A medical reversal occurs when new evidence demonstrates that an accepted medical practice is either inferior to an older practice or worse than doing nothing at all.1 Unfortunately, medical reversals are common: nearly 150 were documented from 2001 through 2010, and they occurred in nearly every field of medicine.2 When faced with studies that contradict accepted practice, members of the affected medical specialty may take a defensive stance, choosing to disbelieve the new evidence on the basis of selective criticism of the studies’ methods, arguments about plausibility, or even financial conflicts of interest.3
In November 2018, we published the findings of a prospective, randomized trial4 and a nationwide, observational study5 that compared disease-free and overall survivals between patients with cervical cancer who were treated with open and minimally invasive radical hysterectomy. Despite a broad consensus,6,7 which was based on data from retrospective studies, that operations performed by a minimally invasive approach would result in oncologic outcomes equivalent to those after operations performed through a laparotomy, the two studies, published in the New England Journal of Medicine, showed that minimally invasive surgery led to a higher risk of recurrence and death. We believe that these studies represent a medical reversal. As in other instances of medical reversal, a contentious debate has ensued: some authors and groups have concluded that open radical hysterectomy should now be the standard of care for women with operable cervical cancer,7,8 but others have suggested that minimally invasive surgery should remain the norm for some or all patients with operable cervical cancer, pending results from additional randomized trials.9,10
The acceptance of minimally invasive surgery as an alternative to open radical hysterectomy was based on the belief—widely held among gynecologic oncologists—that minimally invasive and open surgery represent two approaches to the same operation. This view was reinforced by randomized trials that demonstrated the oncologic safety of minimally invasive surgery for endometrial cancer11,12 and by observational studies in which authors concluded that, compared with open procedures, minimally invasive radical hysterectomy for cervical cancer was associated with similar oncologic outcomes and less surgical morbidity.13-23 The 2015 National Comprehensive Cancer Network guidelines24 codified robotic and laparoscopic radical hysterectomy as standard approaches to the surgical treatment of cervical cancer.
The results of the Laparoscopy Approach for Cervical Cancer (LACC) trial4 have called into question the consensus that minimally invasive and open radical hysterectomy are oncologically equivalent. This randomized, open-label, noninferiority trial was halted by the data and safety monitoring committee after 631 of a planned 740 patients were randomly assigned to open or minimally invasive radical hysterectomy. The committee found that the rate of deaths in the minimally invasive surgery group was unacceptably higher than in the open surgery group. Ultimately, the resulting publication demonstrated a nearly four-fold increase in the risk of recurrence (hazard ratio [HR], 3.7; 95% CI, 1.6 to 8.6) and a six-fold increase in the risk of death (HR, 6.0; 95% CI, 1.77 to 20.3) among women randomly assigned to minimally invasive surgery.4 The excess risk of death was confirmed in the largest observational study conducted to date (to our knowledge),5 which demonstrated that the risk of death was higher for women who had minimally invasive radical hysterectomy than for those who had open procedures (HR, 1.65; 95% CI, 1.22 to 2.22). Furthermore, in an interrupted time series analysis presented in the same paper, adoption of minimally invasive surgical techniques in the management of cervical cancer in the United States was shown to coincide with a decline in survival.
In this perspective, we review the limitations of the pre-2018 evidence that seemed to support the use of minimally invasive radical hysterectomy in cervical cancer, reflect on the biases that contributed to unwarranted optimism about that approach, and consider the impact of the LACC trial on clinical practice and research.
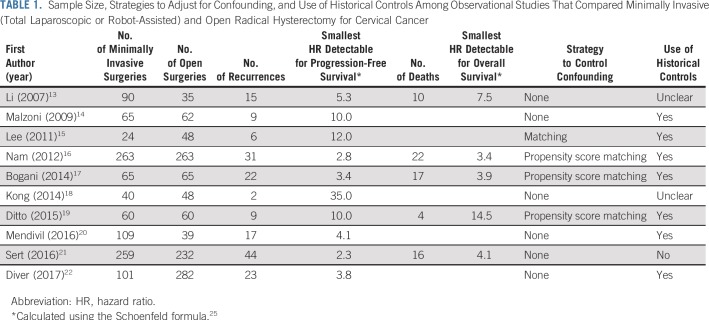
Adoption of minimally invasive radical hysterectomy and arguments that favor its continued use have been based on observational studies that compared recurrence-free or overall survival between women who underwent minimally invasive and open procedures. Review of these studies (Table 1) reveals widespread methodologic limitations, including small sample size, use of historical controls, and lack of adjustment for confounders—although not every study had each of these limitations. These methodologic deficiencies contributed to a biased appraisal of the oncologic efficacy of minimally invasive radical hysterectomy.
