Abstract
Prescriptions for Benzodiazepines (BZDs) have risen continually. According to national statistics, the combination of BZDs with opioids has increased since 1999. BZDs (sometimes called “benzos”) work to calm or sedate a person by raising the level of the inhibitory neurotransmitter GABA in the brain. In terms of neurochemistry, BZDs act at the GABAA receptors to inhibit excitatory neurons, reducing VTA glutaminergic drive to reduce dopamine release at the Nucleus accumbens. Benzodiazepine Use Disorder (BUD) is very difficult to treat, partly because BZDs are used to reduce anxiety which paradoxically induces hypodopaminergia. Considering this, we are proposing a paradigm shift. Instead of simply targeting chloride channel direct GABAA receptors for replacement or substitution therapy, we propose the induction of dopamine homeostasis. Our rationale is supported by the well-established notion that the root cause of drug and non-drug addictions (i.e. Reward Deficiency Syndrome [RDS]), at least in adults, involve dopaminergic dysfunction and heightened stress. This proposition involves coupling the Genetic Addiction Risk Score (GARS) with a subsequent polymorphic matched genetic customized Pro-Dopamine Regulator known as KB220ZPBM (Precision Behavioral Management). Induction of dopamine homeostasis will be clinically beneficial in attempts to combat BUD for at least three reasons: 1) During detoxification of alcoholism, the potential induction of dopamine regulation reduces the need for BZDs; 2) A major reason for BZD abuse is because people want to achieve stress reduction and subsequently, the potential induction of dopamine regulation acts as an anti-stress factor; and 3) BUD and OUD are known to reduce resting state functional connectivity, and as such, potential induction of dopamine regulation enhances resting state functional connectivity. Future randomized placebo-controlled studies will investigate this forward thinking proposed novel modality.
Keywords: benzodiazepines, benzodiazepine use disorder, dopaminergic, reward deficiency syndrome, gars, kb220, dopamine homeostasis
Introduction
Benzodiazepines (BZDs) like Xanax, Valium and Ambien are household names. They are widely popular and widely prescribed. Psych Central reports that in 2013, Xanax, a benzodiazepine, was the most commonly prescribed drug [1]. Other benzodiazepines, Ativan and Valium, came in fifth and ninth in popularity [2]. The Drug Enforcement Agency reports the number of prescriptions written in 2011 for popular benzodiazepines includes: Alprazolam (Xanax): 49 million prescriptions; Lorazepam (Ativan): 27.6 million prescriptions; Clonazepam (Klonopin): 26.9 million prescriptions; Diazepam (Valium): 15 million prescriptions; and Temazepam (Restoril): 8.5 million prescriptions [3,4]. More than 30 percent of overdoses involving opioids also involve benzodiazepines. Benzodiazepines (sometimes called “benzos”) work via different mechanisms (i.e. stimulating GABA synthesis, inhibiting reuptake, mimicking GABA, etc.) to calm or sedate a person by raising the level of the inhibitory neurotransmitter GABA in the brain.
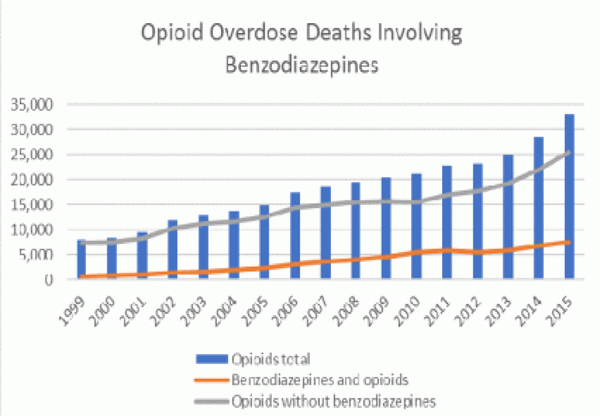
Doctors prescribe both BZDs and opioids simultaneously based on the diagnosed need to medicate their respective symptoms without a deeper understanding of either conflicting or overlapping mechanisms of action. Every day, more than 115 Americans die after overdosing on opioids [5]. However, between 1996 and 2013, the number of adults who filled a benzodiazepine prescription increased by 67%, from 8.1 million to 13.5 million [6]. Unfortunately, the quantity obtained also increased from 1.1 kg to 3.6 kg lorazepam-equivalents per 100,000 adults. Undoubtedly, combining opioids and benzodiazepines can be unsafe because both types of drug sedate users and suppress breathing-the cause of overdose fatality-as well as impair cognitive functions. In 2015, 23% of people who died of an opioid overdose also tested positive for benzodiazepines (Figure 1) [7]. In a study of over 300,000 continuously insured patients receiving opioid prescriptions between 2001 and 2013, the percentage of persons also prescribed benzodiazepines rose to 17% in 2013 from 9% in 2001 [8]. suggested that people concurrently using both drugs are at higher risk of visiting the emergency department or being admitted to a hospital for a drug-related emergency.
Figure 1.
Control and prevention (CDC). Multiple cause of death, 1999–2015. (Source: Centers for Disease)
Previous studies also highlighted the dangers of co-prescribing opioids and benzodiazepines. A cohort study in North Carolina found that the overdose death rate among patients receiving both types of medications was 10 times higher than among those only receiving opioids [9]. Moreover, Gomes et al. [10]. reported that of the overdose deaths in people prescribed opioids for non-cancer pain in Canada, 60% also tested positive for benzodiazepines [10]. Furthermore, Park et al. [11] found that American veterans with an opioid prescription receiving a benzodiazepine prescription were at increased risk of drug overdose death in a dose-response fashion.
In 2016, the Centers for Disease Control and Prevention (CDC) issued new guidelines for the prescribing of opioids [12]. CDC recommends that clinicians avoid prescribing benzodiazepines concurrently with opioids whenever possible. Both prescription opioids and benzodiazepines now carry FDA “black box” warnings on the label highlighting the dangers of using these drugs together.
