Key Points
Question
Is 5-day oral lefamulin noninferior to 7-day oral moxifloxacin in the management of community-acquired bacterial pneumonia?
Findings
In this randomized clinical trial that included 738 patients, the early clinical response at 96 hours (within a 24-hour window) after the first dose of study drug was 90.8% in the lefamulin group and 90.8% in the moxifloxacin group, a difference that met the noninferiority margin of 10%.
Meaning
This study demonstrated the noninferiority of oral lefamulin to oral moxifloxacin for the treatment of community-acquired bacterial pneumonia.
Abstract
Importance
New antibacterials are needed to treat community-acquired bacterial pneumonia (CABP) because of growing antibacterial resistance and safety concerns with standard care.
Objective
To evaluate the efficacy and adverse events of a 5-day oral lefamulin regimen in patients with CABP.
Design, Setting, and Participants
A phase 3, noninferiority randomized clinical trial conducted at 99 sites in 19 countries that included adults aged 18 years or older with a Pneumonia Outcomes Research Team (PORT) risk class of II, III, or IV; radiographically documented pneumonia; acute illness; 3 or more CABP symptoms; and 2 or more vital sign abnormalities. The first patient visit was on August 30, 2016, and patients were followed up for 30 days; the final follow-up visit was on January 2, 2018.
Interventions
Patients were randomized 1:1 to receive oral lefamulin (600 mg every 12 hours for 5 days; n = 370) or moxifloxacin (400 mg every 24 hours for 7 days; n = 368).
Main Outcomes and Measures
The US Food and Drug Administration (FDA) primary end point was early clinical response at 96 hours (within a 24-hour window) after the first dose of either study drug in the intent-to-treat (ITT) population (all randomized patients). Responders were defined as alive, showing improvement in 2 or more of the 4 CABP symptoms, having no worsening of any CABP symptoms, and not receiving any nonstudy antibacterial drug for current CABP episode. The European Medicines Agency coprimary end points (FDA secondary end points) were investigator assessment of clinical response at test of cure (5-10 days after last dose) in the modified ITT population and in the clinically evaluable population. The noninferiority margin was 10% for early clinical response and investigator assessment of clinical response.
Results
Among 738 randomized patients (mean age, 57.5 years; 351 women [47.6%]; 360 had a PORT risk class of III or IV [48.8%]), 707 (95.8%) completed the trial. Early clinical response rates were 90.8% with lefamulin and 90.8% with moxifloxacin (difference, 0.1% [1-sided 97.5% CI, –4.4% to ∞]). Rates of investigator assessment of clinical response success were 87.5% with lefamulin and 89.1% with moxifloxacin in the modified ITT population (difference, –1.6% [1-sided 97.5% CI, –6.3% to ∞]) and 89.7% and 93.6%, respectively, in the clinically evaluable population (difference, –3.9% [1-sided 97.5% CI, –8.2% to ∞]) at test of cure. The most frequently reported treatment-emergent adverse events were gastrointestinal (diarrhea: 45/368 [12.2%] in lefamulin group and 4/368 [1.1%] in moxifloxacin group; nausea: 19/368 [5.2%] in lefamulin group and 7/368 [1.9%] in moxifloxacin group).
Conclusions and Relevance
Among patients with CABP, 5-day oral lefamulin was noninferior to 7-day oral moxifloxacin with respect to early clinical response at 96 hours after first dose.
Trial Registrations
ClinicalTrials.gov Identifier: NCT02813694; European Clinical Trials Identifier: 2015-004782-92
This noninferiority randomized trial compares the effects of oral lefamulin vs oral moxifloxacin on clinical response at 96 hours among adult patients with community-acquired bacterial pneumonia.
Introduction
In the United States, pneumonia is among the most common causes of hospitalization and a leading cause of infectious death.1,2 Patients who recover from pneumonia experience long-term mortality substantially higher than in age- and sex-matched controls, primarily due to comorbidities.3 Older adults are particularly vulnerable to poor outcomes from pneumonia.4,5 Common causative pathogens of community-acquired bacterial pneumonia (CABP) include Streptococcus pneumoniae, Staphylococcus aureus, Haemophilus influenzae, Moraxella catarrhalis, and the atypical pathogens Mycoplasma pneumoniae, Chlamydophila pneumoniae, and Legionella pneumophila. Current first-line CABP treatment includes macrolides, β-lactams, or fluoroquinolones.6,7
Surveillance programs have observed trends of generally decreasing susceptibility among S pneumoniae and S aureus isolates to antimicrobials used to treat CABP, including oral penicillin, macrolides, and folate-pathway inhibitors in S pneumoniae and macrolides and fluoroquinolones in S aureus (particularly methicillin-resistant S aureus).8 High rates of bacterial resistance, combined with increasing fluoroquinolone-associated safety concerns, have created a need for new treatment options.6,8,9
Lefamulin is the first pleuromutilin antibiotic approved for intravenous and oral use in humans.10,11,12 Lefamulin is active against the most common CABP-causing pathogens, including some strains resistant to other antimicrobial classes.8,13,14 A previous phase 3 trial (Lefamulin Evaluation Against Pneumonia 1 [LEAP 1]) in adults with moderate to severe CABP (Pneumonia Outcomes Research Team [PORT] risk class ≥III) demonstrated noninferiority of lefamulin to moxifloxacin when both groups initiated intravenous therapy with an optional switch from intravenous to oral treatment.15 Given these results, LEAP 2 was conducted to compare a 5-day course of oral lefamulin twice daily vs a 7-day course of oral moxifloxacin once daily and the findings from the second trial are reported herein.
Methods
Study Design and Participants
This phase 3, double-blind, double-dummy, parallel-group randomized clinical trial was conducted from August 30, 2016, to January 2, 2018, at 99 sites in 19 countries throughout Europe, North America, South America, Asia, and Africa. The study was designed to evaluate lefamulin treatment over 5 days (minimum treatment duration recommended by CABP treatment guidelines)16 vs moxifloxacin treatment over 7 days (minimum approved treatment duration according to the moxifloxacin prescribing information).17 The trial protocol and statistical analysis plan appear in Supplement 1.
The trial protocol and the informed consent form were approved by the ethics committee or institutional review board at each participating site. The trial was conducted in compliance with the ethical principles of the Declaration of Helsinki18 and the Good Clinical Practice guidelines of the International Conference on Harmonisation. An informed consent form was signed by the patient before initiating any study-related procedures.
