Abstract
PURPOSE
Primary mediastinal B-cell lymphoma (PMBL) is a rare but aggressive non-Hodgkin lymphoma with poor outcomes in patients with relapsed/refractory (R/R) disease. PMBL is characterized by high expression of programmed death-1 ligand and variable expression of CD30. Nivolumab, an anti–programmed death-1 immune checkpoint inhibitor, and brentuximab vedotin (BV), an anti-CD30 antibody–drug conjugate, may have synergistic activity in R/R PMBL.
METHODS
The expansion cohort of the open-label, phase I/II CheckMate 436 study enrolled patients with confirmed R/R PMBL who were previously treated with either autologous hematopoietic cell transplantation or two or more prior chemotherapy regimens if ineligible for autologous hematopoietic cell transplantation. Patients received nivolumab (240 mg intravenously) and BV (1.8 mg/kg intravenously) every 3 weeks until disease progression or unacceptable toxicity. Primary end points were investigator-assessed objective response rate (ORR) per the Lugano 2014 criteria and safety.
RESULTS
Thirty patients with PMBL were treated and evaluable. At a median follow-up of 11.1 months, ORR (95% CI) was 73% (54% to 88%), with a 37% complete remission rate per investigator, and ORR of 70% (51% to 85%), with a 43% complete metabolic response rate per independent review. Median duration of response, median progression-free survival, and median overall survival have not been reached. Eleven responders had consolidation with autologous (n = 5) or allogeneic (n = 6) transplantation. Treatment-related adverse events were reported in 25 patients (83%). Sixteen patients (53%) had grade 3 to 4 treatment-related adverse events; the most common were neutropenia (n = 9), thrombocytopenia (n = 3), and peripheral neuropathy (n = 3). There were no treatment-related deaths.
CONCLUSION
In patients with R/R PMBL, the combination of nivolumab plus BV represents a promising option, with high antitumor activity and a manageable safety profile.
INTRODUCTION
Primary mediastinal B-cell lymphoma (PMBL) is a rare but aggressive lymphoma of thymic B-cell origin, accounting for 2% to 4% of non-Hodgkin lymphomas (NHLs) and up to 10% of diffuse large B-cell lymphomas (DLBCLs).1,2 It occurs predominantly in young adults with a median age of 35 years at diagnosis.1 Approximately 10% to 20% of patients with PMBL are not cured after first-line treatment.3-6 Those with chemosensitive relapse may benefit from autologous hematopoietic cell transplantation (auto-HCT).7 However, patients with relapsed/refractory (R/R) PMBL have poor outcomes. The objective response rate (ORR) to salvage chemotherapy is approximately 25%, and 2-year overall survival (OS) after diagnosis of R/R PMBL is as low as 15%.7 No standard of care for R/R PMBL has been established; it is often treated similarly to other forms of DLBCL.2,8 New therapeutic strategies are urgently needed for patients with R/R PMBL.
Although PMBL has similar histology to DLBCL, the genetic profile of PMBL is distinct and shares many features with classic Hodgkin lymphoma (cHL).2,8 PMBL exhibits 9p24.1 amplification in 45% to 63% of patients, whereas cHL has almost universal copy gain and amplification of the region, and DLBCL shows infrequent alterations.9-12 Genetic alterations at 9p24.1 are associated with increased expression of programmed death-1 (PD-1) ligands, conferring sensitivity to checkpoint inhibitors.9,11,13 Nivolumab, a fully human immunoglobulin G4 anti–PD-1 immune checkpoint inhibitor monoclonal antibody, interrupts PD-1 receptor–ligand interactions and restores T-cell–mediated antitumor immune responses.14 Treatment with another anti–PD-1 antibody, pembrolizumab, as monotherapy has demonstrated a 48% ORR in R/R PMBL.15
Similar to cHL Reed-Sternberg cells, PMBL tumor cells also express CD30, albeit at relatively lower intensities and more heterogeneous levels.16,17 Brentuximab vedotin (BV), an antibody–drug conjugate of monomethyl auristatin E with a CD30 antibody, induces apoptosis of CD30+ tumor cells by disrupting the microtubule network and inhibiting cell division.18 CD30 is also upregulated in intratumoral regulatory T cells (Tregs).19 Thus, BV may affect the tumor microenvironment through depletion of immunosuppressive Tregs in addition to inducing immunogenic cell death, both of which may augment the effect of PD-1 blockade.19-21 In R/R PMBL, BV monotherapy demonstrated a 13% ORR.22
The safety and efficacy of nivolumab combined with BV has been established in studies of R/R cHL.20,23 The complete remission (CR) rate of 67% observed in the phase I/II study suggests potential synergy between these two agents.20,24 Given the common features of PMBL and cHL and the potential synergy of PD-1 inhibition and BV, we evaluated whether the combination of nivolumab and BV was safe and synergistically effective in patients with R/R PMBL. We report the primary efficacy and safety results of the combination therapy in patients with R/R PMBL from the phase II CheckMate 436 study.
METHODS
Study Design and Patients
CheckMate 436 (ClinicalTrials.gov identifier: NCT02581631) is an open-label, multicenter, multicohort, phase I/II study of nivolumab in combination with BV in patients with CD30+ R/R NHL. This study consists of a phase I dose evaluation of the first six treated patients with R/R NHL and phase II efficacy and safety evaluations in expansion cohorts. The phase II expansion cohorts include patients with subtypes of NHLs; results of the R/R PMBL cohort are reported here.
The planned enrollment for the R/R PMBL cohort was 30 patients. Eligible patients were 18 years of age or older, had an Eastern Cooperative Oncology Group performance status of 0 to 1, had a CD30 expression level of 1% or greater in the tumor or tumor-infiltrating lymphocytes by local immunohistochemistry, and had 1 or more measurable sites of disease according to the Lugano 2014 classification.24 Patients were required to have R/R disease after treatment with auto-HCT or have received two or more prior multiagent chemotherapy regimens for those ineligible for auto-HCT. Key exclusion criteria included prior allogeneic HCT (allo-HCT), active autoimmune disease, known history of pancreatitis, CNS involvement, and prior exposure to anti-CD30 treatment or agents targeting T-cell costimulation or checkpoint pathways.
This study was conducted in accordance with Good Clinical Practice guidelines and approved by the institutional review board and independent ethics committee at each study site. All patients provided written informed consent before enrollment.
Treatment and Assessments
The dose-evaluation phase assessed dose-limiting toxicities for 1.8 mg/kg BV (maximum 180 mg) in combination with 240-mg flat-dose nivolumab once every 3 weeks through the first 6 weeks of treatment. Dosages were chosen to streamline the administration of both study drugs. On the basis of clinical data and modeling, the 240-mg flat dose of nivolumab is comparable to the 3-mg/kg dose; after nivolumab once every 3 weeks, PD-1 receptor occupancy of more than 90% is achieved beginning with the first cycle over a range of 0.3 to 10 mg/kg.25,26 In the phase I portion of the study, no patient experienced dose-limiting toxicity; thus, the dose of nivolumab (240-mg flat dose) and BV (1.8 mg/kg) every 3 weeks was chosen for phase II expansion cohorts.
