Abstract
Introduction:
Burnout symptomatology is associated with various negative health consequences; however, the mechanisms underlying these associations remain unclear. One potential pathway involves alterations in the acute stress response. The aims of the present study were to examine burnout-associated alterations in stress-reactivity patterns, during a standardized social stressor compared to a control condition, as well as to examine whether effects associated with greater burnout symptomatology were distinct from other, conceptually overlapping indicators of chronic stress (i.e. depressive symptomatology and elevated hair cortisol concentration [HCC]).
Materials and methods:
In a randomized two-factor design a total of 70 employed males with varying burnout symptoms but without evidence of physical or psychiatric disease were exposed to the Trier Social Stress Test for Groups (TSST-G) or a non-stressful control condition. Acute stress reactivity was assessed using self-report stress measures and non-invasive biomarkers. Associations among acute stress reactivity, burnout and depressive symptoms (assessed with self-report measures), as well as HCC were analysed using repeated measure ANCOVAs and moderation analysis.
Results:
Burnout symptomatology was associated with elevated stress perception independent of the experimental condition. In addition, depressive symptomatology was associated with enhanced anticipatory appraisal, whereas HCC was not related to any subjective stress measure. On a physiological level, burnout and depressive symptomatology, as well as HCC were associated with a pattern of blunted cardiovascular reactivity, however the timing of this effect varied.
Conclusion:
Our results indicate burnout-associated modulations in stress reactivity, which diverge, at least partly, from other indicators of chronic stress.
Keywords: Burnout, Stress reactivity, Depression, Hair cortisol, Chronic stress, TSST
1. Introduction
Recent research suggests growing incidence rates of burnout in western and developing countries with one estimate indicating a life-time prevalence of burnout at 4.2% (Maske et al., 2016). Despite this work, there have been no large epidemiological studies which may allow for more accurate prevalence estimates of burnout owing primarily to the fact that no universally accepted definition of burnout has yet been established. Descriptions of burnout as a state of emotional exhaustion (EE), cynicism (CY), and reduced personal accomplishment (PEr) associated with chronic stress at work, appear to be the common denominator of current scientific conceptualizations. In the absence of comprehensive epidemiological data, the existing preliminary evidence for an increasing prevalence of burnout is nonetheless alarming, given growing evidence of associations between burnout and cardiovascular disease (CVD | Toker et al., 2012), the leading cause of morbidity and mortality in western countries (Lim et al., 2013).
One pathway through which burnout symptoms have been proposed to contribute to heightened CVD risk is by altering the acute stress response. However, results of the four published studies examining potential modulation effects of burnout on reactivity to an acute psychosocial stressor have been inconclusive (de Vente et al., 2015, 2003; Jönsson et al., 2015; Lennartsson et al., 2015a, b), which may be due to differences in burnout operationalization and symptom severity across studies. Most of these studies focused on physiological stress reactivity, namely the hypothalamic-pituitary-adrenal (HPA) axis (mainly salivary cortisol) and the autonomic nervous system (ANS; mainly salivary alpha-amylase [sAA], heart rate [HR], heart rate variability [HRV]). With respect to the HPA axis, there is evidence for burnout-related reductions in HPA responsiveness (de Vente et al., 2015; Jönsson et al., 2015; Lennartsson et al., 2015a, b). Yet, other studies have failed to replicate these effects (de Vente et al., 2003; Jönsson et al., 2015). Regarding the ANS, there is some evidence that burnout modulates basal cardiovascular and humoral functioning (de Vente et al., 2015, 2003), however, none of the previous studies found associations of burnout with modulations in autonomic stress reactivity (de Vente et al., 2015, 2003; Jönsson et al., 2015). Mirroring a general tendency in stress research (Campbell and Ehlert, 2012), compared to physiological processes, less attention has been paid to the effects of burnout symptomatology on subjective emotional stress experiences. The few existing studies have found burnout symptoms to be associated with elevated baseline levels of subjective stress markers (de Vente et al., 2003; Jönsoon et al., 2015), but not with psychological stress reactivity (de Vente et al., 2015, 2003; Jönsoon et al., 2015).
In addition to the identification of burnout-associated modulations of acute stress reactivity, the examination of their discriminability from other consequences of chronic stress remains a critical endeavour.
On the one hand, empirical evidence of differences in modulations of stress reactivity patterns between burnout and depressive symptoms seem to be particularly important (review: Bianchi et al., 2015). While the large overlap between burnout and depressive symptomatology is uncontroversial (Bianchi et al., 2015), previous studies suggest the existence of biological parameters potentially able to differentiate between the two concepts (Bakusic et al., 2017). Beyond others, differences with regard to HPA parameters (Mommersteeg et al., 2006), several markers of immune function (Grossi et al., 2003; Toker et al., 2005), and DNA methylation (Bakusic et al., 2017; Toker et al., 2005) have been suggested.
To date, no studies have attempted to characterize differences in stress reactivity profile as a function of burnout and depression. Despite a tremendous literature, the question regarding specific reactivity patterns associated with depressive symptomatology itself remains unanswered. With respect to both, HPA axis and ANS reactivity, depressive symptoms have been linked to exaggerated (Kibler and Ma, 2004), blunted (Hamilton and Alloy, 2016; Zorn et al., 2016), and undifferentiated (Ciufolini et al., 2014) stress responses. Findings of depression-linked differences in psychological stress reactivity also have been inconclusive, with some evidence for an increased perception of stress associated with depressive symptoms (de Rooij et al., 2010; Ehrenthal et al., 2010), and a number of studies which failed to find any associations between depression and stress perception (Wang et al., 2016; Weinstein et al., 2010). Besides variations in sample compositions (e.g., sex and age), differences regarding depression operationalization between these studies appear to be the most relevant factor to explain the inconsistency, as previous research suggest that depressive symptoms differ in their effects on stress-relevant biological systems (review: Fried and Nesse, 2015). Interestingly, it is the depressive symptomatic with the largest overlap with the core symptom of burnout (i.e., exhaustion) that has been most consistently associated with modulations of stress-relevant biological systems (review: Bassett et al., 2016; Kemp et al., 2010). Therefore, research on the potential of stress reactivity patterns for the discriminability between burnout and depressive symptoms should consider the burnout sub-dimensions.
On the other hand, the consideration of an objective measurable, stable trait-like biological marker of chronic stress, namely hair cortisol concentration (HCC | Russell et al., 2012; Stalder and Kirschbaum, 2012), appears to be particularly relevant in order to differentiate burnout-specific modulations from general chronic-stress associated changes in stress reactivity. Despite the theoretically justifiable assumption of an association between HCC, with both depression and burnout symptoms due to their link to chronic stress, empirical findings supporting these associations are scarce. With respect to burnout, the only existing study revealed elevated HCC in individuals with severe burnout symptomatology (Penz et al., 2017). Regarding depressive symptoms, results are inconsistent, with two studies reporting increased HCC (Dettenborn et al., 2012; Wei et al., 2015), two studies revealing reduced HCC (Gerber et al., 2013; Steudte-Schmiedgen et al., 2017), and three studies (Herane-Vives et al., 2018; Hinkelmann et al., 2013; Janssens et al., 2017), as well as a recently published meta-analysis (Stalder et al., 2017), which did not find any significant associations between HCC and depression.
In summary, previous research suggests burnout-associated modulations of acute stress reactivity. However, no previous studies included a control condition, employed a range of psychological and physiological acute stress markers or attempted to differentiate between patterns of reactivity attributable to burnout compared to other consequences of chronic stress. In addition, the four existing studies on burnout-associated modulations in reactivity to a standardized stress protocol relied on a dichotomous burnout operationalization in the absence of empirically-validated cut-off values (de Vente et al., 2015, 2003; Jönsoon et al., 2015; Lennartsson et al., 2015a, b), with three of them including individuals on sickness absence (de Vente et al., 2015, 2003; Lennartsson et al., 2015a, b). Especially the latter further limits the validity of the revealed results, as certain characteristics of sick leave and hospitalization (e.g., a lack of everyday life obligations, a constant confrontation with the own disease etc.) are very likely to influence relevant psychological and physiological processes.
To begin to address these gaps in research, the present study examined whether burnout symptoms were associated with alterations in the acute stress response during a standardized social stressor compared to a control condition using a variety of physiological (salivary cortisol, sAA, HR, HRV), and psychological stress indices, in a sample of healthy, employed male participants. In addition, we investigated communalities and differences regarding these modulations between different burnout symptoms (sum-score, EE, CY, and PEr), and other conceptually related indicators of chronic stress (i.e., depressive symptoms and HCC). With respect to depressive symptoms, the focus of the present study on a non-clinical population increases the possibility to reveal relevant differences as previous research indicates that burnout-depression overlap enlarges with increasing symptom severity (Ahola et al., 2005).