Adults with PORT risk class II, III, or IV radiographically documented pneumonia, acute illness (≤7 days), and 3 or more CABP symptoms (dyspnea, new or increased cough, purulent sputum production, and chest pain) were eligible for inclusion. Exclusion criteria included receipt of more than 1 dose of a short-acting (having a dosing interval more frequent than every 24 hours) oral or intravenous antibacterial for CABP within 72 hours before randomization, hospitalization for 2 days or longer within 90 days, confirmed or suspected methicillin-resistant S aureus, being at risk for major cardiac events or dysfunction (eg, known prolonged Q-T interval, clinically significant hypokalemia, clinically unstable cardiac disease, complete left bundle branch block), and having significant hepatic disease (eg, known acute hepatitis, history of cirrhosis, manifestation of end-stage liver disease). Complete details regarding the inclusion and exclusion criteria appear in Supplement 1.
Randomization, Stratification, and Blinding
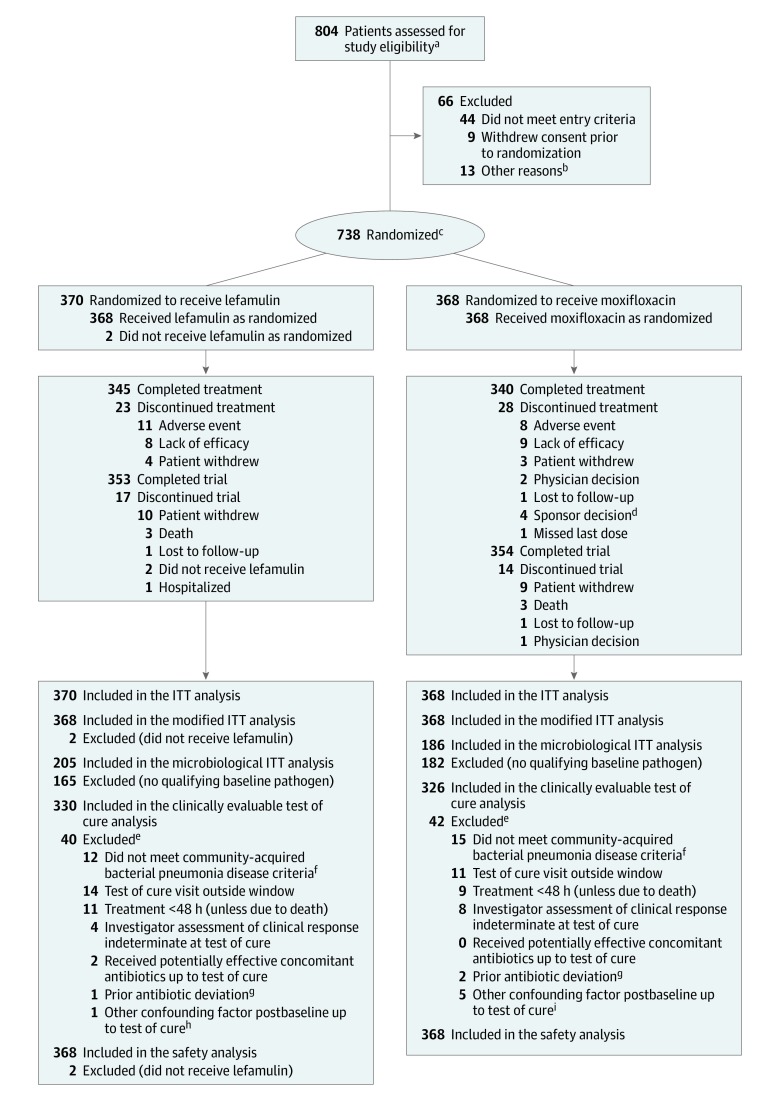
Patients were randomized 1:1 using a computer-generated central randomization schedule with a block size of 6 to receive either 600 mg of oral lefamulin every 12 hours for 5 days or 400 mg of oral moxifloxacin every 24 hours for 7 days (Figure). Patients in the lefamulin group received an oral moxifloxacin placebo every 24 hours for 7 days and patients in the moxifloxacin group received an oral lefamulin placebo every 12 hours for 5 days.
Figure. Patient Recruitment, Randomization, and Follow-up in the LEAP 2 Trial.
ITT indicates intent to treat; LEAP 2, Lefamulin Evaluation Against Pneumonia 2.
aThe number of patients with a Pneumonia Outcomes Research Team (PORT) risk class of II was capped at 50% of the total population; once this cap was reached, these patients were no longer assessed for study eligibility. All patients assessed for study eligibility provided informed consent.
bOne patient did not attend the randomization visit, 1 patient did not receive study drug due to insufficient drug stock at site, 1 patient with PORT risk class II was assessed after the per-protocol cap had been met, 1 patient had prolonged QTcF at the screening visit, 2 patients were excluded by the investigator, 6 patients were excluded due to randomization error, and 1 patient was excluded for an unknown reason.
cThe number of randomized patients who received a single dose of a short-acting antibiotic was capped at 25% of the total population; this cap was not reached and therefore this criterion had no effect on randomization.
dPatients were discontinued from treatment because they met per protocol exclusion or study withdrawal criteria: 1 patient due to baseline and postbaseline QTcF greater than 500 ms, 2 patients due to confirmed Staphylococcus aureus bacteremia, and 1 patient due to a complete left bundle branch block.
eMay have met more than 1 exclusion criterion.
fAs defined by inclusion criteria 3 through 7 and exclusion criteria 3, 5, and 6 in Supplement 1.
gAs defined by exclusion criterion 1 in Supplement 1.
hPatient had a lung abscess diagnosed by computed tomography within hours after randomization.
iOne patient was diagnosed with tuberculosis on study day 1; 1 patient was diagnosed with small cell lung cancer on study day 4; 1 patient was diagnosed with squamous cell carcinoma of the lung on study day 5; 1 patient was diagnosed with tuberculosis effusion on study day 24; and 1 patient was ultimately diagnosed with tuberculosis.
Randomization was stratified by PORT risk class (II vs III or IV), geographic region (United States vs outside the United States), and prior receipt of a single dose of short-acting antibiotic therapy for CABP (yes vs no). Per protocol, 25% or less of the study population could have received a single dose of a short-acting antibiotic, and 50% or more was to have had a PORT risk class of III or IV. Study personnel, patients, and the study sponsor were blinded to treatment allocation unless unblinding was necessary for medical management. A data and safety monitoring committee reviewed tolerability and adverse event data on an ongoing basis.
Race/ethnicity was entered into the case report by the study site using fixed categories to comply with regulatory guidance for a clinical trial.19 The exact method by which patient race/ethnicity was identified (eg, self-reported vs solicited by investigator) and whether the categories were used in a closed-ended or open-ended fashion is unknown and may have varied by study site.
