Abstract
Stingless bee honey produced by Heterotrigona itama from different botanical origins was characterised and discriminated. Three types of stingless bee honey collected from acacia, gelam, and starfruit nectars were analyzed and compared with Apis mellifera honey. The results showed that stingless bee honey samples from the three different botanical origins were significantly different in terms of their moisture content, pH, free acidity, total soluble solids, colour characteristics, sugar content, amino acid content and antioxidant properties. Stingless bee honey was significantly different from Apis mellifera honey in terms of physicochemical and antioxidant properties. The amino acid content was further used in the chemometrics analysis to evaluate the role of amino acid in discriminating honey according to botanical origin. Partial least squares-discriminant analysis (PLS-DA) revealed that the stingless bee honey was completely distinguishable from Apis mellifera honey. Notably, a clear distinction between the stingless bee honey types was also observed. The specific amino acids involved in the distinction of honey were cysteine for acacia and gelam, phenylalanine and 3-hydroxyproline for starfruit, and proline for Apis mellifera honey. The results showed that all honey samples were successfully classified based on amino acid content.
Keywords: stingless bee honey, physicochemical characteristics, antioxidants properties, free amino acids, partial least square-discriminant analysis (PLS-DA), chemometrics analysis
1. Introduction
Stingless bee honey is a valuable product made by stingless bees. It has been reported to have higher nutritional and medicinal values compared to Apis mellifera honey [1]. However, the production of stingless bee honey is limited mainly because of the low quantities produced by stingless bees [2]. Nevertheless, stingless bee honey has been reported to be beneficial for human health due to the high antioxidant content [3]. It is also estimated that the price of stingless bee honey is much higher than Apis mellifera honey [4]. For instance, the market price of stingless bee honey can be as high as AU 50/kg [5]. Recently, there has been an increasing demand for pure and high-quality stingless bee honey as low-quality honey is usually produced with the addition of adulterants such as sweeteners (cane sugar, beet sugar, corn syrup, high fructose or maltose syrup) [6]. This practice is thought to alter its nutritional value and medicinal benefits. The quality of honey can also be characterised by its purity and source of origin. The purity of honey can be determined by its physicochemical properties (moisture content, pH, free acidity, total soluble solids, sugar content, colour characteristics and intensity, 5-hydroxymethylfurfural (5-HMF) content, and amino acid content), while the source of origin is influenced by several factors such as botanical, geographical and entomological origins [7]. Hence, the evaluation of physicochemical properties and authentication of botanical origins is vital to ascertain the quality and authenticity of honey. It is also evident that the quality and source of origin of honey may influence its market price and consumer acceptance, and, hence, it is important to determine these factors to guarantee the consumers’ safety and protect them against fraud.
Pollen analysis, traditionally known as the melissopalynalogical method, has been widely used to determine the botanical origin of honey and is performed through the identification of pollen constituents present in honey. This technique, however, is a very time-consuming and tedious process that relies on the availability of a comprehensive database of pollen grains and requires a well-trained analyst with a good knowledge of pollen morphology [8]. At present, other components in honey such as free amino acids [9], volatile compounds [10], protein content [11], carbohydrates content [12], and phenolic acids content [1] have been confirmed as markers for the identification of botanical and geographical origins of Apis mellifera honey.
In previous studies involving stingless bee honey, numerous parameters have been used to differentiate stingless bee honey types according to their botanical and entomological origins such as compositional features [13], physicochemical and antioxidant properties [14,15], chemical properties and mineral content [16], and a combination of sensorial, physicochemical and sugar content [17]. However, to date, there is no study performed on the use of amino acids as variables in classifying stingless bee honey according to their botanical origins.
Honey contains approximately 1.0 % (w/w) of amino acids derived mainly from the fluids and nectar secretions of the salivary glands and pharynx of honeybees. Pollen, however, has been reported as the main source of amino acids in honey [6]. There are many types of amino acids detected in honey such as alanine, asparagine, glutamine, histidine, glycine, arginine, valine, tyrosine, cysteine, lysine and others [18]. Therefore, these amino acids can be employed as a useful indicator to classify honey based on its botanical origin. Some amino acids have been identified as specific chemical markers for certain types of honey [9,19,20,21].
At present, the determination of botanical and geographical origins of honey is achieved using chemometric techniques to analyse complex data, extract useful information and simplify the analysis [20]. Additionally, support from modern statistical and quantitative data analysis techniques is required to obtain accurate and reliable results. By applying these techniques, the properties of honey and its corresponding constituents can be analysed, identified and classified. In this study, three types of stingless bee honey from acacia, gelam and starfruit nectars were investigated for their physicochemical properties and botanical origins, in which Apis honey was used for comparison. The aims of this study were to (1) assess the impact of botanical origins on the physicochemical properties and (2) affirm the botanical origin of the honey samples based on the amino acids content using chemometric techniques and, additionally, determine the amino acid markers that discriminate them.
2. Results and Discussion
2.1. Physicochemical Properties
The values for moisture content (before and after the drying process), pH, free acidity, total soluble solids (TSS), colour intensity, colour characteristics and 5-hydroxymethylfurfural (5-HMF) are shown in Table 1. Among all these parameters, moisture content, acidity, 5-HMF and sugars content have been stipulated by Codex Alimentarius (2001) [22] as the quality parameters for honey (Apis mellifera). According to Codex Alimentarius (2001), honey (Apis mellifera) must contain moisture content less than 20 g/100 g of honey, acidity value below than 50 meq/kg of honey and 5-HMF content, must less than 80mg/kg of honey. Based on the results obtained, the Apis honey sample met all the requirements. The moisture content, free acidity and 5-HMF content of the Apis honey sample were 14.67 g/100 of g, 39.22 meq/kg of honey and not detected (ND), respectively. This indicates that Apis honey was of a good quality, while all stingless bee honey samples met the requirements set by the Malaysian stingless bee honey standard [23]. According to the Malaysian stingless bee standard, raw stingless bee honey must contain moisture content less than 35 g/100 g of honey, pH less than 3.8 and 5-HMF less than 30 mg/kg of honey.
Table 1.
Physicochemical characteristics of honey samples from different botanical origins.
| Parameters | Unit | Botanical Origins of Honey | Apis | ||
|---|---|---|---|---|---|
| Acacia | Starfruit | Gelam | mellifera | ||
| Moisture content * | g/100 g | 21.52 ± 0.66 c | 24.24 ± 0.19 b | 25.49 ± 0.45 a | 14.67 ± 0.11 d |
| Moisture content ** | g/100 g | 13.86 ± 0.38 b | 15.63 ± 0.41 a | 14.24 ± 0.65 b | NA |
| pH | pH | 3.27 ± 0.03 b | 3.00 ± 0.03 d | 3.18 ± 0.03 c | 3.56 ± 0.02 a |
| Free acidity | meq/kg honey | 107.50 ± 6.45 c | 246.25 ± 9.46 a | 176.25 ± 9.46 b | 39.22 ± 1.50 d |
| TSS | °Brix | 74.65 ± 0.39 bc | 73.88 ± 0.34 c | 74.85 ± 0.73 b | 76.40 ± 0.54 a |
| Colour | mm Pfund | 1.25 ± 0.10 d | 36.85 ± 2.00 c | 46.45 ± 2.45 a | 40.70 ± 0.53 b |
| Colour intensity | mAU | 32.25 ± 3.59 d | 122.50 ± 9.57 c | 280.00 ± 18.26 a | 251.00 ± 2.16 b |
| 5-HMF | mg/kg honey | ND b | 0.07 ± 0.06 a | 0.05 ± 0.02 a | ND b |
* Before dehumidification process; ** After dehumidification process; TSS = Total soluble solid; 5-HMF = 5-Hydroxymethylfurfural; NA = Not available;.ND = Not detected. Mean values in the same row with distinct superscript letters indicate a significant difference at p < 0.05.
Overall, physicochemical results showed that all stingless bee honey had higher (21.52–25.49 g/100 g of honey) moisture content than Apis honey (14.67 g/100 g of honey). Stingless bee honey also had lower pH (3.00–3.27) and higher free acidity (107.50–246.25 meq/kg of honey) values as compared to Apis honey. In terms of total soluble solid (TSS), Apis honey showed higher (76.40 °Brix) TSS than stingless bee honey. In addition to that, 5-HMF of all honey samples studied were low and some honeys such as acacia and Apis honey were free from 5-HMF. This indicates that all honey samples used were fresh.
Stingless bee honey is naturally high in moisture content. The moisture content values of all raw honey samples ranged from 14.67 to 25.49 g/100 g as shown in Table 1. Apis honey had the lowest moisture content of 14.67 g/100 g and was significantly lower compared to all the stingless bee honey samples, which ranged from 21.52 to 25.49 g/100 g. Stingless bee honey also showed significant differences in the moisture content among honey from different botanical origins, with acacia and gelam honey having the highest and lowest moisture content of 21.52 g/100 g and 25.49 g/100 g, respectively. Moisture content is a crucial parameter in the determination of honey quality. Based on the Codex Alimentarius (2001) [22], it is recommended that the moisture content of honey should not surpass 20 g/100 g. This is mainly because honey is vulnerable to fermentation and has low stability against microbes when the moisture content is higher than 20 g/100 g [24]. Generally, stingless bee honey owns an elevated moisture content than Apis honey [14]. This may be due to the different bee species and different preferences of bees towards the plant species used for honey production [25]. Issaro et al. [26] reported that honey from Thailand had lower levels of moisture content compared to our findings, in which the values were 15.73, 13.26 and 14.66 g/100 g for Trigonalaeviceps Smith, Trigona sp. and Trigonapagdenis Schwarz bee species, respectively. In contrast, honey from a Peruvian stingless bee species, Partanoma epiphytophila, possessed a higher moisture content of 45.80 g/100 g [27].
Owing to the high moisture content, stingless bee honey was subjected to the dehumidification process to reduce moisture content and avoid the honey sample from ferment. In this study, the moisture content of stingless bee honey samples obtained after the dehumidification process decreased by approximately 35.52% to 44.13%. The honey moisture content was lower than 20%, and therefore shown to comply with the Malaysian stingless bee honey standard for processed honey [23]. It is believed that honey moisture content levels lower than 20% can facilitate stingless bee honey preservation and prolong its shelf life.