2. Material and methods
2.1. Subjects
Seventy-one healthy, employed male participants between 22 and 67 years of age with varying severity of burnout symptoms were recruited, both from the Dresden Burnout Study (a large-scale longitudinal study designed to investigate the pathogenesis and course of burnout, detailed description see Penz et al., 2018), as well as out of the general population via newspaper advertisements, flyers and social media. Online self-report screenings were used to assess study eligibility. Exclusion criteria included age below 18, no professional occupation (less than 20 h/week), chronic or acute psychiatric or medical illness, regular medication intake, abuse of drugs or alcohol, or smoking more than five cigarettes per day. Moreover, participants were required to be naive to the Trier Social Stress Test (TSST) and similar stress paradigms, as psychosocial stress was induced by the Trier Social Stress Test for Groups (TSST-G | von Dawans et al., 2011). One participant was excluded from analyses because his number of working hours did not meet the inclusion criteria. The final analytic sample (mean age = 38.49 years; SD = 11.39) included 70 participants.1 The study protocol was approved by the ethics committee of the TU Dresden and conducted in accordance with the Declaration of Helsinki. All participants gave written informed consent and received between 15 and 25 € with final compensation depending on their performance during a social-interaction paradigm (SIP | adapted from von Dawans et al., 2012; data not presented here).
2.2. Protocol
Participants were randomly assigned to either a stress condition (TSST-G; n = 35) or a specific control condition (n = 35| von Dawans et al., 2011), using a random number generator. One week before the experiment, participants completed an online questionnaire assessing burnout and depressive symptomatology, respectively. Participants were told to refrain from drinking alcohol, smoking, and medication intake 24 h before the study session. They were told to eat standard meals on the day of participation and then abstain from food two hours prior to the session. The 2.5-hr session took place between 5:00pm and 7:30pm in order to account for diurnal variations in cortisol (Kirschbaum and Hellhammer, 1994).
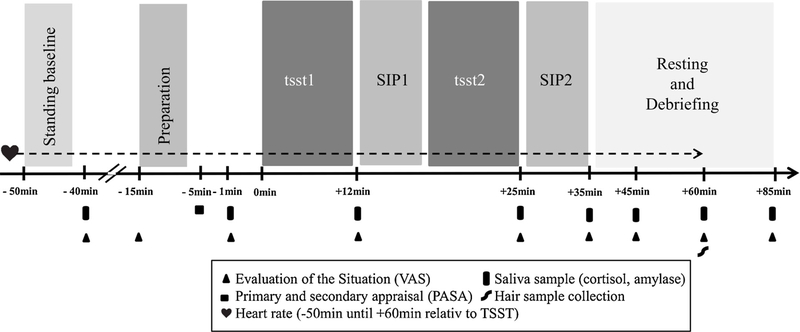
Since we were interested in a comprehensive understanding of the modulations of psychobiological acute stress reactivity, we chose a broad range of stress-related markers of the HPA axis (salivary cortisol), the ANS (sAA, HR, HRV), and subjective ratings of acute stress perception. A timeline of the experimental procedure is depicted in Fig. 1. On arrival at the laboratory, participants were seated in the waiting room and were not allowed to communicate with each other. After reading and signing informed-consent forms, participants were introduced to the saliva-sampling method and were each provided with a heart rate device (Polar RS800TM, Polar Electro, OY, Kempele, Finland). Baseline measures of HR, vagally-mediated HRV (vmHRV), salivary cortisol, sAA, and psychometric variables were then taken ( – 40min; i.e. before instruction for the stress task) with additional measurements taken throughout the experiment (detailed below and in Fig. 1).
Fig. 1.
Sequence of events and timeline for both, TSST-G and control condition. Preparation = preparation of job interview (TSST-G) vs. reading of a popular scientific test (control); SIP1= social interaction paradigm, set 1 (data not reported here); SIP2 = social interaction paradigm, set 2 (data not reported here); tsstl = mock job interview (TSST-G) vs. reading in a low voice (control); tsst2 = serial subtraction (TSST-G) vs. enumerating series of numbers in low voice (control). Figure adapted from von Dawans et al. (2011).
Participants were then provided with the instructions for the TSST-G or a specific control condition and had 6 min to prepare for the mock job interview/group reading. Afterwards, they were guided to the test room, where they received a summary of the procedure.
The TSST-G condition included a mock job interview (tsst1) and a mental arithmetic task (tsst2), whereas the control condition comprised reading in a low voice (tsst1) and enumerating a series of numbers in a low voice (tsst2) (Fig. 1). In both conditions, individuals had to perform in front of a panel of two individuals in groups of six participants, whereas the panel was evaluative only in the TSST-G condition in order to induce stress. A detailed description of the TSST-G and the control condition is provided elsewhere (von Dawans et al., 2011). Independent of assignment to the TSST-G vs. control group, individuals participated in a SIP, which consisted of two sets and was conducted as a part of the larger study, without relevance for the present research question (SIP1 after tsst1; SIP2 after tsst2).
The exact sequence of tasks were as follows: either public-speaking task (TSST-G) or simultaneous group reading of a popular scientific text in a low voice (control condition) (12 min; tsst1), first set of the aforementioned SIP (5 min; SIP1), either mental-arithmetic task (TSST-G) or an easy counting task (control condition) (8 min; tsst2), second set of the SIP (5 min; SIP2). After SIP2, the group was guided back to the waiting room, where additional salivary samples and subjective stress measurements were taken. At the end of the study ( + 85 min after onset of stressor), participants were debriefed and received their reimbursement individually.
2.3. Endocrine and autonomic stress response measures
Saliva samples were collected at eight time points during the stress protocol: baseline (−40min; i.e. before instruction for the stress/control task), immediately before the onset of the speaking/reading out task (−1 min), after the speaking/reading out task ( + 12 min), after the mental arithmetic task/easy counting ( + 25), and then after another 10, 10, 15, and 25 min to cover the cortisol and sAA response recovery phase using a commercially available sampling device (Salivette, Sarstedt, Nurmbrecht, Germany). After each experimental session, samples were stored at −20 °C. For biochemical analyses of salivary cortisol and sAA concentration, salivary samples were spun at 3000 × g for 10 min to obtain 05.−1.0ml of clear saliva with low viscosity. Salivary cortisol and sAA concentrations were determined by commercially available chemiluminescence immunoassay (CLIA; IBL, Hamburg, Germany). Inter- and intrassay coefficients of variation were both < 8%.
Autonomic stress levels were assessed by recording of continuous heart rate (beat-to-beat) intervals at a sampling frequency of 1000 hz, using a wireless chest transmitter and a wrist monitor recorder (Polar RS800CX, Polar Electro OY, Kempele, Finland). We restricted our HR and vmHRV analyses to the data collected during (1) a 6 min standing baseline (−50 min), (2) a 6min preparation phase (−14min), (3) a 30 min acute stress/control condition phase including the aforementioned SIP ( + 0 min) (see Fig. 1). Data was then transferred to the Polar Precision Performance Software (Polar Elector OY, Kempele, Finland) and exported as raw beat-to-beat data for further analyses. The raw beat-to-beat data for determination of vmHRV was processed according to the guidelines of the Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology (1996) using the Kubios HRV package (Tarvainen et al., 2009). In a first step, data were detrended using a smoothness priors algorithm implemented in the Kubios HRV package and visually inspected for abnormal or biologically implausible beats and adjusted or directly excluded. In a second step, the root mean square of successive differences of R-R intervals (RMSSD) and HR were calculated for each period from 2 min of continuous data during standing baseline (total duration: 6 min = 3 intervals), preparation (total duration: 6 min = 3 intervals), and the acute stressor/control condition episode (total duration: 30 min = 15 intervals). 2 min of HR data has been shown to be sufficient to provide reliable estimates and to correlate highly with 5 min recordings (de Rivecourt et al., 2008; Schroeder et al., 2004). RMSSD was used to operationalize vmHRV as it is an approved short-term measure reflecting vagal cardiac influence and has been shown to be less affected by breathing patterns and therefore more robust than other vmHRV indices (Hill et al., 2009; Task Force, 1996). RMSSD values were not normally distributed, thus a log transformation was applied to reduce skewness.
2.4. Psychological stress responses and psychometric measures
In order to quantify subjectively perceived levels of stress, subjects rated tension, anxiety, physical discomfort, avoidance (desire to leave the situation), emotional arousal, and feelings of control using a visual analogue scale (VAS) ranging from 0 (not at all) to 10 (maximum) (von Dawans et al., 2011) at baseline (−40min), after introduction of the TSST-G/control tasks (−15 min), before the TSST-G/control tasks (−1 min), after the speaking task/group reading ( + 12 min), after the mental arithmetic task/easy counting ( + 25 min), and repeatedly after the TSST-G/control tasks to span the recovery period ( + 35, +45, + 60, + 85 min relative to speaking task/group reading). All six VAS scales were aggregated to one mean value for each participant (with the feeling of control scale reverse-coded) in order to operationalize subjective stress (VAS mean stress rating). Anticipatory cognitive stress appraisal was measured after preparation (− 5 min) using the German version of the “Primary Appraisal - Secondary Appraisal” scale (PASA; Gaab et al., 2005). The questionnaire consists of 16 items and was developed in accordance with the model of Lazarus and Folkman (1984). It assesses four anticipatory cognitive appraisal processes: “threat”, “challenge”, “self-concept of own abilities”, and “control expectancy”. Each scale of the PASA comprised four items with a 6-point scale ranging from “strongly disagree” to “strongly agree”. Three secondary subscales can be computed: primary appraisal = threat + challenge; secondary appraisal = self-concept of own ability + control expectancy; stress index = primary appraisal - secondary appraisal.
