Abstract
Background
This is an updated version of the Cochrane review published in 2005 on selective serotonin re‐uptake inhibitors (SSRIs) for preventing migraine and tension‐type headache. The original review has been split in two parts and this review now only regards tension‐type headache prevention. Another updated review covers migraine. Tension‐type headache is the second most common disorder worldwide and has high social and economic relevance. As serotonin and other neurotransmitters may have a role in pain mechanisms, SSRIs and serotonin‐norepinephrine reuptake inhibitors (SNRIs) have been evaluated for the prevention of tension‐type headache.
Objectives
To determine the efficacy and tolerability of SSRIs and SNRIs compared to placebo and other active interventions in the prevention of episodic and chronic tension‐type headache in adults.
Search methods
For the original review, we searched the Cochrane Central Register of Controlled Trials (CENTRAL 2003, Issue 4), MEDLINE (1966 to January 2004), EMBASE (1994 to May 2003), and Headache Quarterly (1990 to 2003). For this update, we revised the original search strategy to reflect the broader type of intervention (SSRIs and SNRIs). We searched CENTRAL (2014, Issue 10) on the Cochrane Library, MEDLINE (1946 to November 2014), EMBASE (1980 to November 2014), and PsycINFO (1987 to November 2014). We also checked the reference lists of retrieved articles and searched trial registries for ongoing trials.
Selection criteria
We included randomised controlled trials comparing SSRIs or SNRIs with any type of control intervention in participants 18 years and older, of either sex, with tension‐type headache.
Data collection and analysis
Two authors independently extracted data (headache frequency, index, intensity, and duration; use of symptomatic/analgesic medication; quality of life; and withdrawals) and assessed the risk of bias of trials. The primary outcome is tension‐type headache frequency, measured by the number of headache attacks or the number of days with headache per evaluation period.
Main results
The original review included six studies on tension‐type headache. We now include eight studies with a total of 412 participants with chronic forms of tension‐type headache. These studies evaluated five SSRIs (citalopram, sertraline, fluoxetine, paroxetine, fluvoxamine) and one SNRI (venlafaxine). The two new studies included in this update are placebo controlled trials, one evaluated sertraline and one venlafaxine. Six studies, already included in the previous version of this review, compared SSRIs to other antidepressants (amitriptyline, desipramine, sulpiride, mianserin). Most of the included studies had methodological and/or reporting shortcomings and lacked adequate power. Follow‐up ranged between two and four months.
Six studies explored the effect of SSRIs or SNRIs on tension‐type headache frequency, the primary endpoint. At eight weeks of follow‐up, we found no difference when compared to placebo (two studies, N = 127; mean difference (MD) ‐0.96, 95% confidence interval (CI) ‐3.95 to 2.03; I2= 0%) or amitriptyline (two studies, N = 152; MD 0.76, 95% CI ‐2.05 to 3.57; I2= 44%).
When considering secondary outcomes, SSRIs reduce the symptomatic/analgesic medication use for acute headache attacks compared to placebo (two studies, N = 118; MD ‐1.87, 95% CI ‐2.09 to ‐1.65; I2= 0%). However, amitriptyline appeared to reduce the intake of analgesic more efficiently than SSRIs (MD 4.98, 95% CI 1.12 to 8.84; I2= 0%). The studies supporting these findings were considered at unclear risk of bias. We found no differences compared to placebo or other antidepressants in headache duration and intensity.
SSRIs or SNRI were generally more tolerable than tricyclics. However, the two groups did not differ in terms of number of participants who withdrew due to adverse events or for other reasons (four studies, N = 257; odds ratio (OR) 1.04; 95% CI 0.41 to 2.60; I2= 25% and OR 1.55, 95% CI 0.71 to 3.38; I2= 0%).
We did not find any study comparing SSRIs or SNRIs with pharmacological treatments other than antidepressants (e.g. botulinum toxin) or non‐drug therapies (e.g. psycho‐behavioural treatments, manual therapy, acupuncture).
Authors' conclusions
Since the last version of this review, the new included studies have not added high quality evidence to support the use of SSRIs or venlafaxine (a SNRI) as preventive drugs for tension‐type headache. Over two months of treatment, SSRIs or venlafaxine are no more effective than placebo or amitriptyline in reducing headache frequency in patients with chronic tension‐type headache. SSRIs seem to be less effective than tricyclic antidepressants in terms of intake of analgesic medications. Tricyclic antidepressants are associated with more adverse events; however, this did not cause a greater number of withdrawals. No reliable information is available at longer follow‐up. Our conclusion is that the use of SSRIs and venlafaxine for the prevention of chronic tension‐type headache is not supported by evidence.
Plain language summary
Selective serotonin reuptake inhibitors (SSRIs) and serotonin‐norepinephrine reuptake inhibitors (SNRIs) for preventing tension‐type headache
Tension‐type headache is a common type of headache that can significantly impair people's quality of life. Individuals who experience frequent or severe headaches may benefit from medications taken before the pain starts. Two classes of medication, the selective serotonin reuptake inhibitors (SSRIs) and serotonin‐norepinephrine reuptake inhibitors (SNRIs), typically used to treat depression, are evaluated in this review.
This is an update of a previous review that included studies on migraine and tension‐type headache. The original review has been split into two separate reviews: this update addresses only studies on tension‐type headache, while a second focuses on migraine. When we updated this review (November 2014), we identified two new studies. Six studies were already included in the previous version of the review. Overall, we analysed a total of 412 adults participants. All the studies had a small number of participants and were conducted over a period of two to four months. Only a few were of high quality.
Results suggest that SSRIs or SNRIs are no better than placebo (sugar pill) in reducing the number of days with tension‐type headache. There were no differences in minor side effects between participants treated with SSRIs or SNRIs versus those treated with placebo. SSRIs and SNRIs do not seem to offer advantages when compared to other active treatments, specifically the tricyclic antidepressant, amitriptyline. The participants treated with SSRIs or SNRIs suffered fewer minor side effects than those who took amitriptyline, however the number of people who stopped taking one drug or the other due to side effects was approximately equal. These results are based on poor quality, small, short‐term trials (no more than four months). We did not find a study comparing SSRIs or SNRIs with other medications (e.g. botulinum toxin) or non‐drug therapies (e.g. psycho‐behavioural treatments, manual therapy, acupuncture).
Summary of findings
Background
This updated systematic review considers the evidence for the efficacy and tolerability of selective serotonin reuptake inhibitors (SSRIs) and serotonin norepinephrine reuptake inhibitors (SNRIs) for the prevention of tension‐type headache. It is an update of a systematic review on SSRIs for the prevention of migraine and tension‐type headache previously published in the Cochrane Database of Systematic Reviews (Cusi 2001; Moja 2005). This original review has been split into two separate reviews: this update addresses only studies on tension‐type headache, while a second focuses on migraine prevention (Banzi 2015).
Description of the condition
Tension‐type headache is the most common type of primary headache in the general population. Its lifetime prevalence estimates vary widely, ranging from 30% to 78% (IHS 2013). The Global Burden of Disease Survey 2010 ranked it as the second most prevalent disorder (Vos 2012). Although typically less disabling than migraine, tension‐type headache has significant impact when it occurs frequently, given the high prevalence in the general population.
Tension‐type headache is typically bilateral, pressing or tightening in quality, and of mild to moderate intensity, lasting hours or days, and it is characterised by an increased pericranial tenderness recorded by manual palpation. According to the attack frequency, tension‐type headache can be classified as episodic or chronic. Episodic tension‐type headache is subdivided into an infrequent subform (less than one day of headache per month) and a frequent subform (one to 14 days of headache per month), the latter being associated with considerable disability. The prevalence of episodic tension‐type headache peaks in the fourth decade of life, with women having a higher prevalence than men (Schwartz 1998). Chronic forms evolve from frequent episodic tension‐type headache, often in relation to medication overuse and occurs more than 15 days per month on average for more than three months, causing greatly decreased quality of life and high disability (IHS 2013). The prevalence of chronic tension‐type headache (more than 15 days per month) is approximately 2% to 3% (Rasmussen 2001; Schwartz 1998).
Tension‐type headache is very often associated with disability and high personal and socioeconomic costs. Some estimations reported that the disability attributable to chronic forms is larger than that due to migraine (Stovner 2007). Costs of the disease for patients and healthcare systems are also an issue, although lower than those due to migraine. A recent cost‐of‐illness survey conducted as part of the Eurolight project in six European countries reported an annual direct and indirect cost of tension‐type headache per person of EUR 303, and a total annual cost for the European countries of EUR 21 billion for adults aged 18 to 65 years (Linde 2012).
For many years tension‐type headache was considered to be primarily psychogenic. More recent studies suggested a neurobiological basis, at least for the more severe subtypes. The exact mechanisms are not known. Peripheral pain mechanisms like the hyperexcitability of peripheral afferent neurons of the head and neck muscles are most likely to play a role in episodic tension‐type headache, whereas central pain mechanisms and generalised increased pain sensitivity are involved in the chronic forms (Ashina 2004;IHS 2013; Loder 2008). Susceptibility to tension‐type headache is influenced by genetic factors (Russell 2006).
Description of the intervention
In general, acute (symptomatic) medications are used in the episodic tension‐type headache, whereas prophylactic drugs should be considered in the frequent episodic and chronic forms (IHS 2004). We were not able to retrieve clear data on the use of preventive therapy in people with tension‐type headache. All major categories of preventive pharmacologic therapies for tension‐type headache were initially developed for other medical indications. Progress in the understanding of tension‐type headache pathophysiology and the clinical observation of the analgesic effect of some antidepressants (Kantor 1991; Max 1992; Sindrup 2000) led to exploring the potential benefits of SSRIs and SNRIs, a class of compounds typically used as antidepressants in the treatment of depression, anxiety disorders, and some personality disorders. SSRIs are believed to increase the extracellular level of the neurotransmitters such as serotonin by inhibiting its reuptake into the presynaptic cell. Depending on their chemical structure, these compounds have varying degrees of selectivity for the other monoamine transporters, with pure SSRIs having only weak affinity for the noradrenaline transporter and non‐selective compounds also blocking the reuptake of noradrenaline and dopamine (Preskorn 2004).
How the intervention might work
Preventive treatment is especially well suited to patients with very frequent or severe headaches. It encompasses mainly chronic tension‐type headache, causing significant headache‐related disability, and resistance to acute therapy (IHS 2013). For patients with frequent headaches, those who respond insufficiently to acute therapies, or those who overuse acute medication, preventive therapies may be indicated (Smitherman 2011). Proposed pharmacological options for prophylaxis of tension‐type headache include Angiotensin‐converting Enzyme (ACE) inhibitors and angiotensin II receptor blockers, antidepressants, beta blockers or antiepileptics. Non‐pharmacological approaches include acupuncture, manual therapies and psychological therapies, exercise and education, and self management (NICE 2012).
Preventive treatments aim to eliminate headache pain without intolerable harms and they are also expected to reduce use of acute drugs and improve quality of life. In clinical practice the choice of a drug, or of one non‐pharmacological approach over another, is based on many drug‐related factors such as familiarity, efficacy, and adverse effects, as well as many patient characteristics such as headache frequency.
Why it is important to do this review
In general, the use of pharmacological preventive strategies for tension‐type headache is controversial (NICE 2012). Although the evidence supporting antidepressants as prophylactic agents is based on sporadic observation in clinical practice and small clinical trials, some guidelines such as those from the European Federation of Neurological Societies recommend amitriptyline as drug of first choice, while mirtazapine and venlafaxine are to be considered as drugs of second choice (Bendtsen 2010).
Objectives
To determine the efficacy and tolerability of SSRIs and SNRIs compared to placebo and other active interventions in the prevention of episodic and chronic tension‐type headache.
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled trials (RCTs) of SSRIs or SNRIs taken regularly to prevent the occurrence of tension‐type headache attacks, or to reduce the severity of those attacks, or both. We included published and unpublished trials in any language provided that enough information about eligibility was available.
Types of participants
Participants of either sex, aged 18 and older who had been diagnosed with chronic tension‐type headache or episodic forms, whenever prophylaxis was considered appropriate. Headache diagnoses were based on the diagnostic criteria of the International Headache Society (IHS) (IHS 2013 and its previous editions IHS 2004; IHS 1988) and the Ad Hoc Committee on the Classification of Headache (Ad Hoc 1962). Where no such criteria were specified, the diagnosis of tension‐type headache had to be based on at least some of its distinctive features (e.g. bilateral location, pressing/tightening (non‐pulsating) quality, mild or moderate intensity, not aggravated by routine physical activity such as walking or climbing stairs). Frequent episodic tension‐type headache is defined if 10 episodes occur more than one but less than 15 days per month for at least three months. Chronic headache is defined when occurring at least 15 days/month (180 days/year), for at least a three‐month period.
We included studies in which participants were described as having 'combination' or 'mixed' tension‐type headache and migraine, only if data on tension‐type headache participants could be extracted. We excluded trials including patients with a secondary headache.
Types of interventions
To be considered for inclusion, trials were required to have at least one treatment arm in which patients were treated with one of the SSRIs or SNRIs commercially available or under development (fluoxetine, paroxetine, fluvoxamine, citalopram, escitalopram, milnacipram, sertraline, venlafaxine, desvenlafaxine, duloxetine, dapoxetine). We considered any dosage or any dosing regimen lasting for at least four weeks. Acceptable comparator groups included placebo, no intervention, other drug treatments, and behavioural or physical therapies. It was expected that patients were free to take medication for acute headache attacks as needed during the trial period.
Types of outcome measures
In this update, we reconsidered the outcome measures, taking into consideration patients' preferences, scientific rigour, and the availability of data. In line with the guidelines for controlled trials of drugs in tension‐type headache issued by the IHS (Bendtsen 2009), we considered the following main outcomes.
Primary outcomes
Headache frequency.
We considered the following ways of measuring headache frequency, listed in the preferred order:
number of attacks per evaluation period;
number of days with tension‐type headache; and
responders, i.e. patients with ≥ 50% reduction in headache frequency.
Secondary outcomes
Headache intensity, measured using numerical or verbal scale.
Headache duration (hours).
Symptomatic/analgesic medication use for acute headache attacks.
Headache index: we preferred those measures that incorporated frequency as a component (along with intensity, or duration, or both), but considered other types of indexes when these were not available. The formula used to calculate the headache index is recorded in the text below and in the table describing the Characteristics of included studies whenever it was reported by investigators.
Quality of life, measured using validated instruments.
Withdrawals from treatment (for any reason and due to adverse events).
Minor adverse events.
We sought headache‐associated symptoms (nausea, photophobia, phonophobia) and other outcome measures (e.g. workdays lost, mood improvement, and cost‐effectiveness).
We initially recorded the outcomes for all the assessment periods reported, then, once all the data had been collected, decided upon which time points to consider in the analysis; we preferred the last periods of the follow‐up, usually eight and 12 weeks. The analyses considered only outcomes obtained directly from the patient, excluding those judged by the treating physician or study personnel.
