This cohort study assesses the association of preoperative patient frailty and varying levels of operative stress during surgical procedures with postoperative mortality.
Key Points
Question
Is frailty associated with increased risk of postoperative mortality across all levels of operative stress?
Findings
In this cohort study of 432 828 unique patients, frailty was associated with increased 30-, 90-, and 180-day mortality across all levels of operative stress. Mortality among patients with frailty after low- and moderate-stress procedures was substantially higher than mortality rates usually associated with high-risk surgical procedures.
Meaning
The findings suggest that even minor surgical procedures are associated with high risk for patients with frailty and that surgeons and referring physicians should consider whether the potential benefits of surgery warrant the increased risk.
Abstract
Importance
Patients with frailty have higher risk for postoperative mortality and complications; however, most research has focused on small groups of high-risk procedures. The associations among frailty, operative stress, and mortality are poorly understood.
Objective
To assess the association between frailty and mortality at varying levels of operative stress as measured by the Operative Stress Score, a novel measure created for this study.
Design, Setting, and Participants
This retrospective cohort study included veterans in the Veterans Administration Surgical Quality Improvement Program from April 1, 2010, through March 31, 2014, who underwent a noncardiac surgical procedure at Veterans Health Administration Hospitals and had information available on vital status (whether the patient was alive or deceased) at 1 year postoperatively. A Delphi consensus method was used to stratify surgical procedures into 5 categories of physiologic stress.
Exposures
Frailty as measured by the Risk Analysis Index and operative stress as measured by the Operative Stress Score.
Main Outcomes and Measures
Postoperative mortality at 30, 90, and 180 days.
Results
Of 432 828 unique patients (401 453 males [92.8%]; mean (SD) age, 61.0 [12.9] years), 36 579 (8.5%) were frail and 9113 (2.1%) were very frail. The 30-day mortality rate among patients who were frail and underwent the lowest-stress surgical procedures (eg, cystoscopy) was 1.55% (95% CI, 1.20%-1.97%) and among patients with frailty who underwent the moderate-stress surgical procedures (eg, laparoscopic cholecystectomy) was 5.13% (95% CI, 4.79%-5.48%); these rates exceeded the 1% mortality rate often used to define high-risk surgery. Among patients who were very frail, 30-day mortality rates were higher after the lowest-stress surgical procedures (10.34%; 95% CI, 7.73%-13.48%) and after the moderate-stress surgical procedures (18.74%; 95% CI, 17.72%-19.80%). For patients who were frail and very frail, mortality continued to increase at 90 and 180 days, reaching 43.00% (95% CI, 41.69%-44.32%) for very frail patients at 180 days after moderate-stress surgical procedures.
Conclusions and Relevance
We developed a novel operative stress score to quantify physiologic stress for surgical procedures. Patients who were frail and very frail had high rates of postoperative mortality across all levels of the Operative Stress Score. These findings suggest that frailty screening should be applied universally because low- and moderate-stress procedures may be high risk among patients who are frail.
Introduction
Research shows that frailty among patients preoperatively is associated with postoperative outcomes.1,2,3,4 Patients who are frail have higher rates of morbidity, mortality, and failure to rescue after major procedures across surgical specialties.5,6,7,8,9,10,11,12,13,14,15,16 Patients who are frail also have higher rates of morbidity after ambulatory procedures typically considered to be minor.17 Thus, frailty is a salient, potentially modifiable patient characteristic in surgical practice.18,19,20,21
Frailty is a global syndrome of decreased physiologic reserve accurately measured by the Risk Analysis Index (RAI).22,23 Surgical stress can exhaust the limited reserve of patients who are frail, leading to decompensation and death. Understanding of whether frailty’s association with poor outcomes varies with the physiologic stress of surgery is inadequate. Patients who undergo physiologically taxing, higher-stress surgical procedures may seem to be at greatest risk, and existing research on surgical risk assessment has focused on such high-risk inpatient surgical procedures.24 Previous work8 showed that frailty was associated with poor outcomes even after procedures with lower risk of mortality, which account for most procedures performed at most hospitals. Many surgical procedures are considered to be so minor that surgeons spend little time considering whether patients can endure the stress of the procedure. However, if frailty is associated with adverse outcomes after such low-risk, ambulatory surgical procedures, it is important to identify and counsel patients who are frail before these relatively minor procedures. Surgeons could then focus on preoperative interventions to mitigate frailty-associated risks. Patients and surgeons could also engage in more informed, shared decision-making to ensure that surgery aligns with patient values.
To address this knowledge gap in the understanding of frailty, we designed this study to examine the association among operative stress, patient frailty, and postoperative mortality. We first used a modified Delphi consensus method to establish a measure of operative stress based on Current Procedural Terminology (CPT) codes (2017 Edition). We applied a score according to the amount of operative stress to a representative sample of surgical procedures across the Veterans Health Administration in which we also assessed frailty and postoperative mortality.