In general, honey is naturally acidic [28]. Acidity has been reported to have a link with moisture content of the honey. Honey with high moisture content vulnerable to ferment and resulted in the high free acidity and low pH values. For instance, stingless bee honey samples had higher moisture content than Apis honey. Therefore, their pH was lower than Apis honey. The results in this study show that the pH and free acidity values obtained for the stingless bee honey samples varied between 3.00 and 3.27 and 107.50 to 246.25 meq/kg, respectively (Table 1). Apis honey, on the other hand, had a significantly higher pH and lower free acidity values compared to the stingless bee honey. Therefore, stingless bee honey was shown to be more acidic as opposed to Apis honey. Our findings are consistent with a previous study by Chuttong et al. [2] who also reported high free acidity values in stingless bee honey obtained from Thailand. Studies have shown that the organic acid content in honey is the main component responsible for the acidity of honey [29].
The total soluble solid has an association with moisture and sugar content in honey [25]. In general, honey with high total soluble solid possesses high sugar content and low moisture content. The total soluble solids of stingless bee honey from different botanical origins varied between 73.88 and 74.85 Brix (Table 1). The Brix value for Apis honey was significantly higher (76.40) than stingless bee honey.
All of the tested honey samples showed low 5-HMF content, ranging from not detected (ND) to 0.07 mg/kg as shown in Table 1. These findings indicate that the honey samples were of good quality as the values were below the maximum limit of 80 mg/kg as specified by the Codex Alimentarius (2001) [22].
The colour of honey is related to the minerals, phenolic compounds, and carotenoids present in honey [30]. Naturally, the colour of honey differs greatly, ranging from yellow to amber, dark amber and black in some cases. In this study, the colour characteristics and colour intensity of stingless bee honey varied between 1.25 and 46.45 mm Pfund, and 32.25 and 280.00 mAU, respectively (Table 1). According to the United States Department of Agriculture (USDA) [31] colour standards, starfruit, gelam and Apis honey types are classified as extra light amber, whereas acacia honey is classified as water white honey. Apis honey, on the other hand, showed significantly lower values for both parameters compared to gelam honey. The differences in colour characteristics and colour intensity were attributed to the different botanical sources used by bees to produce honey.
The sugar concentration profiles of stingless bee and Apis honey are shown in Table 2. The overall mean values of total sugar in stingless bee honey ranged from 70.89 to 73.96 g/100, in which no significant differences were observed between honey samples from different botanical origins. In contrast, Apis honey had the lowest total sugar content with a mean value of 68.80 g/100 g and was shown to be significantly lower compared to stingless bee honey. It is thought that the low total sugar content in Apis honey could be attributed to the difference in bee species.
Table 2.
The concentration of sugar (g/100 g) in honey samples from different botanical origins.
| Parameters | Botanical Origin of Honey | Apis | ||
|---|---|---|---|---|
| Acacia | Starfruit | Gelam | mellifera | |
| Fructose | 22.05 ± 0.85 c | 15.27 ± 0.51 d | 29.06 ± 1.52 b | 33.99 ± 0.31 a |
| Glucose | 21.17 ± 1.50 c | 17.42 ± 0.82 d | 27.54 ± 1.98 b | 32.24 ± 0.38 a |
| Sucrose | 28.44 ± 0.89 b | 37.32 ± 1.14 a | 17.36 ± 1.09 c | 2.57 ± 0.24 d |
| Maltose | ND b | 0.89 ± 0.43 a | ND b | ND b |
| Total sugar | 71.65 ± 2.04 ab | 70.89 ± 0.80 bc | 73.96 ± 2.74 a | 68.80 ± 0.72 c |
ND = not detected. Mean values in the same row with distinct superscript letters indicate a significant difference at p < 0.05.
The concentration of fructose and glucose in stingless bee honey varied from 15.27 to 29.06 g/100 g and 17.42 to 27.54 g/100 g, respectively. However, the concentration of fructose and glucose in Apis honey was 33.99 and 32.24 g/100 g, respectively, and significantly higher than stingless bee honey. In general, fructose is present in high concentrations in honey. However, in this study, only acacia and gelam honey exhibited higher fructose content compared to glucose, while starfruit had a lower fructose but higher glucose content. Previous studies by Tukshita et al. [32] Chuttong et al. [2] and Fuenmayor et al. [4] also found a lower fructose content in Geniotrigona thoracica (Borneo, Malaysia); Tetrigona melanoleuca (Thailand) and Plebeia spp. (Colombia), respectively. In addition, all the honey samples were statistically different in terms of their fructose and glucose contents depending on the type of botanical origin. Apart from fructose and glucose, maltose was also detected in starfruit honey with a concentration of 0.89 g/100 g. Based on several other studies from different countries, the maltose content found in our samples were much lower compared to the findings reported by Tukshita et al. [32], Chuttong et al. [2] and Oddo et al. [33], whereby the values ranged from 20.3 to 53.00 g/100 g.
Sugar composition is directly related to the nectar of blossoms gathered by bees [6]. The enzyme, invertase, is produced by bees to breakdown sucrose into fructose and glucose [34]. Thus, the sucrose content in honey acts as an indicator to determine its maturity and quality [6]. Mature and good quality honey should contain a sucrose content that is lower than 5 g/100 g [22]. The sucrose content detected in stingless bee honey samples varied between 17.36 and 37.32 g/100 g, while, for Apis honey, a lower value of 2.57 g/100 g was observed. All the stingless bee honey samples showed significant differences in terms of their sucrose content as well. These data indicate that the stingless bee honey samples evaluated in this study did not reach the maturity stage during the harvesting process due to the incomplete transformation of sucrose into glucose and fructose. Besides nectar, climate conditions and geographical regions can contribute to the different sugar compositions in honey [6].
2.2. Amino Acid Profile
In this study, gas chromatography flame-ionisation detection (GC-FID) was successfully employed in the separation of amino acids in stingless bee and Apis honey samples. A typical GC-FID chromatogram of amino acids is shown in Figure 1. The limit of detection (LOD) and limit of quantitation (LOQ) for the amino acids in honey samples were 0.01–2.75 mg/kg and 0.02–8.33 mg/kg, respectively. The amino acids profile and concentration of stingless bee and Apis honey are shown in Table 3. All the amino acids analysed for the different honey types were detected, although some amino acids were found to be below the quantitation limit. For example, seven amino acids were detected in acacia, gelam and starfruit honey, while four amino acids were detected in Apis honey. The total amino acid content varied widely between 380.82 and 947.01 mg/kg. Among the stingless bee honey types, starfruit honey had the highest total content of amino acids at 947.01 mg/kg, while acacia honey had the lowest content at 380.82 mg/kg. Significant differences were also observed among the stingless bee honey samples in terms of the total amino acid content. For instance, when compared to Apis honey, starfruit honey samples exhibited a significantly higher value of total amino acid content, while acacia honey had a significantly lower value. Our results also showed a lower amount of total amino acid content compared to chaste honey (1572.90 mg/100 g) from China [20]. Nevertheless, starfruit honey showed a higher total amino acid content in comparison to buckwheat honey (633.50 mg/kg) from Poland and heather honey (655.10 mg/kg) from Estonia [21,35]. On the other hand, gelam and acacia honey had higher total amino acid contents than rape, willow, linden and dandelion honey [21]. These results suggest that the botanical origin can affect the amino acid composition in honey. At present, there is no data available on amino acid composition in stingless bee honey, and, hence, all comparisons were made based on the amino acid content in Apis honey.
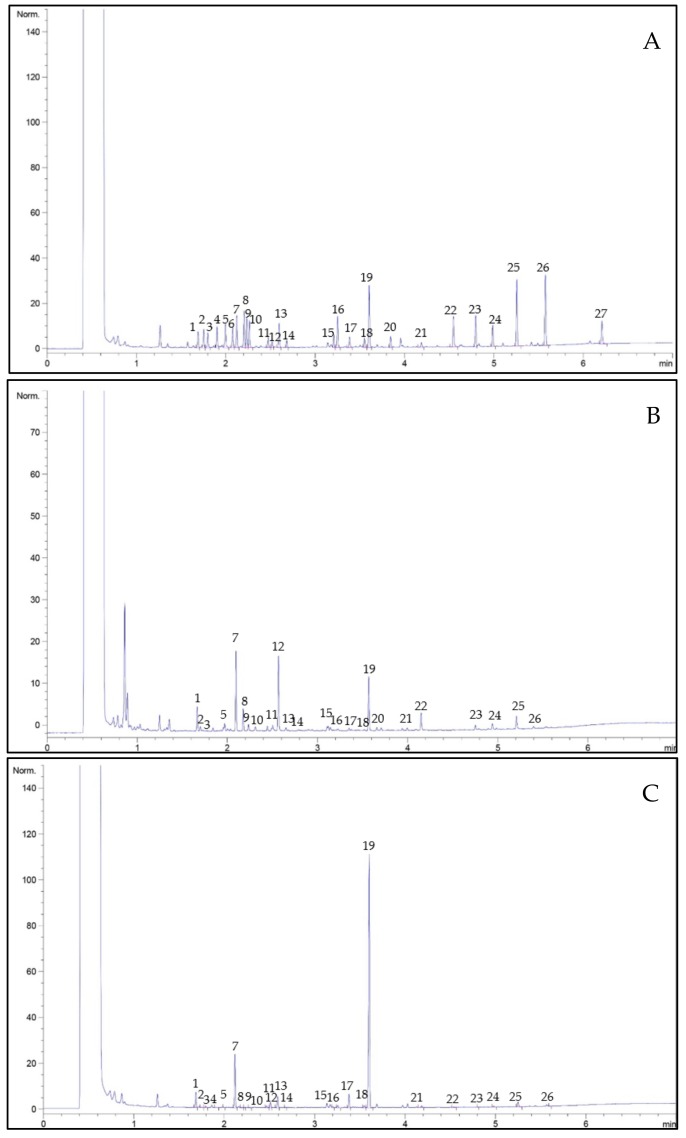
Figure 1.
Representative gas chromatography flame-ionisation detection (GC-FID) chromatogram of amino acids standard mixture (100 umol/L) (A); Apis honey (B) and starfruit honey (C). For peak identification, 1 = Alanine; 2 = sarcosine; 3 = glycine; 4 = α-Aminobutyric acid; 5 = Valine; 6 = β-Aminoisobutyric acid; 7 = IS; 8 = Leucine; 9 = Allo-isoleucine; 10 = Isoleucine; 11 = Threonine; 12 = Serine; 13 = Proline; 14 = Asparagine; 15 = Aspartic acid; 16 = Methionine; 17 = 3-hydroxyproline; 18 = glutamic acid; 19 = Phenylalanine; 20 = α-Aminoadipic acid; 21 = Glutamine; 22 = Ornithine; 23 = Lysine; 24 = Histidine; 25 = Tyrosine; 26 = Tryptophan; 27 = Cysteine.
