Opinion Statement
With no therapy specifically approved for non-clear cell cancers of the kidney, this disease remains an orphan site. Clear cell renal cancers (ccRCC) have seen a flurry of activity with multiple agents gaining Food and Drug Administration (FDA) approval in recent years. Simultaneously, non-clear cell RCC (ncRCC) have also seen a fair share of activity and exploration of new agents in development but no specific FDA approvals. Non-clear cell RCC is a mixed bag of multiple types of tumors originating in the kidney with distinct clinical molecular and genetic characteristics that vary significantly from clear cell carcinoma of the kidney. Conventionally, non-clear cell RCC have been treated with the same therapies as clear cell RCC. The clinical trials are typically conducted in ccRCC and the FDA approval covers non-clear cell cancer as well. Few randomized clinical trials have been conducted specifically for ncRCC. With the advent of molecular and tumor genomic testing, leading to discovery of targets and associated therapies for ncRCC, a specific review of the state of management of this disease is timely and clinically relevant.
Keywords: Non-clear cell, Chromophobe, Papillary, Sarcomatoid, Medullary, Unclassified, Renal cancer
Introduction
Of the 21,052 cases of distant stage kidney cancer between the years 2000 and 2013, 5259 (24.98%) were of non-clear cell histology as reported by the SEER population registry [1]. Although less frequent in incidence, non-clear cell renal cancers (ncRCC) remain an incurable and fatal malignancy in the advanced stages. Some of the histologies such as translocation type, or medullary kidney cancers, can be observed in young patients and the clinical impact can be significant. Kidney cancer incidence increased 2.1-fold between 1990 and 2013, with equal contributions from population growth (35.0%), changes in age structure (34.7%), and increasing incidence rates (37.1%) [2]. Therapeutic development in non-clear cell kidney cancer is gaining momentum as we gain a better understanding of the biology of the disease. However, emerging evidence indicates that each of these rare tumors have a unique pathophysiology and may need to be addressed separately for therapeutic development [3••]. At present, the therapies evaluated in clinical trials conducted in clear cell RCC and approved by the FDA for kidney cancer are routinely used in non-clear cell as well. The efficacy of the same agents in ncRCC is attenuated with decreased response rates and shorter durations of response. This subset of kidney tumors remains lethal in the metastatic setting and a large contributor to the morbidity and mortality of this disease. Due to the rare incidence, any advances in ncRCC continue to require accrual from multiple institutions or cooperative group trial efforts. Recognizing this, priority should be given for rational drug development trials in ncRCC in a collaborative effort.
Genetic or Familial Syndromes
Multiple non-clear cell histologies have associations with specific genetic or familial syndromes (Table 1). Von Hippel-Lindau is typically described and associated with dear cell cancers of kidney. However for ncRCC, hereditary papillary renal carcinoma, Birt-Hogg-Dubé, hereditary leiomyomatosis renal cell carcinoma, succinate dehydrogenase kidney cancer, tuberous sclerosis complex (TSC), and Cowden’s disease have been reported [3••, 4]. Hereditary papillary kidney cancer demonstrates autosomal dominant inheritance with multifocal multiple bilateral type I tumors that tend to be localized. Usually, management consists of careful surveillance and resections as needed prior to tumors attaining a size of about 3 cm. For familial type II papillary cancers, the proto-oncogene MET is frequently mutated usually in the tyrosine kinase domain resulting in possible efficacy of multikinase inhibitors such as vascular endothelial growth factor inhibitors and MET inhibitors [4].
Table 1.
