Abstract
Many dyes and pigments are used in textile and printing industries, and their wastewater has been classed as a top source of pollution. Biodegradation of dyes by fungal laccase has great potential. In this work, the influence of reaction time, pH, temperature, dye concentration, metal ions, and mediators on laccase-catalyzed Remazol Brilliant Blue R dye (RBBR) decolorization were investigated in vitro using crude laccase from the white-rot fungus Ganoderma lucidum. The optimal decolorization percentage (50.3%) was achieved at 35 °C, pH 4.0, and 200 ppm RBBR in 30 min. The mediator effects from syringaldehyde, 1-hydroxybenzotriazole, and vanillin were compared, and 0.1 mM vanillin was found to obviously increase the decolorization percentage of RBBR to 98.7%. Laccase-mediated decolorization percentages significantly increased in the presence of 5 mM Na+ and Cu2+, and decolorization percentages reached 62.4% and 62.2%, respectively. Real-time fluorescence-quantitative PCR (RT-PCR) and protein mass spectrometry results showed that among the 15 laccase isoenzyme genes, Glac1 was the main laccase-contributing gene, contributing the most to the laccase enzyme activity and decolorization process. These results also indicate that under optimal conditions, G. lucidum laccases, especially Glac1, have a strong potential to remove RBBR from reactive dye effluent.
Keywords: decolorization, laccase, Ganoderma lucidum, RT-PCR, isoenzyme genes
1. Introduction
Over 10,000 different chemical dyes and dyeing auxiliaries are used worldwide in the textile and printing industries. Global dye production is estimated to be approximately 800,000 tons per annum, and at least 10% of used dye stuffs are discharged as environmental waste [1]. Due to their complex stable chemical structure and heavy metal content, dye effluent wastewater is relatively resistant to biodegradation. Presently, the removal of dye from wastewater depends mainly on physical or chemical processes, such as absorption, adsorption, coagulation–flocculation, ion exchange, and electrochemical methods. These methods are usually expensive and can produce toxic by-products, which can limit their application [2]. Biodegradation of dyes by microorganisms is an attractive method due to its low cost, high efficiency, absence of hazardous by-products, and environmental friendliness [3].
The application of white-rot fungi for the decolorization of industrial dyes, such as azo, heterocyclic, reactive, and polymeric dyes, has received increasing attention due to their ability to secrete non-specific oxidative enzymes, including lignin peroxidase, manganese peroxidase, and laccase [4,5,6]. Laccases (benzenediol: oxygen oxidoreductase, EC 1.10.3.2) are glycoproteins classified as low-specificity multi-copper oxidases, which can act on o- and p-quinols and catalyze one-electron oxidation of a wide range of organic and inorganic substrates by the reduction of molecular oxygen to water [7,8]. A typical laccase contains four copper ions per molecule, which are arranged into three distinct sites that perform different roles within a laccase domain [9]. Laccases are secreted extracellularly and have a high tolerance to high-concentration pollutants and low/high-pH environments. Therefore, they have attracted interest for detoxifying wastewater produced by pulp-bleaching processes, for industrial wastewater treatment, and for the decolorization of dye-containing effluents [10,11,12].
Ganoderma lucidum is a typical white-rot fungus, and has prominent biotechnological significance due to its medicinal and pharmacological properties. It also has great potential as a laccase producer for industrial dye decolorization [13,14], and plenty of studies have aimed to improve the yield of G. lucidum laccase [15]. Many reports have shown evidence that G. lucidum and its enzymes can be applied in the biodegradation of xenobiotics, including dyes [16], pesticide, and organic compounds [17,18]. Laccase was found to be a major enzyme produced by G. lucidum in most culture conditions [19,20], playing key roles in lignocellulose biomass hydrolysis [21] and has been used in various fields [22,23,24]. Sixteen laccase isoenzymes have been reported within laccase families from G. lucidum [25]. These isoenzymes often present differences in catalytic properties, regulation mechanisms, and localization [26].
The transcriptional profiles and enzymatic activities of laccases are often regulated by many environmental factors, including pH, temperature, substrate concentrations, metals, and mediators. The addition of RBBR inhibits the expression of the Pleurotus ostreatus laccase gene pox1, while Acetyl Yellow G (AYG) addition induces the highest expression of the pox3 and poxa1b genes [27]. Metals can interact with the electron transport system of laccase and then affect its enzyme activity. The laccase from G. lucidum KMK2 was highly sensitive to Fe2+ and its enzyme activity was completely inhibited in the presence of Fe2+ [28]. Mediators can act as electron carriers between laccase and target compounds, making laccase able to oxidize not only phenolic compounds, but also non-phenolic compounds [29,30]. Adding mediators greatly affects the laccase stability, causes some loss of laccase activity, and this effect varies among different mediators [31]. The anthraquinone dye RBBR is one of the most important dyes used in the textile industry, and represents an important class of toxic and recalcitrant organo-pollutants. Various studies have been carried out to investigate RBBR decolorization [19,27,32]. High salt ion concentrations can significantly affect the role of Ganoderma laccase in decolorizing RBBR [33]. pH was also found to be an important parameter for enzymatic RBBR decolorization systems from Lentinus crinitus and Psilocybecastanella [34].
Although there is research reported for the decolorization of dyes by G. lucidum laccase, few studies have analyzed their effects on laccase expression. The main objective of this research was to investigate the optimal conditions for RBBR decolorization by G. lucidum laccase and analyze the main laccase-contributing genes among the 15 laccase isoenzymes.
2. Results and Discussion
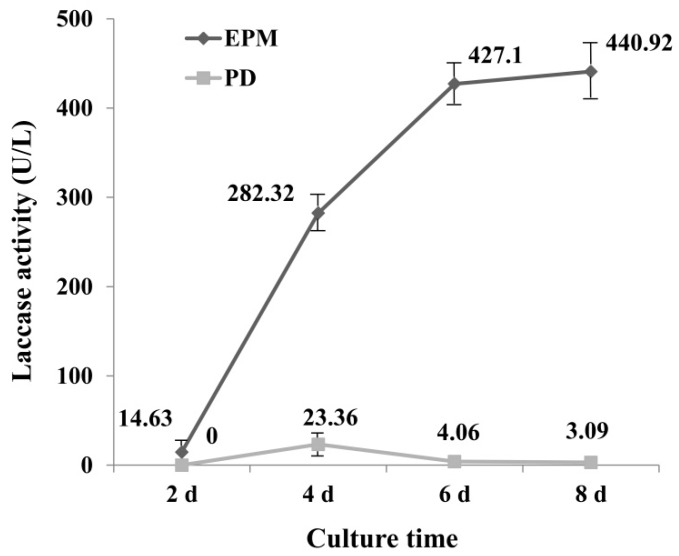
2.1. Laccase Activity in PD and EPM Medium
To select a medium with high laccase enzyme production, the activity of laccase in PD and enzyme-producing medium (EPM) was determined. Results showed that laccase activity from EPM culture was significantly greater than that of PD culture (Figure 1), which may be due to the richer nutrients in EPM medium. The laccase activity reached maximum at 4 d (23.36 U L−1) in PD medium, and then declined, for the EPM culture, the activity of laccase increased with the increase of incubation time, and reached 440.92 U L−1 at day 8. It is interesting to note that the increase in enzyme activity in the first six days was very fast, which was less pronounced after day 6.
Figure 1.
