Abstract
Adipocytes are generally thought to be terminally differentiated cells; however, recent evidence suggests a subset may have greater plasticity in certain contexts. In this issue of Cell Metabolism, Wang et al. (2018) demonstrate a novel capacity for mammary adipocytes to dedifferentiate into preadipocyte-like precursors during lactation and redifferentiate upon weaning.
Adipocytes are abundant in mouse mammary glands, accounting for nearly 90% of tissue volume under normal physiological conditions (Giordano et al., 2014). A significant reduction in the number of mature adipocytes is observed during pregnancy and lactation, as the mammary ductal epithelium rapidly expands to form milk-producing alveolar structures. Upon weaning, secretory epithelial cells undergo a massive wave of apoptosis, alongside a concomitant wave of adipogenesis to regenerate the adipocyte population. What happens to mature adipocytes during lactation and how they are repopulated during involution are poorly understood. To investigate the cellular changes that occur during mammary gland lactation and involution, Morroni et al. have employed electron microscopy and transgenic mouse models to lineage trace secretory epithelial cells and adipocytes, using a reporter under control of the whey acidic protein (WAP) promoter or fatty acid binding protein 4 (FABP4) promoter, respectively (Morroni et al., 2004; Giordano et al., 2014). They found evidence that mammary adipocytes are capable of transdifferentiation into alveolar epithelial cells, and alveolar epithelial cells are also able to transdifferentiate into adipocytes (Morroni et al., 2004; Giordano et al., 2014). Using a similar approach, Giordano et al. (2017) showed that mammary alveolar epithelial cells can also transdifferentiate into brown adipocytes. Another recent study by Marangoni et al. (2015) demonstrated that myofibroblasts in fibrotic skin can derive from adiponectin-positive precursor cells. These studies are intriguing since adipocytes are generally considered to be terminally differentiated cells; however, the origin and fate of adipocytes within the mammary gland is still poorly understood. In the current issue of Cell Metabolism, Wang et al. (2018) provide evidence that mature adipocytes dedifferentiate into preadipocyte-like precursors during lactation, and these same cells redifferentiate back into adipocytes during involution (Figure 1), a process that is repeated during multiple rounds of pregnancies.
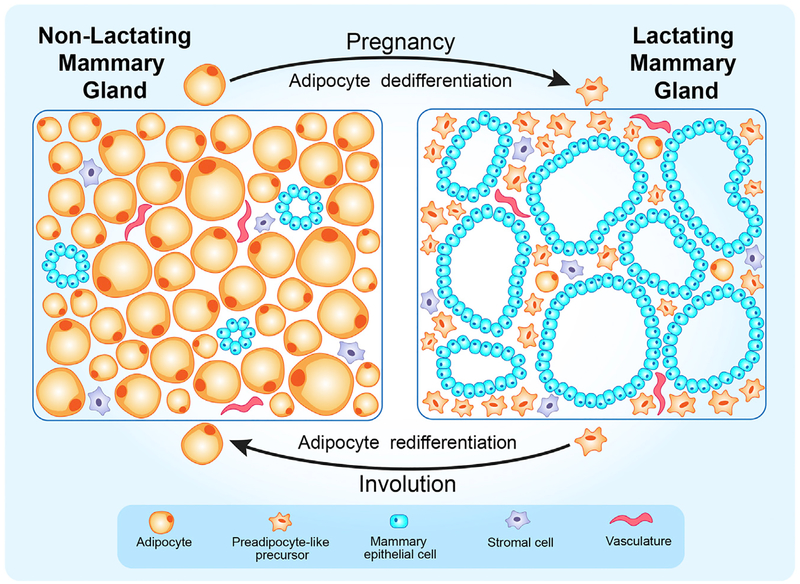
Figure 1. A Schematic Representation of Cyclical Adipocyte Dedifferentiation and Redifferentiation in the Mammary Gland during Reproduction.
Adipocytes account for the majority of the tissue volume in the virgin mammary gland. During pregnancy, mature adipocytes acquire morphological features and transcriptional signatures comparable to preadipocytes, as the mammary epithelium expands to produce milk. Upon weaning, the mammary tissue undergoes involution to a quiescent state similar to the pre-pregnancy mammary gland. During this process, the bulk of secretory epithelium is eliminated by apoptosis, and the preadipocyte-like precursors originally derived from mammary adipocytes repopulate the mammary gland by redifferentiating into mature adipocytes.