Histologic, genetic, and clinical characteristics and management of non-clear cell RCC
| Type of ncRCC |
Clinical | Genetic mutations | Management |
|---|---|---|---|
| Papillary type 1 | Localized, small unlikely to be metastatic | Familial PLRCC: 100% MET mutations Sporadic: 13% MET mutations | Clinical trials of MET inhibitors are preferable Consider cabozantinib as it has MET inhibitory effects |
| Type 2 | Usually high grade and metastatic | Sporadic: usually a mixture of collecting duct and medullary RCC components. No specific gene mutations described. Familial: fumarate hydratase gene mutation if associated with hereditary leiomyomatosis RCC | Consider anti-VEGF therapies, usually sunitinib, pazopanib, or cabozantinib (anti-VEGFand anti-MET) |
| Chromophobe | Varied clinical course | Familial: 96% with FLCN (folliculin) gene mutation when associated with oncocytoma and Birt-Hogg Dube syndrome (renal neoplasms, fibrofolliculomas of skin and pneumatoceles) Sporadic: unknown | Some indication that this subgroup benefited from front-line everolimus |
| Sarcomatoid | Aggressive, rapidly progressive usually metastatic presentation | No familial syndrome noted. Sporadic: 32% biallelic TP53 mutations Exclusive BAP1 and ARID1 mutations PBRM1, SETD2, and PTEN mutations | Chemotherapy with doxorubicin and gemcitabine combination or cisplatin-based chemotherapy. Nivolumab has shown some promise. |
| Translocation type | Initially more indolent than clear cell but frequently metastatic and incurable | Renal/melanoma syndrome: germline mutations of MiTF Sporadic: TFE3 and TFEB mutations | Therapy is the same as that used for clear cell RCC. |
| Medullary | Rapidly progressing, metastatic at presentation | Sickle cell trait association but cases without sickle cell have been reported. 1 case with non-sickle cell had FH gene mutation, 1 had VHL mutation.HIF-1 alpha overexpression noted by IHC | Chemotherapy: typically cisplatin or doxorubicin based. Clinical trials with HIF-1 alpha inhibitors |
| Collecting duct | Rapidly progressing,metastatic at presentation and fatal | No specific gene mutations described.TERT mutations are associated with upper tract urinary tumors. | Chemotherapy: typically cisplatin or doxorubicin based. |
| Unclassified | Rapidly progressing, metastatic at presentation and fatal. | No specific gene mutations described | Chemotherapy is a reasonable strategy. Consider anti-VEGF therapies, usually sunitinib, pazopanib, or cabozantinib |
Treatment of ncRCC
Role of nephrectomy and perioperative therapy
Due to suboptimal systemic therapy, the importance of cytoreductive nephrectomy (CN) in ncRCC is prominent. The studies of CN were conducted in patients with clear cell renal cancers (ccRCC); however, the principle of impacting survival by debulking surgery critically applies to localized and metastatic ncRCC [5, 6]. However, post nephrectomy, the evidence indicates that there is no benefit with adjuvant systemic therapy. ECOG 2805 was a double-blind randomized trial to evaluate the overall survival (OS) benefit of sunitinib or sorafenib versus placebo in patients with localized RCC post nephrectomy. The overall study results showed no benefit with either of the therapies as compared to placebo. In the study, [7], 550 of 1943 (28.3%) enrolled patients had ncRCC histology and no benefit in disease-free survival or OS was noted within this subgroup. The hazard ratios within the non-clear cell histology subgroup in the trial were 1.07 (97.5% confidence interval, 0.69–1.67) for sunitinib versus placebo and 0.94 (97.5% confidence interval, 0.59–1.48) for sorafenib versus placebo. Other reported studies have not included ncRCC. The EVEREST trial randomizing patients to adjuvant everolimus or placebo allowed non-clear cell histology and has completed accrual but results are awaited. In high-risk patients with sarcomatoid, medullary or collecting duct carcinoma, adjuvant chemotherapy with doxorubicin and gemcitabine or cisplatin and gemcitabine should be considered.
In metastatic disease, CN plays an important role. A study evaluating ncRCC cases between 2000 and 2009 in the SEER database noted that 64% of the patients had CN and showed significantly lower cancer-specific mortality (hazard ratio [HR] 0.62, 95% confidence interval [CI] 0.48–0.80, P< 0.001) and all-cause mortality (HR 0.45,95% CI 0.37–0.55, P < 0.001). Our analysis of distant-stage ncRCC cases between 2000 and 2013 from SEER also showed a higher risk of death in patients with ncRCC when compared to ccRCC (HR = 1.2, 95% CI 1.16–1.24, P< 0.001). The median OS was 5 months in ncRCC cases and 7 months in ccRCC [7]. Forty-four percent of cases with distant-stage ncRCC had CN as compared to 34% with ccRCC and, despite the higher incidence of CN, OS outcomes were worse for ncRCC. In metastatic disease, CN should be undertaken if the patient demonstrates adequate performance status (PS) and symptomatic large primary or minimally symptomatic non-bulky metastases. If CN is done in patients with suboptimal PS or bulky metastases, it is likely that the window of opportunity to treat with systemic therapy and achieve significant palliation is lost [5, 6].