Methods
Patient Population and Measures
This retrospective cohort study used data from the Veterans Affairs Surgical Quality Improvement Program (VASQIP). Case sampling methods, the robustness of the data, and available data within VASQIP have been described previously.25 We included all VASQIP records for noncardiac surgical cases between April 1, 2010, and March 31, 2014, for which 1-year postoperative vital status (whether the patient was alive or deceased) was available. The Veterans Affairs Pittsburgh Healthcare System institutional review board, Pittsburgh, Pennsylvania, approved the analysis of the VASQIP data and determined these retrospective, deidentified data to be exempt. The Vanderbilt University Medical Center institutional review board, Nashville, Tennessee, approved the process for the Delphi consensus methodology to generate the Operative Stress Score (OSS).
We assessed patient frailty with the RAI, a validated tool with high predictive power for postoperative mortality that can be applied to quality improvement data sets.22 The RAI is based on the accumulation of deficits model of frailty and uses 14 variables to generate a score from 0 to 81, with higher scores indicating more frailty. The variables include demographic factors (age, sex), comorbidities (presence of disseminated cancer, unintentional weight loss, renal failure, congestive heart failure, poor appetite, and dyspnea at rest), cognitive decline, facility residence, and level of independence in 4 activities of daily living. Revised RAI scores were calculated from VASQIP variables according to procedures described elsewhere.23 The outcomes of interest were 30-day, 90-day, and 180-day all-cause mortality.
Operative Stress Score
We used a modified Delphi consensus method24,26,27 to rate common surgical procedures according to physiologic stress, naming the resulting scale the OSS. The categories are defined as follows: OSS 1, very low stress; OSS 2, low stress; OSS 3, moderate stress; OSS 4, high stress; and OSS 5, very high stress. For pragmatic feasibility, we chose a set of CPT codes comprising 90.0% of all procedures included in our VASQIP cohort. We then recruited a panel of staff-level surgeons and anesthesiologists across the specialty fields covered by these CPT codes. Specialty-specific subpanels included at least 3 surgeons (or anesthesiologists) who we purposively sampled to include early-, mid-, and late-career physicians practicing within 5 years, 5 to 10 years, and more than 10 years, respectively, from completion of residency. Anesthesiologists rated all CPT codes; surgical specialists rated only those CPT codes within their field of practice.
Ratings were solicited and managed using REDCap.28 Survey instructions described the concept of physiologic stress and the purpose of the rating project. Each CPT code was presented along with its verbal description. Respondents were asked to score each code on an integer scale from 1 to 5, with increasing numbers indicating increasing physiologic stress of the surgical procedure. The scoring scale was rooted with exemplars chosen by us (M.C.S., D.E.H.). For each round of scoring, we defined consensus as the modal score when at least 66% of all ratings were identical to the mode, and no score was more than 1-integer unit distant from this mode. Codes that did not reach consensus were presented again in the next round along with the summary of all scores and their mean from the previous round. This consensus process continued for 3 rounds.
Two of us (M.C.S., D.E.H.) independently assessed codes that did not reach consensus after 3 rounds and assigned scores based on comparison with similar procedures that had reached consensus. If these 2 authors agreed, that score was assigned to the procedure; disagreement between the 2 authors resulted in the procedure being presented to the core authorship group (M.C.S., S.A., P.V., R.S., N.N.M., J.M.J., and D.E.H.), who voted to determine the final score.
Statistical Analysis
The RAI scores were categorized into robust (≤20), normal (21-29), frail (30-39), and very frail (≥40) as described elsewhere.23 Point estimates for mortality in each OSS and RAI stratum were calculated for each time point along with their corresponding 95% CIs using exact binomial CIs for proportions. All analyses were performed using Stata Statistical Software, release 14 (StataCorp LLC).
Results
The VASQIP data set contained 480 731 records, each with a single principal CPT code. We focused on the 565 most common CPT codes that defined 90.0% of the sample (n = 432 828). Demographic characteristics of this cohort are presented in Table 1; 92.8% were male (n = 401 453) and 69.3% were white (n = 299 809), with a mean (SD) age of 61.0 (12.9) years and a mean (SD) RAI score of 21.25 (7.34). The codes were grouped according to surgical discipline into 11 specialties: general surgery (n = 151), vascular surgery (n = 70), thoracic surgery (n = 20), plastic surgery (n = 25), gynecology (n = 13), urology (n = 61), otolaryngology (n = 34), hand surgery (n = 15), spine surgery (n = 25), neurosurgery (n = 21), and orthopedics (n = 130).
Table 1. Demographic Characteristics.
| Characteristic | Participants (n = 432 828)a |
|---|---|
| Age, mean (SD), y | 61.0 (12.9) |
| Sex | |
| Female | 31 375 (7.2) |
| Male | 401 453 (92.8) |
| Race | |
| White | 299 809 (69.3) |
| Black | 65 211 (15.1) |
| Otherb | 4696 (1.1) |
| Unknown | 63 112 (14.6) |
| Ethnicity | |
| Not Hispanic or Latino | 379 232 (87.6) |
| Hispanic or Latino | 22 658 (5.2) |
| Missing | 30 938 (7.1) |
| Operative Stress Score | |
| 1 | 44 545 (10.3) |
| 2 | 226 754 (52.4) |
| 3 | 129 846 (30.0) |
| 4 | 28 751 (6.6) |
| 5 | 2932 (0.7) |
| Risk Analysis Index | |
| ≤20 | 199 677 (46.1) |
| 21-29 | 187 459 (43.3) |
| 30-39 | 36 579 (8.5) |
| ≥40 | 9113 (2.1) |
| ASA class | |
| 1 | 10 094 (2.3) |
| 2 | 115 362 (26.6) |
| 3 | 266 402 (61.6) |
| 4 | 39 431 (9.1) |
| 5 | 847 (0.2) |
| Missing | 692 (0.2) |
| Complications, No. | |
| 0 | 401 547 (92.8) |
| 1 | 21 855 (5.0) |
| ≥2 | 9426 (2.2) |
Abbreviation: ASA, American Society of Anesthesiologists.
