Abstract
Objective
To describe the overall risk and factors associated with transitioning to persistent opioid or high-risk use after an initial emergency department (ED) opioid prescription.
Methods
A retrospective cohort study of Washington Medicaid beneficiaries was performed using linked Medicaid and prescription drug monitoring program files. We identified adults who had no record of opioid prescriptions in the prior 12 months, and who filled a new opioid prescription within one day of an ED discharge in 2014. We assessed the risk of persistent opioid use or high-risk prescription fills within 12 months after the index visit. Logistic regression was used to assess the association between pertinent variables and conversion to persistent or high-risk use.
Results
Among 202,807 index ED visits, 23,381 resulted in a new opioid prescription. Of these, 13.7% led to persistent or high-risk opioid prescription fills within 12 months, compared to 3.2% for those who received no opioids at the index visit. Factors associated with increased likelihood of persistent opioid or high-risk prescription fills include a history of skeletal or connective-tissue disorder, neck, back or dental pain, and a history of prescribed benzodiazepines. The highest conversion rates (37.3%) were seen among visits in which ≥350 morphine milligram equivalents were prescribed. Conversion rates remained over 10% even among visits resulting in lower dose opioid prescriptions.
Conclusion
Medicaid recipients are at moderate risk for conversion to persistent or high-risk opioid use after a new ED prescription. Longer or higher dose prescriptions are associated with increased risk for conversion, however, even visits which lead to guideline concordant prescriptions bear some risk for long term or high-risk use.
INTRODUCTION
Background
The U.S. is experiencing an epidemic of prescription drug abuse, according to the Center for Disease Control and Prevention (CDC), with deaths from opioid use now exceeding that from motor vehicle crashes.1,2 Deaths from prescription opioids have quadrupled since 1999.3,4 This epidemic has been associated with increases in opioid sales and prescribing by health care providers, resulting in their expanded availability and frequent diversion for nonmedical use.5–7 The impact of this epidemic is felt in metropolitan and non-metropolitan areas and across all racial and ethnic groups.8
Long term opioid use often starts with treatment for an acute, painful injury or condition.9 In a large representative national sample of patients without cancer who received a new opioid prescription (opioid naïve), the likelihood of persistent opioid use increased with each additional day of medication supplied starting with the third day.10 Approximately 8% of opioid naïve patients who were prescribed opioids within seven days of short-stay surgery were still taking opioids one year later.11 Following a new opioid prescription for wisdom tooth extraction, conversion to persistent use occurred at a rate of 13 per 1,000 patients with private medical insurance.12 Until recently, it was believed that patients prescribed opioids for an acute problem were unlikely to develop drug abuse or addiction.13,14 Systematic reviews indicate that the evidence for that opinion would not meet current scientific standards.15,16
Acute care settings, including emergency departments (EDs), are settings where clinicians and their patients must navigate the line between addressing pain and preventing the misuse of opioid pain medication. Prevention may be the key to addressing the epidemic, as once opioid use disorder occurs, only one in 10 Americans receives treatment4 and current treatment approaches demonstrate low rates of success.17 Emergency providers (EPs) care for victims of opioid overdose, abuse, and misuse every day. Paradoxically, EPs are also among the top prescribers of opioid medication for patients under age 40 years, in terms of number of prescriptions.18 The risk of long term opioid use after a first prescription for acute pain from the ED has been explored: Hoppe and colleagues demonstrated that among opioid naïve patients receiving an ED opioid prescription, 12% had more opioids prescribed at one year.19 Barnett et al, analyzed a cohort of Medicare patient visits and documented a conversion rate to persistent use between 1.2% and 1.5% after a new ED prescription.20 Among young adults, a single “legitimate” prescribed opioid in high school was associated with a 33% increase in the risk of subsequent opioid misuse in a cohort followed to adulthood.21 Additional studies have identified that ED overdose patients and heroin users frequently report that their initial exposure to opioids came from an ED prescription.22,23 With 42% of ED visits related to pain - combined with provider quality measures that include adequacy of pain treatment and patient satisfaction - there has been documented pressure for EPs to prescribe opioids to their patients.24 As safety net providers for a vulnerable population without primary care access or continuity of care, EPs have embraced the responsibility for bridging patients from acute injury to follow up care, including providing pain medications when patients cannot access traditional primary care providers for treatment of pain.
Current policies and guidelines include placing absolute limits on opioid prescription quantities and mandating provider use or enrollment with prescription drug monitoring programs to identify previous, overlapping, or high-risk prescription fills.25 However, these policies do not consider new or low-dose opioid prescriptions. And for some, even small quantity prescriptions can lead to long term or high-risk opioid use.10 Therefore, such policy interventions may not identify or protect patients for whom a new prescription for opioids may pose increased risk for conversion to long term opioid use.
Goal of Study
We sought to describe independent risk factors for transitioning to persistent opioid or high-risk prescription fills after an initial emergency department opioid prescription.
METHODS
Study Design and Setting
A retrospective cohort study of Washington State Medicaid beneficiaries was performed using data collected between Jan 1, 2013 and Dec. 31, 2015. Data included enrollment and medical claims for Medicaid enrollees in Washington State, linked to Prescription Drug Monitoring Program files containing information about all dispensed controlled substances. The creation of this dataset has been described elsewhere.26
Study Population
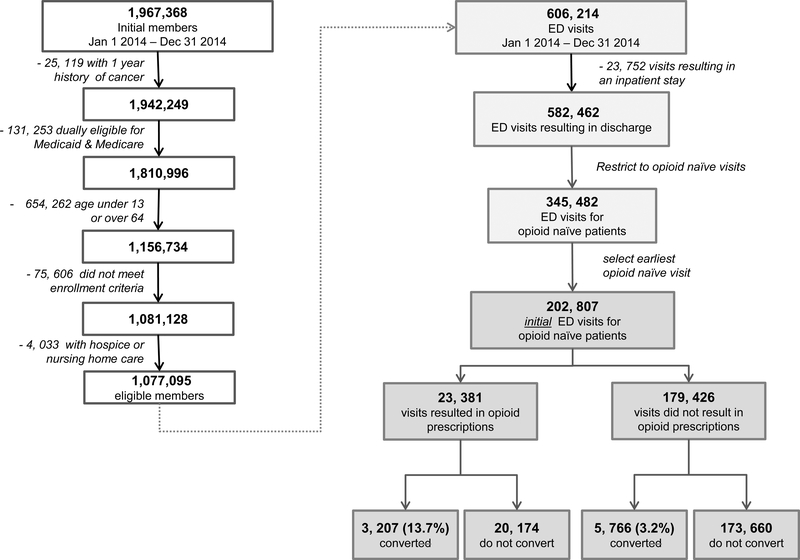
The study population included residents of Washington State who were enrolled in Medicaid between Jan 1, 2014 and Dec 31, 2014. We excluded observations for enrollees with a 1-year history of cancer, those who were also enrolled in Medicare or over age 64, children under the age of 13, and enrollees who received any hospice or nursing home care at any time during the study period. We also excluded members who were enrolled for fewer than 3 of the previous 12 months, to ensure sufficient data for assessing health history. Figure 1 describes the approach to including patient visits in the analyzed sample.