Local therapy
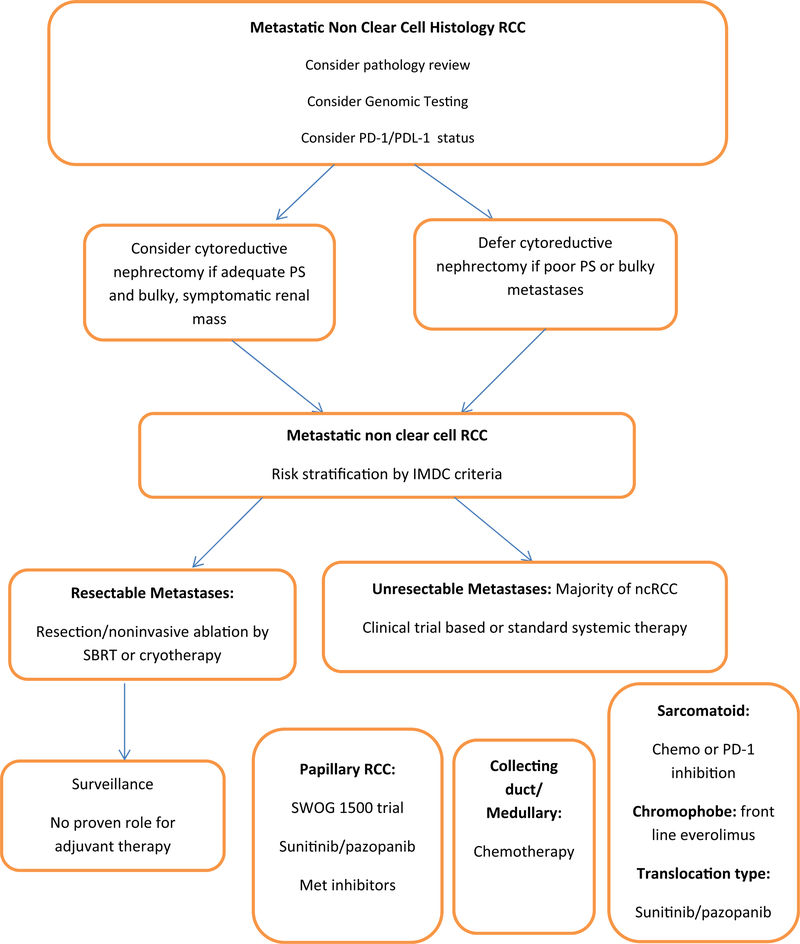
Local therapy, if feasible, should be considered in the management of ncRCC. Metastatectomy to render complete remission constitutes an important strategy in a disease with suboptimal systemic therapy outcomes. Non-invasive local therapies such as stereotactic radiation therapy (SRT) [8], cryotherapy [9], or other ablation techniques should be considered in selected patients with oligometastatic disease (Fig. 1). In a series of 84 patients receiving SRT to a total of 175 sites, the 1-year local control rate was noted to be 91% [8]. A cryotherapy series from our institution that reported on 63 metastatic sites in 29 RCC patients showed an overall disease progression rate of 10% at 11-month follow-up and a local relapse rate of only 1.3% [9]. Although this is a carefully selected oligometastatic disease patient population, the data illustrate that durable remissions in metastatic disease can be achieved with local therapy alone.
Fig. 1.
Management algorithm of advanced non-clear cell RCC.