2.5. Indicators of chronic stress
2.5.1. Burnout symptoms
Burnout symptoms were assessed using the German version (MBI-GS-D; Büssing and Glaser, 1999) of the Maslach Burnout Inventory-General Survey (MBI-GS; Schaufeli et al., 1996), the most frequently used burnout measure in the field. The 16 items of the MBI-GS are rated on a 7-point Likert scale (0 = never, 6 = daily) to form three subscales (EE, CY, PEr). The weighted MBI-GS sum-score ([0.4*EE + 0.3*CY + 0.3*PEr]), introduced by Kalimo et al. (2003), and its three burnout sub-dimensions were used as continuous variables and analyzed separately, as they have been shown to depict high multicollinearity.
2.5.2. Depressive symptoms
Depressive symptoms were measured with the German version (PHQ-9-D; Löwe et al., 2002) of the Patient Health Questionnaire (PHQ-9; Kroenke et al., 2001). The PHQ-9 consists of nine items, which quantify the frequency, over the last two weeks, of each of the nine diagnostic criteria for a depressive disorder of the Diagnostic and Statistical Manual of Mental Disorders (4th ed., text rev.; DSM-IV-TR; American Psychiatric Association, 2000). The items are scored on a 4-point ranking scale (0 = not at all, 3 = nearly every day) and summed to form a continuous variable (PHQ-9 sum-score), with higher scores representing more depressive symptoms.
2.5.3. Hair cortisol analysis
In order to measure hair cortisol we took hair strands (~3 mm diameter) scalp-near from a posterior vertex position. HCC was measured in the proximal 3-cm hair segment reflecting integrated cortisol secretion over the 3 month-period prior to hair sampling (Stalder and Kirschbaum, 2012). HCC was determined by liquid chromatography coupled with tandem mass spectrometry. Detailed information on the analytical protocol is provided elsewhere (Gao et al., 2013).
2.6. Statistical analysis
One-way analysis of variance (ANOVA) was employed to assess whether the TSST-G and control groups differed in terms of baseline levels of acute psychological and physiological stress markers, HCC, burnout or depressive symptoms. Repeated measures ANCOVAs were employed to evaluate the effectiveness of the stress induction in eliciting changes in salivary cortisol, sAA, HR, vmHRV, and subjective stress (VAS mean stress rating). The factors in these analyses were condition (TSST-G, control) and time (repeated factor; 7 for salivary cortisol and sAA2; 6 for HR and vmHRV3; 9 for VAS mean stress rating). Whenever Mauchly’s sphericity test determined heterogeneity of covariance, Greenhouse-Geisser corrected values and associated epsilon (ε) values for ANOVA tests were reported. As they were only sampled once, the influence of condition on the PASA scales was analyzed using MANCOVA with condition as a between groups factor.
In order to examine our primary research question of potential associations between burnout symptoms and psychological and physiological markers of acute stress, we applied a two-step procedure. In a first step, to test for potential main effects, salivary cortisol, sAA, HR, vmHRV, as well as VAS mean stress ratings were analyzed using the aforementioned one-way analyses of covariance (ANCOVAs) with repeated measures including the MBI-GS sum-score as the covariate. In addition, the PASA scales were analyzed using one-factor MANCOVA. In a second step, to examine whether burnout symptomatology moderated the association between condition and stress reactivity, separate moderator analyses were conducted, with condition as predictor and the MBI-GS sum-score as moderator. For saliva cortisol, sAA, and VAS mean stress ratings, areas under the curves with respect to ground (AUCg) were calculated with the trapezoid formula, which allows an aggregated sensitive measure of physiological and psychological changes over time (Pruessner et al., 2003), and used as dependent variables. Analysis of area under the curve with respect to increase (AUCi) were virtually identically and therefore not included in the present manuscript. Because of the reduced response latency, with respect to HR and vmHRV, averages of these parameters for specific study tasks/periods (standing baseline, preparation, tsstl, tsst2) were used as dependent variables. In order to examine our second research question regarding communalities and differences in stress reactivity patterns between burnout symptoms and other consequences of chronic stress, we applied the same previously described two-step procedure for the PHQ-9 sum-score (overlap and differences with depressive symptoms) and HCC (overlap and differences with chronic stress in general), as well as for the burnout sub-dimensions (i.e., EE, CY, PEr). As age has been shown to be associated with alterations in both acute and chronic stress all analyses controlled for age.4 Effect sizes are reported as ηp2, for ANOVAs. All statistical analyses were performed using IBM SPSS Statistics v. 22 (SPSS Inc., Chicago, IL, USA). Moderation analyses were conducted using the SPSS add-on PROCESS version 2.15 (Hayes, 2013).
3. Results
Characteristics of the study sample are depicted in Table 1. According to cut-off values introduced by Kalimo et al. (2003), only 8.6% of the total sample reported serious (MBI-GS sum-score > 3.5), 67.1% reported mild, (MBI-GS sum-score 1.50–3.49), and 22.9% reported no burnout symptoms (MBI-GS sum-score < 1.50), with MBI sum-score ranging between 0.26–4.23 in the present sample. With respect to depressive symptoms, the PHQ-9 sum-scores ranged between 0 and 18. According to the cut-off values introduced by Kroenke et al. (2001), only 7.1% reported moderately severe (PHQ-9 sum-score 15–19), 92.9% reported minimal to mild (PHQ-9 sum-score 0–14), and no individual reported severe depression severity (PHQ-9 sum-score > 15). Depressive and burnout symptoms were significantly positively correlated (r(70) = .66, p < .001), whereas HCC was not associated with either depressive (r(37) = −0.22, p = .18), or burnout (r(37) = −0.13, p = .45) symptoms.
Table 1.
Characteristics of the study sample.
| Characteristics | Control (N = 35) |
TSST-G (N = 35) |
Group comparison |
|---|---|---|---|
| Age (y) | 40.34 (10.76) | 36.63 (11.85) | F(1,68) = 1.89,ns |
| BMI | 24.64 (3.84) | 24.63 (4.08) | F(1,68) < 0.01,ns |
| MBI-GS | 2.32 (0.78) | 2.11 (0.96) | F(1,68) = 0.80,ns |
| PHQ-9 | 7.09 (4.18) | 7.11 (4.54) | F(1,68) = < 0.01,ns |
| HCC | 16.28 (13.36) | 20.49 (18.94) | F(1,35) = 0.63,ns |
| Salivary cortisol (baseline) | 4.89 (3.20) | 6.22 (7.89) | F(1,68) = 0.86,ns |
| sAA (baseline) | 119.80 (77.42) | 147.56 (99.35) | F(1,68) = 1.70,ns |
| HR (seated baseline) | 71.04 (8.83) | 73.19 (10.52) | F(1,68) = 0.85,ns |
| VmHRV (seated baseline) | 35.32 (29.24) | 32.35 (19.55) | F(1,68) = 0.25,ns |
| Mean VAS (baseline) | 1.48 (1.22) | 2.14 (1.61) | F(1,68) = 3.75,ns |
| PASA | |||
| Primary appraisal | 2.71 (0.67) | 3.60 (0.87) | F(1,68) = 22.78,s |
| Secondary | 3.95 (0.66) | 4.40 (0.72) | F(1,68) = 7.32,s |
| appraisal | −1.24 (1.05) | −0.80 (1.21) | F(1,68) = 2.67,ns |
| Stress index | |||
Note. BMI = body mass index; HCC = hair cortisol concentration; HR = mean heart rate; MBI-GS = Maslach Burnout Inventory - General Survey sum-score, burnout symptoms; Mean VAS = mean value of six visual analogue scales; PASA = Primary Appraisal - Secondary Appraisal questionnaire; PHQ-9 = Patient Health Questionnaire sum-score, depressive symptoms; sAA = salivary alpha-amylase; vmHRV vagally-mediated heart rate variability; Cells indicate mean with SD in parentheses.
TSST-G and control groups did not differ in age, body mass index (BMI), burnout and depressive symptoms, HCC, or on any acute physiological or psychological stress markers at baseline (all p-values > .06).
3.1. Stress manipulation
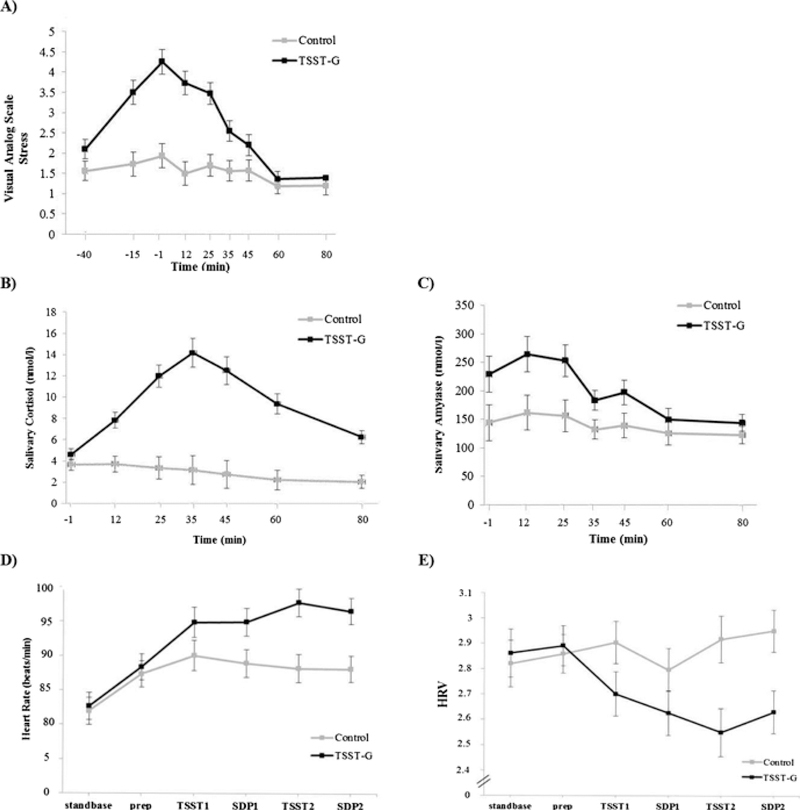
As depicted in Fig. 2, significant effects of the acute stress induction of the TSST-G compared to the control condition were found in the expected direction with respect to salivary cortisol, sAA, HR, HRV, and VAS mean stress ratings (significant condition X time interactions: salivary cortisol, F(6,402) = 31.22, p < .001, ε = .495, ηp2 = .32; sAA, F(6, 402) = 3.65, p = .02, ε = .466, ηp2 = .05; HR, F(5, 315) = 4.47, p = .02, ε = .352, ηp2 = .07; HRV, F(5, 315) = 5.15, p < .01, ε = .514, ηp2 = 0.08; VAS mean stress ratings, F(8, 520) = 15.12, p < .001, ε = .469,ηp2 = .19). Regarding anticipatory cognitive stress appraisal, compared to the control group, the TSST-G group had a higher primary, and secondary appraisal, but no differences regarding the PASA stress index (see Table 1).