Activity of laccase under EPM and PD medium (U L−1). EPM = Enzyme-producing medium; PD = Potato Dextrose medium. Crude laccase was obtained from the supernatant of G. lucidum mycelium culture. The error bars represent the standard deviation of triplicate samples.
It has been reported that G. lucidum is unable to decolorize RBBR in low nitrogen medium [35], suggesting that the medium component has a great influence on decolorization of dye by laccase. Numerous studies have been undertaken to optimize culture conditions for obtaining high-yield laccase production. By optimizing the composition of the culture medium, laccase activities 12 times greater than controls have been achieved, reaching approximately 240 U mL−1 [36]. It was reported G. lucidum 77,002 yielded laccase with a high activity of 141.1 U mL−1 within 6 d when wheat bran and peanut powder were used as nutrient sources [24]. The results of this study were consistent with this result, as EMP medium contains wheat bran, and a higher enzyme activity was obtained in this study from EMP. Culture times greater than 6 d did not lead to a much greater increase in laccase activity, therefore medium from day 6 was selected for further study.
2.2. Effect of Reaction Time, pH, Temperature, and Dye Concentrations on RBBR Decolorization
To explore the optimal RBBR decolorization conditions for G. lucidum laccase, the effects of reaction time, pH, temperature, and dye concentrations on the RBBR decolorization percentage were determined. The supernatant collected from EPM culture was used as a crude laccase. The 3 mL reaction system comprised of 1.5 mL crude laccase concentrated to 2000 U L−1, 1.5 mL 0.1 M acetate buffer (pH 4.5) and 50 ppm RBBR. The basic reaction condition was: 30 min, pH 4.5, 25 °C, and 50 ppm RBBR. Analysis of the following experimental conditions only changes one of these factors and the rest remain unchanged.
2.2.1. The Effect of Reaction Time
The effect of reaction time on decolorization percentage of RBBR by G. lucidum laccase was determined within 150 min. Results (Figure 2a) revealed that RBBR decolorization was associated with an increase in reaction time, which reached a maximum amount of 60.3% at 150 min. During the initial stage of the reaction (20–90 min), the decolorization percentage increased significantly (p < 0.05) and tended to be more stable at later time points. The change of decolorization is very small between the time points 90 min and 150 min. At 30 min, almost 30.8% of RBBR was decolorized, accounting for approximately half of the total decolorization percentage. Therefore, the reaction time of 30 min was chosen for subsequent measurements. It is reported that approximated complete RBBR decolorization was observed by laccases in 240 h [33]. Purified Glac3 took 16 h to reach approximated 60% decolorization percentage for three dyes (AFR, RY, and MV) [37].
Figure 2.
Decolorization percentage of RBBR under different conditions of: (a) time; (b) pH; (c) temperature; and (d) dye concentrations. The final laccase activity of the reaction system was 1000 U L−1. Decolorization percentage was determined by measuring changes in absorbance at 592 nm. The error bars represent the standard deviation of triplicate samples. Different letters (a, b, c, d) above bars indicate statistically significant differences between groups according to one-way ANOVA (n = 3, p < 0.05; refer in-text for test specifics and test statistics).
2.2.2. The Effect of pH
Six pH values (pH 3–7) were chosen and the decolorization percentage of RBBR varied greatly at different pH values (Figure 2b). The highest decolorization percentage (30.8%) appeared at pH 4.5, followed by pH 5.0 (19.0%). At pH values greater or less than 4.5, the decolorization percentages were much less. The decolorization percentage at each pH was significantly different (p < 0.05), which indicates that G. lucidum laccase was sensitive to pH changes and weakly acidic environments (pH 4.5) were promoted G. lucidum laccase activity. Laccase from Trametes pubescens was found to be highly stable and recalcitrant under alkaline conditions (pH 7.0 to 10.0) [38], but the optimum pH of laccase from most other white-rot basidiomycetes is between 2.2–5.0 [39,40], indicating that laccases from different sources have different characteristics. It was reported that optimum pH for RBBR decolorization by G. lucidum was pH 4.0 [19], this may be due to differences in breed or medium.
2.2.3. The Effect of Temperature
Reaction temperature has a strong influence on enzyme activity. Seven temperatures (20–70 °C) were chosen to explore RBBR decolorization by laccase (Figure 2c). Results indicate that the decolorization percentage was low at 20 °C, and was significantly greater at 25 °C (30.8%) (p < 0.05). With the further increase in temperature (25–40 °C), the change in decolorization percentage was not pronounced. When the temperature exceeds 50 °C, the decolorization percentage decline significantly (p < 0.05), especially under 70 °C, the decolorization rate is only 5.3%, indicating temperature over 50 °C is not suitable for laccase enzyme reaction. The optimum reaction temperatures of laccases from other white-rot fungi can reach between 40–70 °C [38,39,40]. According to the report, RBBR decolorization percentage reached the maximum in 60 °C, beyond which the percentage decreased sharply [19].
2.2.4. The Effect of Dye Concentration
Substrate concentration influences the activity of laccase, and excessive concentration inhibits its activity. Six concentrations of RBBR (50–400 ppm) were chosen to test their effect on decolorization. Results show that the maximum decolorization percentage (46.7%) was achieved in 200 ppm RBBR (Figure 2d). The decolorization percentage was significantly increased when 100 ppm RBBR was used (p < 0.05). When higher concentration (>200 ppm) of RBBR was used, decolorization percentage decreased significantly with the increase of concentration, and dropped to 24.7% at 400 ppm (p < 0.05). RBBR strongly inhibited activity of G. lucidum laccases extracted by [19], and the percentages of 17.5% and 1.1% were observed under 200 and 300 ppm RBBR. While increasing the dye concentration of three dyes (AFR, RY, and MV), decolorization percentage by purified Glac3 decreased markedly, and less 40% decolorization percentage was obtained under 100 ppm dyes [37].
To explore the optimal decolorization conditions, a three-factor and three-level experiment was designed based on results. The results of the three-factor and three-level experiment showed that the decolorization percentage varies between 26.8% to 50.3% (Table 1), and the optimal condition was Treatment No. 7: 35 °C, pH 4.0 and 200 ppm in 30 min. Therefore, our research strongly demonstrates the ability of G. lucidum laccases to decolorize the RBBR dye without adding any further component.
Table 1.
Decolorization of RBBR Determined by Three-factor and Three-level Experiments.
| Treat No. | Temperature (°C) | pH | Dye Concentration (ppm) | Decolorization Percentage (%) |
|---|---|---|---|---|
| 1 | 25 | 4.0 | 100 | 39.20 ± 0.34 d |
| 2 | 25 | 4.5 | 150 | 44.00 ± 0.85 c |
| 3 | 25 | 5.0 | 200 | 35.41 ± 0.08 e |
| 4 | 30 | 4.0 | 150 | 47.65 ± 0.84 b |
| 5 | 30 | 4.5 | 200 | 49.95 ± 0.86 a |
| 6 | 30 | 5.0 | 100 | 26.78 ± 1.00 f |
| 7 | 35 | 4.0 | 200 | 50.34 ± 0.30 a |
| 8 | 35 | 4.5 | 100 | 40.88 ± 0.58 d |
| 9 | 35 | 5.0 | 150 | 45.49 ± 1.23 bc |
The bold font is the optimal decolorization condition. Different letters (a, b, c, d, e, f) indicate statistically significant differences between groups according to one-way ANOVA (n = 3, p < 0.05; refer in-text for test specifics and test statistics).