Systemic therapy
The treatment options for ncRCC are dependent on histology; however, overall, randomized studies have demonstrated that anti-VEGF therapy with sunitinib in the front-line setting produces better outcomes than the mammalian target of rapamycin (mTOR) inhibitor everolimus [10••, 11••, 12] (Table 2). Two studies, ASPEN [10••] and ESPN [11••], conducted specifically in ncRCC confirm improved progression-free survival (PFS) and OS with front-line sunitinib therapy. Sequential therapy of sunitinib followed by everolimus or vice versa was evaluated in a trial called RECORD-3 [12]. The clinical outcomes still favored initial therapy with sunitinib. All three studies showed median PFS of 6.1, 7.2, and 8.3 months. This is in contrast to the median PFS observed in clear cell RCC of 11–12 months with sunitinib therapy. A phase II trial of pazopanib in ncRCC reported an 81% disease control rate and partial responses in 10 of 37 (27%) patients [13•]. Median progression-free survival and overall survival were 15.9 and 17.2 months respectively. The International Metastatic RCC Database Consortium evaluated outcomes with anti-VEGF therapy in 337 ncRCC cases [14]. Median OS was 15.7 months in ncRCC and 20.2 months in ccRCC. The intermediate and poor-risk patients predominantly contributed to the differences in OS outcomes between the groups. In the favorable group (37 ncRCC cases), the outcome was no different from favorable ccRCC. Single-agent studies of chemotherapy, everolimus, and c-Met inhibitors have also been conducted demonstrating modest efficacy. Subgroup analysis of chromophobe RCC in the ASPEN trial revealed better clinical outcomes with everolimus therapy [9].
Table 2.
Summary of randomized trial evidence in ncRCC
| Trial (ref) |
Phase | Therapy | Sample size |
ORR | PFS | OS |
|---|---|---|---|---|---|---|
| RECORD-3 [11••] | Phase II randomized (small subset of ncRCC) | Sunitinib 50 mg daily (4 weeks on 2 weeks off) | 35 | NR | 7.2 months | NR |
| Everolimus 10 mg daily | 31 | NR | 5.1 months | NR | ||
| ASPEN [9] | Phase II randomized | Sunitinib 50 mg daily (4 weeks on 2 weeks off) | 51 | NR | Median 8.3 months | Median 31.5 months |
| Everolimus 10 mg daily | 57 | NR | Median 5.6 months HR= 1.41 P = 0.16 | Median 13.2 months | ||
| ESPN [10••] | Phase II randomized | Sunitinib 50 mg daily (4 weeks on 2 weeks off) Everolimus 10 mg daily |
68/108 enrolled Closed after interim analysis | 9.8% | Median 6.1 months | Median 16.2 months |
| 2.8% | Median 4.1 months | Median 14.9 months |
In papillary RCC (the most common histology of ncRCC), c-Met inhibition has been extensively tested in phase II trials. A phase II study of XL-880 or foretinib [15] revealed an ORR of 13.5% and median PFS of 9.3 months in the 74 patients enrolled, but a RR of 50% in patients with germline Met mutations. Savolitinib (NCT02127710/Astra-Zeneca Inc.) 600 mg orally daily was evaluated in a phase II trial conducted in 109 patients and demonstrated an objective RR of 7% and tumor shrinkage in 20%, but in MET-driven papillary RCC, the ORR was noted to be 18% with tumor shrinkage in 61% respectively [16]. Forty-four patients (40%) had MET-driven papillary renal cancer (PRCC), defined by the presence of chromosome 7, MET copy number gain, focal MET, or HGF gene amplification, or MET kinase domain mutations in their tumor samples. The median PFS were 6.2 and 1.4 months, respectively, a difference that was statistically significant and showed a hazard ratio of 0.33 in favor of MET-driven PRCC as compared to the non-MET-driven disease. Now a phase III trial of savolitinib versus sunitinib in papillary RCC with Met mutations is being planned [17]. A randomized trial conducted by the Southwest Oncology Group is evaluating the clinical efficacy of three distinct targeted therapies; savolitinib (c-Met inhibitor), cabozantinib (dual VEGF and c-Met inhibitor), and crizotinib (Alk-1 and c-Met inhibitor) and comparing this to a sunitinib control arm. Both of these studies have the potential to change the current standard therapy of front-line sunitinib in metastatic papillary RCC.
Immunotherapy
High dose interleukin-2 has demonstrated lack of efficacy in ncRCC and should not be considered. Immune checkpoint inhibitors have not been studied but can be utilized due to the FDA approval in advanced kidney cancer. Case reports of PD-1 inhibitors showing efficacy in RCC are noted [18]. PD-1 expression > 1% was noted in 15% of ncRCC studied [19]. A recent report revealed promising results with nivolumab in ncRCC especially sarcomatoid histology [19]. Combinations of PD-1 inhibitors with ipilimumab, epacadostat, or other Ido-1 inhibitors are worthy of evaluation. A phase II trial of a combination of bevacizumab and atezolizumab in ncRCC is currently ongoing at multiple sites across the USA.
