Abstract
The aberrant expression of long non-coding RNAs is closely associated with drug resistance in multiple types of cancer. Long intergenic non-coding RNA 00707 (LINC00707) has previously been reported to be an oncogene able to promote lung adenocarcinoma cell proliferation and metastasis. However, its role in the progression of cisplatin (DDP) resistance in non-small-cell lung cancer (NSCLC) requires further elucidation. In the present study, LINC00707 and microRNA (miR)-145 expression levels were measured using reverse transcription-quantitative PCR (RT-qPCR). MTT and flow cytometric assays were performed to evaluate the IC50 value of DDP and cell apoptosis, respectively. Bcl-2, Bax, multidrug resistance protein 1 (MRP1) and P-glycoprotein (P-gp) mRNA and protein expression were detected using RT-qPCR and western blotting, respectively. The interaction between LINC00707 and miR-145 was explored using a luciferase reporter assay. LINC00707 expression was found to be significantly upregulated in DDP-resistant A549 cells (A549/DDP) cells when compared with that in parental A549 cells. LINC00707 knockdown reduced the IC50 value of DDP, enhanced apoptosis and inhibited Bcl-2, MRP1 and P-gp expression, while promoting Bax expression in A549/DDP cells. miR-145 expression was found to be significantly decreased in A549/DDP cells when compared with in A549 cells. LINC00707 directly interacted with miR-145 and negatively regulated its expression. Furthermore, miR-145 downregulation weakened the effect of LINC00707 knockdown in A549/DDP cells. Therefore, silencing of LINC00707 enhanced DDP sensitivity in A549/DDP cells by sponging miR-145, thereby shedding light on LINC00707 and its corresponding molecular mechanisms involved in the progression of DDP resistance in NSCLC cells.
Keywords: long intergenic non-coding RNA 00707, microRNA-145, cisplatin resistance, non-small-cell lung cancer, competing endogenous RNA
Introduction
Non-small-cell lung cancer (NSCLC) accounts for ~85% of all lung cancer cases, demonstrating a high degree of mortality and poor survival worldwide (1). In recent years, substantial advances have been achieved in NSCLC diagnosis and treatment; however, the 5-year survival rate has remained unchanged at 15% (2). Cisplatin (DDP) is a first-line drug used in NSCLC chemotherapy; however, resistance to chemotherapy drugs administered after surgeries limits the prognosis of patients, as well as the use of DDP in clinical applications (3). Therefore, the molecular mechanisms underlying DDP resistance need to be urgently elucidated in order to improve the survival rate of patients with NSCLC.
Long non-coding RNAs (lncRNAs) are non-protein coding RNAs that are greater than 200 nucleotides in length (3). Numerous studies have indicated that lncRNAs are capable of regulating gene expression at the transcriptional, post-transcriptional and epigenetic levels, and serve an important role in human cancer development, prognosis and drug resistance (4,5). Recent studies have associated certain lncRNAs, including CCAT1 (6) and TRPM2-AS (7), with DDP resistance in lung cancer. Long intergenic non-coding RNA 00707 (LINC00707), located on chromosome 10p14, has also been associated with the development and progression of cancers, including hepatocellular carcinoma (8), gastric cancer (9) and lung cancer (10). However, the function and underlying mechanism of LINC00707 in the progression of DDP resistance in NSCLC remains largely unclear.
Recently, emerging evidence has revealed that lncRNAs are able to serve as competing endogenous RNAs or microRNA (miRNA/miR) sponges, and induce regulatory effects on miRNAs and miRNA-targeted mRNAs (11). miRNAs are a group of evolutionarily conserved, single-stranded, non-coding RNAs (21–25 nucleotides in length) that regulate post-transcriptional gene expression by targeting the 3′-untranslated regions (3′-UTRs) of their target mRNAs (12). Aberrant expression of various miRNAs has been suggested to contribute to DDP resistance in human cancers (13). miR-145, a tumor suppressor miRNA, has been reported to be downregulated in several types of human cancers, including lung cancer (14). Furthermore, Zhan et al (15) reported that miR-145 promoted multidrug resistance protein 1 (MRP1) mRNA degradation and, therefore, sensitized gallbladder cancer cells to DDP. However, whether LINC00707 acts as an miR-145 sponge in order to regulate DDP resistance in NSCLC cells remains to be investigated.
The aim of the present study was to investigate the role and potential regulatory mechanism of LINC00707 in DDP-resistance progression in NSCLC.
Materials and methods
Cell culture and transfection
DDP-resistant A549 cells (A549/DDP) and parental A549 cells were obtained from The Cancer Institute of the Chinese Academy of Sciences. The cells were maintained in RPMI-1640 medium containing 10% FBS (both HyClone; GE Healthcare Life Sciences) and 1% penicillin-streptomycin (Gibco; Thermo Fisher Scientific, Inc.) in a humidified incubator with 5% CO2 at 37°C. To maintain the DDP-resistant phenotype, 2 µM DDP (Sigma-Aldrich; Merck KGaA) was also added to the culture media of A549/DDP cells. LINC00707 siRNA (si-LINC00707; 5′-GCAGGAACAUCACCAUCUUUU-3′), siRNA negative control (si-NC; 5′-UUCUCCGAACGUGUCACGUTT-3′), miR-145 mimic (5′-GUCCAGUUUUCCCAGGAAUCCCU-3′), miRNA negative control (NC, 5′-UCACAACCUCCUAGAAAGAGUAGA-3′), miR-145 inhibitor (5′-AGGGAUUCCUGGGAAAACUGGAC-3′) and negative control (inhibitor NC, 5′-UCUACUCUUUCUAGGAGGUUGUGA-3) were all purchased from Shanghai GenePharma Co., Ltd. The transfection of above siRNAs or miRNA mimics (final concentration: 50 nM) into A549/DDP cells (4×105/per well of 6-well plate) was performed using Lipofectamine® 2000 (Invitrogen; Thermo Fisher Scientific, Inc.) according to the manufacturer's protocol. Cells were collected for further experiments 48 h after transfection.