We included the following outcome measures in Table 1 and Table 2.
Summary of findings for the main comparison. SSRIs or SNRIs versus placebo for tension‐type headache.
| SSRIs or SNRIs compared to placebo for tension‐type headache | ||||||
| Patient or population: patients with tension‐type headache Intervention: SSRIs or SNRIs Comparison: placebo | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No. of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo | SSRIs or SNRIs | |||||
|
Headache frequency
(number of days with headache) Follow‐up: 2 months |
The mean headache frequency ranged across control groups from 13.8 to 21.7 days with headache | The mean headache frequency in the intervention groups was 0.96 lower (3.95 lower to 2.03 higher) | 127 (2 studies) | ⊕⊕⊝⊝ low1 | ||
|
Headache intensity
(score) Follow‐up: 2 months |
The mean headache severity in the control groups was 3.9 | The mean headache severity in the intervention groups was 0.30 lower (1.13 lower to 0.53 higher) | 40 (1 study) | ⊕⊕⊝⊝ low1 | ||
|
Headache duration
(hours) Follow‐up: 2 months |
The mean headache duration in the control groups was 6.57 | The mean headache duration in the intervention groups was 0.07 lower (2.69 lower to 2.55 higher) | 40 (1 study) | ⊕⊕⊝⊝ low1 | ||
|
Symptomatic/analgesic medication for acute headache attacks
(doses/month) Follow‐up: 2 months |
The mean symptomatic/analgesic medication for acute headache attacks ranged across control groups from 1.07 to 34.8 doses | The mean symptomatic/analgesic medication for acute headache attacks in the intervention groups was 1.87 lower (2.09 to 1.65 lower) | 118 (2 studies) | ⊕⊕⊝⊝ low1 | This finding is largely driven by the results reported by a study at high risk of sponsorship bias | |
|
Headache index Follow‐up: 2 months |
The mean headache index ranged across control groups from 18.3 to 877 | The mean headache index in the intervention groups was 0.24 SDs lower (0.59 lower to 0.11 higher) | 128 (2 studies) | ⊕⊝⊝⊝ very low2 | As a rule of thumb, 0.2 SDs represents a small difference, 0.5 moderate and 0.8 large (Cohen 1988) | |
| Quality of life | See comment | See comment | Not estimable | ‐ | See comment | Not measured |
|
Withdrawals due to adverse events Follow‐up: 2 months |
Study population | OR 3.72 (0.79 to 17.53) | 190 (3 studies) | ⊕⊝⊝⊝ very low3 | ||
| 11 per 1000 | 40 per 1000 (9 to 163) | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; OR: Odds ratio; SD: Standard deviation; SNRI: Serotonin‐norepinephrine reuptake inhibitor; SSRI: selective serotonin reuptake inhibitor | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 Limitations in study design, imprecision (insufficient data). 2 Limitations in study design, imprecision (insufficient data), indirectness (lack of generalisability). 3 Limitations in study design, imprecision (insufficient data), inconsistency (heterogeneity).
Summary of findings 2. SSRIs or SNRIs versus other antidepressants for tension‐type headache.
| SSRIs or SNRIs compared to other antidepressants for tension‐type headache | ||||||
| Patient or population: patients with tension‐type headache Intervention: SSRIs or SNRIs Comparison: other antidepressants | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No. of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Other antidepressants | SSRIs or SNRIs | |||||
|
Headache frequency
(number of days with headache) Follow‐up: 2 months |
The mean headache frequency ranged across control groups from 14.4 to 18.6 days with headache | The mean headache frequency in the intervention groups was 0.76higher (2.05 lower to 3.57 higher) | 152 (2 studies) | ⊕⊝⊝⊝ very low1 | Two additional studies (Manna 1994; Oguzhanoglu 1999) evaluated this outcome but were not pooled due to the lack of quantitative data | |
|
Headache intensity
(mean score) Follow‐up: 2 to 4 months |
The mean headache severity score ranged across control groups from 3.0to 3.8 | The mean headache severity score in the intervention groups was 0.32 higher (0.55 lower to 1.19 higher) | 152 (2 studies) | ⊕⊝⊝⊝ very low1 | Four additional studies (Langemark 1993; Manna 1994; Oguzhanoglu 1999; Walker 1997) evaluated this outcome but were not pooled due to the lack of quantitative data | |
|
Headache duration
(hours) Follow‐up: 2 to 4 months |
The mean headache duration ranged across control groups from 5.39 to 6.5 | The mean headache duration in the intervention groups was 1.26 higher (0.06 lower to 2.45 higher) | 152 (2 studies) | ⊕⊕⊝⊝ low2 |
||
|
Symptomatic/analgesic medication for acute headache attacks
(doses/4 weeks) Follow‐up: 2 months |
The mean symptomatic/analgesic medication for acute headache attacks ranged across control groups from 18.9 to 25.3 doses | The mean symptomatic/analgesic medication for acute headache attacks in the intervention groups was 4.98 higher (1.12 lower to 8.84 higher) | 152 (2 studies) | ⊕⊕⊝⊝ low2 | Two additional studies (Langemark 1993; Walker 1997) evaluated this outcome but were not pooled due to the lack of quantitative data | |
| Walker 1997 | ||||||
|
Headache index Follow‐up: 2 months |
The mean headache index ranged across control groups from 11.4 to 616 | The mean headache index in the intervention groups was 0.42 SDs higher (0 to 0.85 higher) | 152 (2 studies) | ⊕⊝⊝⊝ very low3 | As a rule of thumb, 0.2 SDs represents a small difference, 0.5 moderate and 0.8 large (Cohen 1988) | |
| Quality of life | See comment | See comment | Not estimable | ‐ | ⊕⊕⊝⊝ low2 | One study (Walker 1997) reported qualitative results on this outcome |
|
Withdrawals due to adverse events Follow‐up: 2 months |
Study population | OR 1.04 (0.41 to 2.60) | 257 (4 studies) | ⊕⊕⊝⊝ low2 | ||
| 85 per 1000 | 88 per 1000 (37 to 194) | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; OR: Odds ratio; SD: Standard deviation; SNRI: Serotonin‐norepinephrine reuptake inhibitor; SSRI: selective serotonin reuptake inhibitor | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 Limitations in study design, imprecision (insufficient data), inconsistency (heterogeneity). 2 Limitations in study design, imprecision (insufficient data). 3 Limitations in study design, imprecision (insufficient data), indirectness (lack of generalisability).
Headache frequency.
Headache intensity.
Headache duration.
Symptomatic/analgesic medication use for acute headache attacks.
Headache index.
Quality of life.
Withdrawals due to adverse events.
Search methods for identification of studies
The search strategies used for this review are common to a review on SSRIs and SNRIs for migraine prophylaxis in adults (Banzi 2015).
Electronic searches
For the original review, we searched the Cochrane Central Register of Controlled Trials (CENTRAL 2003, Issue 4), MEDLINE (1966 to January 2004), EMBASE (1994 to May 2003), , andHeadache Quarterly (1990 to 2003). For this update, we applied a revised search strategy to reflect the broader type of intervention (SSRIs and SNRIs).
We searched:
CENTRAL (Issue 10 of 12, 2014) on the Cochrane Library;
MEDLINE (1946 to week 1, November 2014) via OVID;
EMBASE (1980 to November 18th 2014),via OVID; and
PsycINFO (1987 to week 2November 2014) via OVID.
Details of search strategies are provided in Appendix 1.
Searching other resources
We also searched trial registries (the metaRegister of controlled trials (mRCT) (www.controlled‐trials.com/mrct), clinicaltrials.gov and the WHO International Clinical Trials Registry Platform (ICTRP) (http://apps.who.int/trialsearch/) for ongoing trials (November 2014). Additional strategies for identifying trials included searching the reference lists of review articles and included studies, searching books related to headache, consulting experts in the field of headache, contacting the authors of trial reports, and contacting pharmaceutical companies to identify additional published or unpublished data.
Data collection and analysis
Compared to the previous version of this review, we revised the assessment of methodological quality of included trials to include the most recent risk of bias approach (Assessment of risk of bias in included studies).
Selection of studies
Two review authors independently screened titles and abstracts from the search and judged whether trials fulfilled the inclusion or exclusion criteria. Disagreements were resolved through discussion with a third author and by contacting the study authors, if needed. Review authors were not blinded to the names of the study authors, their institutions, the journal of publication, or the results. We retrieved all potentially relevant articles for the assessment of the full publication.
Data extraction and management
Two review authors independently abstracted information on study methods (design, duration, randomisation, blinding, withdrawals), participants (age, sex, type of headache, duration of disease, co‐existing depression and other psychiatric illnesses, and concomitant drugs), interventions (type of drug, route of administration, and dosage), outcomes, and adverse events, using specially designed, pre‐tested electronic extraction forms. We resolved disagreements through discussion with a third review author. We entered data into Review Manager (RevMan 2014) for analysis.
When outcomes were reported in dichotomous form (success/failure), we required that the threshold for distinguishing between success and failure be clinically significant (for instance, more than a 50% reduction in frequency or severity).
When outcome data were reported on an ordinal scale, we selected a threshold based on the definition of clinically significant improvement and converted the data into dichotomous form. If categorical data could not be split into dichotomous outcomes meeting our a priori definition, they were not included in the analysis.
When a trial used pre‐ and post‐treatment scores to calculate a change score for each patient and, then, used these within‐patient change scores to calculate a group mean change score, we recorded and analysed the group mean change scores. When only post‐treatment data were available, we used these, relying on allocation to achieve between‐group balance.
Assessment of risk of bias in included studies
Two review authors assessed the risk of bias for each of the included studies using the 'Risk of bias' tool developed by The Cochrane Collaboration (Higgins 2011). This includes five domains of bias: selection, performance, attrition, detection and reporting, as well as an ‘other bias’ category to capture other potential threats to validity.
Selection bias included an assessment of adequate sequence generation as well as allocation concealment. We assessed sequence generation to be at low risk when studies clearly specified a method for generating a truly random sequence. We assessed allocation concealment to be at low risk if the method used to ensure that investigators enrolling participants could not predict group assignment was described. Performance and detection bias were incorporated under the domain blinding in the 'Risk of bias' tool: we did not consider them separately as the large majority of outcomes were self reported by the patients (i.e. using diaries). We assessed this to be low risk for studies that reported blinding of participants and study personnel. We assessed studies as low risk for attrition bias if an adequate description of participant flow through the study was provided, the proportion of missing outcome data was relatively balanced between groups and the reasons for missing outcome data were provided, relatively balanced across groups and considered unlikely to bias the results.
We assessed studies to be at low risk of reporting bias when a published protocol was available and all specified outcomes were included in the study report; we assessed studies without a published protocol as unclear. When an outcome measure was specified and the results were not reported either at baseline or at follow‐up, we considered the study to be at high risk of reporting bias.
Other potential threats to validity were assessed, including early trial discontinuation for benefit and trial sponsorship.
Review authors were not blinded with respect to study authors, institution, or journal. Disagreements were resolved through discussion with a third author.
Measures of treatment effect
In order to assess efficacy, we extracted raw data for outcomes of interest (means and standard deviations for continuous outcomes and number of events for dichotomous outcomes) where available in the published reports.
For dichotomous outcomes, we calculated odds ratios (ORs) along with 95% confidence intervals (CIs). Where outcomes were measured on standard scales, we calculated weighted mean differences (WMDs). Where different scales were used to measure the same or similar outcomes, we calculated standardised mean differences (SMDs).
We calculated number needed to treat to benefit, if possible, although this was a rare circumstance due to the large number of statistically insignificant comparisons. We analysed toxicity for total withdrawals due to adverse events. We calculated number needed to treat to harm, if possible.
Unit of analysis issues
Cross‐over trials
In randomised cross‐over studies, individuals receive each intervention sequentially in a random order. Cross‐over studies usually contain a washout period, which is a stage after the first treatment, but before the second treatment, where time is given for the active effects of the first treatment to wear off before the new treatment begins (that is, to reduce the carry‐over effect). A concern with the cross‐over design is the risk of a carry‐over effect when the first treatment affects the second. Inadequate washouts are seen when the carry‐over effect exceeds the washout period. For this review, we considered an adequate washout period for cross‐over studies to be a minimum of one week. When including cross‐over studies with no indication about washout or an inadequate period we used only the first arm data. Even though this method does not consider all of the information provided, it avoids inappropriate consideration of correlated information. If results are available split by the particular sequence each participant received or adjusted for period effects and the washout is appropriate, we included the effect estimate and its variability in the meta‐analysis using the generic inverse‐variance method.
Cluster trials
We assessed whether the unit of analysis was appropriate for the unit of randomisation. If we were to include cluster‐RCTs, we would use the intra‐class correlation coefficient (ICC) to convert trials to their effective sample size before incorporating them into the meta‐analysis.
Dealing with missing data
We described missing data and the dropouts/attrition for each included study in the Characteristics of included studies. We planned the analysis of outcomes on an intention‐to‐treat basis; in other words, we included all of the participants randomised to each group in the analyses, regardless of whether or not they received the allocated intervention, and irrespective of how the original study authors analysed the data. However, because only a few studies reported these data, we analysed the studies according to an "available case" approach.
We contacted study authors by email to clarify any missing data. For outcomes reported on a continuous scale, we anticipated that many trials would report pre‐ and post‐treatment group means without reporting data on the variance associated with these means. We attempted to calculate or estimate variances based on primary data or test statistics whenever precise P values or test statistics were provided in sufficient detail.
Assessment of heterogeneity
We assessed statistical heterogeneity by examining the I2 statistic (Deeks 2011), a quantity that describes the proportion of variation in point estimates that is due to variability across studies, rather than sampling error.
We interpreted I2 as suggested by the latest version of Higgins 2011:
0% to 40%: might not be important;
30% to 60%: may represent moderate heterogeneity;
50% to 90%: may represent substantial heterogeneity; and
75% to 100%: considerable heterogeneity.
In addition, we used a Chi2 test of homogeneity to determine the strength of evidence that heterogeneity is genuine.
We explored clinical variation across studies by comparing the distribution of important participant factors among trials (for example, age) as well as trial factors (randomisation concealment, blinding of outcome assessment, losses to follow‐up, treatment type, and cointerventions).
Data synthesis
We performed the analyses using Review Manager (RevMan 2014). We assumed a considerable clinical heterogeneity and usually combined the studies using the random‐effects model. When including both parallel and cross‐over studies with an adequate washout period, we used the inverse variance method, as recommended by Elbourne 2002. In the meta‐analysis, the weight of each study is inversely proportional to the variance (one over the square of the standard error) (Deeks 2011).