Genomic Targets for Therapy
The advent of targeted therapies based on next-generation sequencing has been slow in kidney cancer. The heterogeneity of RCC is probably one of the primary reasons why the targets detected are not predictive of therapeutic efficacy. In addition, most of the data is collected from nephrectomy specimens and the concordance with targets expressed by metastases is unknown. As of now, most of the genomic markers identified have shown prognostic value and no predictive benefit [3••, 4]. The c-Met mutations are being explored in clinical trials as a therapeutic target, and clinical trials in metastatic papillary renal cancer are selecting patients with MET mutations to be randomized to sunitinib therapy or the c-Met inhibitor savolitinib [17]. However, a study conducted by Cancer Genome Atlas in 161 papillary cases identified that MET alterations were typically noted in the type 1 disease which is usually not clinically aggressive. In type 2 papillary renal cell carcinomas, alterations such as CDKN2A silencing, SETD2 mutations, TFE3 fusions, and increased expression of the NRF2-antioxidant response element pathway were frequently noted [20]. Mutation of the gene encoding fumarate hydratase was observed in a distinct subgroup of type 2 characterized by poor prognosis [21]. An association between loss of fumarate hydratase and HIF-1 overexpression has been reported and this emphasizes the possibility of evaluating HIF-1 alpha inhibitors in non-clear cell RCC with FH mutations [22]. Chromophobe RCC is typically associated with folliculin gene mutations but has also been associated with Cowden syndrome characterized by PTEN mutations [23]. InTSC1/2 mutations, mTOR inhibition should be the therapy of choice [24]. In sarcomatoid, translocation type, collecting duct, and unclassified and medullary histologies, multiple genomic findings are described and are summarized in Table 1; however, no specific therapeutic recommendations can be made within the limitations of the current level of evidence [25–29].
Conclusions
Non-clear cell kidney cancer represents an unmet need in specific therapeutic development in oncology. Each histology has unique clinical and genomic characteristics and these should be exploited for planning appropriate targeted therapies. Current treatments used in kidney cancer have demonstrated limited efficacy in ncRCC and identification of novel targets, and future rational study design for therapeutic development is needed.
Acknowledgments
Ulka Vaishampayan has received research funding through grants from Exelixis, Pfizer, Novartis, Bristol-Myers Squibb, Genentech, Bayer, and Astellas, and has received compensation from Exelixis, Pfizer, Novartis, Bristol-Myers Squibb, Genentech, Bayer, Astellas, and Sanofifor service as a consultant.
Footnotes
Compliance with Ethical Standards
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
References and Recommended Heading
Papers of particular interest, published recently, have been highlighted as:
• Of importance
•• Of major importance
- 1.Aizer AA, Urun Y, McKay RR Kibel AS, Nguyen PL, Choueiri TK. Cytoreductive nephrectomy in patients with metastatic non-clear-cell renal cell carcinoma (RCC). BJU Int. 2014;113(5b):E67–74. 10.1111/bju.12442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dy GW, Gore JL, Forouzanfar MH, Naghavi M, Fitzmaurice C. Global burden of urologic cancers, 1990–2013. EurUrol. 2017;71(3):437–46. 10.1016/j.eururo.2016.10.008. [DOI] [PubMed] [Google Scholar]
- 3.Linehan WM. Genetic basis of kidney cancer: role of genomics for the development of disease-based therapeutics. Genome Res. 2012;22(11):2089–100. 10.1101/gr.31110.111.••Comprehensive review of genomics of non-clear cell kidney cancer histologies.
