Abstract
Background
This study aimed to discover the effect and mechanism of microRNA-27a-3p (miR-27a-3p) in epilepsy.
Material/Methods
To perform our investigation, in vivo and in vitro models of epilepsy were induced using kainic acid (KA). Expression of miR-27a-3p in the hippocampus of epileptic rats or normal rats or neuronal cells was detected using quantitative reverse transcription polymerase chain reaction (qRT-PCR). Racine score was used to assess seizures in epileptic rats. Cell viability and cell apoptosis were analyzed by 3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2-H-tetrazolium bromide (MTT) assay and flow cytometry. Enzyme-linked immunosorbent assay (ELISA) was performed to detect inflammatory factors expression.
Results
Significantly higher expression of miR-27a-3p in the hippocampus of epileptic rats and in KA-induced neurons was observed. We found that miR-27a-3p inhibitor alleviated seizures in epileptic rats. miR-27a-3p inhibitor also inhibited apoptosis of hippocampal neurons in epileptic rats, promoted Bcl2 expression, and decreased Bax and Caspase3 expression. The results showed that miR-27a-3p inhibitor effectively reduced the expression levels of interleukin-1β (IL-1β), IL-6, and tumor necrosis factor-α (TNF-α) in hippocampal tissues of epileptic rats. Dual luciferase reporter assay showed that mitogen-activated protein kinase 4 (MAP2K4) was a direct target of miR-27a-3p. miR-27a-3p inhibitor significantly promoted the cell viability of KA-induced neurons, inhibited cell apoptosis, promoted the expression of Bcl-2, and decreased Bax and Caspase3 expression, and all these changes were abolished by MAP2K4-siRNA co-transfection.
Conclusions
Our preliminary findings indicated that miR-27a-3p inhibitor protected against epilepsy-induced inflammatory response and hippocampal neuronal apoptosis by targeting MAP2K4.
MeSH Keywords: Apoptosis, Epilepsy, MicroRNAs, Neurons
Background
Epilepsy is a group of chronic heterogeneous neurological disorders due to the abnormally excessive or synchronized neuronal activity in the brain. Epilepsy predisposes an individual to epileptic seizures throughout life and causes serious social discrimination [1–3]. As reported by the World Health Organization (WHO) in 2001, neurological disorders account for 30.8% of all years of life lived with disability, and epilepsy accounts for about 0.5% of the total burden of diseases [4], with a global prevalence of 1.5–14 per 1000 population [5]. Repeated episodes of epilepsy not only cause serious damage to human health, but also cause physical and mental harm to patients. The pathogenesis of the disease has not been fully defined in previous studies, and the disease may be related to the induction of limbic structure and functional damage. Studies have shown that epilepsy involves pathological changes such as germination of mossy fibers and the characteristics of neuronal apoptosis and synaptic plasticity [6]. Epilepsy can lead to the apoptosis of nerve cells, which can be attributed to the activation of apoptosis-related proteases after epilepsy and the production of many free radicals and neurons, leading to cell apoptosis [7]. In recent years, although new anti-epileptic drugs have been developed, some patients are diagnosed as having intractable epilepsy (IE) [8]. The occurrence and development of epilepsy are related to the neuronal apoptosis of patients, so the development of drugs related to neuronal apoptosis in patients with epilepsy will become an important way to treat epilepsy [9]. It has been reported that epilepsy is associated with a cascade of cellular, molecular, and structural alterations, and the dynamic changes in the process of epileptogenesis are still challenges that need to be overcome [10]. Fortunately, many animal models have been proposed and become available for use in searching for the molecular mechanisms of epileptogenesis [11]. KA-induced epilepsy has been widely used in seizure research and in clinical and neuropathological studies of epilepsy [12]. Delayed treatment of KA-induced epilepsy will also result in hippocampal damage. The KA model is the best-characterized animal model of epilepsy for epileptic hippocampus injury research.
MicroRNAs (miRNAs) are small non-coding RNAs with regulatory functions, typically 18–23 nucleotides in length, that inhibit mRNA translation or direct target mRNA degradation [13]. miRNAs may be involved in the development and progression of nervous system diseases, and further research on this topic is needed. Studies have shown that miRNAs play a crucial role in the gene regulatory network of brain development and neural plasticity in humans [14]. miRNAs and their target genes in the hippocampus are now an important research focus. In the present study, we found that many miRNAs are expressed in the mammalian brain, and a large number of miRNAs play an important role in the occurrence and development of neurological diseases such as epilepsy, Parkinson’s disease, and stroke [15]. In addition, miRNA is closely related to the occurrence of epilepsy, and its regulation of gene levels may be involved in the formation of status epilepticus and its pathological process [16]. Agostini et al. showed that miR-27a regulates post-transcriptional lysosomal-associated membrane protein 2 (LAMP-2) protein expression and is a key regulator of autophagy during chronic cerebral perfusion [17]. miR-27a inhibits the abnormal proliferation of cells in esophageal squamous cell carcinoma by targeting Kirsten rat sarcoma viral oncogene homolog (KRAS) [18]. However, the role of miR-27a-3p in the development and progression of epilepsy has been less studied.
miRNA-related epigenetics research is growing in importance. The expression and function of brain-specific miRNAs in epilepsy persistence in TLE patients and mouse models are associated with neuronal development, inflammation, and death. However, the use of animal models to detect pro-apoptotic miRNAs has limited utility in studying the mechanism of epilepsy. In a rat model of status epilepticus (SE) [19], miR-27a had a mediating effect on neuronal death caused by seizures, and its mechanism remains to be further explored.