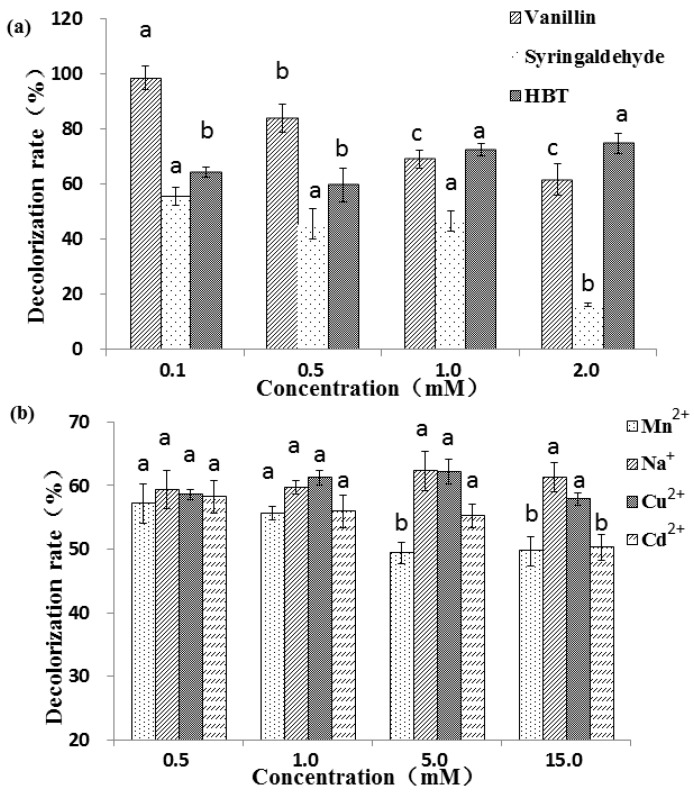
2.3. Effect of Mediators and Metal Ions on RBBR Decolorization
Studies have demonstrated that the presence of mediators can greatly decrease the potential energy of laccase oxidation reactions and significantly increase the oxidation efficiency [41]. There is evidence that RBBR cannot be decolorized without mediators just by pure fungal laccases [42]. Different mechanisms are also involved in the natural and synthetic mediator reactions [43,44]. Aromatic compounds and metal salts can regulate the differential expression of laccase isozyme in G. lucidum [45]. Three different types of copper ions are contained in the active site of laccase, and metal ions as additives can have a great impact on laccase activities. To explore the effects of metals and mediators on G. lucidum laccase activity, we analyzed the effects of three mediators and four metals on RBBR decolorization. Treatment No. 7 (Table 1; pH 4.0, dye concentration 200 ppm, temperature 30 °C, 30 min) was selected for the following experiment. The decolorization percentage (50.0%) indicated for this condition was used as a reference value.
Three mediators, including two natural (vanillin and syringaldehyde) mediators and one synthetic (HBT) mediator, have different effects on the decolorization of RBBR (Figure 3a). For the two natural mediators, the higher the concentration, the lower the decolorization percentage, which decrease significantly from 98.7% (0.1 mM) to 61.7% (2.0 mM) for vanillin (p < 0.05). This may be because the natural mediator is also a substrate for the laccase reaction. The higher the mediator concentration, the more mediators that compete with the substrate, and the laccase involved in the decolorization of dye is reduced, resulting in a decrease in the degree of decolorization. Compared with vanillin, the decolorization effect under syringaldehyde was less obvious. At low concentration ranges (0.1–1 mM), the decolorization percentage did not vary significantly (55.7%–46.6%), but with further increases in syringaldehyde concentration (1.0–2.0 mM), the decolorization varied greatly, decreasing significantly from 46.6% (1.0 mM) to 16.3% (2.0 mM) (p < 0.05). Unlike the two natural mediators, in general, the decolorization percentage associated with the synthetic mediator HBT increased as the concentration increased, and reached maximum (74.8%) at 2.0 mM of HBT. Among the three tested mediators, vanillin showed the greatest decolorization percentages, followed by HBT. The decolorization percentage of adding 1 mM HBT was observably better than that of 0.2 and 0.5 mM [19]. The different results may be caused by different laccase and different action site and reaction efficiency with HBT. Natural mediators assist in laccase-mediated decolorization of industrial dyes, producing better results than without the mediators. For example, 90.0% and 30.0% of Reactive Black 5 and Azure B were decolorized when acetosyringone was used as a laccase mediator, and a longer reaction time (6 h) was required to attain the maximal decolorization percentages with HBT [46]. As the concentration of HBT and ABTS increased, the degradation percentages of anthracene by laccase increased [47]. Two textile industry effluents were successfully detoxified by laccase when using HBT as mediator [48]. Low-toxic acetylacetone was used as a mediator to enhance the detoxification and degradation in a laccase mediator system [49].
Figure 3.
Decolorization percentage of RBBR under three mediators (a) and four metal ions (b). The final laccase activity of the reaction system was 1000 U L−1. Decolorization percentage was determined by measuring changes in absorbance at 592 nm. Reaction condition: pH 4.5, dye concentration 200 ppm, temperature 30 °C, and reaction time 30 min. The error bars represent the standard deviation of triplicate samples. Different letters above bars indicate statistically significant differences between groups according to one-way ANOVA (n = 3, p < 0.05; refer in-text for test specifics and test statistics).
Four metal ions, Mn2+, Na+, Cu2+ and Cd2+, were selected to analyze the effect of metals on RBBR decolorization by laccase. Metal ions have less influence on the decolorization effect than mediator molecules. Results showed that at low concentrations (0.5 and 1.0 mM) the decolorization percentage in the presence four metals were not significantly different (p < 0.05) (Figure 3b). At higher concentrations (5.0 and 10.0 mM), Na+ and Cu2+ increased the decolorization while Mn2+ and Cd2+ decreased discoloration percentage. Copper treatment with appropriate concentration can promote laccase activity [50], and therefore, it is possible to further promote the decolorization effect. The decolorization percentage decreased significantly from 58.2% (0.5 mM) to 50.3% (15 mM) with the specified concentrations of Cd2+ (p < 0.05), suggesting that higher concentrations of Cd2+ may inhibit the activity of laccase. The presence of heavy metal ions in textile effluents may create the problem of low biodegradability, which increases the time required for biological treatment [51]. The addition of Cu2+, Fe2+, Mn2+ and Cd2+ increased laccase activity in vitro, especially Cu2+ (3.86-fold), and synergistic stimulation of metal ions and aromatic compounds further enhanced this effect [52]. For example, laccase was inhibited by NaCl, but not by Na2SO4. Crude laccase completely decolorized RBBR with 1.0 mM Na2SO4 and half decolorization was demonstrated when 0.1 mM NaCl was used [33]. It was reported that the dye decolorization process was inhibited due to the presence of Hg2+, and dye decolorization cannot be achieved without HBT [53].
2.4. Laccase Isoenzyme Electrophoresis and Protein Mass Spectrometry Sequencing
To explore the expression of laccase isoenzymes in EPM medium, crude laccase from the supernatant of G. lucidum cultured for 6 d (relative high laccase activity from Figure 1) was used for isozyme electrophoresis analysis. The BN-PAGE was stained using Coomassie Brilliant Blue (Figure 4, lane 1) and substrate ABTS (Figure 4, lane 2). Only a wide single band can be seen on the gel, which may be due to the small difference in molecular weight of G. lucidum laccases. The corresponding band from the gels stained by Coomassie Brilliant Blue was excised for use in mass spectrometry (MS) sequencing, and the result showed that the main protein contained in crude laccase was Glac1 (GenBank: AHA83584.1).
