Abstract
The study investigated the antimicrobial activity of the essential oil extract of Ocimum gratissimum L. (EOOG) against multiresistant microorganisms in planktonic and biofilm form. Hydrodistillation was used to obtain the EOOG, and the analysis of chemical composition was done by gas chromatography coupled with mass spectrometry (GC/MS) and flame ionization detection (GC/FID). EOOG biological activity was verified against isolates of Staphylococcus aureus and Escherichia coli, using four strains for each species. The antibacterial action of EOOG was determined by disk diffusion, microdilution (MIC/MBC), growth curve under sub-MIC exposure, and the combinatorial activity with ciprofloxacin (CIP) and oxacillin (OXA) were determined by checkerboard assay. The EOOG antibiofilm action was performed against the established biofilm and analyzed by crystal violet, colony-forming unit count, and SEM analyses. EOOG yielded 1.66% w/w, with eugenol as the major component (74.83%). The MIC was 1000 µg/mL for the most tested strains. The growth curve showed a lag phase delay for both species, mainly S. aureus, and reduced the growth level of E. coli by half. The combination of EOOG with OXA and CIP led to an additive action for S. aureus. A significant reduction in biofilm biomass and cell viability was verified for S. aureus and E. coli. In conclusion, EOOG has relevant potential as a natural alternative to treat infections caused by multiresistant strains.
Keywords: antimicrobial activity, multiresistant microorganisms, ciprofloxacin, oxacillin, MDR bacteria, antibiofilm activity
1. Introduction
The rise of infections caused by multidrug-resistant (MDR) bacteria has become a worrying public health problem [1]. The occurrence of these strains is frequently related to strong selective pressure caused by indiscriminate and inadequate use of antibiotics in hospitals [2]. However, this is not a problem restricted to a nosocomial environment, as it can also be related to infections caused by foodborne pathogens [3]. In addition, another important issue is the ability of several bacteria to form a complex multicellular structure, called biofilms [4]. These tridimensional communities are built mainly by bacterial cells embedded into extracellular polysaccharides secreted by themselves [5], and this arrangement reduces the effectiveness of antimicrobial agents [6,7]. Thus, antibiotic resistance reduces the number of therapeutic options and causes an increase in hospitalization costs/time of patients, thus increasing the morbimortality rates [8].
As an alternative therapeutic source, essential oils (EOs) extracted from plants are qualified as an important biotechnological product to be explored for pharmaceuticals and food companies [9,10,11,12]. EOs are constituted by volatile organic products of various classes, such as aldehydes, terpenes, and phenolic compounds, synthesized by a secondary metabolism from different parts of plants [13]. Many studies have documented EOs to be effective antimicrobial agents against several pathogens [14,15,16]. Usually, this EO effect is closely related to the capability of permeabilizing and/or destroying membrane integrity, leading to the leakage of intracellular substances [17,18,19]. Moreover, since the last decade, the antibiofilm activity of EOs has been widely explored and reported in the literature [20,21,22,23].
O. gratissimum L. (EOOG), or clove basil, belongs to the Lamiaceae family, and it is commonly found in Africa, Asia, and South America [24]. This species is included among the 71 plants described in the National List of Medicinal Plants of Interest to the Unified Health System (RENISUS), created in 2009 in Brazil [25]. The use of leaf infusion has already been reported in folk medicine to treat fever, flu, and kidney problems [26]. Other reports have shown that the chemical composition of EOOG depends on the climate zone and soil type wherein the species is cultivated [27], and this feature can influence biological activity, since the major constituents can be altered [28]. Among the biological applications of EOOG, the antimicrobial properties have been studied separately or associated with conventional antibiotics, reestablishing the sensitivity of MDR bacteria [29,30,31]. Therefore, the aim of this study was to characterize the chemical constitution of EOOG extracted from dried leaves and explore in vitro the antibacterial and antibiofilm activity against S. aureus from clinical samples and E. coli isolates from fresh tilapia fillets commercialized in the retail trade of Sobral, Brazil, both of which having multiresistance phenotypes.
2. Results
2.1. Extraction and Chemical Composition of the EOOG
The dried leaves of O. gratissimum L. submitted to hydrodistillation in a Clevenger apparatus showed an EO yield of 1.66% (w/w). The chemical composition of the EOOG and their respective Kovats indices (KIs) are listed in Table 1.
Table 1.
Chemical composition from the essential oil (EO) of the Ocimum gratissimum L. (EOOG) leaves.
| Peak | Compounds a | Chemical Class | KIL b | KIC c | % |
|---|---|---|---|---|---|
| 1 | α-Pinene | HM d | 939 | 943 | 0.08 |
| 2 | Sabinene | HM | 975 | 982 | 0.17 |
| 3 | β-Pinene | HM | 979 | 986 | 0.43 |
| 4 | Myrcene | HM | 990 | 995 | 0.14 |
| 5 | 1,8-Cineole | OM e | 1031 | 1040 | 15.16 |
| 6 | (E)-β-Ocimene | HM | 1050 | 1053 | 0.10 |
| 7 | Linalool | OM | 1096 | 1103 | 0.34 |
| 8 | δ-Terpineol | OM | 1166 | 1174 | 0.12 |
| 9 | Terpinen-4-ol | OM | 1177 | 1184 | 0.16 |
| 10 | α-Terpineol | OM | 1188 | 1196 | 0.31 |
| 11 | Eugenol | PH f | 1359 | 1365 | 74.83 |
| 12 | (E)-Caryophyllene | HS g | 1419 | 1427 | 2.20 |
| 13 | α-Humulene | HS | 1454 | 1461 | 0.32 |
| 14 | γ-Muurolene | HS | 1479 | 1488 | 0.51 |
| 15 | β-Selinene | HS | 1490 | 1493 | 2.82 |
| 16 | α-Selinene | HS | 1498 | 1501 | 0.85 |
| 17 | 7-Epi-α-selinene | HS | 1522 | 1525 | 0.26 |
| 18 | Spathulenol | OS h | 1578 | 1584 | 0.07 |
| 19 | Caryophyllene oxide | OS | 1583 | 1590 | 0.55 |
| Total | 99.42 | ||||
a Compounds ordered by their elution from an HP-5MS column. b Kovats indices from the literature [32]. c Kovats indices calculated against n-alkanes (C9–C30) on an HP-5MS column. d Hydrocarbon monoterpenes. e Oxygenated monoterpenes. f Phenylpropanoid. g Hydrocarbon sesquiterpenes. h Oxygenated sesquiterpenes.
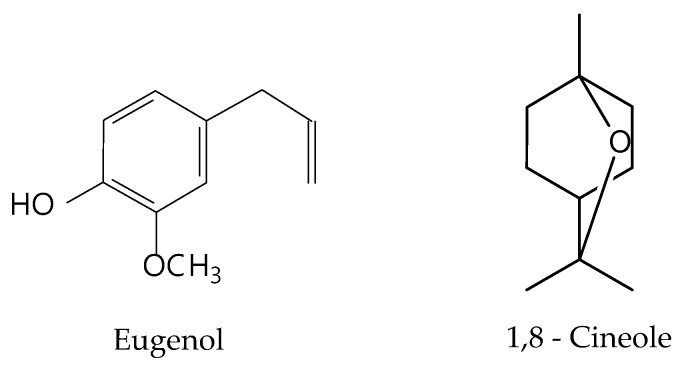
Analysis by gas chromatography coupled with mass spectrometry (GC/MS) and flame ionization detection (GC/FID) resulted in the identification of 19 compounds in the sample, representing 99.42% of the constituents. The most abundant volatile component was eugenol (74.83%), followed by 1,8-cineole (15.16%) (Figure 1). However, several classes of chemical compounds were found, mainly phenylpropanoid (74.83%), oxygenated monoterpenes (16.09%), hydrocarbon sesquiterpenes (6.96%), and minor amounts of hydrocarbon monoterpenes (0.92%) and oxygenated sesquiterpenes (0.62%).
Figure 1.
Chemical structures of the major constituents from the EO of Ocimum gratissimum L. (EOOG) dry leaves.
2.2. Antibacterial Activity
The results demonstrated that EOOG has significant antimicrobial activity against all tested strains. The EO had a large inhibition zone diameter (IZD) against S. aureus cells, among which, 5B and 2B had higher (20 mm) and lower (14 mm) IZD, respectively. However, this promising effect decreased slightly against E. coli cells, and the IZD values were kept around 12–13 mm. According to the microdilution method, the minimum inhibitory concentration (MIC) of EOOG was observed at 1000 µg/mL indistinctly for S. aureus and E. coli cells, with the exception of 5B, which had twice the amount, 2000 µg/mL. It is important that salient E. coli strains had the same bacteriostatic and bactericidal concentration levels (MIC and MBC), at 1000 µg/mL. Therefore, S. aureus strains presented double the quantity (2000 µg/mL) necessary to eliminate the cells (2B, 5B, and 7B). All IZDs, as well as MIC and MBC values of EOOG, are shown in Table 2.