Summary of findings table
We synthesised the main outcome measures (see also ‘Types of outcome measures’) in two ‘Summary of findings’ tables, comparing SSRIs or SNRIs to placebo (Table 1) or to other active comparators (Table 2). Whenever possible, we used the control arm to calculate the ‘assumed risk’ values. We assessed the overall quality of evidence for each outcome using the GRADE approach, as recommended in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011), against five factors: study design and limitations, consistency of results, directness (generalisability), precision (sufficient data), and reporting of the results across all studies that measure that particular outcome. The quality starts at high when high quality RCTs provide results for the outcome, and reduces by a level for each of the factors not met.
High quality evidence: there are consistent findings among at least 75% of RCTs with no limitations of the study design, consistent, direct and precise data and no known or suspected publication biases. Further research is unlikely to change either the estimate or our confidence in the results.
Moderate quality evidence: one of the domains is not met. Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate.
Low quality evidence: two of the domains are not met. Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate.
Very low quality evidence: three of the domains are not met. We are very uncertain about the results.
No evidence: no RCTs were identified that addressed this outcome.
Subgroup analysis and investigation of heterogeneity
We investigated the effects of the following subgroup analyses.
Trials in which patients were depressed (as determined by a rating scale or clinical interview) versus trials in which patients were not depressed.
Trials evaluating the various SSRIs and SNRIs separately.
Sensitivity analysis
We did not plan any sensitivity analysis.
Results
Description of studies
Results of the search
The new electronic search up to November 2014 retrieved a total of 4508 results after discarding duplicates. After retrieving full‐text articles, we included two new studies (120 participants) (Singh 2002; Zissis 2007), along with the six studies already included. We classified one study published only as a poster presentation (Gabrielidou 1998), one Chinese (Zhou 2006), and one Russian (Tarasova 2008) publication as awaiting classification (Characteristics of studies awaiting classification). We did not find ongoing studies by searching clinical trial registries.
See Figure 1 (Liberati 2009).
1.
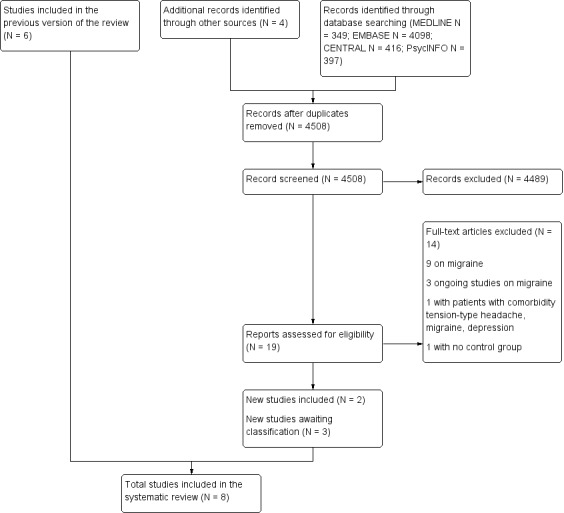
Flow diagram.
Included studies
Overall, we included eight studies published between 1993 and 2007 in this updated review (Bendtsen 1996; Boz 2003; Langemark 1993; Manna 1994; Oguzhanoglu 1999; Singh 2002; Walker 1997; Zissis 2007).
Two trials were multicentric (Langemark 1993; Zissis 2007). The median length of studies was 12 weeks (range: eight to 24).
All but two (Bendtsen 1996; Langemark 1993) were parallel trials. Bendtsen 1996 was a three‐arm cross‐over trial comparing two active interventions (citalopram and amitriptyline) and placebo. In this study patients were treated for an 8‐week period with each treatment, with a washout period (two weeks) between treatments. Carry‐over and time period effects were not present. Therefore, this study was analysed as if it were a parallel‐group trial, combining data from all treatment periods. In Langemark 1993 a response‐conditional cross‐over design was used: only patients considered non‐responders or patients with intolerable adverse events were crossed over to the alternative treatment, without a washout period in between. Since SSRIs and SNRIs are long‐acting agents, we considered 'carry‐over' and 'period' effects to potentially affect the results of this trial. Thus, we used only data from the first period of this cross‐over study.
The washout period was only described in four studies (Bendtsen 1996; Manna 1994; Singh 2002; Zissis 2007).
See Characteristics of included studies.
Participants
The included studies enrolled a total of 412 participants, with women generally more represented than men (68% versus 32%). Singh 2002 only reported that males outnumbered females, but provided no data. Oguzhanoglu 1999 did not report sex of non‐completers. The mean age of the participants ranged from 28 (Singh 2002) to 42 (Langemark 1993) years old.
All the studies included participants affected by chronic forms of tension‐type headache. Seven studies (N = 362) enrolled patients with chronic tension‐type headache diagnosed following the diagnostic criteria of IHS (Bendtsen 1996; Boz 2003; Manna 1994; Oguzhanoglu 1999; Walker 1997; Singh 2002; Zissis 2007). Langemark 1993 (N = 50) did not specify the diagnostic criteria for the selection of participants. Oguzhanoglu 1999 also included a subgroup of patients with episodic tension‐type headache and Zissis 2007 included all subtypes of tension‐type headache.
Two studies (Manna 1994; Walker 1997) included depressed participants but only Walker 1997 compared depressed and non‐depressed subjects. In the remaining studies, depressed subjects were clearly excluded. Only one study excluded participants with acute medication overuse (Bendtsen 1996).
The median number of participants randomised in the included studies was 52 and ranged from 37 (Walker 1997) to 90 (Boz 2003). Losses to follow‐up were moderate, reaching 20% in one trial only (Langemark 1993).
Interventions and controls
Two studies compared two SSRIs (citalopram (Bendtsen 1996); sertraline (Singh 2002)) and one SNRI (venlafaxine (Zissis 2007)) with placebo. The remaining trials compared SSRIs (citalopram (Bendtsen 1996); fluoxetine (Oguzhanoglu 1999; Walker 1997); sertraline (Boz 2003); paroxetine (Langemark 1993); and fluvoxamine (Manna 1994) with other antidepressant agents (amitriptyline) (Bendtsen 1996; Boz 2003; Oguzhanoglu 1999); sulpiride (Langemark 1993); mianserin (Manna 1994); and desipramine (Walker 1997).
Escalation of the SSRI dose was permitted (i.e. paroxetine to a maximum of 30 mg/day; fluvoxamine to a maximum of 100 mg/day; fluoxetine to a maximum of 40 mg/day) in three studies (Langemark 1993; Manna 1994; Walker 1997). The remaining studies used fixed doses of experimental antidepressant. In the active‐comparator trials, the antidepressant dose in the control arm was increased progressively over the first two weeks of treatment.
The only trial evaluating SNRI (Zissis 2007) used an extended release formulation of venlafaxine in a daily dosage of 150 mg (two capsules containing a 75 mg dose).
We did not identify any study comparing SSRIs or SNRIs with a drug treatment other than antidepressants or with a non‐pharmacological treatment (behavioural or physical therapy). We did not find any study comparing two different SSRIs or SNRIs head‐to‐head.
Country and language of publication
Two studies were carried out in Turkey (Boz 2003; Oguzhanoglu 1999), two in Denmark (Bendtsen 1996; Langemark 1993), and one each in Greece (Zissis 2007), the UK (Walker 1997), Italy (Manna 1994), and India (Singh 2002). All were published in English.
Excluded studies
In the original review, we excluded nine studies because they were not randomised, two because they were case reports, and one because the license of the SSRI studied (femoxetine) had been discontinued by drug companies (company communication, Knoll, February 1988, and Martec, February 1990). In the original review, two studies (Saper 1994 and Bussone 1991) were excluded because it was impossible to separate data on patients with migraine from data on patients with chronic daily headache or chronic tension‐type headache. We contacted the authors, who confirmed that study data and analyses are no longer available. In this update, we excluded one study (Rampello 2004) because it recruited patients with comorbidity of depression, migraine, or tension‐type headache.
We excluded a Russian publication previously judged as a study awaiting classification because of the lack of a control group. (Voznesenskaia 1999).
Risk of bias in included studies
The overall risk of bias is presented graphically in Figure 2 and summarised in Figure 3. The majority of included trials had methodological or reporting shortcomings. We cannot exclude the fact that poor reporting could have affected our assessment.
2.
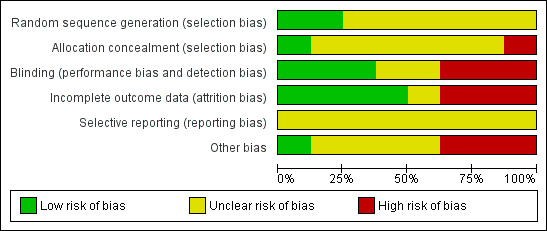
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.
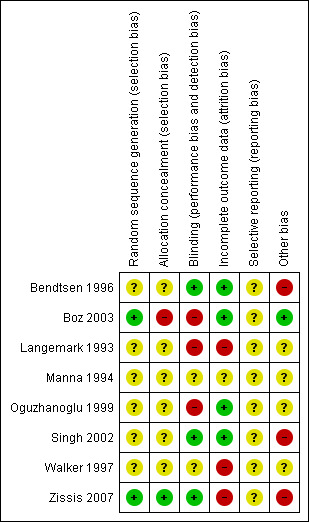
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Only one trial reported an adequate random sequence generation and concealment and was then judged at low risk of selection bias (Zissis 2007). For Boz 2003, we were able to contact the authors and obtain additional information: the generation of sequence was done using computer software (not specified), and there was no attempt to conceal patient assignment. The remaining studies did not provide any information useful to evaluate how the random sequence was generated and concealed, and so we judged these at unclear risk of selection bias.
Blinding
Three studies (33%) were double‐blind and reported information on how participants, physicians, or both, were blinded to the study treatments (Bendtsen 1996; Singh 2002; Zissis 2007). We judged at high risk of bias two open‐label studies (Boz 2003; Oguzhanoglu 1999), and one in which drugs were identical in appearance, but the two groups received different numbers of tablets (Langemark 1993). For the two remaining trials we did not have sufficient details to judge how the blinding was planned and maintained.
Incomplete outcome data
We judged four studies (Bendtsen 1996; Boz 2003; Oguzhanoglu 1999; Singh 2002) at low risk of attrition bias, while three trials (Langemark 1993; Walker 1997; Zissis 2007) were at high risk due to a moderate dropout rate unbalanced across arms and with reasons for drop out not fully reported. Manna 1994 did not report if there were withdrawals after randomisation.
Selective reporting
We did not find any information that allowed us to assess the possible selective reporting of studies and outcomes. None of the trials included in this version of the review are registered nor have a publicly available protocol for consultation. All the studies used multiple outcomes without a predefined primary outcome and multiple time points for the assessment. This suggests possible selective outcome reporting.
Other potential sources of bias
Four studies did not provide any information about financial sponsorship (Langemark 1993; Manna 1994; Oguzhanoglu 1999; Walker 1997). We judged at high risk of bias for commercial sponsorship two studies supported by the manufacturer of the antidepressants being tested (Bendtsen 1996; Singh 2002), and one in which the 'drug company' was responsible for randomisation and blinding (Zissis 2007). Boz 2003 was an independent trial without financial support.
Only one of the included studies reported an adequate sample size calculation (Zissis 2007). Most of the studies were clearly underpowered and, therefore, more prone to be inconclusive (e.g. not enabled to find a statistically significant difference which is true) (Altman 1990; Hotopf 1997; Hotopf 1999). The median sample size was 45 and ranged from 35 to 90. The mean dropout rate was 16% of all randomised patients, leading to much smaller sample sizes across studies.
With respect to the previous version of this review which considered both migraine and tension‐type headache, the median sample size per arm nearly doubled (25 to 45). However, concerns remain about the fact that many studies are likely to be underpowered to detect any difference (Moja 2005).
The lack of statistical power is also reflected in the use of a large number of rating scales to measure outcomes. Furthermore, the majority of trials analysed the multiple outcomes at many different time intervals (four weeks, eight weeks, etc.), increasing exponentially the number of comparisons. Performing multiple comparisons easily leads to detecting statistically significant differences that are spurious (Thornley 1998). Only two studies (Bendtsen 1996; Boz 2003) stated that some form of correction for multiple testing was used (Bonferroni's correction).
The majority of the trials actually conducted per protocol analyses for the participants who completed the study period only. Zissis 2007 described that statistical analysis of patient's withdrawal was performed using the last‐observation‐carried‐forward (LOCF) method. In many trials, the reporting of missing data was unclear.
Effects of interventions
When possible, for each efficacy outcome, we focused on outcomes at two different follow‐up time points in the same analysis graph (eight and 12 weeks).
SSRIs or SNRIs versus placebo
Two new studies (Singh 2002; Zissis 2007) and one study already included in the previous version of this review (Bendtsen 1996) compared SSRIs or SNRIs to placebo. The drugs under investigation were citalopram, sertraline, and venlafaxine. None of these trials reported data on quality of life, workdays lost, or cost‐effectiveness outcomes.
We estimated standard deviations for all continuous outcomes reported by Bendtsen 1996. Zissis 2007 reported efficacy data at different follow‐up periods (three, eight, 12 weeks). Data at eight and 12 weeks follow‐up were similar. We pooled results at eight weeks as Bendtsen 1996 also reported data at this follow‐up.
Table 1 summarises data on this comparison.
Primary outcome
Headache frequency
One new study (Zissis 2007), and one study already included in the previous version of this review (Bendtsen 1996), with a total of 127 participants, reported data on headache frequency, the primary outcome of this review. At eight weeks follow‐up, we found no differences in terms of number of days with headache (MD ‐0.96, 95% CI ‐3.95 to 2.03; I2 = 0%; Analysis 1.1; Figure 4).
1.1. Analysis.
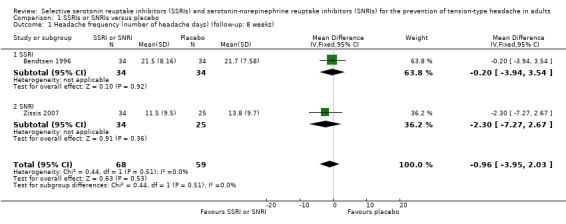
Comparison 1 SSRIs or SNRIs versus placebo, Outcome 1 Headache frequency (number of headache days) (follow‐up: 8 weeks).
4.
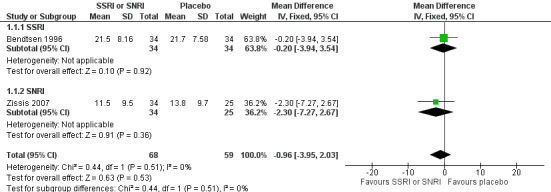
Forest plot of comparison: 1 SSRIs or SNRIs versus placebo, outcome: 1.1 Headache frequency (number of headache days) (follow‐up: 8 weeks).
Secondary outcomes
Headache intensity
Only Bendtsen 1996 reported data on headache intensity (MD ‐0.30, 95% CI ‐1.13 to 0.53).