Table 3.
Amino acids profile and concentration (mg/kg) in honey samples from different botanical origins.
| Amino Acids | Botanical Origin of Honey | |||
|---|---|---|---|---|
| Acacia | Starfruit | Gelam | Apis mellifera | |
| Alanine | 35.04 ± 2.03 c | 46.00 ± 2.06 a | 35.96 ± 2.14 c | 41.34 ± 0.34 b |
| Sarcosine | <LOQ | <LOQ | <LOQ | <LOQ |
| Glycine | <LOQ | <LOQ | <LOQ | <LOQ |
| α-Aminobutyric acid | <LOQ | <LOQ | <LOQ | ND |
| Valine | 1.24 ± 0.77 b | <LOQ b | 5.07 ± 1.65 a | 6.06 ± 1.92 a |
| β-Aminoisobutyric acid | <LOQ | ND | <LOQ | ND |
| Leucine | 24.82 ± 0.64 c | 7.55 ± 0.20 d | 44.50 ± 4.44 a | 32.91 ± 0.26 b |
| Allo-isoleucine | 7.03 ± 1.31 a | 6.19 ± 0.13 a | 6.38 ± 0.03 a | ND b |
| Isoleucine | <LOQ b | <LOQ b | <LOQ b | 7.06 ± 0.84 a |
| Threonine | 4.75 ± 3.67 b | 3.85 ± 1.32 b | 7.07 ± 3.21 ab | 12.08 ± 4.25 a |
| Serine | 13.60 ± 2.46 b | 37.75 ± 5.14 a | 19.07 ± 0.83 b | 19.21 ± 11.45 b |
| Proline | 16.23 ± 5.68 d | 33.01 ± 6.20 c | 48.91 ± 1.44 b | 145.9 ± 3.39 a |
| Asparagine | <LOQ | <LOQ | <LOQ | <LOQ |
| Aspartic acid | <LOQ | <LOQ | <LOQ | <LOQ |
| Methionine | 16.71 ± 0.21 c | 22.62 ± 0.56 a | 19.49 ± 0.19 b | 21.40 ± 2.14 a |
| 3-hydroxyproline | 4.83 ± 3.01 c | 75.53 ± 5.47 a | 29.63 ± 2.28 b | 6.34 ± 0.77 c |
| Glutamic acid | 29.02 ± 4.27 b | 37.29 ± 4.15 a | 29.77 ± 2.31 b | 23.68 ± 0.59 c |
| Phenylalanine | 50.04 ± 4.76 c | 561.10 ± 37.59 a | 237.37 ± 13.73 b | 68.51 ± 0.35 c |
| α-Aminoadipic acid | 4.96 ± 1.00 ab | ND b | ND b | 7.69 ± 5.13 a |
| Glutamine | 42.15 ± 2.73 b | 24.75 ± 4.11 c | 29.32 ± 1.19 c | 134.09 ± 10.81 a |
| Ornithine | 11.26 ± 0.32 ab | 12.15 ± 0.87 a | 11.13 ± 0.50 b | 10.93 ± 0.59 b |
| Lysine | 15.46 ± 1.04 b | 15.17 ± 0.44 b | 14.67 ± 0.04 b | 21.94 ± 0.68 a |
| Histidine | 23.76 ± 4.09 b | 20.27 ± 2.08 bc | 17.82 ± 0.07 c | 30.03 ± 1.00 a |
| Tyrosine | 24.92 ± 0.84 b | 23.31 ± 2.63 b | 19.57 ± 1.97 c | 29.63 ± 0.66 a |
| Tryptophan | 20.32 ± 1.25 a | 20.46 ± 3.16 a | 19.05 ± 0.38 a | 20.58 ± 2.27 a |
| Cysteine | 34.67 ± 2.09 a | ND b | 33.01 ± 2.62 a | ND b |
| TOTAL | 380.82 ± 19.36 c | 947.01 ± 48.42 a | 627.78 ± 21.20 b | 639.47 ± 13.49 b |
<LOQ = below limit of quantitation; ND = not detected; Number of samples used for amino acids analysis; n = 4. Mean values in the same row with distinct superscript letters indicate a significant difference at p < 0.05.
Proline was significantly higher in Apis honey compared to stingless bee honey samples, while allo-isoleucine, cysteine, β-aminoisobutyric acid, and α-aminobutyric acid were not detected in Apis honey. Our findings were consistent with the results reported by Keskes et al. [9] and Qamer et al. [36], in which the authors also revealed a higher proline content in Apis honey. The differences in proline content may be attributed to the different species of bees involved in honey production. It has been reported that proline originates from the bees’ secretion and nectar used to make honey [37].
In acacia, starfruit and gelam honey, phenylalanine was the most abundant amino acid, ranging from 50.04 to 561.10 mg/kg, with starfruit honey having the highest value. Significant differences (p < 0.05) were also observed in the phenylalanine content among the stingless bee honey samples from different botanical origins. Sun et al. [20] reported higher amounts of phenylalanine in chaste honey from China, with a value of 1094.90 mg/100 g. The variation in phenylalanine content may be due to the different botanical and country origins of the honey. Many studies have also demonstrated and affirmed that the botanical origin and location can influence the types and concentration of amino acids present in honey [9,19,20]. The amino acids detected in stingless bee and Apis honey were used as variables in the chemometric analysis to classify the honey samples studied according to their botanical origins as well as to identify possible chemical markers that discriminate them.
2.3. Antioxidant Properties
Antioxidant capacity and activity of honey usually express as total phenolic content (TPC) and total flavonoids content (TFC) and free radical scavenging activity (IC50) and ferric reducing antioxidant power (FRAP) (Table 4). Antioxidant activity of honey mainly associated with the nectar source used by bees to make honey. In addition, phenolic compounds are derived from pollen and propolis constituents present in honey [38,39]. Botanical origin has been reported as the main factor that affect constituents and antioxidant activity of honey. Honey from different botanical origins have different antioxidant activity. The values of TPC detected in stingless bee honey were 61.47 mg GAE/100 g, 84.10 mg GAE/100 g and 114.49 mg GAE/100 g for acacia, starfruit and gelam honey, respectively (Table 4). Gelam and acacia honey had the highest and the lowest TPC values, respectively. Significant differences were also observed in TPC values among the different honey types, in which Apis honey had a significantly lower TPC value (29.05 mg GAE/100 g) compared to stingless bee honey. The variations in TPC value might be due to the different types of phenolic acids present in stingless bee and Apis honey [28]. This finding was consistent with the results reported previously in other studies [28,40].
Table 4.
Antioxidant properties of honey samples from different botanical origins.
| Parameters | Unit | Botanical Origin of Honey | |||
|---|---|---|---|---|---|
| Acacia | Starfruit | Gelam | Apis mellifera | ||
| Total phenolic contents (TPC) | mg GAE/100 g honey | 61.47 ± 3.34 c | 84.10 ± 5.33 b | 114.49 ± 7.31a | 29.05 ± 1.58 d |
| Total flavonoids content (TFC) | mg QAE/100 g honey | 3.63 ± 0.26 c | 11.15 ± 0.55 a | 8.41 ± 0.36 b | 1.57 ± 0.24 d |
| Free radical scavenging activity (IC50) | mg/mL | 58.35 ± 2.45 c | 90.63 ± 5.50 b | 14.29 ± 0.53 d | 202.15 ± 1.60 a |
| Ferric reducing antioxidant power (FRAP) | µmol Fe2SO4.7H2O/100 g honey | 180.59 ± 10.48 b | 263.90 ± 22.1 c | 512.10 ± 47.4 a | 40.22 ± 1.84 d |
GAE = Gallic acid equivalent; QAE = Quercetin acid equivalent; IC50 = concentration of honey solution required to mitigate the initial concentration of DPPH by 50%. Mean values in the same row with distinct superscript letters indicate a significant difference at p < 0.05.
In stingless bee honey samples, the flavonoids content (TFC) varied between 3.63 and 11.15 mg QAE/100 g as shown in Table 4, with significant differences (p < 0.05) observed between the different honey types. Apis honey contained significantly lower TFC values compared to the stingless bee honey types. It is thought that the variations observed in the TFC values could be due to the honey samples used in this study, which is comprised of different botanical origins, locations and bee species. Similar TFC values were observed in M. beecheii honey from Cuba (4.19 mg/100 g) [28] and Trigona spp. honey from Malaysia (4.46 to 7.91 mg QAE/100 g) [24]. On the other hand, Oliviera et al. [38] demonstrated higher TFC values in seven stingless bee honey samples from Bahia state, ranging from 30.24 to 279.73 mg QAE/100 g.
In general, honey with the lowest IC50 value possesses the highest antioxidant activity. The IC50 values found in all stingless bee honey samples analysed in this study ranged from 14.29 to 90.63 mg/mL (Table 4), while the IC50 for Apis honey was significantly higher at 202.15 mg/mL. Among the stingless bee honey types, gelam honey had the lowest IC50, followed by acacia and starfruit honey. These results suggest that gelam honey has the highest antioxidant activity compared to the other honey samples. All three types of stingless bee honey investigated in this study showed statistically different IC50 values. This observation may be due to the variations in phenolic content and types of phenolic compounds present in the honey samples [41]. Nevertheless, it has been reported that the phenolic content is correlated to antioxidant activity in honey [42,43,44]. Apart from phenolic acids, other compounds present in honey such as ascorbic acid, organic acids, amino acids, glucose oxidase, flavonoids and Maillard reaction products can also contribute to the antioxidant activity [45]. Higher IC50 values were detected in seven Melipona species honey samples from Bahia state, with values ranging from 25.39 to 51.44 mg/mL.
The FRAP values for the stingless bee honey investigated in this study ranged between 180.59 and 512.10 µmol Fe2SO4.7H2O/100 g (Table 4). As previously observed for the IC50 values, gelam honey exhibited the highest FRAP value and significant differences were observed in all the honey types. In comparison with other studies, the FRAP values obtained in this study were higher (38.54 µmol Fe2SO4.7H2O/100 g) than Melipona beecheii from Cuba and lower (668.88 µmol Fe2SO4.7H2O/100 g) than Hypotrigona sp. from Nigeria. The different types of phenolic compounds present in honey may influence the antioxidant activity of honey, owing to the difference in reducing potential [46]. Apis honey, however, had a significantly lower FRAP value than stingless bee honey due to the lower phenolic and flavonoids content in Apis honey compared to stingless bee honey.