Benzodiazepine use disorder (BUD)
As stated above, BZD abuse and dependence is a serious clinical problem in not only the methadone maintenance treatment (MMT) population but also with opioids in general. Estimates of the prevalence of BUD among MMT patients range from 21% to 66% [13–20], with roughly half of patients starting BZD use after entering MMT [21]. It is noteworthy that MMT patients with BUD are likely to be using other drugs, including heroin, engage in high risk behaviors, and have increased rates of depression, anxiety, and global psychopathology [22]. It is unfortunate but factual that women in MMT who misuse BZDs during pregnancy have babies of significantly lower birth weight than those who do not [23]. In fact, MMT patients with BUD have an 8-fold likelihood of death compared to other MMT patients, as suggested by Caplehorn and associates [24]. Unfortunately, BUD in MMT programs is widespread and is associated with a negative impact on treatment outcomes [25,26].
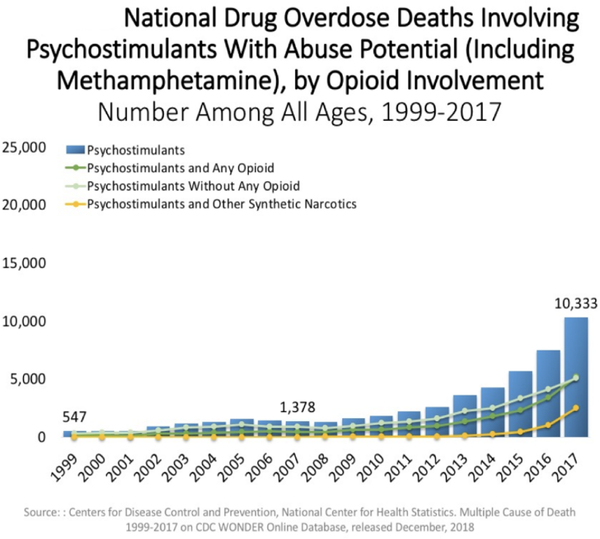
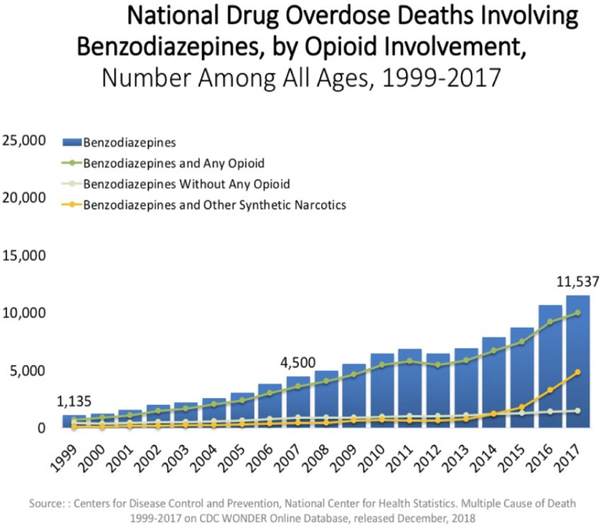
In fact, on a national basis, opioid overdose is increased in either psychostimulant combinations (Figure 2) or even in BZD combination with opioids (Figure 3).
Figure 2.
Oversdose deaths psychostimulants and opioids
Figure 3.
Oversdose deaths Benzodiazepines and opioids
It is well established that Gamma-aminobutyric acid (GABA) type A receptors are a family of ligand-gated ion channels responsible for inhibitory regulation of the central nervous system, especially impacting reward circuitry and attenuation of dopamine release at the VTA-Accumbens [27]. BZDs bind directly to a specific modulatory site that is present on GABAA receptors [28,29], which enhances the effect of the inhibitory neurotransmitter, but does not open the chloride channel in the absence of GABA. Moreover, chronic exposure to BZDs leads to modulation of GABAergic neurotransmission, resulting in symptoms of tolerance, dependence and withdrawal. It is believed that these effects of BZDs are linked directly to GABAA receptor subtypes [30], and the withdrawal syndrome may in part be mediated by calcium channels [31] and glutamatergic [32] mechanisms.
It is well-known that discontinuation of therapeutic BZD treatment has generally supported the approach of a slow, gradual taper [33]. There is a plethora of many small clinical trials involving many pharmaceuticals including: propranolol [34]; buspirone [35]; progesterone [36]; Hydroxyzine [37]; Carbamazepine [38]; Trazodone and valproate [39]; Cyamemazine [40]; and flumazenil [41]. However, no clearly effective pharmacotherapy for the treatment of BZD use disorder has been identified. In addition, even slow outpatient detoxification has not been accepted in clinical practice, and as such, there is no known optimal strategy for BUD globally [42].
One therapeutic approach, which has been studied and is considered to be beneficial clinically to offset BZDS withdrawal and other side effects, is Gabapentin. Briefly, Gabapentin, is a structural analogue of the neurotransmitter GABA [43] and while it is not a GABA-mimetic agent [44], it has been shown to increase synaptic GABA levels [45,46], Gabapentin binds to the α2δ−1 and α2δ−2 subunits of voltage-gated calcium channels [47–49] and inhibits calcium currents [50–52], which leads to attenuation of postsynaptic excitability [53,54], potentially leading to a reduction of dopamine at the Nucleus accumbens (NAc). However, there have been no chronic studies in humans related to the effects of chronic Gabapentin and dopaminergic transmission. There are some studies which suggest that Gabapentin has anxiolytic [55,56] and sedating properties [57].