Reverse transcription-quantitative PCR (RT-qPCR)
Total RNA was isolated from the cells using TRIzol reagent (Invitrogen; Thermo Fisher Scientific, Inc.) according to the manufacturer's protocol. RNA (1 µg) was reversed to cDNA using a High Capacity cDNA Reverse Transcription kit (cat. no. 4368814, Thermo Fisher Scientific, Inc.) according to the manufacturer's protocol. RT-qPCR was performed using the ABI 7500 RT-PCR system (Applied Biosystems; Thermo Fisher Scientific, Inc.) with a SYBR® Premix Ex Taq™ kit and TaqMan miRNA assay (both Takara Biotechnology Co., Ltd.). The primers were synthesized by Shanghai GenePharma Co., Ltd. Primer names and sequences are provided in Table I. 18s rRNA was used as internal references for lncRNA, mRNA and miRNA. U6 small nuclear RNA was used as internal references for miRNA. The relative expression levels were quantified using the 2−∆∆Cq method (16). RT-qPCR reactions were performed in triplicate with the following conditions: 95°C for 2 min; 40 cycles of 95°C for 15 sec and 60°C for 1 min.
Table I.
Primers for reverse transcription-quantitative PCR.
| Gene | Primer sequence (5′ → 3′) |
|---|---|
| LINC00707 | F: GCTGCACATTGAACCAGATA |
| R: ATGTTCCAGTCCAGTCTCAT | |
| Bcl-2 | F: TCATGTGTGTGGAGAGCGTC |
| R: AGCCTCCGTTATCCTGGATC | |
| Bax | F: GATGCGTCCACCAAGAAGCT |
| R: CGGCCCCAGTTGAAGTTG | |
| MRP1 | F: GGCTCAAGGAGTATTCAGAG |
| R: CCATCGATGATGATCTCTCC-3 | |
| P-gp | F: TCATCGAGTCACTGCCTAAT |
| R: CTATGGCAATGCGTTGTTTC | |
| 18s rRNA | F: CCTGGATACCGCAGCTAGGA |
| R: GCGGCGCAATACGAATGCCCC | |
| miR-145 | F: GCGCTCCAGCTGGGGTCCAGTTTTCCCAGGAATC |
| R: CTCAACTGGTGTCGTGGA | |
| U6 | F: CTCGCTTCGGCAGCACA |
| R: AACGCTTCACGAATTTGCGT |
F, forward primer; R, reverse primer; miR, microRNA; MRP1, multidrug resistance protein 1; P-gp, P-glycoprotein; LINC00707, long intergenic non-coding RNA 00707.
Cell proliferation assay
The sensitivity of cells to DDP treatment was determined using a CellTiter 96® Non-Radioactive Cell Proliferation Assay kit (Promega Corporation). In brief, transfected cells were seeded in triplicate into 96-well plates at a density of 4×104 cells/well in 100 µl RPMI-1640 medium (HyClone; GE Healthcare Life Sciences). After 12 h, A549 and A549/DDP cells were treated with different concentrations of DDP (5, 10, 20, 40 and 80 µM) for 1, 2 and 3 days. Subsequently, MTT (10 µl; 5 mg/ml) was added into each well and incubated for 4 h at 37°C. Then 100 µl of dimethyl sulfoxide was added to dissolve the solution and solubilize the crystals. The optical density was detected at 570 nm using a microplate reader (Bio-Rad Laboratories, Inc.). The in vitro DDP activity was expressed in terms of concentrations capable of suppressing cell proliferation by 50% (IC50). This assay was performed in triplicate.
Flow cytometric analysis of apoptosis
The Annexin V-FITC Apoptosis Detection Kit (Nanjing KeyGen Biotech Co., Ltd.) was used to evaluate cell apoptosis. Briefly, A549/DDP cells (106 cells/ml) were harvested 48 h after transfection and washed twice with ice-cold PBS. The cells were then resuspended in 500 µl of binding buffer. Next, the cells were stained with 5 µl of Annexin V-FITC and 5 µl of propidium iodide, and incubated at 25°C for 15 min in the dark according to the manufacturer's protocol. Cell apoptosis was measured via FACSCalibur flow cytometry (BD Biosciences). Results were analyzed using BD FACSDiva software (version 8.0; BD Biosciences). This assay was performed in triplicate. Apoptotic rate was calculated using the sum of early apoptotic and late apoptotic cells.
Western blotting
Total protein was extracted from the cells using RIPA solution containing phenylmethylsulfonyl fluoride (Beyotime Institute of Biotechnology). Protein concentration was measured using BCA reagent (Beyotime Institute of Biotechnology). Total protein (30 µg) was separated via SDS-PAGE (10% gel) and then transferred to PVDF membranes (EMD Millipore). After blocking with 5% non-fat milk at 25°C for 2 h, the membranes were subsequently incubated overnight at 4°C with the following primary antibodies: Anti-Bcl-2 (1:1,000; cat. no. 4223); anti-Bax (1:1,000; cat. no. 5023); anti-MRP1 (1:1,000; cat. no. 14685); anti-P-glycoprotein (P-gp; 1:1,000; cat. no. 12683); and anti-GAPDH (1:5,000; cat. no. 5174) (all purchased from Cell Signaling Technology, Inc.). Next, membranes were washed with TBS containing 1% Tween 20 (TBST), followed by incubation with horseradish peroxidase-linked secondary antibodies (1:5,000; cat. no. 4050-05; Southern Biotech) at 25°C for 2 h. The membranes were then washed with TBST. Protein bands were visualized via chemiluminescence using an ECL kit (Thermo Fisher Scientific, Inc.). Parallel blotting of GAPDH served as the internal reference.