- 4.Linehan WM, Spellman PT, Ricketts CJ, et al. Cancer genome atlas research network, comprehensive molecular characterization of papillary renal-cell carcinoma. N Engl J Med. 2016;374(2):135–45. 10.1056/NEJMoa1505917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Heng DY, Wells JC, Rini BI, et al. Cytoreductive nephrectomy in patients with synchronous metastases from renal cell carcinoma: results from the International Metastatic Renal Cell Carcinoma Database Consortium. Eur Urol. 2014;66(4):704–10. 10.1016/j.eururo.2014.05.034. [DOI] [PubMed] [Google Scholar]
- 6.Vaishampayan UN. The role of nephrectomy for kidney cancer in the era of targeted and immune therapies. Am Soc Clin Oncol Educ Book. 2016;35:e16–20. 10.14694/EDBK_158977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Haas NB, Manola J, Uzzo RG, Flaherty KT, Wood CG, Kane C, et al. Adjuvant sunitinib or sorafenib for highrisk, non-metastatic renal-cell carcinoma (ECOG-ACRIN E2805): a double-blind, placebo-controlled, randomised, phase 3 trial. Lancet. 2016;387(10032):2008–16. 10.1016/S0140-6736(16)00559-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang CJ, Christie A, Lin MH, Jung M, Weix D, Huelsmann L, et al. Safety and efficacy of stereotactic ablative radiation therapy for renal cell carcinoma extracranial metastases. Int J Radiat Oncol Biol Phys. 2017;98(1):91–100. 10.1016/jijrobp.2017.01.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Littrup PJ, Bang HJ, Currier BP, Goodrich DJ, Aoun HD, Heilbrun LK, et al. Soft-tissue cryoablation in diffuse locations: feasibility and intermediate term outcomes. J Vasc Interv Radiol. 2013;24(12):1817–25. 10.1016/j.jvir.2013.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Armstrong AJ, Halabi S, Eisen T, Broderick S, Stadler WM, Jones RJ, et al. Everolimus versus sunitinib for patients with metastatic non-clear cell renal cell carcinoma (ASPEN): a multicentre, open-label, randomised phase 2 trial. Lancet Oncol. 2016;17(3):378–388.•• A randomized trial comparing sunitinib versus everolimus therapy in non-clear cell kidney cancer. https://doi.org/10.1016/S1470-2045(15)00515-X.
- 11.Tannir NM, Jonasch E, Albiges L, Altinmakas E, Ng CS, Matin SF, et al. Everolimus versus sunitinib prospective evaluation in metastatic non-clear cell renal cell carcinoma (ESPN): a randomized multicenter phase 2 trial. EurUrol. 2016;69(5):866–874.•• A randomized trial comparing sunitinib versus everolimus therapy in non-clear cell kidney cancer. https://doi.org/10.1016/j.eururo.2015.10.049.
- 12.Motzer RJ, Barrios CH, Kim TM, Falcon S, Cosgriff T, Harker WG, et al. Phase 2 randomized trial comparing sequential first-line everolimus and second-line sunitinib versus first-line sunitinib and second-line everolimus in patients with metastatic renal cell carcinoma. J Clin Oncol. 2014;32(25):2765–72. 10.1200/JC0.2013.54.6911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Buti S, Bersanelli M, Maines F, Facchini G., Gelsomino F, Zustovich F, Santoni M, Verri E, de Giorgi U, Masini C, Morelli F, Vitale MG, Sava T, Prati G, Librici C, Fraccon AP, Fornarini G, Maruzzo M, Leonardi F, Caffo O First-line PAzopanib in NOn-clear-cell renal cArcinoMA: the Italian Retrospective Multicenter PANORAMA Study. Clin Genitourin Cancer. 2017;15(4):e609–e614.• A single-arm trial establishing the safety and efficacy of pazopanib in non-clear cell kidney cancer, https://doi.org/10.1016/j.clgc.2016.12.024.