Headache duration
Only Bendtsen 1996 reported data on headache duration (hours per day) (MD ‐0.07, 95% CI ‐2.69 to 2.55).
Symptomatic/analgesic medication use for acute headache attacks
One new study (Singh 2002) and one study already included in the previous version of this review (Bendtsen 1996), with a total of 118 participants, reported data on symptomatic/analgesic medication use for acute headache attacks. At eight weeks follow‐up, SSRIs seem to reduce the mean analgesic intake compared to placebo of about two doses per month (MD ‐1.87, 95% CI ‐2.09 to ‐1.65; I2 = 0%; Analysis 1.2; Figure 5). This finding is largely driven by the results of a study at high risk of sponsorship bias (Singh 2002).
1.2. Analysis.
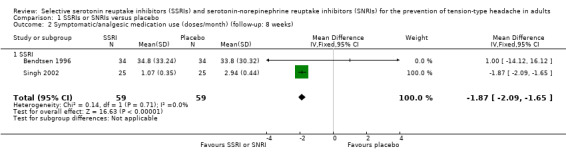
Comparison 1 SSRIs or SNRIs versus placebo, Outcome 2 Symptomatic/analgesic medication use (doses/month) (follow‐up: 8 weeks).
5.
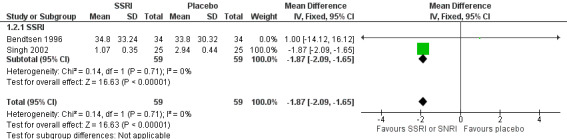
Forest plot of comparison: 1 SSRIs or SNRIs versus placebo, outcome: 1.2 Symptomatic/analgesic medication use (doses/month) (follow‐up: 8 weeks).
Headache index
One new study (Zissis 2007), and one study already included in the previous version of this review (Bendtsen 1996), with a total of 128 participants, reported data on headache indexes (calculated by multiplying the hours with headache by its severity). At eight weeks follow‐up, we found no significant differences in headache index (SMD ‐0.24, 95% CI ‐0.59 to 0.11; I2 = 0%; Analysis 1.3). Singh 2002 also reported data on headache index (calculated as the product of headache frequency per week by severity by duration). However, we did not pool this data in the meta‐analysis, as the reporting on this outcome was unclear and apparently referred only to the within‐group change.
1.3. Analysis.
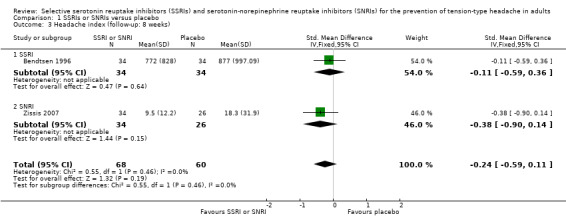
Comparison 1 SSRIs or SNRIs versus placebo, Outcome 3 Headache index (follow‐up: 8 weeks).
Withdrawals from treatment
All three studies provided data on withdrawals from treatment. Bendtsen 1996 reported two dropouts in the citalopram group and three in the placebo, while Zissis 2007 reported nine in the venlafaxine group and 11 in the placebo arm. The reporting of withdrawals in Singh 2002 was unclear: out of the 10 patients who dropped out, we did not consider those excluded in the run‐in period (N = 2), as this is likely to occur before randomisation. The remaining eight dropped out during the follow‐up period (N = 2, and not clear from which arm) and the study treatment (N = 6, five in the placebo arm). The pooled estimate of withdrawals for any reason did not differ between SSRIs or SNRIs and placebo (Peto odds ratio (OR) 0.54, 95% CI 0.25 to 1.19; I2 = 0%; Analysis 1.4).
1.4. Analysis.
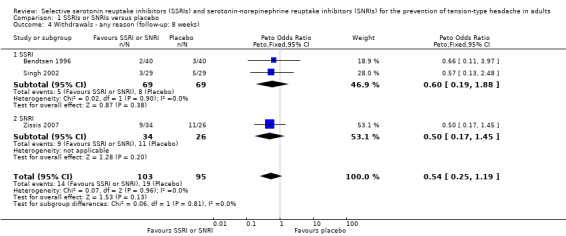
Comparison 1 SSRIs or SNRIs versus placebo, Outcome 4 Withdrawals ‐ any reason (follow‐up: 8 weeks).
Data on patients withdrawing from treatment due to adverse events were few and sparse. Singh 2002 reported that none of the patients had any side effects necessitating withdrawals. We found no evidence of a difference (Peto OR 3.72, 95% CI 0.79 to 17.53; I2 = 69%; Analysis 1.5).
1.5. Analysis.
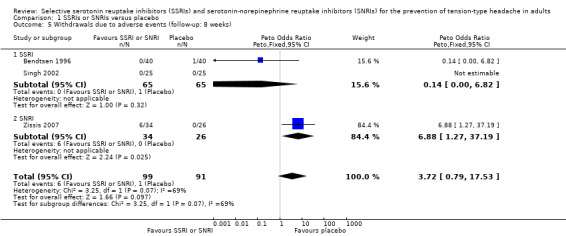
Comparison 1 SSRIs or SNRIs versus placebo, Outcome 5 Withdrawals due to adverse events (follow‐up: 8 weeks).
Minor adverse events
Singh 2002 reported only aggregated data on adverse events. Two studies (Bendtsen 1996; Zissis 2007) contributed to the meta‐analysis (Peto OR 1.57, 95% CI 0.52 to 4.76; I2 = 46%; Analysis 1.6).
1.6. Analysis.
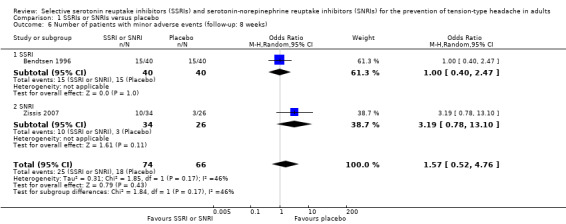
Comparison 1 SSRIs or SNRIs versus placebo, Outcome 6 Number of patients with minor adverse events (follow‐up: 8 weeks).
SSRIs and SNRIs versus another active drug (other antidepressants)
We did not find any new study comparing SSRIs or SNRIs to other antidepressants. Three studies already included in the previous version of this review (Manna 1994; Oguzhanoglu 1999; Walker 1997) provided incomplete data, such that it was not possible to include them in the meta‐analyses of continuous outcome data.
Table 2 summarises data on this comparison.
Primary outcome
Headache frequency
Two studies already included in the previous version of this review (Bendtsen 1996; Boz 2003) with a total of 152 participants reported data on headache frequency (number of days with headache per month) that could be used in the meta‐analysis. At eight weeks, the pooled estimate MD using a random‐effects model was 0.76 (95% CI ‐2.05 to 3.57; I2 = 44%; Analysis 2.1; Figure 6). Heterogeneity was moderate. At 12 weeks, only Boz 2003 reported data (MD 0.80, 95% CI ‐1.29 to 2.89; Analysis 2.1).
2.1. Analysis.

Comparison 2 SSRIs or SNRIs versus other antidepressants, Outcome 1 Headache frequency (number of headache days).
6.

Forest plot of comparison: 2 SSRIs or SNRIs versus another antidepressants, outcome: 2.1 Headache frequency (number of headache days).
Manna 1994 described only within‐group results for the overall study population at eight weeks (both fluvoxamine and mianserin significantly (P < 0.01) reduced the number of days with headache), but stated that fluvoxamine was significantly better than mianserin for this outcome among non‐depressed patients (P < 0.05); no quantitative data were reported. Oguzhanoglu 1999 described only within‐group analyses of data on headache frequency (number of days with headache) and reported no quantitative data. Among patients with chronic tension‐type headache (N = 13), fluoxetine was significantly effective only at eight weeks, while amitriptyline reduced headache frequency significantly at both eight and 12 weeks; among patients with episodic tension‐type headache (N = 19), the situation was reversed: fluoxetine was significantly effective at eight and 12 weeks, while amitriptyline reduced headache frequency only at eight weeks.
Secondary outcomes
Headache intensity
As for the primary outcome, only two studies already included in the previous version of this review (Bendtsen 1996; Boz 2003), with a total of 152 participants, reported data on headache intensity (using a 10‐point scale to assess severity) that could be used in the meta‐analysis. At eight weeks, we found no evidence for a difference (MD: 0.32, 95% CI ‐0.55 to 1.19; I2 = 72%; Analysis 2.2). Heterogeneity between the studies was substantial. At 12 weeks (Boz 2003) the tricyclic antidepressant amitriptyline showed a better efficacy than the SSRI sertraline (MD 1.70, 95% CI 1.06 to 2.34; Analysis 2.2).
2.2. Analysis.
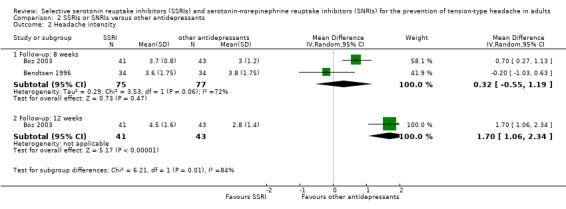
Comparison 2 SSRIs or SNRIs versus other antidepressants, Outcome 2 Headache intensity.
Four additional studies assessed headache intensity but provided data which cannot be pooled in the meta‐analysis: Walker 1997, Langemark 1993 and Manna 1994 used ordinal scales of 10, 5, and 3 points, respectively, to assess headache intensity, while Oguzhanoglu 1999 did not clearly define the severity measure used. Walker 1997 found no differences between fluoxetine and desipramine in change of pain score from baseline to three months; no quantitative data were reported. Langemark 1993 reported that at eight weeks headache severity scores were decreased from baseline levels with both paroxetine (mean change: ‐0.4) and sulpiride (mean change: ‐0.7); there was no difference between the two treatments (P = 0.24). Manna 1994 described only within‐group headache severity results for the overall study population at eight weeks (both fluvoxamine and mianserin reduced headache severity: P < 0.01), but stated that fluvoxamine was "significantly better than mianserin" for this outcome among non‐depressed patients (P < 0.05); no quantitative data were reported. Oguzhanoglu 1999 described only within‐group analyses of results for headache severity and reported no quantitative data. Among patients with chronic tension‐type headache (N = 13), "neither drug was effective against pain intensity." Among patients with episodic tension‐type headache (N = 19), fluoxetine significantly reduced pain severity at eight weeks, but not at 12 weeks; amitriptyline had no significant effect.
Headache duration
Two studies already included in the previous version of this review (Bendtsen 1996; Boz 2003), with a total of 152 participants, reported data on headache duration. Bendtsen 1996 reported duration results as headache hours per four weeks; we recalculated the data as headache hours per day. At eight weeks, the MD was 1.26 (95% CI 0.06 to 2.45; I2 = 0%; Analysis 2.3), slightly favouring amitriptyline. This trend was confirmed at 12 weeks follow‐up (Boz 2003) (MD 1.30, 95% CI ‐0.39 to 2.99; Analysis 2.3).
2.3. Analysis.
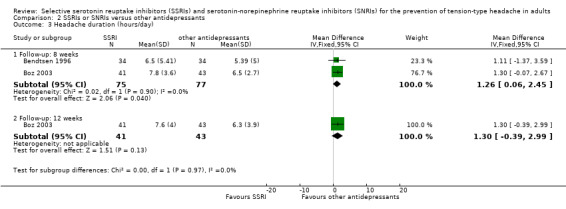
Comparison 2 SSRIs or SNRIs versus other antidepressants, Outcome 3 Headache duration (hours/day).
Symptomatic/analgesic medication use for acute headache attacks
This outcome was considered in four studies already included in the previous version of this review, but only two (Bendtsen 1996; Boz 2003) reported quantitative data. At eight weeks, the pooled estimate MD (doses/four weeks) was 4.98 (95% CI 1.12 to 8.84; I2= 0%; Analysis 2.4), favouring tricyclic antidepressants. At 12 weeks, only Boz 2003 reported data, which again favoured amitriptyline (MD 5.40, 95% CI 1.10 to 9.70; Analysis 2.4).
2.4. Analysis.
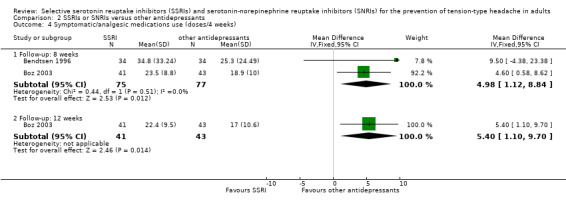
Comparison 2 SSRIs or SNRIs versus other antidepressants, Outcome 4 Symptomatic/analgesic medications use (doses/4 weeks).
Of the two studies that cannot be pooled, Langemark 1993 reported that at eight weeks the number of analgesic tablets taken per day had significantly decreased compared to baseline with both paroxetine (mean change: ‐0.8 tablets; P < 0.05) and sulpiride (mean change: ‐1.3 tablets; P < 0.005); there was no significant difference between the two treatments (P = 0.39). Walker 1997 reported no significant differences between fluoxetine and desipramine in reduction in analgesic intake from baseline to three months; no quantitative data were reported.
Headache index
Two studies already included in the previous version of this review reported results for this outcome: Bendtsen 1996 utilised a migraine headache index that combined intensity and duration of headache, while Boz 2003 calculated the headache index as headache frequency times average intensity times duration divided by 28 days. It was possible to combine results from the two studies only at eight weeks: the pooled estimate included 152 patients, of whom 75 received a SSRI (citalopram or sertraline) and 77 a tricyclic antidepressant (amitriptyline). The SMD when using a random‐effects model was 0.42 (95% CI 0.00 to 0.85; I2 = 42%; Analysis 2.5), favouring the tricyclic antidepressant. Bendtsen 1996 reported that the difference between amitriptyline and placebo was statistically significant (P = 0.002) favouring amitriptyline, whereas the difference between citalopram and placebo was not significant (P = 0.68).
2.5. Analysis.
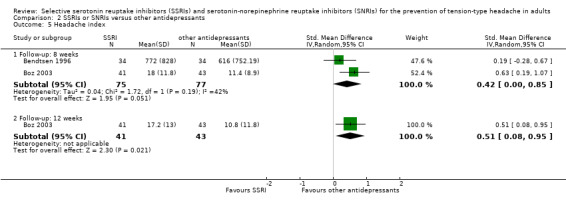
Comparison 2 SSRIs or SNRIs versus other antidepressants, Outcome 5 Headache index.
At 12 weeks, only Boz 2003 reported data: the resulting SMD was 0.51 (95% CI 0.08 to 0.95; Analysis 2.5), again favouring the tricyclic antidepressant. Boz 2003 also reported the number of patients with more than a 50% reduction in headache index after 12 weeks: significantly more patients improved up to the threshold in the amitriptyline group than in the sertraline group (OR 0.30; 95% CI 0.12 to 0.75).