With this rationale, Mariani et al. [58]carried out an eight-week randomized double-blind placebo-controlled outpatient pilot trial using Gabapentin to help treat BUD in methadone patients. They found no significant differences on BZD use outcomes (amount BZD per day (Mann-Whitney U = 27, p = .745), abstinent days per week (U = 28, p = .811)) and CIWA-BZD scale (U = 29.0, p = .913). The authors conclude that Gabapentin was not found to differ from placebo, although the small sample recruited for this trial may have limited the ability to detect a difference.
The need for “dopamine homeostasis”
Despite the risk of BUD on MMT patients, to date there is no clearly effective treatment strategy for managing this clinical issue. Therefore, we are proposing a novel approach we term “Precision Addiction Management” [59] and “Precision Behavioral Management.”
Every day in America, millions of people from all walks of life are unable to combat their fatal romance with getting high. For many caught in all kinds of addictive behaviors, this “high” may be just experiencing feelings of well-being [60]. Importantly, while it is widely accepted that dopamine is a major neurotransmitter implicated in behavioral and substance addictions, including BUD, there remains controversy about how to modulate dopamine clinically. One important question relates to genetic antecedents of hypodopaminergia, placing people at high risk for all addictive behaviors including BUD [61]. We believe that early genetic identification of hypodopaminergia could translate to early intervention. A prudent approach may be biphasic; a short-term blockade followed by long-term dopaminergic upregulation, achieving dopamine homeostasis [62].
The goal of treatment would be to enhance brain reward functional connectivity volume, targeting reward deficiency and the stress-like anti-reward symptomatology of addiction [63,64]. Such phenotypes can be characterized using the Genetic Addiction Risk Score (GARS) [65]. Dopamine homeostasis may thus be achieved via “Precision Addiction Management” (PAM) [58,66], also known as “Precision Behavioral Management” (PBM); the customization of neuronutrient supplementation based on the GARS test result, along with a behavioral intervention.
Dopamine homeostasis and BUD
We are proposing herein that induction of dopamine homeostasis will be clinically beneficial in attempts to combat BUD for at least three reasons: 1) During detoxification of alcoholism, the potential induction of dopamine regulation reduces the need for BZDs; 2) A major reason for BZD abuse is that people want to achieve a stress reduction, and subsequently, potential induction of dopamine regulation acts as an anti-stress factor; and 3) BUD and OUD are known to reduce resting state functional connectivity, and as such, potential induction of dopamine regulation enhances resting state functional connectivity.
Common mechanisms in detoxification
It is well known that a major use of BZDs is to help reduce alcohol-induced withdrawal symptoms because of inhibition of excitatory neuronal activity. Of interest, it is also known that dopamine and morphine significantly reduce alcohol-induced withdrawal symptoms, suggesting common mechanisms [67,68]. In 1988, Blum et al. [69] reported on results of a double-blind investigation of a Pro-dopamine regulator (now identified as ‘KB220’ and evolved variants) for facilitating improvement in a 30-day inpatient alcohol and drug rehabilitation center. KB220 is uniquely designed to elevate levels of enkephalin(s), serotonin, catecholamines, and GABA, which are believed to be functionally deficient in alcoholics. The KB220 patients, as compared to the control group, had a lower building up to drink score (1 vs 2); required no PRN BZDs (0% vs 94%); ceased tremoring at 72 h, as compared to 96 h; and had no severe depression on the MMPI, in contrast to 24% of control group. The take home message here is that KB220, a known Pro-dopamine regulator [70], significantly reduced the need for BZDs in residential treatment for alcoholism. These results suggest the importance of potentially achieving dopamine balance, especially during detoxification as we observed with opioid withdrawal [71].
Dopaminergic anti-stress potential
While it is accepted that up regulation of GABAA receptors results in an attenuation of stress in animal models [72] as well as humans [73], the role of dopamine as an anti-stress molecule is also known [74]. In fact, electrophysiological experiments reveal that aversive stimuli block putative VTA dopamine neuron firing [75–78], Interestingly, microdialysis investigations examining extracellular dopamine and its metabolites found a robust dopaminergic increase during stress in VTA projection targets. Specifically, stressors such as restraint, foot-shock, tail pinch/shock, and social threat, increase extracellular dopamine in the NAc and mPFC [79–82]. Moreover, studies reveal long-lasting neuroadaptive changes on VTA dopamine neurons after a single stress exposure [83]. According to Holly & Miczek [83], these studies highlight that acute stress can modify VTA dopamine neuron responsivity. Understanding the powerful role of dopamine during stressful conditions, and the known fact that hypodopaminergia (possibly genetically) is a culprit for addiction relapse [84,85], and an inability to cope with even family stress in alcoholics [86], provides the rationale for suggesting that KB220 variants could have anti-anxiety effects via dopamine regulation or induction of homeostasis, which could combat BUD. Comings et al. [86] reported that DRD2 A1 genotype–phenotype associations depend on the magnitude of stress exposure, and these results lend support to the view that variability in DRD2 study outcomes may in part be explained by this geneenvironment interaction. Specifically, Volkow’s group showed through PET scans that drug abusers have marked decreases in dopamine D2 receptors and in dopamine release. Moreover, this decrease in dopamine function or hypodopaminergia is associated with reduced regional activity in orbitofrontal cortex (involved in salience attribution; its disruption results in compulsive behaviors), cingulate gyrus (involved in reinstatement of drug abuse) and dorsolateral prefrontal cortex (involved in executive function and decision -making). With brief snapshot of the involvement of Dopamine as an anti-stress molecule, our laboratory found significant evidence regarding the pro-dopamine regulator KB220Z to act as an anti–anxiety agent [87]. Along these lines of investigation, Blum et al., in a published double-blinded placebo-controlled study, found the complex KB220 in alcoholics and polydrug abusers attending an in-patient chemical dependency program, showed significant anti-anxiety effects. Specifically, in 62 alcoholic and polydrug abusers, Blum’s group utilized skin conductance level (SCL) to evaluate stress responses. Patients administered KB220 had a significantly reduced stress response as measured by SCL, compared to patients administered placebo. A two factor ANOVA yielded significant differences as a function of Time (p< 0.01). These results support the notion that regulation of dopamine and possible induction of its homeostasis in chemical dependent patients may improve treatment response in heroin in-patient treatment setting by reducing stress related behaviors and warrants further investigation [87].