- 14.de Velasco G, McKay RR, Lin X, Moreira RB, Simantov R, Choueiri TK.. Comprehensive Analysis of survival outcomes in non-clear cell renal cell carcinoma patients treated in clinical trials. Clin Genitourin Cancer. 2017;(6):652–660.e1. 10.1016/j.clgc.2017.03.004. [DOI] [PubMed] [Google Scholar]
- 15.Choueiri TK, Vaishampayan U, Rosenberg JE, Logan TF, Harzstark AL, Bukowski RM, et al. Phase 2 and biomarker study of the dual MET/VEGFR2 inhibitor foretinib in patients with papillary renal cell carcinoma. J Clin Oncol. 2013;31(2):181–6. 10.1200/JC0.2012.43.3383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Choueiri TK, Plimack E, Arkenau HT, Jonasch E, Heng DYC, Powles T, et al. Biomarker-based phase II trial of savolitinib in patients with advanced papillary renal cell cancer. J Clin Oncol. 2017;35(26):2993–3001. 10.1200/JCO.2017.72.2967. [DOI] [PubMed] [Google Scholar]
- 17.Savolitinib Heads for Phase III Trial in PRCC. Cancer Discov. 2017;7(9):OF4. 10.1158/2159-8290.CD-NB2017-105. [DOI] [PubMed] [Google Scholar]
- 18.Koshkin VS, Barata PC, Vogelzang NJ, Pal SK, Hsu J, Allman KD, Hsu J, Allman KD, Gilligan TD, Rini BI.Nivolumab monotherapy in non clear cell renal cancer. J Clin Oncol 2017;35(4586). [Google Scholar]
- 19.Zhang T, Gong J, Maia MC, Pal SK, et al. Systemic therapy for non-clear cell renal cell carcinoma. Am Soc Clin Oncol Educ Book. 2017;37:337–42. 10.14694/EDBK_175572. [DOI] [PubMed] [Google Scholar]
- 20.Albiges L, Guegan J, Le Formal A, Verkarre V, Rioux-Leclercq N, Sibony M, et al. MET is a potential target across all papillary renal cell carcinomas: result from a large molecular study of pRCC with CGH array and matching gene expression array. Clin Cancer Res. 2014;20(13):3411–21. 10.1158/1078-0432.CCR-13-2173. [DOI] [PubMed] [Google Scholar]
- 21.Isaacs JS, Jung YJ, Mole DR, Lee S, Torres-Cabala C, Chung YL, et al. HIF overexpression correlates with biallelic loss of fumarate hydratase in renal cancer: novel role of fumarate in regulation of HIF stability. Cancer Cell. 2005;8(2):143–53. 10.1016/j.ccr.2005.06.017. [DOI] [PubMed] [Google Scholar]
- 22.Nickerson ML, Warren MB, Toro JR, Matrosova V, Glenn GM, Turner ML, et al. Mutations in a novel gene lead to kidney tumors, lung wall defects, and benign tumors of the hair follicle in patients with the Birt-Hogg-Dube syndrome. Cancer Cell. 2002;2(2):157–64. 10.1016/S1535-6108(02)00104-6. [DOI] [PubMed] [Google Scholar]
- 23.Davis CF, Ricketts CJ, Wang M, Yang L, Cherniack AD, Shen H, Buhay C, Kang H, Kim SC, Fahey CC, Hacker KE, Bhanot G, Gordenin DA, Chu A, Gunaratne PH, Biehl M, Seth S, Kaipparettu BA, Bristow CA, Donehower LA, Wallen EM, Smith AB, Tickoo SK, Tamboli P, Reuter V, Schmidt LS, Hsieh JJ, Choueiri TK, Hakimi AA, Chin L, Meyerson M, Kucherlapati R, Park WY, Robertson AG, Laird PW, Henske EP, Kwiatkowski DJ, Park PJ, Morgan M, Shuch B, Muzny D, Wheeler DA, Linehan WM, Gibbs RA, Rathmell WK, Creighton CJ, Creighton CJ, Davis CF, Morgan M, Gunaratne PH, Donehower LA, Kaipparettu BA, Wheeler DA, Gibbs RA, Signoretti S, Cherniack AD, Robertson AG, Chu A, Choueiri TK, Henske EP, Kwiatkowski DJ, Reuter V, Hsieh JJ, Hakimi AA, Tickoo SK, Ricketts C, Linehan WM, Schmidt LS, Gordenin DA, Bhanot G, Seiler M, Tamboli P, Rathmell WK, Fahey CC, Hacker KE, Smith AB, Wallen EM, Shen H, Laird PW, Shuch B, Muzny D, Buhay C, Wang M, Chao H, Dahdouli M, Xi L, Kakkar N, Reid JG, Downs B, Drummond J, Morton D, Doddapaneni H, Lewis L, English A, Meng Q, Kovar C, Wang Q, Hale W, Hawes A, Kalra D, Walker K, Murray