Luciferase reporter assay
The putative binding sites between LINC00707 and miR-145 were predicted using StarBase v2.0 (http://starbase.sysu.edu.cn/agoClipRNA.php?source=lncRNA). Wild-type (WT) and mutant (MUT) LINC00707 containing putative miR-145 binding sequences were generated and cloned downstream of the luciferase reporter vector, psi-CHECK-2 (Promega Corporation). They were subsequently co-transfected with luciferase plasmids (0.5 µg/per well), and miR-145 mimics (final concentration, 50 nM) or miRNA negative controls (final concentration, 50 nM) using the Lipofectamine 2000 reagent. Luciferase activities were detected 48 h post-transfection using a Dual-Luciferase Reporter Assay System (Promega Corporation). Renilla luciferase activity was normalized to Firefly luciferase activity. This assay was performed in triplicate.
Statistical analysis
Statistical analyses were performed using SPSS software (version 19.0; IBM Corporation). All data are expressed as the mean ± standard deviation from three independent experiments. Differences between two groups were identified using Student's t-test, and differences between more than two groups were identified using ANOVA followed by a least significant difference post hoc test. P<0.05 was considered to indicate a statistically significant difference.
Results
LINC00707 expression is upregulated in A549/DDP cells
To confirm the DDP-resistant phenotype of purchased A549/DDP cells, the half-maximal inhibitory concentration (IC50) value of DDP, and the protein expression levels of multidrug-resistance-related proteins MRP1 and P-gp were examined in A549/DDP and parental A549 cells. The results showed that the IC50 value of DDP in A549/DDP cells was 4.5-fold, 4.6-fold or 5.6-fold higher at 1, 2 or 3 days, respectively, when compared with A549 cells (Fig. 1A). In addition, the protein expression levels of MRP1 and P-gp were markedly increased in A549/DDP cells when compared with those in A549 cells (Fig. 1B). These results verified the DDP-resistant phenotype of A549/DDP cells. The RT-qPCR results showed that LINC00707 expression was significantly upregulated in A549/DDP cells when compared with in A549 cells (Fig. 1C). Therefore, this finding suggested that LINC00707 may contribute to DDP resistance in A549/DDP cells.
Figure 1.
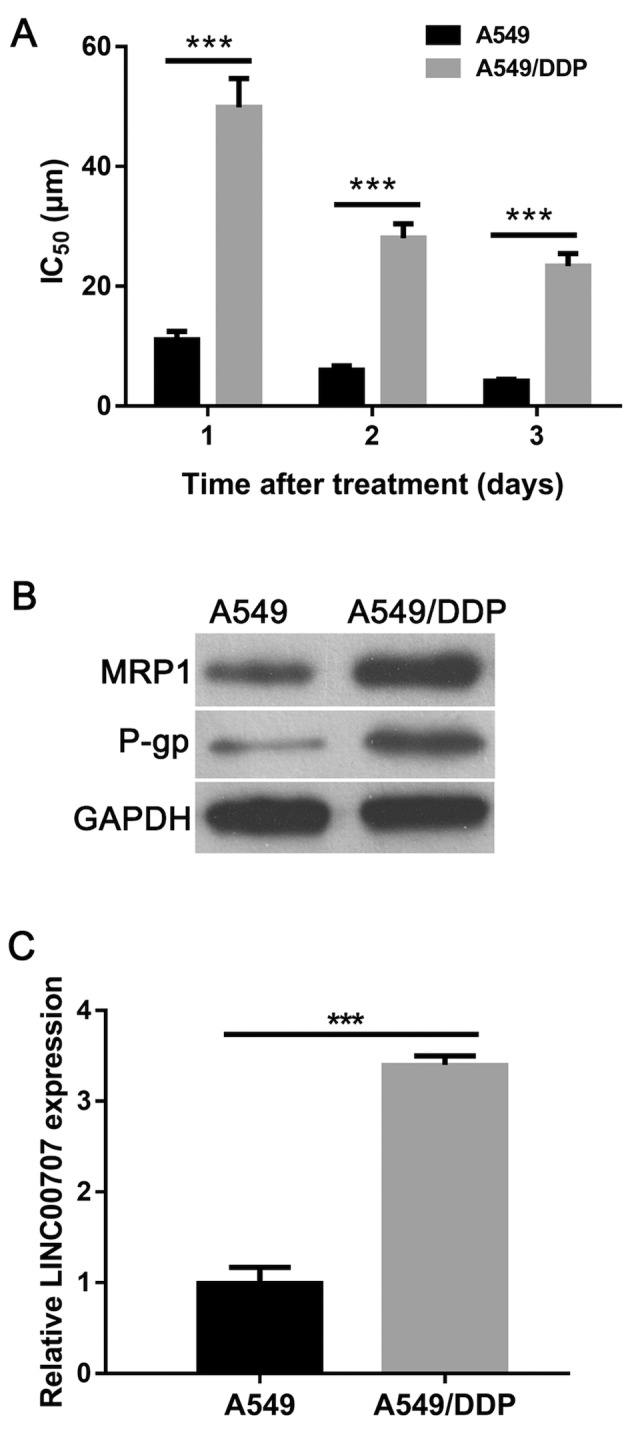
LINC00707 expression is upregulated in A549/DDP cells. (A) Protein expression levels of multidrug-resistance-related proteins MRP1 and P-gp in A549/DDP cells and A549 cells. (B) IC50 value of DDP in A549/DDP cells and parental A549 cells. (C) LINC00707 expression in A549 and A549/DDP cells measured using reverse transcription-quantitative PCR. ***P<0.001 vs. A549. MRP1, multidrug resistance protein 1; P-gp, P-glycoprotein; A549/DDP cells, DDP-resistant A549 cells; IC50, half-maximal inhibitory concentration; LINC00707, long intergenic non-coding RNA 00707.