Fig. 2.
Manipulation check. Mean levels of (A) six VAS ratings, (B) salivary cortisol, (C) salivary alpha amylase, (D) heart rate, and (E) vagally-mediated heart rate variability before, during and after a standardized psychosocial stressor in a group format (Trier Social Stress Test for Groups; TSST-G) and a control condition (Control). Error bars are SE. vmHRV = vagally-mediated heart rate variability.
3.2. Associations between the MBI-GS sum-score and psychological and physiological acute stress markers
With respect to the psychological markers of acute stress, analyses involving VAS mean stress ratings revealed a main effect of the MBI-GS sum-score (Table 2). The MBI-GS sum-score was significantly positively associated with stress perception across the course of the study, irrespective of experimental condition. However, the MBI-GS sum-score had no significant effect on anticipatory appraisal processes, operationalized using the PASA. Moderation analysis also did not reveal any significant interaction effects of the MBI-GS sum-score and condition on psychological stress markers (data not presented).
Table 2.
Main effects of chronic stress constructs predicting psychological stress reactivity by condition.
| MBI-GS |
PHQ-9 |
HCC |
||||
|---|---|---|---|---|---|---|
| F (df) | ηp2 | F (df) | ηp2 | F (df) | ηp2 | |
| Mean VASa | F(1,64) = 8.90** | 0.12 | F(1,64) = 1.61 | 0.03 | F(1,32) = 0.01 | < 0.01 |
| PASAb | ||||||
| Primary Appraisal | F(1,66) = 3.82 | 0.06 | F(1,66) = 4.78* | 0.07 | F(1,33) = 1.30 | 0.04 |
| Secondary Appraisal | F(1,66) = 0.77 | 0.01 | F(1,66) = 2.34 | 0.04 | F(1,33) = 1.15 | 0.03 |
| Stress index | F(1,66) = 3.52 | 0.05 | F(1,66) = 6.10* | 0.09 | F(1,33) = 2.40 | 0.07 |
Note. HCC = hair cortisol concentration, logarithmized; MBI-GS = Maslach Burnout Inventory - General Survey sum-score; burnout symptoms; Mean VAS = mean value of six VAS scales; PASA = Primary Appraisal - Secondary Appraisal questionnaire; PHQ-9 = Patient Health Questionnaire sum-score, depressive symptoms. All models are corrected for age.
p < 0.05
p < 0.01.
Results from repeated measure ANCOVA.
Results from MANCOVA.
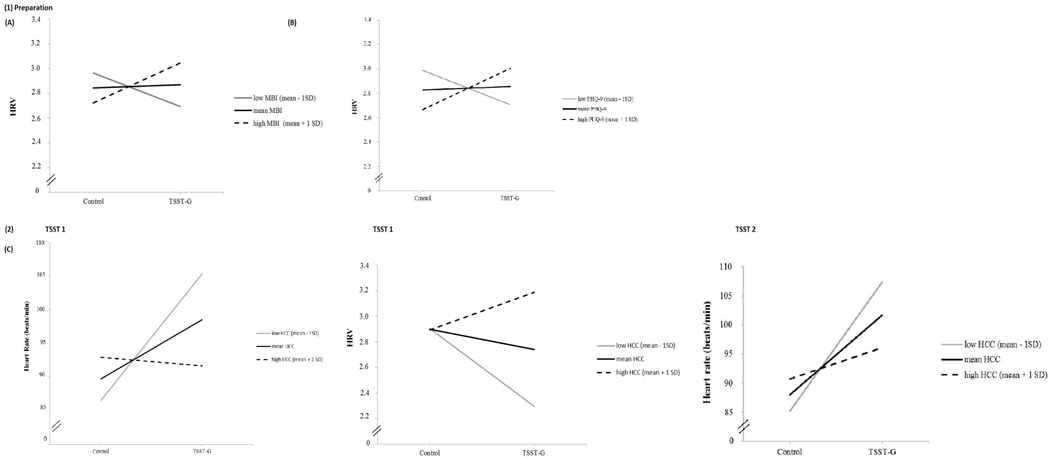
With respect to physiological markers of acute stress, repeated measure ANCOVAs revealed no main effect of the MBI-GS sum-score on any of the physiological markers of acute stress. Moderation analysis revealed a significant interaction effect between the MBI-GS sum-score and condition on vmHRV during preparation (Table 3; F(4,64) = 3.54; p = .01; R2 = 0.18; R2 increase due to interaction [ΔR2] = 0.09; F (1,64) = 7.10; p < .01). As shown in Fig. 3A, higher levels of the MBI-GS sum-score (M + 1SD) were associated with higher vmHRV during the preparation phase only among individuals in the TSST-G condition, but with lower vmHRV among individuals in the control condition. No significant interaction effects of burnout and condition emerged for any other acute physiological stress marker (i.e., HR, salivary cortisol, sAA) or phase of the experiment (all p > .05).
Table 3.
Results of moderation analysis, predicting physiological markers of the ANS and the HPA axis from condition and chronic stress-associated constructs.
| MBI-GS | PHQ-9 | HCC | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Condition | MBI-GS | Condition X MBI-GS |
Condition | PHQ-9 | Condition X PHQ-9 |
Condition | HCC | Condition X HCC |
||
| Salivary Cortisol AUCg | 71.35 (32.35)* | −2.89 (9.36) | 7.12 (13.54) | 71.50 (24.81)** | −1.65 (2.19) | 2.20 (3.07) | 185.07 (66.34)** | 24.14 (18.75) | −32.39 (24.48) | |
| sAA AUCg | 46.29 (34.14) | 7.19 (9.88) | −14.39 (14.30) | 11.71 (26.44) | 0.13 (2.34) | 0.45 (3.27) | 90.08 (61.60) | 9.75 (17.41) | −28.44 (22.73) | |
| HR | base | 2.09 (6.49) | 2.82 (1.88) | −0.23 (2.72) | −2.91 (5.05) | 0.10 (0.45) | 0.57 (0.62) | 19.21 (13.16) | 3.42 (3.72) | −5.19 (4.86) |
| prep | 4.80 (6.65) | −2.13 (1.89) | −1.78 (2.76) | 6.75 (5.02) | −0.26 (0.44) | −0.77 (0.62) | 9.20 (14.24) | 3.92 (3.95) | −4.57 (5.33) | |
| tsstl | 3.49 (7.38) | 2.90 (2.13) | 0.54 (3.09) | −3.66 (5.64) | 0.06 (0.49) | 1.10 (0.69) | 45.56 (14.43) ** | 4.50 (4.08) | −14.03 (5.33)* | |
| tsst2 | 12.72 (7.03) | 2.53 (2.04) | −1.50 (2.95) | 3.04 (5.37) | −0.05 (0.49) | 0.88 (0.67) | 44.20 (14.60) ** | 3.85 (4.22) | −11.71 (5.44)* | |
| VmHRV | base | −0.39 (0.31) | −0.14 (0.09) | 0.20 (0.13) | −0.05 (0.24) | −0.003 (0.02) | 0.02 (0.03) | −0.40 (0.72) | −0.03 (0.20) | 0.16 (0.27) |
| prep | −0.67 (0.28)* | −0.13 (0.08) | 0.31 (0.12)** | −0.49 (0.21)* | −0.04 (0.02) | 0.07 (0.03)** | 0.33 (0.60) | −0.02 (0.17) | −0.03 (0.23) | |
| tsstl | −0.34 (0.36) | −0.19 (0.10) | 0.10 (0.15) | −0.22 (0.28) | −0.02 (0.02) | 0.02 (0.03) | −1.81 (0.81)* | −0.01 (0.23) | 0.63 (0.30)* | |
| tsst2 | −0.42 (0.37) | −0.15 (0.11) | 0.06 (0.15) | −0.20 (0.29) | −0.01 (0.03) | −0.01 (0.04) | −1.88 (0.83)* | −0.06 (0.24) | 0.56 (0.31) | |
Note. AUCg = individual response curves with respect to ground; base = standing baseline; HCC = hair cortisol concentration, logarithmized; HR = mean heart rate; MBI = Maslach Burnout Inventory - General Survey sum-score, burnout symptoms; PHQ-9 = Patient Health Questionnaire sum-score; depressive symptoms; prep = preparation period; sAA = salivary alpha amylase; tsstl = speaking task; tsst2 = number task; vmHRV = vagally-mediated heart rate variability. Cells indicate unstandardized beta values with standard errors in parentheses. All models are corrected for age.
p < 0.05
p < 0.01.