A reliable model for studying epileptic status is a rat model induced by kainic acid SE [16]. We established this model and detected a significant imbalance of miRNA expression in rat hippocampus. In previous studies, miR-27a was found to be upregulated [17], and the present study found that miR-27a is upregulated and induces related neuronal death in the rat hippocampus after status epilepticus. The present study shows that targeting miR-27a offers possible disease-modifying effects in experimental epilepsy and anticonvulsant therapy, which may contribute to the pre-clinical development of an miR-27a inhibitor as a treatment for epilepsy.
Material and Methods
In vivo model establishment
Male Sprague-Dawley (SD) rats (120–140 g, 4–6 weeks old) were supplied by the Qinglong Mountain Breeding Farm (Nanjing, Jiangsu, Permit Number: SCXK(SU)2017-0001) and stored in a specific pathogen-free experimental room (12 h light/dark cycle, (23±2)°C, 50–70% humidity) with free access to food and water.
Epilepsy was induced in rats by intraperitoneal injection of kainic acid. After acclimatization for 7 days, the SD rats were randomly divided into 4 groups with 8 rats each [20] as follows: (1) the control group received intraperitoneal (i.p.) injection of 40 μl/day saline only; (2) the model group received i.p. injection of KA (8.5 mg/kg/day; King Dom Co., Taipei) only; (3) the model+inhibitor control group received i.p. injection of 40 μl/day inhibitor control, 15 min prior to the injection of KA; and (4) the model+miR-27a-3p inhibitor group received the same treatment used in the model+inhibitor control group, but miR-27a-3p inhibitor (40 μl/day) was injected instead of inhibitor control. The animal experiment lasted 7 days.
In this experiment, the severity of convulsions was assessed by the Racine scale, which only includes animals rated 4–5. SE is continuous systemic epilepsy with a duration of not less than 40 min. No seizures were found during the experiment, and intraperitoneal sodium alginate was administered (8.5 mg/kg) once every 30 min, or the seizure activity of the animal was judged to be less than 4 points. The method of terminating seizures was to intraperitoneally inject 10% chloral hydrate (3 ml/kg) into all SE rats. Control rats were injected with an equal amount of physiological saline. All experimental rats had the same living environment and we continuously observed the animal behavior from the time of successful establishment of the SE model to the time of animal death. During the experiment, the state of the epileptic rat was assessed by video recording or directly observing the state of epileptic seizures after 90 episodes. Rats with partial seizures were confirmed by electroencephalogram (EEG) recordings showing high-frequency, high-amplitude, multimodal burst paroxysmal discharges. The animals were killed within 5 h of the occurrence of the last spontaneous seizure, and serum samples were collected from the orbital plexus by using ice-packed tubes. The h hippocampus was removed, separated, and immediately stored at −80°C until analysis. In this experiment, animals were anesthetized by intraperitoneal injection of 10% chloral hydrate (5 ml/kg) and quickly decapitated. Hippocampus tissues were immediately removed from the rat brain and stored in liquid nitrogen for cryopreservation. Other anesthetized animals were first perfused with saline and subsequently perfused with 4% paraformaldehyde.
All animal experiments were reviewed and approved by the Institutional Animal Use and Care Committee of the Brain Hospital of Hunan Province and conformed to the National Institute of Health guidelines on the ethical use of animals. All experiments are designed to alleviate animal suffering.
Isolation and culture of primary rat hippocampal neurons
Hippocampal neuron cells were isolated from rats from the CN, KA, IC, and MI groups by collagenase digestion of hippocampus tissues and cultured in a monolayer as previously described. First-passage neurons were used in the experiments. Human renal epithelial cell line 293T cells were purchased from the American Type Culture Collection (ATCC, Manassas, VA, USA) and grown in Dulbecco’s modified Eagle’s medium (DMEM) with fetal bovine serum (FBS) (37°C, 5% CO2).
Luciferase reporter assay
The binding sites between miR-27a-3p and MAP2K4 were predicted by TargetScan using luciferase reporter assay. The pGV306 luciferase reporter plasmid containing the wild-type (WT) or mutant (MUT) MAP2K4 3′UTR was co-transfected into human renal epithelial cell line 293T cells with the miR-27a-3p mimic or mimic control using Lipofectamine 2000 (Invitrogen). Finally, 48 h after cell transfection, the luciferase reporter gene activity was determined by using a dual luciferase assay system.
Transfection
Hippocampal neuronal cells were seeded in 24-well plates (1×106 cells/well) and cultured for 24 h, and then inhibitor control, miR-27a-3p inhibitor, control-siRNA, miR-27a-3p inhibitor+MAP2K4-siRNA, or MAP2K4-siRNA was transfected into the hippocampal neuronal cells using Lipofectamine 2000 reagent (Invitrogen) following the manufacturer’s instructions. After transfection of hippocampal neuronal cells for 48 h, the hippocampal neuronal cells were treated with kainic acid (50 μm) for 24 h before subsequent studies.
qRT-PCR
First, total RNA in hippocampal tissues or hippocampal neuronal cells was extracted by using Trizol reagent (Invitrogen, Carlsbad, CA, USA). Reverse transcription of total RNA into cDNAs was performed using the Reverse Transcription Kit. The final step was qPCR conduction by using Platinum SYBR Green qPCR Super Mix UDG (Invitrogen, USA). The relative expression levels of the genes were calculated by the 2−ΔΔCt method. U6 for miRNA and GAPDH for mRNA were used as the internal controls.
Flow cytometry analysis
An annexin V-fluorescein isothiocyanate (FITC)/propidium iodide (PI) apoptosis detection kit (Cat no. 70-AP101-100; MultiSciences, Hangzhou, China) was used to evaluate the apoptotic rate of neuronal cells. After 48 h of transfection, neurons were treated with kainic acid (50 μm) for 24 h, and then the cells were collected, washed with PBS, and suspended with 5 μl Annexin V-FITC and 5 μl PI for 30 min in the dark at room temperature. A flow cytometer (BD Biosciences) was used to analyze cell apoptosis, and the cell apoptotic rate was evaluated.
