Abstract
Background
Methamphetamine (METH), a confirmed neurotoxic drug, has also reportedly caused several intestinal inflammatory injury cases. The NLRP3 (Nod-like receptor 3 protein) inflammasome can induce several inflammatory injuries by activating IL-1β and IL-18 when overexpressed. We designed experiments to determine whether METH can cause intestinal inflammatory injury via NLRP3 inflammasome overexpression.
Material/Methods
IEC-6 cells were classified as control, METH (0.5 mM), and METH (0.5 mM)+MCC950 (100 μM) groups. C57BL/6 mice were separated into control, NS, METH (5 mg/kg), and METH (5 mg/kg)+MCC950 (10 mg/kg) groups (n=10). We detected apoptosis, transepithelial electrical resistance (TEER), and proinflammatory factors (IL-6, INF-γ, TNF-α, and NF-κB) in the METH cell model. We also assessed proinflammatory factors (IL-6, INF-γ, TNF-α, and NF-κB) and observed intestinal tissues stained with hematoxylin and eosin (HE) in the METH animal model to explore intestinal inflammatory injury due to METH. After adding MCC950 (an NLRP3 inflammasome inhibitor), we additionally detected NLRP3 inflammasome components (NLRP3, Caspase-1, and ASC), IL-1β, and IL-18 to estimate the relationship of the NLRP3 inflammasome with intestinal inflammatory injury due to METH.
Results
METH can lead apoptosis, increase proinflammatory factors (e.g., IL-6, INF-γ, TNF-α, and NF-κB), and decrease TEER in the METH cell model. In the METH animal model, METH can cause obvious injury and increase proinflammatory factors (e.g., IL-6, INF-γ, TNF-α, and NF-κB). All the intestinal inflammatory changes due to METH depended on overexpression of the NLRP3 inflammasome and could be ameliorated by MCC950, except for ASC and NF-κB.
Conclusions
METH, in addition to being a confirmed neurotoxic drug, can also cause severe intestinal inflammatory injury via NLRP3 inflammasome overexpression. NF-κB may be an activator of the NLRP3 inflammasome in METH intestinal inflammatory injury.
MeSH Keywords: Inflammasomes, Inflammation, Intestines, Methamphetamine
Background
Methamphetamine (METH) has been a crucial component of the popular illegal drug ‘ICE’ since its first synthesis in the early 19th century [1]. Accordingly, METH has spread widely in different regions worldwide, including the Middle East, South Asia, and Europe [2]. Generally, it can be absorbed in vivo by swallowing, injection, snorting, and smoking. Experiments have indicated that it often causes severe detrimental neurotoxic effects by increasing the levels of dopamine, norepinephrine, and serotonin in plasma, while blocking their degradation [3]. Through a literature review, we found that in addition to its neurotoxic effects, METH, orally taken, also has caused severe bleeding and perforation, such as in inflammatory injury of the intestine, in several clinical cases [4–8]. However, there has been no literature on systematic experiments focused on inflammatory intestinal injury due to METH.
Pattern recognition receptors (PRRs), which can detect pathogens, microbes, and cellular stress in vivo and in vitro, are capable of inducing caspases and a series of proinflammatory factors to initiate adaptive inflammation responses to maintain homeostasis [9,10]. NOD-like receptors (NLRs), which are intracellular PRRs, have been discovered to be significant members of the PRRs, and NLRP3 is the most fully characterized NLR. NLRP3 can be combined with ASC (apoptosis associated speck-like protein containing activating and recruitment domain) and Caspase-1 to constitute a multiprotein complex, the NLRP3 inflammasome, which can induce several inflammatory injuries in different tissues and organs by activating and producing IL-1β and IL-18 [11,12]. Therefore, we investigated whether intestinal inflammatory injury caused by METH, if present, is related to overexpression of the NLRP3 inflammasome.
In this study, intestinal cell (IEC-6 cells) and animal models (C57BL/6 mice) of METH exposure were built to observe intestinal inflammatory injury, as both of them are similar to human intestinal tissues in histology and anatomy, and are commonly used in intestinal injury experiments [13–16]. Additionally, by detecting the NLRP3 inflammasome after inhibiting it with MCC950 (NLRP3 inflammasome inhibitor), we observed whether intestinal inflammatory injury due to METH depends on NLRP3 inflammasome overexpression.
Material and Methods
Chemicals
METH (99%) was provided by Yunnan Public Security Bureau, and its purity was tested in Yuxi Police Judicial Expertise Center. It was dissolved in 0.9% to a concentration of 10 mmol/l and then stored at −20°C.
MCC950 (C20H23N2O5S.Na, CP-456773) is an NLRP3 inhibitor that was purchased from Selleck Chemicals Cooperation (Selleck, China). It was dissolved in 0.9% sodium chloride (NaCl) to a concentration of 1 mmol/l and stored at −20°C.
Cells and animals
The intestinal epithelial cell line (IEC-6) was provided by the American Type Culture Collection (ATCC, USA) and cultured in Dulbecco’s modified Eagle’s medium (DMEM, Sigma-Aldrich, USA) supplemented with 5% foetal bovine serum (FBS, Corning, USA), 100 μg/ml bovine insulin (Sigma-Aldrich, USA), 100 U/ml penicillin (Sigma-Aldrich, USA), and 100 mg/ml streptomycin (Sigma-Aldrich, USA) at 37°C, 90% humidity and 5% carbon dioxide (CO2). IEC-6 cells were classified into 3 groups: control, METH, and METH+MCC950. The IEC-6 cells were plated in 6-well plates at a concentration of 1×. After 1 night, cells were added without METH (0.25 mmol/ml) and MCC950 (100 μmol/ml) for 2 h before METH (5 mg/kg) addition. After 36 h, cells were collected for experiments.