TABLE 1.
Sample Size, Strategies to Adjust for Confounding, and Use of Historical Controls Among Observational Studies That Compared Minimally Invasive (Total Laparoscopic or Robot-Assisted) and Open Radical Hysterectomy for Cervical Cancer
Small sample size is a feature of most pre-2018 observational studies that compared open and minimally invasive radical hysterectomies. Of 10 such studies published before 2018 (Table 1), only two included enough events (ie, recurrences or deaths) to permit detection of an HR smaller than 3.0 with a power of 80% and significance level of .05. No study had enough power to permit detection of a difference between minimally invasive and open surgeries, even if minimally invasive surgery doubled the risk of recurrence or death. Although the authors of these small studies concluded that the findings suggested that minimally invasive radical hysterectomy had no deleterious effect on recurrence or death, because survival comparisons yielded P values greater than .05, one must recognize that the absence of statistical significance in these studies is not evidence of equivalent oncologic outcomes but, rather, is the expected consequence of small sample size.
Another common limitation of studies that compared patients who underwent minimally invasive versus open radical hysterectomy is the use of historical controls (Table 1). This practice is problematic, because changes in cervical cancer treatment over time are likely to have contributed to better outcomes in patients who were treated more recently, which leads to bias in favor of minimally invasive surgery. These changes include improvements in adjuvant therapy,26 greater use of cross-sectional imaging to evaluate resectability,27,28 progress in the treatment of recurrent disease,29 and improvements in supportive and end-of-life care.30,31 In addition, the use of historical controls leads to unequal follow-up duration between groups; shorter follow-up among patients who underwent minimally invasive procedures lowers the probability of observing recurrences and deaths in this group.
Finally, less than half of the pre-2018 studies that compared oncologic outcomes between minimally invasive and open radical hysterectomies adjusted for confounding using a statistical method (Table 1). Women who undergo minimally invasive radical hysterectomy tend to have smaller tumors, earlier disease stage, and higher socioeconomic status and are more often white than women treated with open surgery.5 Because each of these factors is associated with a better prognosis, unadjusted or inadequately adjusted survival analyses are likely to be biased in favor of minimally invasive surgery.
To evaluate whether our recently published LACC trial4 and the nationwide observational study5 should change practice, we must critically compare the strengths and limitations of the recently published studies with those of earlier studies. The LACC trial has been the subject of criticism related to the fact that it was stopped early,32 albeit because of safety considerations; concerns about whether surgical quality can ever be ensured in randomized trials33; and questions about the generalizability of the study results to United States practice.34
Although minimally invasive hysterectomies for cervical cancer in the United States are most often performed by fellowship-trained gynecologic oncologists using a robotic platform, only 16% of minimally invasive operations in the LACC trial were performed in this manner. Some gynecologic oncologists believe that robotic-assisted laparoscopic radical hysterectomy is technically easier compared with traditional laparoscopy and that the LACC trial findings, therefore, might have been different if more robotic procedures were performed. Subgroup analysis of the LACC trial does not suggest there is a difference in 4.5-year disease-free survival between patients who received traditional (87.0%; 95% CI, 80.5% to 91.5%) or robotic-assisted (87.2%; 95% CI, 64.0% to 95.9%) laparoscopic surgery. In addition, there are few observational data to suggest a difference in efficacy between traditional and robot-assisted laparoscopic radical hysterectomy.35-37 For example, a meta-analysis of nine studies that compared robotic-assisted and traditional laparoscopic radical hysterectomy found no difference in operative time, lymph node count, or intraoperative complication rates between the procedures.37 Although the few studies that compare recurrence or survival between robotic-assisted and traditional laparoscopic radical hysterectomies are of low quality, oncologic outcomes also seem similar.38-42 Furthermore, in our nationwide observational study,5 in which 80% of minimally invasive operations were performed robotically, women who underwent minimally invasive surgery also experienced inferior survival outcomes, and there was no evidence of heterogeneity of this association between robotic-assisted (HR, 1.61; 95% CI, 1.18 to 2.21) and laparoscopic (HR, 1.50; 95% CI, 0.97 to 2.31) approaches. Although this observational study, which relied on cancer registry data, is limited by the possibility of unmeasured confounding, absence of detailed information about preoperative evaluation, and lack of information about disease recurrence and cause of death in the National Cancer Database, the concordance between randomized trial and real-world findings suggests that the LACC trial is generalizable to patients in the United States.
