ethnopharmacol. 2005;101:238–242. doi: 10.1016/j.jep.2005.04.022. [DOI] [PubMed] [Google Scholar]
- 7.Halpern J.H., Sherwood A.R., Hudson J.I., Yurgelun-Todd D., Pope H.G., Jr Psychological and cognitive effects of long-term peyote use among Native Americans. Biol. Psychiatry. 2005;58:624–631. doi: 10.1016/j.biopsych.2005.06.038. [DOI] [PubMed] [Google Scholar]
- 8.Bullis R.K. Swallowing the scroll: Legal implications of the recent Supreme Court peyote cases. J. Psychoactive Drugs. 1990;22:325–332. doi: 10.1080/02791072.1990.10472556. [DOI] [PubMed] [Google Scholar]
- 9.Csordas T.J., Storck M.J., Strauss M. Diagnosis and distress in Navajo healing. J. Nerv. Ment. Dis. 2008;196:585–596. doi: 10.1097/NMD.0b013e3181812c68. [DOI] [PubMed] [Google Scholar]
- 10.Carstairs S.D., Cantrell F.L. Peyote and mescaline exposures: a 12-year review of a statewide poison center database. Clin. Toxicol. (Phila.) 2010;48:350–353. doi: 10.3109/15563650903586745. [DOI] [PubMed] [Google Scholar]
- 11.Heffter A. Ueber Cacteenalkaloide. (II. Mittheilung). Ber. Dtsch. Chem. Ges. 1896;29:216–227. [Google Scholar]
- 12.Späth E. Über die anhalonium-alkaloide I. Anhalin und mezcalin. Monatsh. Chem. 1919;40:129–154. [Google Scholar]
- 13.Kyzar E.J., Nichols C.D., Gainetdinov R.R., Nichols D.E., Kalueff A.V. Psychedelic Drugs in Biomedicine. Trends Pharmacol. Sci. 2017;38:992–1005. doi: 10.1016/j.tips.2017.08.003. [DOI] [PubMed] [Google Scholar]
- 14.Dyck E., Farrell P. Psychedelics and psychotherapy in Canada: Humphry Osmond and Aldous Huxley. Hist. Psychol. 2018;21:240–253. doi: 10.1037/hop0000088. [DOI] [PubMed] [Google Scholar]
- 15.de Rios M.D., Grob C.S., Baker J.R. Hallucinogens and redemption. J. Psychoactive Drugs. 2002;34:239–248. doi: 10.1080/02791072.2002.10399960. [DOI] [PubMed] [Google Scholar]
- 16.Winkelman M. Psychedelics as medicines for substance abuse rehabilitation: Evaluating treatments with LSD, Peyote, Ibogaine and Ayahuasca. Curr. Drug Abuse Rev. 2014;7:101–116. doi: 10.2174/1874473708666150107120011. [DOI] [PubMed] [Google Scholar]
- 17.Denber H.C. Mescaline and lysergic acid diethylamide: Therapeutic implications of the drug-induced state. Dis. Nerv. Syst. 1969;30(Suppl.):23–27. [PubMed] [Google Scholar]
- 18.Barbosa J., Faria J., Queiros O., Moreira R., Carvalho F. ; Dinis-Oliveira R.J. Comparative metabolism of tramadol and tapentadol: A toxicological perspective. Drug Metab. Rev. 2016;48:577–592. doi: 10.1080/03602532.2016.1229788. [DOI] [PubMed] [Google Scholar]
- 19.Dinis-Oliveira R.J. Metabolic Profile of Flunitrazepam: Clinical and Forensic Toxicological Aspects. Drug Metab. Lett. 2017;11:14–20. doi: 10.2174/1872312811666170407164216. [DOI] [PubMed] [Google Scholar]
- 20.Dinis-Oliveira R.J. Metabolic profile of oxazepam and related benzodiazepines: clinical and forensic aspects. Drug Metab. Rev. 2017;49:451–463. doi: 10.1080/03602532.2017.1377223. [DOI] [PubMed] [Google Scholar]
- 21.Dinis-Oliveira R.J. Metabolic Profiles of Propofol and Fospropofol: Clinical and Forensic Interpretative Aspects. BioMed Res. Int. 2018;2018:6852857. doi: 10.1155/2018/6852857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dinis-Oliveira R.J. Metabolomics of cocaine: Implications in toxicity. Toxicol. Mech. Methods. 2015;25:494–500. [PubMed] [Google Scholar]