Fig. 3.
Simple slopes of condition predicting heart rate, respectively heart rate variability during (1) preparation for high, mean and low levels of (A) burnout symptoms, and (B) depressive symptoms; during (2) speaking task (tsst1), and (3) number task (tsst2) for (C) hair cortisol concentration. High levels represent M + 1SD, mean levels represent M, low levels represent M-1SD. HCC = hair cortisol concentration; MBI-GS = Maslach Burnout Inventory - General Survey sum-score, burnout symptoms; PHQ-9 = Patient Health Questionnaire sum-score, depressive symptoms; vmHRV = vagally-mediated heart rate variability.
3.3. Associations between depressive symptoms, HCC and psychological and physiological acute stress markers
With respect to psychological markers of acute stress, effects of the PHQ-9 sum-score are depicted in Table 3. In contrast to the MBI-GS sum-score, the PHQ-9 sum-score was not associated with elevated VAS mean stress ratings, but was associated with elevated primary appraisal and stress index, operationalized using the PASA, in anticipation of the upcoming stress task.
With respect to physiological markers of acute stress, similar to the MBI-GS sum-score, the PHQ-9 sum-score moderated the effect of condition on vmHRV during preparation (F(4,64) = 3.60; p = .01; R2 = 0.19; R2 increase due to interaction [ΔR2] = 0.09; F(1,64) = 7.44; p < .01), with higher PHQ-9 sum-score values being associated with elevated vmHRV among individuals in the TSST-G condition, compared to controls (see Fig. 3B). There were no other main or interaction effects of the PHQ-9 sum-score on any other psychological or physiological marker of acute stress.
In the sub-sample, composed of individuals with sufficient hair length (n = 37), associations of HCC with psychological and physiological acute stress markers differed substantially from those observed for burnout and depressive symptoms . There were no significant main or moderation effects for HCC on any of the psychological markers of acute stress (see Table 3) . With respect to physiological markers of acute stress, HCC had no main effect on any acute stress marker, but moderated the association between condition and both, HR (F (4.32) = 5.64; p < . 01; R2 = 0 . 41; R2 increase due to interaction [ΔR2] = 0.13; F(1,32) = 6.94; p = . 01) and vmHRV (F(4,32) = 2.87; p = . 04; R2 = 0.26; R2 increase due to interaction [ΔR2] = 0.10; F (1.32) = 4.49; p = . 04) during the speaking task (tsst1). Fig. 3C displays the direction of the moderation effect for the three levels of HCC, indicating that higher levels of HCC (defined as M +1 SD) were associated with reduced HR and enhanced vmHRV during stress, compared to individuals with low levels of HCC In addition, the same moderation effect of HCC was found for HR, during the number task (tsst2: F(4,31) = 5. 84; p < .01; R2 = 0 .41; R2 increase due to interaction [ΔR2] = 0.09; F(1,31) = 4 . 63; p = .04).
3.4. Exploratory analyses on the burnout sub-dimensions and acute stress reactivity
With respect to the psychological markers of acute stress, analyses involving VAS mean stress ratings revealed that EE mirrored the significantly positive association of the MBI-GS sum-score with stress perception, irrespective of the experimental condition (main effect, F (1,64)=7.269, p < .001, ηp²=.11), whereas no significant main or interaction effects of PEr or CY were revealed.
With respect to anticipatory appraisal processes, operationalized using the PASA, CY was associated with an elevated stress index (main effect, F(3,66)=5.77, p = .04, ηp²=.07), whereas analyses including EE or the PEr did not reveal any significant main or interaction effects.
With respect to physiological markers of acute stress, repeated measure ANCOVAs revealed no effect of PEr or EE on any of the biological markers. However, CY was associated with reduced vmHRV (F (1,57)=6.69, p = .01, ηp² = .11), and enhanced vmHR (F (1,57)=4.11, p = .047, ηp² = .07) throughout the experimental session.
Mirroring the effect of the MBI-GS sum score, EE moderated the association between condition and vmHRV (F(4,64)=3.91; p<.01; R² = 0.44; [ΔR²]=0.10; F(1,64)=7.73; p < .01) during preparation. PEr moderated the association between condition and AUCg of sAA (F (4,65)=1.78; p= .14; R²= 0.10; [ΔR²]=0.06; F(1,65)=4.61; p = .04), whereas low PEr was associated with high AUCgs sAA and high PEr wasassociated with low AUCgof sAA. No moderation effects where found for CY on any physiological marker of acute stress.
4. Discussion
The aim of the present study was to examine potential associations between burnout symptoms and the psychological and physiological response to an acute psychological stress task Beyond this, we also were interested in comparing the patterns of stress reactivity associated with burnout symptomatology to those of other consequences of chronic stress (i e depressive symptoms and HCC)
In line with previous research, we found significant negative associations between burnout symptoms and vagal activity (reduced vmHRV) in the control condition during the preparation period (de Vente et al., 2015; Kanthak et al., 2017). In the TSST-G condition, burnout symptoms were positively associated with vagal activity This finding of burnout symptoms being associated with a blunted anticipatory stress response is in line with a growing body of research suggesting blunted cardiovascular reactivity in constructs partially overlapping with burnout such as depression (de Rooij et al., 2010; Ehrenthal et al., 2010; Phillips et al., 2011), and neuroticism (Jonassaint et al., 2009). These findings challenge the widespread reactivity hypothesis (Chida and Steptoe, 2010), which suggests that exaggerated physiological reactivity to stress constitutes a health risk, implicitly stating that blunted physiological reactivity is the generally more adaptive response (Carroll et al., 2009). Our results, however, support notions that both exaggerated and blunted physiological stress reactions may be maladaptive and can have adverse health consequences (Phillips et al., 2011). Beyond this, we are able to extend those previous findings by demonstrating that measures of vagal function show a similar pattern. This finding provides a potentially novel, though somewhat speculative, theoretical approach to explain blunted stress reactivity patterns, by drawing on Obrist’s distinction between active and passive coping (Obrist, 1976). In contrast to active coping, where the individual actively tries to mobilize energy to cope with behavioural demands by increasing sympathetic activity, during passive coping, the individual’s feeling of lacking adequate resources to deal with the situation is accompanied by a vagal dominance of autonomic regulation (Obrist, 1976). Following this line of reasoning, for individuals with high burnout symptomatology the TSST-G could have been seen as a passive coping situation, where they feel unable to cope with the upcoming task and thus disengage, resulting in increased vagal activity, as indexed by increased vmHRV. Individuals with low burnout symptomatology, by contrast, might have approached the upcoming task as an active stressor, and thus remain engaged, as they have adequate resources to cope with the challenge, which is reflected by vagal withdrawal (lower vmHRV). This pattern is exactly what is predicted by Obrist’s active versus passive coping model in which individuals that find themselves in a situation where they find the task too difficult show reduced sympathetic influences on the heart and increased vagal influences (Obrist, 1976, pgs, 95, 102, 103, and 105) whereas those that view the task as not too easy or not too difficult show enhanced sympathetic and reduced vagal influences (Obrist, 1976, pgs, 95 and 103). On a behavioural level, this vagal enhancement in individuals with higher burnout symptomatology seems counterproductive given that vagal withdrawal is an important prerequisite to mobilize sufficient energy necessary to cope with the approaching task. However, since we did not collect data on task performance, we were unable to test for potential differences in performance quality between individuals with high versus low burnout symptomatology.
Our finding of a dissociation between a heightened subjective stress appraisal and a blunted cardiovascular stress response in individuals with high burnout symptomatology is in line with other studies examining conceptually overlapping constructs (de Rooij et al., 2010), and supports our interpretation of the moderation effect mirroring passive vs. active coping instead of, as previously suggested, simply originating from a reduced appraisal of stress. The speculative nature of our interpretation, however, underscores the need for additional research to explicitly test our hypothesis of an increased vagal tone as a result of passive coping being responsible for blunted reactivity observable in individuals with high burnout symptomatology, as well as in other, overlapping constructs.
In contrast to previous findings, we did not find any effect of burnout symptomatology on saliva cortisol. A potential reason for these divergent results could be differences in burnout severity between the studies. Burned-out participants of the previous studies were all patients, either on sick leave (de Vente et al., 2015, 2003), ambulatory treatment (Lennartsson et al., 2015a, b), or recently recovered (Jönsoon et al., 2015) whereas the present study sample consisted of employed individuals, indicating a certain level of functioning. Beyond this, it might be that the vagal system is more sensitive than the HPA system to indicate the effects of chronic stress on acute stress reactivity.