Some authors have questioned whether the findings of the LACC trial are applicable to patients with tumors smaller than 2 cm who undergo radical hysterectomy.43 These patients have a substantially lower risk of recurrence and death than patients who present with tumors measuring 2 to 4 cm, who have been split into a new stage category (IB2) in the newest International Federation of Gynecology and Obstetrics staging system.10 Although women with tumors smaller than 2 cm accounted for 47% of patients enrolled in the LACC study, they experienced only six (15%) of the 40 observed recurrences. The low event rate in this group necessitates that a randomized trial, statistically larger than the LACC trial, would be required to precisely estimate the effect of minimally invasive surgery in women with small tumors. The feasibility of such a study is uncertain, because it is unclear if patients, doctors, and institutional review boards would agree to randomly assign women to minimally invasive surgery, given the results of the LACC trial. Even under the most favorable circumstances, a trial limited to patients with tumors smaller than 2 cm would require international collaboration and many years to complete. Meanwhile, we must consider the best evidence available when we recommend a surgical approach for women with small tumors (ie, International Federation of Gynecology and Obstetrics 2018 stage IA2 to IB1). Among the nearly 300 women with tumors smaller than 2 cm in the LACC trial, the recurrence rate was 0.6% (one of 147 patients) in the open surgery arm compared with 3.3% (five of 150 patients) in the minimally invasive surgery arm. Though this difference may not be statistically significant, it also was not reassuring: on absolute values, the recurrence rate in the minimally invasive group was five times higher. Of additional concern is that the association between minimally invasive surgery and death as a result of any cause did not differ substantially between women with tumors smaller than 2 cm (HR, 1.46; 95% CI, 0.70 to 3.02) and women with tumors 2 cm or larger (HR, 1.66; 95% CI, 1.19 to 2.30) in the nationwide study.5 Taken together, the evidence from our two recent studies does not suggest that minimally invasive surgery is safe among women with smaller tumors.
Another interpretation of the recent evidence is that individual surgeons or practices can review their own outcomes to determine whether minimally invasive radical hysterectomy is harmful in their own hands.43 However, given the low number of radical hysterectomies performed for cervical cancer, even in the highest-volume centers in the United States,44 we believe that the experience of a single surgeon or institution cannot produce estimates that are sufficiently accurate or precise to guide clinical practice. Counseling patients on the basis of single-surgeon or single-center analyses could misrepresent the true risk associated with minimally invasive radical hysterectomy.8
Since the publication of our randomized4 and observational5 studies, several additional investigations, which were based on a national cancer registry in the Britain,45 a population-based cancer registry in Ontario, Canada,46 a multi-institutional cohort in the United States,47 and single-institution studies from high-volume centers in Korea48 and the United States,49 have all detected an increased risk of recurrence or death among women who underwent minimally invasive radical hysterectomy for early-stage cervical cancer. The minimally invasive arms of at least two of these studies47,49 were composed predominately of patients who underwent robot-assisted operations.
In February 2019, the US Food and Drug Administration released a safety communication warning that the efficacy of robot-assisted surgery in the treatment of breast and other cancers has not been evaluated by the agency.50 This warning underscores the importance of evaluating the safety and oncologic efficacy of minimally invasive techniques for specific procedures, cancer sites, and stages whenever possible.