Both drugs were administered intravenously over 30 minutes. In cycle 1, BV was administered on day 1 and nivolumab on day 8. In all subsequent cycles, patients received both drugs on day 1 with at least 30 minutes of rest between BV and nivolumab. Patients were treated until disease progression or unacceptable toxicity.
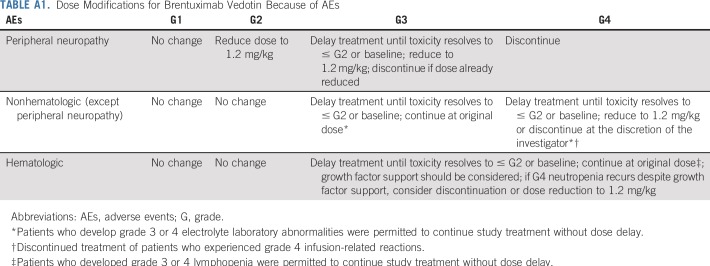
Dose escalation of BV beyond 1.8 mg/kg or reduction below 1.2 mg/kg was not allowed. No dose modification of nivolumab was allowed. Dose delays were permitted for drug-related adverse events (AEs; Appendix, online only; Appendix Table A1, online only; Protocol). If one agent was delayed, the other was also delayed until combination treatment could be safely resumed or one agent was permanently discontinued. Patients who experienced toxicities related to one agent that required treatment discontinuation could continue therapy with the other single agent. Patients who experienced disease progression during treatment were allowed to be treated beyond disease progression until additional progression was observed, as long as they continued to receive investigator-assessed clinical benefit. Patients could receive consolidative HCT at the discretion of the treating physician.
Tumor response was assessed by [18F]fluorodeoxyglucose–positron emission tomography (PET)–computed tomography (CT) at baseline, weeks 6 and 12, every 9 weeks for the following four assessments, and every 12 weeks after the first year until disease progression. Patients with PET-avid lesions at baseline were followed with PET. Those with lesions on CT scans at baseline that were not PET avid were followed with CT scans. CR was confirmed by a negative PET scan score of 1 to 3. Patients treated beyond progression were monitored with radiographic assessment for at least 12 weeks according to Lymphoma Response to Immunomodulatory Therapy Criteria.27
AEs were assessed continuously during the study and for 100 days after the last study treatment using the National Cancer Institute Common Terminology Criteria for Adverse Events (version 4.03).28 Patients who proceeded to HCT were observed for progression and survival on day 100, months 6 and 12, and every year thereafter from the date of stem-cell infusion until the first non-CR response. Patients proceeding to allo-HCT were also assessed for acute and chronic graft versus-host disease (GVHD) at these visits. Health-related quality of life (HRQoL) assessments (EQ-5D 3-level version [EQ-5D-3L] questionnaire) were collected at each visit before study drug administration or via phone during follow-up.
End Points
The primary efficacy end point was investigator-assessed ORR, defined as either CR or partial remission (PR), per Lugano 2014 classification.29 Secondary end points included overall duration of response (DOR), CR rate, duration of CR, and progression-free survival (PFS), all on the basis of investigator assessments, and OS. HRQoL evaluation was an exploratory end point. Tumor response assessed by blinded independent central review (BICR) was a post hoc analysis. Safety end points included incidence of deaths, AEs, serious AEs, AEs leading to discontinuation or dose delay, and specific laboratory abnormalities.
Statistical Analysis
On the basis of historical data, a sample size of 30 patients was chosen with the null hypothesis that the true ORR was 30% or less, to be rejected if 15 or more responses were observed based on an 80% CI. All patients who received at least one dose of the study drugs were included for efficacy and safety analyses.
ORR was summarized by binomial response rate and its corresponding 80% and 95% exact CIs using the Clopper-Pearson method. Descriptive statistics were used to present safety data.
RESULTS
Baseline Patient Characteristics and Disposition
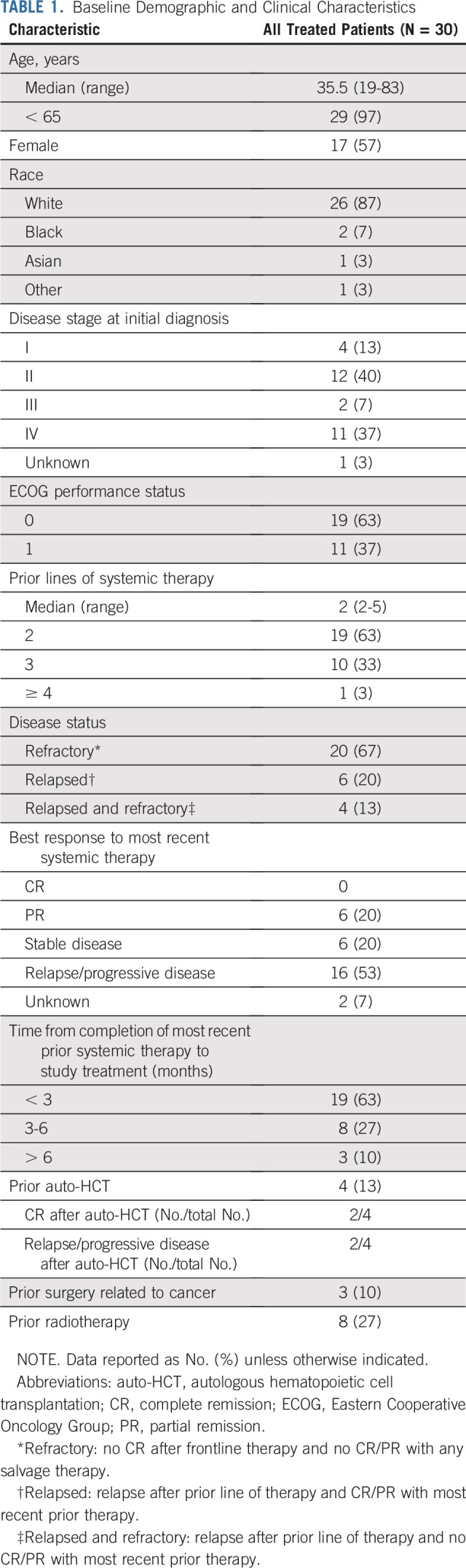
Thirty patients with R/R PMBL were treated in a phase II expansion cohort and are included in this primary analysis. No patients with PMBL were enrolled in the phase I portion of the study. Baseline characteristics are listed in Table 1; the majority of patients (67%) were refractory to any prior line of therapy, and four patients (13%) had prior auto-HCT.
TABLE 1.