Figure 4.
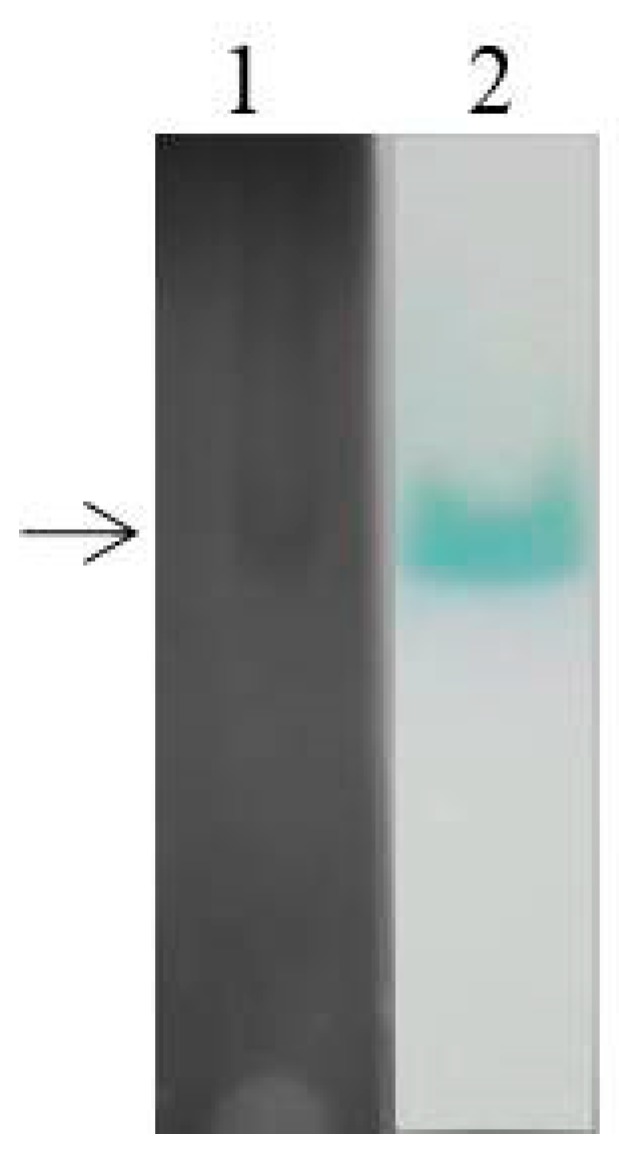
BN-PAGE analysis of laccase isozyme. The crude laccase was used for BN-PAGE. Lane1: Coomassie Brilliant Blue staining, Lane 2: ABTS staining.
The regulation of laccase isoenzymes can due to either the expression of different genes or modifications after the transcription [27]. It was reported that after 1 h incubation, one G. lucidum laccase isozyme was observed in CBB-R 250, RB-5, and RBBR stained gels from nativePAGE results [19]. Four G. lucidum laccase isoforms obtained from potato dextrose agar (PDA) medium were observed by nativePAGE, including Glac3 with a molecular mass of 38.3 kDa [37]. This may be due to different components of the medium that lead to different laccase isozyme expression profiles. The multimeric nature of laccase isozymes in G. lucidum was evident from SDS-PAGE results, and the molecular mass of isozymes was between 40–66 kDa [45]. On day 7, three and two isoenzymes were observed in Pleurotus ostreatus laccases extracts obtained from basal medium and RBBR medium respectively by nativePAGE [27].
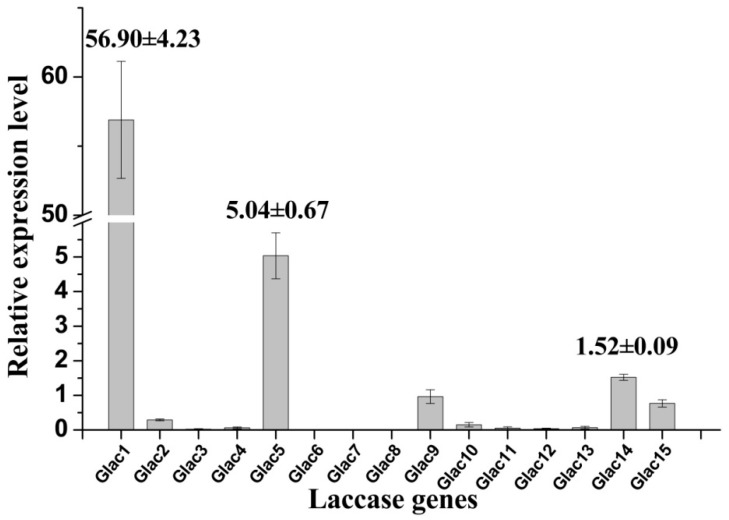
2.5. Transcriptional Analysis of Laccase Genes
High laccase activity was obtained in EMP medium, and transcriptional expression profiles for laccase genes were analyzed by RT-PCR under this culture condition. Results revealed that some laccase genes have higher transcriptional expression levels (e.g., Glac1, Glac5, etc.) relative to others, whereas some genes showed lower expression (e.g., Glac2, Glac10, etc.) or were not expressed (e.g., Glac6-8, etc.) (Figure 5). Consistent with the above MS result, the relative expression of Glac1 was much greater than other laccase genes; this was 10 expression level units greater that of the second expressed genes (Glac5). Results indicate that Glac1 may be the main metabolite from G. lucidum mycelium in EPM medium. The important roles of Glac1 in G. lucidum mycelium has been reported in many previous studies [54,55]. Lcc1 from G. tsugae was reported to play key roles in growth and development of G. tsugae, such as pigmentation and stipe elongation [56]. Lac1, which was cloned from white-rot fungus Cerrena sp., was expressed heterogeneously in yeast [57].
Figure 5.
Relative expression levels of laccase gene of G. lucidum grown in EPM medium. The value represents the expression percentage of laccase gene accounts for the expression of RPL4. The error bars represent the standard deviation of triplicate samples.
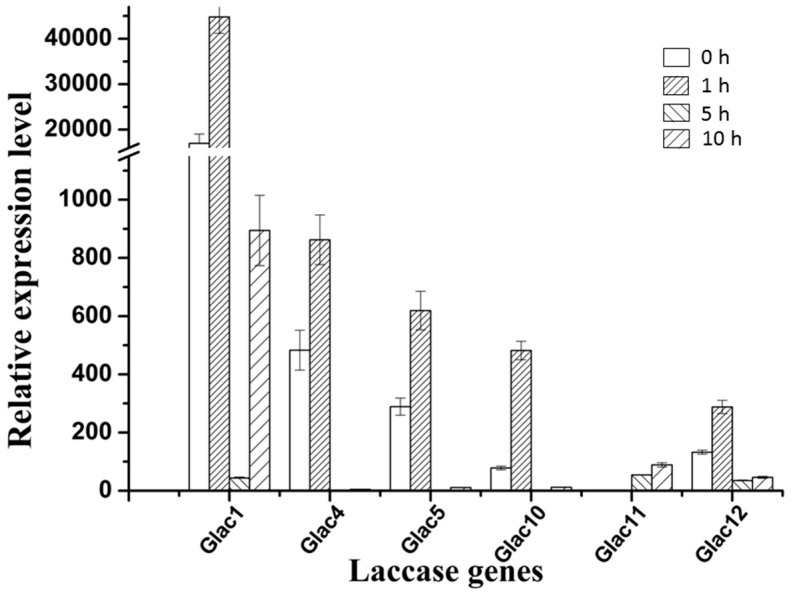
There are two types of fungal laccase: constitutive laccase and inducible laccase. Most of the laccase found so far belong to inducible laccase, and their gene expression is prone to change with the environmental signal [58]. Many factors can regulate laccase genes transcription such as various aromatic compounds related to lignin derivatives, metal ions, nitrogen sources, and carbon sources, etc., which lead different transcriptional profiles not only among different species, but also within the same strain [59,60]. To test whether Glac1 plays a major role in the RBBR decolorization process, in vivo RBBR decolorization by G. lucidum mycelium, and transcriptional expression patterns of laccase genes were analyzed. Results revealed that the transcriptional expression level of laccase genes changed greatly when 200 ppm of RBBR was added to EPM medium after culture for 8 d. Six laccase genes with high expression levels are shown in Figure 6 and the relative expression levels of 9 other genes are shown in Table S1.