Quality of life
Only Walker 1997 reported results for this outcome. Investigators used the Medical Outcomes Study, Short Form‐36 (SF‐36) to assess quality of life. They reported that SF‐36 scores were significantly improved at 12 weeks on all but two subscales ('role functioning‐emotional' and 'social functioning') among patients who completed the trial (N = 25, both treatment groups combined), and that there was no significant difference between the two treatment groups for this outcome. No between‐group P values were reported, and the quantitative data reported were not broken down by treatment group.
Withdrawals from treatment
Four trials already included in the previous version of this review (Bendtsen 1996; Boz 2003; Langemark 1993; Walker 1997) contributed data to these analyses. Bendtsen 1996, a cross‐over trial appropriately designed and executed, reported the dropouts for both periods, therefore we counted the enrolled patients in both arms. Overall, these analyses included 217 participants: 127 received a SSRI and 130 another antidepressant. Among participants receiving a SSRI, 14.2% (18) withdrew from treatment, compared with 10.0% (13) of those receiving other antidepressants. There was no significant difference between the two treatments (Peto OR 1.55, 95% CI 0.71 to 3.38; I2 = 0%; Analysis 2.6). Among the participants receiving a SSRI, 8.7% (11/127) withdrew from treatment because of an adverse event, compared with 8.5% (11/130) of those treated with the other antidepressants. There was no significant difference between the two treatments (Peto OR 1.04, 95% CI 0.41 to 2.60; I2 = 25%; Analysis 2.7). Reasons for withdrawals are specified in the Characteristics of included studies tables.
2.6. Analysis.
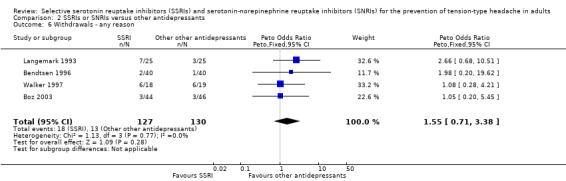
Comparison 2 SSRIs or SNRIs versus other antidepressants, Outcome 6 Withdrawals ‐ any reason.
2.7. Analysis.
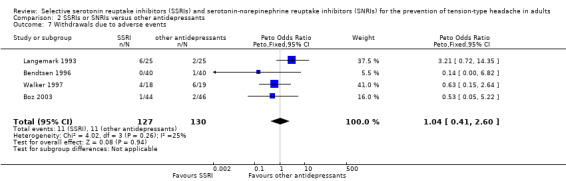
Comparison 2 SSRIs or SNRIs versus other antidepressants, Outcome 7 Withdrawals due to adverse events.
Minor adverse events
Five of the six included trials reported some data on minor adverse effects (Bendtsen 1996; Boz 2003; Langemark 1993; Manna 1994; Walker 1997). Only Bendtsen 1996 reported the overall number of patients with adverse events in a form we could analyse (data were referred to case‐person and not to the overall numbers of adverse effects). In this trial investigators found that amitriptyline induced significantly more minor adverse effects than citalopram (OR 0.13, 95% CI 0.05 to 0.36). The most frequent minor adverse effects in the amitriptyline group were drowsiness and dry mouth. The number needed to treat to harm was eight (95% CI 3 to 20).
Mood improvement
Two studies (Manna 1994; Walker 1997) evaluated the effect on depression, neither of which reported useable quantitative data. Manna 1994 evaluated patients using the Zung Self‐Rating Depression Scale and the Hamilton Depression Rating Scale. At baseline, 12 of 20 patients in the fluvoxamine group were diagnosed with slight or mild mood depression, and 13 of 20 in the mianserin group received the same diagnosis. After eight weeks of treatment, investigators reported that scores on both scales were significantly improved in both treatment groups (P < 0.01), with no significant difference between the two groups on either scale (no P values reported). The data were given only in the form of histograms, and depressed patients were not analysed as a subgroup of interest.
Walker 1997 used the Hospital Anxiety and Depression Scale (HADS) and the Montgomery and Asberg Depression Rating Scale (MADRS) to evaluate patients. Using MADRS, eight of 37 patients (22%) entering the trial (both treatment groups combined) were depressed; using HADS with a cut‐off score of 20, 12 of 37 (32%) had depression. Investigators reported that both MADRS and HADS scores were significantly improved at 12 weeks among patients who completed the trial (N = 25, both treatment groups combined), and that there was no significant difference between the two groups for this outcome. No between‐group P values were reported, and the quantitative data reported were not broken down by treatment group.
Planned subgroup analyses
Two trials (Manna 1994; Walker 1997) enrolled both participants with and without depression, but did not provide subgroup data.
Due to the low number of studies included, we could not analyse the different SSRIs and SNRIs separately.
Prevention of transformation to a chronic headache syndrome
We did not find studies focusing on whether SSRIs or SNRIs can prevent the transformation of episodic into chronic tension‐type headache.
Discussion
Summary of main results
Evidence supporting the use of SSRIs or SNRIs to decrease headache frequency ‐ the most relevant clinical outcome in adults with frequent episodic or chronic tension‐type headache ‐ is scarce. We included two new studies in this update, one on the SSRI sertraline (Singh 2002) and one on the SNRI venlafaxine (Zissis 2007), which did not provide any new substantial evidence in the field. Overall, we identified seven studies comparing SSRIs with placebo and with other antidepressants (amitriptyline, desipramine, mianserin, sulpiride) and one study comparing the SNRI venlafaxine with placebo. We did not find studies comparing SSRIs or SNRIs with non‐antidepressant drug treatments for preventing headache (e.g. tizanidine, botulinum toxin) or with physical or behavioural treatments for headache. SSRIs and SNRIs are not better than placebo or tricyclic antidepressants in reducing the number of days with headache at 8 weeks of follow‐up.
Among secondary outcomes, we found that SSRIs or SNRIs did not reduce headache intensity and duration and did not improve headache index when compared to placebo after 8 or 12 weeks of treatment. One trial at high risk of bias for commercial sponsorship (Singh 2002) suggested that sertraline can reduce the use of symptomatic/analgesic medications by 1.87 doses/week compared to placebo. Amitriptyline, a tricyclic antidepressant, appeared to be more effective in reducing headache intensity and duration especially at longer follow‐up (12 and 16 weeks). Our results also showed a significantly higher intake of symptomatic/analgesic medication in patients treated with SSRIs than in patients treated with tricyclic antidepressants, equivalent to five more doses per month (95% CI 1 to 9; two studies, N = 152). Results may have been confounded by the inclusion of participants with and without acute medication overuse.
The data on the safety profile of SSRIs and SNRIs derived from the included studies were also scarce. The included studies analysed a small number of participants, and thus they lack the power to detect adverse events. Moreover, the reporting of adverse events was generally unclear. No differences in terms of dropouts due to adverse events or minor adverse events were detected compared with placebo or other antidepressants. SSRIs and SNRIs appeared to be better tolerated than the tricyclic antidepressant, amitriptyline, but no conclusions can be drawn on the basis of the included trials.
Tension‐type headache affects many aspects of an individual's life, including both social and occupational roles. We did not find any mention of days off work or any data on cost‐effectiveness. Days off work is a specific, strong measure to assess headache improvement from a more socio‐economic perspective. Only one study analysed quality of life, using the SF‐36 Medical Outcomes Study (Walker 1997), and did not find any significant difference between fluoxetine and desipramine. Several quality of life scales have been validated and are now available. They assess the impact that headache has on activities of daily living and include many items related to general well‐being such as pain and mood states. Many headache indexes used in headache trials replicate some subscale or items already included in quality of life scales. However, the validity of some of the rating tools adopted in the trials is unknown, the clinical relevance questionable, and the use seems rather opportunistic (Moja 2007). Finally, quality of life is a global measure capable of making useful comparisons between adverse events of drugs (Hotopf 1997).
We cannot draw any conclusions about possible differences in efficacy or safety profile between SSRIs and SNRIs. Due to the small amount of evidence in the field and its low quality, it was not possible to explore subgroup differences in efficacy related to different selectivity profiles.
Overall completeness and applicability of evidence
Data that inform this review are few and derived from poorly designed, conducted, and reported trials. Five studies reported data on the most relevant clinical outcome, headache frequency, however only four could be pooled in meta‐analyses, for a total of fewer than 200 participants. All the included studies assessed participants who had been diagnosed with chronic tension‐type headache. Fewer participants with episodic tension‐type headache were included in one study, thus we cannot draw any conclusion on the efficacy and safety of SSRIs and SNRIs in this population. Reporting was often incomplete, making some studies uninformative. The applicability of this scarce evidence is also an issue, mainly because the analysed studies used short follow‐up and outcomes with small clinical value. Having said that, the findings of this review suggest that the use of SSRIs and SNRIs do not provide benefits that may matter to patients.
Quality of the evidence
All the included trials can be considered at unclear or high risk of bias (see Risk of bias in included studies). Six out of eight studies did not report information on the random sequence generation and allocation concealment. Four trials were in fact open‐label in a field in which blinding is highly desirable (Wood 2008). Two studies did not provide information on how blinding was maintained. Another major concern is that almost all of the included trials are likely to be underpowered; only one reported how the sample size was calculated and a reference to the use of an intention‐to‐treat analysis (Zissis 2007). The small sample size is a consistent marker of overestimation of treatment effects. There was a strong inclination to perform multiple testing. Finally, there were frequent ambiguities in presentation of the results of the analyses, emphasising within‐group comparisons. Some readers may find these methodological problems surprising; we did not. Previous methodological work showed that these are common problems in studies of SSRIs and related antidepressants (Hotopf 1997; Hotopf 1999; Thornley 1998) and, more generally, can be found across many medical specialties (Altman 1990). Methodological quality does not seem to have improved in the more recent trials.
Even if we did not formally explore outcome reporting bias, the use of multiple, often overlapping, outcomes without a predefined primary outcome may increase the risk of data dredging and distorted reporting in the attempt to demonstrate post‐hoc differences between interventions (Pocock 1997). This problem is magnified by the limited size of most included trials and the use of composite endpoints. Headache frequency is largely accepted as the most relevant clinical outcome.
We rated the overall quality of evidence for clinically relevant efficacy and safety outcomes as having a ‘low’ or 'very low' level of evidence (Table 1 and Table 2). We downgraded the overall quality of evidence in each outcome because of limitations in the study designs and imprecision. Indirectness and inconsistency also affected some outcomes such as headache frequency, index, and withdrawals due to adverse events.
Agreements and disagreements with other studies or reviews
Other systematic reviews (Tomkins 2001 and its update in Jackson 2010) examined antidepressant medication for tension‐type prophylaxis. Similar to our findings, the authors concluded that tricyclic antidepressants and SSRIs have a similar efficacy, in terms of number of days of tension‐type headache attacks, but tricyclics reduced the number of doses of analgesics taken for acute headache pain in patients with tension‐type headache.
Recent clinical guidelines from the National Institute of Health and Care Excellence (NICE) reported that there was not enough evidence to recommend pharmacological prophylactic treatment for tension‐type headache (NICE 2012). Even amitriptyline is not recommended, as the evidence of its effectiveness, supported by poor quality clinical trials, are traded‐off against relevant side effects. These conclusions can be considered in line with the evidence summarised in this review: tricyclics might be more effective than SSRIs, but their therapeutic role is limited by their scarce tolerability. The guidelines from the European Federation of Neurological Societies advise prevention of tension‐type headache using non‐drug management, although the scientific basis is limited. Concerns about the efficacy and tolerability of prophylactic drugs are raised, however, amitriptyline is still mentioned as the first choice drug, while mirtazapine and venlafaxine are considered second choice options (Bendtsen 2010).
Authors' conclusions
Implications for practice.
Since the last version of this review, we included two new relevant studies which have provided little new evidence on the effectiveness of SSRIs and SNRIs in patients with tension‐type headache. The usefulness of SSRIs and SNRIs to prevent headache attacks is unclear and it is likely that these drugs do not represent an efficacious option for the prevention of chronic tension‐type headache, the population in which pharmacological prophylaxis is more appropriate. We did not find information on the use of these drugs in the prevention of episodic tension‐type headache. Considering the most relevant outcomes (headache frequency, intensity and duration) these new antidepressants did not show a superiority over placebo, and seem to be less efficacious than tricyclic antidepressants, particularly amitriptyline, in terms of duration of the headache and use of symptomatic medications.There is some evidence that SSRIs are better tolerated than other antidepressants with respect to minor adverse events, however, this does not impact on the total number of dropouts.
We cannot draw any conclusions on the place in therapy of antidepressants with respect to other non‐pharmacological prophylactic treatments, or other preventive drug classes, for the prevention of tension‐type headache in adults.
Implications for research.
Overall, the standard in terms of design and reporting still needs to be improved, as does the protection against random error. For example, open design is unacceptable in this context. Headache frequency should be the primary efficacy measure in any new trial (Bendtsen 2009). Because tension‐type headache is often a chronic condition, longer follow‐up and harder outcomes that relate to real life (headache frequency, acute headache medication, days off work and quality of life) should be assessed (Bendtsen 2009). This will also avoid the use of non‐validated indexes, discouraging multiple comparisons at different time points, with the warning that multiple data testing easily results in misleading statistically significant findings that appear by chance (Moja 2005; Thornley 1998). Standardised collection of outcomes as suggested by the COMET (Core Outcome Measures in Effectiveness Trials) Initiative, which is engaged in developing, applying and promoting core outcomes sets (COS), using rigorous consensus methods, for effectiveness trials (Williamson 2012) could be helpful. The sample size should be carefully estimated on the basis of the available evidence and the expected effect, in order to protect the study against random error. Amitriptyline should be considered a reference comparator in terms of efficacy in clinical trials comparing antidepressants for patients with chronic headache disorders, however the value of new studies comparing different antidepressants in this setting is questionable.
During the current update, we noticed a clear reduction in the number of publications testing SSRIs and SNRIs in the prophylaxis of tension‐type headache. Other preventive strategies, especially non‐drug treatments, are likely to be the target of future research. For several clinically relevant outcomes, we reported a low level of evidence. This indicates that “further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate”. However, we think that a small, or large, RCT comparing a SSRI or a SNRI versus another drug or another non‐pharmacological intervention is not a priority and might not exert a significant impact on the overall evidence. In the field of antidepressants, it might be of greater interest exploring the efficacy and safety of drugs belonging to different classes (e.g. mirtazapine) and focus on depressed patients with tension‐type headache, as optimal treatments and the role of weak antidepressants are still debatable.