Baseline Demographic and Clinical Characteristics
At the time of analysis, 29 patients were treated with nivolumab (median, five doses; range, one to 22), and all 30 were treated with BV (median, five doses; range, one to 20). One patient received BV on cycle 1 day 1, but discontinued before the scheduled first dose of nivolumab.
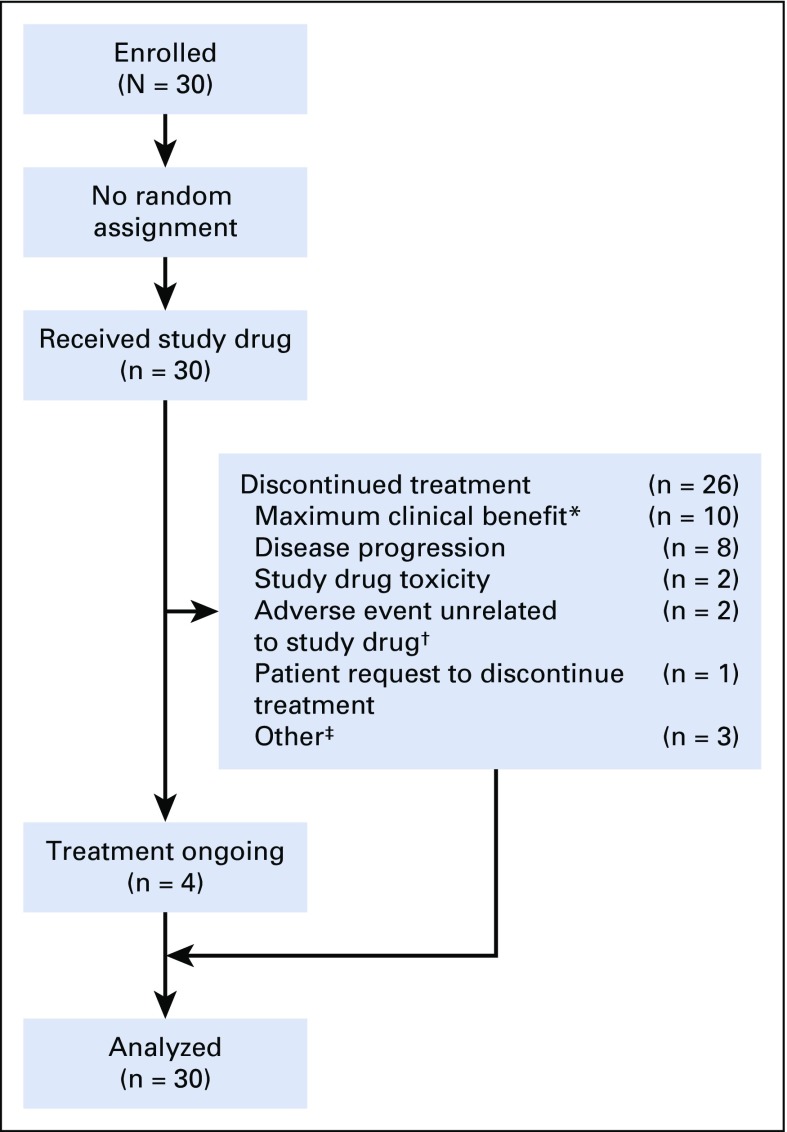
At database lock, four patients continued to receive treatment; the most common reason for discontinuing treatment was maximum clinical benefit (n = 10; Appendix Fig A1, online only). Among patients who were considered to have achieved maximum clinical benefit per investigator, eight received subsequent HCT, one received consolidation radiotherapy, and one received no additional treatment.
Efficacy
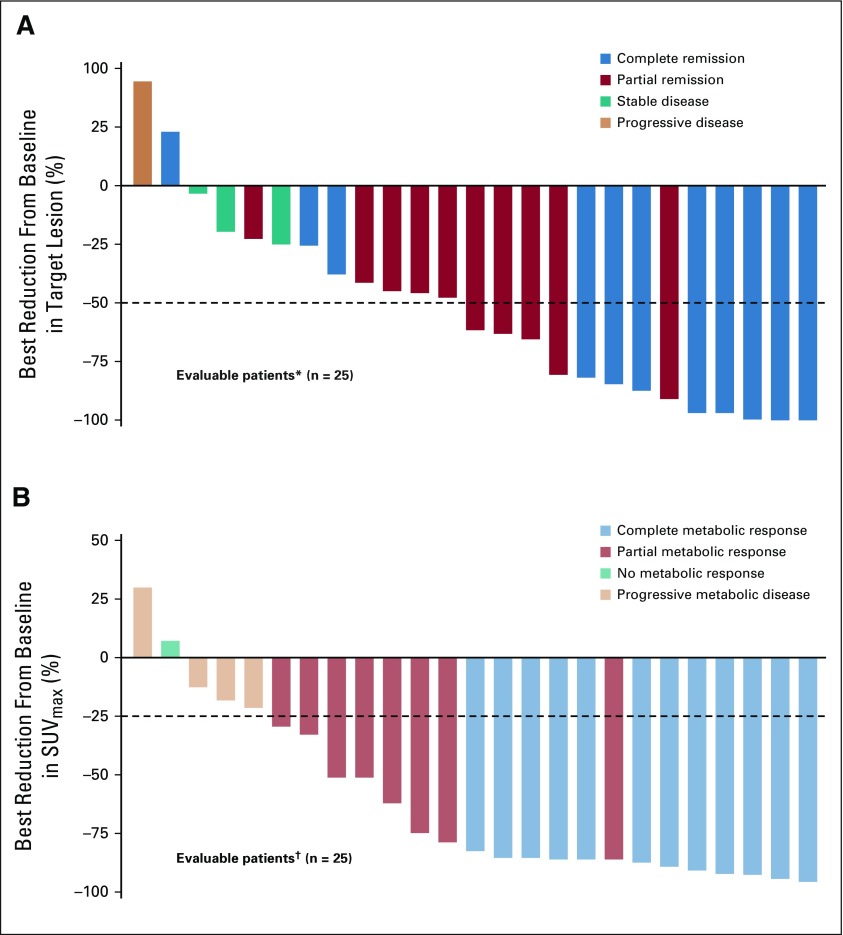
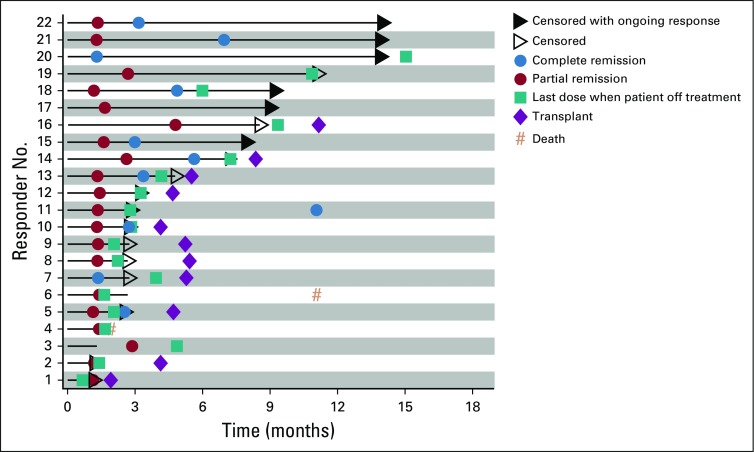
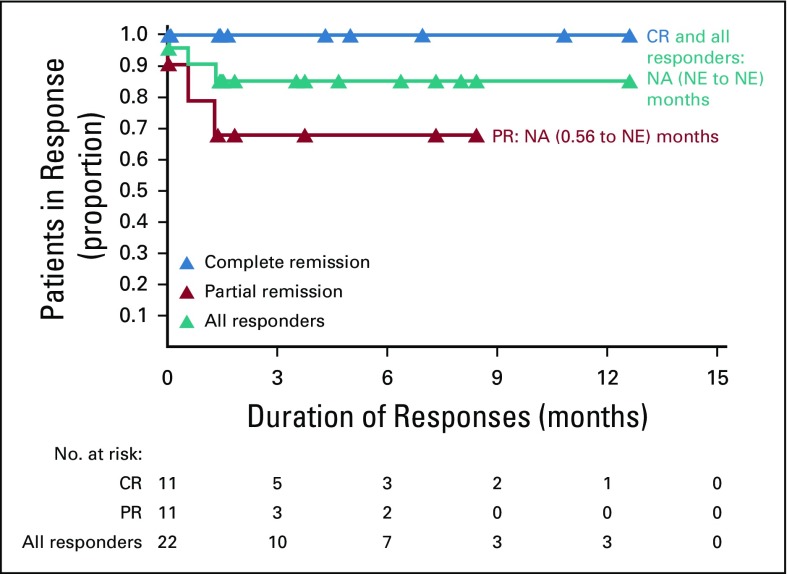
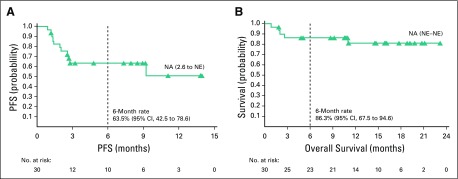
With a median follow-up of 11.1 months, the ORR per investigator was 73% (95% CI, 54 to 88; 80% CI, 60 to 84), with 11 (37%) achieving CR and 11 (37%) achieving PR. Three additional patients (10%) had stable disease. BICR-assessed ORR (95% CI) was 70% (51 to 85), with a CR rate of 43% (Appendix Table A2, online only). Of response-evaluable patients, 52% (13 of 25) had a best reduction in target lesion tumor burden of more than 50%, and 80% (20 of 25) had a best reduction from baseline maximum standardized uptake value of more than 25% (Fig 1).30 Median (interquartile range) time to first objective response was 1.3 (1.3 to 1.6) months and 3.2 (2.5 to 5.6) months to CR. At data cutoff, 12 of the 22 responders had received subsequent anticancer therapies, two experienced disease progression, one died, and seven were in ongoing follow-up (Fig 2). Median DOR and duration of CR were not reached (Fig 3). Eleven responders (50%) received subsequent HCT, including six (three CR and three PR) receiving allo-HCT and five (three CR and two PR) receiving auto-HCT. Four patients underwent radiation consolidation before auto-HCT, and one underwent radiation consolidation before allo-HCT. All five patients who received auto-HCT and five of the patients who received allo-HCT reached the 100-day assessment post-transplantation; all achieved CR. No GVHD was reported in patients receiving subsequent allo-HCT. The 6-month PFS rate was 63.5% (95% CI, 42.5 to 78.6), and the 6-month OS rate was 86.3% (95% CI, 67.5 to 94.6; Fig 4). Median PFS and median OS were not reached.
FIG 1.