Induction of enhanced functional connectivity
Stein’s group from NIDA has clearly established the importance of resting state functional connectivity in addiction medicine [88,89]. Assessing network dynamics via resting-state functional connectivity (rsFC) enables a better understanding of how reductions in rsFC and circuit connectivity link into dopaminergic dysfunction and reward processing and liability to addictive–like behavior. There are many examples showing that drugs of abuse including nicotine, cocaine, alcohol, heroin and even BZDs tend to reduce rsFC [90]. In fact, it was found that oxazepam decreased connectivity between, for example, the modulated amygdala and temporal cortex, whereby L-dopa also altered functional connectivity. With this background it seems prudent that one way to improve clinical outcomes is to target potential enhancement of rsFC in both naïve animals [64] and heroin–abstinent humans [63]. We have learned from the work of Febo et al. [64], that the pro-dopaminergic complex KB220Z significantly enhances, above placebo, functional connectivity between reward and cognitive brain areas in the rat. Importantly, these include the nucleus accumbens, anterior cingulate gyrus, anterior thalamic nuclei, hippocampus, prelimbic and infralimbic loci. Moreover, significant functional connectivity increased brain connectivity volume recruitment (potentially neuroplasticity), and dopaminergic functionality across the brain reward circuitry. Most importantly, increases in functional connectivity were specific to these regions and were not broadly distributed across the brain. In other work from our laboratory, along with Mark Gold and associates from China, we evaluated the role of KB220Z on neural pathways in reward circuitry of abstinent genotyped heroin addicts. In part, the rational was based on the work of Willuhn et al. [91]. who reported cocaine use and even non-substance-related addictive behavior increases as dopaminergic function is reduced? It has been shown by Zhang et al. [92] that rsFC is disruptive in abstinent heroin addicts. We hypothesized that treatment strategies, like dopamine agonist therapy with substances like KB220Z, may be a prudent approach to relapse prevention in abstinent heroin probands. We investigated the effect of KB220Z on reward circuitry of 10 heroin addicts undergoing protracted abstinence (average 16.9 months). Specifically, we found that in a randomized placebo-controlled crossover study of KB220Z, five subjects completed a triple-blinded experiment. In addition, nine subjects were genotyped utilizing the GARS test. KB220Z induced an increase in Blood Oxygen Level Dependent (‘BOLD’) activation in caudate-accumbens-dopaminergic pathways compared to placebo following 1-hour acute administration. Moreover, KB220Z induced an increase in functional connectivity in a putative network that included the dorsal anterior cingulate, medial frontal gyrus, nucleus accumbens, posterior cingulate, occipital cortical areas, and cerebellum.
Summary
Prescriptions for Benzodiazepines (BZDs) have risen continually, and according to national statistics, its combination with opioids has also increased since 1999 up to 2019 (time of this writing). Benzodiazepines (sometimes called “benzos”) work to calm or sedate a person by raising the level of the inhibitory neurotransmitter GABA in the brain. In terms of neurochemistry, BZDs act at the GABAA receptors to inhibit excitatory neurons reducing VTA glutaminergic drive to reduce dopamine release at the accumbens.
While Benzodiazepine Use Disorder (BUD) is very difficult to treat, partly because BZD are used to reduce anxiety and paradoxically induce a hypodopaminergia, we are proposing a paradigm shift. Instead of simply targeting chloride channel direct GABAA receptors for replacement or substitution therapy, we propose the induction of dopamine homeostasis. Our rationale is supported by the well-established notion that the root cause of drug and non-drug addictions (i.e. Reward Deficiency Syndrome [RDS]), at least in adults, involve dopaminergic dysfunction and heightened stress; an epigenetic phenomenon.
This proposition involves coupling the Genetic Addiction Risk Score (GARS) with a subsequent polymorphic matched genetic customized Pro-Dopamine Regulator known as KB220ZPBM. Induction of dopamine homeostasis will be clinically beneficial in attempts to combat BUD for at least three reasons: 1) During detoxification of alcoholism, the potential induction of dopamine regulation reduces the need for BZDs; 2) A major reason for BZD abuse is because people want to achieve stress reduction and subsequently, potential induction of dopamine regulation acts as an anti-stress factor, and; 3) BUD and OUD are known to reduce resting state functional connectivity, and as such, potential induction of dopamine regulation enhances resting state functional connectivity. The experimental support for the neuropharmacological and neurological basis of KB220 can be gleaned from the work of Blum et al. [93]. Future studies will investigate through randomized placebo-controlled studies this forward thinking proposed novel modality.
References
- 1.John G (2015) Top 25 Psychiatric medication prescriptions For 2013. Psych Central. Retrieved 3 April 2015. [Google Scholar]
- 2.Drug Enforcement Agency (2013) “Benzodiazepines.” Jan. 2013. Accessed 13 Sep. 2017.
- 3.Carlisle MJ (2017) Trends in the abuse of prescription drugs. the gulf coast addiction technology transfer center. Nov. 2006. accessed 13 Sep. 2017. [Google Scholar]
- 4.Food and Drug Administration (2011) Xanax. Accessed 13 Sep. 2017.