Analysis Populations
The intent-to-treat (ITT) population included all patients who were randomized. The randomized patients who received any amount of study drug were included in the modified ITT population. The clinically evaluable population included patients who did not have an indeterminate clinical response, received study drug for a total duration of 48 hours or longer (unless patient died prior to 48 hours), did not receive a nonstudy antibacterial potentially effective against CABP pathogens (unless administered due to clinical failure), and had no additional factors that may have confounded efficacy assessment.
The microbiological ITT population included all patients in the ITT population with 1 or more CABP pathogens detected at baseline (defined as any pathogen identified by ≥1 diagnostic method). Detailed microbiological detection and testing methods and baseline pathogen definitions appear in Supplement 1.
Analyses in the ITT population, in the modified ITT population, in the clinically evaluable population, and in the microbiological ITT population were based on the treatment group to which the patient had been randomized. The safety population included all randomized patients who received any amount of study drug, and the analyses were based on the study drug actually received. All patients received the study drug to which they had been randomized.
Primary End Point
The study used CABP end points defined by regulatory bodies in the United States and in Europe.20,21 The US Food and Drug Administration primary efficacy end point was early clinical response at 96 hours (within a 24-hour window) after receipt of first dose of either study drug in the ITT population. Patients were programmatically classified as responders if they were alive, showed improvement in 2 or more of the 4 CABP symptoms, had no worsening of any CABP symptom, and did not receive a nonstudy antibacterial for the current CABP episode. If these criteria were not met, patients were classified as nonresponders. If lost to follow-up or if there were missing data for these criteria, patients were classified as indeterminate.
Coprimary and Secondary End Points
The European Medicines Agency coprimary end points (US Food and Drug Administration secondary end points) were predefined based on the European Medicines Agency guidelines21,22 as investigator assessment of clinical response at test of cure (5-10 days after last dose of study drug) in the modified ITT population and in the clinically evaluable population. Investigator assessment classified patient responses per protocol as a success if CABP was improved or resolved without additional antibacterials, as a failure if the patient died from any cause or a nonstudy antibacterial was required for the current CABP episode, or as indeterminate if the patient was lost to follow-up or these data were missing.
Other secondary analyses reported herein include early clinical response in the microbiological ITT population; investigator assessment of clinical response at test of cure in the microbiological ITT population; early clinical response (ITT population) and investigator assessment of clinical response at test of cure (modified ITT and clinically evaluable populations) in patient subgroups defined by baseline characteristics; and 28-day all-cause mortality in the ITT population. Secondary analyses not reported in this article include investigator assessment of clinical response (patients in the microbiological ITT population who met clinically evaluable criteria [microbiologically evaluable population]) and by pathogen microbiological response (microbiological ITT and microbiologically evaluable populations) at test of cure.
Samples of urine, blood, sputum, oropharyngeal, nasopharyngeal, pleural fluid, or bronchoalveolar lavage were collected at the screening or baseline visit after obtaining informed consent and within 24 hours of the first dose of study drug for eligibility assessment and microbiological testing. Additional assessments included vital signs, 12-lead electrocardiograms, laboratory tests, and treatment-emergent adverse events in the safety population.
Statistical Methods
A sample size of 738 patients randomized 1:1 provided 90% power to establish lefamulin noninferiority to moxifloxacin for early clinical response, using a 79% responder rate in both treatment groups for the ITT population, a 10% noninferiority margin, and 1-sided α level of .025. Assuming an investigator assessment of clinical response success rate of 80% (for the modified ITT population) and 85% (for the clinically evaluable population at test of cure) and a clinical evaluability rate of 80%, this study had 91% power to demonstrate noninferiority of lefamulin to moxifloxacin for investigator assessment of clinical response at test of cure using a 10% noninferiority margin and a 1-sided α level of .025.
Lefamulin noninferiority vs moxifloxacin was concluded if the lower limit of the 1-sided 97.5% CI for the treatment difference (calculated using a continuity-corrected z statistic for early clinical response and the Miettinen and Nurminen method with adjustment for the randomization stratification factors for investigator assessment of clinical response) exceeded –10%. The noninferiority margin of 10% was based on analysis of observational studies comparing no treatment vs antibacterial therapy,23 from which a conservative treatment effect of 20% was estimated. A 10% noninferiority margin preserves 50% of the treatment effect and is consistent with US Food and Drug Administration and European Medicines Agency guidelines.20,21 Because early clinical response and investigator assessment of clinical response were considered primary end points for 2 different regulatory agencies, no adjustment for multiplicity was required.
In the microbiological ITT population, the percentages of patients with an early clinical response or an investigator assessment of clinical response success were determined for each baseline pathogen. Early clinical response (in the ITT population) and investigator assessment of clinical response success (in the modified ITT population and in the clinically evaluable population at test of cure) were also evaluated in subgroups defined by baseline patient characteristics. For these analyses, 2-sided 95% CIs were determined for differences in rates of early clinical response or investigator assessment of clinical response success using a continuity-corrected z statistic.
No inferential testing of these outcomes was completed and the results were interpreted as exploratory descriptive analyses. Because this study was conducted at multiple study sites, post hoc analyses of early clinical response and investigator assessment of clinical response were conducted using hierarchical modeling to evaluate the potential for site effects. Statistical analyses were conducted using version 9.2 or higher of SAS software (SAS Institute Inc).
Results
Patients
Among 738 randomized patients (370 to lefamulin and 368 to moxifloxacin), 685 (345 in the lefamulin group and 340 in the moxifloxacin group) completed treatment, and 707 (95.8%) completed the trial (Figure). The mean duration of exposure to study drug was 5.0 days for lefamulin and 6.7 days for moxifloxacin, which reflects the intended duration of active treatment for each drug per the study design.
The demographics and baseline characteristics were well balanced between treatment groups (Table 1 and eTable 1 in Supplement 2). The ITT population was broadly representative of the patient population with CABP. Overall, 37.5% of the patients were aged 65 years or older, 52.4% were male, and 50.1% had some degree of kidney impairment. Per protocol, the 50% maximum cutoff for patients with PORT risk class II was met; 50.4% of the patients were in PORT risk class II, 37.7% in PORT risk class III, and 11.1% in PORT risk class IV. Common comorbid conditions included hypertension (36.2%), asthma or chronic obstructive pulmonary disease (16.7%), and diabetes (13.4%).