Western blot assay
Proteins from tissues and cells were extracted using RIPA buffer (Beyotime Institute of Biotechnology) with a protease/phosphatase inhibitor cocktail (Cell Signaling Technology). The protein concentrations were detected using a bicinchoninic acid assay kit (BCA; Pierce Biotechnology, Rockford, IL, USA). In the experiment, 30 μg of the protein sample was separated from 12% SDS-PAGE and then transferred to a corresponding polyvinylidene fluoride (PVDF) membrane (Merck Millipore, Billerica, MA). The membrane was blocked with 5% skim milk powder for 1 h at room temperature, followed by incubation with the primary antibodies at 4°C overnight. The second antibody was then incubated with the membrane for another 2 h at room temperature. All bands were visualized using ECL Western blotting detection kits (Millipore). Image J 1.38X software was used to quantify the densitometry of the bands.
ELISA test
After all hippocampal tissue samples were harvested, the levels of IL-1β, IL-6, and TNF-α were measured with ELISA kits (USCN Life Science Co., Wuhan, China) according to the manufacturer’s protocol.
MTT assay
Cell viability was determined using MTT assay. After treatment with kainic acid, hippocampal neuronal cells (1×104 cells/per well) were seeded in 96-well plates and cultured for 24 h. Subsequently, 20 μl of a 0.5-mg/ml MTT solution (Sigma-Aldrich Co., St Louis, MO, USA) was added to the experimental wells in a 96-well plate, followed by incubation of the 96-well plates at 37° C for 4 h. Then, the medium in each well was discarded and 150 μl of dimethyl sulfoxide (DMSO) was added and reacted for 30 min. The absorbance at 570 nm was measured using a FLUOstar® Omega microplate reader.
Statistical analysis
Experimental data were statistically analyzed by SPSS 18.0 (Chicago, IL, USA) software. Differences between groups were analyzed by one-way analysis of variance or t test. Data are expressed as mean ±SD. p<0.05 was considered to be statistically significant.
Results
miR-27a-3p expression in epileptic rats
The qRT-PCR method was used to assess the expression level of miR-27a-3p in a rat model of epilepsy. As shown in Figure 1, the level of miR-27a-3p in the epileptic rat model group was significantly higher relative to the control group.
Figure 1.
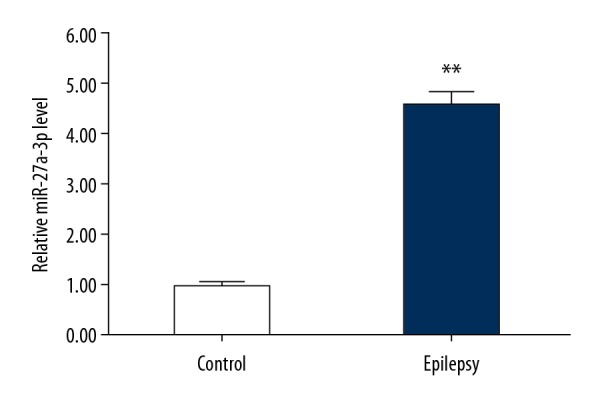
Expression of miR-27a-3p in epileptic rats. Relative miR-27a-3p expression in hippocampus of rats from model group (Epilepsy) or control group (Control) was detected using qRT-PCR. ** p<0.01 vs. Control group.
miR-27a-3p inhibitor relieved epileptic seizures in rats with epilepsy
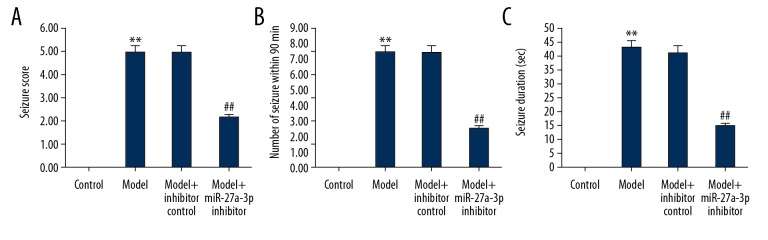
Racine scores were used to assess behavioral seizure performance in animals. As shown in Figure 2, the behavioral seizure response and duration of the epileptic rat model group were significantly higher relative to the control group (Figure 2A–2C). However, the seizure score, the number of seizures in 90 min, and the duration of seizures of epileptic rats were significantly reduced by miR-27a-3p inhibitor treatment.
Figure 2.
Effect of miR-27a-3p inhibitor on epilepsy rats. (A) Racine scores of behavioral seizure performance in animals. (B, C) Behavioral seizure response and duration of epileptic rats, respectively. ** p<0.01 vs. control group; ## p<0.01 vs. model group.
miR-27a-3p inhibitor inhibited apoptosis of hippocampal neurons in epileptic rats
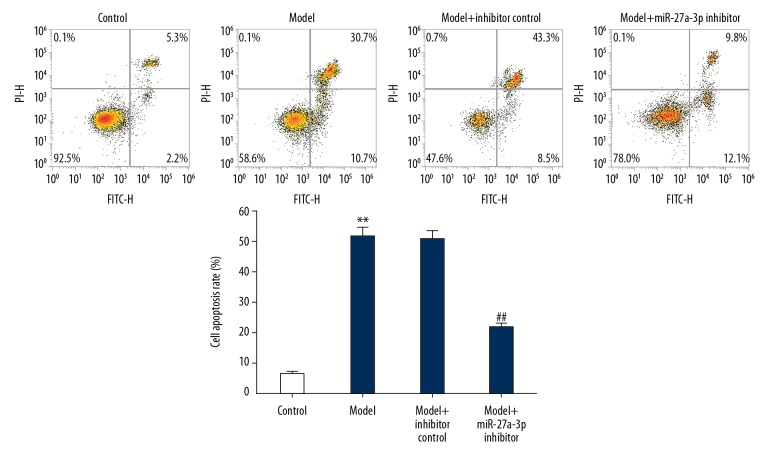
To investigate the effects of miR-27a-3p on cell death of nerve cells in epileptic rats, flow cytometry was performed. Apoptosis of hippocampus neurons was significantly higher in the model group compared with the control group. As expected, miR-27a-3p inhibitor significantly reduced neuronal apoptosis in the hippocampus of rats with epilepsy (Figure 3).