- 5.Centers for Disease Control and Prevention (Cdc) (2017) National vital statistics system, mortality. Cdc wonder online database. Https://Wonder.Cdc.Gov/. Published 2017. Accessed April 4, 2017. [Google Scholar]
- 6.Bachhuber Ma, Hennessy S, Cunningham Co, Starrels J (2016) Increasing benzodiazepine prescriptions and overdose mortality in the united states. Am J Public Health 106: 686–688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Centers for Disease Control and Prevention (Cdc). Multiple Cause of Death, 1999–2015. Cdc Wonder Online Database. Https://Wonder.Cdc.Gov/Mcd-Icd10.Html. Accessed April 4, 2017. [Google Scholar]
- 8.Sun Ec, Dixit A, Humphreys K, Darnall Bd, Baker Lc, et al. (2017) association between concurrent use of prescription opioids and benzodiazepines and overdose: retrospective analysis. BMJ 356: J760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dasgupta N, Funk Mj, Proescholdbell S Hirsch A, Ribisl Km, Marshall S, et al. (2016) Cohort study of the impact of high-dose opioid analgesics on overdose mortality. Pain Med Malden Mass 17: 85–98. [DOI] [PubMed] [Google Scholar]
- 10.Gomes T, Mamdani Mm, Dhalla Ia, Paterson Jm, Juurlink Dn, et al. (2011) Opioid dose and drug-related mortality in patients with nonmalignant pain. Arch Intern Med 171: 686–691. [DOI] [PubMed] [Google Scholar]
- 11.Tw Park, Saitz R, Ganoczy D, Ilgen Ma, Bohnert Asb (2015) Benzodiazepine prescribing patterns and deaths from drug overdose among us veterans receiving opioid analgesics: case-cohort Study. BMJ 350: H2698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dowell D, Haegerich Tm, Chou R (2016) Cdc guideline for prescribing opioids for chronic pain - united states. Mmwr Recomm Rep 65. [DOI] [PubMed] [Google Scholar]
- 13.Darke S, Swift W, Hall W, Ross M (1993) Drug use, hiv risk-taking and psychosocial correlates of benzodiazepine use among methadone maintenance clients. drug alcohol depends 34: 67–70. [DOI] [PubMed] [Google Scholar]
- 14.Iguchi My, Handelsman L, Bickel Wk, Griffiths Rr (1993) Benzodiazepine and sedative use/abuse by methadone maintenance clients. drug alcohol depends 32: 257–266. [DOI] [PubMed] [Google Scholar]
- 15.Kidorf M, Brooner Rk, King Vl, Chutuape Ma, Stitzer M, et al. (1996) concurrent validity of cocaine and sedative dependence diagnoses in opioid-dependent outpatients. drug alcohol depends 42: 117–123. [DOI] [PubMed] [Google Scholar]
- 16.Bleich A, Gelkopf M, Schmidt V, Hayward R, Bodner G, et al. (1999) Correlates of benzodiazepine abuse in methadone maintenance treatment. a 1st year prospective study in an israeli clinic. Addiction (Abingdon, England) 94: 1533–1540. [DOI] [PubMed] [Google Scholar]
- 17.Gelkopf M, Bleich A, Hayward R, Bodner G, Adelson M, et al. (1999) characteristics of benzodiazepine abuse in methadone maintenance treatment patients: a 1st year prospective study in an israeli clinic. Drug Alcohol Depend 55: 63–68. [DOI] [PubMed] [Google Scholar]
- 18.Bleich A, Gelkopf M, Weizman T, Adelson M (2002) Benzodiazepine abuse in a methadone maintenance treatment clinic in israel: characteristics and a pharmacotherapeutic approach. Isr J Psychiatry Relat Sci 39: 104–112. [PubMed] [Google Scholar]
- 19.Specka M, Bonnet U, Heilmann M, Schifano F, Scherbaum N, et al. (2011) Longitudinal patterns of benzodiazepine consumption in a german cohort of methadone maintenance treatment patients. Hum Psychopharmacol 26: 404–411. [DOI] [PubMed] [Google Scholar]
- 20.Usto Ue, Sykora K, Sellers Em (1989) A clinical scale to assess benzodiazepine withdrawal. Journal of Clinical Psychopharmacology 9: 412–416. [PubMed] [Google Scholar]
- 21.Kw Chen, Cc Berger, Dp Forde, D’adamo C, Weintraub E, et al. (2011) Benzodiazepine use and misuse among patients in a methadone program. BMC Psychiatry 11: 90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Condelli Ws, Dunteman Gh (1993) Exposure to methadone programs and heroin use. j drug alcohol abuse 19: 65–78. [DOI] [PubMed] [Google Scholar]
- 23.Mccarthy Je, Siney C, Shaw Nj, Ruben Sm (1999) Outcome predictors in pregnant opiate and polydrug users. Eur J Pediatr 158: 748–749. [DOI] [PubMed] [Google Scholar]
- 24.Caplehorn Jr Dalton Ms, Haldar F, Petrenas Am, Nisbet Jg (1996) Methadone maintenance and addicts’ risk of fatal heroin overdose. Subst Use Misuse 31: 177–196. [DOI] [PubMed] [Google Scholar]
- 25.Brands B, Blake J, Marsh Dc, Sproule B, Jeyapalan R, et al. (2008) The impact of benzodiazepine uses on methadone maintenance treatment outcomes. J Addict Dis 27: 37–48. [DOI] [PubMed] [Google Scholar]
- 26.Eiroa-Orosa Fj, Haasen C, Verthein U, Dilg C, Schafer I, et al. (2010) Benzodiazepine use among patients in heroin-assisted vs. methadone maintenance treatment: findings of the german randomized controlled trial. Drug Alcohol Depend 112: 226–233. [DOI] [PubMed] [Google Scholar]
- 27.Blum K, Oscar-Berman M, Stuller E, Miller D, Giordano J, et al. (2012) Neurogenetics and Nutrigenomics of Neuro-Nutrient Therapy for Reward Deficiency Syndrome (RDS): Clinical Ramifications as a Function of Molecular Neurobiological Mechanisms. Journal of addiction research and therapy 3: 139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schoch P, Mohler H (1983) Purified benzodiazepine receptor retains modulation by gaba. Eur J Pharmacol 95: 323–324. [DOI] [PubMed] [Google Scholar]