- 23.Dinis-Oliveira R.J. Metabolomics of Delta9-tetrahydrocannabinol: Implications in toxicity. Drug Metab. Rev. 2016;48:80–87. doi: 10.3109/03602532.2015.1137307. [DOI] [PubMed] [Google Scholar]
- 24.Dinis-Oliveira R.J. Metabolomics of methadone: Clinical and forensic toxicological implications and variability of dose response. Drug Metab. Rev. 2016;48:568–576. doi: 10.1080/03602532.2016.1192642. [DOI] [PubMed] [Google Scholar]
- 25.Dinis-Oliveira R.J. Metabolomics of Methylphenidate and Ethylphenidate: Implications in Pharmacological and Toxicological Effects. Eur. J. Drug Metab. Pharmacokinet. 2017;42:11–16. doi: 10.1007/s13318-016-0362-1. [DOI] [PubMed] [Google Scholar]
- 26.Nobrega L., Dinis-Oliveira R.J. The synthetic cathinone alpha-pyrrolidinovalerophenone (alpha-PVP): Pharmacokinetic and pharmacodynamic clinical and forensic aspects. Drug Metab. Rev. 2018;50:125–139. doi: 10.1080/03602532.2018.1448867. [DOI] [PubMed] [Google Scholar]
- 27.Dinis-Oliveira R.J. Metabolism and metabolomics of ketamine: A toxicological approach. J. Forensic Sci. 2017;2(1):2–10. doi: 10.1080/20961790.2017.1285219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ogunbodede O., McCombs D., Trout K., Daley P., Terry M. New mescaline concentrations from 14 taxa/cultivars of Echinopsis spp. (Cactaceae) (“San Pedro”) and their relevance to shamanic practice. J. Ethnopharmacol. 2010;131:356–362. doi: 10.1016/j.jep.2010.07.021. [DOI] [PubMed] [Google Scholar]
- 29.Aragane M., Sasaki Y., Nakajima J., Fukumori N., Yoshizawa M., Suzuki Y., Kitagawa S., Mori K., Ogino S., Yasuda I., Nagumo S. Peyote identification on the basis of differences in morphology, mescaline content, and trnL/trnF sequence between Lophophora williamsii and L. diffusa. J. Nat. Med. 2011;65:103–110. doi: 10.1007/s11418-010-0469-7. [DOI] [PubMed] [Google Scholar]
- 30.Carod-Artal F.J., Vazquez-Cabrera C.B. Mescaline and the San Pedro cactus ritual: Archaeological and ethnographic evidence in northern Peru. Rev. Neurol. 2006;42:489–498. [PubMed] [Google Scholar]
- 31.Dasgupta A. Challenges in Laboratory Detection of Unusual Substance Abuse: Issues with Magic Mushroom, Peyote Cactus, Khat, and Solvent Abuse. Adv. Clin. Chem. 2017;78:163–186. doi: 10.1016/bs.acc.2016.07.004. [DOI] [PubMed] [Google Scholar]
- 32.Crosby D.M., McLaughlin J.L. Cactus alkaloids. XIX. Crystallization of mescaline HCl and 3-methoxytyramine HCl from Trichocereus pachanoi. Lloydia. 1973;36:416–418. [PubMed] [Google Scholar]
- 33.Anderson E.F. The cactus family. Oregon: Timber Press; 2001. [Google Scholar]
- 34.Pinto Nde C., Duque A.P., Pacheco N.R., Mendes Rde F., Motta E.V., Bellozi P.M., Ribeiro A., Salvador M.J., Scio E. Pereskia aculeata: A plant food with antinociceptive activity. Pharm. Biol. 2015;53:1780–1785. doi: 10.3109/13880209.2015.1008144. [DOI] [PubMed] [Google Scholar]
- 35.Schlumpberger B.O., Renner S.S. Molecular phylogenetics of Echinopsis (Cactaceae): Polyphyly at all levels and convergent evolution of pollination modes and growth forms. Am. J. Bot. 2012;99:1335–1349. doi: 10.3732/ajb.1100288. [DOI] [PubMed] [Google Scholar]