Figure 1.
Cohort selection flowchart
We analyzed emergency department (ED) visits made by enrollees during 2014, if they met the following criteria: (1) the ED visit did not result in an inpatient admission (2) the patient was opioid naïve at the time of the visit, defined as no history of opioid dispensing during the prior 12 months. If multiple opioid naïve ED visits occurred for a given patient during the study period, we selected the earliest visit (3) the patient filled an opioid prescription, defined as any pharmacy dispensed outpatient prescription for an opioid written within 1 day of the ED visit (defined as prescriptions written on the day of registration or the following calendar day, to account for ED visits that might span midnight). The definition of opioid naïve used was based on the most conservative approach taken by national and international studies, who have defined opioid naive as no prescribed opioids between 60 and 365 days prior to the index visit. 27–29
Measurements
Opioid prescriptions included buprenorphine, codeine, fentanyl, hydrocodone, hydromorphone, levorphanol, meperidine, methadone, morphine, oxycodone, oxymorphone, tapentadol, and tramadol and any combination formulation that included these drugs. We included tablets, syrups/suspensions, films, and transdermal patches, and we excluded other formulations (e.g., sprays). Additionally, we recorded the total outpatient dispensed morphine milligram equivalents (MME) prescribed within 1 day of the index visit registration (to account for ED visits that span midnight). Total MMEs were calculated by multiplying tablet number by the opioid dose per tablet. We used the following conversion factors (mg:mg) to calculate MMEs: buprenorphine patch-12.6, buprenorphine tablet- 30; codeine-0.15, fentanyl patch- 7.2 (mcg/hr), hydrocodone-1, hydromorphone-4,levorphanol-11, meperidine-0.1, methadone-3, morphine-1, oxycodone-1.5, oxymorphone-3, tapentadol-0.4, and tramadol-0.1.30
Covariates were selected based on relevant demographic characteristics and previously described patient and visit level risk factors associated with conversion to persistent opioid or high-risk prescription fills. We also selected variables that would be available to a clinician during an ED visit in order to maximize the potential ease of use during clinical care. Covariates included patient age, sex, race, managed care or fee-for-service Medicaid coverage, and whether the enrollee qualified for Medicaid based on disability or expansion under the Affordable Care Act. We assessed one-year history of physical health conditions, behavioral health conditions, substance use disorder, and benzodiazepine (prescription) use. We also created indicators for the presence of pain conditions at the time of the ED visit,31 and quantity of MMEs prescribed at the index visit. Breakpoints for MME size were determined starting with the high dose category as 350 mme (based on CDC recommendations of no more than 7 days of opioids at 50 mme/day for acute pain) and working backwards assigned categories for each 50 mme prescribed.
Outcomes
The primary outcome was a composite measure of any indicator of long-term opioid use or high-risk prescription fills within 12 months after the index visit. Each measure in the composite has been associated with long term use, opioid use disorder (OUD), or overdose.32–38 The composite measure was defined as the presence of at least one of the following criteria during the 12 months after the index ED visit: at least 1 opioid prescription in every calendar quarter, more than 3 prescribers, more than 4 prescriptions for a DEA scheduled opioid, any prescription for long acting opioids, any prescription medicine for opioid use disorder (MOUD), or any prescription for an average of 100 MME/day or greater. For the 1% of opioid prescription visits in which the days supply was missing in the PDMP dataset, imputation was used to estimate the days supply by calculating the average MME per day using all other prescriptions in the dataset where it was not missing. Prescriptions attributed to the index ED visit were not included in subsequent calculations of high-risk use.
Analysis
A logistic regression model was used to assess the association between measures described above and conversion to persistent or high-risk use. All model results are presented as marginal effects, (i.e. percentage point (p.p.) change in the likelihood of conversion to persistent or high-risk use associated with each measure). All data management and statistical analyses were performed in R version 3.3.2 and STATA MP 14.0. The Washington State Health Care Authority provided Medicaid beneficiary level medical and pharmacy claims data. The Intuitional Review Boards of Washington State and of Oregon Health & Science University approved this study. Sensitivity analyses were conducted using alternative definitions of index visits and primary outcome measurements.
RESULTS
We identified 23,381 emergency department visits made by qualifying opioid naïve Medicaid patients, who filled an opioid prescription that was written within one day of the ED visit. Table 1 describes patient and visit level characteristics stratified by conversion to persistent or high-risk opioid use. The population studied was young (median age 32 years), predominantly between 18 and 39 years old (57.5%), female (57.6%), White (62.0%), and covered by a Medicaid managed care insurance plan (91.7%). A specific pain-related or injury diagnosis was recorded for 59.7% of the index ED visits (N=13,965). Injuries were the most common diagnostic category within the cohort of index visits. This population exhibited moderately high rates of a history tobacco use (10.0%), anxiety (15.4%), and major depression (7.3%). The vast majority (83.2%) of index ED visits that resulted in an opioid prescription were for less than 150 morphine milligram equivalents (mean = 97.2, SD = 83.3). The index prescriptions are described on Table 2. The vast majority of opioid prescriptions filled from the index visit were for short acting hydrocodone (70%) and oxycodone (21%) tablets. Long acting or non-tablet formulations of opioids comprised less than .0016% of the total number The overall conversion rate to persistent opioid or high-risk prescription fills in this cohort after an ED index visit opioid prescription was 13.7%, significantly higher (p<0.001) than the 3.2% conversion rate among the cohort of index ED visits in which no opioid was prescribed (Figure 1). Table 3 describes the frequency that each component of the main composite outcome occurred among those who received an opioid at the index visit. The conversion rate of 13.7% was relatively robust to multiple sensitivity analyses, including 1) using an exact match of the index ED visit date and prescription date to indicate prescriptions written at the index visit (13.8%), 2) removing the criteria of >4 prescriptions for opioids filled in the subsequent year after the index visit from the composite outcome measure (11.7%), 3) removing the criteria of >3 prescribers in the subsequent 12 months, 4) limiting index ED visits to only those with a pain related diagnosis (14.9%), and 5) a combination of all of the above analytic approaches (7.8%). (Supplemental Table S1).The highest conversion rates were seen among visits in which 350 MME or more were prescribed (37.3%, 95% CI=32.3–42.5) and for patients with a history of any substance use disorder, including tobacco, (20.4%, 95% CI= 18.8–22.1), and any non-substance related behavioral health disorder (17.8%, 95% CI= 17.1–18.5).