Data are presented as number (percentage) of participants unless otherwise indicated.
Other includes American Indian or Alaska Native, Asian, and Native Hawaiian or other Pacific Islander.
The Delphi consensus process achieved consensus ratings for 528 of 565 CPT codes (93.4%) after 3 rounds (264 codes [46.7%] in round 1, 209 codes [37.0%] in round 2, and 55 codes [9.7%] in round 3); 32 (5.7%) of the remaining codes were assigned by agreement between 2 of us (M.C.S., D.E.H.), and the final 5 codes (0.9%) were assigned by majority vote of the core authorship group (M.C.S., S.A., P.V., R.S., N.N.M., J.M.J., and D.E.H.). Table 2 shows the 5 most prevalent surgical procedures at each of the 5 levels of the OSS (a full list of CPT codes with associated OSS is available in the eTable in the Supplement). Most of the procedures were classified as low-stress (OSS 2) procedures (240 procedures [42.5%]) or moderate-stress (OSS 3) procedures (179 procedures [31.7%]).
Table 2. Most Prevalent Procedures by OSS.
| OSS Category, Procedure Typea | Patients, No. (%)b |
|---|---|
| Category 1, Very Low Stress | |
| Allc | 44 545 (10.3) |
| 5 Most common procedure types | |
| All | 24 252 (54.4) |
| Cystourethroscopy, with fulguration: minor, small, or mediumd | 15 641 (35.1) |
| Hydrocele | 3212 (7.2) |
| Ganglion cyst excision, wrist | 2189 (4.9) |
| Fasciectomy, palmar | 1534 (3.4) |
| Arthroplasty, hand | 1676 (3.8) |
| Category 2, Low Stress | |
| Alle | 226 754 (52.4) |
| 5 Most common procedure types | |
| All | 140 706 (62.1) |
| Inguinal hernia, laparoscopic or open | 44 542 (19.6) |
| Arthroscopy, knee or shoulder | 64 457 (28.4) |
| Umbilical hernia, laparoscopic or open | 13 450 (5.9) |
| Transurethral prostate incision, excision, or ablation | 12 963 (5.7) |
| Appendectomy, unruptured, laparoscopic or open | 5294 (2.3) |
| Category 3, Moderate Stress | |
| Allf | 129 846 (30.0) |
| 5 Most common procedure types | |
| All | 64 450 (49.8) |
| Cholecystectomy, laparoscopic or open with or without intraoperative cholangiogram | 21 720 (16.7) |
| Arthroplasty, knee, shoulder, or hip | 21 153 (16.3) |
| Amputation, above or below knee | 9069 (7.0) |
| Thromboendarterectomy, carotid, vertebral, or subclavian | 8730 (6.7) |
| Colectomy, laparoscopic | 3971 (3.1) |
| Category 4, High Stress | |
| Allg | 28 751 (6.6) |
| 5 Most common procedure types | |
| All | 23 655 (82.3) |
| Colectomy, open | 11 398 (39.6) |
| Prostatectomy | 4180 (14.5) |
| Lung resection, lobe or segment | 4797 (16.7) |
| Nephrectomy | 2277 (7.9) |
| Aortobifemoral bypass graft | 1003 (3.5) |
| Category 5, Very High Stress | |
| Allh | 2932 (0.7) |
| 5 Most common procedure types | |
| All | 2782 (94.9) |
| Abdominal aortic aneurysm | 1115 (38.0) |
| Pancreaticoduodenectomy | 560 (19.1) |
| Esophagectomy | 685 (23.4) |
| Liver transplant | 264 (9.0) |
| Pneumonectomy | 158 (5.4) |
Abbreviations: CPT, Current Procedural Terminology; OSS, Operative Stress Score.
Procedure types are composed of 1 or more similar CPT codes.
The denominator for percentages of all in each category is the total number of patients (432 828) and for the types is the total number in each category.
Category 1 (n = 88): cystourethroscopy: 52224, 52234, and 52235; hydrocele: 55040, 55041, and 55060; ganglion cyst excision: 25111, 25112; palmar fasciectomy: 26121, 26123; and hand arthroplasty: 25445, 25447, 26530, and 26531.
Minor is defined as tumor measuring less than 0.5 cm; small, tumor measuring from 0.5 to 2.0 cm; and medium, tumor measuring from 2.0 to 5.0 cm.
Category 2 (n = 240): inguinal hernia: 49505, 49507, 49520, 49521, 49525, 49650, and 49651; arthroscopy, knee or shoulder: 27438, 27446, 27447, 27702, 29806, 29807, 29822, 29823, 29824, 29825, 29826, 29827, 29828, 29870, 29871, 29873, 29874, 29875, 29876, 29877, 29879, 29880, 29881, 29882, 29883, 29884, 29891, and 29999; umbilical hernia: 49585, 49652, 49653; transurethral prostate: 52450, 52601, 52630, 52648, 52649, and 55873; and appendectomy: 44950, 44970.