Figure 6.
Relative expression levels of six laccase genes of G. lucidum with high expression levels, which were treated with 200 ppm RBBR in 4 different time periods. The value represents the expression percentage of laccase gene accounts for the expression of RPL4. The error bars represent the standard deviation of triplicate samples.
Except for Glac11: the other five genes in Figure 6 greatly responded at the transcriptional level to RBBR in a short time (1 h). Compared to the 0 h, the fold increase in transcriptional expression level ranged from 2.14 (Glac4) to 6.15 times (Glac10). Although Glac10 had the greatest change in transcriptional response, the background expression level of Glac1 was very high, which was 216.94 (0 h) and 92.97 (1 h) times greater than Glac10. Glac4 was the second gene with transcriptional expression level. Increased transcriptional expression levels of the five laccase genes may produce more laccase proteins that degrade the RBBR in the culture environment. As the RBBR in the environment decreases, the transcriptional expression levels of these five laccase genes gradually decreased (5 h). Compared to the 1 h, the fold decrease in transcriptional expression level ranged from 1.3 (Glac12) to 20.5 times (Glac1). As the culture time increased beyond 5h, the transcriptional expression level began to rise again, implying that after a period of adaptation, RBBR was degraded and the strain gradually returned to normal laccase secretion levels. The transcriptional expression profile of Glac11 was opposite to that of the other five genes, with no response at the time of addition, and as the culture time increased, the transcriptional expression level increased, indicating there was a later response of this gene to RBBR. Differential expression levels of the laccase genes are associated with induction of different dyes [27]. The other 9 genes had relatively low expression levels (Table S1), probably because they are not expressed in the mycelial stage, and involved in some other life activities of G. lucidum. The expression of some laccase genes is associated with different developmental stages of fungi [61].
3. Material and Methods
3.1. Microorganism and Culture Conditions
The G. lucidum strain, Meizhi, was obtained from the Microorganism Department of Sichuan Agricultural University, Chengdu, China. It was cultured on sterile PDA medium for 8 days at 28 °C. When plates were fully covered with the mycelia, mycelial plugs (5 mm diameter) were used as inoculum. EPM was composed of corn flour 2.0%, glucose 1.0%, bran 2.0%, soy flour 1.0%, KH2PO4 0.3%, and pH 6.0. Three mycelial plugs were inoculated into 250 mL Erlenmeyer flasks containing 50 mL EPM or PD medium (PDA without agar) and shaking at 28 °C, 150 rpm. Samples were collected every two days (2, 4, 6, and 8 d). To test the effect of RBBR (Rhawn, Shanghai, China) on G. lucidum laccase, 50 ppm RBBR was added to the medium after culturing for 8 d, and samples were collected at four time intervals: 0, 1, 5, and 10 h. Experiments were performed in triplicate.
3.2. Enzyme Assays
Samples were centrifuged at 12,000 rpm, and supernatants were used for the laccase activity test. Laccase activity was determined by measuring changes in absorbance at 420 nm with the extinction coefficient ɛ420 = 36,000 M−1cm−1, using ABTS (BioDuly, Nanjing, China) as the substrate. The assay mixture contained 2900 μL of substrate (0.5 mM ABTS in 0.1 M acetate buffer, pH 4.5) and 100 μL supernatant and was incubated at 30 °C for 3 min. Activities are expressed in international units (U mL−1). 1 μM ABTS oxidized by laccase within 1 min is defined as an enzyme unit.
3.3. Dye Decolorization
The culture collected from EPM medium was centrifuged, and the supernatant was concentrated to one-half volume using an ultra-filtration centrifuge tube (30 kDa). The concentrated supernatant was diluted to an enzyme activity of 2000 U L−1 with 0.1 M acetate buffer (pH 4.5). The dye decolorization was measured by monitoring the decrease in absorbance at 592 nm in a UV-Vis Spectrophotometer. The reaction system was 3 mL, which contained 1.5 mL crude enzyme (2000 U L−1) and 1.5 mL dye solution prepared in acetate buffer (pH 4.5). Various reaction conditions, including reaction time (0, 10, 20, 30, 60, 90, and 120 min), dye concentration (100, 150, 200, 300, and 400 ppm), pH (3.0, 4.0, 5.0, 6.0, and 7.0), reaction temperature (20, 25, 30, 40, 50, 60, and 70 °C), mediators (HBT, vanillin, and syringaldehyde, purchased from Rhawn, Shanghai, China) and metal ions (Mn2+, Na+, Cu2+, and Cd2+) were chosen for analysis of the decolorization percentage. The decolorization percentage was determined in terms of percentage decrease in absorbance by using the following equation:
| (1) |
where D was the decolorization of the dye in percentage (%), C1 was the OD of initial dye system, and C2 was the OD of dye system after incubation. When the dye is completely decolorized, the OD value at 592 nm is zero, and the decolorization percentage of RBBR is 100%. Decolorization was expressed in percentages. Control samples without microorganism were separately maintained in parallel to experimental samples.
3.4. Laccase Isoenzyme Electrophoresis and Protein Mass Spectrometry Sequencing
Native polyacrylamide gel electrophoresis was used for the analysis of laccase isoenzyme. Same volumes of laccase samples were loaded into 2 sample wells of a 12.0% NativePAGE Bis-Tris gel (Sangon Biotech, Shanghai, China), and run at a constant voltage of 150 V. After electrophoresis, the two laccase samples on the gel were separated; one was stained with the substrate (ABTS) and the other with Coomassie Brilliant Blue. A BioDocAnalyze system (Biometra, Goettingen, Germany) was used to generate digital image of the stained gel. The sample stained with Coomassie Brilliant Blue was excised for protein MS sequencing (Sangon Biotech, Shanghai, China).
3.5. RNA Extraction and Real-time PCR Analysis
The total RNA of G. lucidum mycelium was isolated using Trizol reagent (Sangon Biotech, Shanghai, China) following the manufacturer’s instructions. An amount of 1.5 μg total RNA was synthesized to cDNA by a reverse transcription kit (Tiangen, Beijing, China), and the synthesized cDNAs served as templates in subsequent PCR reactions.