We used 8-week-old male C57BL/6 mice (Kunming Medical University, China) with an average body weight of 22–25 g, kept at 22±2°C and 30–70% relative humidity with room lighting for 12 h/12 h of light and dark (light, 7: 00–19: 00). All experiments were approved by the Animal Care and Use Committee of Kunming Medical University and performed according to the National Regulation of Experimental Animals of the Chinese Association for Laboratory Animal Sciences. Mice were separated into 4 groups – control, NS (normal saline), METH, and METH+MCC950 – and each group contained 10 mice. Mice received no injection, intraperitoneal injections of the same volume of NS, intraperitoneal injections of METH (5 mg/kg) for 8 days, or intraperitoneal injections of MCC950 (10 mg/kg) before METH (5 mg/kg) injection for 8 days. Subsequently, all the mice were sacrificed, and 2–3 cm of terminal ileum was collected for experiments.
Cell Counting Kit-8 Assay (CCK8)
The concentration of methamphetamine was tested based on cell viability, which was ascertained by Cell Counting Kit-8 assay (CCK-8, Sigma-Aldrich, USA). IEC-6 cells with a density of 1×cells/well in 100 μl of complete culture medium were cultured in 96-well plates. After the cells were cultured for 1 night, the medium was replaced with fresh media containing METH at concentrations ranging from 0 to 2 mmol/ml and then incubated in a humidified incubator at 37°C for 12, 24, 36, or 48 h. After incubation, 10 μl of CCK-8 was added to each well for 1 h at 37°C. The optical density (OD) was recorded at 450 nm using a microplate reader (Dojindo Molecular Technology, USA).
When the working concentration of METH was confirmed, MCC950 (NLRP3 inflammasome inhibitor) was ascertained using Cell Counting Kit-8 assay (CCK-8, Sigma Chemical Corporation) under the confirmed concentration of METH (0.5 mmol/ml) and time (36 h), ranging from 0 to 100 μmol/ml for 36 h. MCC950 was added 2 h before methamphetamine treatment.
Annexin V-FITC/PI staining
The apoptosis percentage was detected by flow cytometry using Annexin V-FITC/PI (BioLegend, USA) staining. The control, METH, and METH+MCC950 groups of IEC-6 cells were washed twice with PBS and centrifuged at approximately 1000×g at 4°C for 5 min. After being resuspended in 100 μl of binding buffer, the cells were placed on ice with 5 μl of Annexin V-FITC for 15 min. Finally, 1 μl of Propidine Iodide (PI) was added to the cells at ambient temperature for 2 min. A flow cytometer was used to assess the percentage of apoptotic cells.
Transepithelial electrical resistance (TEER) measurement
Intestinal inflammatory injury of tight junctions was detected by TEER measurement. The control, NS, METH, and METH+MCC950 IEC-6 cells were plated in a 24-well Transwell chamber, with 3 wells each. The culture medium was changed every 2 days for 1 week and then each day for 1 week. The resistance value of each well was measured each time by a Milli Cell ERS-2 (Millipore, USA). After 2 weeks, the resistance value was stable at nearly 200Ω.m2. Subsequently, we added the determined METH (0.5 mmol/ml) and MCC950 (100 μmol/ml) for 36 h. Next, we obtained the TEER=(sample resistance-blank resistance)×0.33Ω.m2 of the control, NS, METH, and METH+MCC950 groups.
Enzyme-linked immunosorbent assay (ELISA) of IL-6, TNF-α, NF-κB, and INF-γ
Proinflammatory factors were detected using an ELISA kit (Mmbio, China). Cell culture supernatants were collected and centrifuged for 20 min at approximately 1000×g. Mouse intestinal tissue was collected, ground, and then centrifuged for 5–10 min at nearly 500×g. Protein levels of IL-6, TNF-α, INF-γ, and NF-κB in culture supernatants and mouse intestinal tissues were ascertained using a commercial ELISA kit (Mmbio, China) following the manufacturer’s instructions.
Western blot analysis of NLRP3, Caspase-1, ASC, IL-1β, and IL-18
The NLRP3 inflammasome components (NLRP3, Caspase-1, and ASC) and IL-1β and IL-18 were detected by Western blot. Total protein was extracted from IEC-6 cells and mouse intestinal tissues using RIPA lysis buffer containing protease and phosphatase inhibitors. To produce cell lysates, the lysis solution was centrifuged at 10 000 rpm for 10 min. A bicinchoninic acid (BCA) assay was performed to evaluate the protein concentrations for each sample. Proteins were subsequently separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDSPAGE) and transferred to a polyvinylidene difluoride (PVDF) membrane (Millipore, USA). Immunoblotting was performed using an anti-NLRP3 antibody (rabbit; 1: 1000; Abcam, UK), anti-caspase-1 antibody (rabbit; 1: 1000; Abcam, UK), anti-ASC antibody (rabbit; 1: 1000; Abcam, UK), anti-IL-1 antibody (rabbit; 1: 1000; Abcam, UK), anti-IL-18 antibody (rabbit; 1: 1000; Abcam, UK), and anti-β actin antibody (rabbit; 1: 5000; Abcam, UK). β-actin was used as an internal standard.
Real-time quantitative PCR (qRT-PCR) for NLRP3, Caspase-1, ASC, IL-1β, and IL-18
The NLRP3 inflammasome components (NLRP3, Caspase-1, and ASC) and IL-1β and IL-18 were detected by qRT-PCR. Total RNA was extracted from the cultured IEC-6 cells and mouse intestinal tissues with TRIzol reagent and reversed transcribed into cDNA using a TaqMan microRNA Reverse Transcription Kit (Biosystems, USA) following the manufacturer’s instructions. The qRT-PCRs were performed using Fast Start Universal SYBR Green Master Mix (Roche Applied Science, GER) and a 7500 Real-Time system (Biosystems, USA) following the manufacturer’s protocols. Fold changes were calculated by the 2−ΔΔCt method. Primer sequences used were designed as follows:
NLRP3 forward, 5′-ATCAACAGGCGAGACCTCTG-3′;
NLRP3 reverse, 5′-GTCCTCCTGGCATACCATAGA-3′;
Caspase-1 forward, 5′-AATACAACCACTCGTACACGTC-3′;
Caspase-1 reverse, 5′-AGCTCCAACCCTCGGAGAAA-3′;
ASC forward, 5′-AATTCGGATCCAACGGCAGCAGG-3′;
ASC reverse, 5′-TCAAAAATTGTGTATACAAAGTC-3′;
IL-1β forward, 5′-GTCCTGATGAGAGCATCCAG-3′;
reverse: 5′-CGGGAAAGACACAGGTAGC-3′;
IL-18 forward, 5′-CCAGGCCCGTGCTAAAAATG-3′;
reverse: 5′-TGTAGGCATACTGGCAACAGG-3′;
β-actin forward, 5′-GAGGGAAATCGTGCGTGAC-3′; and
β-actin reverse, 5′-GCATCGGAACCGCTCATT-3′.