Figure 3.
Effect of miR-27a-3p inhibitor on hippocampal neuron cell apoptosis in epileptic rats. Apoptosis and expression of apoptotic cells in rat hippocampal neurons were assessed by FCM. ** p<0.01 vs. control group; ## p<0.01 vs. model group.
miR-27a-3p inhibitor inhibited inflammatory response in rats with epilepsy
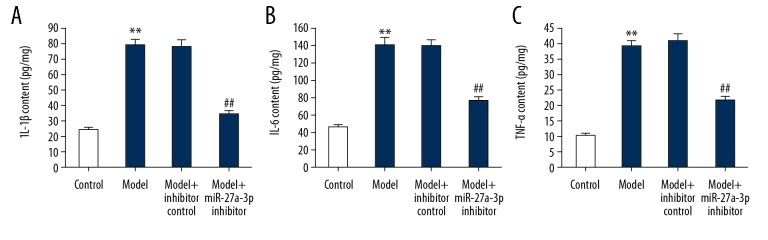
Statistical results (Figure 4A–4C) showed that compared with the control group, the inflammatory factors of IL-1β, IL-6, and TNF-α in hippocampal tissues of rats from the model group were significantly increased. Compared with the model group, miR-27a-3p inhibitor significantly reduced the levels of IL-1β, IL-6, and TNF-α in the hippocampal tissues of epileptic rats. These results indicate that miR-27a-3p plays an important role in the pathogenesis of epilepsy, and its mechanism of action was then studied at the cellular and molecular levels.
Figure 4.
Effect of miR-27a-3p inhibitor on inflammatory response in epileptic rats. The levels of IL-1β (A), IL-6 (B), and TNF-α (C) in hippocampal tissues of rats were assessed by ELISA. ** p<0.01 vs. control group; ## p<0.01 vs. model group.
miR-27a-3p was significantly upregulated in KA-induced neuronal cells
We then assessed the expression of miR-27a-3p in KA-induced neuronal cells using qRT-PCR, showing that the level of miR-27a-3p was significantly upregulated in KA-induced neuronal cells (Figure 5).
Figure 5.
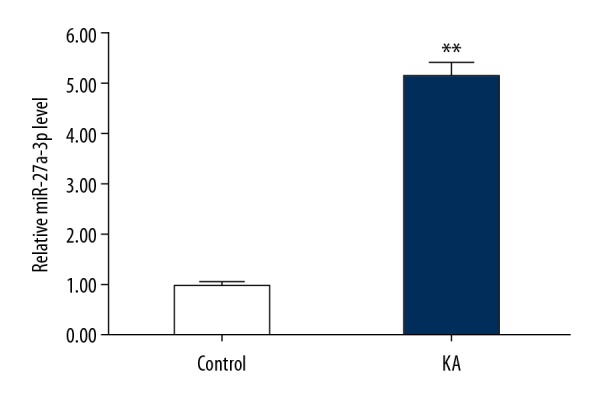
The expression of miR-27a-3p in kainic acid-treated neurons. Neurons were treated with (KA) or without (Control) 50 μm kainic acid for 24 h, then the relative miR-27a-3p expression in neurons was detected using qRT-PCR. Data are displayed as mean ±SD. ** p<0.01 vs. control group.
MAP2K4 was a direct target of miR-27a-3p
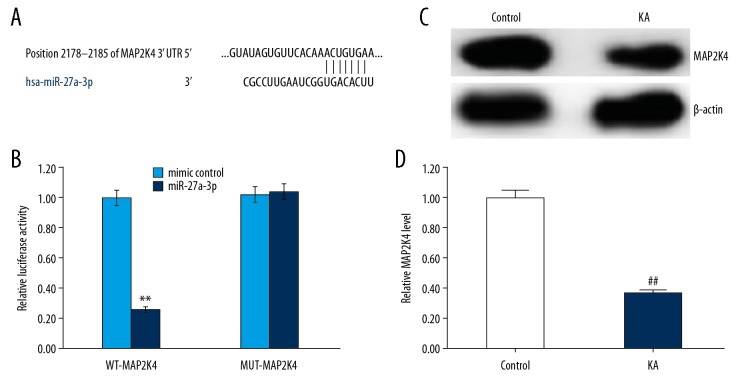
Potential target sites for miR-27a-3p were predicted by the bioinformatics tool TargetScan (http://www.targetscan.org/vert_72/), indicating the binding sites between miR-27a-3p and MAP2K4 (Figure 6A). Subsequently, to confirm whether miR-27a-3p directly modulated MAP2K4 expression via interaction with potential binding sites, luciferase reporter assay was performed using 293T cells transfected with vectors harboring the wild-type or mutated 3′-UTR of MAP2K4, in the presence or absence of the miR-27a-3p mimic or mimic control. As shown in Figure 6B, compared with cells co-transfected with MAP2K4-WT and mimic control, luciferase activity was markedly decreased by co-transfection with MAP2K4-WT and miR-27a-3p mimic, but no significant differences in luciferase activity were observed in cells co-transfected with MAP2K4-MUT and mimic control and co-transfected with MAP2K4-MUT and miR-27a-3p mimic. These results indicated that MAP2K4 was a direct target of miR-27a-3p. We also found that the level of MAP2K4 was significantly lower in KA-induced neuronal cells (Figure 6C, 6D).
Figure 6.