Regarding our secondary research question on the specificity of burnout-associated reactivity patterns, we found distinctions from patterns associated with depressive symptoms only with respect to psychological markers of acute stress, as depressive symptomatology mirrored the moderation effects of burnout symptoms on physiological markers, discussed in the previous section. In contrast to burnout symptoms, depressive symptoms were associated with a bias towards anticipatory cognitive threat and stress appraisal before the actual task (independent of condition), but not with a generally elevated perception of stress throughout the experiment. This finding replicates previous findings of burnout and depressive symptoms being associated with dysfunctional, inflexible environmental perceptions (de Rooij et al., 2010; de Vente et al., 2003; Jönsoon et al., 2015; Wekenborg et al., accepted). Our results indicate, however, that this rigid, inflexible perception varies between the two conditions, a finding which might explain deviations in behavioral patterns typically associated with the respective symptomatology. Burnout symptomatology on the one hand, has been associated with a reduced ability to retrieve oneself from stressful environments to the point of full exhaustion (Edelwich and Brodsky, 1980) whereas depressed symptomatology has been associated with avoidance behavior as a way of reacting to challenges that are perceived as impossible (Gruenberg and Goldstein, 2003), as well as low approach motivation and general pessimism (Dickson et al., 2016). Therefore, the inability of protecting oneself from stressful environments associated with burnout symptomatology could be due to a general tendency of the individual to perceive their environment as stressful, independent of actual situational demands, reducing the motivation to seek for recovery options. With respect to depressive symptoms, on the other hand, our findings suggest that those individuals with high depressive symptoms tend to appraise specific tasks as more threatening or stressful, independent of the actual nature of the task, resulting in the well-known avoidance behavior associated with depressive disorders. This interpretation is, however, very tentative. Nevertheless, our results challenge the often postulated indivisibility of burnout and depressive symptomatology.
In order to enhance the understanding of the differences between burnout and depressive symptomatology, we also examined the more exploratory question of whether or not specific burnout sub-dimensions differ in their overlap with reactivity patterns associated with depressive symptoms. With respect to markers of physiological stress reactivity, EE mirrored the effects of depressive symptoms, whereas CY and PEr did not show any overlap. This is in line with previous research suggesting that exhaustion is both, the burnout sub-dimension with the largest overlap with depression (Bianchi et al., 2015), as well as a psychological symptom which has consistently been associated with modulations of markers of acute stress reactivity (Chida and Hamer, 2008; Kudielka et al., 2006). Given the large overlap between depressive symptoms and EE, CY and PEr might be of special relevance in order to further differentiate burnout and depression. Thus, the observed association between PEr and sAA may be an important starting point for future research, as previous research suggest a special impact of PEr on humoral markers of stress (Penz et al., 2017). The picture grows more complicated, however, when considering our findings for the markers of acute psychological stress, as CY and not EE mirrored the effects of depressive symptoms on primary stress appraisal. Overall, while these exploratory analyses on the role of the burnout sub-dimensions provide insights for further research to disentangle burnout and depressive symptoms, in view of the large number of statistical test in this relatively small sample, the present results should be interpreted with caution.
The associations with acute stress markers in the present study differed considerable between subjective and objectively measured indicators of chronic stress. HCC had no associations with any of the psychological stress markers. This finding indicates that the perception of acute stress is more strongly associated with subjective compared to objective measures of consequences of chronic stress. With respect to acute physiological stress reactivity, associations with HCC paralleled the direction of findings for depressive and burnout symptomatology, suggesting that blunted cardiovascular reactivity during stressful conditions may not be specific to burnout symptomatology but rather a more general effect of chronic stress load. However, the timing of the described interaction effect differed between the psychological stress constructs and HCC. In contrast to the former, HCC showed its influence not during preparation but during acute stress. One potential explanation for this difference in timing might be that burnout (Verkuil et al., 2011) and depressive symptomatology (Nolen-Hoeksema, 1991) are, in contrast to HCC, associated with increased perseveration, which has been described as a common pathway underlying poor mental and physical health (Zawadzki et al., 2018; Ottaviani et al., 2016). Following this line of reasoning, the absence of a concrete distraction such as an explicit task or a direct interaction with anyone during preparation could have resulted in a default state of perseverative cognition, characterized by worry and rumination; whereas, the concrete task of the mock job interview, as well as the mathematical task may have distracted individuals with high burnout and depressive symptomatology from perseverating. The results regarding our second research question emphasize the advantage of including objectively measurable indicators of chronic stress (HCC), given their differential effects on acute stress reactivity when compared to subjectively measurable indicators of chronic stress (burnout and depressive symptomatology).
There are also some limitations of our study to be considered. First, even though we were able to demonstrate that the TSST-G induced acute psychological and physiological stress, other potential influences of the deviation from the standardized TSST-G procedure due to the introduction of the SIP (e.g. impairment of recovery) cannot be completely ruled out. Second, in order to minimize other potential explanatory influences on stress reactivity, the study sample included only currently employed individuals. This contributed to a restriction of variance in clinically relevant burnout symptomatology. Taken together with the relatively small sample size and the inclusion of males only, the results may have limited generalizability. Third, as we used self-report measures instead of comprehensive psychiatric diagnostic procedures we cannot completely rule out that certain subjects did not meet inclusion criteria (i.e. psychiatric disease). Fourth, given the cross-sectional nature of the study, no assertions can be made regarding causality.
5. Conclusions
In sum, our results indicate that burnout symptomatology is associated with modulations of psychological and physiological markers of acute stress reactivity. The extent to which these modulations are either causal or merely reflect reinforcement of burnout symptomatology remains to be determined. Beyond this, our results suggest that modulations in stress reactivity vary between subjectively and objectively measurable indicators of chronic stress making stress reactivity patterns a valuable starting point for the ongoing search for objective differential diagnostic biomarkers.
Acknowledgement
We thank Susan Lindenlaub and Julia Reeder for assistance during data collection.
Funding details
This work was supported by the TU Dresden’s Institutional Strategy (“The Synergetic University”), which is funded by the Excellence Initiative of the German Federal and State Governments. M.K.W. was supported by the German Academic Scholarship Foundation. L.K.H was supported by funding from the National Institutes of Health/ National Heart, Lung, and Blood Institute (HL121708).
Footnotes
Conflict of interests
The authors report no conflicts of interest.
HR data for single experimental intervals were not analysable for 4 participants due to poor data quality and randomly occurring artefacts. Visual analogue scale data was missing for 2 participants. Hair samples were collected for 37 participants’ only, as the other participants did not have 3 cm hair length. All statistical analyses were conducted without elimination of outliers (values > 2 SD of group means), as reported findings were the same regardless of whether outliers were excluded or not.
The following saliva samples were used: collected immediately before the onset of the speaking task (−1min), after the speaking task (+12 min), after the mental arithmetic task/easy counting (+25), and then after another 10, 10, 15, and 25 minute.
The following HR/vmHRV measures were included: standing baseline, preparation, tsst1, SIP1, tsst2, SIP2.
Except for analyses of potential differences of baseline levels of acute psychological and physiological stress markers, HCC, burnout or depressive symptoms.