- 36.Clement B.A., Goff C.M., Forbes T.D.A. Toxic amines and alkaloids from Acacia berlandieri. Phytochemistry. 1997;46:249–254. [Google Scholar]
- 37.Neal J.M., Sato P.T., Howald W.N., McLaughlin J.L. yote Alkaloids: Identification in the Mexican ictus Pelecyphora aselliformis Ehrenberg. Science. 1972;176:1131–1133. doi: 10.1126/science.176.4039.1131. [DOI] [PubMed] [Google Scholar]
- 38.Gomez-Coronado N., Walker A.J., Berk M., Dodd S. Current and Emerging Pharmacotherapies for Cessation of Tobacco Smoking. Pharmacotherapy. 2018;38:235–258. doi: 10.1002/phar.2073. [DOI] [PubMed] [Google Scholar]
- 39.Tutka P., Zatonski W. Cytisine for the treatment of nicotine addiction: from a molecule to therapeutic efficacy. Pharmacol. Rep. 2006;58(6):777–798. [PubMed] [Google Scholar]
- 40.Walker N., Howe C., Glover M., McRobbie H., Barnes J., Nosa V., Parag V., Bassett B., Bullen C. Cytisine versus nicotine for smoking cessation. N. Engl. J. Med. 2014;371:2353–2362. doi: 10.1056/NEJMoa1407764. [DOI] [PubMed] [Google Scholar]
- 41.Terry M., Mauseth J.D. Root-shoot anatomy and post-harvest vegetative clonal development in Lophophora williamsii (Cacta-ceae: Cacteae): Implications for conservation. 2006;22:565–592. [Google Scholar]
- 42.Spinella M. The psychopharmacology of herbal medicine: Plant drugs that alter mind, brain, and behavior. Massachusetts: MIT Press; 2001. [Google Scholar]
- 43.Kapadia G.J., Fayez M.B. Peyote constituents: chemistry, biogenesis, and biological effects. J. Pharm. Sci. 1970;59:1699–1727. doi: 10.1002/jps.2600591202. [DOI] [PubMed] [Google Scholar]
- 44.Štarha R., Kuchiňa J. Analysis of Mexican Populations of Lophophora (Cactaceae). Universitas Ostraviensis Acta Facultatis Rerum Naturalium. Physica-Chemia. 1996;156:67–70. [Google Scholar]
- 45.Heffter A. Ueber Pellote. Ein Betrag zur pharmakologischen Kenntnis der Cacteen. Naunyn Schmiedebergs Arch. Pharmacol. 1894;34:65–86. [Google Scholar]
- 46.Lundstrom J., Agurell S. Biosynthesis of mescaline and anhalamine in peyote. IIa. Tetrahedron Lett. 1968;9:4437–4440. doi: 10.1016/s0040-4039(01)99153-1. [DOI] [PubMed] [Google Scholar]
- 47.Klein M.T., Kalam M., Trout K., Fowler N., Terry M. Mescaline concentrations in three principal tissues of Lophophora Williamsii (Cactaceae): Implications for sustainable harvesting practices. Haseltonia. 2015;2015(20):34–42. [Google Scholar]
- 48.Steiner I., Brauers G., Temme O., Daldrup T. A sensitive method for the determination of hordenine in human serum by ESI(+) UPLC-MS/MS for forensic toxicological applications. Anal. Bioanal. Chem. 2016;408:2285–2292. doi: 10.1007/s00216-016-9324-3. [DOI] [PubMed] [Google Scholar]
- 49.Frank M., Weckman T.J., Wood T., Woods W.E., Tai C.L., Chang S.L., Ewing A., Blake J.W., Tobin T. Hordenine: Pharmacology, pharmacokinetics and behavioural effects in the horse. Equine Vet. J. 1990;22:437–441. doi: 10.1111/j.2042-3306.1990.tb04312.x. [DOI] [PubMed] [Google Scholar]