MAP2K4 is a target of miR-27a-3p and MAP2K4 expression in kainic acid-treated neurons. (A) Interaction between miR-27a-3p and 3′UTR of MAP2K4 was predicted using microRNA target site prediction software; (B) Luciferase activity of a reporter containing a wild-type MAP2K4 3′UTR or a mutant MAP2K4 3′ UTR are presented. “MAP2K4-MUT” indicates the MAP2K4 3′ UTR with a mutation in the miR-27a-3p binding site. UTR, untranslated region. (C, D) Neurons were treated with (KA) or without (Control) 50 μm kainic acid for 24 h, then the mRNA and protein expression of MAP2K4 in neurons was detected using qRT-PCR and Western blotting, respectively. Data are displayed as mean ±SD. ** p<0.01 vs. mimic control; ## p<0.01 vs. control group.
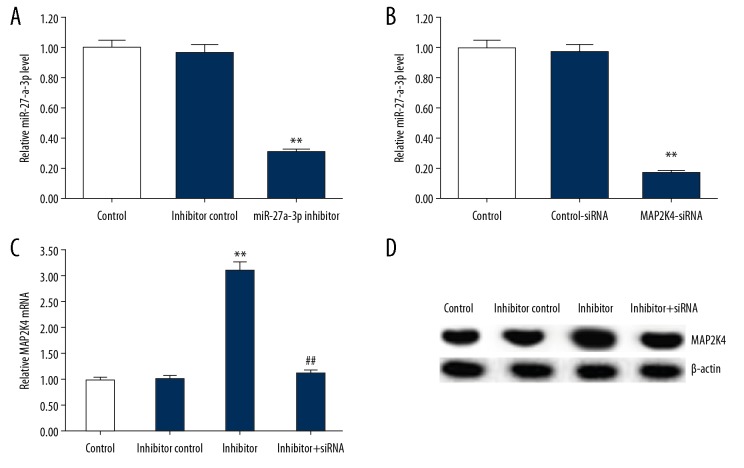
miR-27a-3p inhibitor positively regulated MAP2K4 expression in neuronal cells
To further investigate the impact of miR-27a-3p on MAP2K4 expression in neuron cells, the cells were transfected with inhibitor control, miR-27a-3p inhibitor, control-siRNA, MAP2K4-siRNA, or miR-27a-3p inhibitor+MAP2K4-siRNA for 48 h. As shown in Figure 7A, the miR-27a-3p inhibitor significantly reduced the level of miR-27a-3p in neuronal cells relative to the control group, and MAP2K4-siRNA significantly reduced the mRNA expression of MAP2K4 in neuronal cells relative to the control group (Figure 7B). In addition, miR-27a-3p inhibitors significantly increased the mRNA and protein expression of MAP2K4 in neuronal cells, and this effect was reversed by MAP2K4-siRNA (Figure 7C, 7D).
Figure 7.
Effect of miR-27a-3p inhibitor on the expression of MAP2K4 in neurons. (A) After transfection with inhibitor control or miR-27a-3p inhibitor for 48 h, the relative miR-27a-3p expression in neurons was detected using qRT-PCR. (B) After transfection with control-siRNA or MAP2K4-siRNA for 48 h, the mRNA expression of MAP2K4 in neurons was detected using qRT-PCR. (C, D) After transfection with inhibitor control, miR-27a-3p inhibitor, or miR-27a-3p inhibitor+MAP2K4-siRNA for 48 h, the mRNA and protein expression of MAP2K4 in neurons was detected using qRT-PCR and Western blotting, respectively. All data are presented as the mean ±SD of 3 independent experiments. ** p<0.01 vs. control group; ## p<0.01 vs. inhibitor group.
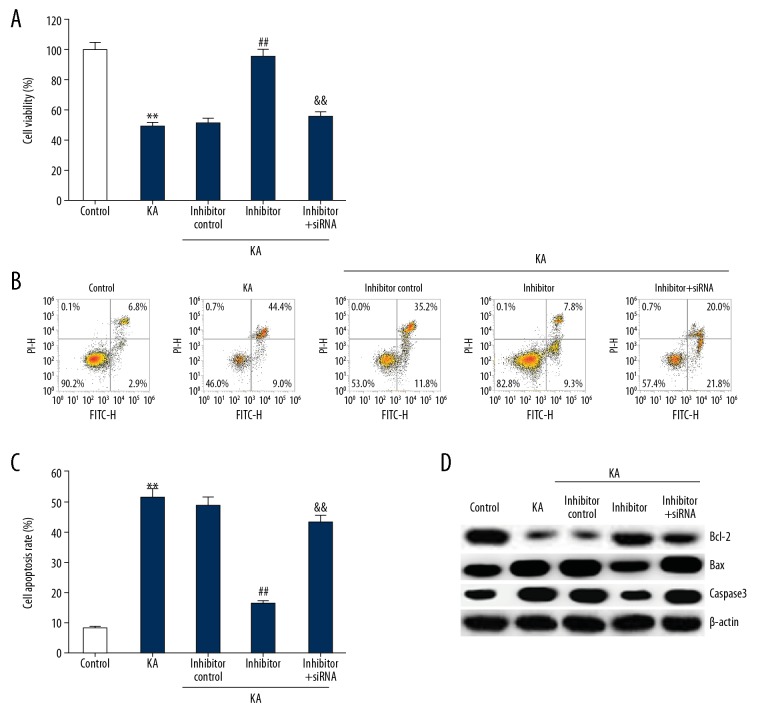
miRNA-27a-3p inhibitor promoted cell viability of KA-induced neuronal cells
MTT assay was performed to assess the viability of neuronal cells. Compared with the control group, KA significantly reduced the viability of neuronal cells, while miR-27a-3p inhibitor significantly improved the viability of neuronal cells, which was eliminated by MAP2K4-siRNA co-transfection (Figure 8A).