To investigate the fate of the mature adipocytes that “disappear” during pregnancy and lactation and later reappear during involution, Wang et al. (2018) used AdipoChaser-LacZ mice to lineage trace adipocytes throughout these processes (Wang et al., 2013). This inducible reporter irreversibly labels all cells that express adiponectin (i.e., adipocytes) with LacZ upon doxycycline activation. LacZ staining of mammary gland tissue collected at different time points during reproduction revealed a significant number of LacZ-positive cells during lactation that did not resemble adipocytes; these cells were significantly smaller than adipocytes and did not contain lipid droplets. Adipocytes that repopulated the mammary gland during involution were nearly all LacZ positive, indicating that they were derived from preexisting mature adipocytes. Furthermore, they did not observe LacZ-positive cells within the secretory epithelial structures, suggesting that adipocytes do not transdifferentiate. This result confiicts with observations made by Marroni et al., who demonstrated that mammary adipocytes transdifferentiate into alveolar epithelial cells; this discrepancy may be due to use of different promoters: FABP4 by Marroni et al. and adiponectin in the current study (Morroni et al., 2004; Wang et al., 2018). Together, these findings demonstrate that adipocytes transition to another cell type that resembles neither adipocytes nor secretory mammary epithelial cells and that these cells are capable of redifferentiation into mammary adipocytes during involution. Interestingly, repopulated adipocytes following two rounds of pregnancies were still nearly all LacZ positive, indicating that they were derived from adipocytes labeled prior to breeding rather than de novo adipogenesis from other precursors. These data suggest that mammary adipocytes cyclically dedifferentiate and redifferentiate through reproductive cycles.
The LacZ-positive cells observed during lactation were next characterized to investigate whether these cells dedifferentiated or transdifferentiated into other cell types or whether they simply lost their lipid content. After fiuorescence-activated cell sorting (FACS) of preadipocytes from mammary tissue of lactating AdipoChaser-LacZ mice and exposure to adipogenic stimuli in culture, nearly all of the resulting adipocytes (87%) stained positive for LacZ, indicating that they arose from preexisting adipocytes. These data suggest that the LacZ-positive cells revert into adipogenic precursors rather than transdifferentiating into secretory epithelial cells or other cell types. The LacZ-positive cells also acquire expression of PDGFRα, a well-established preadipocyte marker, suggesting that mammary adipocytes regress to preadipocytes during lactation rather than simply forming lipid-depleted adipocytes.
To further characterize this precursor pool, the researchers used AdipoChaser-mT/mG mice to permanently label all adipocytes with GFP (Ye et al., 2015; Vishvanath et al., 2016). Cells collected from lactating mice that are GFP positive are therefore derived from adipocytes despite lacking morphological features of adipocytes. FACS analysis of this population confirmed a high proportion of PDGFRα-positive cells, further supporting a regression to a precursor state rather than transdifferentiation. The GFP+/PDGFRα+ cells were then subjected to single-cell RNA sequencing in comparison with preadipocytes isolated from the mammary gland of virgin mice and with cultured mature brown adipocytes. The transcriptional signature of adipocyte-derived GFP+/PDGFRα+ cells was highly similar to mammary preadipocytes from virgin mice and quite distinct from mature adipocytes as evidenced by high expression of preadipocyte markers, including Pdgfra and Pdgfrb, and low expression of adipogenic markers, such as adiponectin (Adipoq), Fabp4, and peroxisome proliferator activated receptor gamma (Pparg). These analyses provide further support that mature adipocytes lose their characteristic markers and acquire preadipocyte-like features during lactation.