Category 3 (n = 179): cholecystectomy: 47562, 47563, 47564, 47600, 47605, and 47610; arthroplasty, knee, shoulder, or hip: 23470, 23472, 27125, 27130, 27132, 27134, 27137, 27138, 27486, and 27487; amputation, above or below knee: 27590, 27592, 27594, 27596, 27598, 27880, 27881, 27882, and 27886; thromboendarterectomy: 35301; and laparoscopic colectomy: 44204, 44205, 44206, and 44207.
Category 4 (n = 47): open colectomy: 44140, 44141, 44143, 44144, 44145, 44146, 44150, 44155, 44160, 45110, 45111, and 45395; prostatectomy: 55810, 55842, and 55845; lung resection, lobe or segment: 32480, 32482, 32484, 32505, 32663; nephrectomy: 50230, 50234, and 50240; and aortobifemoral bypass graft: 35540, 35646.
Category 5 (n = 11): abdominal aortic aneurysm: 35081, 35091, and 35102; pancreaticoduodenectomy: 48150, 48153; esophagectomy: 43107, 43112, and 43117; liver transplant: 47135; and pneumonectomy: 32440.
Table 3 shows 30-, 90-, and 180-day mortality stratified by OSS. The unadjusted mortality rates at all 3 time points were lower for OSS 2 surgical procedures compared with OSS 1 surgical procedures. However, mortality rate increased as OSS increased (eg, 180-day mortality for OSS 1, 2.4%; OSS 2, 1.5%; OSS 3, 5.7%; OSS 4, 8.0%; and OSS 5, 10.2%).
Table 3. The 30-, 90-, and 180-Day Mortality by OSSa.
| Mortality | OSS 1 (n = 44 545) | OSS 2 (n = 226 754) | OSS 3 (n = 129 846) | OSS 4 (n = 28 751) | OSS 5 (n = 2932) |
|---|---|---|---|---|---|
| 30 d | |||||
| Deaths, No. | 168 | 762 | 2541 | 905 | 103 |
| Mortality rate by RAI, % (95% CI)b | |||||
| ≤20 | 0.04 (0.01-0.08) | 0.09 (0.07-0.11) | 0.30 (0.25-0.35) | 0.88 (0.70-1.09) | 2.03 (1.08-3.44) |
| 21-29 | 0.22 (0.16-0.30) | 0.29 (0.26-0.33) | 0.91 (0.83-0.99) | 1.89 (1.67-2.12) | 2.83 (2.08-3.76) |
| 30-39 | 1.55 (1.20-1.97) | 1.85 (1.62-2.11) | 5.13 (4.79-5.48) | 6.98 (6.20-7.83) | 6.14 (4.15-8.70) |
| ≥40 | 10.34 (7.73-13.48) | 10.12 (8.71-11.67) | 18.74 (17.72-19.80) | 22.26 (19.99-24.66) | 7.77 (4.42-12.95) |
| Overall mortality rate, % (95% CI) | 0.38 (0.32-0.44) | 0.34 (0.31-0.36) | 1.96 (1.88-2.03) | 3.15 (2.95-3.36) | 3.51 (2.88-4.24) |
| 90 d | |||||
| Deaths, No. | 529 | 1963 | 5132 | 1631 | 188 |
| Mortality rate by RAI, % (95% CI)b | |||||
| ≤20 | 0.22 (0.16-0.30) | 0.22 (0.19-0.24) | 0.58 (0.52-0.65) | 1.64 (1.39-1.92) | 4.06 (2.67-5.89) |
| 21-29 | 0.84 (0.72-0.82) | 0.76 (0.70-0.82) | 2.05 (1.93-2.16) | 3.73 (3.43-4.05) | 5.10 (4.09-6.29) |
| 30-39 | 4.79 (4.17-5.48) | 5.28 (4.88-5.69) | 10.95 (10.47-11.44) | 12.98 (11.94-14.08) | 11.65 (8.90-14.90) |
| ≥40 | 22.84 (19.10-26.93) | 22.63 (20.65-24.72) | 34.04 (32.80-35.31) | 34.74 (32.11-37.44) | 12.44 (8.13-17.94) |
| Overall mortality rate, % (95% CI) | 1.19 (1.09-1.29) | 0.87 (0.83-0.90) | 3.95 (3.85-4.06) | 5.67 (5.41-5.95) | 6.41 (5.55-7.36) |
| 180 d | |||||
| Deaths, No. | 1076 | 3469 | 7429 | 2312 | 298 |
| Mortality rate by RAI, % (95% CI)b | |||||
| ≤20 | 0.54 (0.44-0.65) | 0.43 (0.40-0.46) | 1.03 (0.94-1.12) | 2.69 (2.37-3.04) | 5.93 (4.23-8.05) |
| 21-29 | 1.97 (1.78-2.17) | 1.45 (1.37-1.53) | 3.29 (3.15-3.44) | 5.78 (5.41-6.18) | 8.61 (7.29-10.08) |
| 30-39 | 9.40 (8.54-10.32) | 9.19 (8.68-9.72) | 16.22 (15.65-16.80) | 18.03 (16.83-19.28) | 18.43 (15.04-22.23) |
| ≥40 | 34.91 (30.58-39.44) | 31.92 (29.68-34.21) | 43.00 (41.69-44.32) | 41.97 (39.23-44.75) | 17.10 (12.07-23.17) |
| Overall mortality rate, % (95% CI) | 2.42 (2.27-2.56) | 1.53 (1.48-1.58) | 5.72 (5.60-5.85) | 8.04 (7.73-8.36) | 10.2 (9.09-11.31) |
Abbreviations: OSS, Operative Stress Score; RAI, Risk Analysis Index.