Figure 8.
Effect of miR-27a-3p inhibitor on KA-induced neuron cell viability and apoptosis. After transfection with inhibitor control, miR-27a-3p inhibitor, or miR-27a-3p inhibitor+MAP2K4-siRNA for 48 h, neurons were treated with KA for 24 h, then cell viability was analyzed using MTT assay (A); cell apoptosis was determined using FCM (B, C); Western blotting detection of protein levels of Bcl-2, Bax, and Caspase3 (D). ** p<0.01 vs. control group; ## p<0.01 vs. KA group; && p<0.01 vs. inhibitor group.
miR-27a-3p inhibitor inhibited apoptosis of KA-induced neuronal cells
Neuronal apoptosis was assessed by flow cytometry. Bcl-2, Bax and Caspase3 protein levels were assessed by Western blotting. As shown in Figure 8B–8D, KA significantly promoted neuronal cell apoptosis, reduced the expression of Bcl-2, and increased the expression of Bax and Caspase3, compared to the control group. miR-27a-3p inhibitor significantly inhibited cell apoptosis, increased Bcl-2 protein level, and reduced Bax and Caspase3 protein expression (Figure 8B–8D). All these changes were eliminated by MAP2K4-siRNA co-transfection.
Discussion
The most important feature of chronic brain disease epilepsy is the recurrent transient disease caused by abnormal nerve discharge in the brain [9]. Seizures are not the main cause of damage in the disease, but also include damage to memory, intelligence, language, and ability to act, and also increase economic and psychological pressure on society and patients with epilepsy [21]. However, the pathogenesis of epilepsy is still unclear, and in recent years, although the diagnosis and treatment of epilepsy has made great progress [22–24], the effect is still not satisfactory. Therefore, the main challenges are the discovery of new anti-epileptic drugs and elucidating the molecular mechanism underlying the onset of epilepsy.
Neuronal apoptosis and inflammatory response play critical roles in the development of epilepsy [6,25]. Chronic inflammation is a common component of hippocampal sclerosis-associated temporal lobe epilepsy [26]. miRNA is a novel regulator of the pathogenesis of temporal lobe epilepsy, which can have important effects on neuronal apoptosis, as well as affecting immune and inflammatory processes [15].
In this study, the relationship between epilepsy and miR-27a-3p was explored by establishing a rat model of epilepsy and by treatment with miR-27a-3p inhibitor. We found that miR-27a-3p was significantly upregulated in hippocampal expression of epileptic rats. miR-27a-3p inhibitor relieved epileptic seizures in rats with epilepsy. In addition, miR-27a-3p inhibitor inhibited apoptosis of hippocampal neurons in epileptic rats, and inhibited inflammatory response, as evidenced by reduced levels of IL-1β, IL-6, and TNF-α in serum of epileptic rats.
The occurrence and development of epilepsy has a regulatory effect on gene expression and protein translation, and re-constructs the hippocampal network [27,28]. miRNAs have been reported to be post-transcriptional regulators of the pathogenesis of epilepsy [28,29].
Dual luciferase reporter gene test results verified that MAP2K4 is the target gene of miR-27a-3p. MAP2K4, a type of early gene associated with changes in neuronal activity, contains neuronal structures and functions in advanced animal nervous systems [30]. It is worth noting that MAP2K4 is significantly expressed in the neuron population during the development and progression of status epilepticus [31]. In our study, the results suggested that MAP2K4 was downregulated in KA-induced hippocampal neuronal cells. The effects of miR-27a-3p downregulation on KA-induced hippocampal neuronal cells were reversed by MAP2K4 silencing.
This study further validates the possible underlying mechanisms by which miR-27a regulates downstream targets. It has been reported that miR-27a-3p promotes apoptosis of tumor cells. There are few relevant studies on proteins, so we further analyzed proteins such as Bcl-2, caspase-3, and Bax. Among them, the Bcl-2 family is a group of proteins that may have the ability to promote or prevent apoptosis [32]. Changes in the expression ratio of Bax to Bcl-2 can activate caspase-3-like proteases in hippocampal neurons, leading to cell death [33]. Among them, Bcl-2 and Bax have the effects of preventing and promoting apoptosis, respectively [34,35]. In addition, long-term seizures can activate caspase and bcl-2 family proteins in animals [36]. It is worth noting that acute epilepsy can cause ischemia, hypoxia, and hippocampal edema in the body; activate caspase protein; induce excitatory amino acids in cells; and then generate free radicals and nitric oxide synthase to degrade or inactivate cells. The skeleton eventually leads to the selective death of a large number of hippocampal neurons, which is also the basis for recurrent seizures [37]. miR-27a-3p is an epigenetic factor that plays an important role in the regulation of apoptosis-related genes such as caspase and Bcl-2. The experimental results in the present study indicated that proapoptotic miR-27a-3p is associated with caspase-3 expression levels. After miR-27a-3p inhibitor treatment of rat hippocampus after epilepticus, seizure-induced neuronal death was not significant. The reason for neuroprotection was that the inhibition of Bcl-2 translation was weak due to the decreased expression of miR-27a-3p, which led to a decrease in the expression level of caspase-3 protein, followed by a decrease in neuronal death caused by seizures.
In summary, our research showed for the first time that miR-27a-3p was upregulated in rats with epilepsy and in KA-induced neuronal cells. Downregulation of miR-27a-3p might inhibit the occurrence and development of epileptic seizures by inhibition of hippocampal neuronal apoptosis. These findings strongly suggest that miR-27a-3p/MAP2K4 are bio-markers of epilepsy and might be promising therapeutic targets for the treatment of patients diagnosed with epilepsy. However, this was only a preliminary study of the role of miR-27a-3p in epilepsy. To further define the role of miR-27a-3p in epilepsy, more in-depth research is needed. For example, the effect of miR-27a-3p upregulation on epileptic rats and KA-induced neuronal cells should be investigated. The expression of MAP2K4 in epileptic rats and its role in epileptic rats and KA-induced neuronal cells need to be explored. In addition, the relationship between the expression of miR-27a-3p/MAP2K4 and the clinical features of patients with epilepsy need further study. We intend to explore these topics in future research.