Real-time amplification reactions were run in triplicate with the iCycler iQ5 thermocycler (Bio-Rad, Hercules, CA, USA). The reaction volume contained (10 μL) 12.5 μg cDNA, 5 μL SsoFast™ EvaGreen® Supermix (Bio-Rad, USA), 400 nM of each primer, and nuclease-free water to a final volume of 10 μL. The same primer sequence of laccase genes (Table S2) from Shen’s research [62] were used in this study. Reactions were performed under the following conditions: 95 °C for 2 min, followed by 40 cycles of 95 °C for 15 s, 60 °C for 15 s, and then 72 °C for 20 s. A melting curve was created to confirm that a single product was generated by each reaction. A negative control (water) was included in each run. RT-PCR products were verified by agarose gel electrophoresis and sequencing. The G. lucidum constitutive gene ribosomal protein L4 (RPL4) was used as the internal reference gene. The relative expression levels of laccase gene were indicated as percentage to the expression of internal control gene RPL4 and the following formula was used to calculate the relative expression level of each Glac gene:
| (2) |
where ΔCt represents the differences in the cycle threshold value of the target Glac gene and the control RPL4 products. Mean values were obtained from three biological replicates.
4. Conclusions
Results from this study have shown that a higher laccase production was obtained from the more nutritious EPM medium than PD medium. Reaction time, pH, temperature, and dye concentration had a strong effect on laccase-mediated degradation of RBBR, and optimal decolorization conditions were 35 °C, pH 4.0 and 200 ppm in 30 min. Moreover, mediator (vanillin and HBT) and metal ions (Na+ and Cu2+) enhanced the ability of laccase to decolorize RBBR. MS and transcriptional expression analysis results showed that Glac1 may be the main laccase-contributing gene in the decolorization system, which is of worth for further studies.
Supplementary Materials
The following are available online. Table S1: Relative expression levels of other nine G. Lucidum laccase genes under RBBR; Table S2 GenBank accession number of Glac genes.
Author Contributions
This study was conceived and designed by Q.X. and P.Q. All experiments in this study were performed by P.Q., Y.W., J.W., and Y.G. Data were analyzed by P.Q., X.Y., K.Z., and X.Z. The guidance for this study was provided by Q.X., M.M., Q.C., and X.C. This manuscript was written by P.Q., Q.X., B.A., and Z.Z. All authors contributed to manuscript revision, read and approved the submitted version.
Funding
This work was supported by the Breeding Research Project from the Science & Technology Department of Sichuan Province (grant numbers 2016NYZ0040), Sichuan Mushroom Innovational Team of Industry Technology System of Modern Agriculture and the Double support program of Sichuan Agricultural University.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Sample Availability: Samples of the compounds are available from the authors.
References
- 1.Palmieri G., Cennamo G., Sannia G. Remazol brilliant blue r decolourisation by the fungus pleurotus ostreatus and its oxidative enzymatic system. Enzym. Microb. Technol. 2005;36:17–24. doi: 10.1016/j.enzmictec.2004.03.026. [DOI] [Google Scholar]
- 2.Moreira M.T., Feijoo G., Lema J.M. Evaluation of different fungal strains in the decolorisation of synthetic dyes. Biotechnol. Lett. 2000;22:1499–1503. doi: 10.1023/A:1005606330152. [DOI] [Google Scholar]
- 3.Li L., Dai W.K., Yu P., Zhao J., Qu Y.B. Decolorisation of synthetic dyes by crude laccase from Rigidoporus lignosus W1. J. Chem. Technol. Biot. 2009;84:399–404. doi: 10.1002/jctb.2053. [DOI] [Google Scholar]
- 4.Eichlerová I., Homolka L., Benada O., Kofroňová O., Hubálek T., Nerud F. Decolorization of Orange G and Remazol Brilliant Blue R by the white rot fungus Dichomitus squalens: Toxicological evaluation and morphological study. Chemosphere. 2007;69:795–802. doi: 10.1016/j.chemosphere.2007.04.083. [DOI] [PubMed] [Google Scholar]
- 5.Chander M., Arora D.S. Evaluation of some white-rot fungi for their potential to decolourise industrial dyes. Dye. Pigment. 2007;72:192–198. doi: 10.1016/j.dyepig.2005.08.023. [DOI] [Google Scholar]
- 6.Asgher M., Bhatti H.N., Ashraf M., Legge R.L. Recent developments in biodegradation of industrial pollutants by white-rot fungi and their enzyme system. Biodegradation. 2008;19:771–783. doi: 10.1007/s10532-008-9185-3. [DOI] [PubMed] [Google Scholar]
- 7.Arora D.S., Sharma R.K. Ligninolytic fungal laccases and their biotechnological applications. Appl. Biochem. Biotechnol. 2010;160:1760–1788. doi: 10.1007/s12010-009-8676-y. [DOI] [PubMed] [Google Scholar]
- 8.Rajeeva S., Lele S.S. Three-phase partitioning for concentration and purification of laccase produced by submerged cultures of Ganoderma sp. WR-1. Biochem. Eng. J. 2011;54:103–110. doi: 10.1016/j.bej.2011.02.006. [DOI] [Google Scholar]
- 9.Shraddha Shekher R., Sehgal S., Kamthania M., Kumar A. Laccase: Microbial sources, production, purification, and potential biotechnological applications. Enzym. Res. 2011:1–11. doi: 10.4061/2011/217861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Michniewicz A., Ledakowicz S., Ullrich R., Hofrichter M. Kinetics of the enzymatic decolorization of textile dyes by laccase from Cerrena unicolor. Dye. Pigment. 2008;77:295–302. doi: 10.1016/j.dyepig.2007.05.015. [DOI] [Google Scholar]
- 11.Majeau J.A., Brar S.K., Tyagi R.D. Laccases for removal of recalcitrant and emerging pollutants. Bioresour. Technol. 2010;101:2331–2350. doi: 10.1016/j.biortech.2009.10.087. [DOI] [PubMed] [Google Scholar]
- 12.Kerovuo J.S., Haremza S., Koch O., Habicher T., Robertson D.E., Desantis G., McCann R., Luginbuhl P. Laccases for Bio-Bleaching. No. 20160237411. U.S. Patent. 2013 Apr 28;
- 13.Fang Q.H., Zhong J.J. Submerged fermentation of higher fungus Ganoderma lucidum for production of valuable bioactive metabolites-ganoderic acid and polysaccharide. Biochem. Eng. J. 2002;10:61–65. doi: 10.1016/S1369-703X(01)00158-9. [DOI] [Google Scholar]
- 14.Liu J., Shimizu K., Konishi F., Noda K., Kumamoto S., Kurashiki K., Kondo R. Anti-androgenic activities of the triterpenoids fraction of Ganoderma lucidum. Food Chem. 2007;100:1691–1696. doi: 10.1016/j.foodchem.2006.01.003. [DOI] [Google Scholar]
- 15.Yao Q., Gong Z.Y., Gao X.G., Ren P.F., Ren H.X., Li J., Qu L., Wang Z.X., Liu Y. Fermentation Process for Improving Yield of Lucid Ganoderma Laccase. No. 101928699. China Patent. 2010 Apr 20;