β-actin was used as the internal standard.
Histological analysis
Mouse intestinal tissues were maintained in 10% buffered formalin, embedded in paraffin, and stained with HE (hematoxylin and eosin) to assess the degree of inflammatory injury by Chui’s score. Histological scoring was ascertained separately and in a blinded way.
Data management and statistics
Data are denoted as the means±SEMs. Statistical significance was ascertained using one-way analysis of variance (ANOVA) followed by post hoc Bonferroni correction using SPSS Statistics 22 software. A p-value of less than 5% (p<0.05) was considered statistically significant.
Results
Determination of optimal concentrations of METH and MCC950
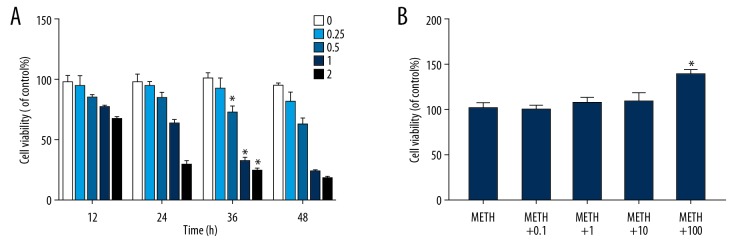
To determine the optimum concentration of METH and MCC950 for cells, we evaluated IEC-6 cells treated with METH concentrations ranging from 0 to 2 mmol/ml and incubated for 12, 24, 36, or 48 h and treated with MCC950 concentrations ranging from 0 to 100 μmol/ml for 36 h using a Cell Counting Kit-8 assay. With the increase in METH concentration and incubation time, the cell viability of METH-treated cells showed a downward trend, for which the optimum METH concentration was 0.5 mmol/ml and the optimum incubation time was 36 h (Figure 1A, P=0.000, P<0.05). After the MTH concentration and incubation time were determined, we found that the cell viability of METH+MCC950 cells increased slightly with increasing MCC950 concentration, with the optimum MCC950 concentration being 100 μmol/ml (Figure 1B, P=0.000, P<0.05).
Figure 1.
Determine the optimum concentration and incubated time of Methamphetamine and optimum concentration of MCC950. (A) Effects of varying Methamphetamine concentrations (0, 0.25, 0.5, 1 and 2 mmol/L) on IEC-6 cell viability for 12, 24, 36 and 48 h, * P<0.05 compared with that of non-METH group. (B) Effects of varying MCC950 concentrations (0, 0.1, 1, 10 and 100 μmol/L) combined with confirmed concentration of Methamphetamine(0.5 mmol/L) on IEC-6 cell viability in confirmed time (36 h). * P<0.05 compared with that of non-MCC950 group.
METH induces intestinal inflammatory injury
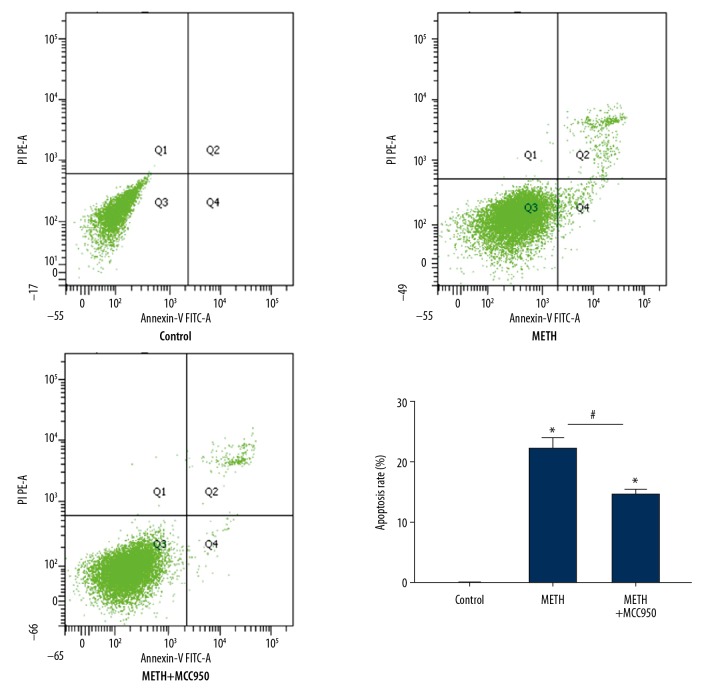
In the METH cell model, we detected apoptosis and TEER of IEC-6 cells induced by METH. We collected IEC-6 cells stained by Annexin V-FITC and detected them using flow cytometry. The apoptosis percentage of the METH group was evidently higher than that of the control group (Figure 2, P=0.000, P<0.05). We established single layers of IEC-6 cells in Transwell plates and detected TEER by a Milli cell ERS-2 (Millipore, USA) after adding METH. The TEER value in the METH group was sharply reduced compared with that in the control group (Figure 3, P=0.026, P<0.05).
Figure 2.
Methamphetamine induces necroptosis. Flow cytometry analysis of apoptosis by Annexin V-FITC/PI double staining in IEC-6 cells with each group (Control, METH and METH+MCC950). Cells in the right lower and right upper quadrant were considered as the early and late apoptotic cells, respectively. * P<0.05 compared with that of Control group. # P<0.05 compared with that of METH group.
Figure 3.