Table 2.
Antibacterial activity of the EOOG against S. aureus and E. coli strains by the paper disk diffusion test and the microdilution method.
| Staphylococcus aureus | IZD (mm) 1 | MIC (µg/mL) 2 | MBC (µg/mL) 3 |
| ATCC 6538 | 17 | 1000 | 1000 |
| 2B | 14 | 1000 | 2000 |
| 5B | 20 | 2000 | 2000 |
| 7B | 15 | 1000 | 2000 |
| Escherichia coli | |||
| ATCC 11303 | 12 | 1000 | 1000 |
| P12 | 13 | 1000 | 1000 |
| P25 | 12 | 1000 | 1000 |
| P36 | 13 | 1000 | 1000 |
Notes: IZD 1: Inhibition zones diameter using 6 mm disks. MIC 2: Minimum inhibitory concentration. MBC 3: Minimum bactericidal concentration.
2.3. Bacterial Growth Curve
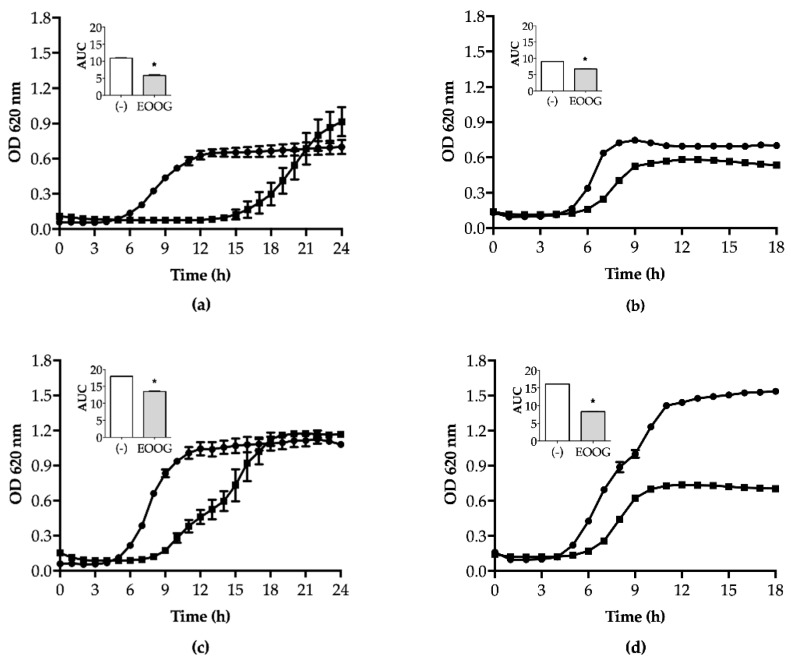
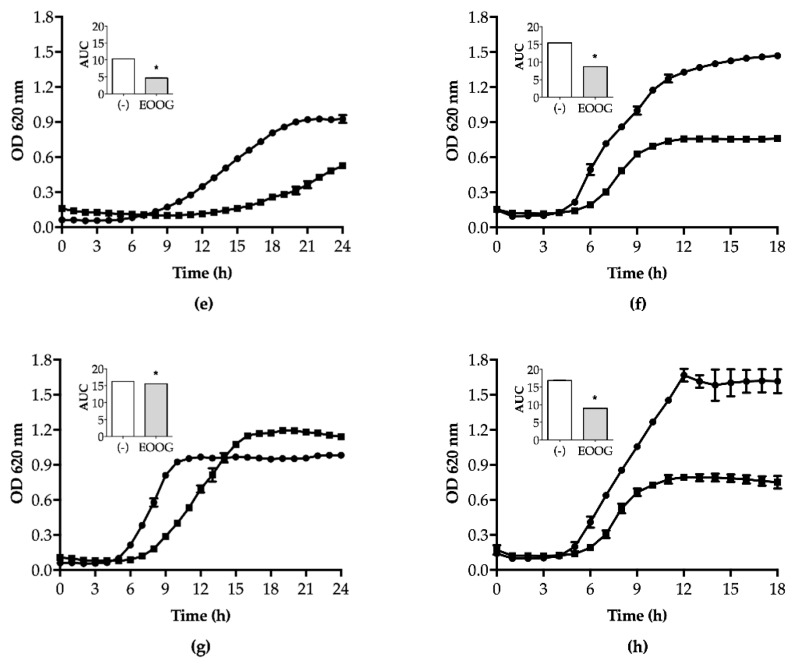
The majority of strains in the control group, media broth only, clearly presented different growth, adaptation (lag), exponential (log), and stationary phases, as expected. However, the presence of EOOG (1/2 MIC) interfered with the performance of these microorganisms during curve development, as shown in Figure 2.
Figure 2.
Effect of EOOG (1/2 MIC) on the bacterial growth curve (line graph) of S. aureus (a) ATCC 6538, (c) 2B, (e) 5B, and (g) 7B and of E. coli, (b) ATCC 11303, (d) P12, (f) P25, and (h) P36. The growth level of the EOOG-treated (■/grey bar) and -untreated (●/white bar) cells was quantified by the area under the curve (AUC). * Statistically different by ANOVA (p < 0.01) compared to the control group.
S. aureus cells left the lag phase after 5 h of incubation, which was followed by a 5 h exponential phase before reaching the stationary stage, which consisted of 12 h of development. The presence of EOOG delayed the beginning of the multiplication step, prolonging the lag phase. However, at the end of growth (24 h), the optical density (OD) was similar between the control and the EOOG group. However, S. aureus 5B reached the stationary phase only after 21 h of incubation. The EOOG reduced the bacterial growth to 50% of the control OD at the end of the curve (Figure 2).
All E. coli displayed three growth phases after 18 h. Except for the ATCC strain, they presented a short lag and an expressive log growth, reaching a high level (>1,500 UA) of absorbance at the end of the curve. The influence of EOOG on E. coli strains caused a reduction in cell density, which was determined by OD values. The establishment of the stationary phase was at least 50% below the control curve, except for the ATCC strain, which had a 20% reduction only. The growth level was determined by the area under the curve (AUC), and the treated groups showed a significant reduction (p < 0.01) for all tested strains, as shown by bar graphs in Figure 2.
2.4. Combination between EOOG and Antibiotics
The 5B and P12 strains, which presented resistance profiles by the VITEK®2 system (BioMérieux, Marcy-l’Étoile, France), were used to evaluate the EOOG and antibiotics interaction performed by checkerboard assay. S. aureus (5B) and E. coli (P12) obtained the same MIC for ciprofloxacin (CIP), 62.50 µg/mL. The OXA bacteriostatic concentration was determined only against 5B, which was 2000 µg/mL, since this kind of antibiotic is not clinically recommended to treat E. coli-related illnesses.
The combination of the EOOG with antibiotics, CIP and OXA had fractional inhibitory concentration index (FICi) of 0.516 and 0.562, respectively, showing additive effect interaction between them (Table 3). In both cases the combination with antibiotics allowed to decrease by half the EO concentration, only. Furthermore, the CIP and OXA doses were 16- and 64-fold less, respectively, than individual use. In relation to the P12 strain, the effect of the EOOG/CIP combination was antagonistic (FICi = 2). The individual and combined MIC of the EO and antibiotic, as well as the FICi interpretation and reduction in drug concentration, are shown in Table 3.
Table 3.
Fractional inhibitory concentrations index (FICi) of OXA and CIP combined with EOOG for S. aureus (5B) and E. coli (P12) microorganisms.
| Microorganisms | Combination | MIC (µg/mL) | FICi | Interpretation | Drug Reduction | |
|---|---|---|---|---|---|---|
| Individual | Combined | |||||
| S. aureus (5B) | EOOG | 2000 | 1000 | 0.516 | Additive | 2x |
| OXA | 2000 | 31.25 | 64x | |||
| EOOG | 2000 | 1000 | 0.562 | Additive | 2x | |
| CIP | 62.50 | 3.90 | 16x | |||
| E. coli (P12) | EOOG | 1000 | 1000 | 2.000 | Antagonistic | NR |
| CIP | 62.50 | 62.50 | NR | |||
Notes: NR: no reduction. OXA: oxacillin. CIP: ciprofloxacin.
2.5. Activity on Preformed Biofilm
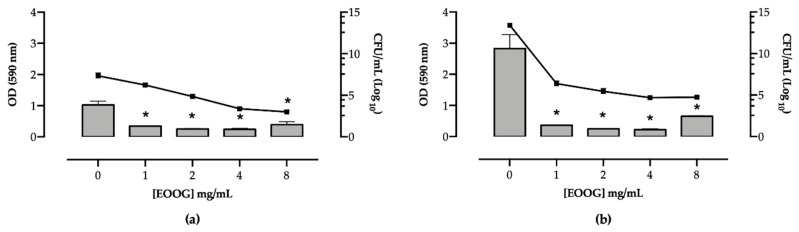
The EOOG activity against the biofilm of S. aureus (5B) and E. coli (P12), pre-established for 24 and 12 h, respectively, was evaluated according to biomass by the crystal violet method and cell enumeration by CFU count (Figure 3).