The current study unravels one more layer of the remarkable plasticity within the mammary gland during pregnancy, lactation, and involution. The finding that mature adipocytes can undergo cycles of temporary reversion to a precursor state suggests exciting new possibilities for adipocyte plasticity. However, molecular mechanisms underlying these cellular transitions remain unclear. Signals initiating dedifferentiation or promoting subsequent adipogenic reversion are unknown, though it is likely that they are regulated in part by pregnancy hormones and/or paracrine signals from developing parenchymal cells. The full capacity of mature adipocyte plasticity remains to be explored but may yield new insights. Do the dedifferentiated cells contain unique characteristics compared to preadipocytes from other depots? Are they capable of differentiating into other cell types, or do they remain committed to the adipocyte lineage? Are these cellular transitions unique to mammary gland involution, or do they occur in other physiological contexts? Could similar transitions occur in bone marrow adipose tissue, where regulated marrow adipocytes are lost during lactation and reappear upon weaning (Bornstein et al., 2014; Scheller et al., 2015)? Could future insight into these processes be used to develop novel treatments for obesity or lipodystrophy? The current study by Wang et al. (2018) sets the stage to address these questions in future investigations to reveal the full extent of adipocyte plasticity.
ACKNOWLEDGMENTS
This work was supported by funds from the National Institute of Health (R24 DK092759, O.A.M.; RO1 DK62876, O.A.M.; and T32 DK101357, C.A.S.C.) and from the American Diabetes Association (1-18-PDF-064, C.A.S.C.).
REFERENCES
- Bornstein S, Brown SA, Le PT, Wang X, De-Mambro V, Horowitz MC, MacDougald O, Baron R, Lotinun S, Karsenty G, et al. (2014). FGF-21 and skeletal remodeling during and after lactation in C57BL/6J mice. Endocrinology 155, 3516–3526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giordano A, Smorlesi A, Frontini A, Barbatelli G, and Cinti S (2014). White, brown and pink adipocytes: the extraordinary plasticity of the adipose organ. Eur. J. Endocrinol 170, R159–R171. [DOI] [PubMed] [Google Scholar]
- Giordano A, Perugini J, Kristensen DM, Sartini L, Frontini A, Kajimura S, Kristiansen K, and Cinti S (2017). Mammary alveolar epithelial cells convert to brown adipocytes in post-lactating mice. J. Cell. Physiol 232, 2923–2928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marangoni RG, Korman BD, Wei J, Wood TA, Graham LV, Whitfield ML, Scherer PE, Tourtellotte WG, and Varga J (2015). Myofibroblasts in murine cutaneous fibrosis originate from adiponectin-positive intradermal progenitors. Arthritis Rheumatol. 67, 1062–1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morroni M, Giordano A, Zingaretti MC, Boiani R, De Matteis R, Kahn BB, Nisoli E, Tonello C, Pisoschi C, Luchetti MM, et al. (2004). Reversible transdifferentiation of secretory epithelial cells into adipocytes in the mammary gland. Proc. Natl. Acad. Sci. USA 101, 16801–16806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheller EL, Doucette CR, Learman BS, Cawthorn WP, Khandaker S, Schell B, Wu B, Ding SY, Bredella MA, Fazeli PK, et al. (2015). Region-specific variation in the properties of skeletal adipocytes reveals regulated and constitutive marrow adipose tissues. Nat. Commun 6, 7808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vishvanath L, MacPherson KA, Hepler C, Wang QA, Shao M, Spurgin SB, Wang MY, Kusminski CM, Morley TS, and Gupta RK (2016). Pdgfrβ+ mural preadipocytes contribute to adipocyte hyperplasia induced by high-fat-diet feeding and prolonged cold exposure in adult mice. Cell Metab. 23, 350–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang QA, Tao C, Gupta RK, and Scherer PE (2013). Tracking adipogenesis during white adipose tissue development, expansion and regeneration. Nat. Med 19, 1338–1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang QA, Song A, Chen W, Schwalie PC, Zhang F, Vishvanath L, Jiang L, Ye R, Shao M, Tao C, et al. (2018). Reversible de-differentiation of mature white adipocytes into preadipocyte- like precursors during lactation. Cell Metab. 28, this issue, 282–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye R, Wang QA, Tao C, Vishvanath L, Shao M, McDonald JG, Gupta RK, and Scherer PE (2015). Impact of tamoxifen on adipocyte lineage tracing: inducer of adipogenesis and prolonged nuclear translocation of Cre recombinase. Mol. Metab 4, 771–778. [DOI] [PMC free article] [PubMed] [Google Scholar]