LINC00707 knockdown reduces the IC50 value of DDP in A549/DDP cells
LINC00707 expression levels were observed to be significantly decreased in A549/DDP cells transfected with si-LINC00707 when compared with cells transfected with si-NC in the control group (Fig. 2A). An MTT assay revealed that si-LINC00707 significantly reduced the IC50 value of DDP in A549/DDP cells compared with the si-NC group (Fig. 2B). Thus, these results indicated that LINC00707 knockdown induces DDP sensitivity in A549/DDP cells.
Figure 2.
LINC00707 knockdown decreases the IC50 values of DDP in A549/DDP cells. (A) LINC00707 expression levels were measured in A549/DDP cells transfected with either si-LINC00707 or si-NC using reverse transcription-quantitative PCR. (B) IC50 values of DDP in either si-LINC00707- or si-NC-transfected A549/DDP cells were measured using an MTT assay. **P<0.01; ***P<0.001 vs. si-NC. IC50, half-maximal inhibitory concentration; LINC00707, long intergenic non-coding RNA 00707; A549/DDP cells, DDP-resistant A549 cells; si-LINC00707, LINC00707 siRNA; si-NC, siRNA negative control.
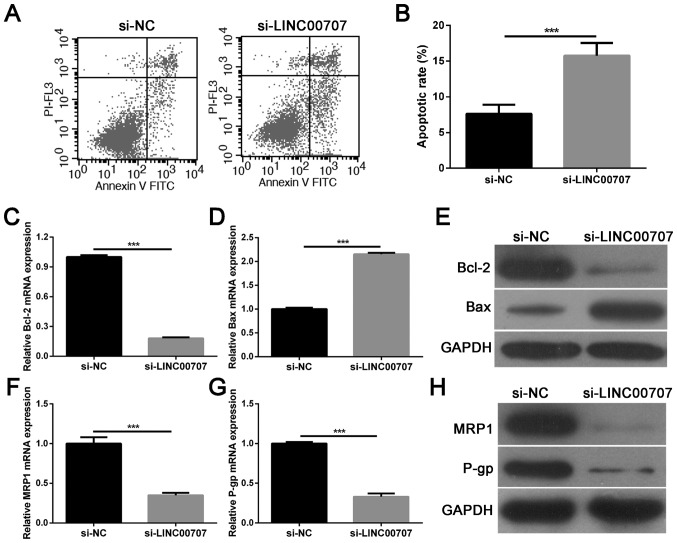
LINC00707 knockdown enhances apoptosis and influences the expression of multidrug-resistance-related proteins in A549/DDP cells
Flow cytometric analysis demonstrated that LINC00707 knockdown significantly accelerated A549/DDP cell apoptosis when compared with the si-NC group (Fig. 3A and B). Furthermore, the expression levels of the apoptosis-associated proteins Bcl-2 and Bax were analyzed in A549/DDP cells transfected with si-LINC00707 or si-NC using RT-qPCR and western blotting. Results showed that LINC00707 knockdown significantly inhibited Bcl-2 mRNA and protein expression, and promoted Bax mRNA and protein expression in A549/DDP cells when compared with the si-NC group (Fig. 3C-E). Moreover, multidrug-resistance-related proteins, MRP1 and P-gp, were selected as detection indexes to analyze the effect of LINC00707 knockdown on DDP resistance in A549/DDP cells. The results showed that LINC00707 knockdown significantly decreased MRP1 and P-gp mRNA and protein levels in A549/DDP cells when compared with the si-NC group (Fig. 3F-H). Thus, these results indicated that LINC00707 knockdown enhances apoptosis and DDP sensitivity in A549/DDP cells.
Figure 3.
LINC00707 knockdown enhances apoptosis and DDP sensitivity in A549/DDP cells. (A and B) Apoptotic cell rates of A549/DDP cells transfected with either si-LINC00707 or si-NC were measured using flow cytometry. (C) Relative mRNA expression levels of Bcl-2 in A549/DDP cells transfected with either si-LINC00707 or si-NC were measured using RT-qPCR. (D) Relative mRNA expression levels of Bax in A549/DDP cells transfected with either si-LINC00707 or si-NC were measured using RT-qPCR. (E) Relative protein expression levels of Bcl-2 and Bax in A549/DDP cells transfected with either si-LINC00707 or si-NC were measured using western blotting. (F) Relative mRNA expression levels of MRP1 in A549/DDP cells transfected with either si-LINC00707 or si-NC were measured using RT-qPCR. (G) Relative mRNA expression levels of P-gp in A549/DDP cells transfected with either si-LINC00707 or si-NC were measured using RT-qPCR. (H) Relative protein expression levels of MRP1 and P-gp in A549/DDP cells transfected with either si-LINC00707 or si-NC were measured using western blotting. ***P<0.001 vs. si-NC. MRP1, multidrug resistance protein 1; P-gp, P-glycoprotein; LINC00707, long intergenic non-coding RNA 00707; RT-qPCR, reverse transcription-quantitative PCR; A549/DDP cells, DDP-resistant A549 cells; PI, propidium iodide; si-LINC00707, LINC00707 siRNA; si-NC, siRNA negative control.