Figure 3.
Antibiofilm activity of different concentrations of EO of Ocimum gratissimum L. against preformed biofilms of S. aureus 5B (a) and E. coli P12 (b), respectively. Biomass quantification by crystal violet staining (OD 590 nm, bars) and cell enumeration by colony count (log10 CFU/mL, lines). * Statistically different by ANOVA (p < 0.01) compared to untreated cells.
The strains tested have an equally high capacity for biofilm formation. However, E. coli produced 2.75-fold more biomass and an additional six log units of viable cells into the biofilm than S. aureus, even when less time (12 h) is given for biofilm development. After EOOG treatment (4 h), these features decreased significantly for both 5B and P12 strains. At a lower concentration, 1 mg/mL, the EOOG-treated biofilm presented a great reduction, mainly for the E. coli strain, which decreased the biomass by 12-fold, and declined in the number of viable cells in the biofilm by eight log10 units. According to S. aureus, these values were discretely lower, the biomass reduced 4-fold, and the CFU count decreased by four log10 units. In both strains, the EOOG was unable to eradicate the biofilm, even at a higher concentration, where some remaining viable cells were detectable.
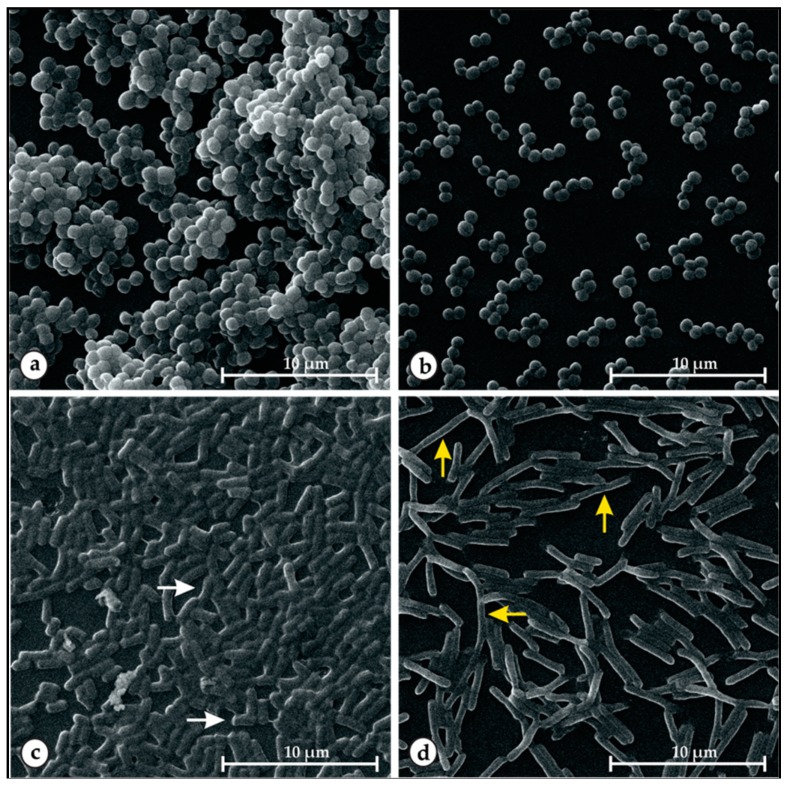
2.6. Scanning Electron Microscopy
The preformed biofilm structure of S. aureus 5B (24 h) and E. coli P12 (12 h), treated and untreated with EOOG, were evaluated through scanning electron microscopy (SEM). Comparative analysis of SEM showed a significant reduction in cell density in the biofilm after 4 h of EOOG treatment when compared to control conditions in both strains (Figure 4).
Figure 4.
Scanning electron microscopy images of preformed biofilms of S. aureus (5B) and E. coli (P12) treated with the EO of Ocimum gratissimum L. (EOOG) at MIC doses. Untreated 5B (a) and P12, (c) and EOOG-treated 5B, (b) and P12 (d) cells. In the control condition, E. coli presented rod-shaped (white arrow) cells, and after EOOG treatment, the P12 cells displayed an elongated (yellow arrow) morphology status.
In control groups, it is possible to verify cell clusters forming a well-defined three-dimensional structure, mainly for 5B. EOOG treatment at MIC doses was able to disrupt the bacterial community of both strains, reducing the number of adsorbed cells in the biofilm. EOOG-treated P12 cells were clearly found elongated compared to the cells in the control condition, as shown in Figure 4c,d, respectively.
3. Discussion
In the present study, it was found that dried leaves of O. gratissimum L. collected from Alto da Pipira, located in Maranhão State, Brazil, obtained yield of 1.66% w/w. Similar amount (1.12% w/w) was extracted from dry aerial parts of O. gratissimum cultivated in Londrina, State of Paraná, Brazil [33]. Yield of 0.21% w/w was also found using fresh leaves and branches obtained from Uganda, East African country [34]. Thus, the type of raw material used for extraction process, dry or fresh samples, may have impact on the essential oil yield. It was possible to identify 99.42% of the constituents, corresponding to 19 compounds, among which, eugenol (74.83%) and 1,8-cineole (15.16%) were the most abundant in the EOOG composition (Table 1). Other studies have also reported eugenol as the main constituent of this oil; however, the second most present component has been different, such as methyl eugenol, germacrene-D [35], terpinolene [36], citronellal [37], and α-ocimene [38].
It has been proven that vegetative stages can influence the constitution of EOs [39]. EOOG obtained in the Republic of Benin, West Africa, showed a difference in the quantification of prevalent constituents in relation to vegetative, pre-flowering, and flowering stages. In addition, diverging from the findings in the present research, other major constituents, p-cymene and thymol, were detected [40]. However, as seen above, a range of variables, such as seasonality, circadian rhythm, temperature, water availability, incidence of ultraviolet radiation, nutrient availability, altitude, and pathogen attack to plants, can interfere with a plant’s secondary metabolism and can considerably modify the OE composition [41].
Currently, due to the emergence and spread of MDR bacteria, it is necessary to search for new alternatives to combat this kind of pathogen, and to reverse the deficiency of antibiotic options against these infections [42]. The present investigation corroborated the promising applications of EOs as an alternative therapy against bacterial isolates, such as S. aureus and E. coli. Initially, the EOOG antimicrobial activity was accessed by disk diffusion and microdilution techniques to verify the sensibility profile of the tested strains. Thus, EOOG was effective mainly at concentrations between 1000 and 2000 µg/mL. However, different EOs or chemotypes from the same EO may have a wide MIC variety (from 50 to 6400 μg/mL) against foodborne pathogens [43].
Although the literature has shown Gram-negative bacteria to be more resistant to EOs compared to Gram-positive [44,45], it was possible to verify that EOOG had a greater effect against E. coli than S. aureus strains, as also demonstrated by the SEM images (Figure 4). This differential EO action on microbial species was also observed in the bacterial growth curve under sub-MIC EOOG exposure. The treated cells clearly had a lag phase extended compared to the control. However, the exponential growth was recovered, and S. aureus easily reached optical density measurements similar to the control, while E. coli had its growth level reduced by half, as shown by the AUC graphs in Figure 2.
The microbial behavior in response to EO exposure could be due to several reasons, one of which is the ability of small molecules, such as eugenol and 1,8-cineole (Figure 1), major compounds of EOOG, to interact with the outer surface of the cells [44,46,47]. In addition, some bacteria have an adaptive capacity to protect itself against the stress caused by OE attacks that alter the fatty acid profile of the cytoplasmic membrane [48,49]. Therefore, the main bactericidal action of the EO is attributed to the ability to interact with the cell membrane, altering its permeability, causing extravasation of intracellular constituents, and resulting in bacterial death [50]. Furthermore, EOs are made up of various compounds, and it is still believed that this makes it difficult to develop bacterial resistance when compared to antibiotics that have only one cell target, suggesting that EOs can be used to fight MDR bacteria [51].
Natural products also fit as components of interest for combinatorial therapies against MDR bacterial infections [52,53]. Previous studies have reported interactions between EO constituents and conventional antibiotics against E. coli MDR [54,55,56]. Oliva et al. (2018), using terpinen-4-ol-rich EO of Melaleuca alternifolia, found synergistic activity with OXA against methicillin-resistant S. aureus (MRSA) [57]. This effect might be attributed to the ability of the EO compound, such as eugenol/thymol, to increase the antibiotic permeation into the bacterial cell [55,58]. Although many researchers seek to demonstrate a synergism between EO and antibiotics [59,60,61,62], it should be considered that combinations that result in additive effects may be as effective as synergistic effects because, with minor amounts of antibiotics, they provide satisfactory effects [63], as shown by the 16- and 64-fold decreases in CIP and OXA concentrations, respectively, against the 5B strain. Concerning the P12 strain, EOOG combined with CIP was unable to alter the strain resistance, showing an antagonistic effect. However, EOOG in combination with other antibiotics should not be ruled out, since the majority EOOG compound (eugenol) has been explored in previous studies and has demonstrated a synergistic effect using penicillin and colistin [49,54].