Table 1:
Characteristics of ED visits for opioid naïve patients who received an opioid prescription
| Converted to Persistent or High-Risk Use N = 3,207 (13.7%) |
Did not convert N = 20,174 (86.3%) |
|
|---|---|---|
| Patient demographics | ||
| Age (yrs) – N(%) | ||
| 13–17 | 78 (2.4) | 2,468 (12.2) |
| 18–39 | 1,826 (56.9) | 11,616 (57.6) |
| 40–64 | 1,303 (40.6) | 6,090 (30.2) |
| Female sex – N(%) | 1,783 (55.6) | 11,676 (57.9) |
| Race-ethnicity – N(%) | ||
| White | 2,215 (69.1) | 12,288 (60.9) |
| Hispanic | 302 (9.4) | 2,668 (13.2) |
| Black | 243 (7.6) | 1,738 (8.6) |
| American Indian/Alaska Native | 110 (3.4) | 582 (2.9) |
| Asian/Hawaiian or other Pacific Islander | 67 (2.1) | 714 (3.5) |
| Other/unknown | 270 (8.4) | 2,184 (10.8) |
| Insurance | ||
| Qualified for Medicaid under disability – N(%) | 858 (26.8) | 3,532 (17.5) |
| Qualified for Medicaid by income under expansion – N(%) | 916 (28.6) | 4,900 (24.3) |
| Coverage type – N(%) | ||
| Managed Care | 2,928 (91.3) | 18,515 (91.8) |
| Fee-for-service | 279 (8.7) | 1,659 (8.2) |
| History of physical health conditions – N(%) | ||
| Skeletal and connective | 1,086 (33.9) | 4,212 (20.9) |
| Cardiovascular | 759 (23.7) | 3,039 (15.1) |
| Pulmonary | 582 (18.1) | 2,597 (12.9) |
| Gastrointestinal | 541 (16.9) | 2,260 (11.2) |
| Skin | 432 (13.5) | 2,357 (11.7) |
| Diabetes | 282 (8.8) | 1,180 (5.8) |
| Nervous system | 230 (7.2) | 845 (4.2) |
| Genital | 219 (6.8) | 1,046 (5.2) |
| Pregnancy | 172 (5.4) | 1,261 (6.3) |
| Metabolic | 174 (5.4) | 676 (3.4) |
| Infectious | 193 (6.0) | 672 (3.3) |
| Renal | 124 (3.9) | 481 (2.4) |
| Hematologic | 52 (1.6) | 210 (1.0) |
| Other1 | 66 (2.1) | 400 (2.0) |
| History of substance use disorder – N(%) | ||
| Opioids | 81 (2.5) | 212 (1.1) |
| Cannabis | 69 (2.2) | 313 (1.6) |
| Amphetamines | 74 (2.3) | 288 (1.4) |
| Tobacco | 477 (14.9) | 1,860 (9.2) |
| Other2 | 261 (8.1) | 1,066 (5.3) |
| History of other behavioral health disorder – N(%) | ||
| Anxiety | 730 (22.8) | 2,863 (14.2) |
| Dysthymia or other depression | 579 (18.1) | 2,467 (12.2) |
| Major depression | 351 (10.9) | 1360 (6.7) |
| Alcohol disorder | 287 (8.9) | 1,100 (5.5) |
| Bipolar disorder | 189 (5.9) | 779 (3.9) |
| Disorders originating in childhood | 108 (3.4) | 820 (4.1) |
| Schizophrenia or other non-mood disorder | 79 (2.5) | 343 (1.7) |
| Other3 | 744 (23.2) | 2,981 (14.8) |
| Pain-related or injury diagnoses at visit – N(%) | ||
| Any pain or injury diagnosis | 2,080 (64.9) | 11,885 (58.9) |
| Arthritis/joint pain | 479 (14.9) | 2,135 (10.6) |
| Back pain | 488 (15.2) | 2,316 (11.5) |
| Non-traumatic dental pain | 331 (10.3) | 1967 (9.8) |
| Neck pain | 190 (5.9) | 909 (4.5) |
| Kidney stone | 87 (2.7) | 609 (3.0) |
| Injury | 1,222 (38.1) | 7,600 (37.7) |
| Other4 | 210 (6.5) | 1,107 (5.5) |
| Medications | ||
| History of benzodiazepine use – N(%) | 115 (3.6) | 302 (1.5) |
| Total MME prescribed at visit – N(%) | ||
| under 150 | 2416 (75.3) | 17029 (84.4) |
| 150–350 | 665 (20.7) | 2933 (14.5) |
| 350 and over | 126 (3.9) | 212 (1.1) |
Notes: history of physical, behavioral, and substance use disorders, as well as history of benzodiazepine use, were evaluated using the prior year of claims data. Pain-related and injury diagnoses were assessed at the index visit, and total prescribed MME was evaluated for prescriptions written within 1 day of the index visit.
eye, cerebrovascular, and developmental disability
barbiturate, cocaine, hallucinogen, and antidepressant abuse, and miscellaneous
personality disorder, adjustment disorder, and miscellaneous
gallstone, headache, and miscellaneous
Table 2.