Table 1. Demographic and Baseline Characteristics of the Intent-to-Treat Population.
| Characteristic | Lefamulin (n = 370) | Moxifloxacin (n = 368) |
|---|---|---|
| Demographics | ||
| Age, mean (SD), y | 57.4 (16.4) | 57.7 (16.2) |
| Age group, No. (%) | ||
| <65 y | 234 (63.2) | 227 (61.7) |
| 65-74 y | 78 (21.1) | 79 (21.5) |
| ≥75 y | 58 (15.7) | 62 (16.8) |
| Sex, No. (%) | ||
| Male | 207 (55.9) | 180 (48.9) |
| Female | 163 (44.1) | 188 (51.1) |
| Body mass index, mean (SD)a | 26.5 (5.7) | 26.5 (5.8) |
| Race, No. (%)b | ||
| White | 274 (74.1) | 270 (73.4) |
| Asian | 48 (13.0) | 52 (14.1) |
| American Indian or Alaskan native | 24 (6.5) | 16 (4.3) |
| Black or African American | 19 (5.1) | 22 (6.0) |
| Other | 5 (1.4) | 8 (2.2) |
| Hispanic or Latino ethnicity, No. (%)b | 45 (12.2) | 38 (10.3) |
| Vital Signs, Mean (SD) | ||
| Pulse rate, beats/min | 95.3 (18.4) | 94.4 (18.0) |
| Blood pressure, mm Hg | ||
| Systolic | 118.6 (21.2) | 115.9 (20.6) |
| Diastolic | 74.3 (12.0) | 72.8 (11.2) |
| Temperature, °C | 38.7 (1.0) | 38.6 (1.1) |
| Respiratory rate, breaths/min | 24.8 (4.3) | 24.8 (4.4) |
| Disease Severity, No. (%) | ||
| PORT risk classc | ||
| I (least severe) | 1 (0.3) | 2 (0.5) |
| II | 183 (49.5) | 189 (51.4) |
| III | 145 (39.2) | 133 (36.1) |
| IV | 40 (10.8) | 42 (11.4) |
| V (most severe) | 1 (0.3) | 2 (0.5) |
| Met American Thoracic Society severity criteria | ||
| Minord | 31 (8.4) | 37 (10.1) |
| Modifiede | 20 (5.4) | 22 (6.0) |
| Met SIRS criteriaf | 353 (95.4) | 342 (92.9) |
| Bacteremia | 6 (1.6) | 9 (2.4) |
| Multilobar pneumonia | 88 (23.8) | 101 (27.4) |
| Received systemic antibacterial medication within 72 h prior to randomization | 78 (21.1) | 74 (20.1) |
| Comorbidities, No. (%) | ||
| Vascular disorders | 138 (37.3) | 145 (39.4) |
| Kidney impairment | ||
| Any (creatinine clearance <90 mL/min) | 180 (48.6) | 190 (51.6) |
| Moderate to severe (creatinine clearance <60 mL/min) | 68 (18.4) | 73 (19.8) |
| Diabetes | 48 (13.0) | 51 (13.9) |
| Cardiac disorders | 46 (12.4) | 53 (14.4) |
| Chronic obstructive pulmonary disease | 38 (10.3) | 33 (9.0) |
| Asthma | 23 (6.2) | 29 (7.9) |
| Current or previous smoker | 163 (44.1) | 135 (36.7) |
| Location, No. (%) | ||
| Eastern Europe | 236 (63.8) | 218 (59.2) |
| Latin America and Mexicog | 38 (10.3) | 34 (9.2) |
| Western Europe | 17 (4.6) | 19 (5.2) |
| United States | 11 (3.0) | 12 (3.3) |
| Asia and Africa | 68 (18.4) | 85 (23.1) |
Abbreviations: PORT, Pneumonia Outcomes Research Team; SIRS, systemic inflammatory response syndrome.
Calculated as weight in kilograms divided by height in meters squared.
Data on race and ethnicity were entered into the case report by the study site using fixed categories. The method by which patients were asked their identified race/ethnicity and whether the categories were used in a closed-ended or open-ended fashion is unknown and may have varied by investigator, study coordinator, and study site.
A clinical prediction rule used to calculate risk of morbidity and mortality among patients presenting with community-acquired pneumonia taking into consideration age, history of comorbid conditions, physical examination findings, and laboratory or radiographic results.24 The specific methods used to calculate and determine PORT risk class in this trial were described in supplement methods 1 in the article by File et al.15
Defined as presence of 3 or more of the following 9 criteria at baseline: respiratory rate of 30/min or greater, oxygen saturation less than 90% or Pao2 less than 60 mm Hg, blood urea nitrogen level of 20 mg/dL or greater, white blood cell count less than 4000/mm3, confusion, multilobar infiltrates, platelet level less than 100 000 cells/mm3, temperature lower than 36°C, or systolic blood pressure less than 90 mm Hg.
Defined as presence of 3 or more of the following 6 criteria at baseline: respiratory rate of 30/min or greater, oxygen saturation as measured by pulse oximetry (Spo2) divided by fraction of inspired oxygen (Fio2) of less than 274 where Spo2 divided by Fio2 equals 64 plus 0.84 (Pao2/Fio2), blood urea nitrogen level of 20 mg/dL or greater, confusion, age of 65 years or older, or multilobar infiltrates.
Defined as 2 or more of the following 4 symptoms at baseline: temperature less than 36°C or greater than 38°C; heart rate greater than 90/min; respiratory rate greater than 20/min; white blood cell count less than 4000 cells/mm3, white blood cell count greater than 12 000 cells/mm3, or immature polymorphonuclear neutrophils greater than 10%. The proportion of patients who met individual SIRS criteria appear in eTable 1 in Supplement 2.
Latin America includes patients from the countries of Argentina, Chile, and Peru.
In the microbiological ITT population (n = 391 [205 in the lefamulin group and 186 in the moxifloxacin group]), the most commonly isolated baseline pathogens were S pneumoniae (63.7% [249/391]) and H influenzae (26.6% [104/391]), followed by the atypical pathogens M pneumoniae, L pneumophila, and C pneumoniae (22.3% [87/391]; eTable 2 in Supplement 2). Most infections were monomicrobial (70.8% [277/391]). The remaining 29.2% (114/391) of infections were polymicrobial. Across all pathogens, the resistance rates to moxifloxacin were low (eTable 3 in Supplement 2).
The minimum inhibitory concentrations of lefamulin required to inhibit 50%/90% of baseline isolates were as follows: 0.25/0.25 μg/mL for overall, penicillin-resistant, multidrug-resistant, and macrolide-resistant strains of S pneumoniae; 1/2 μg/mL for H influenzae, 0.12/0.12 μg/mL for S aureus, and 0.001/0.001 μg/mL or lower for M pneumoniae.