Another applicability of EOs is related to biofilm disruption [64]. Since biofilm formation is a virulence factor for many microorganisms, such as S. aureus and E. coli, and increases tolerance to antimicrobial drugs, new strategies to combat them are sought [65,66]. Budzyńska et al. (2017) described the antibiofilm activity of EOs from cloves, which have a high eugenol percentage (86.2%), and were able to reduce S. aureus mono- and dual-species with a Candida albicans biofilm [67]. In other case, the EO from bay leaves, cloves, and berry peppers with more than 60% eugenol in their constitution show important activities against antibiotic-resistant biofilms of E. coli O157:H7 [68]. Combined with these results, this study shows that eugenol-rich EOOG is able to reduce biomass and the number of viable cells in biofilms from S. aureus and E. coli MDR.
The mechanism of action against these established cell clusters may be through the disruption of 3D structures and/or the direct killing of the bacteria in the biofilm [69,70,71]. Through SEM images (Figure 4), it can be noted that the cellular damage to S. aureus was less significant than that to E. coli, since the Gram-negative cell showed a considerable difference in cellular dimensions between EOOG-treated and -untreated bacteria. As shown before, this morphological status could be related to interference in the cell division process due to stress responses, including DNA damage and the inhibition of replication [72,73]. Previous research has also associated antibiofilm activity with the ability of these natural compounds to disturb the bacterial communication mechanism, quorum sensing, and the expression of virulence factors that are responsible for the survival characteristics of the biofilm [74,75,76].
4. Materials and Methods
4.1. Plant Material
Fresh leaves of O. gratissimum L. were collected in the morning at Alto da Pipira (5°26′4.07″ S, 47°17′45.83″ W), in the State of Maranhão, in the northeast region of Brazil. The botanical identification was performed by Prof. Dr. Eduardo Bezerra de Almeida Júnior, and the voucher specimen (No. 11175) was deposited and cataloged in the collection of the Maranhão Herbarium (MAR) of the Department of Biology (CCBS) of the Federal University of Maranhão (UFMA), Maranhão, Brazil.
4.2. Essential Oil Extraction
The fresh leaves of O. gratissimum were dried in room temperature for four days. Then, the dried material (76.84 g) was mixed with 2.5 L of distilled water and subjected to hydrodistillation in a Clevenger-type apparatus for 3 h to afford a pale yellow oil. The isolated oil, after drying over anhydrous sodium sulfate (Na2SO4) and filtration, was stored in sealed glass vials and maintained under refrigeration until further analysis. Total oil yield was expressed as a percentage (g per 100 g of dried leaves).
4.3. Chemical Composition of EOOG
Qualitative analysis of the chemical composition of the essential oil was performed with a gas chromatograph (GC) coupled with a mass spectrometer (MS), Agilent Model GC-7890B/MSD-5977A (quadrupole), with an electron impact at 70 eV, an HP-5MS methylpolysiloxane column (30 m × 0.25 mm × 0.25 μm, Agilent), a 1 mL/min flowing helium carrier gas, an injector temperature of 250 °C, a detector temperature of 150 °C, and a transfer line at 280 °C. The chromatographic oven was programmed as follows: an initial temperature of 70 °C, with a heating ramp of 4 °C/min to 180 °C, and an increase of 10 °C/min to 250 °C at the end of the run (34.5 min).
Quantitative analysis of the chemical composition of the oil was carried out by GC coupled to a flame ionization detector (FID), Shimadzu Model CG-2010 Plus instrument, with an RTX-5 methylpolysiloxane column (30 m × 0.25 mm × 0.25 μm), a 1:30 flow split injection mode, a 1.00 mL/min flow nitrogen carrier gas, an injector temperature of 250 °C, and a detector temperature of 280 °C. The programming of the chromatographic oven was similar to that used in the GC/MS analysis.
The constituents’ percentages were calculated by the integral area of their respective peaks, related to the total area of all the constituents of the sample. The various constituents of the EO were identified by visually comparing their mass spectra with those in the literature [32] and the spectra provided by the equipment database (NIST11), and by comparing retention rates with those in the literature [32]. A standard solution of n-alkanes (C9–C30) was injected using the same chromatographic conditions of the sample to calculate the retention index for each peak, as described by Dool and Kratz (1963) [77].
4.4. Preparation of Antimicrobial Solutions
EOOG stock solution was prepared in Brain Heart Infusion broth (BHI, Acumedia®, Michigan, USA) with 1% tween 80 sterile (VETEC Fine Chemistry LTDA, Rio de Janeiro, Brazil) at 16 mg/mL. From the oil density (1.0074 g/mL), it was firstly mixed EOOG (16 μL) with tween 80 (10 μL) and bring volume to 1 mL with fresh BHI (974 μL). It was considered as control group (untreated cells) in the experimental assays, only broth media plus tween 80 in an equivalent amount for each EOOG concentration tested. Oxacillin sodium (OXA, Blau Pharmaceutical SA, Sao Paulo, Brazil) and ciprofloxacin (CIP, Fresenius Laboratory Kabi, Sao Paulo, Brazil) antibiotics, obtained commercially, were prepared according to manufacture note, and the concentration was adjusted to 32 mg/mL.
4.5. Bacterial Strains and Culture Conditions
EOOG activity was evaluated against two representatives, Gram-negative and -positive bacterial cells, four strains of S. aureus and E. coli species. The standard strains from the American Type Culture Collection (ATCC, USA) were used as sensitive antibiotic strains. The clinical S. aureus were isolated and identified in the Laboratory of Microbiology of the Santa Casa de Misericórdia de Sobral, CE, Brazil, as part of the hospital routine. E. coli strains were isolated from fresh tilapia fillets (Oreochromis niloticus) obtained from the retail trade of Sobral, CE, Brazil [78]. The confirmatory identification and resistance profile of bacterial strains were determined by the VITEK®2 system (BioMérieux, Marcy- L’Etoile, France). The source and resistance profile of the microorganisms selected are described below (Table 4).
Table 4.
Antibiotic resistance profile obtained by the VITEK®2 system.
| Staphylococcus aureus | Source | Antibiotic Resistance |
| Standard | ATCC 6538 | Sensitive |
| 2B | Soft tissues | ERT, CLIN e BZP |
| 5B | Human blood | ERT, CLIN, CIP, NOR, MOX, BZP, OXA e RIP (I) |
| 7B | Human blood | BZP e OXA |
| Escherichia coli | ||
| Standard | ATCC 11303 | Sensitive |
| P12 | Fish fillet | AMP, CFL, CIP, NOR, NAL e AMC (I) |
| P25 | Fish fillet | AMP, CFL (I) e AMC (I) |
| P36 | Fish fillet | AMP, CFL (I) e AMC (I) |
Notes: AMC: Amoxicillin/clavulanic acid. AMP: Ampicillin. BZP: Benzylpenicillin. CFL: Cefelotin. CIP: Ciprofloxacin. CLIN: Clindamycin. ERT: Erythromycin. MOX: Moxifloxacin. NAL: Nalidixic acid. NOR: Norfloxacin. OXA: Oxacillin. (I): Intermediate resistance.
Separately, cells were cultured from stock culture and maintained at −80 °C in 5 mL of BHI broth at 37 °C overnight before each experimental procedure. Afterward, 50 µL were inoculated into the same culture media (1:10) and grown at 37 °C until late in the exponential phase. Prior to biological assays, the turbidity of bacterial suspension was adjusted against the standard 0.5 McFarland (108 CFU/mL) and diluted with fresh BHI to reach an appropriate cell concentration for each method.
4.6. Antimicrobial Activity of EOOG
The antibacterial activity of the EO was firstly determined by the paper disk diffusion method. Briefly, a sterile swab was dipped into each tested bacterial suspension adjusted to 108 CFU/mL in saline, rotated against the tube wall, and rubbed in several directions on the whole surface of Mueller–Hinton agar (MHA) plates (Acumedia®, Michigan, USA). Afterward, three disks 6 mm in diameter (Biomérieux, Marcy-l’Etoile, France) and impregnated with 5 µL of pure EOOG were placed equidistantly on the MHA surface. After 24 h of incubation at 37 °C, the antibacterial effect was evaluated by measuring the diameter of inhibitory zones (DIZ) in millimeters, and the results were expressed as means from three determinations.