Opioid prescription from initial ED visit for opioid naïve patients
| Form | Opioid Class | Frequency | Percent |
|---|---|---|---|
| Tablet | Hydrocodone SA | 16699 | 69.55 |
| Tablet | Oxycodone SA | 5080 | 21.16 |
| Tablet | Codeine | 956 | 3.98 |
| Tablet | Tramadol SA | 861 | 3.59 |
| Solution | Codeine | 130 | 0.54 |
| Tablet | Hydromorphone SA | 122 | 0.51 |
| Solution | Hydrocodone SA | 83 | 0.35 |
| Solution | Oxycodone SA | 26 | 0.11 |
| Capsule | Codeine | 16 | 0.07 |
| Film | Buprenorphine | 9 | 0.04 |
| Tablet, Extended Release | Morphine LA | 6 | 0.02 |
| Tablet, Extended Release | Oxycodone LA | 6 | 0.02 |
| Capsule | Oxycodone SA | 6 | 0.02 |
| Tablet | Morphine SA | 4 | 0.02 |
| Tablet | Methadone | 3 | 0.01 |
| Tablet | Meperidine | 1 | 0.00 |
| Tablet | Tapentadol SA | 1 | 0.00 |
| Tablet, Extended Release | Tramadol LA | 1 | 0.00 |
Table 3.
Breakdown of individual components in the composite measure of long-term or high-risk use
| Criteria | Frequency N = 3,207 (%) |
|---|---|
| At least 1 opioid prescription in every calendar quarter | 1,032 (32.2) |
| More than 3 prescribers | 1,969 (61.4) |
| More than 4 prescriptions for a DEA scheduled opioid | 2,432 (75.8) |
| Any prescription for long acting opioids | 294 (9.2) |
| Any prescription medicine for opioid use disorder (MOUD) | 147 (4.6) |
| Any prescription for an average of 100 MME/day or greater | 734 (22.9) |
The marginal effects for each covariate, adjusting for all other factors, are presented in Table 4. For Washington State Medicaid patients prescribed opioids at the index emergency department visit, notable factors associated with an independent increased likelihood of persistent opioid or high-risk prescription fills use include: a history of skeletal and connective tissue disorder (7.9 percentage point (pp) increase, 95% CI= 6.6–9.1 pp), history of opioid use disorder (4.6 percentage point increase, 95% CI= 0.5–8.6 pp), history of anxiety (3.0 percentage point increase, 95% CI= 1.6–4.4pp), an index visit diagnosis of neck pain (2.7 percentage point increase, 95% CI= 0.6–4.9 pp), diagnosis of nontraumatic dental pain (2.5 percentage point increase, 95% CI= 0.9–4.2 pp), and history of prescribed benzodiazepines prior to the index visit (7.1 percentage point increase, 95% CI= 3.6–10.5 pp).
Table 4.
Regression marginal effects estimates
| Characteristic | Marginal Effect Estimate |
|---|---|
| (95% CI) | |
| Age | |
| 13–17 | −10.83 (−11.87, −9.80) |
| 18–39 | ref |
| 40–64 | 0.27 (−0.84, 1.38) |
| Male gender | 1.15 (0.19, 2.11) |
| Race | |
| White | ref |
| Black | −2.4 (−3.92, −0.88) |
| Hispanic | −2.48 (−3.86, −1.11) |
| Asian/Hawaiian or other Pacific Islander | −5.47 (−7.59, −3.35) |
| American Indian/Alaska Native | −0.18 (−2.86, 2.49) |
| Other/Unknown | −3.03 (−4.42, −1.63) |
| Qualified for Medicaid under disability | 1.78 (0.53, 3.04) |
| Qualified for Medicaid under expansion | 1.68 (0.55, 2.80) |
| Coverage type | |
| Fee-for-service | ref |
| Managed care | −2.43 (−4.24, −0.62) |
| History of physical health conditions | |
| Cardiovascular | 1.78 (0.51, 3.04) |
| Skeletal and connective tissue | 7.85 (6.59, 9.11) |
| Nervous System | 0.98 (−0.92, 2.87) |
| Pulmonary | 2.1 (0.81, 3.40) |
| Gastrointestinal | 1.46 (0.13, 2.78) |
| Diabetes | 1.34 (−0.45, 3.14) |
| Skin | 1.45 (0.05, 2.85) |
| Renal | 2.27 (−0.42, 4.96) |
| Genital | 4.77 (2.56, 6.98) |
| Metabolic | 1.94 (−0.32, 4.20) |
| Pregnancy | 0.31 (−1.68, 2.30) |
| Hematologic | 1.9 (−2.11, 5.90) |
| Infectious | 1.93 (−0.27, 4.14) |
| Other Physical Health Condition | −3.86 (−6.26, −1.47) |
| History of other behavioral health | |
| Alcohol disorder | 1.51 (−0.28, 3.30) |
| Anxiety | 3.03 (1.62, 4.44) |
| Bipolar disorder | −0.39 (−2.34, 1.56) |
| Disorders originating in childhood | −2.16 (−4.29, −0.02) |
| Dysthymia or other depression | 0.8 (−0.53, 2.14) |
| Major depression | 0.84 (−0.81, 2.49) |
| Schizophrenia or other non-mood disorder | −1.92 (−4.57, 0.73) |
| Other Behavioral health disorders | 3.28 (2.00, 4.57) |
| Pain-related or injury diagnoses at visit | |
| Kidney stone | −1.87 (−4.27, 0.54) |
| Injury | −0.83 (−1.83, 0.18) |
| Back pain | 2.28 (0.91, 3.65) |
| Neck pain | 2.74 (0.56, 4.92) |
| Arthritis/joint pain | 2.57 (1.16, 3.99) |
| Non-traumatic dental pain | 2.5 (0.85, 4.16) |
| Other pain | 0.95 (−0.93, 2.82) |
| History of substance use disorder | |
| Opioids | 4.56 (0.53, 8.59) |
| Cannabis | −1.72 (−4.62, 1.19) |
| Amphetamines | −1.79 (−4.70, 1.12) |
| Tobacco | 2.01 (0.56, 3.46) |
| Other Substance Use Disorders | 1.09 (−0.86, 3.05) |
| Medications | |
| History of prescribed benzodiazepines | 7.05 (3.56, 10.54) |
| Total MME prescribed at visit | |
| under 150 | ref |
| 150–350 | 4.47 (3.20, 5.74) |
| 350 and over | 19.32 (14.64, 24.00) |
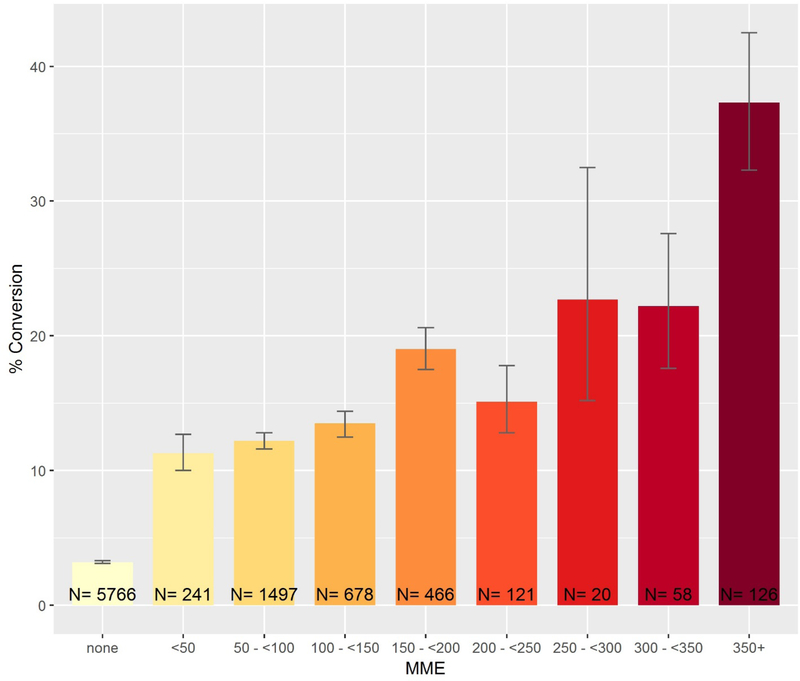
The size (total MME) of the index opioid prescription was associated with the largest significant increase in conversion rates to persistent opioid or high-risk filled prescriptions fills. After adjustment, patient index visits in which >350 MME of opioids were prescribed were associated with a 19.3-percentage point increase in the likelihood of conversion compared to those visits with under 150 MME. Figure 2 displays the unadjusted conversion rates for index visits with opioid prescriptions of differing sizes. While patients who received over 350 MME have the highest conversion rate, there does not appear to be a threshold effect, and notably patients who received lower dose opioid prescriptions (<50 mme, 50–100 mme and 100–150mme) also have relatively high conversion rates (all over 10%).
Figure 2.
Frequency of persistent or high-risk opioid conversion by quantity of morphine milligram equivalent prescribed at the index ED visit