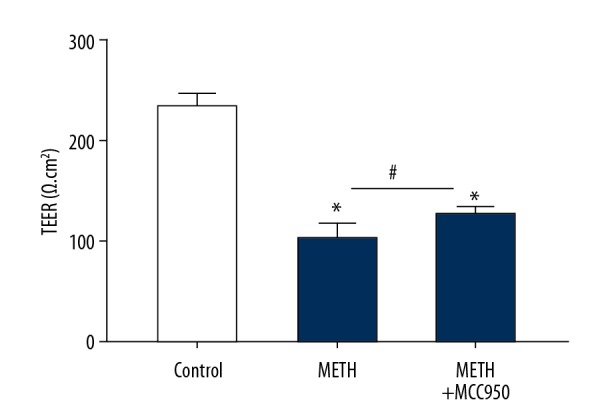
Methamphetamine induces IEC-6 cells’ descend of TEER. Milli cell ERS-2 detect TEER in IEC-6 cells with each group (Control, METH and METH+MCC950). * P<0.05 compared with that of Control group. # P<0.05 compared with that of METH group.
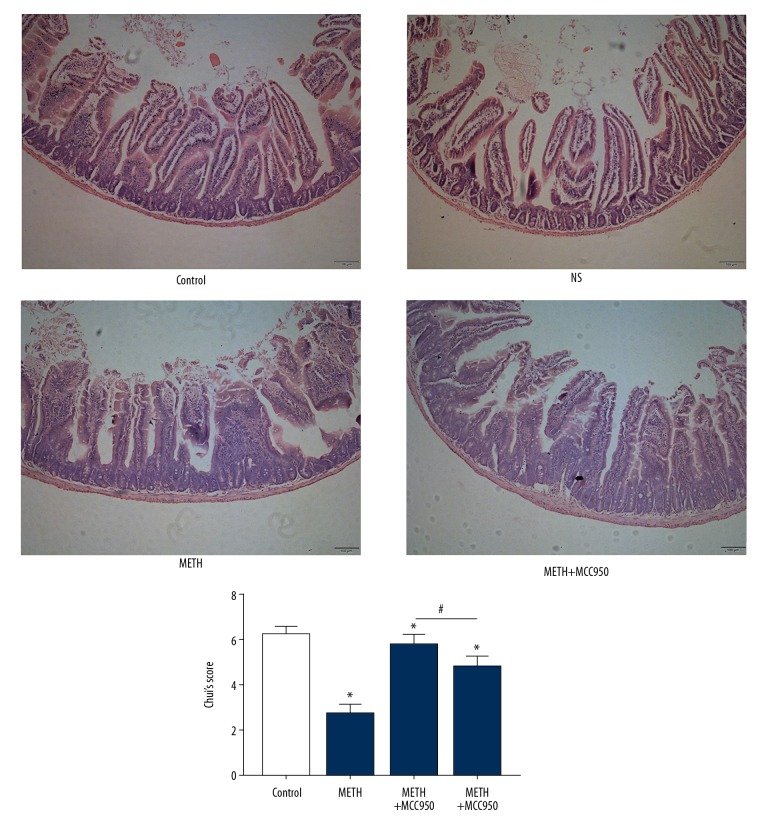
In the METH animal model, we observed pathological sections of HE-stained mouse intestinal tissues. We judged the degree of mouse intestinal tissue damage by Chui’s score (Table 1). We found more obvious intestinal injury signs in the METH group, with more inflammatory cells, obtuse intestinal villi, disintegration of the lamina propria, ulceration, and higher Chui’s score, than in the control group (Figure 4, P=0.000, P<0.05).
Table 1.
Chui’s score of intestinal injury.
| Sore | Standard |
|---|---|
| 1 | Normal intestinal villus |
| 2 | Interval under top submucosa membrane of intestinal villus, capillaries congestion |
| 3 | Interval under submucosa of intestinal villus amplifies, isolation between mucous and submucosa membrane |
| 4 | Isolation between mucous and submucosa membrane spread to side of intestinal villus |
| 5 | Obstuse intestinal villus, exposed vessels of lamina propria, infiltration of inflammatory cells |
| 6 | Disintegration of lamina propria, blood or ulceration |
Figure 4.
Methamphetamine induces Mice intestinal tissues’ injury. HE stained biopsy of Mice’s intestinal tissues of each group. The degree of inflammatory injury was determined by Chui’s score. ← was intestinal mucosa injury including isolation between mucous and submucosa membrane, infiltration of inflammatory cells, disintegration of lamina propria and so on * P<0.05 compared with that of Control group. # P<0.05 compared with that of METH group.
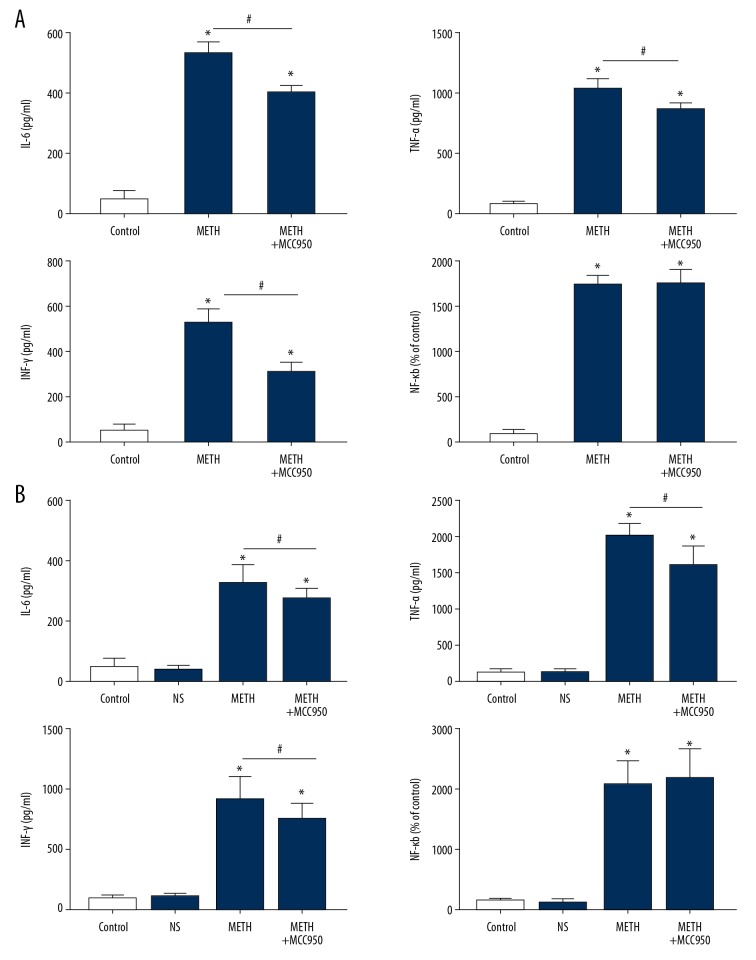
We collected IEC-6 supernatants and mouse intestinal tissue from both groups to detect the levels of IL-6, TNF-α, INF-γ, and NF-κB by ELISA. The levels of IL-6, TNF-α, and INF-γ were all increased obviously in the METH group compared with those in the control group (Figure 5A, 5B, P=0.000, P<0.05).