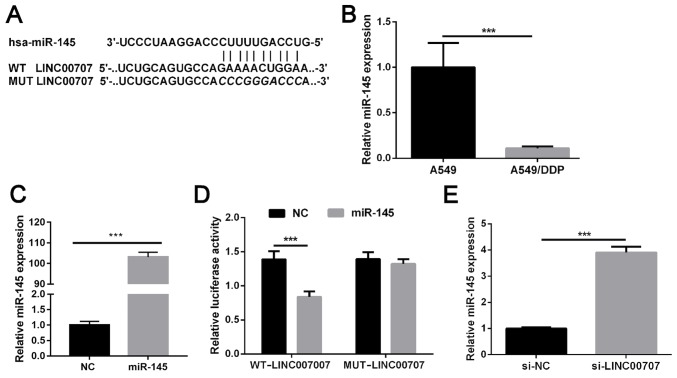
LINC00707 functions as a molecular miR-145 sponge in A549/DDP cells
Bioinformatics analysis was used to determine the putative binding sites between LINC00707 and miR-145, and the results revealed a miR-145 binding site in LINC00707 (Fig. 4A). RT-qPCR demonstrated that miR-145 expression was significantly decreased in A549/DDP cells when compared with in parental A549 (Fig. 4B). As shown in Fig. 1C, LINC00707 expression was significantly upregulated in A549/DDP cells when compared with that in parental A549 cells. Therefore, it was hypothesized that LINC00707 may lead to this result by sponging miR-145. miR-145 levels were significantly upregulated in A549/DDP cells following transfection with miR-156 mimics (Fig. 4C). Dual-luciferase reporter assay results indicated that cells co-transfected with WT-LINC00707 and miR-145 mimics exhibited significantly weakened luciferase activity, whereas no significant difference was identified in luciferase activity in cells co-transfected with MUT-LINC00707 and miR-145 mimics compared with the NC group (Fig. 4D). LINC00707 knockdown markedly promoted miR-145 expression in A549/DDP cells (Fig. 4E). Overall, these results suggest that LINC00707 may function as a molecular sponge by competitively binding to miR-145.
Figure 4.
LINC00707 functions as a molecular miR-145 sponge in A549/DDP cells. (A) Predicted binding sites between LINC00707 and miR-145. (B) Relative expression of miR-145 in A549/DDP cells and parental A549 cells was measured using RT-qPCR. ***P<0.001 vs. A549. (C) miR-145 was upregulated in cells transfected with miR-145 mimics. ***P<0.001 vs. NC. (D) Luciferase activity was measured in cells co-transfected with either WT-LINC00707 or MUT-LINC00707, and miRNA negative control or miR-145 mimics. ***P<0.001 vs. WT-LINC00707. (E) Relative expression levels of miR-145 in A549/DDP cells transfected with either si-LINC00707 or si-NC were measured using RT-qPCR. ***P<0.001 vs. si-NC. LINC00707, long intergenic non-coding RNA 00707; miR, microRNA; RT-qPCR, reverse transcription-quantitative PCR; A549/DDP cells, DDP-resistant A549 cells; si-LINC00707, LINC00707 siRNA; si-NC, siRNA negative control; WT, wild-type; MUT, mutant; NC, negative control.
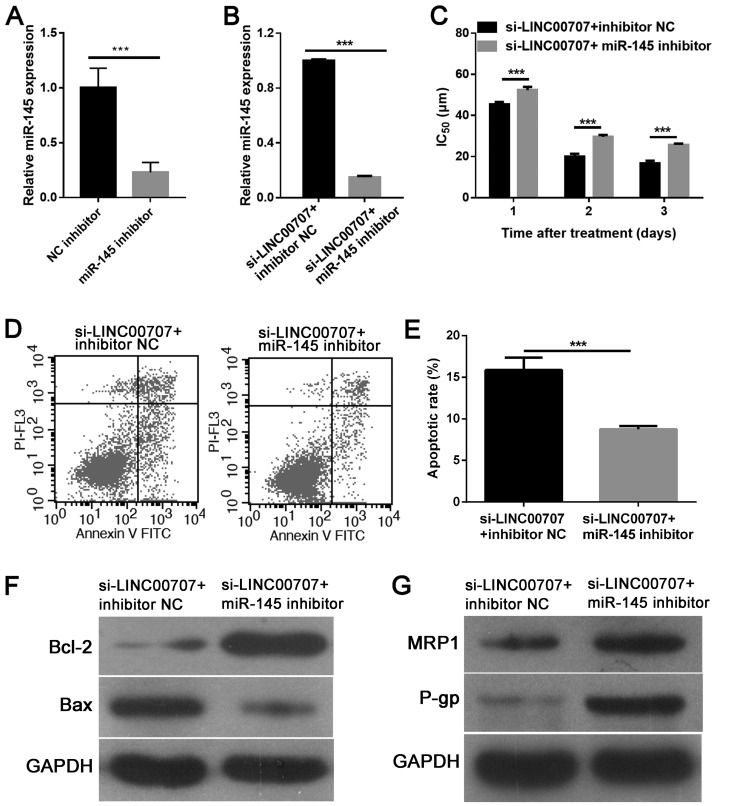
miR-145 downregulation weakens the effect of LINC00707 knockdown in A549/DDP cells
To gain insight into the mechanism via which LINC00707 knockdown enhanced DDP sensitivity in A549/DDP cells, an miR-145 inhibitor or inhibitor NC was further transfected into A549-DDP cells transfected with si-LINC00707. RT-qPCR analysis revealed that miR-145 inhibitor significantly downregulated miR-145 expression in A549/DDP cells compared with inhibitor NC (Fig. 5A). Additionally, it was demonstrated that the miR-145 inhibitor significantly downregulated miR-145 expression in A549-DDP cells transfected with si-LINC00707 when compared with the control group (Fig. 5B). It was further explored whether miR-145 downregulation reversed the effect of LINC00707 knockdown in A549/DDP cells. An MTT assay showed that miR-145 downregulation significantly increased the IC50 value of DDP in A549/DDP cells transfected with si-LINC00707 (Fig. 5C). Flow cytometric analysis also demonstrated that miR-145 downregulation inhibited apoptosis in A549/DDP cells transfected with si-LINC00707 (Fig. 5D and E). Furthermore, western blotting results indicated that miR-145 downregulation promoted Bcl-2, MRP1 and P-gp expression, while reducing Bax expression in A549/DDP cells transfected with si-LINC00707 and miR-145 inhibitor (Fig. 5F and G). Thus, these results indicated that miR-145 downregulation may reverse the effect of LINC00707 knockdown in A549/DDP cells.