- 29.Schofield Pr, Darlison Mg, Fujita N, Burt Dr, Stephenson Fa, et al. (1987) sequence and functional expression of the gaba a receptor shows a ligand-gated receptor superfamily. Nature 328: 221–227. [DOI] [PubMed] [Google Scholar]
- 30.Wafford Ka (2005) gabaa receptor subtypes: any clues to the mechanism of benzodiazepine dependence? Current Opinion in Pharmacology. 5: 47–52. [DOI] [PubMed] [Google Scholar]
- 31.Xiang K, Tietz Ei (2007) benzodiazepine-induced hippocampal ca1 neuron alpha-amino-3-hydroxy-5-methylisoxasole-4-propionic acid (ampa) receptor plasticity linked to severity of withdrawal anxiety: differential role of voltage-gated calcium channels and n-methyl-d-aspartic acid receptors. Behavioural Pharmacology 18: 447–460. [DOI] [PubMed] [Google Scholar]
- 32.Song J, Shen G, Greenfield Lj, Tietz Ei (2007) benzodiazepine withdrawal-induced glutamatergic plasticity involves up-regulation of glur1-containing alpha-amino-3-hydroxy-5-methylisoxazole-4-propionic acid receptors in hippocampal ca1 neurons. J Pharmacol Exp Ther. 322: 569–581. [DOI] [PubMed] [Google Scholar]
- 33.Nardi Ae, Freire Rc, Valenca Am, Amrein R, De Cerqueira Ac, et al. (2010) Tapering clonazepam in patients with panic disorder after at least 3 years of treatment. J Clin Psychopharmacol 30: 290–293. [DOI] [PubMed] [Google Scholar]
- 34.Hallstrom C, Crouch G, Robson M, Shine P (1988) The treatment of tranquilizer dependence by propranolol. Postgraduate Medical Journal 64(Suppl 2): 40–44. [PubMed] [Google Scholar]
- 35.Lader M, Olajide D (1987) A comparison of buspirone and placebo in relieving benzodiazepine withdrawal symptoms. J Clin Psychopharmacol 7: 11–15. [PubMed] [Google Scholar]
- 36.Schweizer E, Case Wg, Garcia-Espana F, Greenblatt Dj, Rickels K, et al. (1995) Progesterone co-administration in patients discontinuing long-term benzodiazepine therapy: effects on withdrawal severity and taper outcome. Psychopharmacology (Berl) 117: 424–429. [DOI] [PubMed] [Google Scholar]
- 37.Lemoine P, Touchon J, Billardon M (1997) Comparison Of 6 different methods for lorazepam withdrawal. a controlled study, hydroxyzine versus placebo. L’encephale 23: 290–299. [PubMed] [Google Scholar]
- 38.Schweizer E, Rickels K, Case Wg, Greenblatt Dj (1991) Carbamazepine treatment in patients discontinuing long-term benzosdiazepine therapy. effects on withdrawal severity and outcome. Arch Gen Psychiatry 48: 448–452. [DOI] [PubMed] [Google Scholar]
- 39.Rickels K, Schweizer E, Garcia Espana F, Case G, Demartinis N, et al. (1999) trazodone and valproate in patients discontinuing long-term benzodiazepine therapy: effects on withdrawal symptoms and taper outcome. Psychopharmacology (Berl) 141: 1–5. [DOI] [PubMed] [Google Scholar]
- 40.Lemoine P, Kermadi I, Garcia-Acosta S, Garay Rp, Dib M, et al. (2006) Double-blind, comparative study of cyamemazine vs. bromazepam in the benzodiazepine withdrawal syndrome. Prog Neuropsychopharmacol Biol Psychiatry 30: 131–137. [DOI] [PubMed] [Google Scholar]
- 41.Hood S, O’neil G, Hulse G (2009) The role of flumazenil in the treatment of benzodiazepine dependence: physiological and psychological profiles. J Psychopharmacol 23: 401–409. [DOI] [PubMed] [Google Scholar]
- 42.Mcduff Dr, Schwartz Rp, Tommasello A, Tiegel S, Donovan T, et al. (1993) Outpatient benzodiazepine detoxification procedure for methadone patients. J Subst Abuse Treat 10: 297–302. [DOI] [PubMed] [Google Scholar]
- 43.Taylor Cp, Gee Ns, Su Tz, Kocsis Jd, Welty Df, et al. (1998) A summary of mechanistic hypotheses of gabapentin pharmacology. Epilepsy Res 29: 233–249. [DOI] [PubMed] [Google Scholar]
- 44.Maneuf Yp, Gonzalez Mi, Sutton Ks, Chung Fz, Pinnock Rd, et al. (2003) Cellular and molecular action of the putative gaba-mimetic, gabapentin. Cell Mol Life Sci 60: 742–750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Errante Ld, Williamson A, Spencer Dd, Petroff Oa (2002) Gabapentin and vigabatrin increase gaba in the human neocortical slice. Epilepsy Res 49: 203–210. [DOI] [PubMed] [Google Scholar]
- 46.Petroff Oa, Rothman Dl, Behar Kl, Lamoureux D, Mattson Rh, et al. (1996) The effect of gabapentin on brain gamma-aminobutyric acid in patients with epilepsy. Annals of Neurology 39: 95–99. [DOI] [PubMed] [Google Scholar]
- 47.Gee Ns, Brown Jp, Dissanayake Vu, Offord J, Thurlow R, et al. (1996) The novel anticonvulsant drug, gabapentin (neurontin), binds to the alpha2delta subunit of a calcium channel. The journal of biological chemistry 271: 5768–5776. [DOI] [PubMed] [Google Scholar]
- 48.Wang M, Offord J, Oxender Dl, Su Tz (1999) Structural requirement of the calcium-channel subunit alpha2delta for gabapentin binding. The Biochemical Journal 342: 313–320. [PMC free article] [PubMed] [Google Scholar]
- 49.Marais E, Klugbauer N, Hofmann F (2001) Calcium channel alpha(2)delta subunits-structure and gabapentin binding. Mol Pharmacol 59: 1243–1248. [DOI] [PubMed] [Google Scholar]