(A) Best change from baseline in target lesion by best overall response per investigator. (B) Best reduction from baseline in maximum standardized uptake value (SUVmax) by best overall response per blinded independent central review. Target lesion was measured by sum of the product of the diameters, on the basis of computed tomography scan. SUVmax was measured by [18F]fluorodeoxyglucose–positron emission tomography. Horizontal reference line in (A) indicates the 50% reduction consistent with a response per Lugano Classification 2014. Horizontal reference line in (B) indicates the 25% reduction in SUVmax consistent with a response per Lugano positron emission tomography staging criteria.30 (*) Five patients were nonevaluable: two died before any postbaseline tumor measurements were obtained, one had inconsistent tumor codes at screening and week 6, and two had measurements after progression per investigator. (†) Five patients were nonevaluable: two had no baseline SUVmax measurements, and three had no postbaseline SUVmax measurements.
FIG 2.
Event chart for investigator-assessed tumor response, progression, duration of therapy, transplantation, and death in all responders.
FIG 3.
Duration of response by best overall response per investigator. Symbols represent censored observations. Values are median (95% CI) unless stated otherwise. CR, complete remission; NA, not available; NE, not estimable; PR, partial remission.
FIG 4.
(A) Progression-free survival (PFS) per investigator and (B) overall survival. Symbols represent censored observations. Values are median (95% CI) unless stated otherwise. NA, not available; NE, not estimable.
Health-Related Quality of Life
HRQoL data are reported in Appendix Table A3 (online only).
Safety
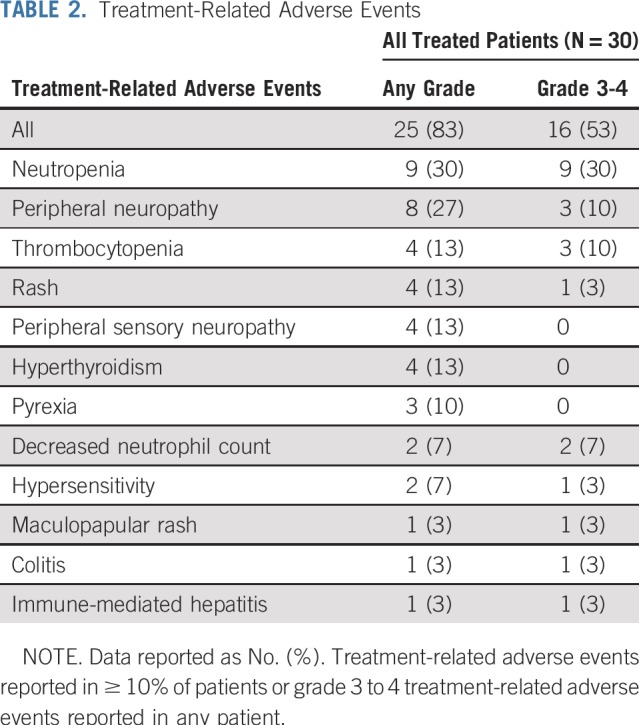
Treatment-related AEs (TRAEs) were reported in 25 patients (83%); 16 (53%) experienced grade 3 to 4 TRAEs (Table 2) after a median of five cycles. The most frequently reported TRAEs were neutropenia (30%) and peripheral neuropathy (27%). All nine TRAEs of neutropenia and three (10%) each of thrombocytopenia and peripheral neuropathy were grade 3 to 4. Four patients (13%) had treatment-related serious AEs: one patient had grade 3 colitis and maculopapular rash, one had grade 4 immune-mediated hepatitis, one had grade 2 acute kidney injury, and one had a grade 2 fall. TRAEs that led to discontinuation of treatment included one grade 4 immune-mediated hepatitis and one grade 3 rash. Three patients discontinued BV because of grade 3 peripheral neuropathy; all of these patients continued therapy with nivolumab and were still receiving treatment at database lock.