Figure 5.
miR-145 downregulation reverses the effect of LINC00707 knockdown in A549/DDP cells. (A) miR-145 was downregulated in A549/DDP cells transfected with miR-145 inhibitor. (B) Relative expression levels of miR-145 in A549/DDP cells transfected with si-LINC00707 + inhibitor NC or si-LINC00707 + miR-145 inhibitor were measured using RT-qPCR. (C) IC50 values of DDP in A549/DDP cells transfected with either si-LINC00707 + inhibitor NC or si-LINC00707 + miR-145 inhibitor were measured using an MTT assay. (D-E) Apoptotic cell rates of A549/DDP cells transfected with either si-LINC00707 + inhibitor NC or si-LINC00707 + miR-145 inhibitor were measured using flow cytometry. Protein expression levels of (F) Bcl-2 and Bax, and (G) MRP1 and P-gp in A549/DDP cells transfected with either si-LINC00707 + inhibitor NC or si-LINC00707 + miR-145 inhibitor were measured using western blotting. ***P<0.001 vs. si-LINC00707 + inhibitor NC. MRP1, multidrug resistance protein 1; P-gp, P-glycoprotein; IC50, half-maximal inhibitory concentration; LINC00707, long intergenic non-coding RNA 00707; miR, microRNA; si-LINC00707, LINC00707 siRNA; NC, negative control; A549/DDP cells, DDP-resistant A549 cells; RT-qPCR, reverse transcription-quantitative PCR.
Discussion
DDP is considered to be a classical chemotherapeutic drug used for treating patients with NSCLC. However, DDP resistance among patients with NSCLC presents a significant barrier towards successful chemotherapy (17). Therefore, there is an urgent need to elucidate the molecular and biological mechanisms underlying the development of DDP resistance. In the present study, LINC00707 expression was found to be significantly upregulated in A549/DDP cells. Correspondingly, LINC00707 knockdown enhanced A549/DDP cell sensitivity towards DDP. Moreover, it was demonstrated that miR-145 was a target of LINC00707 and that miR-145 downregulation was capable of reversing the effect of LINC00707 knockdown in A549/DDP cells.
A number of studies have shown that lncRNA dysregulation fuels drug resistance in human cancers, including NSCLC (18,19). For example, lncRNA maternally expressed 3 was shown to be downregulated in DDP-resistant NSCLC cells, and its overexpression enhanced DDP sensitivity in NSCLC cells in vitro (18). Therefore, comprehensive elucidation of lncRNA regulatory mechanisms in drug resistance may provide a promising therapeutic strategy for the treatment of NSCLC. LINC00707 was previously identified to be an oncogene in various cancers. It was shown to be upregulated in hepatocellular carcinoma cells, thereby promoting hepatocellular carcinoma progression (8). Its expression was also shown to be highly upregulated in gastric cancer tissues and cells, thus promoting their proliferation and metastasis by interacting with human antigen R (9). In lung cancer, Ma et al (10) found that LINC00707 expression was clearly upregulated in lung adenocarcinoma tissues and cells; notably, LINC00707 promoted lung adenocarcinoma progression by regulating cell division control protein 42. However, the role of LINC00707 in the progression of DDP resistance in NSCLC still remains unclear. Herein, it was revealed that LINC00707 expression was highly upregulated in A549/DDP cells. LINC00707 knockdown reduced the IC50 value of DDP in A549/DDP cells. In addition, LINC00707 knockdown enhanced the percentage of apoptotic A549/DDP cells, inhibited the expression of anti-apoptotic protein Bcl-2 and promoted the expression of pro-apoptotic protein Bax in A549/DDP cells. These results indicated that LINC00707 knockdown enhances the DDP sensitivity of A549/DDP cells. By investigating the underlying mechanism, it was demonstrated that LINC00707 knockdown inhibited the expression of MRP1 and P-gp. The official full name of MRP1 is ATP binding cassette subfamily C member 1. The official full name of P-gp is ATP binding cassette subfamily B member 1. Both MRP1 and P-gp are members of the superfamily of ATP-binding cassette transporters, which is involved in multidrug resistance. The increased expression of MRP1 and P-gp usually represents the enhancement of multidrug resistance (20). Therefore, the results of the present study suggest that LINC00707 knockdown enhances DDP sensitivity by weakening multidrug resistance.