- 50.Ghansah E., Kopsombut P., Maleque M.A., Brossi A. Effects of mescaline and some of its analogs on cholinergic neuromuscular transmission. Neuropsychopharmacol. 1993;32:169–174. doi: 10.1016/0028-3908(93)90097-m. [DOI] [PubMed] [Google Scholar]
- 51.Bruhn J.G., El-Seedi H.R., Stephanson N., Beck O., Shulgin A.T. Ecstasy analogues found in cacti. J. Psychoactive Drugs. 2008;40:219–222. doi: 10.1080/02791072.2008.10400635. [DOI] [PubMed] [Google Scholar]
- 52.da Silva D.D., Silva E., Carvalho F., Carmo H. Mixtures of 3,4-methylenedioxymethamphetamine (ecstasy) and its major human metabolites act additively to induce significant toxicity to liver cells when combined at low, non-cytotoxic concentrations. J. Appl. Toxicol. 2014;34:618–627. doi: 10.1002/jat.2885. [DOI] [PubMed] [Google Scholar]
- 53.Millan M.J., Marin P., Bockaert J., Mannoury la Cour C. Signaling at G-protein-coupled serotonin receptors: Recent advances and future research directions. Trends Pharmacol. Sci. 2008;29:454–464. doi: 10.1016/j.tips.2008.06.007. [DOI] [PubMed] [Google Scholar]
- 54.Landolt H.P., Wehrle R. Antagonism of serotonergic 5-HT2A/2C receptors: Mutual improvement of sleep, cognition and mood? Eur. J. Neurol. 2009;29:1795–1809. doi: 10.1111/j.1460-9568.2009.06718.x. [DOI] [PubMed] [Google Scholar]
- 55.Urbán L., Patel V.F., Vaz R.J. Antitargets and drug safety. Weinheim: Wiley-VCH; 2015. [Google Scholar]
- 56.Aghajanian G.K., Marek G.J. Serotonin and hallucinogens. Neuropsychopharmacology. 1999;21:16s–23s. doi: 10.1016/S0893-133X(98)00135-3. [DOI] [PubMed] [Google Scholar]
- 57.Monte A.P., Waldman S.R., Marona-Lewicka D., Wainscott D.B., Nelson D.L., Sanders-Bush E., Nichols D.E. Dihydrobenzofuran analogues of hallucinogens. 4. Mescaline derivatives. J. Med. Chem. 1997;40:2997–3008. doi: 10.1021/jm970219x. [DOI] [PubMed] [Google Scholar]
- 58.Freedman D.X., Gottlieb R., Lovell R.A. Psychotomimetic drugs and brain 5-hydroxytryptamine metabolism. Biochem. Pharmacol. 1970;19:1181–1188. [Google Scholar]
- 59.Tilson H.A., Sparber S.B. Studies on the concurrent behavioral and neurochemical effects of psychoactive drugs using the push-pull cannula. J. Pharmacol. Exp. Ther. 1972;181:387–398. [PubMed] [Google Scholar]
- 60.Trulson M.E., Crisp T., Henderson L.J. Mescaline elicits behavioral effects in cats by an action at both serotonin and dopamine receptors. Eur. J. Pharmacol. 1983;96:151–154. doi: 10.1016/0014-2999(83)90544-7. [DOI] [PubMed] [Google Scholar]
- 61.Freedman D.X. The psychopharmacology of hallucinogenic agents. Annu. Rev. Med. 1969;20:409–418. doi: 10.1146/annurev.me.20.020169.002205. [DOI] [PubMed] [Google Scholar]
- 62.van Amsterdam J., Opperhuizen A., van den Brink W. Harm potential of magic mush2room use: A review. Regul. Toxicol. Pharmacol. 2011;59:423–429. doi: 10.1016/j.yrtph.2011.01.006. [DOI] [PubMed] [Google Scholar]
- 63.Dasgupta A. Advances in clinical chemis-try. Vol. 78. Makowski, G.S., Ed. Elsevier; 2017. Chapter Five - Challenges in Laboratory Detection of Unusual Substance Abuse: Issues with Magic Mushroom, Peyote Cactus, Khat, and Solvent Abuse. pp. 63–186. [DOI] [PubMed] [Google Scholar]
- 64.Mokrasch L.C., Stevenson I. The metabolism of mescaline with a note on correlations between metabolism and psychological effects. J. Nerv. Ment. Dis. 1959;129:177–183. doi: 10.1097/00005053-195908000-00007. [DOI] [PubMed] [Google Scholar]