Figure 5.
Methamphetamine induce elevation of inflammatory factors. (A) ELISA of pro-inflammatory factors (IL-6, TNF-α, NF-κB and INF-γ) of IEC-6 cells of each group (Control, METH and METH+MCC950). (B) ELISA of pro-inflammatory factors (IL-1β, IL-18, IL-6, TNF-α, NF-κB and INF-γ) of mice intestinal tissues of each group (Control, NS, METH and METH+MCC950). * P<0.05 compared with that of Control group. # P<0.05 compared with that of METH group.
METH intestinal inflammatory injury depended on NLRP3 inflammasome overexpression and was partially alleviated after inhibition by MCC950
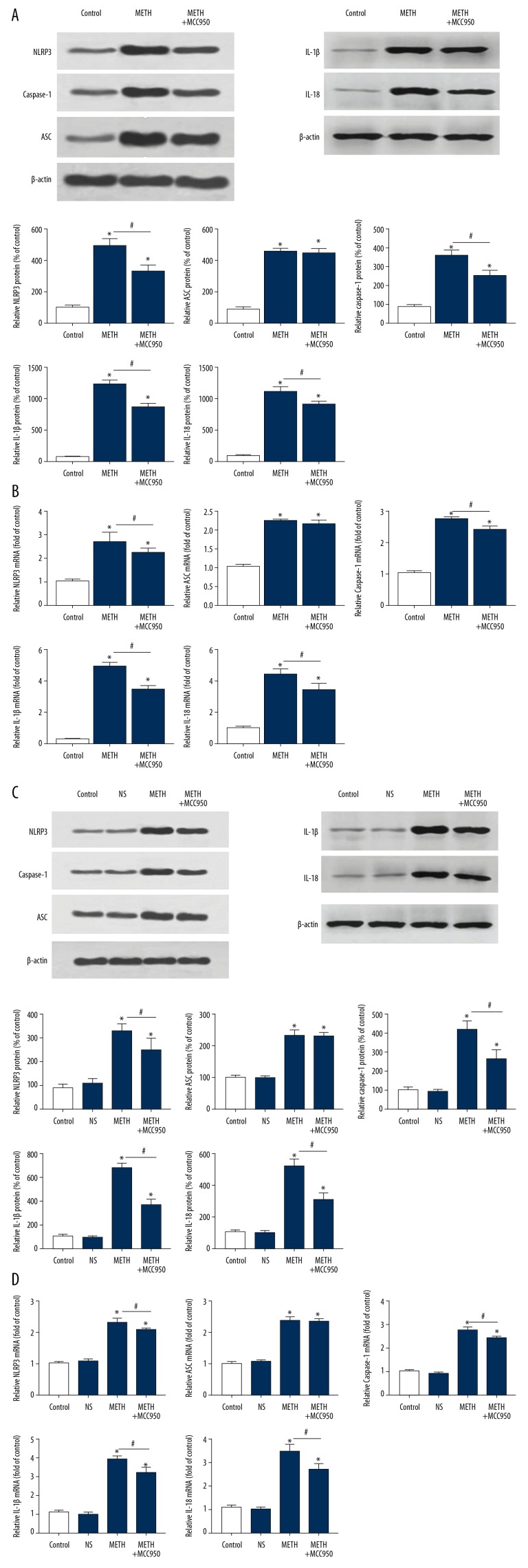
To detect the NLRP3 inflammasome, we collected IEC-6 cells and mouse intestinal tissues to ascertain both protein and mRNA levels of the NLRP3 inflammasome (NLRP3, Caspase-1 and ASC) and the direct proinflammatory factors (IL-1β and IL-18) activated by the NLRP3 inflammasome by Western blot and qRT-PCR (Figure 6A–6D, P=0.000, P<0.05). We detected cleaved Caspase-1 (P20), which is an active form of Caspase-1. Accompanied with intestinal inflammatory injury due to METH in both IEC-6 cells and mice, the level of the NLRP3 inflammasome components (NLRP3, Caspase-1 and ASC) was obviously upregulated in the METH group compared with that in the control group, as were the levels of the direct proinflammatory factors (IL-1β and IL-18), which were all obviously upregulated in the METH group compared with those in the control group. After inhibiting the NLRP3 inflammasome with MCC950, the levels of NLRP3, Caspase-1, and the direct proinflammatory factors (IL-1β and IL-18) were slightly downregulated in the METH+MCC950 group compared with those in the METH group.
Figure 6.