The minimum inhibitory concentrations (MICs) were determined using a microdilution method following the standard CLSI (Clinical and Laboratory Standards Institute, M07-A10) protocol [79]. Serial two-fold dilutions of 100 µL were prepared with BHI broth in 96-well microtiter plates (KASVI, Paraná, Brazil), obtaining EOOG concentrations from 250 to 8000 μg/mL. For antibiotic CIP and OXA, concentrations ranging from 0.25 to 8000 µg/mL and from 0.25 to 256 µg/mL, respectively, were used. Afterward, 100 µL of the bacterial suspension (106 CFU/mL) were added to each well, yielding a final cell concentration of 5 × 105, and plates were incubated for 24 h at 37 °C. The lowest concentration capable of inhibiting the visible bacterial growth was defined as the MIC. To determine minimum bactericidal concentrations (MBCs), 10 μL were collected from each well that contained no bacterial growth and were plated on the BHI agar. The MBC was recorded as the lowest concentration capable of inhibiting bacterial growth on the agar surface after 24 h of incubation at 37 °C. All experiments were performed in triplicate, with three independent repeats.
4.7. Kinetic Growth Assay
The growth curve assay was performed for all bacterial strains in 96-well polystyrene microtiter plates, as described by Field et al. (2010), with modifications [80]. The bacterial suspension adjusted (106 CFU/mL) and EOOG at sub-inhibitory concentration (1/2MIC) (1:1, v/v) was added to each well, and the kinetic growth was determined over a time course. Optical density (OD620) was measured every hour to evaluate bacterial density for a period of 24 and 18 h for S. aureus and E. coli strains, respectively. The growth level was quantified by the area under the curve (AUC), which was calculated as a metric absorbance (620 nm) distribution as a function of time. For each condition, an average value from six replicas was given. It is important to highlight that experimental protocols were carried out with selected strains from each bacterial species with a multiresistance profile (5B and P12).
4.8. Checkerboard Assay
The combination activity of antimicrobial compounds was determined by checkerboard assay in 96-well microtiter plates [81]. The combinations used were as follows: EOOG + OXA/CIP for S. aureus (5B) and EOOG + CIP for E. coli (P12). First, serial two-fold dilutions of drugs were prepared with BHI broth separately. Using a 96-well microtiter plate, 50 μL of each substance (1:1 v/v) were added in rows (EOOG), in ascending concentrations, and the antibiotics (CIP or OXA) were similarly distributed among the columns. Thus, each well held a unique combination of concentrations of the two substances. Afterward, 100 μL of bacterial inoculum (106 CFU/mL) were added to the wells and incubated at 37 °C for 24 h. The analysis of the interaction results was performed by the fractional inhibitory concentration index (FICi), defined as the sum of the MIC of the combined substances divided by the MIC of the isolated substances and and categorized as: Synergism (FICi ≤ 0.5), additive (FICi > 0.5 to ≤ 1), indifferent (FICi > 1 to < 2), or antagonism (FICi ≥ 2), according to European Committee for Antimicrobial Susceptibility Testing (EUCAST) [82].
4.9. Antibiofilm Activity
The EOOG antibiofilm assay was verified on preformed biofilms, 12 and 24 h for E. coli (P12) and S. aureus (5B), respectively, using 96-well polystyrene microtiter plates, according to Yadav et al. (2015), with modifications [69]. After biofilm formation, the wells were washed three times with PBS (Phosphate-Saline Buffer 0.1 M; pH 7.4) to remove the planktonic and weakly attached cells. Afterward, the biofilm was treated with 200 µL of essential oil at decreasing concentrations (8000 to 1000 µg/mL) for 4 h at 37 °C. The biofilm was quantified according to biomass and the number of viable cells by crystal violet staining (CV) and colony forming units (CFU), respectively. Each technique was performed in triplicate, with three independent experiments.
Firstly, 200 μL of methanol PA (Dinâmica, São Paulo, Brazil) were added to treated biofilm wells for 15 min to fix the adhered cells. Afterward, it was removed, and the microplate was left to dry at room temperature. Thus, 200 μL of 0.1% crystal violet (Synth®, Sao Paulo, Brazil) were added over a period of 10 min. After washing the wells using distilled water, the biofilm-bound dye was solubilized in 200 μL of 33% glacial acetic acid (Dinâmica, Sao Paulo, Brazil). After that, the absorbance was measured using an optical density reader (SpectraMax® Paradigm® Molecular Devices, San Jose, California, USA) at 590 nm. Secondly, to determine the viable number of cells in the EOOG-treated biofilm, 200 µL of PBS were added to each well, and the microplate was subjected to ultrasound (GNATUS, São Paulo, Brazil) for 5 min to detach the biofilm-embedded bacteria. Serial ten-fold dilutions were then prepared from that cell suspension, and 10 μL were plated in BHI agar. After 18 h of incubation at 37 °C, the total of enumerated cells was expressed in log10 CFU/mL from the average of the number of CFUs of three different wells from the same replicate.
4.10. Scanning Electron Microscopy
Structural arrangement changes of S. aureus (5B) and E. coli (P12) biofilms induced by EOOG (1xMIC) treatment were evaluated by scanning electron microscopy (SEM—Inspect S50—FEI Company®, Oregon, USA), according to Yadav et al. (2015), with modifications [69]. The biofilm was formed from a cell suspension (106 cells/mL) in fresh BHI for 24 h (5B) and 12 h (P12) at 37 °C in 24-well plates containing glass slides (1 × 1 cm). The slides were then washed three times with 0.1 M PBS, and surface adsorbed cells were treated with EOOG at MIC values against 5B and P12, 2000 and 1000 µg/mL, respectively, for 4 h at 37 °C. For the control group, only fresh BHI with tween 80 was used. Before SEM analysis, the cells were pre-fixed with 2% glutaraldehyde, dehydrated with an alcohol solution at 10, 30, 50, 70, 90, and 100% for 20 min each, and dried at room temperature. The slides were placed on carbon tape on the aluminum sample holder (stubs), coated with gold (Emitech Q150T, Lewes, UK), and viewed via SEM at 20 kW.
4.11. Statistical Analysis
Antimicrobial and antibiofilm activity assays performed in triplicate were presented as the mean ± standard deviations (SD). Data were statistically analyzed using GraphPad Prism 8.0 software (GraphPad Software, Inc., San Diego, CA, USA) applying analysis of variance (ANOVA) with Tukey’s post hoc test. Differences between treated and control groups were considered significant when p < 0.01. The graphs generated in the growth curve test were used to determine the area under the curve (AUC).
5. Conclusions
The EO from dried leaves of O. gratissimum L. obtained in Maranhão State, Brazil, demonstrated antibacterial activity against representative Gram-negative and -positive strains, S. aureus and E. coli, with a multidrug resistance profile. In addition, the ability of EO combined with clinical antibiotics to increase the sensitivity of S. aureus to OXA and CIP was determined. It was possible to observe the action against established biofilms as well as the reduction in bacterial load within S. aureus and E. coli biofilms. Considering all the results obtained, the EOOG can be used as an alternative for new therapies against MDR bacteria, for instance, like formulations of antibacterial creams or gels containing OEs for topical applications or even to produce EO-based wound dressings.
Acknowledgments
The LaBAM team is grateful to the Coordination for the Improvement of Higher Education Personnel (CAPES) for the financial support, and to the University Center INTA (UNINTA) for encouragement to carry out the research. We are also thankful to Center for Analytical Microscopy from Federal University of Ceará (UFC) for assistance with SEM images, which improved the manuscript significantly.
Author Contributions
Conceptualization: V.A.C. and F.E.A.C.J.; data curation: R.S.M., Á.M.A.A. and V.A.C.; formal analysis: R.S.M.; Á.M.A.A., A.M.G.P. and M.N.C.M.; funding acquisition: H.S.d.S. and F.E.A.C.J.; investigation: R.S.M., Á.M.A.A., A.M.G.P., R.R.R., R.M.B.C., M.N.C.M., P.H.R.L., G.A.G., T.H.S.R., I.L.P., R.A.C. and G.S.B.; methodology: R.S.M., Á.M.A.A. and G.A.G.; project administration: F.E.A.C.J.; resources: G.A.G.; supervision: V.A.C.; Writing—Original draft: R.S.M., Á.M.A.A. and G.A.G.; Writing—Review & edition: R.A.C. and V.A.C.
Funding
This research was funded by the Foundation for Support for Research and Scientific and Technological Development of Maranhão, (FAPEMA-Brazil), grant number 00986/17 – Universal Program.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Sample Availability: Samples of the compounds EOOG, Ocimum gratissimum EO, are available from the authors.