- 65.Cochin J., Woods L.A., Seevers M.H. The absorption, distribution and urinary excretion of mescaline in the dog. J. Pharmacol. Exp. Ther. 1951;101:205–209. [PubMed] [Google Scholar]
- 66.Palenicek T., Balikova M., Bubenikova-Valesova V., Horacek J. Mescaline effects on rat behavior and its time profile in serum and brain tissue after a single subcutaneous dose. Psychopharmacology (Berl.) 2008;196:51–62. doi: 10.1007/s00213-007-0926-5. [DOI] [PubMed] [Google Scholar]
- 67.Halpern J.H. Hallucinogens and dissociative agents naturally growing in the United States. Pharmacol. Ther. 2004;102:131–138. doi: 10.1016/j.pharmthera.2004.03.003. [DOI] [PubMed] [Google Scholar]
- 68.Charalampous K.D., Walker K.E., Kinross-Wright J. Metabolic fate of mescaline in man. Psychopharmacology (Berl.) 1966;9:48–63. doi: 10.1007/BF00427703. [DOI] [PubMed] [Google Scholar]
- 69.Harley-Mason J., Laird A.H., Smythies J.R. The metabolism of mescalin in the human; Delayed clinical reactions to mescalin. Confin. Neurol. 1958;18:152–155. doi: 10.1159/000105047. [DOI] [PubMed] [Google Scholar]
- 70.Shah N.S., Green C. Tissue levels of mescaline in mice: Influence of chlorpromazine on repeated administration of mescaline. Eur. J. Pharmacol. 1973;24:334–340. doi: 10.1016/0014-2999(73)90159-3. [DOI] [PubMed] [Google Scholar]
- 71.Seiler N., Demisch L. Oxidative metabolism of mescaline in the central nervous system-III: Side chain degradation of mescaline and formation of 3,4,5-trimethoxy-benzoic acid In vivo. Biochem. Pharmacol. 1974;23:259–271. doi: 10.1016/0006-2952(74)90417-1. [DOI] [PubMed] [Google Scholar]
- 72.Daly J., Axelrod J., Witkop B. Methylation and demethylation in relation to the in vitro metabolism of mescaline. Ann. N. Y. Acad. Sci. 1962;96:37–43. doi: 10.1111/j.1749-6632.1962.tb50099.x. [DOI] [PubMed] [Google Scholar]
- 73.Kovacic P., Somanathan R. Novel, unifying mechanism for mescaline in the central nervous system: Electrochemistry, catechol redox metabolite, receptor, cell signaling and structure activity relationships. Oxid. Med. Cell. Longev. 2009;2:181–190. doi: 10.4161/oxim.2.4.9380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Shah N.S., Himwich H.E. Study with mescaline-8-C14 in mice: Effect of amine oxidase inhibitors on metabolism. Neuropsychopharmacol. 1971;10:547–556. doi: 10.1016/0028-3908(71)90020-7. [DOI] [PubMed] [Google Scholar]
- 75.Hilliker K.S., Roth R.A. Prediction of mescaline clearance by rabbit lung and liver from enzyme kinetic data. Biochem. Pharmacol. 1980;29:253–255. doi: 10.1016/0006-2952(80)90337-8. [DOI] [PubMed] [Google Scholar]
- 76.Scheline R.R. Handbook of mammalian metabolism of plant compounds (1991). Boca Raton: CRC Press; 2017. [Google Scholar]
- 77.Friedhoff A.J., Goldstein M. New developments in metabolism of mescaline and related amines. Ann. N. Y. Acad. Sci. 1962;96:5–13. doi: 10.1111/j.1749-6632.1962.tb50097.x. [DOI] [PubMed] [Google Scholar]