2.4. Correlation Coefficients
A significant positive correlation coefficient of 0.981 was observed between the FRAP and TPC values as shown in Table 5, thereby indicating that the phenolic content may influence the reducing power activity of honey. However, no significant correlations were found between IC50 and TPC (−0.886) or TFC (−0.572) values, indicating that the antioxidant activity is not solely dependent on the phenolic and flavonoids content as other antioxidant compounds present in honey may also be involved. Previous studies by Ahmed et al. [47] and Idris et al. [41] also found similar results to those reported in this study. Likewise, there were no significant correlations between the colour of the honey and TPC (0.165) or TFC (−0.010) values. These results suggest that colour is not influenced by TPC or TFC in Malaysian stingless bee honey as previously reported in two studies involving honeybee honey (Apis mellifera) [44,48]. In these studies, the authors observed that dark honey contained high levels of phenolic and flavonoid content.
Table 5.
Correlation coefficients (r) between antioxidants properties and colour intensity of honey.
| Parameters | TPC | TFC | IC50 | FRAP | Colour Intensity |
|---|---|---|---|---|---|
| TPC | 1.000 | ||||
| TFC | 0.802 | 1.000 | |||
| IC50 | −0.886 | −0.572 | 1.000 | ||
| FRAP | 0.981 * | 0.691 | −0.863 | 1.000 | |
| Colour intensity | 0.165 | −0.010 | 0.182 | 0.303 | 1.000 |
TPC = Total phenolic contents, TFC = Total flavonoids content, IC50 = Free radical scavenging activity, FRAP = Ferric reducing antioxidant power. (r) near to +1 or −1 indicates strong relationship and near to 0 indicates weak/no relationship. *Correlation is significant at p < 0.05.
2.5. Chemometric Analysis
2.5.1. Partial Least Squares-Discriminant Analysis (PLS-DA)
PLS-DA is a supervised method commonly used to enhance the separation of groups through the identification of variables that focus on class separation [49]. Based on the PLS-DA results, all four principal components (PC) obtained in this study contributed to 97.77% of the variance, thereby indicating that PLS-DA provides a better classification than Principle Component Analysis (PCA) (data not shown). The PLS-DA model also had a goodness of fit value of 0.877 (R2Xcum) and 0.978 (R2Ycum) and predictive value of 0.955 (Q2cum), with no outliers observed.
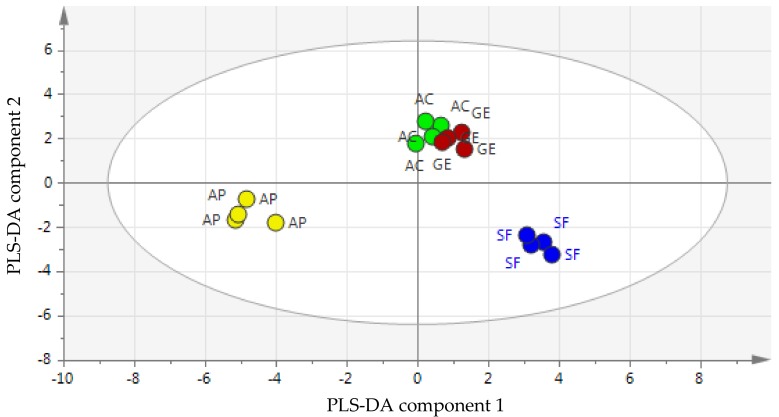
The PLS-DA score plot represented in Figure 2 showed a clear separation between Apis honey and the stingless bee honey sample groups. These results demonstrated that amino acid content is an important discriminator of Apis honey from stingless bee honey. Based on PC1, starfruit, acacia, and gelam honey types were clearly distinguished from Apis honey, while the separation on PC2 showed that starfruit and Apis honey can be further differentiated from gelam and acacia honey. Thus, all the honey samples in this study were well-separated into four different groups, thereby indicating that amino acid content can be used as a discriminant to classify honey samples from different botanical origins.
Figure 2.
The Partial Least Squares-Discriminant Analysis (PLS-DA) score plot of amino acid data. Coloured circles are represented by AC = acacia honey (green); GE = gelam honey (red); SF = starfruit honey (blue) and AP = Apis honey (yellow).
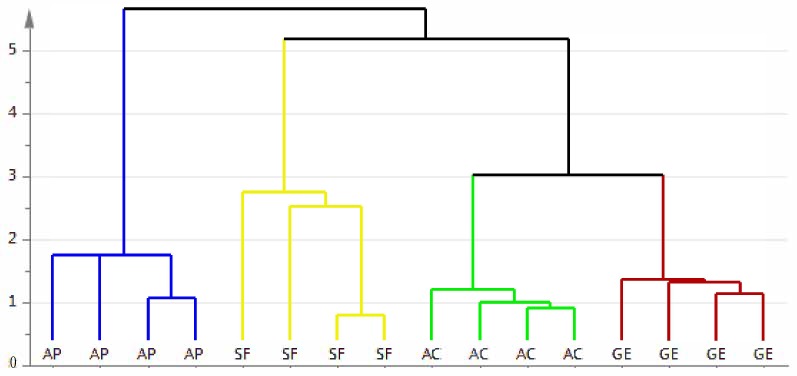
Cluster analysis (CA) was further performed to evaluate the role of amino acids in classifying honey samples from different botanical origins (Figure 3) based on similarity. The results showed that honey from the same botanical origin are placed in the same group. There were no samples being assigned to the wrong group. This indicates that amino acids can be used as indicators to authenticate the botanical origin of honey.
Figure 3.
The dendrogram of the cluster analysis of stingless bee honey and Apis mellifera honey. AC = acacia honey; SF = starfruit honey; GE = gelam honey; AP = Apis mellifera honey.
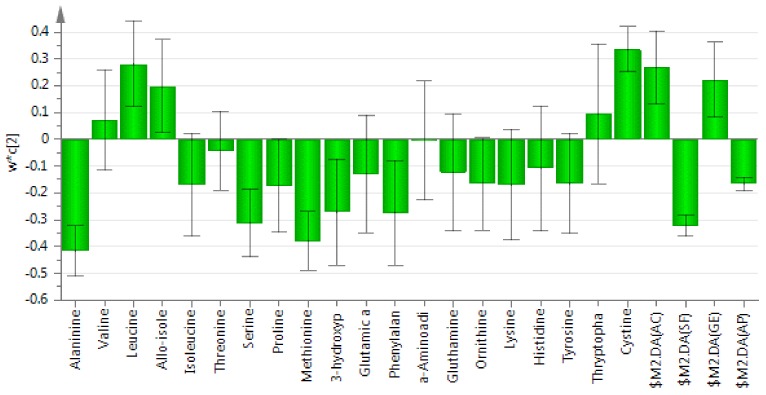
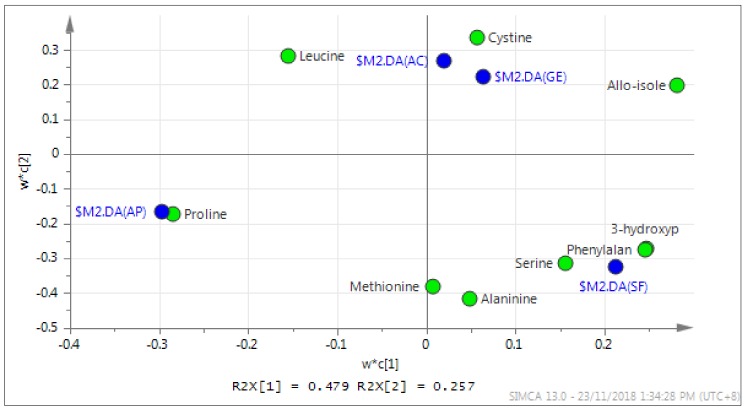
Figure 4 shows the PLS-DA loading column plot which highlights the variables responsible for the separation of honey samples. According to the loading column plot of PC2, three amino acids (leucine, allo-isoleucine, cysteine) contributed to the discrimination of acacia and gelam honey from starfruit and Apis honey. The starfruit and Apis honey samples are located on the negative side of the plot, with six amino acids (alanine, serine, proline, methionine, 3-hydroxyproline, phenylalanine) identified as discriminant variables. However, some variables were not considered as discriminants as their error bar exceeded zero [7]. These amino acids were identified as follows: valine, isoleucine, tryptophan, threonine, glutamic acid, α-aminoadipic acid, glutamine, ornithine, lysine, histidine, and tyrosine.
Figure 4.
The Partial Least Squares-Discriminant Analysis (PLS-DA) loading column plot of amino acids in honey samples from different botanical origins. AC = acacia honey, SF = starfruit honey, GE = gelam honey and AP = Apis honey.
The score scatter plot was performed to determine the most discriminatory amino acids responsible for grouping as well as identify potential markers for the honey samples (Figure 5). All amino acids that were not considered as discriminants were excluded from the score scatter plot. It was observed that some of the quantified amino acids were strongly associated with certain honey samples, in which amino acids that are located close to the honey sample have a strong discriminatory power compared to those located at a distance on the plot [50]. For instance, cysteine was a stronger discriminator compared to leucine and allo-isoleucine, and it can be used as a possible marker for acacia and gelam honey types. In addition, phenylalanine and 3-hydroxyproline were identified as possible markers for starfruit honey, while proline can be used to distinguish Apis honey from the stingless bee honey samples.
Figure 5.
The score scatter plot of amino acids for all honey samples. The blue circle indicates the type of honey. AC = acacia honey, GE = gelam honey, SF = starfruit honey and AP = Apis honey.