- 50.Stefani A, Spadoni F, Bernardi G (1998) Gabapentin inhibits calcium currents in isolated rat brain neurons. Neuropharmacology 37: 83–91. [DOI] [PubMed] [Google Scholar]
- 51.Alden Kj, Garcia J (2001) Differential effect of gabapentin on neuronal and muscle calcium currents. j pharmacol exp ther 297: 727–735. [PubMed] [Google Scholar]
- 52.Sutton Kg, Martin Dj, Pinnock Rd, Lee K, Scott Rh, et al. (2002) Gabapentin inhibits high-threshold calcium channel currents in cultured rat dorsal root ganglion neurones. Br J Pharmacol 135: 257–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sills Gj (2006) The mechanisms of action of gabapentin and pregabalin. current opinion in pharmacology 6: 108–113. [DOI] [PubMed] [Google Scholar]
- 54.Cheng Jk, Chiou Lc (2006) Mechanisms of the antinociceptive action of gabapentin. journal of pharmacological sciences 100: 471–486. [DOI] [PubMed] [Google Scholar]
- 55.Pande Ac, Davidson Jr, Jefferson Jw, Janney Ca, Katzelnick Dj, et al. (1999) Treatment of social phobia with gabapentin: a placebo-controlled study. j clin psychopharmacol 19: 341–348. [DOI] [PubMed] [Google Scholar]
- 56.Pande Ac, Pollack Mh, Crockatt J, Greiner M, Chouinard G, et al. (2000) Placebo-controlled study of gabapentin treatment of panic disorder. j clin psychopharmacol 20: 467–471. [DOI] [PubMed] [Google Scholar]
- 57.Karam-Hage M, Brower Kj (2000) Gabapentin treatment for insomnia associated with alcohol dependence. Am J Psychiatry 157: 151. [DOI] [PubMed] [Google Scholar]
- 58.Mariani JJ, Malcolm RJ, Mamczur AK, Choi JC, Brady R, et al. (2016) Pilot trial of gabapentin for the treatment of benzodiazepine abuse or dependence in methadone maintenance patients. The american journal of drug and alcohol abuse 42: 333–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Blum K, Gondre-Lewis MC, Baron D, Thanos PK, Braverman ER, et al. (2018) Introducing precision addiction management of reward deficiency syndrome, the construct that underpins all addictive behaviors. Frontiers in psychiatry 9: 548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Blum L, Giordano J, Morse S, Bowirrat A, Madigan M, et al. (2010) Understanding the hugh mind: humans are still evolving genetically. Tne IIOAB 1: 1–14 (2010). [Google Scholar]
- 61.Blum K, Modestino EJ, Gondre-Lewis M, Downs BW, Baron D, et al. (2017) Dopamine homeostasis” requires balanced polypharmacy: Issue with destructive, powerful dopamine agents to combat America’s drug epidemic. Journal of systems and integrative neuroscience 3: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Blum K, Chen AL, Chen TJ, Braverman ER, Reinking J, et al. (2008) Activation instead of blocking mesolimbic dopaminergic reward circuitry is a preferred modality in the long-term treatment of reward deficiency syndrome (RDS): a commentary. Theoretical biology and medical modelling 5: 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Blum K, Liu Y, Wang W, Wang Y, Zhang Y, et al. (2014) rsfMRI effects of KB220Z™ on neural pathways in reward circuitry of abstinent genotyped heroin addicts. Postgraduate medicine 127: 232–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Febo M, Blum K, Badgaiyan RD, Perez PD, Colon-Perez LM, et al. (2017) Enhanced functional connectivity and volume between cognitive and reward centers of naïve rodent brain produced by pro-dopaminergic agent KB220Z. PloS one 12: e0174774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Blum K, Madigan MA, Fried L, Braverman ER, Giordano J, et al. (2017) Coupling genetic addiction risk score (gars) and pro dopamine regulation (kb220) to combat substance use disorder (sud). Global Journal of addiction and rehabilitation medicine 1: 555556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Blum, Modestino EJ, Neary J, Gondre-Lewis MC, Siwicki D, et al. (2018) Promoting precision addiction management (pam) to combat the global opioid crisis. Biomedical journal of scientific and technical research 2: 1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Blum K, Eubanks JD, Wallace JE, Schwertner HA (1976) Suppression of ethanol withdrawal by dopamine. Experientia 32: 493–495. [DOI] [PubMed] [Google Scholar]
- 68.Blum K, Wallace JE, Schwerter HA, Eubanks JD (1976) Morphine suppression of ethanol withdrawal in mice. Experientia 32: 79–82. [DOI] [PubMed] [Google Scholar]
- 69.Blum K, Trachtenberg MC, Ramsay JC (1988) Improvement of inpatient treatment of the alcoholic as a function of neurotransmitter restoration: a pilot study. Int J Addict 23: 991–998. [DOI] [PubMed] [Google Scholar]