Methamphetamine induce elevation and MCC950 induce descend of NLRP3 inflammasome (NLRP3, Caspase-1 and ASC) and IL-1β, IL-18. (A) The Western Blot of NLRP3 inflammasome (NLRP3, Caspase-1 and ASC) and IL-1β, IL-18 of IEC-6 Cells of each group (Control, METH and METH+MCC950). (B) The q-PCR of NLRP3 inflammasome (NLRP3, Caspase-1 and ASC) and IL-1β, IL-18 of IEC-6 Cells of each group (Control, METH and METH+MCC950). (C) The Western Blot of NLRP3 inflammasome (NLRP3, Caspase-1 and ASC) and IL-1β, IL-18 of mice intestinal cells of each group (Control, NS, METH and METH+MCC950). (D) The q-PCR of NLRP3 inflammasome (NLRP3, Caspase-1 and ASC) and IL-1β, IL-18 of mice intestinal cells of each group (Control, NS, METH and METH+MCC950). β-actin was used as a loading control. * P<0.05 compared with that of Control group. # P<0.05 compared with that of METH group.
With inhibition of the NLRP3 inflammasome, we found that the TEER value was obviously increased in the METH+MCC950 group compared with that in the METH group (Figure 3, P=0.000, P<0.05), that there was obvious notable mitigation in pathological sections in the METH+MCC950 group compared with those in the METH group (Figure 4, P=0.000, P<0.05), and the levels of IL-6, TNF-α and INF-γ were downregulated (Figure 5A, 5B, P=0.000, P<0.05).
Inhibition of the NLRP3 inflammasome by MCC950 did not change the NF-κB level
After inhibiting the NLRP3 inflammasome with MCC950 in cells and mice, the level of the proinflammatory factor NF-κB did not change in the METH+MCC950 group compared with that in the METH group (Figure 5A, 5B, P=0.000, P<0.05).
MCC950 did not inhibit all components of the NLRP3 inflammasome
In inhibiting the NLRP3 inflammasome with MCC950 in cells and animals, we observed that the level of ASC, a constituent of the NLRP3 inflammasome, did not change in the METH+MCC950 group compared with that in the METH group (Figure 6A–6D, P=0.000, P<0.05).
Discussion
Generally, METH has been considered a neurotoxic drug, and previous studies of METH have focused mostly on the cerebral nervous system (CNS). However, when METH is taken via the most common route, the oral route, the bioavailability of METH can reach approximately 64–70% [2], and the small intestine, as the most important absorption section in the digestive system, can be easily damaged by METH. Attaran reported a case of fatal small intestinal ischemia and distal ileum perforation in a patient with a METH drug use history [17]. Carlson et al. reported another case of a paralytic ileus patient who was taking METH the night before [4]. Xiaojing Zou et al. reported another individual with a history of chronic METH abuse with abdominal pain and hematochezia caused by small intestinal infarction [5]. The above cases all indicate that METH can have detrimental effects on the digestive system, especially on the small intestine. However, there have been no previous systematic experiments to verify the intestinal inflammatory injury of METH. Therefore, we chose normal IEC-6 small intestinal cells and the distal ileal tissues of C57BL/6 mice, which are similar to human intestinal tissues in histology and anatomy, exposed to METH to observe whether METH can cause intestinal inflammatory injury. We did not observe the intestinal inflammatory injury of METH in human intestinal tissues due to ethical and legal concerns. In our METH exposure IEC-6 cell model, we found that METH can lead to IEC-6 cell inflammatory injury, with an elevation of apoptosis percentage and the levels of several proinflammatory factors (IL-6, TNF-α, INF-γ, and NF-κb) and a decrease in TEER. In our METH exposure mouse model, we found that METH can lead to obvious inflammatory injury in ileal tissues, as well as elevation of several proinflammatory factors (IL-6, TNF-α, INF-γ, and NF-κb).
PRRs are expressed in macrophages, monocytes, and epithelial cells, and have recognized pathogen-associated molecular patterns (PAMPs) and damage-associated molecular patterns (DAMPs). NLRP3, a significant member of the PRRs, combines with ASC and Caspase-1 to form a multiprotein called the NLRP3 inflammasome, which can then promote the maturation of Caspase-1 [7,18]. Mature Caspase-1 can finally promote the maturation and production of the proinflammatory factors IL-1β and IL-18, which leads to inflammatory injury in different organs [9,10,19]. NLRP3 has been verified to be widely expressed in the gastrointestinal tract and can be found in epithelial cells at mucosal sites, which is important in maintaining intestinal homeostasis [11]. However, overexpression of the NLRP3 inflammasome often causes severe inflammatory diseases, such as inflammatory bowel diseases (e.g., Crohn’s disease and ulcerative colitis) or enteroinvasive bacterial infections, and blockage of NLRP3 inflammasome activation can partially protect against inflammatory damage [12,20,21]. Therefore, we wondered whether METH caused this kind of intestinal inflammatory injury via overexpression of the NLRP3 inflammasome. In this study, IEC-6 cell changes and mouse intestinal inflammatory injury caused by METH depended on the elevation of NLRP3 inflammasome (NLRP3, ASC, and Caspase-1) levels. Because of the inhibition of the NLRP3 inflammasome by MCC950, the percentage of apoptosis, the relative level of proinflammatory factors (IL-6, TNF-α, and INF-γ, but not NF-κB), and the TEER of IEC cells and mice were evidently reduced, and the inflammatory injury in mouse intestinal tissues was obviously ameliorated. Therefore, the NLRP3 inflammasome plays a vital role in intestinal inflammatory injury caused by METH. In addition, we found that inhibiting the NLRP3 inflammasome did not affect the expression of NF-κB. It has been indicated that ATP, ROS, and K+ efflux can activate the NLRP3 inflammasome [22]. NF-κB has also been reported to activate and regulate the NLRP3 inflammasome. Bauernfeind et al. found that priming of the NLRP3 inflammasome was dose-dependently reduced by a specific inhibitor of NF-κB (Bay11-7082) and that LPS failed to induce NLRP3 or IL-1β in cells with NF-κB inhibition [23]. Different experiments have shown that blocking NF-κB can reduce the NLRP3 inflammasome level to ameliorate different inflammatory diseases [24–26]. However, the activation mechanisms of the NLRP3 inflammasome are intensely debated. Therefore, we assume that NF-κB may be important for NLRP3 inflammasome activation in intestinal inflammatory injury due to METH, and we will further pursue this hypothesis.