Recent studies have proposed that lncRNAs, including LINC00707, may serve as molecular miRNA sponges, thus affecting their target gene expression indirectly. For example, Jia et al (21) reported that LINC00707 sponged miR-370-3p to promote the osteogenesis of human bone marrow-derived mesenchymal stem cells by increasing WNT2B. In addition, Tu et al (22) found that LINC00707 contributed to hepatocellular carcinoma progression by sponging miR-206, which led to the upregulation of CDK14. In the present study, miR-145 was identified as a target of LINC00707. Changes in LINC00707 expression resulted in corresponding changes in miR-145 expression, thereby indicating that miR-145 expression was negatively regulated by LINC00707. miR-145, a known tumor suppressor, has been commonly reported to be downregulated in various types of human cancers, including colorectal cancer (23), breast cancer (24) and NSCLC (25), and has been shown to suppress tumor cell proliferation, apoptosis, migration and invasion (26). In the present study, it was revealed that miR-145 downregulation markedly reversed LINC00707 knockdown-induced cell dysfunction in A549-DDP cells. This was determined by the increased IC50 value of DDP, reduced apoptosis, increased Bcl-2, MRP1 and P-gp protein expression levels, and attenuated Bax protein expression levels in A549/DDP cells. Interestingly, Zhan et al (15) reported that miR-145 promoted MRP1 mRNA degradation by directly targeting its 3′-UTR, thereby sensitizing gallbladder cancer cells to DDP. Similarly, in the present study, it was demonstrated that miR-145 downregulation led to upregulated MRP1 protein expression, thus suggesting that miR-145 downregulation may enhance DDP activity in A549/DDP cells by regulating the expression of MRP1. These results indicated that silencing of LINC00707 enhances DDP sensitivity in NSCLC cells by sponging miR-145.
There are certain limitations in this study. Firstly, the effect of LINC00707 knockdown-induced DDP resistance was analyzed only in A549/DDP cells; additional NSCLC cells were not included. Furthermore, although miR-145 was identified as a target of LINC00707, its target genes were not identified. Finally, the effect of LINC00707 knockdown on A549/DDP cells was not verified in vivo. Despite these limitations, the present study indicated that LINC00707 contributed to the progression of DDP resistance in NSCLC cells.
In conclusion, LINC00707 was identified to be highly expressed in A549/DDP cells, and its knockdown was in turn found to significantly enhance DDP sensitivity in A549/DDP cells by sponging miR-145. Therefore, these findings suggest that LINC00707 may be a potential target in the treatment of DDP-resistant NSCLC patients.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the present study are available from the corresponding author.
Authors' contributions
HZ was involved in study design, conducting all experiments and preparing the manuscript. YL was involved in data collection and literature analysis. WX was responsible for performing the cell culture. KL and CL were responsible for performing the western blot analysis. All of the authors read and approved the final manuscript.
Ethics approval and consent to participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66:7–30. doi: 10.3322/caac.21332. [DOI] [PubMed] [Google Scholar]
- 2.Zhou K, Wang L, Cheng R, Liu X, Mao S, Yan Y. Elemene increases autophagic apoptosis and drug sensitivity in human cisplatin (DDP)-resistant lung cancer cell line SPC-A-1/DDP By inducing beclin-1 expression. Oncol Res. 2017 doi: 10.3727/096504017X14954936991990. (Epub ahead of print) [DOI] [PubMed] [Google Scholar]
- 3.Hu Y, Zhu QN, Deng JL, Li ZX, Wang G, Zhu YS. Emerging role of long non-coding RNAs in cisplatin resistance. OncoTargets Ther. 2018;11:3185–3194. doi: 10.2147/OTT.S158104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhang S, Ma H, Zhang D, Xie S, Wang W, Li Q, Lin Z, Wang Y. LncRNA KCNQ1OT1 regulates proliferation and cisplatin resistance in tongue cancer via miR-211-5p mediated Ezrin/Fak/Src signaling. Cell Death Dis. 2018;9:742. doi: 10.1038/s41419-018-0793-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wu F, Li J, Du X, Zhang W, Lei P, Zhang Q. Long noncoding RNA AB019562 promotes cell proliferation and metastasis in human hepatocellular carcinoma. Mol Med Rep. 2017;16:69–74. doi: 10.3892/mmr.2017.6612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hu B, Zhang H, Wang Z, Zhang F, Wei H, Li L. LncRNA CCAT1/miR-130a-3p axis increases cisplatin resistance in non-small-cell lung cancer cell line by targeting SOX4. Cancer Biol Ther. 2017;18:974–983. doi: 10.1080/15384047.2017.1385679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ma LY, Xie XW, Ma L, Pang JL, Xiong XM, Zheng HD, Shen XL, Wen ZG, Wang HY. Downregulated long non-coding RNA TRPM2-AS inhibits cisplatin resistance of non-small cell lung cancer cells via activation of p53- p66shc pathway. Eur Rev Med Pharmacol Sci. 2017;21:2626–2634. [PubMed] [Google Scholar]