Efficacy Outcomes
Clinical Response and Success Determined by Early Clinical Response or Investigator Assessment
The early clinical response rate in the ITT population was 90.8% with lefamulin vs 90.8% with moxifloxacin (difference, 0.1% [1-sided 97.5% CI, −4.4% to ∞]; Table 2). The rate of investigator assessment of clinical response at test of cure in the modified ITT population was 87.5% with lefamulin vs 89.1% with moxifloxacin (difference, −1.6% [1-sided 97.5% CI, –6.3% to ∞]). Similar rates of investigator assessment of clinical response at test of cure were recorded for the clinically evaluable population (89.7% with lefamulin vs 93.6% with moxifloxacin; difference, −3.9% [1-sided 97.5% CI, −8.2% to ∞]).
Table 2. Early Clinical Response for the Intent-to-Treat (ITT) Population and Investigator Assessment of Clinical Response at Test of Cure in Modified ITT and Clinically Evaluable Populations.
| End Points | No. (%) | Between-Group Difference, % (1-Sided 97.5% CI)a |
|
|---|---|---|---|
| Lefamulin | Moxifloxacin | ||
| US FDA Primary End Point | |||
| Early clinical response in ITT population, No. of patients | 370 | 368 | |
| Responder | 336 (90.8) | 334 (90.8) | 0.1 (–4.4 to ∞) |
| Nonresponder | 29 (7.8) | 31 (8.4) | |
| Indeterminate | 5 (1.4) | 3 (0.8) | |
| EMA Coprimary End Points and US FDA Secondary End Points | |||
| Investigator assessment of clinical response at test of cure in modified ITT population, No. of patients | 368 | 368 | |
| Success | 322 (87.5) | 328 (89.1) | –1.6 (–6.3 to ∞) |
| Failure | 44 (12.0) | 32 (8.7) | |
| Indeterminate | 2 (0.5) | 8 (2.2) | |
| Investigator assessment of clinical response at test of cure in clinically evaluable population, No. of patients | 330 | 326 | |
| Success | 296 (89.7) | 305 (93.6) | –3.9 (–8.2 to ∞) |
| Failure | 34 (10.3) | 21 (6.4) | |
Abbreviations: EMA, European Medicines Agency; FDA, Food and Drug Administration.
Calculated using a continuity-corrected z statistic for early clinical response and the Miettinen and Nurminen method with adjustment for the randomization stratification factors for investigator assessment of clinical response. The margin for noninferiority was –10%; a lower bound of the CI that did not exceed this margin indicated noninferiority for lefamulin vs moxifloxacin.
Post hoc hierarchical modeling analyses identified 1 study site as having a potential effect on study results; however, exclusion of this site from the analyses did not affect early clinical response in the ITT population or investigator assessment of clinical response in the modified ITT and clinically evaluable populations, and lefamulin remained noninferior to moxifloxacin (eTable 4 and eText in Supplement 2).
Clinical Response and Success Determined by Baseline Pathogen
Lefamulin and moxifloxacin demonstrated high rates of early clinical response and investigator assessment of clinical response at test of cure across all baseline CABP pathogens (microbiological ITT population; Table 3), including multidrug-resistant pathogens. Specifically, the early clinical response rate for multidrug-resistant S pneumoniae was 100% (8/8) in the lefamulin group vs 83.3% (10/12) in the moxifloxacin group and the investigator assessment of clinical response success rate was 100% (8/8) in the lefamulin group vs 91.7% (11/12) in the moxifloxacin group. Among difficult to treat pathogens, the early clinical response rate for L pneumophila was 81.3% (13/16) in the lefamulin group vs 94.1% (16/17) in the moxifloxacin group and the investigator assessment of clinical response success rate was 81.3% (13/16) in the lefamulin group vs 88.2% (15/17) in the moxifloxacin group.
Table 3. Prespecified Secondary End Points by Baseline Community-Acquired Bacterial Pneumonia (CABP) Pathogen in Microbiological Intent-to-Treat Population.
| Baseline CABP Pathogena | Early Clinical Response, No. Successfully Treated/Total No. |
Treatment Difference, % (2-Sided 95% CI) |
Investigator Assessment of Clinical Response, No. Successfully Treated/Total No. |
Treatment Difference, % (2-Sided 95% CI) |
||
|---|---|---|---|---|---|---|
| Lefamulin (n = 205) |
Moxifloxacin (n = 186) |
Lefamulin (n = 205) |
Moxifloxacin (n = 186) |
|||
| Overall response | 186 (90.7) | 173 (93.0) | –2.3 (–8.2 to 3.6) | 176 (85.9) | 163 (87.6) | –1.8 (–8.7 to 5.1) |
| Streptococcus pneumoniae | 110/123 (89.4) | 115/126 (91.3) | 105/123 (85.4) | 108/126 (85.7) | ||
| Penicillin susceptible | 20/26 (76.9) | 36/38 (94.7) | 20/26 (76.9) | 38/38 (100) | ||
| Penicillin resistant | 5/5 (100) | 4/4 (100) | 5/5 (100) | 3/4 (75.0) | ||
| Macrolide resistantb | 7/8 (87.5) | 9/11 (81.8) | 7/8 (87.5) | 10/11 (90.9) | ||
| Multidrug resistantc | 8/8 (100) | 10/12 (83.3) | 8/8 (100) | 11/12 (91.7) | ||
| Staphylococcus aureus | 13/13 (100) | 6/6 (100) | 12/13 (92.3) | 5/6 (83.3) | ||
| Methicillin susceptible | 9/9 (100) | 2/2 (100) | 8/9 (88.9) | 2/2 (100) | ||
| Methicillin resistant | 2/2 (100) | 1/1 (100) | 2/2 (100) | 0/1 | ||
| Haemophilus influenzae | 50/56 (89.3) | 44/48 (91.7) | 52/56 (92.9) | 40/48 (83.3) | ||
| Moraxella catarrhalis | 18/21 (85.7) | 11/11 (100) | 17/21 (81.0) | 11/11 (100) | ||
| Mycoplasma pneumoniae | 20/20 (100) | 14/14 (100) | 19/20 (95.0) | 14/14 (100) | ||
| Legionella pneumophila | 13/16 (81.3) | 16/17 (94.1) | 13/16 (81.3) | 15/17 (88.2) | ||
| Chlamydophila pneumoniae | 15/16 (93.8) | 12/12 (100) | 12/16 (75.0) | 10/12 (83.3) | ||
A patient could have had more than 1 pathogen. Multiple isolates of the same species from the same patient were counted once for each phenotype and once for the overall tabulation of the genus and species. Phenotypes were only determined for pathogens identified from cultures and with susceptibility testing results.
Resistant to azithromycin or erythromycin.