What's new
| Date | Event | Description |
|---|---|---|
| 15 November 2019 | Review declared as stable | See Published notes. |
History
Review first published: Issue 5, 2015
| Date | Event | Description |
|---|---|---|
| 8 April 2016 | Amended | Affiliation added for LM. |
| 30 April 2015 | Review declared as stable | This review will be assessed for further updating in 2020. |
| 24 November 2014 | New citation required and conclusions have changed | This is the update of the 'Selective serotonin re‐uptake inhibitors (SSRIs) for preventing migraine and tension‐type headaches' review. However, the following major changes have been implemented:
We included two new studies (120 participants) in this update ( Singh 2002; Zissis 2007). Overall, we included eight studies and 412 participants. We recommend previous readers of the review should re‐read this update. |
| 7 April 2014 | New search has been performed | We have updated this review to include the results of a new search and to include a new class of antidepressants (serotonin‐norepinephrine reuptake inhibitors). We have added a study flow chart, 'Risk of bias' tables, and 'Summary of findings' tables. |
| 10 August 2009 | Amended | Contact details updated. |
| 28 October 2008 | Amended | Converted to new review format. |
Notes
We performed a restricted updated search in October 2019, but we did not identify any potentially relevant studies likely to change the conclusions. The research area is no longer active and we do not expect new RCTs for this intervention and population to be published. Therefore, this review has now been stabilised following discussion with the authors and editors. The review will be reassessed for updating in five years. If appropriate, we will update the review before this date if new evidence likely to change the conclusions is published, or if standards change substantially which necessitate major revisions.
Acknowledgements
The authors gratefully acknowledge Joanne Abbott and Jane Hayes for the search strategies; Claudio Canepari for the handsearching; Svetlana Kobrina for translations; Valentina Pecoraro and Koren Kwag for manuscript revision; and the Cochrane Pain, Palliative and Supportive Care (PaPaS) Review Group for its helpful assistance during all the phases of the preparation of the review.
Cochrane Review Group funding acknowledgement: The National Institute for Health Research (NIHR) is the largest single funder of the Cochrane PaPaS Group. Disclaimer: The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the NIHR, National Health Service (NHS) or the Department of Health.
Appendices
Appendix 1. Search strategies for identification of studies
CENTRAL (The Cochrane Library)
#1 MeSH descriptor Headache, this term only
#2 MeSH descriptor Headache Disorders explode all trees
#3 (headach* or migrain* or cephalgi* or cephalalgi*)
#4 (#1 OR #2 OR #3)
#5 MeSH descriptor Serotonin Uptake Inhibitors explode all trees
#6 (SSRI* or SNRI*)
#7 (serotonin* and (reuptake or re‐uptake) and inhibitor*)
#8 (citalopram or dapoxetin* or escitalopram or fluoxetin* or fluvoxamin* or paroxetin* or sertralin* or desvenlafaxin* or duloxetin* or milnacipran or venlafaxin*)
#9 (#5 OR #6 OR #7 OR #8)
#10 (#4 AND #9)
Medline (Ovid)
1 Headache/
2 exp Headache Disorders/
3 (headach* or migrain* or cephalgi* or cephalalgi*).mp.
4 1 or 2 or 3
5 exp Serotonin Uptake Inhibitors/
6 (SSRI* or SNRI*).mp.
7 (serotonin* and (reuptake or re‐uptake) and inhibitor*).mp.
8 (citalopram or dapoxetin* or escitalopram or fluoxetin* or fluvoxamin* or paroxetin* or sertralin* or desvenlafaxin* or duloxetin* or milnacipran or venlafaxin*).mp.
9 5 or 6 or 7 or 8
10 4 and 9
11 randomized controlled trial.pt.
12 controlled clinical trial.pt.
13 randomized.ab.
14 placebo.ab.
15 clinical trials as topic.sh.
16 randomly.ab.
17 trial.ti.
18 11 or 12 or 13 or 14 or 15 or 16 or 17
19 10 and 18
20 exp animals/ not humans.sh.
21 19 not 20
key:
mp=title, abstract, original title, name of substance word, subject heading word, protocol supplementary concept, rare disease supplementary concept, unique identifier
pt=publication type
ab=abstract
fs=floating subheading
Embase (Ovid)
1 exp "headache and facial pain"/
2 (headach* or migrain* or cephalgi* or cephalalgi*).mp.
3 1 or 2
4 exp serotonin uptake inhibitor/
5 exp serotonin noradrenalin reuptake inhibitor/
6 (SSRI* or SNRI*).mp.
7 (serotonin* and (reuptake or re‐uptake) and inhibitor*).mp.
8 (citalopram or dapoxetin* or escitalopram or fluoxetin* or fluvoxamin* or paroxetin* or sertralin* or desvenlafaxin* or duloxetin* or milnacipran or venlafaxin*).mp.
9 4 or 5 or 6 or 7 or 8
10 3 and 9
11 crossover procedure/
12 double‐blind procedure/
13 randomized controlled trial/
14 single‐blind procedure/
15 random*.mp.
16 factorial*.mp.
17 (crossover* or cross over* or cross‐over*).mp.
18 placebo*.mp.
19 (double* adj blind*).mp.
20 (singl* adj blind*).mp.
21 assign*.mp.
22 allocat*.mp.
23 volunteer*.mp.
24 11 or 12 or 13 or 14 or 15 or 16 or 17 or 18 or 19 or 20 or 21 or 22 or 23
25 10 and 24
26 (exp Animal/ or Nonhuman/ or exp animal Experiment/) not Human/
27 25 not 26
key: [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer, device trade name, keyword]
PsycINFO Ovid
1 exp headache/
2 (headach* or migrain* or cephalgi* or cephalalgi*).mp.
3 1 or 2
4 exp serotonin reuptake inhibitors/
5 exp serotonin norepinephrine reuptake inhibitors/
6 (SSRI* or SNRI*).mp.
7 (serotonin* and (reuptake or re‐uptake) and inhibitor*).mp.
8 (citalopram or dapoxetin* or escitalopram or fluoxetin* or fluvoxamin* or paroxetin* or sertralin* or desvenlafaxin* or duloxetin* or milnacipran or venlafaxin*).mp.
9 4 or 5 or 6 or 7 or 8
10 3 and 9
key: [mp=title, abstract, heading word, table of contents, key concepts, original title, tests & measures]
Data and analyses
Comparison 1. SSRIs or SNRIs versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Headache frequency (number of headache days) (follow‐up: 8 weeks) | 2 | 127 | Mean Difference (IV, Fixed, 95% CI) | ‐0.96 [‐3.95, 2.03] |
| 1.1 SSRI | 1 | 68 | Mean Difference (IV, Fixed, 95% CI) | ‐0.20 [‐3.94, 3.54] |
| 1.2 SNRI | 1 | 59 | Mean Difference (IV, Fixed, 95% CI) | ‐2.30 [‐7.27, 2.67] |
| 2 Symptomatic/analgesic medication use (doses/month) (follow‐up: 8 weeks) | 2 | 118 | Mean Difference (IV, Fixed, 95% CI) | ‐1.87 [‐2.09, ‐1.65] |
| 2.1 SSRI | 2 | 118 | Mean Difference (IV, Fixed, 95% CI) | ‐1.87 [‐2.09, ‐1.65] |
| 3 Headache index (follow‐up: 8 weeks) | 2 | 128 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.24 [‐0.59, 0.11] |
| 3.1 SSRI | 1 | 68 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.11 [‐0.59, 0.36] |
| 3.2 SNRI | 1 | 60 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.38 [‐0.90, 0.14] |
| 4 Withdrawals ‐ any reason (follow‐up: 8 weeks) | 3 | 198 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.54 [0.25, 1.19] |
| 4.1 SSRI | 2 | 138 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.60 [0.19, 1.88] |
| 4.2 SNRI | 1 | 60 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.50 [0.17, 1.45] |
| 5 Withdrawals due to adverse events (follow‐up: 8 weeks) | 3 | 190 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 3.72 [0.79, 17.53] |
| 5.1 SSRI | 2 | 130 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.14 [0.00, 6.82] |
| 5.2 SNRI | 1 | 60 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 6.88 [1.27, 37.19] |
| 6 Number of patients with minor adverse events (follow‐up: 8 weeks) | 2 | 140 | Odds Ratio (M‐H, Random, 95% CI) | 1.57 [0.52, 4.76] |
| 6.1 SSRI | 1 | 80 | Odds Ratio (M‐H, Random, 95% CI) | 1.0 [0.40, 2.47] |
| 6.2 SNRI | 1 | 60 | Odds Ratio (M‐H, Random, 95% CI) | 3.19 [0.78, 13.10] |
Comparison 2. SSRIs or SNRIs versus other antidepressants.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Headache frequency (number of headache days) | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.1 Follow‐up: 8 weeks | 2 | 152 | Mean Difference (IV, Random, 95% CI) | 0.76 [‐2.05, 3.57] |
| 1.2 Follow‐up: 12 weeks | 1 | 84 | Mean Difference (IV, Random, 95% CI) | 0.80 [‐1.29, 2.89] |
| 2 Headache intensity | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 2.1 Follow‐up: 8 weeks | 2 | 152 | Mean Difference (IV, Random, 95% CI) | 0.32 [‐0.55, 1.19] |
| 2.2 Follow‐up: 12 weeks | 1 | 84 | Mean Difference (IV, Random, 95% CI) | 1.70 [1.06, 2.34] |
| 3 Headache duration (hours/day) | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 3.1 Follow‐up: 8 weeks | 2 | 152 | Mean Difference (IV, Fixed, 95% CI) | 1.26 [0.06, 2.45] |
| 3.2 Follow‐up: 12 weeks | 1 | 84 | Mean Difference (IV, Fixed, 95% CI) | 1.30 [‐0.39, 2.99] |
| 4 Symptomatic/analgesic medications use (doses/4 weeks) | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 4.1 Follow‐up: 8 weeks | 2 | 152 | Mean Difference (IV, Fixed, 95% CI) | 4.98 [1.12, 8.84] |
| 4.2 Follow‐up: 12 weeks | 1 | 84 | Mean Difference (IV, Fixed, 95% CI) | 5.40 [1.10, 9.70] |
| 5 Headache index | 2 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 5.1 Follow‐up: 8 weeks | 2 | 152 | Std. Mean Difference (IV, Random, 95% CI) | 0.42 [‐0.00, 0.85] |
| 5.2 Follow‐up: 12 weeks | 1 | 84 | Std. Mean Difference (IV, Random, 95% CI) | 0.51 [0.08, 0.95] |
| 6 Withdrawals ‐ any reason | 4 | 257 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.55 [0.71, 3.38] |
| 7 Withdrawals due to adverse events | 4 | 257 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.04 [0.41, 2.60] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Bendtsen 1996.
| Methods | Single‐centre, double‐blind, randomised, three‐arm cross‐over study: citalopram vs amitriptyline vs placebo No control for symptomatic/analgesic medications use | |
| Participants | Country: Denmark
N = 40; Sex: 15 male, 25 female
Mean age 40 (range 18 ‐ 60) Diagnosis: Chronic tension‐type headache according to the International Headache Society Criteria (IHS 1988) Exclusion criteria: previous participation in a clinical trial, migraine more than 1 day a month, serious somatic or psychiatric disease including depression, intake of opiates or benzodiazepines, overuse of analgesic drug (> 2 g aspirin a day), previous treatment with antidepressant drugs Recruitment: outpatients from the Headache Clinic of Glostrup Hospital, Copenhagen |
|
| Interventions | Citalopram 20 mg/day Amitriptyline to a maximum of 75 mg/day Placebo Active treatment: total of 24 weeks (3 treatment periods each of 8 weeks, with a 2‐week washout period) | |
| Outcomes | 1. Headache Index (duration * severity) 2. Headache severity (scale '0 = free condition', '5 = moderate headache', '10 = worst headache imaginable') 3. Headache duration (hours/28 days) 4. Headache frequency (days/28 days) 5. Symptomatic/analgesic drug consumption (doses/28 days) | |
| Notes | 6 dropouts (15%):
Per protocol analysis No sample size calculation |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Allocation sequence generated in blocks of 6 patients, method not stated |
| Allocation concealment (selection bias) | Unclear risk | No information |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Drugs identical in appearance |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Moderate dropout rate, balanced, reasons fully reported |
| Selective reporting (reporting bias) | Unclear risk | No information |
| Other bias | High risk | Financial support provided by Lundbeck Foundation |
Boz 2003.
| Methods | Single‐centre, open‐label, randomised parallel study: sertraline vs amitriptyline Control for symptomatic/analgesic medications use | |
| Participants | Country: Turkey
N = 90; Sex: 11 male, 79 female
Mean age: 37.8 (SD 12.2) sertraline, 40.4 (SD 11.4) amitriptyline Diagnosis: Chronic tension‐type headache according to the International Headache Society Criteria (IHS 1988) Exclusion criteria: presence of major depression or depression symptoms, antidepressant use in the previous year, score > 15 Hamilton Depression Scale or > 13 Beck Depression Inventory I‐II Scale, severe concomitant neurological and medical disorders, breast feeding and pregnancy Recruitment: first visit at the outpatient clinic |
|
| Interventions | N = 46 amitriptyline to a maximum of 25 mg/day
N = 44 sertraline 25 mg/day Active treatment: 12 weeks |
|
| Outcomes | 1. Headache frequency (number of attacks/28 days) 2. Duration (hours/day) 3. Headache severity (scored on a 10‐point visual analogue scale) 4. Headache index (headache frequency * average severity * duration/28 days) 5. Number of patients with more than a 50% reduction in the headache index 6. Symptomatic/analgesic drug consumption | |
| Notes | 22 patients met the criteria for co‐existing migraine (8 in Sertraline and 14 in Amitriptyline) 6 dropouts (7%):
Per protocol analyses No sample size calculation |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated (supplemental communication by authors) |
| Allocation concealment (selection bias) | High risk | Patients sequentially allocated depending on time of presentation |
| Blinding (performance bias and detection bias) All outcomes | High risk | Open‐label design |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Low dropout rate, balanced, reasons fully reported |
| Selective reporting (reporting bias) | Unclear risk | No information |
| Other bias | Low risk | No financial support provided by drug companies |
Langemark 1993.