TABLE 2.
Treatment-Related Adverse Events
In total, 17 (59%) and 16 (53%) patients had one or more cycle of treatment delayed with nivolumab and BV, respectively. Treatment cycles were delayed in 14% and 15% of total cycles with nivolumab and BV, respectively. Of the total number of treatment cycles received, 11% (22 of 197) and 12% (21 of 182) of cycles were delayed because of AEs from treatment with nivolumab and BV, respectively. The most common AEs that led to dose delay or reduction were neutropenia in nine patients (seven with grade 3 and two with grade 2) and peripheral neuropathy in five patients (all grade 2). Grade 2 peripheral neuropathy (n = 5) and peripheral sensory neuropathy (n = 1) led to dose reduction of BV. No instances were completely resolved: one patient improved to grade 1, and three became grade 3, which led to discontinuation of BV.
A total of 15 immune-mediated AEs were reported in 10 patients, including four events of hyperthyroidism (grade 1 and 2), three events of rash (grade 2 and 3) in the same patient, two events each of acute kidney injury (grade 2 and 5) and hypothyroidism (grade 1), and one each of colitis (grade 3), maculopapular rash (grade 3), infusion-related reaction (grade 2), and thyroiditis (grade 1). Immune-modulating medication was given to treat 60% of immune-mediated AEs.
Five patients died during the study follow-up, four as a result of disease progression and one as a result of sepsis that was not considered treatment related. None of the deaths was considered treatment related by the investigator.
DISCUSSION
In this phase II study, the combination of nivolumab plus BV was highly active in patients with R/R PMBL, with an investigator-assessed ORR of 73% and a CR rate of 37%. The safety of the combination therapy was acceptable and consistent with the known safety profiles of nivolumab and BV alone.14,18 No new safety signals were identified compared with previous studies in other hematologic malignancies.20
The efficacy results of nivolumab plus BV combination therapy showed substantial clinical benefits in this population of patients with highly refractory disease. In our study, patients had received a median of two prior lines of systemic therapy, 67% of patients had disease that was refractory to any prior line of therapy, only 20% had PR, and none achieved CR from their most recent systemic therapies. The response rate to salvage chemotherapy is generally lower for patients with primary refractory disease than for those with relapsed disease.7 In a retrospective study of second-line salvage chemotherapy in R/R PMBL, the ORR was 15% for patients with primary refractory disease and 38% for those who relapsed after first-line treatment, a striking contrast to the high ORR observed in the current study.7 In our study, six patients were chemosensitive at study entry, among whom three achieved CR, two achieved PR, and one achieved stable disease with study treatment.
Our results compare favorably with the outcomes of PD-1 inhibition alone. A phase II study evaluating pembrolizumab monotherapy in 53 patients with R/R PMBL showed an ORR of 45% and a CR rate of 13% per BICR using International Working Group 2007 criteria (21% by Lugano criteria). After a median follow-up of 12.5 months, median DOR and median OS were not reached, and median PFS was 5.5 months. Grade 3 to 4 treatment-related neutropenia was reported in 13% of patients.15,31 In an open-label phase I trial of pembrolizumab in 21 patients with R/R PMBL, the investigator-assessed ORR was 48%, with 33% achieving CR per International Working Group 2007 criteria.15 Median DOR was not reached at a median follow-up of 29.1 months. Median PFS was 10.4 months, and median OS was 31.4 months.15 The patient population of that study was slightly younger (median age, 30 years), more heavily treated (median, three prior lines of therapy), but less refractory (44% refractory to first-line therapy).32
Interestingly, in a phase II trial of patients with R/R PMBL treated with BV monotherapy, ORR was low, at 13% (two of 15), with both being PR, and the trial was terminated early.22 This result was surprising given that most PMBLs express variable levels of CD30. Results from a planned retrospective post hoc analysis of CD30 expression in patients in the BV monotherapy trial may offer additional insights. It is also possible that BV exerts its antitumor activities in PMBL through depletion of Tregs and induction of immunogenic cell death, both of which further boost antitumor immunity potentiated by PD-1 blockade.19-21 Indeed, the combination of nivolumab and BV seems to have synergistic antitumor activity in cHL, a disease that shares similar genetic features with PMBL.9,17,20
In our study, the responses were durable, and the median DOR was not reached. Importantly, patients who achieved CR maintained their response. Of the patients (n = 5) who achieved CR and did not receive additional consolidation with transplantation, two had ongoing CR of 12.6 and 10.8 months at the time of data cutoff. Longer follow-up is needed to assess the durability of disease control and long-term survival outcomes with the combination of nivolumab plus BV.
The results from our study are promising in R/R PMBL, where no randomized studies have been performed and no standard of care has been established. The ability to perform PMBL-specific studies has been difficult, given the rare nature of R/R PMBL and poor survival at relapse. Many older studies were small, retrospective, and likely included patients misclassified with PMBL as defined by current standards.2 The National Comprehensive Cancer Network guidelines recommend that patients with R/R disease who are ineligible for or nonresponsive to HCT should consider enrollment in clinical trials or receive second-line regimens, palliative radiation, or supportive care. Nevertheless, the current treatment options for these predominantly young patients with R/R PMBL are limited and include mostly chemotherapies.8 Minimizing chemotherapy exposure and long-term toxicity is particularly important in this patient population. On the basis of results from phase I and II studies, pembrolizumab and chimeric antigen receptor T-cell therapy are listed as treatment options for R/R PMBL in the National Comprehensive Cancer Network guidelines.8,33
The majority of patients in our study were auto-HCT ineligible at enrollment; only four patients had prior auto-HCT. Yet, 11 patients were successfully bridged to HCT after nivolumab plus BV combination therapy; all 10 patients who had reached the 100-day post-transplantation assessment, including five who received auto-HCT, achieved and remained in CR. These outcomes suggest that stem cell mobilization and auto-HCT are feasible after treatment with nivolumab plus BV, and auto-HCT may still be a viable option for patients who achieve objective responses after the combination therapy, even in those with chemotherapy-refractory disease.
Furthermore, as patients with R/R PMBL can receive consolidative allo-HCT for curative intent, more effective and less toxic bridging therapies are critical. Given the high response rate of this combination regimen in R/R PMBL, we believe this is a promising strategy, particularly for patients with chemotherapy-refractory disease.