Resistant to 2 or more of the following: oral penicillin, moxifloxacin, ceftriaxone, clindamycin, azithromycin or erythromycin, doxycycline, or trimethoprim/sulfamethoxazole.
Among patients with penicillin-susceptible S pneumoniae, clinical response rates were lower with lefamulin vs moxifloxacin (early clinical response rate: 76.9% [20/26] in the lefamulin group vs 94.7% [36/38] in the moxifloxacin group; and investigator assessment of clinical response rate: 76.9% [20/26] in the lefamulin group vs 100% [38/38] in the moxifloxacin group). Of note, within this patient subgroup, a greater proportion of patients in the lefamulin group had PORT risk class severity scores of III or IV (69.2% [18/26]) compared with the moxifloxacin group (36.8% [14/38]).
Among the 1.6% (6/370) of patients randomized to lefamulin with baseline bacteremia (Table 1), 4 patients had baseline pathogens covered by lefamulin (3 with S pneumoniae and 1 with S aureus); of these, 2 patients achieved early clinical response and had an outcome of investigator assessment of clinical response success at test of cure. Of patients randomized to moxifloxacin, 2.4% (9/368) had baseline bacteremia (Table 1); of the 7 patients who had common CABP pathogens at baseline (5 with S pneumoniae and 2 with S aureus), all achieved early clinical response and 6 had an outcome of investigator assessment of clinical response success at test of cure.
Clinical Response and Success by Subpopulations
Although the study was not designed for statistical inference in subpopulations, lefamulin and moxifloxacin demonstrated high rates of early clinical response and investigator assessment of clinical response across all CABP severity indices (eFigure in Supplement 2).
Adverse Events and Tolerability
The overall incidence of treatment-emergent adverse events was 32.6% with lefamulin and 25.0% with moxifloxacin; most reported treatment-emergent adverse events were mild or moderate in severity (Table 4). The most common treatment-emergent adverse events were diarrhea (12.2%), nausea (5.2%), and vomiting (3.3%) in the lefamulin group and nausea (1.9%), headache (1.6%), and urinary tract infection (1.6%) in the moxifloxacin group.
Table 4. Treatment-Emergent Adverse Events (TEAEs) in Safety Population.
| Type of Adverse Event | No. (%) | |
|---|---|---|
| Lefamulin (n = 368) | Moxifloxacin (n = 368) | |
| Total TEAEs | 120 (32.6) | 92 (25.0) |
| Severe | 13 (3.5) | 10 (2.7) |
| Moderate | 44 (12.0) | 27 (7.3) |
| Mild | 63 (17.1) | 55 (14.9) |
| TEAEs occurring in >1% in either group | ||
| Diarrheaa | 45 (12.2) | 4 (1.1) |
| Nauseab | 19 (5.2) | 7 (1.9) |
| Vomiting | 12 (3.3) | 3 (0.8) |
| Hypertension | 5 (1.4) | 5 (1.4) |
| Respiratory tract viral infection | 5 (1.4) | 1 (0.3) |
| Headache | 4 (1.1) | 6 (1.6) |
| Gastritis | 4 (1.1) | 2 (0.5) |
| Pneumonia | 4 (1.1) | 1 (0.3) |
| Chronic obstructive pulmonary disease | 4 (1.1) | 0 |
| Urinary tract infection | 3 (0.8) | 6 (1.6) |
| ALT increased | 3 (0.8) | 4 (1.1) |
| AST increased | 2 (0.5) | 4 (1.1) |
| Anemia | 0 | 4 (1.1) |
| Insomnia | 0 | 4 (1.1) |
| Treatment-related TEAEs | 58 (15.8) | 29 (7.9) |
| Diarrhea | 34 (9.2) | 3 (0.8) |
| Nausea | 15 (4.1) | 6 (1.6) |
| Serious TEAEs | 17 (4.6) | 18 (4.9) |
| Treatment-related serious TEAEs | 0 | 1 (0.3) |
| TEAEs leading to death | 5 (1.4)c | 3 (0.8) |
| 28-d all-cause mortalityd | 3 (0.8) | 3 (0.8) |
| TEAEs leading to discontinuation of study drug | 12 (3.3) | 9 (2.4) |
| TEAEs leading to withdrawal from study | 5 (1.4) | 5 (1.4) |
Abbreviations: ALT, alanine aminotransferase; AST, aspartate aminotransferase.
In the lefamulin group, diarrhea was mild in 32 patients and moderate in 13 patients; in the moxifloxacin group, diarrhea was mild in 4 patients.
In the lefamulin group, nausea was mild in 16 patients and moderate in 3 patients; in the moxifloxacin group, nausea was mild in 6 patients and moderate in 1 patient.
Two deaths occurred beyond the 28-day window (details appear in the Adverse Events and Tolerability section).
Assessed in the intent-to-treat population (370 in the lefamulin group vs 368 in the moxifloxacin group).
Gastrointestinal-related treatment-emergent adverse events occurred in 17.9% of patients who received lefamulin and 7.6% of patients who received moxifloxacin. The higher incidence of gastrointestinal-related treatment-emergent adverse events with lefamulin was driven primarily by diarrhea (median duration, 2 days). No cases of diarrhea led to study drug discontinuation. Vomiting led to study drug discontinuation in 2 patients randomized to lefamulin and in 1 patient randomized to moxifloxacin. One case of Clostridium difficile infection was reported. The event occurred in a patient successfully treated with lefamulin who remained hospitalized, with onset approximately 1 week after completing 5 days of active lefamulin treatment. The event resolved after treatment with oral vancomycin.
Both treatment groups reported low treatment-emergent adverse event rates for hepatobiliary events (1.1% in the lefamulin group and 0.5% in the moxifloxacin group) and cardiac events (2.2% in the lefamulin group and 2.4% in the moxifloxacin group). Changes in laboratory values, including liver enzyme tests, are reported in eTable 5 in Supplement 2. No patient met criteria for Hy’s law.25
On day 4 postdose (steady state), the mean change from baseline in QTcF interval was 9.5 ms with lefamulin and 11.6 ms with moxifloxacin. One patient randomized to lefamulin discontinued the study drug because of a mild treatment-emergent adverse event of electrocardiogram Q-T prolongation; the patient’s QTc interval increased from 440.3 ms at baseline to a maximum of 490 ms on day 5. No associated cardiac arrhythmias were observed.
Study drug discontinuation due to treatment-emergent adverse events occurred in 3.3% of patients who received lefamulin and 2.4% of patients who received moxifloxacin, and serious treatment-emergent adverse events were experienced by 4.6% and 4.9% of patients, respectively (Table 4). One patient in the moxifloxacin group had a serious treatment-emergent adverse event considered by the investigator to be possibly related to the study drug (acute non-Q wave anterolateral and septal myocardial infarction); the patient recovered from the event.