An unanticipated consequence of the LACC trial is its potential impact on ongoing randomized trials. The SHAPE study (Radical Versus Simple Hysterectomy and Pelvic Node Dissection in Patients With Low-Risk Early Stage Cervical Cancer; ClinicalTrials.gov identifier: NCT01658930) is an ongoing, open-label noninferiority trial in which 700 women with small (≤ 2 cm) cervical cancers are being randomly assigned to simple versus radical hysterectomy and pelvic lymphadenectomy to determine if the more extensive surgery is necessary for women with these small tumors. The SHAPE study protocol does not dictate a surgical approach, nor is the random assignment stratified by use of minimally invasive surgery (Marie Plante, personal communication, April 2019). Because the mechanism by which minimally invasive surgery increases the risk of recurrence in cervical cancer is unknown, it is difficult to predict how the use of minimally invasive techniques will affect the results of the SHAPE study. For example, if the deleterious effect of minimally invasive surgery proceeds through a mechanism that equally affects simple and radical hysterectomy, then the SHAPE study’s conclusion would be valid, provided that the rate of minimally invasive surgery is similar in the treatment arms. If, however, minimally invasive surgery increases recurrence risk by a mechanism that is specific to radical hysterectomy (for example, by limiting parametrial or uterosacral resection), then a conclusion of noninferiority of simple hysterectomy in the SHAPE trial would be suspect, because mandating open radical hysterectomy would have resulted in better outcomes in the radical hysterectomy arm. Likewise, there are at least two ongoing trials (ClinicalTrials.gov identifiers: NCT02595554 and NCT00193739) in which women with locally advanced cervical cancer are randomly assigned to definitive chemoradiotherapy versus neoadjuvant chemotherapy followed by radical hysterectomy; use of minimally invasive surgery could bias the results of these trials against the surgery arm.
Medical reversals are difficult for affected practitioners. It is uncomfortable to confront evidence that suggests that a treatment previously thought to be beneficial is, in fact, harmful. Denial can be a natural first instinct. But denial is the wrong approach for our patients. Unless and until a modified approach to minimally invasive radical hysterectomy is developed and shown to achieve adequate cancer control in a phase III trial, we should consider open abdominal radical hysterectomy to be the standard of care for the surgical management of early cervical cancer.51 The experience with minimally invasive radical hysterectomy underscores an old and often ignored precept of evidence-based medicine: our enthusiasm for new treatments must not outpace the gathering of evidence to support their use.
AUTHOR CONTRIBUTIONS
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Minimally Invasive Radical Hysterectomy for Cervical Cancer: When Adoption of a Novel Treatment Precedes Prospective, Randomized Evidence
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/site/ifc.
Pedro T. Ramirez
Honoraria: Johnson & Johnson
Research Funding: Pacira Pharmaceuticals (Inst)
No other potential conflicts of interest were reported.
REFERENCES
- 1.Prasad V, Cifu A. Ending Medical Reversal: Improving Outcome, Saving Lives. Baltimore, MD: John Hopkins University Press; 2015. [Google Scholar]
- 2.Prasad V, Vandross A, Toomey C, et al. A decade of reversal: An analysis of 146 contradicted medical practices. Mayo Clin Proc. 2013;88:790–798. doi: 10.1016/j.mayocp.2013.05.012. [DOI] [PubMed] [Google Scholar]
- 3.Wang MT, Gamble G, Grey A. Responses of specialist societies to evidence for reversal of practice. JAMA Intern Med. 2015;175:845–848. doi: 10.1001/jamainternmed.2015.0153. [DOI] [PubMed] [Google Scholar]
- 4.Ramirez PT, Frumovitz M, Pareja R, et al. Minimally invasive versus abdominal radical hysterectomy for cervical cancer. N Engl J Med. 2018;379:1895–1904. doi: 10.1056/NEJMoa1806395. [DOI] [PubMed] [Google Scholar]