In conclusion, for patients with R/R PMBL, nivolumab plus BV treatment demonstrated a high and sustained investigator-assessed ORR of 73%, with a 37% CR rate. TRAEs were consistent with the safety profiles of nivolumab and BV treatment alone. The combination of nivolumab and BV may be synergistic and is highly active in patients with R/R PMBL, serving as a potential bridge to other consolidative therapies of curative intent.
ACKNOWLEDGMENT
The authors thank all co-investigators and the patients and families who participated in the trial. Medical writing and editorial support was provided by Janice Zhou, Caudex, funded by Bristol-Myers Squibb.
APPENDIX
Methods
For nivolumab, no dose modification was allowed. Criteria for dose delay for all drug-related adverse events (AEs) included:
Any grade 2 or greater nonskin drug-related AE, except grade 2 drug-related fatigue or laboratory abnormalities
-
Any grade 3 drug-related laboratory abnormality except for lymphopenia and leukopenia, and the following:
° For patients with normal baseline AST, ALT, or total bilirubin, delay dosing for drug-related grade 2 or greater toxicity
° For patients with grade 1 baseline AST, ALT, or total bilirubin, delay dosing for drug-related grade 3 or greater toxicity
Any AE, laboratory abnormality, or intercurrent illness at the investigator’s discretion
Health-Related Quality-of-Life Results
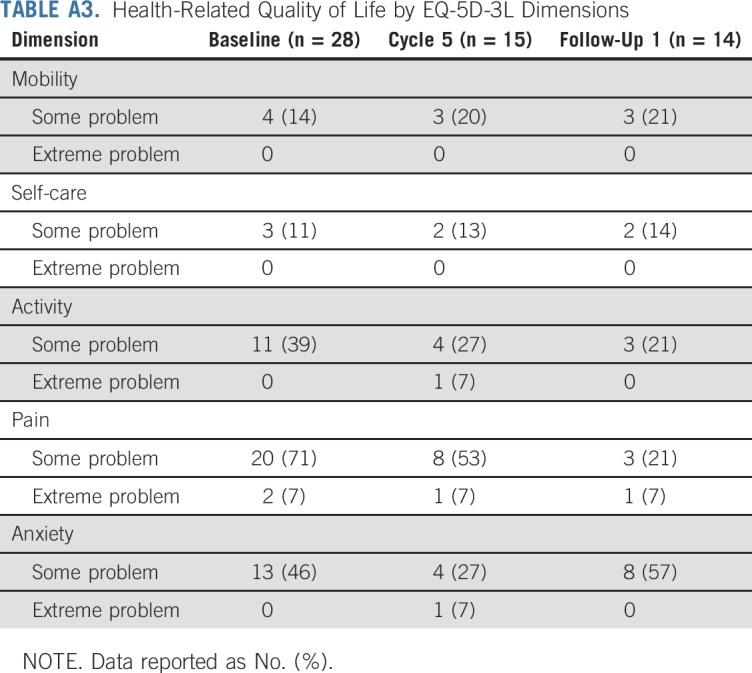
At baseline, most patients (71%) reported some problems, and two (7%) had extreme problems in the pain domain of the EQ-5D-3L questionnaire. Some problems were also reported in the Activity (39%) and Anxiety (46%) domains, but few had problems with Mobility (14%) or Self-care (11%). During subsequent study visits and at follow-up visits, fewer patients reported pain or activity-related problems compared with baseline (Appendix Table A3).
FIG A1.
Patient disposition. (*) Maximum clinical benefit was determined by the investigator, including eight patients with subsequent transplantation, one patient with consolidation radiotherapy, and one patient with no additional treatment. (†) Adverse events unrelated to the study drug included one patient with GI perforation that led to hospitalization and one patient with sepsis. (‡) Other reasons included three patients proceeding to transplantation.
TABLE A1.
Dose Modifications for Brentuximab Vedotin Because of AEs
TABLE A2.
Objective and Best Overall Response
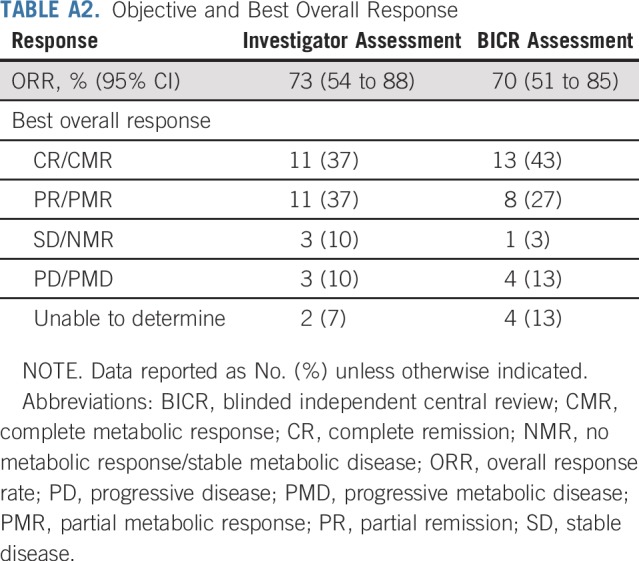
TABLE A3.
Health-Related Quality of Life by EQ-5D-3L Dimensions
Footnotes
Supported by Bristol-Myers Squibb, in collaboration with Seattle Genetics. Direct funding was provided by Bristol-Myers Squibb through the joint financial support of Bristol-Myers Squibb and Seattle Genetics.
An interim analysis of CheckMate 436 was presented at the 60th American Society of Hematology Annual Meeting, San Diego, CA, December 1-4, 2018. Results from the primary analyses were presented at the 24th European Hematology Association Annual Meeting, Amsterdam, the Netherlands, June 13-16, 2019, and the International Conference on Malignant Lymphoma, Lugano, Switzerland, June 18-22, 2019.
Clinical trial information: NCT02581631.
AUTHOR CONTRIBUTIONS
Conception and design: Pier Luigi Zinzani, Stephen Francis, Manish Sharma
Provision of study materials or patients: Armando Santoro, Giuseppe Gritti, Pauline Brice, John Kuruvilla, Nathalie A. Johnson, Neha Mehta-Shah
Collection and assembly of data: Pier Luigi Zinzani, Giuseppe Gritti, John Kuruvilla, Nathalie A. Johnson, Neha Mehta-Shah, Manish Sharma, Alison J. Moskowitz
Data analysis and interpretation: Pier Luigi Zinzani, Armando Santoro, Giuseppe Gritti, Pauline Brice, Paul M. Barr, John Kuruvilla, David Cunningham, Justin Kline, Neha Mehta-Shah, Thomas Manley, Stephen Francis, Manish Sharma, Alison J. Moskowitz
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Nivolumab Combined With Brentuximab Vedotin for Relapsed/Refractory Primary Mediastinal Large B-Cell Lymphoma: Efficacy and Safety From the Phase II CheckMate 436 Study
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/site/ifc.