BA, Sougnez C, Saksena G, Carter SL, Schumacher SE, Tabak B, ZackT I, Getz G, Beroukhim R, Gabriel SB, Meyerson M, Ally A, Balasundaram M, Birol I, Brooks D, Butterfield YSN, Chuah E, Clarke A, Dhalla N, Guin R, Holt RA, Kasaian K, Lee D, Li HI, Lim E, Ma Y, Mayo M, Moore RA, Mungall AJ, Schein JE, Sipahimalani P, Tam A, Thiessen N, Wong T, Jones SJM, Marra MA, Auman JT, Tan D, Meng S, Jones CD, Hoadley KA, Mieczkowski PA, Mose LE, Jefferys SR, Roach J, Veluvolu U, Wilkerson MD, Waring S, Buda E, Wu J, Bodenheimer T, Hoyle AP, Simons JV, Soloway MG, Balu S, Parker JS, Hayes DN, Perou CM, Weisenberger DJ, Bootwalla MS, Triche T Jr, Lai PH, van den Berg DJ, Baylin SB, Chen F, Coarfa C, Noble MS, DiCara D, Zhang H, Cho J, Heiman DI, Gehlenborg N, Voet D, Lin P, Frazer S, Stojanov P, Liu Y, Zou L, Kim J, Lawrence MS, Chin L, Yang L, Seth S, Bristow CA, Protopopov A, Song X, Zhang J, Pantazi A, Hadjipanayis A, Lee E, Luquette LJ, Lee S, Parfenov M, Santoso N, Seidman J, Xu AW, Kucherlapati R, Park PJ, Kang H, Lee J, Roberts SA, Klimczak LJ, Fargo D, Lang M, Choi YL, Kim SC, Lee JK, Park WY, Wang W, Fan Y, Ahn J, Akbani R, Weinstein JN, Haussler D, Ma S, Radenbaugh A, Zhul J, Biehl M, Lichtenberg TM, Zmuda E, Black AD, Hanf B, Ramirez NC, Wise L, Bowen J, Leraas KM, Hall TM, Gastier-Foster JM, Kaelin WG, Thorne L, Boice L, Huang M, Vocke C, Peterson J, Worrell R, Merino M, Czerniak BA, Aldape KD, Wood CG, Spellman PT, Atkins MB, Cheville J, Thompson RH, Jensen MA, Pihl T, Wan Y, Ayala B, Baboud J, Velaga S, Walton J, Liu J, Chudamani S, Wu Y, Sheth M, Mills Shaw K.R., Demchok JA, Davidsen T, Yang L, Wang Z, Tarnuzzer RW, Zhang J, Eley G, Felau I, Zenklusen JC, Hutter CM, Guyer MS, Ozenberger BA, Sofia HJ The somatic genomic landscape of chromophobe renal cell carcinoma. Cancer Cell 2014; 26: 319–330, 3, DOI: 10.1016/j.ccr.2014.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Habib SL, Al-Obaidi NY, Nowacki M, Pietkun K, Zegarska B, Kloskowski T, et al. Is mTOR inhibitor good enough for treatment all tumors in TSC patients? J Cancer. 2016;7(12):1621–31. 10.7150/jca.14747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bi M, Zhao S, Said JW, Merino MJ, Adeniran AJ, Xie Z, Nawaf CB, Choi J, Belldegrun AS, Pantuck AJ, Kluger HM, Bilguvar K, Lifton RP, Shuch B. Genomic characterization of sarcomatoid transformation in clear cell renal cell carcinoma. Proc Natl Acad Sci U S A. 2016;113(8):2170–5. 10.1073/pnas.1525735113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang K, Liu T, Liu L, Liu J, Liu C, Wang C, et al. TERT promoter mutations in renal cell carcinomas and upper tract urothelial carcinomas. Oncotarget. 2014;5(7):1829–36. 10.18632/oncotarget.1829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hu ZY, Pang LJ, Qi Y, Kang XL, Hu JM, Wang L, et al. Unclassified renal cell carcinoma: a clinicopathological, comparative genomic hybridization, and whole-genome exon sequencing study. Int J Clin Exp Pathol. 2014;7(7):3865–75. [PMC free article] [PubMed] [Google Scholar]
- 28.Gatalica Z, Lilleberg SL, Monzon FA, Koul MS, Bridge JA, Knezetic J, et al. Renal medullary carcinomas: histopathologic phenotype associated with diverse genotypes. Hum Pathol. 2011;42(12):1979–88. 10.1016/j.humpath.2011.02.026. [DOI] [PubMed] [Google Scholar]
- 29.Durinck S, Stawiski EW, Pavia-Jimenez A, et al. Spectrum of diverse genomic alterations define non-clear cell renal carcinoma subtypes. Nat Genet. 2015;47(1):13–21. 10.1038/ng.3146. [DOI] [PMC free article] [PubMed] [Google Scholar]