Limitations
We sought to limit the study to opioid-naïve ED visits during which a new opioid prescription was written and subsequently filled. We acknowledge that it is possible some of the index ED visit prescriptions did not originate at the index ED visit. We attempted to minimize this potential misclassification by limiting index prescriptions to those that were written within one day after the ED visit and by conducting sensitivity analyses that included exact match on index prescription date (supplemental table S1). We only had access to outpatient prescription data, so it is possible that patients were misclassified and were not truly opioid naïve, for example if they were using diverted prescription opioids that were not prescribed to them or illicit opioids, such as heroin or fentanyl. In determining associations with conversion to high risk prescriptions, we recognize that administrative data cannot capture all clinically and socially relevant data. Moreover, the linkage of the Medicaid claims and PMP data, while highly specific, demonstrate modest sensitivity. The false negative matching is thought to occur at random due to mismatches for elements like name and birthdate. The high specificity of the matching increases our confidence that we correctly identified patients who filled opioid prescriptions at the index and on follow up visits. The matching algorithm and limitations have been discussed elsewhere.26 Finally, these data were derived from a single state and therefore may not be generalizable to Medicaid enrollees or other patients from different regions. We attempted to account for many of these inherent limitations of observational and claims-data analyses by using recommended best practices for opioid safety research as described by Ranapurala and colleagues, including using multiple data sources including linked PMP and claims data as well as conducting sensitivity analyses to examine the level of confounding, selection or misclassification. 39
Discussion
In this study of a large cohort of Medicaid patients, 13.7% of patients who filled a new opioid prescription within 1 day of an ED visit converted to persistent or high-risk opioid prescription fills within 12 months. This conversion rate stands in stark contrast to the 3.2% conversion rate among visits in which no opioids were prescribed. Patient level characteristics such as a history of skeletal and connective tissue disorder, a history of opioid use disorder, a history of anxiety, a diagnosis of neck pain, a diagnosis of dental pain and previous history of prescribed benzodiazepines were each independently, but modestly, associated with increased risk of persistent or high-risk use. Notably, patient visits which resulted in higher dose opioid prescriptions were the most likely to convert to persistent opioid or high-risk prescription fills.
Other published research demonstrated that rates of conversion to persistent opioid use after initial opioid prescriptions in multiple settings (including the emergency department) ranged from 1% among general Medicare recipients to 13% among disabled publicly insured patients. 20,37 These studies have been limited by use of claims-only data which does not capture uninsured and cash purchased prescriptions. Moreover, conversion to persistent or high-risk prescriptions have not previously been studied in a Medicaid population which includes younger and disabled patients compared to Medicare and commercial insurance samples.
This study aligns with previously reported risk factors for persistent opioid use after an initial therapeutic opioid exposure. 40 Neck pain, diagnosis and/or treatment for anxiety, tobacco use, and a history of substance use disorder have each been described as independent risk factors for persistent opioid use after a new prescription in surgical settings.40,41 Similarly, in primary care settings, past or current nicotine use, or a history of substance use was shown to be associated with persistent opioid use after an index prescription.42 There are limited published descriptions of independent risk factors for conversion to persistent or high-risk use for patients with acute pain in emergency department settings for opioid naïve patients. The Opioid Risk Tool (ORT)43 and the Screener and Opioid Assessment for Patients with Pain-Revised (SOAPP-R) 44,45both predict possible OUD in chronic pain patients, but not acute pain, ED, or opioid naive patients. Weiner et al compared SOAPP-R scores to real time ED PMP queries and identified that high scoring SOAPP-R scores are associated with evidence of high concurrent or past high-risk use.46 However this study did not assess the ability to risk stratify opioid naïve ED patients for conversion to persistent or high-risk use.
We found that the size of the initial ED opioid prescription was strongly associated with conversion to persistent opioid or high-risk filled prescriptions. This finding has been identified in previous studies broadly across many clinical settings47, and specifically in emergency departments.20,48 Our findings suggest that the independent likelihood of persistent or high risk opioid use increases with increasing size of the initial ED prescription, but that there is no automatically safe opioid threshold below which an ED prescription spares all patients from the hazard of risky or long- term use. Notably almost 1500 opioid naïve visits in which the initial ED prescription was between 50 and 100 MMEs were associated with high risk conversion. These visits, despite being compliant with CDC acute pain guidelines49, represented the largest absolute count of conversions within our sample.