- 5.Melamed A, Margul DJ, Chen L, et al. Survival after minimally invasive radical hysterectomy for early-stage cervical cancer. N Engl J Med. 2018;379:1905–1914. doi: 10.1056/NEJMoa1804923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Colombo N, Carinelli S, Colombo A, et al. Cervical cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2012;23:vii27–vii32. doi: 10.1093/annonc/mds268. [DOI] [PubMed] [Google Scholar]
- 7.Koh WJ, Abu-Rustum NR, Bean S, et al. Cervical cancer, version 3.2019: NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2019;17:64–84. doi: 10.6004/jnccn.2019.0001. [DOI] [PubMed] [Google Scholar]
- 8.Pennington KP, Urban RR, Gray HJ. Revisiting minimally invasive surgery in the management of early-stage cervical cancer. J Natl Compr Canc Netw. 2019;17:86–90. doi: 10.6004/jnccn.2018.7263. [DOI] [PubMed] [Google Scholar]
- 9.Leitao MM., Jr The LACC trial: Has minimally invasive surgery for early-stage cervical cancer been dealt a knockout punch? Int J Gynecol Cancer. 2018;28:1248–1250. doi: 10.1097/IGC.0000000000001342. [DOI] [PubMed] [Google Scholar]
- 10.Bhatla N, Aoki D, Sharma DN, et al. Cancer of the cervix uteri. Int J Gynaecol Obstet. 2018;143:22–36. doi: 10.1002/ijgo.12611. [DOI] [PubMed] [Google Scholar]
- 11.Walker JL, Piedmonte MR, Spirtos NM, et al. Recurrence and survival after random assignment to laparoscopy versus laparotomy for comprehensive surgical staging of uterine cancer: Gynecologic Oncology Group LAP2 study. J Clin Oncol. 2012;30:695–700. doi: 10.1200/JCO.2011.38.8645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Janda M, Gebski V, Davies LC, et al. Effect of total laparoscopic hysterectomy vs total abdominal hysterectomy on disease-free survival among women with stage I endometrial cancer: A randomized clinical trial. JAMA. 2017;317:1224–1233. doi: 10.1001/jama.2017.2068. [DOI] [PubMed] [Google Scholar]
- 13.Li G, Yan X, Shang H, et al. A comparison of laparoscopic radical hysterectomy and pelvic lymphadenectomy and laparotomy in the treatment of Ib-IIa cervical cancer. Gynecol Oncol. 2007;105:176–180. doi: 10.1016/j.ygyno.2006.11.011. [DOI] [PubMed] [Google Scholar]
- 14.Malzoni M, Tinelli R, Cosentino F, et al. Total laparoscopic radical hysterectomy versus abdominal radical hysterectomy with lymphadenectomy in patients with early cervical cancer: Our experience. Ann Surg Oncol. 2009;16:1316–1323. doi: 10.1245/s10434-009-0342-7. [DOI] [PubMed] [Google Scholar]
- 15.Lee EJ, Kang H, Kim DH. A comparative study of laparoscopic radical hysterectomy with radical abdominal hysterectomy for early-stage cervical cancer: A long-term follow-up study. Eur J Obstet Gynecol Reprod Biol. 2011;156:83–86. doi: 10.1016/j.ejogrb.2010.12.016. [DOI] [PubMed] [Google Scholar]
- 16.Nam JH, Park JY, Kim DY, et al. Laparoscopic versus open radical hysterectomy in early-stage cervical cancer: Long-term survival outcomes in a matched cohort study. Ann Oncol. 2012;23:903–911. doi: 10.1093/annonc/mdr360. [DOI] [PubMed] [Google Scholar]
- 17.Bogani G, Cromi A, Uccella S, et al. Laparoscopic versus open abdominal management of cervical cancer: Long-term results from a propensity-matched analysis. J Minim Invasive Gynecol. 2014;21:857–862. doi: 10.1016/j.jmig.2014.03.018. [DOI] [PubMed] [Google Scholar]
- 18.Kong TW, Chang SJ, Lee J, et al. Comparison of laparoscopic versus abdominal radical hysterectomy for FIGO stage IB and IIA cervical cancer with tumor diameter of 3 cm or greater. Int J Gynecol Cancer. 2014;24:280–288. doi: 10.1097/IGC.0000000000000052. [DOI] [PubMed] [Google Scholar]
- 19.Ditto A, Martinelli F, Bogani G, et al. Implementation of laparoscopic approach for type B radical hysterectomy: A comparison with open surgical operations. Eur J Surg Oncol. 2015;41:34–39. doi: 10.1016/j.ejso.2014.10.058. [DOI] [PubMed] [Google Scholar]
- 20.Mendivil AA, Rettenmaier MA, Abaid LN, et al. Survival rate comparisons amongst cervical cancer patients treated with an open, robotic-assisted or laparoscopic radical hysterectomy: A five-year experience. Surg Oncol. 2016;25:66–71. doi: 10.1016/j.suronc.2015.09.004. [DOI] [PubMed] [Google Scholar]