MCC950 (C20H23N2O5S.Na, CP-456773), a selective NLRP3 inflammasome inhibitor that blocks NLRP3-induced ASC oligomerization and canonical and non-canonical NLRP3 activation, can inhibit NLRP3 inflammasome synthesis to reduce IL-1β secretion and affect Caspase-1 processing [27]. Therefore, MCC950 has been proven to ameliorate inflammatory diseases caused by the NLRP3 inflammasome [28–30]. In our study, we found that MCC950 decreased levels of NLRP3, caspase-1, IL-1β, and IL-18, but not ASC levels. IL-1β and IL-18, which are regulated and directly activated by mainly the NLRP3 inflammasome, are IL-1 family members that can activate intracellular inflammatory signalling cascades and play instructive roles in driving both innate and adaptive inflammatory responses. Recent experiments even shown that IL-1β plays an important role in regulating TH17 cells and the activation of IL-17 secretion [31,32], which control the transport of dimeric immunoglobulin A and pentameric immunoglobulin M across the intestinal epithelium, which is essential for intestinal homeostasis [33]. Therefore, inhibiting the NLRP3 inflammasome can directly decrease the production of IL-1β and IL-18, indirectly decrease other proinflammatory factors, and finally ameliorate intestinal inflammatory injury. According to the present results, MCC950 cannot not affect ASC. ASC, as a link between NLRP3 and Caspase-1, can condense into a large speck once NLRP3 is activated, which can be inhibited by MCC950. Therefore, MCC950 cannot change the expression of ASC, which is consistent with our study [29]. However, some experiments also showed that MCC950 can also reduce the expression of ASC [34,35], but they indicated that decreased ASC expression might depend on different cell lines [29]; however, this hypothesis still needs additional experiments to prove.
Conclusions
We found that METH can cause severe intestinal inflammatory injury via NLRP3 inflammasome overexpression with an elevation of apoptosis percentage and the levels of several proinflammatory factors (IL-6, TNF-α, INF-γ, and NF-κb), a decrease in TEER, and obvious inflammatory injury in intestinal tissues, which can be alleviated by MCC950 (NLRP3, Caspase-1, except ASC). NF-κB, which remained unchanged after inhibiting by MCC950, may be essential for NLRP3 inflammasome activation in intestinal inflammatory injury due to METH, but this needs further verification.
Footnotes
Source of support: Departmental sources
Conflicts of interests
None.
References
- 1.Vearrier D, Greenberg MI, Miller SN, et al. Methamphetamine: History, pathophysiology, adverse health effects, current trends, and hazards associated with the clandestine manufacture of methamphetamine. Dis Mon. 2012;58:37–39. doi: 10.1016/j.disamonth.2011.09.004. [DOI] [PubMed] [Google Scholar]
- 2.Chomchaia C, Chomchaib S. Global patterns of methamphetamine use. Curr Opin Psychiatry. 2015;28(4):269–74. doi: 10.1097/YCO.0000000000000168. [DOI] [PubMed] [Google Scholar]
- 3.Attaran H. Fatal small intestinal ischemia due to methamphetamine intoxication: Report of a case with autopsy results. Acta Med Iran. 2017;5:344–47. [PubMed] [Google Scholar]
- 4.Carlson TL, Plackett TP, Gagliano RA, Jr, Smith RR. Methamphetamine-induced paralytic ileus. Hawaii J Med Public Health. 2012;71:44–45. [PMC free article] [PubMed] [Google Scholar]
- 5.Zou X, Huang H, Yang L, et al. Methamphetamine consumption and life-threatening abdominal complications: A case report. Medicine (Baltimore) 2018;97(18):e0647. doi: 10.1097/MD.0000000000010647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Takeuchi O, Akira S. Pattern recognition receptors and inflammation. Cell. 2015;140:805–20. doi: 10.1016/j.cell.2010.01.022. [DOI] [PubMed] [Google Scholar]
- 7.Kayagaki N, Warming S, Lamkanfi M, et al. Non-canonical inflammasome activation targets caspase-11. Nature. 2011;479:117–21. doi: 10.1038/nature10558. [DOI] [PubMed] [Google Scholar]
- 8.Abu-Elsaad N, El-Karef A. Protection against nonalcoholic steatohepatitis through targeting IL-18 and IL-1alpha by luteolin. Pharmacol Rep. 2019;15:688–94. doi: 10.1016/j.pharep.2019.03.009. [DOI] [PubMed] [Google Scholar]
- 9.Funayama H, Tashima I, Okada S, et al. Effects of zoledronate on local and systemic production of IL-1β, IL18, and TNF-α in mice and augmentation by lipopolysaccharide. Biol Pharm. 2019;42:929–36. doi: 10.1248/bpb.b18-00923. [DOI] [PubMed] [Google Scholar]
- 10.Zhu Q, Kannegant T-D. Distinct regulatory mechanisms control proinflammatory cytokines IL-18 and IL-1β. J Immunol. 2017;198:4210–15. doi: 10.4049/jimmunol.1700352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kummer JA, Broekhuizen R, Everett H, et al. Inflammasome components NALP 1 and 3 show distinct but separate expression profiles in human tissues suggesting a site-specific role in the inflammatory response. J Histochem Cytochem. 2007;55:443–52. doi: 10.1369/jhc.6A7101.2006. [DOI] [PubMed] [Google Scholar]
- 12.Villani AC, Lemire M, Fortin G, et al. Common variants in the NLRP3 region contribute to Crohn’s disease susceptibility. Nat Genet. 2009;41:71–76. doi: 10.1038/ng285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.He S, Guo Y, Zhao J, et al. Ferulic acid protects against heat stress-induced intestinal epithelial barrier dysfunction in IEC-6cells via the PI3K/Akt-mediated Nrf2/HO-1 signaling pathway. Int J Hyperthermia. 2019;35:112–21. doi: 10.1080/02656736.2018.1483534. [DOI] [PubMed] [Google Scholar]