- 8.Wang J, Luo Z, Yao T, Li W, Pu J. LINC00707 promotes hepatocellular carcinoma progression through activating ERK/JNK/AKT pathway signaling pathway. J Cell Physiol. 2019;234:6908–6916. doi: 10.1002/jcp.27449. [DOI] [PubMed] [Google Scholar]
- 9.Xie M, Ma T, Xue J, Ma H, Sun M, Zhang Z, Liu M, Liu Y, Ju S, Wang Z, De W. The long intergenic non-protein coding RNA 707 promotes proliferation and metastasis of gastric cancer by interacting with mRNA stabilizing protein HuR. Cancer Lett. 2019;443:67–79. doi: 10.1016/j.canlet.2018.11.032. [DOI] [PubMed] [Google Scholar]
- 10.Ma T, Ma H, Zou Z, He X, Liu Y, Shuai Y, Xie M, Zhang Z. The long intergenic noncoding RNA 00707 promotes lung adenocarcinoma cell proliferation and migration by regulating Cdc42. Cell Physiol Biochem. 2018;45:1566–1580. doi: 10.1159/000487693. [DOI] [PubMed] [Google Scholar]
- 11.Yuan Y, Haiying G, Zhuo L, Ying L, Xin H. Long non-coding RNA LINC00339 facilitates the tumorigenesis of non-small cell lung cancer by sponging miR-145 through targeting FOXM1. Biomed Pharmacother. 2018;105:707–713. doi: 10.1016/j.biopha.2018.06.022. [DOI] [PubMed] [Google Scholar]
- 12.Ambros V. The functions of animal microRNAs. Nature. 2004;431:350–355. doi: 10.1038/nature02871. [DOI] [PubMed] [Google Scholar]
- 13.Liu J, Xing Y, Rong L. miR-181 regulates cisplatin-resistant non-small cell lung cancer via downregulation of autophagy through the PTEN/PI3K/AKT pathway. Oncol Rep. 2018;39:1631–1639. doi: 10.3892/or.2018.6268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu K, Chen H, You Q, Ye Q, Wang F, Wang S, Zhang S, Yu K, Li W, Gu M. miR145 inhibits human nonsmall-cell lung cancer growth by dual-targeting RIOK2 and NOB1. Int J Oncol. 2018;53:257–265. doi: 10.3892/ijo.2018.4393. [DOI] [PubMed] [Google Scholar]
- 15.Zhan M, Zhao X, Wang H, Chen W, Xu S, Wang W, Shen H, Huang S, Wang J. miR-145 sensitizes gallbladder cancer to cisplatin by regulating multidrug resistance associated protein 1. Tumour Biol. 2016;37:10553–10562. doi: 10.1007/s13277-016-4957-6. [DOI] [PubMed] [Google Scholar]
- 16.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 17.Thatcher N, Hirsch FR, Luft AV, Szczesna A, Ciuleanu TE, Dediu M, Ramlau R, Galiulin RK, Bálint B, Losonczy G, et al. Necitumumab plus gemcitabine and cisplatin versus gemcitabine and cisplatin alone as first-line therapy in patients with stage IV squamous non-small-cell lung cancer (SQUIRE): An open-label, randomised, controlled phase 3 trial. Lancet Oncol. 2015;16:763–774. doi: 10.1016/S1470-2045(15)00021-2. [DOI] [PubMed] [Google Scholar]
- 18.Wang P, Chen D, Ma H, Li Y. LncRNA MEG3 enhances cisplatin sensitivity in non-small cell lung cancer by regulating miR-21-5p/SOX7 axis. Onco Targets Ther. 2017;10:5137–5149. doi: 10.2147/OTT.S146423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen QN, Wei CC, Wang ZX, Sun M. Long non-coding RNAs in anti-cancer drug resistance. Oncotarget. 2017;8:1925–1936. doi: 10.18632/oncotarget.12461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tekchandani P, Kurmi BD, Paliwal SR. Nanomedicine to deal with cancer cell biology in multi-drug resistance. Mini Rev Med Chem. 2017;17:1793–1810. doi: 10.2174/1389557516666160219123222. [DOI] [PubMed] [Google Scholar]
- 21.Jia B, Wang Z, Sun X, Chen J, Zhao J, Qiu X. Long noncoding RNA LINC00707 sponges miR-370-3p to promote osteogenesis of human bone marrow-derived mesenchymal stem cells through upregulating WNT2B. Stem Cell Res Ther. 2019;10:67. doi: 10.1186/s13287-019-1161-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tu J, Zhao Z, Xu M, Chen M, Weng Q, Wang J, Ji J. LINC00707 contributes to hepatocellular carcinoma progression via sponging miR-206 to increase CDK14. J Cell Physiol. 2019;234:10615–10624. doi: 10.1002/jcp.27737. [DOI] [PubMed] [Google Scholar]
- 23.Sheng N, Tan G, You W, Chen H, Gong J, Chen D, Zhang H, Wang Z. MiR-145 inhibits human colorectal cancer cell migration and invasion via PAK4-dependent pathway. Cancer Med. 2017;6:1331–1340. doi: 10.1002/cam4.1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ding Y, Zhang C, Zhang J, Zhang N, Li T, Fang J, Zhang Y, Zuo F, Tao Z, Tang S, et al. miR-145 inhibits proliferation and migration of breast cancer cells by directly or indirectly regulating TGF-β1 expression. Int J Oncol. 2017;50:1701–1710. doi: 10.3892/ijo.2017.3945. [DOI] [PubMed] [Google Scholar]
- 25.Li JC, Zheng JQ. Effect of microRNA-145 on proliferation and apoptosis of human non-small cell lung cancer A549 cells by regulating mTOR signaling pathway. J Cell Biochem. 2017 doi: 10.1002/jcb.26629. (Epub ahead of print) [DOI] [Google Scholar]
- 26.Zhou X, Yue Y, Wang R, Gong B, Duan Z. MicroRNA-145 inhibits tumorigenesis and invasion of cervical cancer stem cells. Int J Oncol. 2017;50:853–862. doi: 10.3892/ijo.2017.3857. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the present study are available from the corresponding author.