The categories are defined as follows: OSS 1, very low stress; OSS 2, low stress; OSS 3, moderate stress; OSS 4, high stress; and OSS 5, very high stress.
The Risk Analysis Index is based on the accumulation of deficits model of frailty and uses 14 variables to generate a score from 0 to 81, with higher scores indicating more frailty.
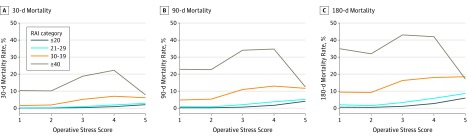
Figure 1 shows the association between OSS and mortality stratified by different levels of frailty. Among patients without frailty, mortality gradually increased as operative stress increased. Patients who were frail had higher mortality across all procedure types. For example, for OSS 1, 30-day mortality was 1.6% (95% CI, 1.2%-2.0%) for patients who were frail and 10.3% (95% CI, 7.7%-13.5%) for patients who were very frail, whereas it was 0.22% (95% CI, 0.16%-0.30%) for patients without frailty. Similar patterns occurred at 90 and 180 days. By 180 days after OSS 1 to OSS 3 surgical procedures, the mortality rates among patients who were frail was 16.22% (95% CI, 15.65%-16.80%) and among those who were very frail was 43.00% (95% CI, 41.69%-44.32%). A marked decrease in mortality in OSS 5 compared with OSS 4 surgical procedures for patients who were very frail at each time point was found.
Figure 1. Mortality at 30, 90, and 180 Days Stratified by Risk Analysis Index (RAI).
The categories of Operative Stress Score are defined as follows: 1, very low stress; 2, low stress; 3, moderate stress; 4, high stress; and 5, very high stress. The RAI is based on the accumulation of deficits model of frailty and uses 14 variables to generate a score from 0 to 81, with higher scores indicating more frailty.
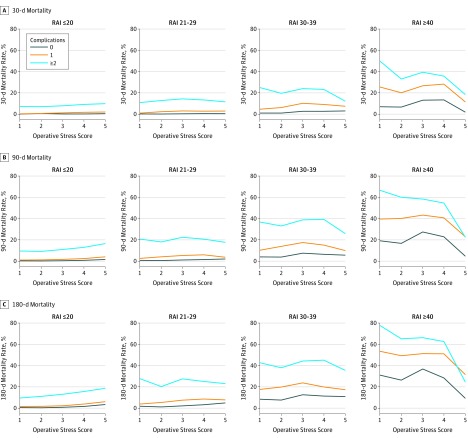
Figure 2 further explores the association between frailty and mortality by adding the number of postoperative complications as a fourth dimension, including failure to rescue (ie, mortality following a complication).8 Regardless of the number of complications, RAI was significantly correlated with mortality at all 3 time points (maximum R2: 21.6% for 30-day mortality; 23.9% for 90-day mortality, and 23.4% for 180-day mortality). At every level of OSS, presence of more complications was associated with higher mortality. Patients who were frail and very frail without any complications had substantial mortality at all OSS levels, and complications among the frail were associated with even higher mortality rates.
Figure 2. Mortality at 30, 90, and 180 Days Stratified by Frailty and Number of Complications.
The categories of Operative Stress Score are defined as follows: 1, very low stress; 2, low stress; 3, moderate stress; 4, high stress; and 5, very high stress. The Risk Analysis Index (RAI) is based on the accumulation of deficits model of frailty and uses 14 variables to generate a score from 0 to 81, with higher scores indicating more frailty.
Discussion
We used a Delphi consensus method to develop the OSS, a new rating system categorizing common surgical CPT codes according to the degree of physiologic stress. In contrast to other surgical risk scoring systems that incorporate specific operative factors,29,30,31,32 the OSS provides a consistent, global assessment of operative stress. The development of the OSS allowed us to compare the association of frailty across a diverse array of surgical procedures with differing levels of operative stress. The face validity of this taxonomy was supported by mortality rates that increased with increasing OSS.
Our findings have important implications for whether operative risk should be conceptualized in terms of high-risk procedures vs high-risk patients. Although OSS 5 surgical procedures are widely recognized as high risk, patients who are frail and very frail who underwent lower-stress procedures (OSS 1-3) had mortality rates exceeding those typically reported for the highest-risk surgical procedures. Although there is no universally accepted definition of high-risk surgery, in-hospital or 30-day mortality rates greater than 1% have been used to identify high-risk procedures.8,24 By this metric, procedures at all levels of OSS are high risk for patients who are frail and very frail. Seib et al17 recently showed that frailty was associated with increased rates of complications after common ambulatory surgical procedures, and Shah et al8 showed that frailty was associated with mortality after high- and low-mortality risk surgical procedures. These results suggest that low-stress procedures are not low risk for patients who are frail. Our data indicate that there are no low-risk procedures among patients who are frail.