- 16.Murugesan K., Yang I.H., Kim Y.M., Jeon J.R., Chang Y.S. Enhanced transformation of malachite green by laccase of Ganoderma lucidum in the presence of natural phenolic compounds. Appl. Microbiol. Biot. 2009;82:341–350. doi: 10.1007/s00253-008-1819-1. [DOI] [PubMed] [Google Scholar]
- 17.Jong-Rok J., Kumarasamy M., Young-Mo K., Eun-Ju K., Yoon-Seok C. Synergistic effect of laccase mediators on pentachlorophenol removal by Ganoderma lucidum laccase. Appl. Microbiol. Biot. 2008;81:783–790. doi: 10.1007/s00253-008-1753-2. [DOI] [PubMed] [Google Scholar]
- 18.Coelho J.S., Souza C.G.M., Oliveira A.L., Bracht A., Costa M.A.F., Peralta R.M. Comparative removal of bentazon by Ganoderma lucidum in liquid and solidstate cultures. Curr. Microbiol. 2010;60:350–355. doi: 10.1007/s00284-009-9548-y. [DOI] [PubMed] [Google Scholar]
- 19.Murugesan K., Nam I.H., Kim Y.M., Chang Y.S. Decolorisation of rective dyes by a thermostable laccase produced by Ganoderma lucidum in solid state culture. Enzym. Microb. Technol. 2007;40:1662–1672. doi: 10.1016/j.enzmictec.2006.08.028. [DOI] [Google Scholar]
- 20.Songulashvili G., Elisashvilli V., Wasser S.P., Nevo E., Hadar Y. Basidiomycetes laccase and manganese peroxidase activity in submerged fermentation of food industry wastes. Enzym. Microb. Technol. 2007;41:57–61. doi: 10.1016/j.enzmictec.2006.11.024. [DOI] [Google Scholar]
- 21.Sitarz A., Mikkelsen J.D., Meyer A.M.B.S., Lezyk M. A Novel lLaccase from Ganoderma Lucidum Capable of Enhancing Enzymatic Degradation of Lignocellulolytic Biomass. No. 2895599. Europe Patent. 2013 Oct 10;
- 22.Liao C.S., Yuan S.Y., Hung B.H., Chang B.V. Removal of organic toxic chemicals using the spent mushroom compost of Ganoderma lucidum. J. Env. Monit. 2012;14:1983–1988. doi: 10.1039/c2em10910g. [DOI] [PubMed] [Google Scholar]
- 23.Pundir C.S., Rawal R., Chawla S., Renuka, Kuhad R.C. Development of an amperometric polyphenol biosensor based on fungal laccase immobilized on nitrocellulose membrane. Artif. Cell. Blood Sub. 2012;40:163–170. doi: 10.3109/10731199.2011.637926. [DOI] [PubMed] [Google Scholar]
- 24.Fang Z., Liu X., Chen L., Shen Y., Zhang X., Fang W., Wang X., Bao X., Xiao Y. Identification of a laccase Glac15 from Ganoderma lucidum 77002 and its application in bioethanol production. Biotechnol. Biofuels. 2015;8:54. doi: 10.1186/s13068-015-0235-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu D.B., Gong J., Dai W.K., Kang X.C., Huang Z., Zhang H.M., Liu W., Liu L., Ma J.P., Xia Z.L., et al. The genome of Ganderma lucidum provide insights into triterpense biosynthesis and wood degradation. Plos ONE. 2012;7:1932–6203. doi: 10.1371/journal.pone.0036146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Campos P.A., Levin L.N., Wirth S.A. Heterologous production, characterization and dye decolorization ability of a novel thermostable laccase isoenzyme from Trametestrogii BAFC 463. Process. Biochem. 2016;51:895–903. doi: 10.1016/j.procbio.2016.03.015. [DOI] [Google Scholar]
- 27.Garrido-Bazán V., Téllez-Téllez M., Herrera-Estrella A., Díaz-Godínez G., Nava-Galicia S., Villalobos-López M., Arroyo-Becerra A., Bibbins-Martínez M. Effect of textile dyes on activity and differential regulation of laccase genes from Pleurotus ostreatus grown in submerged fermentation. Amb. Express. 2016;6:93–111. doi: 10.1186/s13568-016-0263-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Murugesan K., Kim Y.M., Jeon J.R., Chang Y.S. Effect of metal ions on reactive dye decolorization by laccase from Ganoderma lucidum. J. Hazard. Mater. 2009;168:523–529. doi: 10.1016/j.jhazmat.2009.02.075. [DOI] [PubMed] [Google Scholar]
- 29.Bourbonnais R., Paice M.G. Oxidation of non-phenolic substrates: An expanded role of laccase in lignin biodegradation. FEBS Lett. 1990;267:99–102. doi: 10.1016/0014-5793(90)80298-W. [DOI] [PubMed] [Google Scholar]
- 30.Call H.P., Mucke I. History, overview and applications of mediated lignolytic systems, especially laccase-mediator systems (LignozymR process) J. Biotechnol. 1997;53:163–202. doi: 10.1016/S0168-1656(97)01683-0. [DOI] [Google Scholar]
- 31.Ashe B., Nguyen L.N., Hai F.I., Lee D.J., Van D.M.J.P., Leusch F.D.L. Impacts of redox-mediator type on trace organic contaminants degradation by laccase: Degradation efficiency, laccase stability and effluent toxicity. Int. Biodeter. Biodegr. 2016;113:169–176. doi: 10.1016/j.ibiod.2016.04.027. [DOI] [Google Scholar]
- 32.Boer C.G., Obici L., Souza C.G.M.D., Peralta R.M. Decolorization of synthetic dyes by solid state cultures of Lentinula (Lentinus) edodes producing manganese peroxidase as the main ligninolytic enzyme. Bioresour. Technol. 2004;94:107–112. doi: 10.1016/j.biortech.2003.12.015. [DOI] [PubMed] [Google Scholar]
- 33.Zilly A., Jaqueline C.M., Bracht A., Giatti M.D.S.C., Carvajal A.E., Koehnlein E.A. Influence of NaCl and Na2SO4 on the kinetics and dye decolorization ability of crude laccase from Ganoderma lucidum. Int. Biodeter. Biodegr. 2011;65:340–344. doi: 10.1016/j.ibiod.2010.12.007. [DOI] [Google Scholar]
- 34.Moreira N.S.L., Matheus D.R., Machado K.M.G. Influence of pH on the growth, laccase activity and RBBR decolorization by tropical basidiomycetes. Braz. Arch. Biol. Technol. 2009;52:1075–1082. doi: 10.1590/S1516-89132009000500003. [DOI] [Google Scholar]
- 35.Eichlerová I., Homolka L., Lisá L., Nerud F. Orange G and Remazol Brilliant Blue R decolorization by white rot fungi Dichomitus squalens, Ischnoderma resinosum and Pleurotus calyptratus. Chemosphere. 2005;60:398–404. doi: 10.1016/j.chemosphere.2004.12.036. [DOI] [PubMed] [Google Scholar]
- 36.Teerapatsakul C., Parra R., Bucke C., Chitradon L. Improvement of laccase reduction from Ganoderma sp. KU-ALK4 by medium engineering. World J. Microb. Biot. 2007;23:1519–1527. doi: 10.1007/s11274-007-9396-5. [DOI] [Google Scholar]
- 37.Manavalan T., Manavalan A., Thangavelu K.P., Heese K. Characterization of optimized production, purification and application of laccase from Ganoderma lucidum. Biochem. Eng. J. 2013;70:106–114. doi: 10.1016/j.bej.2012.10.007. [DOI] [Google Scholar]
- 38.Si J., Peng F., Cui B. Purification, biochemical characterization and dye decolorization capacity of an alkali-resistant and metal-tolerant laccase from Trametes pubescens. Bioresour. Technol. 2013;128:49–57. doi: 10.1016/j.biortech.2012.10.085. [DOI] [PubMed] [Google Scholar]