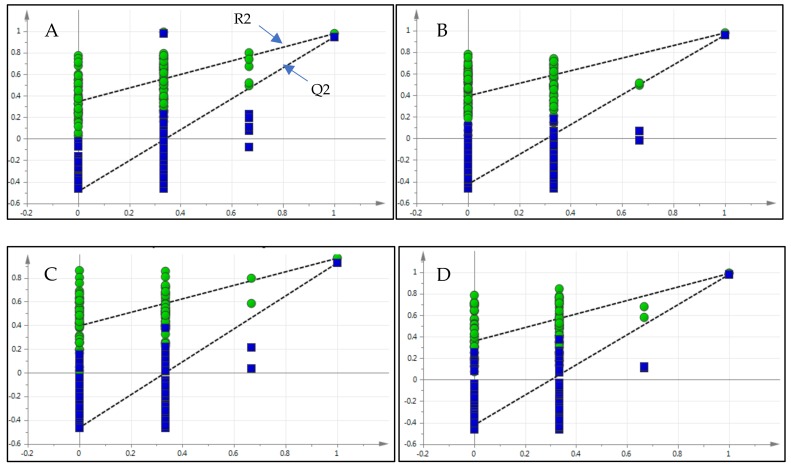
2.5.2. Validation of the PLS-DA Model
To validate the PLS-DA model, permutation tests were performed for all the honey samples. The model is successfully validated when the R2-intercept and Q2-intercept do not exceed 0.3–0.4 and 0.05, respectively [49]. The permutation test results in Figure 6 exhibited Y-intercept and Q2-intercept pair values at 0.346 (R2) and –0.491 (Q2), 0.395 (R2) and –0.464 (Q2), 0.394 (R2) and –0.426 (Q2) and 0.346 (R2) and –0.491 (Q2) for acacia, gelam, starfruit and Apis honey types, respectively, thereby demonstrating that the PLS-DA classification model was successfully validated.
Figure 6.
Graphs displaying the permutation tests for acacia (A); starfruit (B); gelam (C); and Apis (D) honeys.
2.6. Potential Uses of Stingless Bee Honey
Honeybee and stingless bee honey have been reported to have ability to reduce food-borne toxicant formed during cooking meat and has a therapeutic effect due to their antioxidant activity. Previous studies have proved the role of honey in reducing heterocyclic amines (HCAs) in cooked meat [51,52]. In terms of therapeutic effect, honey has been used mainly to treat diseases related to the intestine and wounds [53]. For instance, applications of honey on wound can stimulate the healing process by stimulating tissue regeneration and reducing inflammation [53]. Apart from antioxidants, other honey constituents such as sugars (glucose and fructose), vitamins, minerals, proteins, hydrogen peroxide, flavonoids, phenolic acids, high acidity and high-water content may be involved in the wound healing process [54]. Currently, honey has been reported to prevent human diseases related to oxidative stress, such as cancer, cardiovascular disease, hypertension, diabetes mellitus and atherosclerosis [55]. Stingless bee honey has higher antioxidant activity than Apis mellifera honey [17]. Due to this reason, it is expected that stingless bee honey is always a better honey to treat wounds and all diseases related to the oxidative stress.
3. Materials and Methods
3.1. Standards and Chemicals
Sodium hydroxide (NaOH) was supplied by Sigma-Aldrich (St. Louis, MO, USA). The EZ:faast™ amino acid analysis kit was obtained from Phenomenex (Torrance, CA, USA). All reagents and standards for amino acids measured were provided within the kit. The water was filtered using an ELGA Pure Lab Classic system (ELGA, Woodridge, IL, USA). Methanol and acetonitrile were obtained from Merck (Darmstadt, Germany). All of the chemicals and solvents utilized were of analytical grade while of the High-performance Liquid Chromatography (HPLC) grade for HPLC analysis.
3.2. Geographical and Botanical Description of Honey Samples
Three natural honey samples produced by Heterotrigona itama were acquired directly from different beekeepers in Malaysia (Pahang, Terengganu and Malacca). The samples were gathered during flowering season between February and May 2017 to reduce variations from nectar of other flowers and guarantees the monofloral character of honey. Three honey samples from varying botanical origins, namely starfruit (Averrhoa carambola L), acacia (Acacia mangium) and gelam (Meleleuca cajaputi Powell) were used (Table 6). Acacia honey produced by honeybee honey (Apis mellifera) was used as a control in this study. Currently, there is no gelam and starfruit farms in Malaysia that produced both stingless bee and Apis honey. Only the acacia farm produced both stingless and Apis honey. Owing to this limitation, Apis honey from gelam and starfruit were not used in this study.
Table 6.
Stingless bee honey samples from varying botanical origins.
| Sample | Scientific Name | Geographical Origin | Bee Species |
|---|---|---|---|
| Gelam Acacia Starfruit |
Meleleuca cajaputi Powell Acacia mangium Averrhoa carambola L |
Malacca Johor Pahang |
Heterotrigona itama |
| Acacia | Acacia mangium | Johor | Apis mellifera |
All honey samples were obtained from a local stingless bee farm. The identification of botanical origin was performed based on their geographical foraging area and floral availability where bee hives are located.
Gelam honey was collected from a stingless bee farm in Kuala Linggi Malacca, Malaysia. This farm was planted with three acres of gelam trees (Meleleuca cajaputi Powell) from Myrtaceae family and commonly known as kayu putih in Malaysia [56]. Honey samples were produced mainly from nectar of gelam flowers, while starfruit honey was gathered from a farm planted with two hectares of starfruit trees in Lanchang, Temerloh, Pahang, Malaysia. The major nectar collected by the bees is from the plant Averrhoa carambola L. from Oxalidaceae family [57,58]. Acacia honey was collected from a stingless bee farm located in the middle of the acacia forest in Sedili, Kota Tinggi, Johor, Malaysia. This farm consisting of acacia trees (Acacia mangium) that grow naturally in the forest with the land area is about two hectares. Acacia belongs to Fabaceae family and is called forest mangrove [59]. The bees collected the nectar mainly from Acacia mangium sap and flowers.
All samples were extracted from honey pots using an electric vacuum pump (Rocker 300, Kaohsiung, Taiwan). All stingless bee honey samples were submitted to the dehumidification process (40 °C) to reduce moisture content around 13–15 mg/100 g honey since the maximum limit is below than 20 mg/100 g honey in order to provide a better preservation of honey. Prior to dehumidification process, the water content of raw honey samples was evaluated. The processed honey samples were stored at 4 ± 2 °C in airtight plastic containers until further examination [60].
3.3. Physicochemical Analyses
3.3.1. Moisture Content
The AOAC official method 969.38 [61] was referred to determine the moisture content of honey sample. Briefly, each honey sample was placed in vacuum oven at temperature of 60±2 °C with pressure at ≤50 mm Hg for 6 h. The moisture content was computed by dividing the weight of honey after drying with weight of honey before drying and time with 100%.
3.3.2. Total Soluble Solid
A digital refractometer (ATAGO, Tokyo, Japan) was used to determine the total soluble solid of honey samples as described by Colucci et al. [62]. Approximately 0.3 mL of honey was spotted onto the glass prism of the refractometer and the measure was recorded at 25 °C in Brix.
3.3.3. pH and Free Acidity
The AOAC official method 962.19 [61] was employed to determine pH and free acidity of honey samples. The pH and acidity were measured using a calibrated pH meter (calibrated at pH 4.00, 7.00 and 9.00 using buffer solutions). The pH was recorded after diluting a 10 g honey in 75 mL distilled water (pH 8.50). Then, 0.1 M NaOH was titrated into honey solution until the pH reached 8.50. The acidity was calculated based on differences in the volume of NaOH used in honey solution and distilled water. The results were expressed in milliequivalents (meq) of acid per kg of honey.
3.3.4. Honey Colour Characteristics and Intensity
Colour characteristic [47] and colour intensity [63] of all honey samples were determined by using spectrophotometric analysis. The absorbance of diluted and filtered honey sample (1 g honey in 10 mL (w/v) distilled water) was measured using GENESYSTM 10S UV-Vis spectrophotometry from Thermo Fisher Scientific (Waltham, MA, USA) at 636 nm. Then, the absorbance data were recorded in Pfund value by utilizing this equation: mm Pfund = -38.7 + 371.39 × Abs [63]. For colour characteristics, the same diluted honey sample was measured for its absorbance at 450 nm and 720 nm. The distinction of the absorbance was reported in mAU.
3.3.5. 5-hydroxymethylfurfural (5-HMF)
The 5-HMF content in the honey samples was measured using reversed-phase high-performance liquid chromatography (HPLC-RP) according to the approach established by Harmonised Methods of the International Honey Commission (HMIHC) [64]. The 5-HMF solution was extracted from honey samples according to Gokmen and Acer [65]. Subsequently, the 20 µL of 5-HMF solution was injected into a reversed-phase high-performance liquid chromatography (WATERS, Milford, MA, USA) coupled with a photodiode array detector (WATERS 2996, Milford, MA, USA). The separation of 5-HMF was facilitated by a mixture of mobile phase, water (90%) and methanol (10%) using a reversed-phase column, Luna® C18(2) 100 Å (250 mm × 4.6 mm × 5 µm) from Phenomenex Inc. (Torrance, CA, USA). A continual flow rate of 1.0 mL/min was applied for 30 min. A 5-HMF standard was used to determine the 5-HMF in the samples by comparing the corresponding peak of the samples and 5-HMF standard. A standard curve of five different concentrations of 5-HMF was constructed to compute the amount of 5-HMF in honey samples and express in mg/kg honey.
3.3.6. Sugar Profile
The detection and quantitation of sugar in the honey samples were performed using high-performance liquid chromatography integrated with a refractive index detector (HPLC-RI) as established by Harmonised Methods of the International Honey Commission (HMIHC) [64] with some modifications. Separation of sugars in the honey sample was carried out using an amino column Luna® NH2 100 Å (250 mm × 4.6 mm × 5 µm) from Phenomenex Inc. (Torrance, CA, USA) with acetonitrile:water (80:20) as a mobile phase. The temperature of column was set at 40 °C to obtain an efficient separation. A volume of 10 µL honey solution (0.5 g of honey in 10 mL of distilled water) and the flow rate of 1 mL/min was used. The analysis was observed for 20 min. Each sugar was distinguished by comparing the retention time of genuine standard. An equation from the calibration curve of each sugar standard was used to calculate sugar concentrations and expressed in gram sugar per 100 g of honey.