Previously published work from this team has described how emergency medicine providers and patients seek more information about individual opioid risks when choosing a pain treatment. In particular, we found that providers did not seem to know or explain the individual risks of opioid medications when discussing pain treatment.50 Similarly, providers expressed frustration regarding not have good tools to understand and communicate tradeoffs to patients when discussing pain management.51,52 It is known that providers frequently prescribe opioids to patients with known or potential risk factors for abuse53, therefore an overall understanding of both the general risk of conversion to persistent or high risk opioid use for ED patients as well as individual patient-level risk factors could allow for better understanding and communication of risk to patients during pain treatment discussions.
It is important to note the policy and care delivery landscape focused on opioid prescribing in Washington State before and during the study period. Of note, most of the prescribing policy changes occurred in the state prior to the study period. In Washington State, opioid prescribing increased 500% from 1997 to 2006. By 2006, 10,000 Washington patients with public health insurance were prescribed at least 100 MME/day. Between 2005–2012, Medicaid implemented a Narcotic Review Program in Washington in which included communication with providers and prior authorization for some long-term prescriptions. In 2007, Washington created a prescribing guideline which recommended limits to MME/day prescriptions and CME presentations to provider groups. In 2011, the Washington chapter of the American College of Emergency Physicians (ACEP) adopted an ED prescribing guideline which included limited prescriptions for chronic pain and limiting intravenous opioid medication for acute pain and using an electronic information exchange that existed across all state EDs.54 In 2012, WA legislature mandated the adoption of ED prescribing guidelines and required all ED providers to register for the state prescription drug monitoring program. During the study period (2013–2015), no significant policy changes reflecting ED prescriptions were made, however most of the earlier policies were still being implemented and scaled-up. 55,56
This study is, to our knowledge, the only analysis of opioid related outcomes after an initial prescription within a cohort of Medicaid beneficiaries. Our findings of an overall 13.7% conversion rate to persistent or high-risk opioid use, represents a significantly higher rate of conversion among an adult population than has been previously described among Medicare and commercial insurance populations. Medicaid recipients represent a unique and understudied population as it relates to health care delivery and outcomes. Medicaid enrollees are younger, and more likely to have poorer health and disabilities than privately insured patients. It has also been demonstrated that patients with Medicaid have a harder time accessing routine and specialty health care services.57 Moreover, due to the Affordable Care Act, Medicaid is the most rapidly expanding sector of the health insurance market nationally and many policy initiatives are currently occurring at the state Medicaid level. Medicaid beneficiaries, have a ten-fold higher rate of fatal prescription opioid overdose compared to privately insured populations.58 These patients may also be at risk for being undertreated for serious pain, and these findings should not prevent the use of suitable analgesia, including opioids, when appropriate. This study provides an opportunity to address prescription policies and practices for this important and vulnerable population of patients.
In summary Medicaid recipients are at moderate risk for conversion to persistent or high-risk opioid use after a new ED opioid prescription. Longer or higher dose prescriptions are associated with increased risk for conversion, however even patients who receive guideline concordant prescriptions are at risk. Specific patient and visit level characteristics increase the likelihood of high-risk use and should be considered by providers, patients, and policy makers in decision making for acute pain treatment.
Supplementary Material
Bibliography
- 1.Paulozzi L, Baldwin G, Franklin G, et al. CDC Grand Rounds: Prescription Drug Overdoses - a U.S. Epidemic. MMWR Morb Mortal Wkly Rep. 2012;61(1):10–13. doi:mm6101a3 [pii]. [PubMed] [Google Scholar]
- 2.Paulozzi L, Dellinger A, Degutis L. Lessons from the past. Inj Prev. 2012;18(1):70. doi: 10.1136/injuryprev-2011-040294. [DOI] [PubMed] [Google Scholar]
- 3.CDC. NCHS. Underlying Cause of Death 1999–2014 on CDC WONDER Online Database. Underlying Cause Death 1999–2014 CDC WONDER Online Database. 2015. http://wonder.cdc.gov/ucd-icd10.html. [Google Scholar]
- 4.Levi, Jeffrey Segal, Laura M. Miller A. Prescription Drug Abuse: Strategies to Stop the Epidemic 2013. Prescr Drug Abus Inj Policy Rep Ser. 2013:1–64. www.healthyamericans.org. [Google Scholar]
- 5.Califf RM, Woodcock J, Ostroff S. A Proactive Response to Prescription Opioid Abuse. N Engl J Med. 2016. doi: 10.1056/NEJMsr1601307. [DOI] [PubMed] [Google Scholar]
- 6.Holman JE, Stoddard GJ, Higgins TF. Rates of prescription opiate use before and after injury in patients with orthopaedic trauma and the risk factors for prolonged opiate use. J Bone Joint Surg Am. 2013;95:1075–1080. doi: 10.2106/JBJS.L.00619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Manchikanti L, Fellows B, Ailinani H, Pampati V. Therapeutic use, abuse, and nonmedical use of opioids: A ten-year perspective. Pain Physician. 2010;13(5):401–435. http://ovidsp.ovid.com/ovidweb.cgi?T=JS&CSC=Y&NEWS=N&PAGE=fulltext&D=emed9&AN=2010576242;%5Cnhttp://gw2jh3xr2c.search.serialssolutions.com?url_ver=Z39.88-2004&rft_val_fmt=info:ofi/fmt:kev:mtx:journal&rfr_id=info:sid/Ovid:emed9&rft.genre=article&rft_id=inf. [PubMed] [Google Scholar]