- 39.Yan J.P., Chen D.D., Yang E., Niu J.Z., Chen Y.H., Chagan I. Purification and characterization of a thermo tolerant laccase isoform in Trametes trogii strain and its potential in dye decolorization. Int. Biodeter. Biodegr. 2014;93:186–194. doi: 10.1016/j.ibiod.2014.06.001. [DOI] [Google Scholar]
- 40.Patel H., Gupte S., Gahlout M., Gupte A. Purification and characterization of an extracellular laccase from solid-state culture of Pleurotus ostreatus HP-1. 3 Biotech. 2014;4:77–84. doi: 10.1007/s13205-013-0129-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chao J., Hou J., Kai W., Zhang Y., Chen V. Biocatalytic degradation of carbamazepine with immobilized laccase-mediator membrane hybrid reactor. J. Membr. Sci. 2016;502:11–20. [Google Scholar]
- 42.Graça M.B.S., Amorim M.T.P.D., Costa-Ferreira M. Use of laccase together with redox mediators to decolourize Remazol Brilliant Blue, R. J. Biotechnol. 2001;89:123–129. doi: 10.1016/s0168-1656(01)00302-9. [DOI] [PubMed] [Google Scholar]
- 43.Cantarella G., Galli C., Gentili P. Oxidation of non-phenolic substrates with the laccase/n-hydroxyacetanilide system: Structure of the key intermediate from the mediator and mechanistic insight. NewJ. Chem. 2004;28:366–372. doi: 10.1039/b311907f. [DOI] [Google Scholar]
- 44.Galli C., Gentili P. Chemical messengers: Mediated oxidations with the enzyme laccase. J. Phys. Org. Chem. 2010;17:12–16. doi: 10.1002/poc.812. [DOI] [Google Scholar]
- 45.Amit K., Deepti S., Sharma K.K., Sakshi A., Singh A.K., Gill S.S., Singhal B. Gel-based purification and biochemical study of laccase isozymes from Ganoderma sp. and its role in enhanced cotton callogenesis. Front. Microbiol. 2017;8:674–682. doi: 10.3389/fmicb.2017.00674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Camarero S., Ibarra D., Martínez Á.T., Romero J., Gutiérrez A., Del R.J.C. Paper pulp delignification using laccase and natural mediators. Enzym. Microb. Technol. 2007;40:1264–1271. doi: 10.1016/j.enzmictec.2006.09.016. [DOI] [Google Scholar]
- 47.Vandertol-Vanier H.A., Vazquez-Duhalt R., Tinoco R., Pickard M.A. Enhanced activity by poly (ethylene glycol) modification of Coriolopsis gallica laccase. J. Ind. Microbiol. Biot. 2002;29:214–220. doi: 10.1038/sj.jim.7000308. [DOI] [PubMed] [Google Scholar]
- 48.Benzina O., Daassi D., Zouari-Mechichi H., Frikha F., Woodward S., Belbahri L., Rodriguez-Couto S., Mechichi T. Decolorization and detoxification of two textile industry effluents by the laccase/1-hydroxybenzotriazole system. Eniviron. Sci. Pollut. R. 2013;20:5177–5187. doi: 10.1007/s11356-013-1491-6. [DOI] [PubMed] [Google Scholar]
- 49.Yang H., Sun H., Zhang S., Wu B., Pan B. Potential of acetylacetone as a mediator for Trametes versicolor laccase in enzymatic transformation of organic pollutants. Eniviron. Sci. Pollut. R. 2015;22:10882–10889. doi: 10.1007/s11356-015-4312-2. [DOI] [PubMed] [Google Scholar]
- 50.Tychanowicz G.K., Souza D.F.D., Souza C.G.M., Kadowaki M.K., Peralta R.M. copper improves the production of laccase by the white- rot fungus pleurotus pulmonarius in solid state fermentation. Braz. Arch. Biol. Technol. 2006;49:699–704. doi: 10.1590/S1516-89132006000600002. [DOI] [Google Scholar]
- 51.Lorenzo M., Moldes D., Rodríguez Couto S., Sanromán M.A. Inhibition of laccase activity from Trametes versicolor by heavy metals and organic compounds. Chemosphere. 2005;60:1124–1128. doi: 10.1016/j.chemosphere.2004.12.051. [DOI] [PubMed] [Google Scholar]
- 52.Zhuo R., Yuan P., Yang Y., Zhang S., Ma F.Y., Zhang X.Y. Induction of laccase by metal ions and aromatic compounds in Pleurotus ostreatus HAUCC 162 and decolorization of different synthetic dyes by the extracellular laccase. Biochem. Eng. J. 2017;117:62–72. doi: 10.1016/j.bej.2016.09.016. [DOI] [Google Scholar]
- 53.Rodríguez C.S., Sanromán M.A., Gübitz G.M. Influence of redox mediators and metal ions on synthetic acid dye decolourization by crude laccase from Trametes hirsuta. Chemosphere. 2005;58:417–422. doi: 10.1016/j.chemosphere.2004.09.033. [DOI] [PubMed] [Google Scholar]
- 54.Das N., Sengupta S., Mukherjee M. Importance of laccase in vegetative growth of Pleurotus florida. Appl. Environ. Microbiol. 1997;63:4120–4122. doi: 10.1128/aem.63.10.4120-4122.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Matcham S.E., Jordan B.R., Wood D.A. Estimation of fungal biomass in a soil substrate by three independent methods. Appl. Microbiol. Biot. 1985;21:108–112. [Google Scholar]
- 56.Jin W.S., Li J.H., Feng H.C., You S., Zhang L.Y., Norvienyeku J., Hu K.H., Sun S.J., Wang Z.H. Importance of a laccase gene (Lcc1) in the development of Ganode rmatsugae. Int. J. Mol. Sci. 2018;19:471. doi: 10.3390/ijms19020471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yang J., Ng T.B., Lin J., Ye X. A novel laccase from basidiomycete Cerrena sp.: Cloning, heterologous expression, and characterization. Int. J. Biol. Macromol. 2015;77:344–349. doi: 10.1016/j.ijbiomac.2015.03.028. [DOI] [PubMed] [Google Scholar]
- 58.Kim S.I., Ha B.S., Kim M.S., Park M., Ro H.S. Evaluation of copper-inducible fungal laccase promoter in foreign gene expression in Pichia pastoris. Biotechnol. Bioproc. Eng. 2016;21:53–59. doi: 10.1007/s12257-015-0567-1. [DOI] [Google Scholar]
- 59.Piscitelli A., Giardina P., Lettera V., Pezzella C., Sannia G., Vincenza F. Induction and transcriptional regulation of laccases in fungi. Curr. Genom. 2011;12:104–112. doi: 10.2174/138920211795564331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Pezzella C., Lettera V., Piscitelli A., Giardina P.S., Sannia G. Transcriptional analysis of Pleurotus ostreatus laccase genes. Appl. Microbiol. Biotechnol. 2013;97:705–717. doi: 10.1007/s00253-012-3980-9. [DOI] [PubMed] [Google Scholar]
- 61.Courty P.E., Hoegger P.J., Kilaru S., Kohler A., Buée M., Garbaye J., Martin F., Kües U. Phylogenetic analysis, genomic organization, and expression analysis of multi-copper oxidases in the ectomycorrhizal basidiomycete Laccaria bicolor. New Phytol. 2009;182:736–750. doi: 10.1111/j.1469-8137.2009.02774.x. [DOI] [PubMed] [Google Scholar]
- 62.Shen K.Y., Zhang X.B., Qin P., Cheng Q., Xiang Q.J. Enzymatic activity and transcription of Ganoderma lucidum laccases following treatment with four heavy metals. Chin. J. Appl. Environ. Biol. 2017;23:42–47. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.