3.3.7. Amino Acid Profile
The amino acids profile was analysed using gas chromatography with flame ionization detection as reported by Nozal et al. [19] and Mustafa et al. [66]. All honey samples were subjected to derivatization process prior to gas chromatography analysis. About 100 mg honey was dissolved with 500 uL distilled water and the solution was homogenised using a vortex mixer. The Phenomenex EZ:faast™ amino acid analysis kit (Phenomenex, Torrance, CA, USA) was used to extract and derivatize honey solution (30 uL) prior to GC-FID analysis. The separation of derivatised amino acids were performed using Agilent 7890A GC-FID (Agilent Technologies, Wilmington, DE, USA). The column used was Zebron ZB-AAA capillary GC column (10 m × 0.25 mm id, Phenomenex, Torrance, CA, USA). The column oven temperature program as follows: 110 to 320 °C at 32 °C/min. The FID detector temperature was 320 °C and 1 μL of each sample was injected at an injection temperature of 250 °C and a split level of 1:15. The carrier gas was helium at a pressure of 3 kPa/min (a flow rate of 1.5 ml/min). Identification of chromatographic peaks were performed by comparing the retention times of the standards and the samples components. Quantification was carried out based on the standard curve obtained from five amino acids standards mix with known concentrations (50–400 µmol/L). The limit of detection (LOD) and limit of quantitation (LOQ) values were computed utilizing these formulas, 3.3*standard deviation of blank response/slope and 10*standard deviation of blank response/slope separately [67]. The LOD and LOQ of amino acids were varied from 0.01 to 2.36 mg/kg of honey and 0.02 to 7.15 mg/kg of honey, respectively.
3.4. Analyses of Antioxidant Properties
3.4.1. Total Phenolic Content (TPC)
The Folin–Ciocalteu method [68] with slight modification was used to estimate the concentration of total phenolic content (TPC). Initially, the honey solution was prepared by mixing 100 mg of honey (dry basis) with 3 mL methanol. Then, approximately 200 µL of honey solution was added with 1000 µL of Folin–Ciocalteu (FC) reagent (dilution ratio of FC reagent, 1:10 v/v), and incubated for 6 min in the dark. Afterwards, a 7.5% sodium carbonate solution (800 µL) was added, shaken and incubated in the dark for 2 h. Later, the absorbance was read at 740 nm against a methanol blank (GENESYSTM 10S UV-Vis spectrophotometry, Waltham, MA, USA). The TPC of the honey samples was computed according to the equation gained from a calibration curve of a standard solutions gallic acid and expressed in gallic acid equivalent (GAE/100 g honey).
3.4.2. Total Flavonoids Content (TFC)
The spectrophotometric approach was employed to estimate the total flavonoids content (TFC) in honey samples while quercetin was used as a reference [69]. Briefly, a mixture of 1 mL of honey solution (500 mg honey (dry basis) in 2 mL methanol) and 0.3 mL of 5% NaNO2 solution was mixed for 5 min and 0.3 mL of 10% AlCl3 was added. The mixture was stirred for 6 min and the solution was neutralized by adding 2 mL of 1 M NaOH. The absorbance of each sample was measured at 510 nm against a methanol blank by utilizing a UV-VIS spectrophotometer (GENESYSTM 10S UV-Vis spectrophotometry) from Thermo Fisher Scientific (Waltham, MA, USA). The TFC was computed according to the equation obtained from a calibration curve of a standard quercetin acid solutions and expressed in mg quercetin acid equivalent (QAE)/100 g of honey.
3.4.3. Free Radical Scavenging Activity (DPPH Assay)
The DPPH assay was used to estimate the radical scavenging activity of honey sample based on the method reported by Meda et al. [70] with a slight alteration. The radical scavenging activity was expressed as IC50 (concentration of honey solution required to mitigate the initial concentration of DPPH by 50%). Shortly, five serials of methanolic honey dilution were prepared. Later, 1.5 mL (0.02 mg/mL) of a methanolic solution of DPPH was added to the 0.75 mL methanolic honey dilution and mixed thoroughly. The mixtures were maintained in the dark for 15 min at room temperature. The absorbance of each solution was read at 517 nm against a methanol blank using a UV-VIS spectrophotometer (GENESYSTM 10S UV-Vis spectrophotometry) from Thermo Fisher Scientific (Waltham, MA, USA). The radical scavenging activity was calculated as the percentage of radical scavenging activity (RSA) using the following formula: Radical scavenging activity = ((ADPPH − As) / ADPPH) × 100, where As is the absorbance of the sample solution and ADPPH is the absorbance of the DPPH solution.
3.4.4. Ferric Reducing Antioxidant Power (FRAP Assay)
FRAP assay was carried out to evaluate the ability of antioxidants in honey sample to reduce ferric ion (Fe3+) to ferrous ion (Fe2+) according to Khalil et al. [71] with some modifications. The FRAP value is expressed as micromoles of ferrous equivalent (µM Fe (II)) per kilogram of honey. Initially, 200 µL of honey solution (100 mg of honey (dry basis) in 11 mL methanol) was mixed with 1.5 mL of FRAP reagent. After incubation at 37 °C in a water bath for 4 min, the absorbance was measured at 593 nm against a methanol blank using a UV-VIS spectrophotometer (GENESYSTM 10S UV-Vis spectrophotometry) from Thermo Fisher Scientific (Waltham, MA, USA). Fresh FRAP reagent was prepared by mixing 10 mL of 300 mM/L acetate buffer (pH 3.6) with 1 mL of 10 mmol 2,4,6-tris(1-pyridyl)-1,3,5-triazine (TPTZ) and 1 mL of 40 mM/L HCl containing 20 mM ferric chloride (FeCl3 6H2O). Then, the FRAP reagent solution was pre-warmed at 37 °C prior to use. The quantification of antioxidant activity was done by constructing a calibration curve of standard ferrous sulphate (FeSO4 7H2O) solutions against the concentration of honey solution.
3.5. Chemometrics Analysis
All four honey samples were subjected to chemometrics analysis. In chemometrics analysis, the amino acid profile was used as variables. A total of 20 amino acids from each honey sample were selected by the SIMCA software (version, Manufacturer, City, US State abbrev. if applicable, Country) and used to develop a partial least squares discriminant analysis (PLS-DA) model. The cluster analysis (CA), score plot and the score scatter plot were performed to discriminate honey samples according to their botanical origin. Loading column plot was performed to determine the correlation between variables and sample. The quality of the model was explained by goodness of fit (R2Xcum and R2Ycum) and predictive values (Q2cum). The model was further validated with the permutation test [72,73].
3.6. Statistical Analysis
All experiments were conducted in four replicates and the results were presented as mean ± standard deviation. The difference between samples was analysed using one-way analysis of variance (ANOVA) at p < 0.05 using MINITAB software Version 17.0 (Manufacturer, Sydney, NSW, Australia). A correlation test was performed using Pearson’s correlation at p < 0.05. SIMCA software Version 13.0 (MKS Data Analytics Solutions, Umeå, Sweden) was used to evaluate the relationship between amino acids and honey classification based on their botanical origin.
4. Conclusions
The physicochemical and antioxidant properties of stingless bee honey from acacia, starfruit, and gelam produced by Heterotrigona itama bees were characterized. The findings revealed that the physicochemical properties of the honey samples investigated were highly dependent on their botanical origins. The results also showed that the physicochemical properties and antioxidant activity of stingless bee honey were significantly different compared to Apis mellifera honey (honeybee honey). Moreover, the PLS-DA evaluation indicated that stingless bee honey was clearly distinguishable from Apis mellifera honey, and within the stingless bee honey types, based on the amino acid profile. The amino acid profile coupled with chemometrics analysis has successfully classified stingless bee honey and proved the possibility of using chemometrics analysis to classify and identify biomarkers in stingless bee honey. In addition, stingless bee honey was shown to have a high antioxidant activity and therefore it is good for human health.
Acknowledgments
The authors also would like to thank MOE for the HICoE rendered to the Institute of Tropical Agriculture and Food Security (ITAFoS), Universiti Putra Malaysia (UPM), and the Faculty of Food Science and Technology, UPM for the facilities rendered.
Author Contributions
Conceptualization, S.S. and J.S.; Methodology, S.S.; Formal Analysis, S.S.; Investigation, S.S., J.S., M.S., A.K. and N.N.J.; Resources, J.S.; Data Curation, S.S.; Writing—Original Draft Preparation, S.S.; Writing—Review and Editing, S.S., J.S., M.S., A.K. and N.N.J.; Visualization, S.S.; Supervision, J.S., M.S., S.B.A.R., N.N.J. and A.K.; Project Administration, J.S.; Funding Acquisition, J.S.
Funding
This project was supported by the Ministry of Education Malaysia (MOE) under UPM/700-2/1/FRGS/MRSA/5524985.
Conflicts of Interest
The authors declare that there is no conflict of interest for this study.