- 78.Seiler N., Demisch L. Oxidative metabolism of mescaline in the central nervous system. II. Oxidative deamination of mescaline and 2,3,4-trimethoxy-beta-phenylethylamine by different mouse brain area in vitro. Biochem. Pharmacol. 1971;20:2485–2493. doi: 10.1016/0006-2952(71)90249-8. [DOI] [PubMed] [Google Scholar]
- 79.Steensholt G. On an amine oxidase in rabbit’s liver. Acta Physiol. Scand. 1947;14:356–362. doi: 10.1111/j.1748-1716.1947.tb00471.x. [DOI] [PubMed] [Google Scholar]
- 80.Demisch L., Seiler N. Oxidative metabolism of mescaline in the central nervous system-V: In vitro deamination of mescaline to 3,4,5-trimethoxy-benzoic acid. Biochem. Pharmacol. 1975;24:575–580. doi: 10.1016/0006-2952(75)90176-8. [DOI] [PubMed] [Google Scholar]
- 81.Carvalho M., Carmo H., Costa V.M., Capela J.P., Pontes H., Remiao F., Carvalho F., Bastos Mde L. Toxicity of amphetamines: An update. Arch. Toxicol. 2012;86:1167–1231. doi: 10.1007/s00204-012-0815-5. [DOI] [PubMed] [Google Scholar]
- 82.Demisch L., Seiler N. Oxidative metabolism of mescaline in the central nervous system-V. In vitro deamination of mescaline to 3,4,5-trimethoxy-benzoic acid. Biochem. Pharmacol. 1975;24:575–580. doi: 10.1016/0006-2952(75)90176-8. [DOI] [PubMed] [Google Scholar]
- 83.Musacchio J., Goldstein M. The metabolism of mescaline-14C in rats. Biochem. Pharmacol. 1967;16:963–970. doi: 10.1016/0006-2952(67)90268-7. [DOI] [PubMed] [Google Scholar]
- 84.Axelrod J. The enzymic cleavage of aromatic ethers. Biochem. J. 1956;63:634–639. doi: 10.1042/bj0630634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Friedhoff A.J., Hollister L.E. Comparison of the metabolism of 3,4-dimethoxyphenylethylamine and mescaline in humans. Biochem. Pharmacol. 1966;15:269–273. doi: 10.1016/0006-2952(66)90298-x. [DOI] [PubMed] [Google Scholar]
- 86.Wu D., Otton S.V., Inaba T., Kalow W., Sellers E.M. Interactions of amphetamine analogs with human liver CYP2D6. Biochem. Pharmacol. 1997;53:1605–1612. doi: 10.1016/s0006-2952(97)00014-2. [DOI] [PubMed] [Google Scholar]
- 87.Demisch L., Kaczmarczyk P., Seiler N. 3,4,5-Trimethoxybenzoic acid, a new mescaline metabolite in humans. Drug Metab. Dispos. 1978;6:507–509. [PubMed] [Google Scholar]
- 88.Goldstein M., Contrera J.F. The substrate specificity of phenylamine-beta-hydroxylase. J. Biol. Chem. 1962;237:1898–1902. [PubMed] [Google Scholar]
- 89.Hermle L., Funfgeld M., Oepen G., Botsch H., Borchardt D., Gouzoulis E., Fehrenbach R.A., Spitzer M. Mescaline-induced psychopathological, neuropsychological, and neurometabolic effects in normal subjects: Experimental psychosis as a tool for psychiatric research. Biol. Psychiatry. 1992;32:976–991. doi: 10.1016/0006-3223(92)90059-9. [DOI] [PubMed] [Google Scholar]
- 90.Hardman H.F., Haavik C.O., Seevers M.H. Relationship of the structure of mescaline and seven analogs to toxicity and behavior in five species of laboratory animals. Toxicol. Appl. Pharmacol. 1973;25:299–309. doi: 10.1016/s0041-008x(73)80016-x. [DOI] [PubMed] [Google Scholar]
- 91.Schultes R.E. Hallucinogens of plant origin. Science. 1969;163:245–254. doi: 10.1126/science.163.3864.245. [DOI] [PubMed] [Google Scholar]