- 14.Wong J, Garcia-Carbonell R, et al. RIPK1 mediates TNF-induced intestinal crypt apoptosis during chronic NF-κB activation. Cell Mol Gastroenterol Hepatol. 2019;19:30138–39. doi: 10.1016/j.jcmgh.2019.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Venkateswaran K, Shrivastava A, Agrawala PK, et al. Mitigation of radiation-induced gastro-intestinal injury by the polyphenolic acetate 7, 8-diacetoxy-4-methylthiocoumarin in mice. Sci Rep. 2019;9:14134. doi: 10.1038/s41598-019-50785-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cai B, Wan P, Chen H, et al. Composition characterization of oyster polysaccharides from Crassostrea hongkongensis and their protective effect against H2O2-induced oxidative damage in IEC-6 cells. Int J Biol Macromol. 2019;124:246–54. doi: 10.1016/j.ijbiomac.2018.11.154. [DOI] [PubMed] [Google Scholar]
- 17.Attaran H. Fatal small intestinal ischemia due to methamphetamine intoxication: Report of a case with autopsy results. Acta Med Iran. 2017;55:344–47. [PubMed] [Google Scholar]
- 18.Takeuchi O, Akira S. Pattern recognition receptors and inflammation. Cell. 2015;140:805–20. doi: 10.1016/j.cell.2010.01.022. [DOI] [PubMed] [Google Scholar]
- 19.Abu-Elsaad N, El-Karef A. Protection against nonalcoholic steatohepatitis through targeting IL-18 and IL-1alpha by luteolin. Pharmacol Rep. 2019;71:688–94. doi: 10.1016/j.pharep.2019.03.009. [DOI] [PubMed] [Google Scholar]
- 20.Dinarello CA. IL-1: Discoveries, controversies and future directions. Eur J Immunol. 2010;40:599–606. doi: 10.1002/eji.201040319. [DOI] [PubMed] [Google Scholar]
- 21.Maeda S, Hsu LC, Liu H, et al. Nod2 mutation in Crohn’s disease potentiates NF-kappaB activity and IL-1beta processing. Science. 2005;81:734–38. doi: 10.1126/science.1103685. [DOI] [PubMed] [Google Scholar]
- 22.Allen IC, Wilson JE, Schneider M, et al. NLRP12 suppresses colon inflammation and tumorigenesis through the negative regulation of noncanonical NF-kappaB signaling. J Immunol. 2012;36:742–54. doi: 10.1016/j.immuni.2012.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bauernfeind FG, Horvath G, Stutz A, et al. NF-κB activating pattern recognition and cytokine receptors license NLRP3 inflammasome activation by regulating NLRP3 expression. J Immunol. 2009;183:787–91. doi: 10.4049/jimmunol.0901363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Matias ML, Gomes VJ, Romao-Veiga M, et al. Downregulates the NF-κB pathway and NLRP1/NLRP3 inflammasomes in monocytes from pregnant women with preeclampsia. Molecules. 2019;19:24–28. doi: 10.3390/molecules24081548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Niu X, Yao Q, Li W, et al. Harmine mitigates LPS-induced acute kidney injury through inhibition of the TLR4-NF-κB/NLRP3inflammasome signalling pathway in mice. Eur J Pharmacol. 2019;849:160–69. doi: 10.1016/j.ejphar.2019.01.062. [DOI] [PubMed] [Google Scholar]
- 26.Yan Y, Lu K, Ye T, et al. MicroRNA 223 attenuates LPS induced inflammation in an acute lung injury model via the NLRP3inflammasome and TLR4/NF κB signaling pathway via RHOB. Int J Mol Med. 2019;43:1467–77. doi: 10.3892/ijmm.2019.4075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang Q, Wei LW, Xiao HQ, et al. Methamphetamine induces hepatotoxicity via inhibiting cell division, arresting cell cycle and activating apoptosis: In vivo and in vitro studies. Food Chem Toxicol. 2017;105:1061–72. doi: 10.1016/j.fct.2017.03.030. [DOI] [PubMed] [Google Scholar]
- 28.Guarda G, Zenger M, Yazdi AS, et al. Differential expression of NLRP3 among hematopoietic cells. J Immunol. 2011;186:2529–34. doi: 10.4049/jimmunol.1002720. [DOI] [PubMed] [Google Scholar]
- 29.Coll RC, Robertson AA, Chae JJ, et al. A small-molecule inhibitor of the NLRP3 inflammasome for the treatment of inflammatory diseases. Nat Med. 2015;21:248–55. doi: 10.1038/nm.3806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Primiano MJ, Lefker BA, Bowman MR, et al. Efficacy and pharmacology of the NLRP3 inflammasome inhibitor CP-456,773 (CRID3) in murine models of dermal and pulmonary inflammation. J Immunol. 2016;197:2421–33. doi: 10.4049/jimmunol.1600035. [DOI] [PubMed] [Google Scholar]
- 31.Shaw MH, Kamada N, Kim YG, Núñez G. Microbiota-induced IL-1, but not IL-6, is critical for the development of steady-state TH17 cells in the intestine. J Exp Med. 2012;209:251–58. doi: 10.1084/jem.20111703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Duan J, Chung H, Troy E, Kasper DL. Microbial colonization drives expansion of IL-1 receptor 1-expressing and IL-17-Producing gamma/delata T Cells. Cell Host Microbe. 2010;7:140–50. doi: 10.1016/j.chom.2010.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cao AT, Yao S, Gong B, et al. Th17 cells upregulate polymeric Ig receptor and intestinal IgA and contribute to intestinal homeostasis. J Immunol. 2012;189:4666–73. doi: 10.4049/jimmunol.1200955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kuwar R, Rolfe A, Di L, et al. A novel small molecular NLRP3 inflammasome inhibitor alleviates neuroinflammatory response following traumatic brain injury. J Neuroinflammation. 2019;16:81–85. doi: 10.1186/s12974-019-1471-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ismael S, Nasoohi S, Ishrat T. MCC950, the selective inhibitor of nucleotide oligomerization domain-like receptor protein-3 inflammasome, protects mice against traumatic brain injury. J Nerotrauma. 2018;35:1294–303. doi: 10.1089/neu.2017.5344. [DOI] [PMC free article] [PubMed] [Google Scholar]