The lower mortality for patients who are frail and very frail after OSS 5 surgical procedures compared with OSS 4 surgical procedures deserves special comment. Similar results were seen by McIsaac et al,6 who have shown that the association of patient frailty with outcomes differs by surgical procedure, with patient frailty having a stronger association with certain minor surgical procedures (eg, appendectomy) than with major surgical procedures (eg, pancreaticoduodenectomy).6,33 Physicians may be more attuned to patient-level risk factors when considering procedures that they perceive as high risk, leading to more careful selection and more vigorous efforts to mitigate postoperative morbidity and mortality. This hypothesis suggests that for these rare OSS 5 procedures, physicians are effectively selecting patients based on factors not captured by the RAI that indicate more favorable outcomes. If correct, this hypothesis indicates an opportunity to improve outcomes through systematic efforts to improve patient selection and optimization among patients who are frail and very frail and who consider undergoing categories OSS 1 to OSS 4 surgical procedures.
We contend that frailty should be assessed for any patient considering surgery and that frailty-associated risks be discussed with patients in a robust process of shared decision-making. Many patients may still elect to pursue surgical intervention to manage symptoms or preserve independence because data show that older patients value quality of life at least as much as survival.34 Regardless of the indication for a given procedure (ie, palliative or therapeutic), the substantial frailty-associated risk identified here should be considered and factored into the decision-making shared between the care team and the patient.
Beyond the specific results presented here, this study may have wider implications for surgical outcomes research and practice. This study adds to the growing body of literature showing the usefulness of the RAI as an indicator of postoperative complications and mortality across a number of different time points, patient populations, and stratifications of operative risk.8,22,35,36,37,38,39 Although these data report RAI scores calculated retrospectively from registry data, the RAI can be calculated with a survey instrument administered to patients at the point of care to inform real-time, patient-centered, shared decision-making.37 The information from the RAI, which is easily collected, can help physicians risk stratify their patients, and this information in the future could be incorporated with other risk models (such as the VASQIP risk calculator) to develop even more refined models of stratification. Moreover, the OSS (eTable in the Supplement) could be used in future studies as a means of stratifying the stress of operations.
Limitations
This study has limitations. These data derive from veteran patients, and the results may not generalize to other patient populations, especially because women are underrepresented in the sample. Future studies are needed to examine the association among frailty, operative stress, and mortality in nonveteran populations. Moreover, VASQIP data do not distinguish deaths directly related to the surgical procedure from deaths from unrelated causes. However, as a global measure of physiologic reserve, the RAI identifies risk for all-cause mortality, and the likelihood of medium-term mortality likely shifts the risk-benefit ratio because patients who are frail may not live long enough to accrue the benefits of surgery. In addition, the OSS is a physician-rated measure and does not include objective physiologic criteria. However, it is a first attempt to delineate global procedural risk across a broad range of surgical specialties and may pave the way for future research in the magnitude of surgical stress and frailty. The OSS may be particularly useful for disease-agnostic analyses of system-level factors shared across all procedure types. Mortality is not the only or even the most important outcome for patients who are frail, and our data set does not include critically important patient-reported outcome measures, such as quality of life, loss of independence, or human flourishing.40,41,42
Conclusions
These findings suggest that frailty is associated with postoperative mortality across all types of surgical procedures regardless of operative stress, and thus patients who are frail may not have the outcomes that are typical for the general population. Surgeons, anesthesiologists, and referring clinicians may wish to consider frailty to determine whether a surgical procedure is appropriate and what can be done to optimize the outcomes for these patients. There is a substantial opportunity to leverage frailty assessment to inform patient selection and optimization for the medium- and low-stress surgical procedures, which constitute most of the surgical volume at most hospitals. Although the rates of mortality for these lower-stress surgical procedures were lower compared with high-stress surgical procedures, their higher volume translates to more overall mortality. Furthermore, by 180 days, patients who were frail and very frail experienced high rates of mortality after low-stress surgical procedures even when they experienced no complications. Efforts to screen patients for frailty should not only focus on high-stress surgical procedures but should also focus on the low-stress surgical procedures, which are also risky among patients who are frail.