References
- 1.Vivas R., Barbosa A.A.T., Dolabela S.S., Jain S. Multidrug-resistant bacteria and alternative methods to control them: An overview. Microb. Drug. Resist. 2019;25:890–908. doi: 10.1089/mdr.2018.0319. [DOI] [PubMed] [Google Scholar]
- 2.Karam G., Chastre J., Wilcox M.H., Vincent J.L. Antibiotic strategies in the era of multidrug resistance. Crit. Care. 2016;20:136. doi: 10.1186/s13054-016-1320-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ma D.S.L., Tan L.T.H., Chan K.G., Yap W.H., Pusparajah P., Chuah L.H., Ming L.C., Khan T.M., Lee L.H., Goh B.H. Resveratrol-potential antibacterial agent against foodborne pathogens. Front. Pharmacol. 2018;9:102. doi: 10.3389/fphar.2018.00102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Davies S.K., Fearn S., Allsopp L.P., Harrison F., Ware E., Diggle S.P., Filloux A., McPhail D.S., Bundy J.G. Visualizing antimicrobials in bacterial biofilms: Three-dimensional biochemical imaging using TOF-SIMS. mSphere. 2017;2:e00211–e00217. doi: 10.1128/mSphere.00211-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Makovcova J., Babak V., Kulich P., Masek J., Slany M., Cincarova L. Dynamics of mono- and dual-species biofilm formation and interactions between Staphylococcus aureus and Gram-negative bactéria. Microb. Biotechnol. 2017;10:819–832. doi: 10.1111/1751-7915.12705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Thurlow L.R., Hanke M.L., Fritz T., Angle A., Aldrich A., Williams S.H., Engebretsen I.L., Bayles K.W., Horswill A.R., Kielian T. Staphylococcus aureus biofilms prevent macrophage phagocytosis and attenuate inflammation in vivo. J. Immunol. 2011;186:6585–6596. doi: 10.4049/jimmunol.1002794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lebeaux D., Ghigo J.M., Beloin C. Biofilm-related infections: Bridging the Gap between clinical management and fundamental aspects of recalcitrance toward antibiotics. Microbiol. Mol. Biol. Rew. 2014;78:510–543. doi: 10.1128/MMBR.00013-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.van Duin D., Paterson D. Multidrug resistant bacteria in the community: Trends and lessons learned. Infect. Dis. Clin. N. Am. 2016;30:377–390. doi: 10.1016/j.idc.2016.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Elshafie H.S., Camele I. An overview of the biological effects of some Mediterranean essential oils on human health. Biomed Res. Int. 2017;2017:9268468. doi: 10.1155/2017/9268468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sharifi-Rad J., Sureda A., Tenore G.C., Daglia M., Sharifi-Rad M., Valussi M., Tundis R., Sharifi-Rad M., Loizzo M.R., Ademiluyi A.O., et al. Biological activities of essential oils: From plant chemoecology to traditional healing systems. Molecules. 2016;22:70. doi: 10.3390/molecules22010070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ju J., Xie Y., Guo Y., Cheng Y., Qian H., Yao W. Application of edible coating with essential oil in food preservation. Crit. Rev. Food Sci. Nutr. 2018;59:2467–2480. doi: 10.1080/10408398.2018.1456402. [DOI] [PubMed] [Google Scholar]
- 12.Blowman K., Magalhães M., Lemos M.F.L., Cabral C., Pires I.M. Anticancer properties of essential oils and other natural products. Evid. Based Complement. Alternat. Med. 2018;2018:3149362. doi: 10.1155/2018/3149362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Swamy M.K., Akhtar M.S., Sinniah U.R. Antimicrobial properties of plant essential oils against human pathogens and their mode of action: An updated review. Evid. Based Complement. Alternat. Med. 2016;2016:3012462. doi: 10.1155/2016/3012462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Solórzano-Santos F., Miranda-Novales M.G. Essential oils from aromatic herbs as antimicrobial agents. Curr. Opin. Biotechnol. 2012;23:136–141. doi: 10.1016/j.copbio.2011.08.005. [DOI] [PubMed] [Google Scholar]
- 15.Man A., Santacroce L., Jacob R., Mare A., Man L. Antimicrobial activity of six essential oils against a group of human pathogens: A comparative study. Pathogens. 2019;8:15. doi: 10.3390/pathogens8010015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ebani V.V., Nardoni S., Bertelloni F., Pistelli L., Mancianti F. Antimicrobial activity of five essential oils against bacteria and fungi responsible for urinary tract infections. Molecules. 2018;23:1668. doi: 10.3390/molecules23071668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tariqa S., Wania S., Rasoola W., Shafia K., Bhata M.A., Prabhakarb A., Shallaa A.H., Rathera M.A. A comprehensive review of the antibacterial, antifungal and antiviral potential of essential oils and their chemical constituents against drugresistant microbial pathogens. Microb. Pathog. 2019;103580:134. doi: 10.1016/j.micpath.2019. [DOI] [PubMed] [Google Scholar]
- 18.Li Z.H., Cai M., Liu Y.S., Sun P.L., Luo S.L. Antibacterial activity and mechanisms of essential oil from Citrus medica L. Var. Sarcodactylis. Molecules. 2019;24:1577. doi: 10.3390/molecules24081577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zomorodian K., Moein M., Pakshir K., Karami F., Sabahi Z. Chemical composition and antimicrobial activities of the essential oil from Salvia mirzayanii leaves. Evid. Based Complement. Alternat. Med. 2017;22:770–776. doi: 10.1177/2156587217717414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Porfírio E.M., Melo H.M., Pereira A.M.G., Cavalcante T.T.A., Gomes G.A., de Carvalho M.G., Costa R.A., Catunda Júnior F.E.A. In vitro antibacterial and antibiofilm activity of Lippia alba essential oil, citral, and carvone against Staphylococcus aureus. Sci. World. J. 2017;2017:4962707. doi: 10.1155/2017/4962707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vasconcelos S.E.C.B., Melo H.M., Cavalcante T.T.A., Catunda Júnior F.E.A., de Carvalho M.G., Menezes F.G.R., de Sousa O.V., Costa R.A. Plectranthus amboinicus essential oil and carvacrol bioactive against planktonic and biofilm of oxacillin- and vancomycinresistant Staphylococcus aureus. BMC Complement. Altern. Med. 2017;17:462. doi: 10.1186/s12906-017-1968-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Firmino D.F., Cavalcante T.T.A., Gomes G.A., Firmino N.C.S., Rosa L.D., de Carvalho M.G., Catunda Júnior F.E.A. Antibacterial and antibiofilm activities of Cinnamomum Sp. essential oil and cinnamaldehyde: Antimicrobial activities. Sci. World J. 2018;2018:7405736. doi: 10.1155/2018/7405736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lagha R., Abdallah F.B., AL-Sarhan B.O., Al-Sodany Y. Antibacterial and biofilm inhibitory activity of medicinal plant essential oils against Escherichia coli isolated from UTI patients. Molecules. 2019;24:1161. doi: 10.3390/molecules24061161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mohr F.B.M., Lermen C., Gazim Z.C., Gonçalves J.E., Alberton O. Antifungal activity, yield, and composition of Ocimum gratissimum essential oil. Genet. Mol. Res. 2017;16:1–10. doi: 10.4238/gmr16019542. [DOI] [PubMed] [Google Scholar]
- 25.Brasil Ministério da Saúde. Plantas Medicinais de Interesse ao SUS–Renisus. [(accessed on 14 August 2019)];2009 Available online: http://www.saude.gov.br/acoes-e-programas/programa-nacional-de-plantas-medicinais-e-fitoterapicos-ppnpmf/politica-e-programa-nacional-de-plantas-medicinais-e-fitoterapicos/plantas-medicinais-de-interesse-ao-sus-renisus.