| Methods | Multicentre, double‐blind, randomised, response‐conditional cross‐over study: paroxetine vs sulpiride No control for symptomatic/analgesic medications use | |
| Participants | Country: Denmark
N = 50; Sex: 20 male, 30 female
Mean age: 42 (range 20 ‐ 70) Diagnosis: Chronic tension‐type headache (not defined) of at least 6 months duration and no more than 14 headache‐free days per months Exclusion criteria: patients suffering migraine more than one day per month; moderate or severe cardiovascular disease or other chronic disease Recruitment: mailed questionnaire among patients from a private neurology clinic and a neurological hospital department |
|
| Interventions | N = 25 first treated with paroxetine to a maximum of 30 mg/day N = 25 first treated with sulpiride to a maximum of 100 mg/day Active treatment: 16 weeks (8 weeks + 8 weeks, no in‐between washout period if patients crossed over) | |
| Outcomes | 1. Severity Index (5 ordinal scale: a. 'no headache'; b. 'slight'; c. 'moderate'; d. 'very troublesome'; e. 'worst possible') 2. Patient's global Evaluation drug performance (5 ordinal scale: a. 'worthless'; b. 'poor'; c. 'fair'; d. 'good'; e. 'excellent') 3. Observer's Global Evaluation drug performance (5 ordinal scale: a. 'worthless'; b. 'poor'; c. 'fair'; d. 'good'; e. 'excellent') 4. Symptomatic/analgesic drug consumption 5. Side effects (4 ordinal scale: a. 'none'; b. 'slight'; c. 'moderate'; d. 'severe = discontinues drug') | |
| Notes | First 8 weeks: 10 dropouts (20%):
13 patients crossed over to sulpiride after paroxetine for no response or intolerable side effects, while 10 crossed over to paroxetine after sulpiride Per protocol analyses |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information |
| Allocation concealment (selection bias) | Unclear risk | No information |
| Blinding (performance bias and detection bias) All outcomes | High risk | Drugs identical in appearance (but the two groups received different numbers of tablets) |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Moderate dropout rate, unbalanced, reasons partially reported |
| Selective reporting (reporting bias) | Unclear risk | No information |
| Other bias | Unclear risk | Financial support not reported |
Manna 1994.
| Methods | Single‐centre, double‐blind, randomised parallel study: fluvoxamine vs mianserin Control for symptomatic/analgesic medications use | |
| Participants | Country: Italy
N = 40; Sex : 15 male, 25 female
Mean age: 34.30 (SD 8.68) fluvoxamine; 38.15 (SD 10.24) mianserin Diagnosis: chronic tension‐type headache according to the International Headache Society Criteria (IHS 1988), age between 20 and 52 years Exclusion criteria: post‐traumatic headache, disease of facial or cranial structures, use or exposure or withdrawal to any substance with effects on headache symptoms Recruitment: outpatient, no other information provided |
|
| Interventions | N = 20 fluvoxamine to a maximum of 100 mg/day
N = 20 mianserin to a maximum of 60 mg/day Active treatment: 8 weeks |
|
| Outcomes | 1. Headache frequency (days with headache/28 days) 2. Pain severity (4‐point scale: '0 = no headache' to '4 = bed rest') 3. Symptomatic/analgesic drug consumption 4. Depression: Zung Self‐Rating Depression Scale 5. Depression: Hamilton Depression Rating Scale | |
| Notes | 50 patients admitted to the study, 10 excluded before randomisation
It is not clearly stated if there were no withdrawals after randomisation No sample size calculation |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Assignment was stratified to ensure balanced distribution of patients for sex, age, length of clinical history and age at the onset of headache, no other information |
| Allocation concealment (selection bias) | Unclear risk | No information |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | The study was defined as "double‐blind" but did not report any information on how blinding was performed and maintain. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No information |
| Selective reporting (reporting bias) | Unclear risk | No information |
| Other bias | Unclear risk | Financial support not reported |
Oguzhanoglu 1999.
| Methods | Single‐centre, open‐label randomised, parallel study: fluoxetine vs amitriptyline No control for symptomatic/analgesic medications use | |
| Participants | Country: Turkey
Overall: N = 52; Sex: 6 male, 41 female (sex not reported for dropouts). Chronic tension‐type headache and episodic tension‐type headache groups: N = 35; Sex: 3 male, 29 female (sex not reported for dropouts); mean age: 38.5 chronic tension‐type headache, 31.5 episodic tension‐type headache Diagnosis: migraine (n = 17), chronic tension‐type headache (n = 14) and episodic tension‐type headache (n = 21), all defined according to International Headache Society Criteria (IHS 1988) Exclusion criteria: antidepressant use in the previous year, score > 17 Hamilton Depression Scale Recruitment: from November 1996 to September 1997, no other information |
|
| Interventions | N = 22 amitriptyline to a maximum of 50 mg/day
N = 25 fluoxetine 20 mg/day Duration of active treatment: unclear |
|
| Outcomes | 1. Headache frequency (number of days with headache/30 days) 2. Pain intensity (not defined) 3. Headache duration (not defined) | |
| Notes | Overall, 5 dropouts (10%) for side effects, three in the chronic tension‐type headache and episodic tension‐type headache groups Per protocol analysis. No sample size calculation | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information |
| Allocation concealment (selection bias) | Unclear risk | No information |
| Blinding (performance bias and detection bias) All outcomes | High risk | Open‐label |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Low dropout rate (3/35, 8.6%), reasons reported |
| Selective reporting (reporting bias) | Unclear risk | No information |
| Other bias | Unclear risk | Financial support not reported |
Singh 2002.
| Methods | Single‐centre, randomised, double‐blind, placebo controlled: sertraline vs placebo | |
| Participants | Country: India N = 60 (50 completed the study) Mean age: 28.7 (SD 1.60) sertraline; 27.2 (SD 1.66) placebo Diagnosis: chronic tension‐type headache according to the International headache society criteria (IHS 1988) Exclusion criteria: diabetes, hypertension, migraine, and psychiatric illness Symptomatic/analgesic medications allowed |
|
| Interventions | Sertraline 100 mg/day; placebo | |
| Outcomes | 1. Pain severity (0: no pain; 3: headache requiring bed rest) 2. Headache index (headache frequency per week x severity of pain x duration of pain in hours) 3. Number of analgesic medication 4. Anxiety and depression (Hamilton rating scale) |
|
| Notes | Two patients dropped out in the run‐in period; 2 in the follow‐up; 6 during drug treatment (5 in placebo) Per protocol analysis No sample size calculation |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information |
| Allocation concealment (selection bias) | Unclear risk | A sealed envelope was kept with the principle investigator |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Drug and placebo were identical in shape, size, weight, and colour |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Low dropout rate (16%), reasons reported, 5 dropouts for lack of efficacy in the placebo group |
| Selective reporting (reporting bias) | Unclear risk | No information |
| Other bias | High risk | Solus Pharm. is acknowledged for having provided drug, placebo, and relevant literature |
Walker 1997.
| Methods | Single‐centre, single‐blind, randomised parallel study: fluoxetine vs desipramine No control for symptomatic/analgesic medications use | |
| Participants | Country: United Kingdom
N = 37; Sex: 7 male, 30 female Mean age: 35 Diagnosis: Chronic tension‐type headache according to the Headache International Society Criteria (IHS 1988) Exclusion criteria: already treated with an antidepressant or other psychotropic medication |
|
| Interventions | N = 18 fluoxetine to a maximum of 40 mg/day
N = 19 desipramine to a maximum of 150 mg/day Active treatment: 12 weeks |
|
| Outcomes | 1. Headache severity (10‐point scale: '0 = no pain' to '10 = max. pain') 2. Symptomatic/analgesic drug consumption 3. Hospital Anxiety and Depression Scale (HADS) 4. Montgomery and Asberg Depression Rating Scale (MADRS) 5. SF‐36 (Medical Outcomes Study Health Survey Short Form) | |
| Notes | 12 dropouts (32%):
Depression: 8 patients (22%) were depressed with the MADRS (score > 20); 12 (32%) were depressed with the HADS (score not provided) Anxiety: 22 (59 %) were anxious with the HADS (score > 8) Per protocol analysis |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information |
| Allocation concealment (selection bias) | Unclear risk | No information |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Only the assessing physician was blinded to treatment |
| Incomplete outcome data (attrition bias) All outcomes | High risk | High dropout rate, balanced, reasons fully reported |
| Selective reporting (reporting bias) | Unclear risk | No information |
| Other bias | Unclear risk | Financial support not reported |
Zissis 2007.
| Methods | Six‐centre, randomised, double‐blind: venlafaxine XR vs placebo | |
| Participants | Country: Greece N = 60. Sex: 11 male 49 female Mean age: 40.8 (SD 14.5) Diagnosis: all subtypes of tension‐type headache according to International Headache Society Criteria (IHS 2004) Exclusion criteria: patients < 18 years old, patients with headache for < 5 days/month, patients with < 21 in the Migraine Disability Assessment (MIDAS) questionnaire. No current depression or anxiety disorders |
|
| Interventions | N = 34 venlafaxine XR to a maximum of 150 mg/day N = 26 Placebo Active treatment: 12 weeks |
|
| Outcomes | 1. Number of days with headache 2. Number of hours with headache 3. Total headache intensity index (duration x severity) 4. Clinical global impression improvement |
|
| Notes | 40 completers, 25 venlafaxine XR, 15 placebo Adequate sample size calculation, intention‐to‐treat analysis using the last‐observation‐carried‐forward method |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random numbers table |
| Allocation concealment (selection bias) | Low risk | Sealed envelopes |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Venlafaxine XR and placebo were supplied as identically appearing capsules; the drug was dispensed by the hospital pharmacies |
| Incomplete outcome data (attrition bias) All outcomes | High risk | High dropout rate (20/60 = 33%), unbalanced (venlafaxine XR 9/34 = 26.5%, placebo 11/26 = 42.3%) |
| Selective reporting (reporting bias) | Unclear risk | No information |
| Other bias | High risk | Financial support not reported but the randomisation and the blinding were done by the drug company |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Bermejo 2010 | Not randomised, one group only |
| Bittman 1992 | Not randomised |
| Bonazzi 1991 | Not randomised |
| Bussone 1991 | Data presented aggregating patients with migraine and with chronic tension‐type headache |
| Diamond 1989 | Not randomised |
| Foster 1994 | Not randomised |
| Iannacchero 1999 | Not randomised |
| Joffe 1997 | Case report |
| Karageorgiou 1996 | Not randomised |
| Lampl 1995 | Not randomised |
| Rampello 2004 | Data presented aggregating patients with comorbidity of depression, migraine, tension‐type headache |
| Sandrini 1991 | Not randomised |
| Saper 1994 | Data presented aggregating patients with migraine and with chronic daily headache |
| Sjaastad 1983 | Selective serotonin reuptake inhibitor (femoxetine) no longer produced by drug company |
| Sosin 1993 | Case report |
| Voznesenskaia 1999 | One group only |
Characteristics of studies awaiting assessment [ordered by study ID]
Gabrielidou 1998.
| Methods | Randomised, double‐blind cross‐over study Country: Greece |
| Participants | 20 subjects (16 female; 4 male) with tension‐type headache according to the International Headache Society Criteria (IHS 2004); mean age 38 ± 18 More than 10 attacks per month, significant disturbance in quality of life, refractory headache attacks, ability to keep a diary |
| Interventions | 1. Amitriptyline 50 mg/day 2. Venlafaxine 100 mg/day Active treatment duration: 8 weeks |
| Outcomes | Headache frequency, intensity, and duration Quality of life Hamilton and Beck Anxiety and Depression Rating Scales |
| Notes | Washout period: one week |
Tarasova 2008.
| Methods | Randomised controlled trial Country: Russia |
| Participants | Chronic daily headache |
| Interventions | Fluvoxamine Amitriptyline Transcranial electrostimulation |
| Outcomes | Not clear from the abstract |
| Notes | No full text available; article in Russian |
Zhou 2006.
| Methods | Randomised, double‐blind cross‐over study Country: China |
| Participants | 123 patients with chronic tension‐type headache |
| Interventions | 1. Venlafaxine 50 mg/day 2. Naproxen Active treatment duration: 2 weeks (?) |
| Outcomes | Quality of life |
| Notes | Article in Chinese |
Differences between protocol and review
This 2015 update excludes migraine. A separate review on migraine has been published (Banzi 2015). We have added a study flow chart, 'Risk of bias' tables, and 'Summary of findings' tables.
Contributions of authors
Study concept, protocol and first version publication: Lorenzo Moja (LM), Cristina Cusi (CC), Roberto Sterzi (RS). Selection of studies: Rita Banzi (RB), CC, Concetta Randazzo (CR), Dario Tedesco (DT). Acquisition of data: RB, CC, CR, DT. Risk of bias assessment: RB, CC, CR, DT. Analysis of data: RB, LM. Drafting of the manuscript: RB. Interpretation and critical revision of the manuscript for important intellectual content: RB, CC, CR, DT, RS, LM.
Sources of support
Internal sources
Cochrane Neurological Network, Other.
Italian Cochrane Centre, Italy.
Fondazione Bancaria Monte di Lombardia, Italy.
External sources
International Headache Society (for administrative costs associated with editorial review and peer review), Other.
Declarations of interest
RB, CC, CR, DT, RS, LM have no relevant conflicts of interest to declare.
Stable (no update expected for reasons given in 'What's new')
References
References to studies included in this review
Bendtsen 1996 {published data only}
- Bendtsen L, Jensen R, Olesen J. A non‐selective (amitriptyline), but not a selective (citalopram), serotonin reuptake inhibitor is effective in the prophylactic treatment of chronic tension‐type headache. Journal of Neurology, Neurosurgery and Psychiatry 1996;61(3):285‐90. [DOI] [PMC free article] [PubMed] [Google Scholar]
Boz 2003 {published data only}
- Boz C, Altunayoglu V, Velioglu S, Ozmenoglu M. Sertraline versus amitriptyline in the prophylactic therapy of non‐depressed chronic tension‐type headache patients. Journal of Headache and Pain 2003;4(2):72‐8. [Google Scholar]
Langemark 1993 {published data only}
- Langemark M, Olesen J. Sulpiride and paroxetine in the treatment of chronic tension‐type headache. An explanatory double‐blind trial. Headache 1994;34(1):20‐4. [DOI] [PubMed] [Google Scholar]
Manna 1994 {published data only}
- Manna V, Bolino F, Cicco L. Chronic tension‐type headache, mood depression and serotonin: therapeutic effects of fluvoxamine and mianserin. Headache 1994;34(1):44‐9. [DOI] [PubMed] [Google Scholar]
Oguzhanoglu 1999 {published data only}
- Oguzhanoglu A, Sahiner T, Kurt T, Akalin O. Use of amitriptyline and fluoxetine in prophylaxis of migraine and tension‐type headaches. Cephalalgia 1999;19(5):531‐2. [DOI] [PubMed] [Google Scholar]
Singh 2002 {published data only}
- Singh N, Misra S. Sertraline in chronic tension‐type headache. Journal of the Association of Physicians of India 2002;50:873‐8. [PubMed] [Google Scholar]
Walker 1997 {published data only}
- Walker Z, Walker RWH, Robertson MM, Stansfeld S. Antidepressant treatment of chronic tension‐type headache: a comparison between fluoxetine and desipramine. Headache 1998;38(7):523‐8. [DOI] [PubMed] [Google Scholar]
Zissis 2007 {published data only}
- Zissis NP, Harmoussi S, Vlaikidis N, Mitsikostas D, Thomaidis T, Georgiadis G, et al. A randomized, double‐blind, placebo‐controlled study of venlafaxine XR in out‐patients with tension‐type headache. Cephalalgia 2007;27:315–24. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Bermejo 2010 {published data only}
- Bermejo PE, Toribio‐Diaz ME, Zea‐Sevilla MA. Duloxetine prophylaxis in chronic tension type headache. Journal of Headache and Pain. 2010; Vol. Conference: 2nd European Headache and Migraine Trust International Congress ‐ EHMTIC Nice, France:S84.