eTable. Operative Stress Scores (OSS) for 565 CPT Codes
References
- 1.Makary MA, Segev DL, Pronovost PJ, et al. Frailty as a predictor of surgical outcomes in older patients. J Am Coll Surg. 2010;210(6):-. doi: 10.1016/j.jamcollsurg.2010.01.028 [DOI] [PubMed] [Google Scholar]
- 2.Robinson TN, Wu DS, Pointer L, Dunn CL, Cleveland JC Jr, Moss M. Simple frailty score predicts postoperative complications across surgical specialties. Am J Surg. 2013;206(4):544-550. doi: 10.1016/j.amjsurg.2013.03.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lin HS, Watts JN, Peel NM, Hubbard RE. Frailty and post-operative outcomes in older surgical patients: a systematic review. BMC Geriatr. 2016;16(1):157. doi: 10.1186/s12877-016-0329-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Robinson TN, Wallace JI, Wu DS, et al. Accumulated frailty characteristics predict postoperative discharge institutionalization in the geriatric patient. J Am Coll Surg. 2011;213(1):37-42. doi: 10.1016/j.jamcollsurg.2011.01.056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kim SW, Han HS, Jung HW, et al. Multidimensional frailty score for the prediction of postoperative mortality risk. JAMA Surg. 2014;149(7):633-640. doi: 10.1001/jamasurg.2014.241 [DOI] [PubMed] [Google Scholar]
- 6.McIsaac DI, Bryson GL, van Walraven C. Association of frailty and 1-year postoperative mortality following major elective noncardiac surgery: a population-based cohort study. JAMA Surg. 2016;151(6):538-545. doi: 10.1001/jamasurg.2015.5085 [DOI] [PubMed] [Google Scholar]
- 7.McIsaac DI, Taljaard M, Bryson GL, et al. Frailty as a predictor of death or new disability after surgery: a prospective cohort study [published ahead of print July 24, 2018]. Ann Surg. 2018. doi: 10.1097/SLA.0000000000002967 [DOI] [PubMed] [Google Scholar]
- 8.Shah R, Attwood K, Arya S, et al. Association of frailty with failure to rescue after low-risk and high-risk inpatient surgery. JAMA Surg. 2018;153(5):e180214. doi: 10.1001/jamasurg.2018.0214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Joseph B, Phelan H, Hassan A, et al. The impact of frailty on failure-to-rescue in geriatric trauma patients: a prospective study. J Trauma Acute Care Surg. 2016;81(6):1150-1155. doi: 10.1097/TA.0000000000001250 [DOI] [PubMed] [Google Scholar]
- 10.Arya S, Kim SI, Duwayri Y, et al. Frailty increases the risk of 30-day mortality, morbidity, and failure to rescue after elective abdominal aortic aneurysm repair independent of age and comorbidities. J Vasc Surg. 2015;61(2):324-331. doi: 10.1016/j.jvs.2014.08.115 [DOI] [PubMed] [Google Scholar]
- 11.Saxton A, Velanovich V. Preoperative frailty and quality of life as predictors of postoperative complications. Ann Surg. 2011;253(6):1223-1229. doi: 10.1097/SLA.0b013e318214bce7 [DOI] [PubMed] [Google Scholar]
- 12.Augustin T, Burstein MD, Schneider EB, et al. Frailty predicts risk of life-threatening complications and mortality after pancreatic resections. Surgery. 2016;160(4):987-996. doi: 10.1016/j.surg.2016.07.010 [DOI] [PubMed] [Google Scholar]
- 13.Farhat JS, Velanovich V, Falvo AJ, et al. Are the frail destined to fail? frailty index as predictor of surgical morbidity and mortality in the elderly. J Trauma Acute Care Surg. 2012;72(6):1526-1530. doi: 10.1097/TA.0b013e3182542fab [DOI] [PubMed] [Google Scholar]
- 14.Suskind AM, Walter LC, Jin C, et al. Impact of frailty on complications in patients undergoing common urological procedures: a study from the American College of Surgeons National Surgical Quality Improvement database. BJU Int. 2016;117(5):836-842. doi: 10.1111/bju.13399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Adams P, Ghanem T, Stachler R, Hall F, Velanovich V, Rubinfeld I. Frailty as a predictor of morbidity and mortality in inpatient head and neck surgery. JAMA Otolaryngol Head Neck Surg. 2013;139(8):783-789. doi: 10.1001/jamaoto.2013.3969 [DOI] [PubMed] [Google Scholar]
- 16.George EM, Burke WM, Hou JY, et al. Measurement and validation of frailty as a predictor of outcomes in women undergoing major gynaecological surgery. BJOG. 2016;123(3):455-461. doi: 10.1111/1471-0528.13598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Seib CD, Rochefort H, Chomsky-Higgins K, et al. Association of patient frailty with increased morbidity after common ambulatory general surgery operations. JAMA Surg. 2018;153(2):160-168. doi: 10.1001/jamasurg.2017.4007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rumer KK, Saraswathula A, Melcher ML. Prehabilitation in our most frail surgical patients: are wearable fitness devices the next frontier? Curr Opin Organ Transplant. 2016;21(2):188-193. doi: 10.1097/MOT.0000000000000295 [DOI] [PubMed] [Google Scholar]
- 19.Halloway S, Buchholz SW, Wilbur J, Schoeny ME. Prehabilitation interventions for older adults: an integrative review. West J Nurs Res. 2015;37(1):103-123. doi: 10.1177/0193945914551006 [DOI] [PubMed] [Google Scholar]
- 20.Gillis C, Li C, Lee L, et al. Prehabilitation versus rehabilitation: a randomized control trial in patients undergoing colorectal resection for cancer. Anesthesiology. 2014;121(5):937-947. doi: 10.1097/ALN.0000000000000393 [DOI] [PubMed] [Google Scholar]