- 70.Blum K, Febo M, Fried L, Li M, Dushaj K, et al. (2016) Hypothesizing that neuropharmacological and neuroimaging studies of glutaminergic-dopaminergic optimization complex (kb220z) are associated with “dopamine homeostasis” in reward deficiency syndrome (rds). Substance use and misuse 52: 535–547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Blum K, Whitney D, Fried L, Febo M, Waite RL, et al. (2016) Hypothesizing that a Pro-Dopaminergic Regulator (KB220z™ Liquid Variant) can Induce “Dopamine Homeostasis” and Provide Adjunctive Detoxification Benefits in Opiate/Opioid Dependence. Clinical medical reviews and case reports 3: 125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Price TJ, Cervero F, Gold MS, Hammond DL, Prescott SA, et al. (2008) Chloride regulation in the pain pathway. Brain research reviews 60: 149–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Tan KR, Rudolph U, Luscher C (2011) Hooked on benzodiazepines: GABAA receptor subtypes and addiction. Trends in neurosciences 34: 188–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Holly EN, Miczek KA (2015) Ventral tegmental area dopamine revisited: effects of acute and repeated stress. Psychopharmacology 233: 163–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Mantz J, Thierry AM, Glowinski J (1989) Effect of noxious tail pinch on the discharge rate of mesocortical and mesolimbic dopamine neurons: selective activation of the mesocortical system. Brain Res 476: 377–381. [DOI] [PubMed] [Google Scholar]
- 76.Mirenowicz J, Schultz W (1996) Preferential activation of midbrain dopamine neurons by appetitive rather than aversive stimuli. Nature 379: 449–451. [DOI] [PubMed] [Google Scholar]
- 77.Schultz W, Romo R (1987) Responses of nigrostriatal dopamine neurons to high-intensity somatosensory stimulation in the anesthetized monkey. J Neurophysiol 57: 201–217. [DOI] [PubMed] [Google Scholar]
- 78.Ungless MA, Magill PJ, Bolam JP (2004) Uniform inhibition of dopamine neurons in the ventral tegmental area by aversive stimuli. Science 303: 2040–2042. [DOI] [PubMed] [Google Scholar]
- 79.Imperato A, Puglisi-Allegra S, Casolini P, Zocchi A, Angelucci L (1989) Stress-induced enhancement of dopamine and acetylcholine release in limbic structures: role of corticosterone. Eur J Pharmacol 165: 337–338. [DOI] [PubMed] [Google Scholar]
- 80.Jedema HP, Grace AA (2003) Chronic exposure to cold stress alters electrophysiological properties of locus coeruleus neurons recorded in vitro. Neuropsychopharmacology 28: 63–72. [DOI] [PubMed] [Google Scholar]
- 81.Abercrombie ED, Keefe KA, DiFrischia DS, Zigmond MJ (1989) Differential effect of stress on in vivo dopamine release in striatum, nucleus accumbens, and medial frontal cortex. J Neurochem 52: 1655–1658. [DOI] [PubMed] [Google Scholar]
- 82.Wu YL, Yoshida M, Emoto H, Tanaka M (1999) Psychological stress selectively increases extracellular dopamine in the ‘shell’, but not in the ‘core’ of the rat nucleus accumbens: a novel dual-needle probe simultaneous microdialysis study. Neurosci Lett 275: 69–72. [DOI] [PubMed] [Google Scholar]
- 83.Holly EN, Miczek KA (2015) Ventral tegmental area dopamine revisited: effects of acute and repeated stress. Psychopharmacology 233: 163–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Moore H, Rose HJ, Grace AA (2001) Chronic cold stress reduces the spontaneous activity of ventral tegmental dopamine neurons. Neuropsychopharmacology 24: 410–419. [DOI] [PubMed] [Google Scholar]
- 85.Volkow ND (2008) Imaging dopamine’s role in drug abuse and addiction. Neuropharmacology 56: 3–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Madrid GA, MacMurray J, Lee JW, Anderson BA, Comings DE, et al. (2001) Stress as a mediating factor in the association between the DRD2 TaqI polymorphism and alcoholism. Alcohol 23: 117–122. [DOI] [PubMed] [Google Scholar]
- 87.Blum K, Trachtenberg MC, Elliott CE, Dingler ML, Sexton RL, et al. (1988) Enkephalinase inhibition and precursor amino acid loading improves inpatient treatment of alcohol and polydrug abusers: double-blind placebo-controlled study of the nutritional adjunct SAAVE. Alcohol 5: 481–493. [DOI] [PubMed] [Google Scholar]
- 88.Pariyadath V, Gowin JL, Stein EA (2016) Resting state functional connectivity analysis for addiction medicine: From individual loci to complex networks. Prog Brain Res 224: 155–173. [DOI] [PubMed] [Google Scholar]
- 89.Sutherland MT, McHugh MJ, Pariyadath V, Stein EA (2012) Resting state functional connectivity in addiction: Lessons learned and a road ahead. Neuroimage 62: 2281–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Flodin P, Gospic K, Petrovic P, Fransson P (2012) Effects of L-dopa and oxazepam on resting-state functional magnetic resonance imaging connectivity: a randomized, cross-sectional placebo study. Brain Connect 2: 246–253. [DOI] [PubMed] [Google Scholar]
- 91.Willuhn I, Burgeno LM, Groblewski PA, Phillips PE (2014) Excessive cocaine use results from decreased phasic dopamine signaling in the striatum. Nature neuroscience 17: 704–709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Li Q, Wang Y, Zhang Y, Li W, Zhu J, et al. (2013) Assessing cue-induced brain response as a function of abstinence duration in heroin-dependent individuals: an event-related fMRI study. PloS one 8: e62911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Blum K (2018) Pro-Dopamine Regulator (KB220) A Fifty Year Sojourn to Combat Reward Deficiency Syndrome (RDS): Evidence Based Bibliography (Annotated). CPQ Neurology and Psychology 1: 01–23. [PMC free article] [PubMed] [Google Scholar]