References
- 1.Zhao J., Du X., Cheng N., Chen L., Xue X., Wu L., Cao W. Identification of monofloral honeys using HPLC–ECD and chemometrics. Food Chem. 2016;194:167–174. doi: 10.1016/j.foodchem.2015.08.010. [DOI] [PubMed] [Google Scholar]
- 2.Chuttong B., Chanbang Y., Sringarm K., Burgett M. Physicochemical profiles of stingless bee (Apidae: Meliponini) honey from South east Asia (Thailand) Food Chem. 2016;192:149–155. doi: 10.1016/j.foodchem.2015.06.089. [DOI] [PubMed] [Google Scholar]
- 3.Amin Z., Aina F., Sabri S., Mohammad S.M., Ismail M., Chan K.W., Ismail N., Norhaizan M.E., Zawawi N. Therapeutic Properties of Stingless Bee Honey in Comparison with European Bee Honey. Adv. Pharmacol. Sci. 2018;2018:6179596. doi: 10.1155/2018/6179596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fuenmayor C.A., Diaz-Moreno A.C., Zuluaga-Dominguez C.M., Quicazan M.C. Honey of Colombian stingless bees: Nutritional characteristics and physicochemical quality indicators. In: Patricia V., Silvia R.M.P., David R., editors. Pot-Honey: A Legacy of Stingless Bee. 1st ed. Springer; London, UK: New York, NY, USA: 2013. [Google Scholar]
- 5.Kelly N., Farisya M.S.N., Kumara T.K., Marcela P. Species Diversity and External Nest Characteristics of Stingless Bees in Meliponiculture. Pertanika J. Trop. Agric. Sci. 2014;37:293–298. [Google Scholar]
- 6.da Silva P.M., Gauche C., Gonzaga L.V., Costa A.C.O., Fett R. Honey: Chemical composition, stability and authenticity. Food Chem. 2016;196:309–323. doi: 10.1016/j.foodchem.2015.09.051. [DOI] [PubMed] [Google Scholar]
- 7.Razali M., Zainal Z., Maulidiani M., Shaari K., Zamri Z., Mohd Idrus M., Khatib A., Abas F., Ling Y.S., Rui L.L., et al. Classification of Raw Stingless Bee Honeys by Bee Species Origins Using the NMR-and LC-MS-Based Metabolomics Approach. Molecules. 2018;23:2160. doi: 10.3390/molecules23092160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kaškonienė V., Venskutonis P.R. Floral markers in honey of various botanical and geographic origins: A review. Compr. Rev. Food Sci. Food Saf. 2010;9:620–634. doi: 10.1111/j.1541-4337.2010.00130.x. [DOI] [PubMed] [Google Scholar]
- 9.Kečkeš J., Trifković J., Andrić F., Jovetić M., Tešić Ž., Milojković-Opsenica D. Amino acids profile of Serbian unifloral honeys. J. Sci. Food Agric. 2013;93:3368–3376. doi: 10.1002/jsfa.6187. [DOI] [PubMed] [Google Scholar]
- 10.Aliferis K.A., Tarantilis P.A., Harizanis P.C., Alissandrakis E. Botanical discrimination and classification of honey samples applying gas chromatography/mass spectrometry fingerprinting of headspace volatile compounds. Food Chem. 2010;121:856–862. doi: 10.1016/j.foodchem.2009.12.098. [DOI] [Google Scholar]
- 11.Wang J., Kliks M.M., Qu W., Jun S., Shi G., Li Q.X. Rapid determination of the geographical origin of honey based on protein fingerprinting and barcoding using MALDI TOF MS. J. Agric. Food Chem. 2009;57:10081–10088. doi: 10.1021/jf902286p. [DOI] [PubMed] [Google Scholar]
- 12.Nozal M.J., Bernal J.L., Toribio L., Alamo M., Diego J.C., Tapia J. The use of carbohydrate profiles and chemometrics in the characterization of natural honeys of identical geographical origin. J. Agric. Food Chem. 2005;53:3095–3100. doi: 10.1021/jf0489724. [DOI] [PubMed] [Google Scholar]
- 13.Vit P., Oddo L.P., Marano M.L., de Mejias E.S. Venezuelan stingless bee honeys characterized by multivariate analysis of physicochemical properties. Apidologie. 1998;29:377–389. doi: 10.1051/apido:19980501. [DOI] [Google Scholar]
- 14.Duarte A.W.F., dos Santos Vasconcelos M.R., de Menezes A.P.D., da Silva S.C., Oda-souza M., López A.M.Q. Composition and antioxidant activity of honey from Africanized and stingless bees in Alagoas (Brazil): A multivariate analysis. J. Apic. Res. 2012;51:23–35. doi: 10.3896/IBRA.1.51.1.04. [DOI] [Google Scholar]
- 15.Kek S.P., Chin N.L., Yusof Y.A., Tan S.W., Chua L.S. Classification of entomological origin of honey based on its physicochemical and antioxidant properties. Int. J. Food Prop. 2017;20:S2723–S2738. doi: 10.1080/10942912.2017.1359185. [DOI] [Google Scholar]
- 16.Kek S.P., Chin N.L., Tan S.W., Yusof Y.A., Chua L.S. Classification of honey from its bee origin via chemical profiles and mineral content. Food Anal. Methods. 2017;10:19–30. doi: 10.1007/s12161-016-0544-0. [DOI] [Google Scholar]
- 17.Souza B.A., Roubik D.W., Barth O.M., Heard T.A., Enríquez E., Carvalho C., Villas-Boas J., Marchini L., Locatelli J., Persano-Oddo L., et al. Composition of stingless bee honey: Setting quality standards. Interciencia. 2006;31:867–875. [Google Scholar]
- 18.Hermosín I., Chicon R.M., Cabezudo M.D. Free amino acid composition and botanical origin of honey. Food Chem. 2003;83:263–268. doi: 10.1016/S0308-8146(03)00089-X. [DOI] [Google Scholar]
- 19.Nozal M.J., Bernal J.L., Toribio M.L., Diego J.C., Ruiz A. Rapid and sensitive method for determining free amino acids in honey by gas chromatography with flame ionization or mass spectrometric detection. J. Chromatogr. A. 2004;1047:137–146. doi: 10.1016/j.chroma.2004.07.013. [DOI] [PubMed] [Google Scholar]
- 20.Sun Z., Zhao L., Cheng N., Xue X., Wu L., Zheng J., Cao W. Identification of botanical origin of Chinese unifloral honeys by free amino acid profiles and chemometric methods. J. Pharm. Anal. 2017;7:317–323. doi: 10.1016/j.jpha.2017.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rebane R., Herodes K. Evaluation of the botanical origin of Estonian uni-and polyfloral honeys by amino acid content. J. Agric. Food Chem. 2008;56:10716–10720. doi: 10.1021/jf8018968. [DOI] [PubMed] [Google Scholar]
- 22.Codex Alimentarius Commission . Draft Revised Standard for Honey (at Step 10 of the Codex Procedure) Volume 25. Codex Alimentarius Commission, FAO; Rome, Italy: 2001. pp. 19–26. [Google Scholar]
- 23.Malaysian Standard . Kelulut (Stingless Bee) Honey-Specification: MS 2683:2017. Department of Standard Malaysia; Selangor, Malaysia: 2017. [Google Scholar]
- 24.Keng C.B., Haron H., Talib R.A., Subramaniam P. Physical Properties, Antioxidant Content and Anti-Oxidative Activities of Malaysian Stingless Kelulut (Trigona spp.) Honey. J. Agric. Sci. 2017;9:32–40. [Google Scholar]
- 25.Biluca F.C., Braghini F., Gonzaga L.V., Costa A.C.O., Fett R. Physicochemical profiles, minerals and bioactive compounds of stingless bee honey (Meliponinae) J. Food Compos. Anal. 2016;50:61–69. doi: 10.1016/j.jfca.2016.05.007. [DOI] [Google Scholar]
- 26.Issaro N., Weerakul T., Machana S., Ornnim P., Phanudulkitti C., Srijan T., Laiwattanaphaisal J., Pattarapanich C. Stingless bee honey II: Qualitative and quantitative studies on honey produced by three stingless bee species collected from a mangosteen garden in Chantaburi province, Thailand. Thai J. Pharm. Sci. 2013;38:16–18. [Google Scholar]
- 27.Rodriguez-Malaver A.J., Rasmussen C., Gutierrez M.G., Gil F., Nieves B., Vit P. Properties of honey from ten species of Peruvian stingless bees. Nat. Prod. Commun. 2009;4:1221–1226. doi: 10.1177/1934578X0900400913. [DOI] [PubMed] [Google Scholar]
- 28.Alvarez-Suarez J.M., Giampieri F., Brenciani A., Mazzoni L., Gasparrini M., González-Paramás A.M., Santos-Buelga C., Morroni G., Simoni S., Forbes-Hernandez T.Y., et al. Apis mellifera vs Melipona beecheii Cuban polifloral honeys: A comparison based on their physicochemical parameters, chemical composition and biological properties. LWT. 2018;87:272–279. doi: 10.1016/j.lwt.2017.08.079. [DOI] [Google Scholar]
- 29.de Almeida-Muradian L.B., Stramm K.M., Estevinho L.M. Efficiency of the FT-IR ATR spectrometry for the prediction of the physicochemical characteristics of M elipona subnitida honey and study of the temperature’s effect on those properties. Int. J. Food Sci. Technol. 2014;49:188–195. doi: 10.1111/ijfs.12297. [DOI] [Google Scholar]
- 30.Moniruzzaman M., Khalil M.I., Sulaiman S.A., Gan S.H. Physicochemical and antioxidant properties of Malaysian honeys produced by Apis cerana, Apis dorsata and Apis mellifera. BMC Complement. Altern. Med. 2013;13:43. doi: 10.1186/1472-6882-13-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.USDA, Agricultural Marketing Services Fruit and Vegetable Division Processed Products Branch . United State Standards for Grades of Extracted Honey. US Department of Agriculture; Washington, DC, USA: 1985. [Google Scholar]
- 32.Tuksitha L., Chen Y.L.S., Chen Y.L., Wong K.Y., Peng C.C. Antioxidant and antibacterial capacity of stingless bee honey from Borneo (Sarawak) J. Asia Pac. Entomol. 2018;21:563–570. doi: 10.1016/j.aspen.2018.03.007. [DOI] [Google Scholar]
- 33.Oddo L.P., Heard T.A., Rodríguez-Malaver A., Pérez R.A., Fernández-Muiño M., Sancho M.T., Vit P. Composition and antioxidant activity of Trigona carbonaria honey from Australia. J. Med. Food. 2008;11:789–794. doi: 10.1089/jmf.2007.0724. [DOI] [PubMed] [Google Scholar]
- 34.Olaitan P.B., Adeleke O.E., Iyabo O.O. Honey: A reservoir for microorganisms and an inhibitory agent for microbes. Afr. Health Sci. 2007;7:159–165. doi: 10.5555/afhs.2007.7.3.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Janiszewska K., Aniołowska M., Nowakowski P. Free amino acids content of honeys from Poland. Pol. J. Food Nutr. Sci. 2012;62:85–89. doi: 10.2478/v10222-011-0041-5. [DOI] [Google Scholar]
- 36.Qamer S., Ehsan M., Nadeem S., Shakoori A.R. Free amino acids content of Pakistani unifloral honey produced by Apis mellifera. Pak. J. Zool. 2007;39:99–102. [Google Scholar]