- 8.Rudd RA, Aleshire N, Zibbell JE, Gladden RM. Increases in Drug and Opioid Overdose Deaths — United States, 2000–2014. MMWR Morb Mortal Wkly Rep. 2015;64:1–5. [DOI] [PubMed] [Google Scholar]
- 9.Edlund MJ, Martin BC, Russo JE, Devries A, Braden JB, Sullivan MD. The Role of Opioid Prescription in Incident Opioid Abuse and Dependence Among Individuals with Chronic Non-cancer Pain. Clin J Pain. 2013:1. doi: 10.1097/AJP.0000000000000021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shah A, Hayes CJ, Martin BC. Characteristics of Initial Prescription Episodes and Likelihood of Long-Term Opioid Use — United States, 2006–2015. MMWR Morb Mortal Wkly Rep. 2017;66(10):265–269. doi: 10.15585/mmwr.mm6610a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Alam A, Gomes T, Zheng H, et al. Long-term Analgesic Use After Low-Risk Surgery: A Retrospective Cohort Study. Arch Intern Med. 2012;172(5):425–430. doi: 10.1001/archinternmed.2011.1827. [DOI] [PubMed] [Google Scholar]
- 12.Harbaugh CM, Nalliah R, Hu HM, Englesbe M, Walijee J, Brummett CM. Persistent Opioid Use AfterWisdom Tooth Extraction. Ann Intern Med. 2010. doi: 10.7326/0003-4819-152-2-201001190-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Portenoy RK, Foley KM. Chronic use of opioid analgesics in non-malignant pain: Report of 38 cases. Pain. 1986;25(2):171–186. doi: 10.1016/0304-3959(86)90091-6. [DOI] [PubMed] [Google Scholar]
- 14.P J, J H. Addiction Rare in Patients Treated with Narcotics. NEJM. 1980;302(2):123. doi: 10.1056/NEJM198001103020221. [DOI] [PubMed] [Google Scholar]
- 15.Ballantyne JC. “Safe and effective when used as directed”: the case of chronic use of opioid analgesics. J Med Toxicol. 2012;8(4):417–423. http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3550253&tool=pmcentrez&rendertype=abstract. Accessed February 9, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Harden. Chronic opioid therapy: another reappraisal. APS Bull. 2002;12(1):8. [Google Scholar]
- 17.Weiss RD, Potter JS, Fiellin DA, et al. Adjunctive counseling during brief and extended buprenorphine-naloxone treatment for prescription opioid dependence: a 2-phase randomized controlled trial. Arch Gen Psychiatry. 2011;68(12):1238–1246. doi: 10.1001/archgenpsychiatry.2011.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Volkow ND, McLellan TA, Cotto JH, Karithanom M, Weiss SRB. Characteristics of opioid prescriptions in 2009. JAMA. 2011;305(13):1299–1301. doi: 10.1001/jama.2011.401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hoppe J a., Kim H, Heard K. Association of Emergency Department Opioid Initiation With Recurrent Opioid Use. Ann Emerg Med. 2014. doi: 10.1016/j.annemergmed.2014.11.015. [DOI] [PubMed] [Google Scholar]
- 20.Barnett ML, Olenski AR, Jena AB. Opioid-Prescribing Patterns of Emergency Physicians and Risk of Long-Term Use. N Engl J Med. 2017;376(7):663–673. doi: 10.1056/NEJMsa1610524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Miech R, Johnston L, O’Malley PM, et al. Prescription Opioids in Adolescence and Future Opioid Misuse. Pediatrics. 2015;136(5):e1169-e1177. doi: 10.1542/peds.2015-1364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Butler MM, Ancona RM, Beauchamp GA, et al. Emergency Department Prescription Opioids as an Initial Exposure Preceding Addiction. In: Annals of Emergency Medicine.Vol 68; 2016. doi: 10.1016/j.annemergmed.2015.11.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ancona RM, Butler MM, Beauchamp GA, et al. ED prescription opioids are a frequent initial exposure preceding addiction. Acad Emerg Med. 2015. doi: 10.1016/j.annemergmed.2015.11.033.ED. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cantrill S V, Brown MD, Carlisle RJ, et al. Clinical policy: Critical issues in the prescribing of opioids for adult patients in the emergency department. Ann Emerg Med. 2012;60(4):499–525. doi: 10.1016/j.annemergmed.2015.11.033.ED. [DOI] [PubMed] [Google Scholar]
- 25.National Conference of State Legislatures. Prescribing Policies: States Confront Opioid Overdose Epidemic.; 2017. http://www.ncsl.org/Portals/1/Documents/Health/prescribingOpioids_final01-web.pdf.
- 26.Sun BC, Lupulescu-Mann N, Charlesworth CJ, et al. Does Prescription Opioid Shopping Increase Overdose Rates in Medicaid Beneficiaries? Annals of Emergency Medicine. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bateman BT, Franklin JM, Bykov K, et al. Persistent opioid use following cesarean delivery: patterns and predictors among opioid-naïve women. Am J Obstet Gynecol. 2016. doi: 10.1016/j.ajog.2016.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Brat GA, Agniel D, Beam A, et al. Postsurgical prescriptions for opioid naive patients and association with overdose and misuse: Retrospective cohort study. BMJ. 2018. doi: 10.1136/bmj.j5790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jena AB, Goldman D, Karaca-Mandic P. Hospital Prescribing of Opioids to Medicare Beneficiaries. JAMA Intern Med. 2016;176(7):990. doi: 10.1001/jamainternmed.2016.2737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Opioid Morphine Equivalent Conversion Factors. 2017. https://www.cms.gov/Medicare/Prescription-Drug-Coverage/PrescriptionDrugCovContra/Downloads/Opioid-Morphine-EQ-Conversion-Factors-Aug-2017.pdf. Accessed October 1, 2018.