- 26.Penido A.B., de Morais S.M., Ribeiro A.B., Silva A.Z. Ethnobotanical study of medicinal plants in Imperatriz, State of Maranhão, Northeastern Brazil. Acta Amazon. 2016;46:345–354. doi: 10.1590/1809-4392201600584. [DOI] [Google Scholar]
- 27.Pandey A.K., Singh P., Tripathi N.N. Chemistry and bioactivities of essential oils of some Ocimum species: An overview. Asian Pac. J. Trop. Biomed. 2014;4:682–694. doi: 10.12980/APJTB.4.2014C77. [DOI] [Google Scholar]
- 28.Castro J.A.M., Monteiro O.S., Coutinho D.F., Rodrigues A.A.C., da Silva J.K.R., Maia J.G.S. Seasonal and circadian study of a thymol/γ-terpinene/p-cymene type oil of Ocimum gratissimum L. and Its antioxidant and antifungal effects. J. Braz. Chem. Soc. 2019;30:930–938. doi: 10.21577/0103-5053.20180237. [DOI] [Google Scholar]
- 29.Iwalokun B.A., Gbenle G.O., Adewole T.A., Smith S.I., Akinsinde K.A., Omonigbehin E.O. Effects of Ocimum gratissimum L essential oil at subinhibitory concentrations on virulent and multidrug-resistant Shigella strains from Lagos, Nigeria. Apmis. 2003;111:477–482. doi: 10.1034/j.1600-0463.2003.1110405.x. [DOI] [PubMed] [Google Scholar]
- 30.Silva N.C.C., Fernandes Júnior A. Biological properties of medicinal plants: A review of their antimicrobial activity. J. Venom. Anim. Toxins Incl. Trop. Dis. 2010;16:402–413. doi: 10.1590/S1678-91992010000300006. [DOI] [Google Scholar]
- 31.Intorasoot A., Chornchoem P., Sookkhee S., Intorasoot S. Bactericidal activity of herbal volatile oil extracts against multidrug-resistant Acinetobacter baumannii. J. Ethnopharmacol. 2017;6:218–222. doi: 10.5455/jice.20170411091159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Adams R.P. Identification of Essential Oil Components by Gas Chromatography/Mass Spectrometry. 4th ed. Allured Publishing Corporation; Carol Stream, IL, USA: 2007. [Google Scholar]
- 33.Faria T.J., Ferreira R.S., Yassumoto L., de Souza J.R.P., Ishikawa N.K., Barbosa A.M. Antifungal activity of essential oil isolated from Ocimum gratissimum L. (eugenol chemotype) against phytopathogenic fungi. Braz. Arch. Biol. Technol. 2006;49:867–871. doi: 10.1590/S1516-89132006000700002. [DOI] [Google Scholar]
- 34.Ocheng F., Bwanga F., Joloba M., Softrata A., Azeem M., Pütsep K., Borg-Karlson A.K., Obua C., Gustafsson A. Essential oils from Ugandan aromatic medicinal plants: Chemical composition and growth inhibitory effects on oral pathogens. Evid. Based Complement. Alternat. Med. 2015;2015:230832. doi: 10.1155/2015/230832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Matasyoha L.G., Matasyoh J.C., Wachira F.N., Kinyua M.G., Muigai A.W.T., Mukiama T.K. Antimicrobial activity of essential oils of Ocimum gratissimum L. from different populations of Kenya. Afr. J. Tradit. Complement. Altern. Med. 2008;5:187–193. doi: 10.4314/ajtcam.v5i2.31272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Joshi R.K. Chemical composition, in vitro antimicrobial and antioxidant activities of the essential oils of Ocimum gratissimum, O. sanctum and their major constituents. Indian J. Pharm. Sci. 2013;75:457–462. doi: 10.4103/0250-474X.119834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Saha S., Dhar T.N., Sengupta C., Ghosh P. Biological activities of essential oils and methanol extracts of five Ocimum species against pathogenic bacteria. Czech J. Food Sci. 2013;31:194–202. doi: 10.17221/234/2012-CJFS. [DOI] [Google Scholar]
- 38.Tangpao T., Chung H.H., Sommano S.R. Aromatic profiles of essential oils from five commonly used Thai Basils. Foods. 2018;7:175. doi: 10.3390/foods7110175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kim D.Y., Won K.J., Hwang D., Park S.M., Kim B., Lee H.M. Chemical composition, antioxidant and anti-melanogenic activities of essential oils from Chrysanthemum boreale MAKINO at different harvesting stages. Chem. Biodivers. 2018;15:e1700506. doi: 10.1002/cbdv.201700506. [DOI] [PubMed] [Google Scholar]
- 40.Kpadonou Kpoviessi B.G.H., Ladekana E.Y., Kpoviessia D.S.S., Gbaguidib F., Yehouenoud B., Quetin-Leclercq J., Figueredoe G., Moudachiroub M., Accrombessi G.C. Chemical variation of essential oil constituents of Ocimum gratissimum L. from Benin, and impact on antimicrobial properties and toxicity against Artemia salina LEACH. Chem. Biodivers. 2012;9:139–150. doi: 10.1002/cbdv.201100194. [DOI] [PubMed] [Google Scholar]
- 41.Gobbo-Neto L., Lopes N.P.L. Plantas medicinais: Fatores de influência no conteúdo de metabólitos secundários. Quim. Nova. 2007;30:374–381. doi: 10.1590/S0100-40422007000200026. [DOI] [Google Scholar]
- 42.Yamani H.A., Pang E.C., Mantri N., Deighton M.A. Antimicrobial activity of Tulsi (Ocimum tenuiflorum) essential oil and their major constituents against three species of bacteria. Front. Microbiol. 2016;7:681. doi: 10.3389/fmicb.2016.00681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Thielmann J., Muranyi P., Kazman P. Screening essential oils for their antimicrobial activities against the foodborne pathogenic bacteria Escherichia coli and Staphylococcus aureus. Heliyon. 2019;5:e01860. doi: 10.1016/j.heliyon.2019.e01860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Trombetta D., Castelli F., Sarpietro M.G., Venuti V., Cristani M., Daniele C., Saija A., Mazzanti G., Bisignano G. Mechanisms of antibacterial action of three monoterpenes. Antimicrob. Ag. Chemother. 2005;49:2474–2478. doi: 10.1128/AAC.49.6.2474-2478.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Guo J.J., Gao J.P., Xia J.L., Ritenour M.A., Li G.Y., Shan Y. Comparative analysis of chemical composition, antimicrobial and antioxidant activity of citrus essential oils from the main cultivated varieties in China. LWT. 2018;97:825–839. doi: 10.1016/j.lwt.2018.07.060. [DOI] [Google Scholar]
- 46.Cristani M., D’Arrigo M., Mandalari G., Castelli F., Sarpietro M.G., Micieli D., Venuti V., Bisignano G., Saija A., Trombetta D. Interaction of four monoterpenes contained in essential oils with model membranes: Implications for their antibacterial activity. J. Agric. Food Chem. 2007;55:6300–6308. doi: 10.1021/jf070094x. [DOI] [PubMed] [Google Scholar]
- 47.Lins L., Dal Maso S., Foncoux B., Kamili A., Laurin Y., Genva M., Jijakli H.M., De Clerck C., Fauconnier M.L., Deleu M. Insights into the relationships between herbicide activities, molecular structure and membrane interaction of cinnamon and citronella essential oils components. Int. J. Mol. Sci. 2019;20:4007. doi: 10.3390/ijms20164007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Di Pasqua R., Hoskins N., Betts G., Mauriello G. Changes in membrane fatty acids composition of microbial cells induced by addiction of thymol, carvacrol, limonene, cinnamaldehyde, and eugenol in the growing media. J. Agric. Food Chem. 2006;54:2745–2749. doi: 10.1021/jf052722l. [DOI] [PubMed] [Google Scholar]
- 49.Wang Y.M., Kong L.C., Liu J., Ma H.X. Synergistic effect of eugenol with colistin against clinical isolated colistin-resistant Escherichia coli strains. Antimicrob. Resist. Infect. Control. 2018;7:17. doi: 10.1186/s13756-018-0303-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chimnoi N., Reuk-ngam N., Chuysinuan P., Khlaychan P., Khunnawutmanotham N., Chokchaichamnankit D., Thamniyom W., Klayraung S., Mahidol C., Techasakul S. Characterization of essential oil from Ocimum gratissimum leaves: Antibacterial and mode of action against selected gastroenteritis pathogens. Microb. Pathogenesis. 2018;118:290–300. doi: 10.1016/j.micpath.2018.03.041. [DOI] [PubMed] [Google Scholar]
- 51.Yap P.S.X., Yiap B.C., Ping H.C., Lim S.H.E. Essential oils, a new horizon in combating bacterial antibiotic resistance. Open Microbiol. J. 2014;8:6–14. doi: 10.2174/1874285801408010006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Palaniappan K., Holley R.A. Use of natural antimicrobials to increase antibiotic susceptibility of drug resistant bacteria. Int. J. Food. Microbiol. 2010;140:164–168. doi: 10.1016/j.ijfoodmicro.2010.04.001. [DOI] [PubMed] [Google Scholar]
- 53.Bhardwaj M., Singh B.R., Sinha D.K., Kumar V., Prasanna Vadhana O.R., Varan Singh S., Nirupama K.R., Pruthvishree, Archana Saraf B.S. Potential of herbal drug and antibiotic combination therapy: A new approach to treat multidrug resistant bacteria. Pharm. Anal. Acta. 2016;7:1000523. doi: 10.4172/2153-2435.1000523. [DOI] [Google Scholar]
- 54.Gallucci N., Casero C., Oliva M., Zygadlo J., Demo M. Interaction between terpenes and penicillin on bacterial strains resistant to betalactam antibiotics. Mol. Med. Chem. 2006;10:30–32. [Google Scholar]