Bittman 1992 {published data only}
- Bittman B, Emanuele S. Fluoxetine: side effects and efficacy in a headache population. Headache Quarterly 1992;3(1):82‐5. [Google Scholar]
Bonazzi 1991 {published data only}
- Bonazzi A, Giagnorio F. Fluoxetine vs. amitriptyline in the prophylaxis of tension type headache. Cephalalgia 1991;11 Suppl 11:341‐2. [Google Scholar]
Bussone 1991 {published data only}
- Bussone G, Sandrini G, Patruno G, Ruiz L, Tassorelli C, Nappi G. Effectiveness of fluoxetine on pain and depression in chronic headache disorders. In: Nappi G, Bono G, Sandrini G, Martignoni E, Micieli G editor(s). Headache and depression: serotonin pathways as a common clue. New York: Raven Press, 1991:265‐72. [Google Scholar]
Diamond 1989 {published data only}
- Diamond S, Freitag FG. The use of fluoxetine in the treatment of headache. Clinical Journal of Pain 1989;5(2):200‐1. [PubMed] [Google Scholar]
Foster 1994 {published data only}
- Foster CA, Bafaloukos J. Paroxetine in the treatment of chronic daily headache. Headache 1994;34(10):587‐9. [DOI] [PubMed] [Google Scholar]
Iannacchero 1999 {published data only}
- Iannacchero R, Ambrosio A, Rose F, Flippo A, Ambrosio LA. Efficacy and tolerability of Citalopram in chronic headache syndrome prophylaxis [Efficacia e tollerabilita'del Citaloprom nella profilassi delle sindromi cefalalgiche cronicizzate: comorbilita' emicrania senz'aura e depressione]. Psichiatria 1999:73‐7. [Google Scholar]
Joffe 1997 {published data only}
- Joffe RT, Sokolov ST. Co‐administration of fluoxetine and sumatriptan: the Canadian experience. Acta Psychiatrica Scandinavica 1997;95(6):551‐2. [DOI] [PubMed] [Google Scholar]
Karageorgiou 1996 {published data only}
- Karageorgiou CE, Spantideas A, Triantopoulou M, Lekkou G, Robotis A, Papatriantaphylou J. A pilot study of paroxetin a serotonin reuptake inhibitor and amitriptyline in the treatment of chronic tension type headache. Functional Neurology 1996;11(2‐3):157. [Google Scholar]
Lampl 1995 {published data only}
- Lampl C, Klingler D. Chronic and episodic tension‐type headache: prophylactic therapy with citalopram. Neuropsychiatrie 1995;9(1):15‐9. [Google Scholar]
Rampello 2004 {published data only}
- Rampello L, Alvano A, Chiechio S, Malaguarnera M, Raffaele R, Vecchio I, et al. Evaluation of the prophylactic efficacy of amitriptyline and citalopram, alone or in combination, in patients with comorbidity of depression, migraine, and tension‐type headache. Neuropsychobiology 2004;50:322‐8. [DOI] [PubMed] [Google Scholar]
Sandrini 1991 {published data only}
- Sandrini G, Ruiz L, Patruno G, Bussone G, Verri AP, Nappi G. Antalgic and antidepressant activities of fluoxetine in daily chronic headache. Cephalalgia 1991;11 Suppl 11:280‐1. [Google Scholar]
Saper 1994 {published data only}
- Saper JR, Silberstein SD, Lake AE 3rd, Winters ME. Double‐blind trial of fluoxetine: chronic daily headache and migraine. Headache 1994;34(9):497‐502. [DOI] [PubMed] [Google Scholar]
Sjaastad 1983 {published data only}
- Sjaastad O. So‐called "tension headache" ‐ the response to a 5‐HT uptake inhibitor: femoxetine. Cephalalgia 1983;3(1):53‐60. [DOI] [PubMed] [Google Scholar]
Sosin 1993 {published data only}
- Sosin D. Clinical efficacy of fluoxetine vs sertraline in a headache clinic population. Headache 1993;33(5):284. [Google Scholar]
Voznesenskaia 1999 {published data only}
- Voznesenskaia TG. Prozac treatment of chronic tension headache [Lechenie prozakom khronicheskikh golovnykh bolei napriazheniia]. Zhurnal Nevrologii i Psikhiatr ii Imeni S.S. Korsakova 1999;99(1):34‐6. [PubMed] [Google Scholar]
References to studies awaiting assessment
Gabrielidou 1998 {published data only}
- Gabrielidou P, Lekkou G, Robotis A, Papatriantafillou J, Manias P, Karagiannis V, et al. Venlafaxine versus amitriptyline for the prophylaxis of tension type headache. 4th European Headache Congress. 1998.
Tarasova 2008 {published data only}
- Tarasova SV, Amelin AV, Skoromets AA. Fluvoxamine, amitriptyline and transcranial electrostimulation of the brain in the treatment of chronic daily headache. Zhurnal Nevropatoliogii I Psikhiatrii Imeni SS Korsakova 2009;108:43‐6. [PubMed] [Google Scholar]
Zhou 2006 {published data only}
- Zhou HY, Zhou D. Influence of venlafaxine hydrochloride on the quality of life in patients with chronic tension headache. [Chinese]. Chinese Journal of Clinical Rehabilitation 2006;10(10):26‐8. [Google Scholar]
Additional references
Ad Hoc 1962
- Ad Hoc Committee on the Classification of Headache of the National Institute of Neurological Diseases and Blindness. Classification of headache. JAMA 1962;179(9):717‐8. [Google Scholar]
Altman 1990
- Altman DG, Dore CJ. Randomisation and baseline comparisons in clinical trials. Lancet 1990;335(8682):149‐53. [DOI] [PubMed] [Google Scholar]
Ashina 2004
- Ashina M. Neurobiology of chronic tension‐type headache. Cephalalgia 2004;24:161‐72. [DOI] [PubMed] [Google Scholar]
Banzi 2015
- Banzi R, Cusi C, Randazzo C, Sterzi R, Tedesco D, Moja L. Selective serotonin reuptake inhibitors (SSRIs) and serotonin‐norepinephrine reuptake inhibitors (SNRIs) for the prevention of migraine in adults. Cochrane Database of Systematic Reviews 2015, Issue 4. [DOI: 10.1002/14651858.CD002919.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Bendtsen 2009
- Bendtsen L, Bigal ME, Cerbo R, Diener HC, Holroyd K, Lampl C, et al. International Headache Society Clinical Trials Subcommittee. Guidelines for controlled trials of drugs in tension‐type headache: Second edition. Cephalalgia 2009;30(1):1–16. [DOI] [PubMed] [Google Scholar]
Bendtsen 2010
- Bendtsen L, Evers S, Linde M, Mitsikostas DD, Sandrini G, Schoenen J. EFNS guideline on the treatment of tension‐type headache ‐ Report of an EFNS task force. European Journal of Neurology 2010;17:1318–25. [DOI] [PubMed] [Google Scholar]
Cohen 1988
- Cohen J. Statistical Power Analysis in the Behavioral Sciences. 2nd Edition. New Jersey: Hillsdale: Lawrence Erlbaum Associates, Inc, 1988. [Google Scholar]
Deeks 2011
- Deeks JJ, Higgins JPT, Altman DG (editors). Chapter 9: Analysing data and undertaking meta‐analyses. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org. The Cochrane Collaboration, 2011. Available at www.cochrane.org.
Elbourne 2002
- Elbourne DR, Altman DG, Higgins JPT, Curtin F, Worthington HV, Vail A. Meta‐analyses involving cross‐over trials: methodological issues. International Journal of Epidemiology 2002;31(1):140‐9. [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Altman DG, Sterne JAC (editors). Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org. The Cochrane Collaboration, 2011. Available at www.cochrane.org.
Hotopf 1997
- Hotopf M, Lewis G, Normand C. Putting trials on trial ‐ the costs and consequences of small trials in depression: a systematic review of methodology. Journal of Epidemiology and Community Health 1997;51(4):354‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hotopf 1999
- Hotopf M, Churchill R, Lewis G. Pragmatic randomised controlled trials in psychiatry. British Journal of Psychiatry 1999;175:217‐23. [DOI] [PubMed] [Google Scholar]
IHS 1988
- Headache Classification Committee of the International Headache Society. Classification and diagnostic criteria for headache disorders, cranial neuralgias and facial pain. Cephalalgia 1988;8(Suppl 7):1‐96. [PubMed] [Google Scholar]
IHS 2004
- Headache Classification Subcommittee of the International Headache Society (IHS). The International Classification of Headache Disorders, 2nd edition. Cephalalgia 2004;24(Suppl 1):9‐160. [DOI] [PubMed] [Google Scholar]
IHS 2013
- Headache Classification Committee of the International Headache Society (IHS). The International Classification of Headache Disorders, 3rd edition (beta version). Cephalalgia 2013;33(9):629‐808. [DOI] [PubMed] [Google Scholar]
Jackson 2010
- Jackson JL, Shimeall W, Sessums L, DeZee KJ, Becher D, Diemer M, et al. Tricyclic antidepressants and headaches: systematic review and meta‐analysis. BMJ 2010;341:c5222. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kantor 1991
- Kantor TG. The pharmacological control of musculoskeletal pain. Canadian Journal of Physiology and Pharmacology 1991;69(5):713‐8. [DOI] [PubMed] [Google Scholar]
Liberati 2009
- Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, et al. The PRISMA Statement for Reporting Systematic Reviews and Meta‐Analyses of Studies That Evaluate Health Care Interventions: Explanation and Elaboration. PLoS Med 2009;6(7):e1000100. [DOI] [PMC free article] [PubMed] [Google Scholar]
Linde 2012
- Linde M, Gustavsson A, Stovner LJ, Steiner TJ, Barré J, Katsarava Z, et al. The cost of headache disorders in Europe: the Eurolight project. European Journal of Neurology 2012;19(5):703‐11. [DOI] [PubMed] [Google Scholar]
Loder 2008
- Loder E, Rizzoli P. Tension‐type headache. BMJ 2008;336:88‐92. [DOI] [PMC free article] [PubMed] [Google Scholar]
Max 1992
- Max MB, Lynch SA, Muir J, Shoaf SE, Smoller B, Dubner R. Effects of desipramine, amitriptyline, and fluoxetine on pain in diabetic neuropathy. New England Journal of Medicine 1992;326(19):1250‐6. [DOI] [PubMed] [Google Scholar]
Moja 2007
- Moja L, Virgili G, Liberati A, Gensini GF, Gusinu R, Conti AA. Scales to climb borderline personalities: when science goes nowhere. Internal and Emergency Medicine 2007;2(4):315‐7. [DOI] [PubMed] [Google Scholar]
NICE 2012
- National Institute of Health and Care Excellence. Headaches: diagnosis and management of headaches in young people and adults. Clinical guidelines, CG150 September 2012:187‐214.
Pocock 1997
- Pocock SJ. Clinical trials with multiple outcomes: a statistical perspective on their design, analysis, and interpretation. Controlled Clinical Trials 1997;18(6):530‐45. [DOI] [PubMed] [Google Scholar]
Preskorn 2004
- Preskorn SH, Ross R, Stanga CY. Selective serotonin reuptake inhibitors. In: Preskorn SH, Feighner HP, Stanga CY, Ross R editor(s). Antidepressants: Past, Present and Future. Berlin: Springer, 2004. [Google Scholar]
Rasmussen 2001
- Rasmussen BK. Epidemiology of headache. Cephalgia 2001;21(7):774‐7. [DOI] [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Russell 2006
- Russell MB, Saltyte‐Benth J, Levi N. Are infrequent episodic, frequent episodic and chronic tension‐type headache inherited?. Journal of Headache Pain 2006;7:119‐26. [DOI] [PMC free article] [PubMed] [Google Scholar]
Schwartz 1998
- Schwartz BS, Stewart WF, Simon D, Lipton RB. Epidemiology of tension‐type headache. JAMA 1998;279(5):381‐3. [DOI] [PubMed] [Google Scholar]
Sindrup 2000
- Sindrup SH, Jensen TS. Pharmacologic treatment of pain in polyneuropathy. Neurology 2000;55(7):915‐20. [DOI] [PubMed] [Google Scholar]
Smitherman 2011
- Smitherman TA, Walters B, Maizels M, Penzien DB. The use of antidepressants for headache prophylaxis. CNS Neuroscience and Therapeutics 2011;17:462–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Stovner 2007
- Stovner LJ, Hagen K, Jensen R, Katsarava Z, Lipton R, Scher A, et al. The global burden of headache: a documentation of headache prevalence and disability worldwide. Cephalalgia 2007;27(3):193‐210. [DOI] [PubMed] [Google Scholar]
Thornley 1998
- Thornley B, Adams C. Content and quality of 2000 controlled trials in schizophrenia over 50 years. BMJ 1998;317(7167):1181‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Tomkins 2001
- Tomkins GE, Jackson JL, O'Malley PG, Balden E, Santoro JE. Treatment of chronic headache with antidepressants: a meta‐analysis. American Journal of Medicine 2001;111(1):54‐63. [DOI] [PubMed] [Google Scholar]
Vos 2012
- Vos T, Flaxman AD, Naghavi M, Lozano R, Michaud C, Ezzati M, et al. Years lived with disability (YLD) for 1160 sequelae of 289 diseases and injuries 1990–2010: a systematic analysis for the global burden of disease study 2010. Lancet 2012;380(9859):2163–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
Williamson 2012
- Williamson PR, Altman DG, Blazeby JM, Clarke M, Devane D, Gargon E, et al. Developing core outcome sets for clinical trials: issues to consider. Trials 2012;13:132. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wood 2008
- Wood L, Egger M, Gluud LL, Schulz KF, Jüni P, Altman DG, et al. Empirical evidence of bias in treatment effect estimates in controlled trials with different interventions and outcomes: meta‐epidemiological study. BMJ 2008;336(7644):601‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to other published versions of this review
Cusi 2001
- Cusi C, Sterzi R, Canepari C. Selective serotonin re‐uptake inhibitors (SSRIs) for preventing migraine and tension‐type headaches. Cochrane Database of Systematic Reviews 2001, Issue 1. [DOI: 10.1002/14651858.CD002919] [DOI] [PubMed] [Google Scholar]
Moja 2005
- Moja PL, Cusi C, Sterzi RR, Canepari C. Selective serotonin re‐uptake inhibitors (SSRIs) for preventing migraine and tension‐type headaches. Cochrane Database of Systematic Reviews 2005, Issue 3. [DOI: 10.1002/14651858.CD002919.pub2] [DOI] [PubMed] [Google Scholar]