Pier Luigi Zinzani
Consulting or Advisory Role: Sanofi, Verastem, Celltrion, Gilead Sciences, Janssen-Cilag, Bristol-Myers Squibb, Servier, Sandoz, MSD, Immune Design, Celgene, Portola Pharmaceuticals, Roche, EUSA Pharma, Kyowa Hakko Kirin
Speakers' Bureau: Verastem, Celltrion, Gilead Sciences, Janssen-Cilag, Bristol-Myers Squibb, Servier, Sandoz, MSD, Immune Design, Celgene, Portola Pharmaceuticals, Roche, EUSA Pharma, Kyowa Hakko Kirin
Armando Santoro
Consulting or Advisory Role: Bristol-Myers Squibb, Servier, Gilead Sciences, Pfizer, Eisai, Bayer, MSD
Speakers' Bureau: Takeda, Roche, AbbVie, Amgen, Celgene, AstraZeneca, ArQule, Lilly, Sandoz, Novartis
Giuseppe Gritti
Consulting or Advisory Role: Autolus, BiovelocITA
Speakers' Bureau: Amgen
Research Funding: Gilead Sciences
Travel, Accommodations, Expenses: Roche, AbbVie, Becton Dickinson
Pauline Brice
Research Funding: Takeda, BMS, Roche, Amgen, AbbVie/Genentech
Paul M. Barr
Consulting or Advisory Role: Pharmacyclics, AbbVie, Seattle Genetics, Genentech, Celgene, Novartis, Infinity Pharmaceuticals, Janssen, Merck, TG Therapeutics
Research Funding: Pharmacyclics (Inst)
John Kuruvilla
Honoraria: AbbVie, Bristol-Myers Squibb, Amgen, AstraZeneca, Celgene, Gilead, Janssen, Karyopharm Therapeutics, Merck, Novartis, Roche, Seattle Genetics
Consulting or Advisory Role: AbbVie, Bristol-Myers Squibb, Gilead Sciences, Karyopharm Therapeutics, Merck, Roche, Seattle Genetics
Research Funding: Janssen, Roche
David Cunningham
Research Funding: AstraZeneca (Inst), Amgen (Inst), Sanofi (Inst), Merrimack (Inst), Celgene (Inst), MedImmune (Inst), Bayer (Inst), 4SC (Inst), Clovis Oncology (Inst), Lilly (Inst), Janssen (Inst), Merck (Inst)
Justin Kline
Honoraria: Cardinal Health
Consulting or Advisory Role: Merck, Seattle Genetics
Speakers' Bureau: Kite/Gilead
Research Funding: Merck, ITeos Therapeutics
Travel, Accommodations, Expenses: Merck, Bristol-Myers Squibb, ITeos Therapeutics
Nathalie A. Johnson
Honoraria: Genentech, Merck
Consulting or Advisory Role: Genentech, Merck, Bristol-Myers Squibb, AbbVie
Research Funding: AbbVie
Neha Mehta-Shah
Consulting or Advisory Role: Kyowa Hakko Kirin
Research Funding: Bristol-Myers Squibb (Inst), Genentech (Inst), Celgene (Inst), Verastem (Inst)
Thomas Manley
Employment: Seattle Genetics
Stock and Other Ownership Interests: Seattle Genetics
Patents, Royalties, Other Intellectual Property: I am listed as a co-inventor on multiple patent applications from Seattle Genetics (Inst)
Stephen Francis
Employment: Bristol-Myers Squibb
Stock and Other Ownership Interests: Bristol-Myers Squibb
Manish Sharma
Employment: Regeneron
Stock and Other Ownership Interests: Regeneron
Alison J. Moskowitz
Honoraria: Seattle Genetics
Consulting or Advisory Role: Seattle Genetics, Erytech Pharma, Bristol-Myers Squibb, Cell Medica, ADC Therapeutics, Takeda, miRagen, Kyowa Hakko Kirin
Research Funding: Incyte (Inst), Seattle Genetics (Inst), Merck (Inst), Bristol-Myers Squibb (Inst)
No other potential conflicts of interest were reported.
REFERENCES
- 1. National Cancer Institute Surveillance, Epidemiology, and End Results Program: Primary mediastinal (thymic) large B-cell lymphoma. https://seer.cancer.gov/seertools/hemelymph/51f6cf56e3e27c3994bd5318/
- 2.Dunleavy K, Wilson WH. Primary mediastinal B-cell lymphoma and mediastinal gray zone lymphoma: Do they require a unique therapeutic approach? Blood. 2015;125:33–39. doi: 10.1182/blood-2014-05-575092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zinzani PL, Stefoni V, Finolezzi E, et al. Rituximab combined with MACOP-B or VACOP-B and radiation therapy in primary mediastinal large B-cell lymphoma: A retrospective study. Clin Lymphoma Myeloma. 2009;9:381–385. doi: 10.3816/CLM.2009.n.074. [DOI] [PubMed] [Google Scholar]
- 4.Rieger M, Osterborg A, Pettengell R, et al. Primary mediastinal B-cell lymphoma treated with CHOP-like chemotherapy with or without rituximab: Results of the Mabthera International Trial Group study. Ann Oncol. 2011;22:664–670. doi: 10.1093/annonc/mdq418. [DOI] [PubMed] [Google Scholar]
- 5.Giulino-Roth L, O’Donohue T, Chen Z, et al. Outcomes of adults and children with primary mediastinal B-cell lymphoma treated with dose-adjusted EPOCH-R. Br J Haematol. 2017;179:739–747. doi: 10.1111/bjh.14951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dunleavy K, Pittaluga S, Maeda LS, et al. Dose-adjusted EPOCH-rituximab therapy in primary mediastinal B-cell lymphoma. N Engl J Med. 2013;368:1408–1416. doi: 10.1056/NEJMoa1214561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kuruvilla J, Pintilie M, Tsang R, et al. Salvage chemotherapy and autologous stem cell transplantation are inferior for relapsed or refractory primary mediastinal large B-cell lymphoma compared with diffuse large B-cell lymphoma. Leuk Lymphoma. 2008;49:1329–1336. doi: 10.1080/10428190802108870. [DOI] [PubMed] [Google Scholar]
- 8. doi: 10.6004/jnccn.2022.0015. National Comprehensive Cancer Network: NCCN clinical practice guidelines in oncology: B-cell lymphomas. Version 2.2019, 2019. https://www.nccn.org/professionals/physician_gls/ [DOI] [PubMed]
- 9.Green MR, Monti S, Rodig SJ, et al. Integrative analysis reveals selective 9p24.1 amplification, increased PD-1 ligand expression, and further induction via JAK2 in nodular sclerosing Hodgkin lymphoma and primary mediastinal large B-cell lymphoma. Blood. 2010;116:3268–3277. doi: 10.1182/blood-2010-05-282780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lenz G, Wright GW, Emre NC, et al. Molecular subtypes of diffuse large B-cell lymphoma arise by distinct genetic pathways. Proc Natl Acad Sci USA. 2008;105:13520–13525. doi: 10.1073/pnas.0804295105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Roemer MG, Advani RH, Ligon AH, et al. PD-L1 and PD-L2 genetic alterations define classical Hodgkin lymphoma and predict outcome. J Clin Oncol. 2016;34:2690–2697. doi: 10.1200/JCO.2016.66.4482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ansell SM, Minnema MC, Johnson P, et al. Nivolumab for relapsed/refractory diffuse large B-cell lymphoma in patients ineligible for or having failed autologous transplantation: A single-arm, phase II study. J Clin Oncol. 2019;37:481–489. doi: 10.1200/JCO.18.00766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Twa DD, Chan FC, Ben-Neriah S, et al. Genomic rearrangements involving programmed death ligands are recurrent in primary mediastinal large B-cell lymphoma. Blood. 2014;123:2062–2065. doi: 10.1182/blood-2013-10-535443. [DOI] [PubMed] [Google Scholar]
- 14. Bristol-Myers Squibb: Opdivo (nivolumab) prescribing information. https://packageinserts.bms.com/pi/pi_opdivo.pdf.