Serious treatment-emergent adverse events resulted in the death of 5 patients in the lefamulin group (3 died within the 28-day window, 1 each due to acute respiratory distress syndrome [study day 2], myocardial infarction [study day 3], and pulmonary edema [study day 1]; 2 died beyond the 28-day window, 1 due to subacute aortic valve endocarditis [day 57] and 1 due to acute myeloid leukemia [day 271]) and in the death of 3 patients in the moxifloxacin group (all within the 28-day window; 1 each due to respiratory failure [study day 4], natural causes [study day 12], and cerebral infarction [study day 18]). None of these events was considered related to the study drug.
Discussion
This trial demonstrated the noninferiority of 5 days of oral lefamulin to 7 days of oral moxifloxacin among adults with CABP. Both agents were associated with a high clinical response, including analyses by PORT risk class, typical and atypical pathogens, polymicrobial infections, and demographic and baseline characteristics.
These efficacy results are consistent with those obtained in the LEAP 1 trial in which lefamulin was noninferior to moxifloxacin when both groups initiated intravenous therapy with an option to switch from intravenous to oral treatment.15 The early clinical response rates were high in both trials (90.8% in the lefamulin group and 90.8% in the moxifloxacin group in this trial vs 87.3% in the lefamulin group and 90.2% in the moxifloxacin group in LEAP 1), and notably higher than those reported in other antimicrobial trials of CABP.26,27 In terms of CABP severity, the greatest overlap between LEAP 1 and this trial was among patients with PORT risk class III, in whom early clinical response rates were high (91.0% in the lefamulin group and 90.2% in the moxifloxacin group in this trial vs 89.3% in the lefamulin group and 93.0% in the moxifloxacin group in LEAP 1).
Oral lefamulin was generally well tolerated; however, a greater percentage of patients receiving lefamulin reported gastrointestinal-related treatment-emergent adverse events compared with moxifloxacin. All gastrointestinal-related treatment-emergent adverse events in the lefamulin group were mild to moderate in severity, manageable, and rarely led to study drug discontinuation. Mild or moderate diarrhea was reported in 12.2% of patients in the lefamulin group and in 1.1% of patients in the moxifloxacin group. Gastrointestinal-related treatment-emergent adverse events are the most notable adverse event category associated with lefamulin, particularly when starting with oral therapy.
Lefamulin has a 5-day oral treatment option, has no cross-resistance to other classes,8 covers typical and atypical CABP pathogens, and the option to switch from intravenous to oral administration may provide an alternative approach for the treatment of vulnerable patients (eg, older patients with comorbid conditions).
The strengths of this study include its randomized design, evaluation of oral only short-course therapy, a high proportion of patients with more severe forms of CABP, limited use of prior antibiotics, and low drug and study discontinuation rates. The use of moxifloxacin, a highly effective antibiotic for treatment of CABP, resulted in assay sensitivity such that noninferiority evaluations were valid. The use of multiple diagnostic modalities, including real-time polymerase chain reaction with conservative cutoff limits, standard culture, urinary antigen testing, and serology, increased the yield of baseline pathogen identification.
Limitations
This trial has several limitations. First, the extensive list of exclusion criteria may have limited the generalizability of these results to patient subpopulations with major diseases. Second, many baseline pathogens were identified using nonculture methods, limiting the collection of minimum inhibitory concentration data. Third, samples were not tested for viral co-pathogens. Fourth, patients with suspected methicillin-resistant S aureus were excluded per protocol due to poor coverage with moxifloxacin (although 3 patients with a baseline pathogen of methicillin-resistant S aureus were enrolled). Fifth, the overall recovery of resistant pathogens was low. Sixth, race/ethnicity designation may have been misclassified, given that the methods (eg, self-reported vs solicited by investigator in an open-ended or closed-ended fashion) to collect these data may not have been consistent across sites.
Conclusions
Among patients with CABP, 5-day oral lefamulin was noninferior to 7-day oral moxifloxacin with respect to early clinical response at 96 hours after first dose.
Study protocol and statistical analysis plan
eTable 1. Patients who met SIRS criteria at baseline (ITT population)
eTable 2. Baseline CABP pathogen distribution (microITT population)
eTable 3. Minimum inhibitory concentrations for key cultured CABP pathogens (microITT population)
eTable 4. Results from hierarchical modeling analysis
eTable 5. Results for selected laboratory parameters (safety analysis set)
eFigure. Early clinical response by baseline variables in the ITT population (A) and investigator assessment of clinical response by baseline variables in the mITT population at test of cure (B)
eText. Post hoc hierarchical modeling analysis
Data sharing statement
References
- 1.Xu J, Murphy SL, Kochanek KD, Bastian B, Arias E Deaths: final data for 2016. https://www.cdc.gov/nchs/data/nvsr/nvsr67/nvsr67_05.pdf. Accessed August 26, 2019. [PubMed]
- 2.McDermott KW, Elixhauser A, Sun R. Trends in hospital inpatient stays in the United States, 2005-2014 In: Healthcare Cost and Utilization Project Statistical Brief No. 225. Rockville, MD: Agency for Healthcare Research and Quality; 2017. [Google Scholar]
- 3.Bruns AH, Oosterheert JJ, Cucciolillo MC, et al. . Cause-specific long-term mortality rates in patients recovered from community-acquired pneumonia as compared with the general Dutch population. Clin Microbiol Infect. 2011;17(5):763-768. doi: 10.1111/j.1469-0691.2010.03296.x [DOI] [PubMed] [Google Scholar]
- 4.Jain S, Self WH, Wunderink RG, et al. ; CDC EPIC Study Team . Community-acquired pneumonia requiring hospitalization among US adults. N Engl J Med. 2015;373(5):415-427. doi: 10.1056/NEJMoa1500245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hines AL, Heslin KC, Jiang HH, Coffey R. Trends in observed adult inpatient mortality for high-volume conditions, 2002-2012 In: Healthcare Cost and Utilization Project Statistical Brief No. 194. Rockville, MD: Agency for Healthcare Research and Quality; 2015. [PubMed] [Google Scholar]
- 6.Peyrani P, Mandell L, Torres A, Tillotson GS. The burden of community-acquired bacterial pneumonia in the era of antibiotic resistance. Expert Rev Respir Med. 2019;13(2):139-152. doi: 10.1080/17476348.2019.1562339 [DOI] [PubMed] [Google Scholar]
- 7.Pfaller MA, Farrell DJ, Sader HS, Jones RN. AWARE Ceftaroline Surveillance Program (2008-2010): trends in resistance patterns among Streptococcus pneumoniae, Haemophilus influenzae, and Moraxella catarrhalis in the United States. Clin Infect Dis. 2012;55(suppl 3):S187-S193. doi: 10.1093/cid/cis561 [DOI] [PubMed] [Google Scholar]
- 8.Paukner S, Gelone SP, Arends SJR, Flamm RK, Sader HS. Antibacterial activity of lefamulin against pathogens most commonly causing community-acquired bacterial pneumonia: SENTRY antimicrobial surveillance program (2015-2016). Antimicrob Agents Chemother. 2019;63(4):e021610-e021618. doi: 10.1128/AAC.02161-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sakoulas G. Adverse effects of fluoroquinolones: where do we stand? https://www.jwatch.org/na48248/2019/02/13/adverse-effects-fluoroquinolones-where-do-we-stand. Accessed August 26, 2019.