Conclusions
miR-27a-3p downregulation inhibited the occurrence and development of epileptic seizures of hippocampus and temporal epilepsies by inhibition of hippocampal neuronal apoptosis and inflammatory response.
Footnotes
Source of support: Departmental sources
Conflict of interests
None.
References
- 1.Espinola-Nadurille M, Crail-Melendez D, Sanchez-Guzman MA. Stigma experience of people with epilepsy in Mexico and views of health care providers. Epilepsy Behav. 2014;32:162–69. doi: 10.1016/j.yebeh.2013.12.007. [DOI] [PubMed] [Google Scholar]
- 2.Kanner AM, Meador KJ. Remember: There is more to epilepsy than seizures! Neurology. 2015;85:1094–95. doi: 10.1212/WNL.0000000000001985. [DOI] [PubMed] [Google Scholar]
- 3.Fisher RS, Acevedo C, Arzimanoglou A, et al. ILAE official report: A practical clinical definition of epilepsy. Epilepsia. 2014;55:475–82. doi: 10.1111/epi.12550. [DOI] [PubMed] [Google Scholar]
- 4.Matin N, Tabatabaie O, Falsaperla R, et al. Epilepsy and innate immune system: A possible immunogenic predisposition and related therapeutic implications. Hum Vaccin Immunother. 2015;11:2021–29. doi: 10.1080/21645515.2015.1034921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mac TL, Tran DS, Quet F, et al. Epidemiology, aetiology, and clinical management of epilepsy in Asia: A systematic review. Lancet Neurol. 2007;6:533–43. doi: 10.1016/S1474-4422(07)70127-8. [DOI] [PubMed] [Google Scholar]
- 6.Peng WF, Wang X, Hong Z, et al. The anti-depression effect of Xylaria nigripes in patients with epilepsy: A multicenter randomized double-blind study. Seizure. 2015;29:26–33. doi: 10.1016/j.seizure.2015.03.014. [DOI] [PubMed] [Google Scholar]
- 7.Schröder J, Brückner K, Fischer A, et al. Efficacy of a psychological online intervention for depression in people with epilepsy: A randomized controlled trial. Epilepsia. 2014;55:2069–76. doi: 10.1111/epi.12833. [DOI] [PubMed] [Google Scholar]
- 8.Baulac M, Patten A, Giorgi L. Long-term safety and efficacy of zonisamide versus carbamazepine monotherapy for treatment of partial seizures in adults with newly diagnosed epilepsy: Results of a phase III, randomized, double-blind study. Epilepsia. 2014;55:1534–43. doi: 10.1111/epi.12749. [DOI] [PubMed] [Google Scholar]
- 9.Kasteleijn-Nolst Trenité DG, Groenwold RH, et al. Single dose efficacy evaluation of two partial benzodiazepine receptor agonists in photosensitive epilepsy patients: A placebo-controlled pilot study. Epilepsy Res. 2016;122:30–36. doi: 10.1016/j.eplepsyres.2016.02.003. [DOI] [PubMed] [Google Scholar]
- 10.Pitkanen A, Lukasiuk K, Dudek FE, Staley KJ. Epileptogenesis. Cold Spring Harb Perspect Med. 2015;5 doi: 10.1101/cshperspect.a022822. pii: a022822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Grone BP, Baraban SC. Animal models in epilepsy research: Legacies and new directions. Nat Neurosci. 2015;18:339–43. doi: 10.1038/nn.3934. [DOI] [PubMed] [Google Scholar]
- 12.Lo WY, Tsai FJ, Liu CH, et al. Uncaria rhynchophylla upregulates the expression of MIF and cyclophilin A in kainic acid-induced epilepsy rats: A proteomic analysis. Am J Chin Med. 2010;38:745–59. doi: 10.1142/S0192415X10008214. [DOI] [PubMed] [Google Scholar]
- 13.Wang L, Song LF, Chen XY, et al. MiR-181b inhibits P38/JNK signaling pathway to attenuate autophagy and apoptosis in juvenile rats with kainic acid-induced epilepsy via targeting TLR4. CNS Neurosci Ther. 2019;25:112–22. doi: 10.1111/cns.12991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li Q, Li QQ, Jia JN, et al. Sodium valproate ameliorates neuronal apoptosis in a kainic acid model of epilepsy via enhancing PKC-dependent GABAAR gamma2 serine 327 phosphorylation. Neurochem Res. 2018;43:2343–52. doi: 10.1007/s11064-018-2659-8. [DOI] [PubMed] [Google Scholar]
- 15.Alsharafi W, Xiao B. Dynamic expression of microRNAs (183, 135a, 125b, 128, 30c and 27a) in the rat pilocarpine model and temporal lobe epilepsy patients. CNS Neurol Disord Drug Targets. 2015;14:1096–102. doi: 10.2174/1871527314666150317225945. [DOI] [PubMed] [Google Scholar]
- 16.An N, Zhao W, Liu Y, et al. Elevated serum miR-106b and miR-146a in patients with focal and generalized epilepsy. Epilepsy Res. 2016;127:311–16. doi: 10.1016/j.eplepsyres.2016.09.019. [DOI] [PubMed] [Google Scholar]
- 17.Che H, Yan Y, Kang XH, et al. MicroRNA-27a promotes inefficient lysosomal clearance in the hippocampi of rats following chronic brain hypoperfusion. Mol Neurobiol. 2017;54:2595–610. doi: 10.1007/s12035-016-9856-8. [DOI] [PubMed] [Google Scholar]