- 15.Armand P, Rodig S, Melnichenko V, et al. Pembrolizumab in patients with relapsed or refractory primary mediastinal large B-Cell lymphoma (PMBCL): Data from the Keynote-013 and Keynote-170 studies. Blood. 2018;132:228. [Google Scholar]
- 16.Higgins JP, Warnke RA. CD30 expression is common in mediastinal large B-cell lymphoma. Am J Clin Pathol. 1999;112:241–247. doi: 10.1093/ajcp/112.2.241. [DOI] [PubMed] [Google Scholar]
- 17.Steidl C, Gascoyne RD. The molecular pathogenesis of primary mediastinal large B-cell lymphoma. Blood. 2011;118:2659–2669. doi: 10.1182/blood-2011-05-326538. [DOI] [PubMed] [Google Scholar]
- 18. Seattle Genetics: Adcetris (brentuximab vedotin) prescribing information. https://www.seattlegenetics.com/application/files/6515/4238/3292/adcetris_USPI.pdf.
- 19.Heiser RA, Grogan BM, Manlove LS, et al. CD30+ T regulatory cells, but not CD30+ CD8 T cells, are impaired following brentuximab vedotin treatment in vitro and in vivo. Cancer Res. 78, 2018 (suppl 13; abstr1789) [Google Scholar]
- 20.Herrera AF, Moskowitz AJ, Bartlett NL, et al. Interim results of brentuximab vedotin in combination with nivolumab in patients with relapsed or refractory Hodgkin lymphoma. Blood. 2018;131:1183–1194. doi: 10.1182/blood-2017-10-811224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Cao AT, Law C-L, Gardai SJ, et al: Brentuximab vedotin-driven immunogenic cell death enhances antitumor immune responses, and is potentiated by PD1 inhibition in vivo. Cancer Res 77, 2017(suppl 13; abstr 5588)
- 22.Zinzani PL, Pellegrini C, Chiappella A, et al. Brentuximab vedotin in relapsed primary mediastinal large B-cell lymphoma: Results from a phase 2 clinical trial. Blood. 2017;129:2328–2330. doi: 10.1182/blood-2017-01-764258. [DOI] [PubMed] [Google Scholar]
- 23. Diefenbach CS, Hong F, David KA, et al: A phase I study with an expansion cohort of the combination of ipilimumab and nivolumab and brentuximab vedotin in patients with relapsed/refractory Hodgkin lymphoma: A trial of the ECOG-ACRIN Cancer Research Group (E4412 Arms D and E). Blood 128:1106, 2016 (suppl 1) [Google Scholar]
- 24.Advani R, Moskowitz A, Bartlett N, et al. Phase 1/2 study of brentuximab vedotin in combination with nivolumab in patients with relapsed or refractory classic Hodgkin lymphoma: Part 3 (concurrent dosing) results and updated progression-free survival results from parts 1 and 2 (staggered dosing). Blood 128:1635, 2018 (suppl 1)
- 25.Zhao X, Suryawanshi S, Hruska M, et al. Assessment of nivolumab benefit-risk profile of a 240-mg flat dose relative to a 3-mg/kg dosing regimen in patients with advanced tumors. Ann Oncol. 2017;28:2002–2008. doi: 10.1093/annonc/mdx235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. European Medicines Agency: Assessment report: Opdivo. https://www.ema.europa.eu/en/documents/assessment-report/opdivo-epar-public-assessment-report_en.pdf.
- 27.Cheson BD, Ansell S, Schwartz L, et al. Refinement of the Lugano classification lymphoma response criteria in the era of immunomodulatory therapy. Blood. 2016;128:2489–2496. doi: 10.1182/blood-2016-05-718528. [DOI] [PubMed] [Google Scholar]
- 28. US Department of Health and Human Services: Common Terminology Criteria for Adverse Events (CTCAE), v5.0. https://ctep.cancer.gov/protocolDevelopment/electronic_applications/ctc.htm#ctc_50.
- 29.Cheson BD, Fisher RI, Barrington SF, et al. Recommendations for initial evaluation, staging, and response assessment of Hodgkin and non-Hodgkin lymphoma: The Lugano classification. J Clin Oncol. 2014;32:3059–3068. doi: 10.1200/JCO.2013.54.8800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Van Heertum RL, Scarimbolo R, Wolodzko JG, et al. Lugano 2014 criteria for assessing FDG-PET/CT in lymphoma: An operational approach for clinical trials. Drug Des Devel Ther. 2017;11:1719–1728. doi: 10.2147/DDDT.S136988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Zinzani PL, Thieblemont C, Melnichenko V, et al: Efficacy and safety of pembrolizumab in relapsed/refractory primary mediastinal large B-cell lymphoma (rrPMBCL): Interim analysis of the KEYNOTE-170 phase 2 trial. Presented at the International Conference on Malignant Lymphoma. Lugano, Switzerland. June 14–17, 2017. [Google Scholar]
- 32.Zinzani PL, Ribrag V, Moskowitz CH, et al. Safety and tolerability of pembrolizumab in patients with relapsed/refractory primary mediastinal large B-cell lymphoma. Blood. 2017;130:267–270. doi: 10.1182/blood-2016-12-758383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Locke FL, Ghobadi A, Jacobson CA, et al. Long-term safety and activity of axicabtagene ciloleucel in refractory large B-cell lymphoma (ZUMA-1): A single-arm, multicentre, phase 1-2 trial. Lancet Oncol. 2019;20:31–42. doi: 10.1016/S1470-2045(18)30864-7. [DOI] [PMC free article] [PubMed] [Google Scholar]