- 21.Sert BM, Boggess JF, Ahmad S, et al. Robot-assisted versus open radical hysterectomy: A multi-institutional experience for early-stage cervical cancer. Eur J Surg Oncol. 2016;42:513–522. doi: 10.1016/j.ejso.2015.12.014. [DOI] [PubMed] [Google Scholar]
- 22.Diver E, Hinchcliff E, Gockley A, et al. Minimally invasive radical hysterectomy for cervical cancer is associated with reduced morbidity and similar survival outcomes compared with laparotomy. J Minim Invasive Gynecol. 2017;24:402–406. doi: 10.1016/j.jmig.2016.12.005. [DOI] [PubMed] [Google Scholar]
- 23.Gil-Moreno A, Carbonell-Socias M, Salicru S, et al. Radical hysterectomy: Efficacy and safety in the dawn of minimally invasive techniques. J Minim Invasive Gynecol. 2018 doi: 10.1016/j.jmig.2018.06.007. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 24.Koh WJ, Greer BE, Abu-Rustum NR, et al. Cervical cancer, version 2.2015. J Natl Compr Canc Netw. 2015;13:395–404. doi: 10.6004/jnccn.2015.0055. quiz 404. [DOI] [PubMed] [Google Scholar]
- 25.Schoenfeld DA. Sample-size formula for the proportional-hazards regression model. Biometrics. 1983;39:499–503. [PubMed] [Google Scholar]
- 26.Peters WA, III, Liu PY, Barrett RJ, II, et al. Concurrent chemotherapy and pelvic radiation therapy compared with pelvic radiation therapy alone as adjuvant therapy after radical surgery in high-risk early-stage cancer of the cervix. J Clin Oncol. 2000;18:1606–1613. doi: 10.1200/JCO.2000.18.8.1606. [DOI] [PubMed] [Google Scholar]
- 27.Mitchell DG, Snyder B, Coakley F, et al. Early invasive cervical cancer: Tumor delineation by magnetic resonance imaging, computed tomography, and clinical examination, verified by pathologic results, in the ACRIN 6651/GOG 183 Intergroup Study. J Clin Oncol. 2006;24:5687–5694. doi: 10.1200/JCO.2006.07.4799. [DOI] [PubMed] [Google Scholar]
- 28.Wright JD, Dehdashti F, Herzog TJ, et al. Preoperative lymph node staging of early-stage cervical carcinoma by [18F]-fluoro-2-deoxy-D-glucose-positron emission tomography. Cancer. 2005;104:2484–2491. doi: 10.1002/cncr.21527. [DOI] [PubMed] [Google Scholar]
- 29.Tewari KS, Sill MW, Long HJ, III, et al. Improved survival with bevacizumab in advanced cervical cancer. N Engl J Med. 2014;370:734–743. doi: 10.1056/NEJMoa1309748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hicks-Courant K, Melamed A, Worley MJ, Jr, et al. Trends in place of death among patients with gynecologic cancer in the United States. Obstet Gynecol. 2018;131:1111–1120. doi: 10.1097/AOG.0000000000002614. [DOI] [PubMed] [Google Scholar]
- 31.Temel JS, Greer JA, Muzikansky A, et al. Early palliative care for patients with metastatic non–small-cell lung cancer. N Engl J Med. 2010;363:733–742. doi: 10.1056/NEJMoa1000678. [DOI] [PubMed] [Google Scholar]
- 32.Guyatt GH, Briel M, Glasziou P, et al. Problems of stopping trials early. BMJ. 2012;344:e3863. doi: 10.1136/bmj.e3863. [DOI] [PubMed] [Google Scholar]
- 33.McLeod RS. Issues in surgical randomized controlled trials. World J Surg. 1999;23:1210–1214. doi: 10.1007/s002689900649. [DOI] [PubMed] [Google Scholar]
- 34.Naumann RW. Minimally invasive radical hysterectomy has many benefits compared with open radical hysterectomy: Will the LACC trial cause the premature demise of this procedure? J Minim Invasive Gynecol. 2019;26:379–380. doi: 10.1016/j.jmig.2019.01.002. [DOI] [PubMed] [Google Scholar]
- 35.Nezhat FR, Datta MS, Liu C, et al. Robotic radical hysterectomy versus total laparoscopic radical hysterectomy with pelvic lymphadenectomy for treatment of early cervical cancer. JSLS. 2008;12:227–237. [PMC free article] [PubMed] [Google Scholar]
- 36.Kruijdenberg CB, van den Einden LC, Hendriks JC, et al. Robot-assisted versus total laparoscopic radical hysterectomy in early cervical cancer: A review. Gynecol Oncol. 2011;120:334–339. doi: 10.1016/j.ygyno.2010.12.342. [DOI] [PubMed] [Google Scholar]
- 37.Shazly SA, Murad MH, Dowdy SC, et al. Robotic radical hysterectomy in early stage cervical cancer: A systematic review and meta-analysis. Gynecol Oncol. 2015;138:457–471. doi: 10.1016/j.ygyno.2015.06.009. [DOI] [PubMed] [Google Scholar]