- 31.Sullivan MD, Edlund MJ, Fan MY, DeVries A, Brennan Braden J, Martin BC. Trends in use of opioids for non-cancer pain conditions 2000–2005 in Commercial and Medicaid insurance plans: The TROUP study. Pain. 2008. doi: 10.1016/j.pain.2008.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jena AB, Goldman D, Weaver L, Karaca-Mandic P. Opioid prescribing by multiple providers in Medicare: Retrospective observational study of insurance claims. BMJ. 2014;348. doi: 10.1136/bmj.g1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dunn KM, Saunders KW, Rutter CM, et al. Opioid prescriptions for chronic pain and overdose: A cohort study. Ann Intern Med. 2010;152(2):85–92. doi: 10.7326/0003-4819-152-2-201001190-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Franklin GM, Mai J, Wickizer T, Turner JA, Fulton-Kehoe D, Grant L. Opioid dosing trends and mortality in Washington State Workers’ Compensation, 1996–2002. In: American Journal of Industrial Medicine.Vol 48; 2005:91–99. doi: 10.1002/ajim.20191. [DOI] [PubMed] [Google Scholar]
- 35.Bohnert ASB. Association Between Opioid Prescribing Patterns and Opioid Overdose-Related Deaths. JAMA. 2011;305(13):1315. doi: 10.1001/jama.2011.370. [DOI] [PubMed] [Google Scholar]
- 36.Braden JB, Russo J, Fan M-Y, et al. Emergency department visits among recipients of chronic opioid therapy. Arch Intern Med. 2010;170(16):1425–1432. doi: 10.1001/archinternmed.2010.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jeffery MM, Hooten WM, Hess EP, et al. Opioid Prescribing for Opioid-Naive Patients in Emergency Departments and Other Settings: Characteristics of Prescriptions and Association With Long-Term Use. Ann Emerg Med. 2018;71(3):326–336.e19. doi: 10.1016/j.annemergmed.2017.08.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Carey CM, Jena AB, Barnett ML. Patterns of Potential Opioid Misuse and Subsequent Adverse Outcomes in Medicare, 2008 to 2012. Ann Intern Med. 2018;168(12):837. doi: 10.7326/M17-3065. [DOI] [PubMed] [Google Scholar]
- 39.Ranapurwala SI, Naumann RB, Austin AE, Dasgupta N, Marshall SW. Methodologic limitations of prescription opioid safety research and recommendations for improving the evidence base. Pharmacoepidemiol Drug Saf. 2019;28(1):4–12. doi: 10.1002/pds.4564. [DOI] [PubMed] [Google Scholar]
- 40.Brummett CM, Waljee JF, Goesling J, et al. New Persistent Opioid Use After Minor and Major Surgical Procedures in US Adults. JAMA Surg. 2017;152(6). doi: 10.1001/jamasurg.2017.0504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sekhri S, Arora NS, Cottrell H, et al. Probability of Opioid Prescription Refilling After Surgery: Does Initial Prescription Dose Matter? Ann Surg. 2018. doi: 10.1097/SLA.0000000000002308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hooten WM, St Sauver JL, McGree ME, Jacobson DJ, Warner DO. Incidence and Risk Factors for Progression From Short-term to Episodic or Long-term Opioid Prescribing: A Population-Based Study. Mayo Clin Proc. 2015. doi: 10.1016/j.mayocp.2015.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Webster LR, Webster RM. Predicting aberrant behaviors in opioid-treated patients: Preliminary validation of the opioid risk tool. Pain Med. 2005;6(6):432–442. doi: 10.1111/j.1526-4637.2005.00072.x. [DOI] [PubMed] [Google Scholar]
- 44.Butler SF, Fernandez K, Benoit C, Budman SH, Jamison RN. Validation of the Revised Screener and Opioid Assessment for Patients With Pain (SOAPP-R). J Pain. 2008;9(4):360–372. doi: 10.1016/j.jpain.2007.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Butler SF, Budman SH, Fernandez KC, Fanciullo GJ, Jamison RN. Cross-validation of a screener to predict opioid misuse in chronic pain patients (SOAPP-R). J Addict Med. 2009;3(2):66–73. doi: 10.1097/ADM.0b013e31818e41da. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Weiner SG, Griggs CA, Mitchell PM, et al. Clinician Impression Versus Prescription Drug Monitoring Program Criteria in the Assessment of Drug-Seeking Behavior in the Emergency Department. Ann Emerg Med. 2013;62(4):281–289. doi: 10.1016/j.annemergmed.2013.05.025. [DOI] [PubMed] [Google Scholar]
- 47.Shah A, Hayes CJ, Martin BC. Characteristics of Initial Prescription Episodes and Likelihood of Long-Term Opioid Use — United States, 2006–2015. MMWR Morb Mortal Wkly Rep. 2017. doi: 10.15585/mmwr.mm6610a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jeffery MM, Hooten WM, Hess EP, et al. Opioid Prescribing for Opioid-Naive Patients in Emergency Departments and Other Settings: Characteristics of Prescriptions and Association With Long-Term Use. In: Annals of Emergency Medicine; 2018. doi: 10.1016/j.annemergmed.2017.08.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dowell D, Haegerich TM, Chou R. CDC Guideline for Prescribing Opioids for Chronic Pain—United States, 2016. JAMA. 2016;315(15):1624. doi: 10.1001/jama.2016.1464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Smith RJRJ, Rhodes K, Paciotti B, Kelly S, Perrone J, Meisel ZFZF. Patient Perspectives of Acute Pain Management in the Era of the Opioid Epidemic. Ann Emerg Med. 2015;66(3):1–8. doi: 10.1016/j.annemergmed.2015.03.025. [DOI] [PubMed] [Google Scholar]
- 51.Sinnenberg LE, Wanner KJ, Perrone J, Barg FK, Rhodes K V., Meisel ZF. What Factors Affect Physicians’ Decisions to Prescribe Opioids in Emergency Departments? MDM Policy Pract. 2017;2(1):2381468316681006. doi: 10.1177/2381468316681006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kilaru AS, Gadsden SM, Perrone J, Paciotti B, Barg FK, Meisel ZF. How do physicians adopt and apply opioid prescription guidelines in the emergency department? A qualitative study. Ann Emerg Med. 2014;64(5):482–489. doi: 10.1016/j.annemergmed.2014.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Charumilind S, Mednez-Escobar E, Latkovic T. Ten Insights on the US Opioid Crisis from Claims Data Analysis.; 2018. https://www.mckinsey.com/industries/healthcare-systems-and-services/our-insights/ten-insights-on-the-us-opioid-crisis-from-claims-data-analysis.
- 54.Franklin G, Sabel J, Jones CM, et al. A Comprehensive Approach to Address the Prescription Opioid Epidemic in Washington State: Milestones and Lessons Learned. Am J Public Health. 2015;105(3):463–469. doi: 10.2105/AJPH.2014.302367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sun BC, Lupulescu-Mann N, Charlesworth CJ, et al. Impact of Hospital “Best Practice” Mandates on Prescription Opioid Dispensing After an Emergency Department Visit. Acad Emerg Med. 2017;24(8):905–913. doi: 10.1111/acem.13230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sun BC, Charlesworth CJ, Lupulescu-Mann N, et al. Effect of Automated Prescription Drug Monitoring Program Queries on Emergency Department Opioid Prescribing. Annals of Emergency Medicine. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Polsky D, Richards M, Basseyn S, et al. Appointment Availability after Increases in Medicaid Payments for Primary Care. N Engl J Med. 2015. doi: 10.1056/NEJMsa1413299. [DOI] [PubMed] [Google Scholar]
- 58.Ghate SR, Haroutiunian S, Winslow R, McAdam-Marx C. Cost and comorbidities associated with opioid abuse in managed care and medicaid patients in the United Stated: A comparison of two recently published studies. J Pain Palliat Care Pharmacother. 2010. doi: 10.3109/15360288.2010.501851. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.