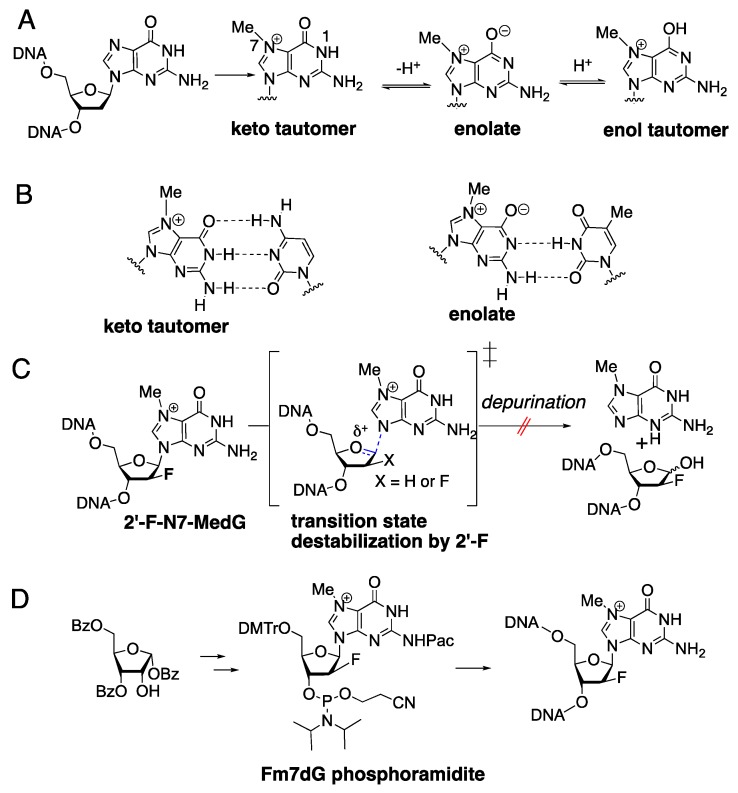
Figure 1.
Base-pairing properties of N7-methyl-2-deoxyguanosine (m7dG). (A) Methylation of guanine and tautomers of m7dG. (B) Base pairings of the keto tautomer and the enolate of m7dG with dC and dT, respectively. (C) Prevention of the cleavage of the C–N glycosidic bond of m7dG via transition state destabilization. (D) Synthesis of N7-methyl-2′-fluorine-2′-deoxyguanosine (Fm7dG)-containing DNA from a ribose derivative. The 2′-fluorination prevented spontaneous depurination of Fm7dG and allowed site-specific incorporation of the lesion into DNA through solid-phase DNA synthesis and ultramild deprotection (50 mM K2CO3 in methanol at 25 °C).