- 18.Jiang Y, Duan Y, Zhou H. MicroRNA-27a directly targets KRAS to inhibit cell proliferation in esophageal squamous cell carcinoma. Oncol Lett. 2015;9:471–77. doi: 10.3892/ol.2014.2701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Trinka E, Cock H, Hesdorffer D, et al. A definition and classification of status epilepticus – Report of the ILAE Task Force on Classification of Status Epilepticus. Epilepsia. 2015;56:1515–23. doi: 10.1111/epi.13121. [DOI] [PubMed] [Google Scholar]
- 20.Li Q, Li QQ, Jia JN, et al. Sodium valproate ameliorates neuronal apoptosis in a kainic acidmodel of epilepsy via enhancing PKC-dependent GABAAR γ2 serine327 phosphorylation. Neurochem Res. 2018;43:2343–52. doi: 10.1007/s11064-018-2659-8. [DOI] [PubMed] [Google Scholar]
- 21.Klein P, Herr D, Pearl PL, et al. Results of phase II pharmacokinetic study of levetiracetam for prevention of post-traumatic epilepsy. Epilepsy Behav. 2012;24:457–61. doi: 10.1016/j.yebeh.2012.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nenadic-Baranasic N, Gjergja-Juraski R, Lehman I, et al. Overnight video-polysomnographic studies in children with intractableepileptic encephalopathies. Med Sci Monit. 2018;24:5405–11. doi: 10.12659/MSM.908911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fu P, Wei L, Zhang F, et al. Added value of NeuroGam Software Analysis in single photon emissioncomputed tomography localization diagnosis of epilepsy in interictal stage. Med Sci Monit. 2018;24:1494–501. doi: 10.12659/MSM.908437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lezaic N, Gore G, Josephson CB, et al. The medical treatment of epilepsy in the elderly: A systematic review and meta-analysis. Epilepsia. 2019;60:1325–40. doi: 10.1111/epi.16068. [DOI] [PubMed] [Google Scholar]
- 25.Rana A, Musto AE. The role of inflammation in the development of epilepsy. J Neuroinflammation. 2018;15:144. doi: 10.1186/s12974-018-1192-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gales JM, Prayson RA. Chronic inflammation in refractory hippocampal sclerosis-relatedtemporal lobe epilepsy. Ann Diagn Pathol. 2017;30:12–16. doi: 10.1016/j.anndiagpath.2017.05.009. [DOI] [PubMed] [Google Scholar]
- 27.Kinjo ER, Higa GS, Santos BA, et al. Pilocarpine-induced seizures trigger differential regulation of microRNA-stability related genes in rat hippocampal neurons. Sci Rep. 2016;6:20969. doi: 10.1038/srep20969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Xiang L, Ren Y, Li X, et al. MicroRNA-204 suppresses epileptiform discharges through regulating TrkB-ERK1/2-CREB signaling in cultured hippocampal neurons. Brain Res. 2016;1639:99–107. doi: 10.1016/j.brainres.2016.02.045. [DOI] [PubMed] [Google Scholar]
- 29.Rajman M, Metge F, Fiore R, et al. A microRNA-129-5p/Rbfox crosstalk coordinates homeostatic downscaling of excitatory synapses. EMBO J. 2017;36:1770–87. doi: 10.15252/embj.201695748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Watanabe Y, Johnson RS, Butler LS, et al. Null mutation of c-fos impairs structural and functional plasticities in the kindling model of epilepsy. J Neurosci. 1996;16:3827–36. doi: 10.1523/JNEUROSCI.16-12-03827.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sinel’Nikova VV, Shubina LV, Gol’Tiaev MV, et al. [Detection of c-fos expression in animal brain in a pilocarpine model of temporal lobe epilepsy]. Zh Vyssh Nerv Deiat Im I P Pavlova. 2012;62:497–505. [in Russian] [PubMed] [Google Scholar]
- 32.Daido S, Tamiya T, Ono Y, et al. Expression of Bcl-2, Bcl-x, and Bax proteins in astrocytomas in relation to patient survival. Brain Tumor Pathol. 2001;18:123–29. doi: 10.1007/BF02479425. [DOI] [PubMed] [Google Scholar]
- 33.Tamatani M, Ogawa S, Nunez G, Tohyama M. Growth factors prevent changes in Bcl-2 and Bax expression and neuronal apoptosis induced by nitric oxide. Cell Death Differ. 1998;5:911–19. doi: 10.1038/sj.cdd.4400439. [DOI] [PubMed] [Google Scholar]
- 34.Wang Y, Bai C, Guan H, et al. Subchronic exposure to arsenic induces apoptosis in the hippocampus of the mouse brains through the Bcl-2/Bax pathway. J Occup Health. 2015;57:212–21. doi: 10.1539/joh.14-0226-OA. [DOI] [PubMed] [Google Scholar]
- 35.Shinoura N, Satou R, Yoshida Y, et al. Adenovirus-mediated transfer of Bcl-X(L) protects neuronal cells from Bax-induced apoptosis. Exp Cell Res. 2000;254:221–31. doi: 10.1006/excr.1999.4751. [DOI] [PubMed] [Google Scholar]
- 36.Prabowo AS, Iyer AM, Veersema TJ, et al. Expression of neurodegenerative disease-related proteins and caspase-3 in glioneuronal tumours. Neuropathol Appl Neurobiol. 2015;41:e1–e15. doi: 10.1111/nan.12143. [DOI] [PubMed] [Google Scholar]
- 37.Yang J, He F, Meng Q, et al. Inhibiting HIF-1alpha decreases expression of TNF-alpha and Caspase-3 in specific brain regions exposed kainic acid-induced status epilepticus. Cell Physiol Biochem. 2016;38:75–82. doi: 10.1159/000438610. [DOI] [PubMed] [Google Scholar]