References
- Ahola K, Honkonen T, Isometsä E, Kalimo R, Nykyri E, Aromaa A, Lönnqvist J, 2005. The relationship between job-related burnout and depressive disorders - results from the Finnish Health 2000 Study. J. Affect Disord. 88 (1), 55–62. 10.1016/j.jad.2005.06.004. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association, 2000. Diagnostic and Statistical Manual of Mental Disorders, fourth ed Author, Washington, DC: 10.1176/appi.books.9780890423349. [DOI] [Google Scholar]
- Bakusic J, Schaufeli W, Claes S, Godderis L, 2017. Stress, burnout and depression: a systematic review on DNA methylation mechanisms. J. Psychosom. Res. 92, 34–44. 10.1016/j.jpsychores.2016.11.005. [DOI] [PubMed] [Google Scholar]
- Bassett D, Bear N, Nutt D, Hood S, Bassett S, Hans D, 2016. Reduced heart rate variability in remitted bipolar disorder and recurrent depression. Aust. N. Z. J. Psychiatr. 50 (8), 793–804. 10.1177/0004867416652734. [DOI] [PubMed] [Google Scholar]
- Bianchi R, Schonfeld IS, Laurent E, 2015. Burnout-depression overlap: a review. Clin. Psychol. Rev. 36, 28–41. 10.1016/j.cpr.2015.01.004. [DOI] [PubMed] [Google Scholar]
- Büssing A, Glaser J, 1999. Deutsche Fassung des Maslach Burnout Inventory-General Survey (MBI-GS-D). Technische Universität, Lehrstuhl für Psychologie, München. [Google Scholar]
- Campbell J, Ehlert U, 2012. Acute psychosocial stress: does the emotional stress response correspond with physiological responses? Psychoneuroendocrinology 37 (8), 1111–1134. 10.1016/j.psyneuen.2011.12.010. [DOI] [PubMed] [Google Scholar]
- Carroll D, Lovallo WR, Phillips AC, 2009. Are large physiological reactions to acute psychological stress always bad for health? Soc. Personal. Psychol. 3 (5), 725–743. 10.1111/j.1751-9004.2009.00205.x. [DOI] [Google Scholar]
- Chida Y, Hamer M, 2008. Chronic psychosocial factors and acute physiological responses to laboratory-induced stress in healthy populations: a quantitative review of years of investigations. Psychol. Bull. 134 (6), 829–885. 10.1037/a0016852. [DOI] [PubMed] [Google Scholar]
- Chida Y, Steptoe A, 2010. Greater cardiovascular responses to laboratory mental stress are associated with poor subsequent cardiovascular risk status: a meta-analysis of prospective evidence. Hypertension 55 (4), 1026–1032. 10.1161/HYPERTENSI0NAHA.109.146621. [DOI] [PubMed] [Google Scholar]
- Ciufolini S, Dazzan P, Kempton MJ, Pariante C, Mondelli V, 2014. HPA axis response to social stress is attenuated in schizophrenia but normal in depression: evidence from a meta-analysis of existing studies. Neurosci. Biobehav. Rev. 47, 359–368. 10.1016/j.neubiorev.2014.09.004. [DOI] [PubMed] [Google Scholar]
- De Rivecourt M, Kuperus MN, Post WJ, Mulder LJM, 2008. Cardiovascular and eye activity measures as indices for momentary changes in mental effort during simulated flight. Ergonomics 51 (9), 1295–1319. 10.1080/00140130802120267. [DOI] [PubMed] [Google Scholar]
- De Rooij SR, Schene AH, Phillips DI, Roseboom TJ, 2010. Depression and anxiety: associations with biological and perceived stress reactivity to a psychological stress protocol in a middle-aged population. Psychoneuroendocrinology 35 (6), 866–877. 10.1016/j.psyneuen.2009.11.011. [DOI] [PubMed] [Google Scholar]
- De Vente W, Olff M, Van Amsterdam JGC, Kamphuis JH, Emmelkamp PMG, 2003. Physiological differences between burnout patients and healthy controls: blood pressure, heart rate, and cortisol responses. Int. J. Occup. Environ. Med. 60 (Suppl. 1), i54–i61. 10.1136/oem.60.suppl_1.i54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Vente W, van Amsterdam JG, Off M, Kamphuis JH, Emmelkamp PM, 2015. Burnout is associated with reduced parasympathetic activity and reduced HPA axis responsiveness, predominantly in males. Biomed Res. Int. 431725. 10.1155/2015/431725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dettenborn L, Muhtz C, Skoluda N, Stalder T, Steudte S, Hinkelmann K, et al. , 2012. Introducing a novel method to assess cumulative steroid concentrations: increased hair cortisol concentrations over 6 months in medicated patients with depression. Stress 15 (3), 348–353. 10.3109/10253890.2011.619239. [DOI] [PubMed] [Google Scholar]
- Dickson JM, Moberly NJ, O’Dea C, Field M, 2016. Goal fluency, pessimism and disengagement in depression. PLoS One 11 (11), e0166259. 10.1371/journal.pone.0166259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edelwich J, Brodsky A, 1980. Burn-out: Stages of Disillusionment in the Helping Professions Vol. 1 Human Sciences Press, New York. [Google Scholar]
- Ehrenthal JC, Herrmann-Lingen C, Fey M, Schauenburg H, 2010. Altered cardiovascular adaptability in depressed patients without heart disease. World J. Biol. Psychiatry 11 (3), 586–593. 10.3109/15622970903397714. [DOI] [PubMed] [Google Scholar]
- Gaab J, Rohleder N, Nater UM, Ehlert U, 2005. Psychological determinants of the cortisol stress response: the role of anticipatory cognitive appraisal. Psychoneuroendocrinology 30 (6), 599–610. 10.1016/j.psyneuen.2005.02.001. [DOI] [PubMed] [Google Scholar]
- Gao W, Stalder T, Foley P, Rauh M, Deng H, Kirschbaum C, 2013. Quantitative analysis of steroid hormones in human hair using a column-switching LC-APCI-MS/ MS assay. J. Chromatogr. B 928, 1–8. 10.1016/j.jchromb.2013.03.008. [DOI] [PubMed] [Google Scholar]
- Gerber M, Jonsdottir IH, Kalak N, Elliot C, Puhse U, Holsboer-Trachsler E, Brand S, 2013. Objectively assessed physical activity is associated with increased hair cortisol content in young adults. Stress 16 (6), 593–599. 10.3109/10253890.2013.823599. [DOI] [PubMed] [Google Scholar]
- Grossi G, Perski A, Evengård B, Blomkvist V, Orth-Gomér K, 2003. Physiological correlates of burnout among women. J. Psychosom. Res. 55 (4), 309–316. 10.1016/S0022-3999(02)00633-5. [DOI] [PubMed] [Google Scholar]
- Gruenberg AM, Goldstein RD, 2003. Mood disorders: depression In: Tasman AJ, Kay JJ, Lieberman JA (Eds.), Psychiatry, second ed John Wiley, New York, pp. 1207–1213. [Google Scholar]
- Hamilton JL, Alloy LB, 2016. Atypical reactivity of heart rate variability to stress and depression across development: systematic review of the literature and directions for future research. Clin. Psychol. Rev. 50, 67–79. 10.1016/j.cpr.2016.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes AF, 2013. Introduction to mediation, moderation, and conditional process analysis: a regression-based approach. Guilford Press, New York: 10.1111/jedm.12050. [DOI] [Google Scholar]
- Herane-Vives A, de Angel V, Papadopoulos A, Wise T, Chua KC, Strawbridge R, et al. , 2018. Short-term and long-term measures of cortisol in saliva and hair in atypical and non-atypical depression. Acta Psychiatr. Scand. 137 (3), 216–230. 10.1111/acps.12852. [DOI] [PubMed] [Google Scholar]
- Hill LK, Siebenbrock A, Sollers JJ, Thayer JF, 2009. Are all measures created equal? Heart rate variability and respiration. Biomed. Sci. Instrum. 45, 71–76. [PubMed] [Google Scholar]
- Hinkelmann K, Muhtz C, Dettenborn L, Agorastos A, Moritz S, Wingenfeld K, et al. , 2013. Association between cortisol awakening response and memory function in major depression. Psychol. Med. 43 (11), 2255–2263. 10.1017/S0033291713000287. [DOI] [PubMed] [Google Scholar]
- Janssens H, Clays E, Fiers T, Verstraete A, De Bacquer D, Braeckman L, 2017. Hair cortisol in relation to job stress and depressive symptoms. Occup. Med. 67 (2), 114–120. 10.1093/occmed/kqw114. [DOI] [PubMed] [Google Scholar]
- Jonassaint C, Why Y, Bishop G, Tong E, Diong S, Enkelmann H, et al. , 2009. The effects of neuroticism and extraversion on cardiovascular reactivity during a mental and an emotional stress task. Int. J. Psychophysiol. 74 (3), 274–279. 10.1016/j.ijpsycho.2009.09.012. [DOI] [PubMed] [Google Scholar]
- Jönsoon P, Österberg K, Wallergård M, Hansen ÅM, Garde AH, Johansson G, Karlson B, 2015. Exhaustion-related changes in cardiovascular and cortisol reactivity to acute psychosocial stress. Physiol. Behav. 151, 327–337. 10.1016/j.physbeh.2015.07.020. [DOI] [PubMed] [Google Scholar]
- Kalimo R, Pahkin K, Mutanen P, Topipinen-Tanner S, 2003. Staying well or burning out at work: work characteristics and personal resources as long-term predictors. Work Stress 17, 109–122. 10.1080/0267837031000149919. [DOI] [Google Scholar]
- Kanthak MK, Stalder T, Hill LK, Thayer JF, Penz M, Kirschbaum C, 2017. Autonomic dysregulation in burnout and depression: evidence for the central role of exhaustion. Scand. J. Work Environ. Health 43 (5), 475–481. 10.5271/sjweh.3647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemp AH, Quintana DS, Gray MA, Felmingham KL, Brown K, Gatt JM, 2010. Impact of depression and antidepressant treatment on heart rate variability: a review and meta-analysis. Biol. Psychiatry 67 (11), 1067–1074. 10.1016/j.biopsych.2009.12.012. [DOI] [PubMed] [Google Scholar]
- Kibler JL, Ma M, 2004. Depressive symptoms and cardiovascular reactivity to laboratory behavioral stress. Int. J. Behav. Med. 11 (2), 81–87. 10.1207/s15327558ijbm1102_3. [DOI] [PubMed] [Google Scholar]
- Kirschbaum C, Hellhammer DH, 1994. Salivary cortisol in psychoneuroendocrine research: recent developments and applications. Psychoneuroendocrinology 19 (4), 313–333. 10.1016/0306-4530(94)90013-2. [DOI] [PubMed] [Google Scholar]