- 38.Cantrell LA, Mendivil A, Gehrig PA, et al. Survival outcomes for women undergoing type III robotic radical hysterectomy for cervical cancer: A 3-year experience. Gynecol Oncol. 2010;117:260–265. doi: 10.1016/j.ygyno.2010.01.012. [DOI] [PubMed] [Google Scholar]
- 39.Chen CH, Chiu LH, Chang CW, et al. Comparing robotic surgery with conventional laparoscopy and laparotomy for cervical cancer management. Int J Gynecol Cancer. 2014;24:1105–1111. doi: 10.1097/IGC.0000000000000160. [DOI] [PubMed] [Google Scholar]
- 40.Nie JC, Yan AQ, Liu XS. Robotic-assisted radical hysterectomy results in better surgical outcomes compared with the traditional laparoscopic radical hysterectomy for the treatment of cervical cancer. Int J Gynecol Cancer. 2017;27:1990–1999. doi: 10.1097/IGC.0000000000001101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tinelli R, Malzoni M, Cosentino F, et al. Robotics versus laparoscopic radical hysterectomy with lymphadenectomy in patients with early cervical cancer: A multicenter study. Ann Surg Oncol. 2011;18:2622–2628. doi: 10.1245/s10434-011-1611-9. [DOI] [PubMed] [Google Scholar]
- 42.Zhang SS, Ding T, Cui ZH, et al. Efficacy of robotic radical hysterectomy for cervical cancer compared with that of open and laparoscopic surgery: A separate meta-analysis of high-quality studies. Medicine (Baltimore) 2019;98:e14171. doi: 10.1097/MD.0000000000014171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Leitao MM., Jr The change in landscape after a new landmark is constructed: Radical hysterectomy for early cervical cancer and minimally invasive surgery. Gynecol Oncol. 2019;153:1–2. doi: 10.1016/j.ygyno.2019.03.009. [DOI] [PubMed] [Google Scholar]
- 44.Wright JD, Lewin SN, Deutsch I, et al. The influence of surgical volume on morbidity and mortality of radical hysterectomy for cervical cancer. Am J Obstet Gynecol. 2011;205:225.e1–225.e7. doi: 10.1016/j.ajog.2011.04.014. [DOI] [PubMed] [Google Scholar]
- 45.National Cancer Registration and Analysis Service Comparisons of overall survival in women diagnosed with early stage cervical cancer during 2013-2016, treated by radical hysterectomy using minimal access or open approach. https://bgcs.org.uk/news/ncras-cervical-cancer-radical-hysterectomy-analysis.html.
- 46.Cusimano MC, Baxter NN, Gien LT, et al. Impact of surgical approach on oncologic outcomes in women undergoing radical hysterectomy for cervical cancer. Am J Obstet Gynecol. [epub ahead of print on July 6, 2019] [DOI] [PubMed]
- 47.Uppal S, Gehrig P, Vetter MH, et al. Recurrence rates in cervical cancer patients treated with abdominal versus minimally invasive radical hysterectomy: A multi-institutional analysis of 700 cases. J Clin Oncol. 2019;37 doi: 10.1200/JCO.19.03012. (suppl; abstr 5504) [DOI] [PubMed] [Google Scholar]
- 48.Kim SI, Cho JH, Seol A, et al. Comparison of survival outcomes between minimally invasive surgery and conventional open surgery for radical hysterectomy as primary treatment in patients with stage IB1-IIA2 cervical cancer. Gynecol Oncol. 2019;153:3–12. doi: 10.1016/j.ygyno.2019.01.008. [DOI] [PubMed] [Google Scholar]
- 49.Doo DW, Kirkland CT, Griswold LH, et al. Comparative outcomes between robotic and abdominal radical hysterectomy for IB1 cervical cancer: Results from a single high-volume institution. Gynecol Oncol. 2019;153:242–247. doi: 10.1016/j.ygyno.2019.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.US Federal Drug Administration Caution when using robotically-assisted surgical devices in women's health including mastectomy and other cancer-related surgeries: FDA safety communication. https://www.fda.gov/medical-devices/safety-communications/caution-when-using-robotically-assisted-surgical-devices-womens-health-including-mastectomy-and.
- 51.Uppal S, Spencer R. Modify or abandon: Minimally invasive radical hysterectomy for early-stage cervical cancer. Int J Gynecol Cancer. 2019;29:843–844. doi: 10.1136/ijgc-2019-000574. [DOI] [PubMed] [Google Scholar]