- 92.Bressloff P.C., Cowan J.D., Golubitsky M., Thomas P.J., Wiener M.C. What geometric visual hallucinations tell us about the visual cortex. Neural Comput. 2002;14:473–491. doi: 10.1162/089976602317250861. [DOI] [PubMed] [Google Scholar]
- 93.Golembiowska K., Jurczak A., Kaminska K., Noworyta-Sokolowska K., Gorska A. Effect of Some Psychoactive Drugs Used as ‘Legal Highs’ on Brain Neurotransmitters. Neurotox. Res. 2016;29:394–407. doi: 10.1007/s12640-015-9569-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Shulgin A.T. Mescaline: The chemistry and pharmacology of its analogs. Lloydia. 1973;36:46–58. [PubMed] [Google Scholar]
- 95.Schultes R.E. The Appeal of Peyote (Lophophora Williamsii) as a Medicine. Am. Anthropol. 1938;40:698–715. [Google Scholar]
- 96.McLaughlin J.L. Peyote: An introduction. Lloydia. 1973;36:1–8. [PubMed] [Google Scholar]
- 97.Halpern J.H. The Use of Hallucinogens in the Treatment of Addiction. Addict. Res. 1996;4:177–189. [Google Scholar]
- 98.Gilmore H.T. Peyote use during pregnancy. S. D. J. Med. 2001;54:27–29. [PubMed] [Google Scholar]
- 99.Hardaway R., Schweitzer J., Suzuki J. Hallucinogen Use Disorders. Child Adolesc. Psychiatr. Clin. N. Am. 2016;25:489–496. doi: 10.1016/j.chc.2016.03.006. [DOI] [PubMed] [Google Scholar]
- 100.Brown R.T., Braden N.J. Hallucinogens. Pediatr. Clin. North Am. 1987;34:341–347. doi: 10.1016/s0031-3955(16)36219-8. [DOI] [PubMed] [Google Scholar]
- 101.Stevenson I., Mokrasch L.C. A further note on the mechanism of the antidotal action of sodium succinate in the mescaline psychosis. Am. J. Psychiatry. 1958;114:1038–1039. doi: 10.1176/ajp.114.11.1038. [DOI] [PubMed] [Google Scholar]
- 102.Stevenson I., Sanchez A.J., Jr The antidotal action of sodium succinate in the mescaline psychosis. Am. J. Psychiatry. 1957;114:328–332. doi: 10.1176/ajp.114.4.328. [DOI] [PubMed] [Google Scholar]
- 103.Nolte K.B., Zumwalt R.E. Fatal peyote ingestion associated with Mallory-Weiss lacerations. West. J. Med. 1999;170:328. [PMC free article] [PubMed] [Google Scholar]
- 104.Hashimoto H., Clyde V.J., Parko K.L. Botulism from peyote. N. Engl. J. Med. 1998;339:203–204. doi: 10.1056/nejm199807163390316. [DOI] [PubMed] [Google Scholar]
- 105.McCleary J.A., Sypherd P.S., Walkington D.L. Antibiotic activity of an extract of peyote (Lophophora Williamii (Lemaire) coulter). Econ. Bot. 1960;14:247–249. [Google Scholar]
- 106.Lumholtz C. Unknown Mexico. London: MacMillan and Co., Limited; 1903. [Google Scholar]
- 107.Cassels B.K., Saez-Briones P. Dark classics in chemical neuroscience: mescaline. ACS Chem. Neurosci. 2018;9(10):2448–2458. doi: 10.1021/acschemneuro.8b00215. [DOI] [PubMed] [Google Scholar]
- 108.Rucker J.J.H., Iliff J., Nutt D. J. Psychiatry & the psychedelic drugs. Past, present & future. Neuropsychopharmacol. 2017;142:200–218. doi: 10.1016/j.neuropharm.2017.12.040. [DOI] [PubMed] [Google Scholar]
- 109.Rickli A., Moning O.D., Hoener M.C., Liechti M.E. Receptor interaction profiles of novel psychoactive tryptamines compared with classic hallucinogens. Eur. Neuropsychopharmacol. 2016;26:1327–1337. doi: 10.1016/j.euroneuro.2016.05.001. [DOI] [PubMed] [Google Scholar]
- 110.Shulgin A.T., Shulgin A. PIHKAL: A chemical love story. Berkeley: Transform Press; 1991. [Google Scholar]