- 21.Carli F, Awasthi R, Gillis C, Kassouf W. Optimizing a frail elderly patient for radical cystectomy with a prehabilitation program. Can Urol Assoc J. 2014;8(11-12):E884-E887. doi: 10.5489/cuaj.2025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hall DE, Arya S, Schmid KK, et al. Development and initial validation of the Risk Analysis Index for measuring frailty in surgical populations. JAMA Surg. 2017;152(2):175-182. doi: 10.1001/jamasurg.2016.4202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Arya S, Varley P, Youk A, et al. Recalibration and external validation of the Risk Analysis Index: a surgical frailty assessment tool [published online March 19, 2019]. Ann Surg. 2019. doi: 10.1097/SLA.0000000000003276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schwarze ML, Barnato AE, Rathouz PJ, et al. Development of a list of high-risk operations for patients 65 years and older. JAMA Surg. 2015;150(4):325-331. doi: 10.1001/jamasurg.2014.1819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Massarweh NN, Kaji AH, Itani KMF. Practical guide to surgical data sets: Veterans Affairs Surgical Quality Improvement Program (VASQIP). JAMA Surg. 2018;153(8):768-769. doi: 10.1001/jamasurg.2018.0504 [DOI] [PubMed] [Google Scholar]
- 26.Powell C. The Delphi technique: myths and realities. J Adv Nurs. 2003;41(4):376-382. doi: 10.1046/j.1365-2648.2003.02537.x [DOI] [PubMed] [Google Scholar]
- 27.Jones J, Hunter D. Consensus methods for medical and health services research. BMJ. 1995;311(7001):376-380. doi: 10.1136/bmj.311.7001.376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)—a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377-381. doi: 10.1016/j.jbi.2008.08.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Copeland GP, Jones D, Walters M. POSSUM: a scoring system for surgical audit. Br J Surg. 1991;78(3):355-360. doi: 10.1002/bjs.1800780327 [DOI] [PubMed] [Google Scholar]
- 30.Scott S, Lund JN, Gold S, et al. An evaluation of POSSUM and P-POSSUM scoring in predicting post-operative mortality in a level 1 critical care setting. BMC Anesthesiol. 2014;14:104. doi: 10.1186/1471-2253-14-104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Haga Y, Ikei S, Ogawa M. Estimation of Physiologic Ability and Surgical Stress (E-PASS) as a new prediction scoring system for postoperative morbidity and mortality following elective gastrointestinal surgery. Surg Today. 1999;29(3):219-225. doi: 10.1007/BF02483010 [DOI] [PubMed] [Google Scholar]
- 32.Haga Y, Ikejiri K, Wada Y, et al. A multicenter prospective study of surgical audit systems. Ann Surg. 2011;253(1):194-201. doi: 10.1097/SLA.0b013e3181f66199 [DOI] [PubMed] [Google Scholar]
- 33.McIsaac DI, Moloo H, Bryson GL, van Walraven C. The association of frailty with outcomes and resource use after emergency general surgery: a population-based cohort study. Anesth Analg. 2017;124(5):1653-1661. doi: 10.1213/ANE.0000000000001960 [DOI] [PubMed] [Google Scholar]
- 34.Fried TR, Bradley EH, Towle VR, Allore H. Understanding the treatment preferences of seriously ill patients. N Engl J Med. 2002;346(14):1061-1066. doi: 10.1056/NEJMsa012528 [DOI] [PubMed] [Google Scholar]
- 35.van der Windt DJ, Bou-Samra P, Dadashzadeh ER, Chen X, Varley PR, Tsung A. Preoperative Risk Analysis Index for frailty predicts short-term outcomes after hepatopancreatobiliary surgery. HPB (Oxford). 2018;20(12):1181-1188. doi: 10.1016/j.hpb.2018.05.016 [DOI] [PubMed] [Google Scholar]
- 36.Esses G, Andreopoulos E, Lin HM, Arya S, Deiner S. A comparison of three frailty indices in predicting morbidity and mortality after on-pump aortic valve replacement. Anesth Analg. 2018;126(1):39-45. doi: 10.1213/ANE.0000000000002411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hall DE, Arya S, Schmid KK, et al. Association of a frailty screening initiative with postoperative survival at 30, 180, and 365 days. JAMA Surg. 2017;152(3):233-240. doi: 10.1001/jamasurg.2016.4219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Isharwal S, Johanning JM, Dwyer JG, Schimid KK, LaGrange CA. Preoperative frailty predicts postoperative complications and mortality in urology patients. World J Urol. 2017;35(1):21-26. doi: 10.1007/s00345-016-1845-z [DOI] [PubMed] [Google Scholar]
- 39.Ernst KF, Hall DE, Schmid KK, et al. Surgical palliative care consultations over time in relationship to systemwide frailty screening. JAMA Surg. 2014;149(11):1121-1126. doi: 10.1001/jamasurg.2014.1393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Berian JR, Mohanty S, Ko CY, Rosenthal RA, Robinson TN. Association of loss of independence with readmission and death after discharge in older patients after surgical procedures. JAMA Surg. 2016;151(9):e161689. doi: 10.1001/jamasurg.2016.1689 [DOI] [PubMed] [Google Scholar]
- 41.VanderWeele TJ. On the promotion of human flourishing. Proc Natl Acad Sci U S A. 2017;114(31):8148-8156. doi: 10.1073/pnas.1702996114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.VanderWeele TJ, McNeely E, Koh HK. Reimagining health-flourishing. JAMA. 2019;321(17):1667-1668. doi: 10.1001/jama.2019.3035 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable. Operative Stress Scores (OSS) for 565 CPT Codes