- Kroenke K, Spitzer RL, Williams JBW, 2001. The PHQ-9: validity of a brief depression severity measure. J. Gen. Intern. Med. 16, 606–613. 10.1046/j.1525-1497.2001.016009606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kudielka B, Bellingrath S, Hellhammer D, 2006. Cortisol in burnout and vital exhaustion: an overview. G. Ital. Med. Lav. Ergon. 28 (1 Suppl. 1), 34–42. [PubMed] [Google Scholar]
- Lazarus RS, Folkman S, 1984. Stress, Appraisal, and Coping. Springer, New York. [Google Scholar]
- Lennartsson A-K, Sjörs A, Jonsdottir IH, 2015a. Indication of attenuated DHEA-s response during acute psychosocial stress in patients with clinical burnout. J. Psychosom. Res. 79 (2), 107–111. 10.1016/j.biopsycho.2012.03.003. [DOI] [PubMed] [Google Scholar]
- Lennartsson A-K, Sjörs A, Ljung T, Währborg P, Jonsdottir I, 2015b. Burnout and hypocortisolism-a matter of severity? A study on ACTH and cortisol responses to acute psychosocial stress. Front. Psychiatry 6 (8). 10.3389/fpsyt.2015.00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim SS, Vos T, Flaxman AD, Danaei G, Shibuya K, Adair-Rohani H, et al. , 2013. A comparative risk assessment of burden of disease and injury attributable to 67 risk factors and risk factor clusters in 21 regions, 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet 380 (9859), 2224–2260. 10.1016/S0140-6736(12)61766-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Löwe BLSR, Spitzer RL, Zipfel S, Herzog W, 2002. Gesundheitsfragebogen für Patienten (PHQ-9) Manual und Testunterlagen, 2. Auflage PRIME MD Patient Health Questionnaire (PHQ) - German version, second ed Pfitzer, Karlsruhe. [Google Scholar]
- Maske UE, Riedel-Heller SG, Seiffert I, Jacobi F, Hapke U, 2016. Häufigkeit und psychiatrische Komorbiditäten von selbstberichtetem diagnostiziertem Burnout- Syndrom. Psychiatr. Prax. 43 (01), 18–24. 10.1055/s-0034-1387201. [DOI] [PubMed] [Google Scholar]
- Mommersteeg PMC, Heijnen CJ, Verbraak MJ, van Doornen LJ, 2006. Clinical burnout is not reflected in the cortisol awakening response, the day-curve or the response to a low-dose dexamethasone suppression test. Psychoneuroendocrinology 31 (2), 216–225. 10.1016/j.psyneuen.2005.07.003. [DOI] [PubMed] [Google Scholar]
- Nolen-Hoeksema S, 1991. Responses to depression and their effects on the duration of depressive episodes. J. Abnorm. Psychol. 100 (4), 569–582. 10.1037/0021-843X.100.4.569. [DOI] [PubMed] [Google Scholar]
- Obrist PA, 1976. The cardiovascular-behavioral interaction—as it appears today. Psychophysiology 13 (2), 95–107. 10.1111/jM469-8986.1976.tb00081.x. [DOI] [PubMed] [Google Scholar]
- Ottaviani C, Thayer JF, Verkuil B, Lonigro A, Medea B, Couyoumdjian A, Brosschot JF, 2016. Physiological concomitants of perseverative cognition: a systematic review and meta-analysis. Psychol. Bull. 142 (3), 231–259. 10.1037/bul0000036. [DOI] [PubMed] [Google Scholar]
- Penz M, Stalder T, Miller R, Ludwig VM, Kanthak MK, Kirschbaum C, 2017. Hair cortisol as a biological marker for burnout symptomatology. Psychoneuroendocrinology 87, 218–221. 10.1016/j.psyneuen.2017.07.485. [DOI] [PubMed] [Google Scholar]
- Penz M, Wekenborg MK, Pieper L, Beesdo-Baum K, Walther A, Miller R, et al. , 2018. The Dresden Burnout Study: protocol of a prospective cohort study for the bio-psychological investigation of burnout. Int. J. Methods Psychiatr. Res. 27 (2), e1613. 10.1002/mpr.1613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips AC, Hunt K, Der G, Carroll D, 2011. Blunted cardiac reactions to acute psychological stress predict symptoms of depression five years later: evidence from a large community study. Psychophysiology 48 (1), 142–148. 10.1111/j.1469-8986.2010.01045.x. [DOI] [PubMed] [Google Scholar]
- Pruessner JC, Kirschbaum C, Meinlschmid G, Hellhammer DH, 2003. Two formulas for computation of the area under the curve represent measures of total hormone concentration versus time-dependent change. Psychoneuroendocrinology 28 (7), 916–931. 10.1016/S0306-4530(02)00108-7. [DOI] [PubMed] [Google Scholar]
- Russell E, Koren G, Rieder M, Van Uum S, 2012. Hair cortisol as a biological marker of chronic stress: current status, future directions and unanswered questions. Psychoneuroendocrinology 37 (5), 589–601. 10.1016/j.psyneuen.2011.09.009. [DOI] [PubMed] [Google Scholar]
- Schaufeli WB, Leiter MP, Maslach C, Jackson SE, 1996. Maslach burnout inventory-general survey In: Maslach C, Jackson SE, Leiter MP (Eds.), The Maslach Burnout Inventory-Test Manual, third ed Consulting Psychologists Press, Palo Alto (CA). [Google Scholar]
- Schroeder EB, Whitsel EA, Evans GW, Prineas RJ, Chambless LE, Heiss G, Repeatability of heart rate variability measures. J. Electrocard. 37 (3), 163–172. 10.1016/j.jelectrocard.2004.04.004. [DOI] [PubMed] [Google Scholar]
- Stalder T, Kirschbaum C, 2012. Analysis of cortisol in hair-State of the art and future directions. Brain Behav. Immun. 26 (7), 1019–1029. 10.1016/j.bbi.2012.02.002. [DOI] [PubMed] [Google Scholar]
- Stalder T, Steudte-Schmiedgen S, Alexander N, Klucken T, Vater A, Wichmann S, et al. , 2017. Stress-related and basic determinants of hair cortisol in humans: a meta-analysis. Psychoneuroendocrinology 77, 261–274. 10.1016/j.psyneuen.2016.12.017. [DOI] [PubMed] [Google Scholar]
- Steudte-Schmiedgen S, Wichmann S, Stalder T, Hilbert K, Muehlhan M, Lueken U, Beesdo-Baum K, 2017. Hair cortisol concentrations and cortisol stress reactivity in generalized anxiety disorder, major depression and their comorbidity. Psychiatry Res. 84, 184–190. 10.1016/j.jpsychires.2016.09.024. [DOI] [PubMed] [Google Scholar]
- Tarvainen MP, Niskanen JP, Lipponen JA, Ranta-Aho PO, Karjalainen PA, 2009. Kubios HRV—a software for advanced heart rate variability analysis. 4th European Conference of the International Federation for Medical and Biological Engineering 1022–1025. 10.1007/978-3-540-89208-3_243. [DOI] [Google Scholar]
- Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology, 1996. Heart rate variability: standards of measurement, physiological interpretation and clinical use. Circulation 93 (5), 1043–1065. 10.1161/01.CIR.93.5.1043. [DOI] [PubMed] [Google Scholar]
- Toker S, Shirom A, Shapira I, Berliner S, Melamed S, 2005. The association between burnout, depression, anxiety, and inflammation biomarkers: C-reactive protein and fibrinogen in men and women. J. Occup. Health Psychol. 10 (4), 344–362. 10.1037/1076-8998.10.4.344. [DOI] [PubMed] [Google Scholar]
- Toker S, Melamed S, Berliner S, Zeltser D, Shapira I, 2012. Burnout and risk of coronary heart disease: a prospective study of 8838 employees. Psychosom. Med. 74 (8), 840–847. 10.1097/PSY.0b013e31826c3174. [DOI] [PubMed] [Google Scholar]
- Verkuil B, Brosschot JF, Korrelboom K, Reul-Verlaan R, Thayer JF, 2011. Pretreatment of worry enhances the effects of stress management therapy: a randomized clinical trial. Psychother. Psychosom. 80 (3), 189–190. 10.1159/000320328. [DOI] [PubMed] [Google Scholar]
- Von Dawans B, Kirschbaum C, Heinrichs M, 2011. The Trier Social Stress Test for Groups (TSST-G): a new research tool for controlled simultaneous social stress exposure in a group format. Psychoneuroendocrinology 36 (4), 514–522. 10.1016/j.psyneuen.2010.08.004. [DOI] [PubMed] [Google Scholar]
- VonDawans B, Fischbacher U, Kirschbaum C, Fehr E, Heinrichs M, 2012. The social dimension of stress reactivity acute stress increases prosocial behavior in humans. Psychol. Sci. 23 (6), 651–660. 10.1177/0956797611431576. [DOI] [PubMed] [Google Scholar]
- Wang M-Y, Chiu C-H, Lee H-C, Su C-T, Tsai P-S, 2016. Cardiovascular reactivity in patients with major depressive disorder with high-or low-level depressive symptoms: a cross-sectional comparison of cardiovascular reactivity to laboratory-induced mental stress. Biol. Res. Nurs. 18 (2), 221–229. 10.1177/1099800415596227. [DOI] [PubMed] [Google Scholar]
- Wei J, Sun G, Zhao L, Yang X, Liu X, Lin D, et al. , 2015. Analysis of hair cortisol level in first-episodic and recurrent female patients with depression compared to healthy controls. J. Affect. Disord. 75, 299–302. 10.1016/j.jad.2015.01.023. [DOI] [PubMed] [Google Scholar]
- Weinstein AA, Deuster PA, Francis JL, Bonsall RW, Tracy RP, Kop WJ, 2010. Neurohormonal and inflammatory hyper-responsiveness to acute mental stress in depression. Biol. Psychol. 84 (2), 228–234. 10.1016/j.biopsycho.2010.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wekenborg MK, Hill LB, Miller R, Thayer JF, Penz M, Kirschbaum C, 2018. Reduced self-regulation mirrors the distorting effects of burnout symptomatology on task difficulty perception during an inhibition task. Stress 21 (6). 10.1080/10253890.2018.1479393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zawadzki MJ, Sliwinski MJ, Smyth JM, 2018. Perseverative cognitions and stress exposure: comparing relationships with psychological health across a diverse adult sample. Ann. Behav. Med. 52 (12), 1060–1072. 10.1093/abm/kay009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zorn JV, Schür RR, Boks MP, Kahn RS, Joels M, Vinkers CH, 2016. Cortisol stress reactivity across psychiatric disorders: a systematic review and meta-analysis. Psychoneuroendocrinology 77, 25–36. 10.1016/j.psyneuen.2016.11.036. [DOI] [PubMed] [Google Scholar]