- 10.US Food and Drug Administration Xenleta (lefamulin): full prescribing information. https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/211672s000,211673s000lbl.pdf. Accessed August 26, 2019.
- 11.Nabriva Therapeutics Nabriva Therapeutics receives US FDA approval of Xenleta (lefamulin) to treat community-acquired bacterial pneumonia (CABP). http://investors.nabriva.com/news-releases/news-release-details/nabriva-therapeutics-receives-us-fda-approval-xenleta. Accessed August 26, 2019.
- 12.US Food and Drug Administration FDA approves new antibiotic to treat community-acquired bacterial pneumonia. https://www.fda.gov/news-events/press-announcements/fda-approves-new-antibiotic-treat-community-acquired-bacterial-pneumonia. Accessed August 26, 2019.
- 13.Paukner S, Sader HS, Ivezic-Schoenfeld Z, Jones RN. Antimicrobial activity of the pleuromutilin antibiotic BC-3781 against bacterial pathogens isolated in the SENTRY antimicrobial surveillance program in 2010. Antimicrob Agents Chemother. 2013;57(9):4489-4495. doi: 10.1128/AAC.00358-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Waites KB, Crabb DM, Duffy LB, Jensen JS, Liu Y, Paukner S. In vitro activities of lefamulin and other antimicrobial agents against macrolide-susceptible and macrolide-resistant Mycoplasma pneumoniae from the United States, Europe, and China. Antimicrob Agents Chemother. 2017;61(2):e02008-e020016. doi: 10.1128/AAC.02008-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.File TM Jr, Goldberg L, Das A, et al. . Efficacy and safety of intravenous-to-oral lefamulin, a pleuromutilin antibiotic, for the treatment of community-acquired bacterial pneumonia: the phase III Lefamulin Evaluation Against Pneumonia (LEAP 1) trial [published February 4, 2019]. Clin Infect Dis. doi: 10.1093/cid/ciz090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mandell LA, Wunderink RG, Anzueto A, et al. ; Infectious Diseases Society of America; American Thoracic Society . Infectious Diseases Society of America/American Thoracic Society consensus guidelines on the management of community-acquired pneumonia in adults. Clin Infect Dis. 2007;44(suppl 2):S27-S72. doi: 10.1086/511159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.US Food and Drug Administration Avelox (moxifloxacin hydrochloride): full prescribing information. https://www.accessdata.fda.gov/drugsatfda_docs/label/2016/021085s063lbl.pdf. Accessed August 26, 2019.
- 18.World Medical Association World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. 2013;310(20):2191-2194. doi: 10.1001/jama.2013.281053 [DOI] [PubMed] [Google Scholar]
- 19.US Food and Drug Administration Guidance for industry and Food and Drug Administration staff: collection of race and ethnicity data in clinical trials. https://www.fda.gov/media/75453/download. Accessed August 26, 2019.
- 20.US Food and Drug Administration Guidance for industry: community-acquired bacterial pneumonia: developing drugs for treatment. https://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM123686.pdf. Accessed August 26, 2019.
- 21.European Medicines Agency Committee for Human Medicinal Products Addendum to the guideline on the evaluation of medicinal products indicated for treatment of bacterial infections. https://www.ema.europa.eu/documents/scientific-guideline/addendum-guideline-evaluation-medicinal-products-indicated-treatment-bacterial-infections_en.pdf. Accessed August 26, 2019.
- 22.European Medicines Agency Committee for Human Medicinal Products Guideline on the evaluation of medicinal products indicated for treatment of bacterial infections. https://www.ema.europa.eu/en/documents/scientific-guideline/draft-guideline-evaluation-medicinal-products-indicated-treatment-bacterial-infections-revision-2_en.pdf. Accessed September 3, 2019.
- 23.Talbot GH, Powers JH, Fleming TR, Siuciak JA, Bradley J, Boucher H; CABP-ABSSSI Project Team . Progress on developing endpoints for registrational clinical trials of community-acquired bacterial pneumonia and acute bacterial skin and skin structure infections: update from the Biomarkers Consortium of the Foundation for the National Institutes of Health. Clin Infect Dis. 2012;55(8):1114-1121. doi: 10.1093/cid/cis566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fine MJ, Auble TE, Yealy DM, et al. . A prediction rule to identify low-risk patients with community-acquired pneumonia. N Engl J Med. 1997;336(4):243-250. doi: 10.1056/NEJM199701233360402 [DOI] [PubMed] [Google Scholar]
- 25.US Food and Drug Administration Guidance for industry drug-induced liver injury: premarketing clinical evaluation. https://www.fda.gov/downloads/guidances/UCM174090.pdf. Accessed August 26, 2019.
- 26.US Food and Drug Administration Antimicrobial Drugs Advisory Committee Solithromycin for the treatment of community acquired bacterial pneumonia. https://www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/Drugs/Anti-InfectiveDrugsAdvisoryCommittee/UCM527691.pdf. Accessed August 26, 2019.
- 27.US Food and Drug Administration Nuzyra (omadacycline): full prescribing information. https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/209816_209817lbl.pdf. Accessed August 26, 2019.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Study protocol and statistical analysis plan
eTable 1. Patients who met SIRS criteria at baseline (ITT population)
eTable 2. Baseline CABP pathogen distribution (microITT population)
eTable 3. Minimum inhibitory concentrations for key cultured CABP pathogens (microITT population)
eTable 4. Results from hierarchical modeling analysis
eTable 5. Results for selected laboratory parameters (safety analysis set)
eFigure. Early clinical response by baseline variables in the ITT population (A) and investigator assessment of clinical response by baseline variables in the mITT population at test of cure (B)
eText. Post hoc hierarchical modeling analysis
Data sharing statement