- 55.Pei R.S., Zhou F., Ji B.P., Xu J. Evaluation of combined antibacterial effects of eugenol, cinnamaldehyde, thymol, and carvacrol against E. coli with an improved method. J. Food Sci. 2009;74:M379–M383. doi: 10.1111/j.1750-3841.2009.01287.x. [DOI] [PubMed] [Google Scholar]
- 56.De Oliveira A.D.L., Rodrigue F.F.G., Coutinho H.D.M., da Costa J.G.M., de Menezes I.R.A. Chemical composition, modulatory bacterial resistance and antimicrobial activity of essential oil the Hyptis martiusii benth. by direct and gaseous contact. Jundishapur J. Nat. Pharm. Prod. 2014;9:e13521. doi: 10.17795/jjnpp-13521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Oliva A., Costantini S., de Angelis M., Garzoli S., Božović M., Mascellino M.T., Vullo V., Ragno R. High potency of Melaleuca alternifolia essential oil against multi-drug resistant gram-negative bacteria and methicillin-resistant Staphylococcus aureus. Molecules. 2018;23:2584. doi: 10.3390/molecules23102584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Miladi H., Zmantar T., Kouidhi B., Chaabouni Y., Mahdouani K., Bakhrouf A., Chaieb K. Use of carvacrol, thymol, and eugenol for biofilm eradication and resistance modifying susceptibility of Salmonella enterica serovar Typhimurium strains to nalidixic acid. Microb. Pathog. 2017;104:56–63. doi: 10.1016/j.micpath.2017.01.012. [DOI] [PubMed] [Google Scholar]
- 59.Fadli M., Saada A., Sayadi S., Chevalier J., Mezrioui N.E., Pagès J.M., Hassani L. Antibacterial activity of Thymus maroccanus and Thymus broussonetii essential oils against nosocomial infection – bacteria and their synergistic potential with antibiotics. Phytomedicine. 2012;19:464–471. doi: 10.1016/j.phymed.2011.12.003. [DOI] [PubMed] [Google Scholar]
- 60.Magi G., Marini E., Facinelli B. Antimicrobial activity of essential oils and carvacrol, and synergy of carvacrol and erythromycin, against clinical, erythromycin-resistant Group A Streptococci. Front. Microbiol. 2015;6:165. doi: 10.3389/fmicb.2015.00165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Rao H., Lai P., Gao Y. Chemical composition, antibacterial activity, and synergistic effects with conventional antibiotics and nitric oxide production inhibitory activity of essential oil from Geophila repens (L.) I.M. Johnst. Molecules. 2017;22:1561. doi: 10.3390/molecules22091561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Aelenei P., Rimbu C.M., Guguianu E., Dimitriu G., Aprotosoaie A.C., Brebu M., Horhogea C.E., Miron A. Coriander essential oil and linalool–interactions with antibiotics against Gram-positive and Gram-negative bacteria. Lett. Appl. Microbiol. 2019;68:156–164. doi: 10.1111/lam.13100. [DOI] [PubMed] [Google Scholar]
- 63.Yang S.K., Yusoff K., Mai C.W., Lim W.M., Yap W.S., Lim S.H.E., Lai K.S. Additivity vs. synergism: Investigation of the additive interaction of cinnamon bark oil and meropenem in combinatory therapy. Molecules. 2017;22:1733. doi: 10.3390/molecules22111733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Rubini D., Banu S.F., Nisha P., Murugan R., Thamotharan S., Percino M.J., Subramani P., Nithyanand P. Essential oils from unexplored aromatic plants quench biofilm formation and virulence of Methicillin resistant Staphylococcus aureus. Microb. Pathog. 2018;122:162–173. doi: 10.1016/j.micpath.2018.06.028. [DOI] [PubMed] [Google Scholar]
- 65.Koo H., Allan R.N., Howlind R.P., Hall-Stoodleye L., Stoodley P. Targeting microbial biofilms: Current and prospective therapeutic strategies. Nat. Rev. Microbiol. 2017;15:740–755. doi: 10.1038/nrmicro.2017.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Carneiro V.A., Santos H.S., Arruda F.V.S., Bandeira P.N., Albuquerque M.R.J.R., Pereira M.O., Henriques M., Cavada B.S., Teixeira E.H. Casbane diterpene as a promising natural antimicrobial agent against biofilm-associated infections. Molecules. 2011;16:190–201. doi: 10.3390/molecules16010190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Budzyńska A., Rożalska S., Sadowska B., Rożalska B. Candida albicans/Staphylococcus aureus dual-species biofilm as a target for the combination of essential oils and fluconazole or mupirocin. Mycopathologia. 2017;182:989–995. doi: 10.1007/s11046-017-0192-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kim Y.G., Lee J.H., Gwon G., Kim S.I., Park J.G., Lee J. Essential oils and eugenols inhibit biofilm formation and the virulence of Escherichia coli O157:H7. Sci. Rep. 2016;6:36377. doi: 10.1038/srep36377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Yadav M.K., Chae S.W., Im G.J., Chung J.W., Song J.J. Eugenol: A phyto-compound effective against methicillin-resistant and methicillin-sensitive Staphylococcus aureus clinical strain biolms. PLoS ONE. 2015;10:e0119564. doi: 10.1371/journal.pone.0119564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Marchese A., Barbieri R., Coppo E., Orhan I.E., Daglia M., Nabavi F.S., Izadi M., Abdollahi M., Nabavi S.M., Ajami M. Antimicrobial activity of eugenol and essential oils containing eugenol: A mechanistic viewpoint. Crit. Rev. Microbiol. 2017;43:668–689. doi: 10.1080/1040841X.2017.1295225. [DOI] [PubMed] [Google Scholar]
- 71.Rathinam P., Viswanathan P. Anti-virulence potential of eugenol-rich fraction of Syzygium aromaticum against multidrug resistant uropathogens isolated from catheterized patients. Avicenna J. Phytomed. 2018;8:416–431. [PMC free article] [PubMed] [Google Scholar]
- 72.Ghosh T., Das A.B., Jena B., Pradhan C. Antimicrobial effect of silver zinc oxide (Ag-ZnO) nanocomposite particles. Front. Life Sci. 2014;8:47–54. doi: 10.1080/21553769.2014.952048. [DOI] [Google Scholar]
- 73.Mojsoska B., Carretero G., Larsen S., Mateiu R.V., Jenssen H. Peptoids successfully inhibit the growth of gram negative E-coli causing substantial membrane damage. Sci. Rep. 2017;14:42332. doi: 10.1038/srep42332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Husain F.M., Ahmad I., Khan M.S., Ahmad E., Tahseen Q., Khan M.S., Alshabib N.A. Sub-MICs of Mentha piperita essential oil and menthol inhibits AHL mediated quorum sensing and biofilm of Gram-negative bacteria. Front. Microbiol. 2015;6:420. doi: 10.3389/fmicb.2015.00420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Poli J.P., Guinoiseau E., Serra D.R., Sutour S., Paoli M., Tomi F., Quilichini Y., Berti L., Lorenzi V. Anti-quorum sensing activity of 12 essential oils on Chromobacterium violaceum and specific action of cis-cis-p-menthenolide from Corsican Mentha suaveolens ssp. Insularis. Molecules. 2018;23:2125. doi: 10.3390/molecules23092125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lu L., Hu W., Tian Z., Yuan D., Yi G., Zhou1 Y., Cheng Q., Zhu1 J., Li M. Developing natural products as potential anti-biofilm agents. Chin. Med. 2019;14:11. doi: 10.1186/s13020-019-0232-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Dool H.D., Kratz P.D. A generalization of the retention index system including linear temperature programmed gas—liquid partition chromatography. J. Chromatogr. A. 1963;11:463–471. doi: 10.1016/S0021-9673(01)80947-X. [DOI] [PubMed] [Google Scholar]
- 78.Almeida M.V.A., Cangussú Í.M., Carvalho A.L.S., Brito I.L.P., Costa R.A. Drug resistance, AmpC-β-lactamase and extended-spectrum β-lactamase-producing Enterobacteriaceae isolated from fish and shrimp. Rev. Inst. Med. Trop. São Paulo. 2017;59:e70. doi: 10.1590/s1678-9946201759070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.CLS . Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria that Grow Aerobically: Approved Standard. 10th ed. Clinical and Laboratory Standards Institute; Wayne, PA, USA: 2015. CLSI document M07-A10. [Google Scholar]
- 80.Field D., Quigley L., O’Connor P.M., Rea M.C., Daly K., Cotter P.D., Hill C., Ross R.P. Studies with bioengineered Nisin peptides highlight the broad-spectrum potency of Nisin V. Microb. Biotechnol. 2010;3:473–486. doi: 10.1111/j.1751-7915.2010.00184.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.White R., Burgess D.S., Manduru M., Bosso J.A. Comparison of three different in vitro methods of detecting synergy: Time-kill, checkerboard, and E test. Antimicrob. Ag. Chemother. 1996;40:1914–1918. doi: 10.1128/AAC.40.8.1914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.European Committee for Antimicrobial Susceptibility Testing (EUCAST) of the European Society of Clinical Microbiology and Infectious Diseases . Terminology Relating to Methods for the Determination of Susceptibility of Bacteria to Antimicrobial Agents; EUCAST Definitive Document E. Def 1.2. European Committee for Antimicrobial Susceptibility Testing (EUCAST) of the European Society of Clinical Microbiology and Infectious Diseases (ESCMID); Basel, Switzerland: 2000. [DOI] [PubMed] [Google Scholar]