- 37.Anklam E. A review of the analytical methods to determine the geographical and botanical origin of honey. Food Chem. 1998;63:549–562. doi: 10.1016/S0308-8146(98)00057-0. [DOI] [Google Scholar]
- 38.Oliveira R.G.D., Jain S., Luna A.C., Freitas L.D.S., Araújo E.D.D. Screening for quality indicators and phenolic compounds of biotechnological interest in honey samples from six species of stingless bees (Hymenoptera: Apidae) Food Sci. Technol. 2017;37:552–557. doi: 10.1590/1678-457x.25716. [DOI] [Google Scholar]
- 39.da Silva G.R., da Natividade T.B., Camara C.A., da Silva E.M.S., dos Santos F.D.A.R., Silva T.M.S. Identification of sugar, amino acids and minerals from the pollen of Jandaíra stingless bees (Melipona subnitida) Food Nutr. Sci. 2014;5:1015–1021. [Google Scholar]
- 40.Ranneh Y., Ali F., Zarei M., Akim A.M., Hamid H.A., Khazaai H. Malaysian stingless bee and Tualang honeys: A comparative characterization of total antioxidant capacity and phenolic profile using liquid chromatography-mass spectrometry. LWT. 2018;89:1–9. doi: 10.1016/j.lwt.2017.10.020. [DOI] [Google Scholar]
- 41.Idris Y.M.A., Mariod A.A., Hamad S.I. Physicochemical properties, phenolic contents and antioxidant activity of Sudanese honey. Int. J. Food Prop. 2011;14:450–458. doi: 10.1080/10942910903243673. [DOI] [Google Scholar]
- 42.Al-Mamary M., Al-Meeri A., Al-Habori M. Antioxidant activities and total phenolics of different types of honey. Nutr. Res. 2002;22:1041–1047. doi: 10.1016/S0271-5317(02)00406-2. [DOI] [Google Scholar]
- 43.Aljadi A.M., Kamaruddin M.Y. Evaluation of the phenolic contents and antioxidant capacities of two Malaysian floral honeys. Food Chem. 2004;85:513–518. doi: 10.1016/S0308-8146(02)00596-4. [DOI] [Google Scholar]
- 44.Baek Y., Kim Y.J., Baik M.Y., Kim D.O., Lee H. Total phenolic contents and antioxidant activities of Korean domestic honey from different floral sources. Food Sci. Biotechnol. 2015;24:1453–1457. doi: 10.1007/s10068-015-0187-8. [DOI] [Google Scholar]
- 45.Nweze J.A., Okafor J.I., Nweze E.I., Nweze J.E. Evaluation of physicochemical and antioxidant properties of two stingless bee honeys: A comparison with Apis mellifera honey from Nsukka, Nigeria. BMC Res. Notes. 2017;10:566. doi: 10.1186/s13104-017-2884-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Can Z., Yildiz O., Sahin H., Turumtay E.A., Silici S., Kolayli S. An investigation of Turkish honeys: Their physico-chemical properties, antioxidant capacities and phenolic profiles. Food Chem. 2015;180:133–141. doi: 10.1016/j.foodchem.2015.02.024. [DOI] [PubMed] [Google Scholar]
- 47.Ahmed M., Imtiaz Shafiq M., Khaleeq A., Huma R., Abdul Qadir M., Khalid A., Samad A. Physiochemical, Biochemical, Minerals Content Analysis, and Antioxidant Potential of National and International Honeys in Pakistan. J. Chem. 2016;2016:8072305. doi: 10.1155/2016/8072305. [DOI] [Google Scholar]
- 48.Alzahrani H.A., Alsabehi R., Boukraâ L., Abdellah F., Bellik Y., Bakhotmah B.A. Antibacterial and antioxidant potency of floral honeys from different botanical and geographical origins. Molecules. 2012;17:10540–10549. doi: 10.3390/molecules170910540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Eriksson L., Byrne T., Johansson E., Trygg J., Vikström C. Multi-and Megavariate Data Analysis Basic Principles and Applications. Umetrics Academy; Umeå, Sweden: 2013. [Google Scholar]
- 50.Umetrics M. User Guide to SIMCA. MKS Umetrics AB; Malmö, Sweden: 2013. [Google Scholar]
- 51.Shin H.S., Ustunol Z. Influence of honey-containing marinades on heterocyclic aromatic amine formation and overall mutagenicity in fried beef steak and chicken breast. J. Food Sci. 2004;69:FCT147–FCT153. doi: 10.1111/j.1365-2621.2004.tb13350.x. [DOI] [Google Scholar]
- 52.Shin H.S., Strasburg G.M., Ustunol Z. Influence of different unifloral honeys on heterocyclic aromatic amine formation and overall mutagenicity in fried ground-beef patties. J. Food Sci. 2003;68:810–815. doi: 10.1111/j.1365-2621.2003.tb08247.x. [DOI] [PubMed] [Google Scholar]
- 53.Eteraf-Oskouei T., Najafi M. Traditional and modern uses of natural honey in human diseases: A review. Iran. J. Basic Med. Sci. 2013;16:731–742. [PMC free article] [PubMed] [Google Scholar]
- 54.Jalil M.A.A., Kasmuri A.R., Hadi H. Stingless bee honey, the natural wound healer: A review. Skin Pharmacol. Physiol. 2017;30:66–75. doi: 10.1159/000458416. [DOI] [PubMed] [Google Scholar]
- 55.Erejuwa O.O., Sulaiman S.A., Ab Wahab M.S. Honey: A l novel antioxidant. Molecules. 2012;17:4400–4423. doi: 10.3390/molecules17044400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Jamilah M.S., Nur-Atiqah M.H., Mohamad Hafis M., Seriza M.R. Potential climate change mitigation through carbon stock accumulation by melaleuca cajuputi powell (gelam) Int. J. Agric. For. Plant. 2017;5:92–98. [Google Scholar]
- 57.Muthu N., Lee S.Y., Phua K.K., Bhore S.J. Nutritional, medicinal and toxicological attributes of star-fruits (Averrhoa Carambola L.): A review. Bioinformation. 2016;12:420–424. doi: 10.6026/97320630012420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Khanam Z., Sam K.H., Zakaria N.H.B.M., Ching C.H., Bhat I.U.H. Determination of polyphenolic content, HPLC analyses and DNA cleavage activity of Malaysian Averrhoa carambola L. fruit extracts. J. King Saud Univ. Sci. 2015;27:331–337. doi: 10.1016/j.jksus.2015.01.004. [DOI] [Google Scholar]
- 59.Ismaili G., Bakar B.H.A., Rahim K.K.A. Evaluation of Acacia Mangium in Structural Size at Green Condition. J. Civ. Eng. Sci. Technol. 2011;2:17–22. doi: 10.33736/jcest.90.2011. [DOI] [Google Scholar]
- 60.Silva T.M.S., dos Santos F.P., Evangelista-Rodrigues A., da Silva E.M.S., da Silva G.S., de Novais J.S., Camara C.A. Phenolic compounds, melissopalynological, physicochemical analysis and antioxidant activity of jandaíra (Melipona subnitida) honey. J. Food Compos. Anal. 2013;29:10–18. doi: 10.1016/j.jfca.2012.08.010. [DOI] [Google Scholar]
- 61.Association of Official Analytical Chemists (AOAC) Official Method of Analysis. 19th ed. AOAC; Washington, DC, USA: 2012. [Google Scholar]
- 62.Colucci G., De Vito V., Varricchio E., De Cunzo F., Coccia E. Identification of Traceability Markers in Italian Unifloral Honeys of different Botanical Origin. J. Nutr. Food Sci. 2016;6:462. [Google Scholar]
- 63.de Sousa J.M.B., de Souza E.L., Marques G., de Toledo Benassi M., Gullón B., Pintado M.M., Magnani M. Sugar profile, physicochemical and sensory aspects of monofloral honeys produced by different stingless bee species in Brazilian semi-arid region. LWT-Food Sci. Technol. 2016;65:645–651. doi: 10.1016/j.lwt.2015.08.058. [DOI] [Google Scholar]
- 64.Bogdanov S. Harmonised Methods of the International Honey Commission. International Honey Commission; Bern, Switzerland: 2009. [(accessed on 10 April 2018)]. Available online: http://www.ihc-platform.net/ihcmethods2009.pdf. [Google Scholar]
- 65.Gökmen V., Acar J. Simultaneous determination of 5-hydroxymethylfurfural and patulin in apple juice by reversed-phase liquid chromatography. J. Chromatogr. A. 1999;847:69–74. doi: 10.1016/S0021-9673(99)00133-8. [DOI] [PubMed] [Google Scholar]
- 66.Mustafa A., Åman P., Andersson R., Kamal-Eldin A. Analysis of free amino acids in cereal products. Food Chem. 2007;105:317–324. doi: 10.1016/j.foodchem.2006.11.044. [DOI] [Google Scholar]
- 67.Shrivastava A., Gupta V.B. Methods for the determination of limit of detection and limit of quantitation of the analytical methods. Chron. Young Sci. 2011;2:21–25. doi: 10.4103/2229-5186.79345. [DOI] [Google Scholar]
- 68.Garjanovic S.Z., Alvarez-Suarez J.M., Novakovic M.M., Pastor F.T., Pezo L., Battino M., Suznjevic D.Z. Comparative analysis of antioxidant activity of honey of different floral sources using recently developed polarographic and various spectrophotometric assays. J. Food Compos. Anal. 2013;30:13–18. doi: 10.1016/j.jfca.2012.12.004. [DOI] [Google Scholar]
- 69.Kamboj R., Bera M.B., Nanda V. Evaluation of physicochemical properties, trace metal content and antioxidant activity of Indian honeys. Int. J. Food Sci. Technol. 2013;48:578–587. doi: 10.1111/ijfs.12002. [DOI] [Google Scholar]
- 70.Meda A., Lamien C.E., Romito M., Millogo J., Nacoulma O.G. Determination of the total phenolic, flavonoid and proline contents in Burkina Fasan honey, as well as their radical scavenging activity. Food Chem. 2005;91:571–577. doi: 10.1016/j.foodchem.2004.10.006. [DOI] [Google Scholar]
- 71.Khalil M.I., Alam N., Moniruzzaman M., Sulaiman S.A., Gan S.H. Phenolic acid composition and antioxidant properties of Malaysian honeys. J. Food Sci. 2011;76:C921–C928. doi: 10.1111/j.1750-3841.2011.02282.x. [DOI] [PubMed] [Google Scholar]
- 72.Javadi N., Abas F., Hamid A.A., Simoh S., Shaari K., Ismail I.S., Mediani A., Khatib A. GC-MS-Based Metabolite Profiling of Cosmos caudatus Leaves Possessing Alpha-Glucosidase Inhibitory Activity. J. Food Sci. 2014;79:C1130–C1136. doi: 10.1111/1750-3841.12491. [DOI] [PubMed] [Google Scholar]
- 73.Murugesu S., Ibrahim Z., Ahmed Q.U., Nik Yusoff N.I., Uzir B.F., Perumal V., Abas F., Saari K., El-Seedi H., Khatib A. Characterization of α-glucosidase inhibitors from clinacanthus nutans lindau leaves by gas chromatography-mass spectrometry-based metabolomics and molecular docking simulation. Molecules. 2018;23:2402. doi: 10.3390/molecules23092402. [DOI] [PMC free article] [PubMed] [Google Scholar]