Abstract
Background
Hyperuricemia has a pathogenic role in the development of hypertension and other cardiovascular diseases (CVD). Uric acid has been reported to activate Nod-like receptor protein 3 (NLRP3)-inflammasome and alter vascular smooth muscle cells (VSMC). However, the potential mechanisms underlying this association are still not understood. The aim of this study was to investigate the role and potential mechanisms of uric acid in proliferation of VSMC.
Material/Methods
Cell Counting Kit-8 (CCK-8) proliferation assay and colony formation assay were performed to determine the proliferative ability of VSMC under uric acid stimulation. Immunofluorescence microscopy was carried out to determine the expression of Alpha-smooth muscle actin (α-SMA). In addition, real-time PCR and Western blot were used to detect the expression of NLRP3-inflammasome, and ELISA was performed to measure the levels of IL-18 and IL-1β.
Results
The results showed that uric acid increases the proliferation of VSMC and induces α-SMA accumulation. We also found that uric acid increases the level of NLRP3 and induces NLRP3-inflammasome activation. The expressions of uric acid-induced inflammatory markers IL-1β and IL-18 were decreased by the inhibitor MCC950.
Conclusions
Our findings revealed that uric acid induces inflammation through NLRP3-inflammasome-mediated VSMC proliferation. NLRP3 may be a new therapeutic target for hypertension.
MeSH Keywords: Hyperuricemia; Muscle, Smooth, Vascular; Nod Signaling Adaptor Proteins; Uric Acid
Background
Hyperuricemia usually involves an unusually high level of uric acid in the blood. In the body, uric acid mainly exists as urate, and the balance is dependent on the synthesis of purines [1]. Hyperuricemia has been connected with hypertension, coronary heart disease, and other CVDs [2,3]. Biologically active uric acid can stimulate endothelial dysfunction, inflammation, oxidative stress, and vasoconstriction [4–6]. High uric acid levels usually occur when the kidneys cannot balance uric acid levels, resulting in slow removal of uric acid.
Common causes of hyperuricemia include a high-purine diet, obesity, diabetes, use of diuretics, and drinking too much alcohol [7–10]. High levels of uric acid crystals stimulate Nod-like receptor protein 3(NLRP3)-inflammasome, mainly through aggravated phagocytosis, to produce inflammatory cytokines in VSMCs [11,12]. The protein NLRP3 is encoded by the NLRP3 gene located on the long arm of chromosome 1 in humans [13]. In addition, uric acid crystals can damage cellular membranes without the association of any known cellular receptor, which leads to accumulation of inflammatory cytokines [14]. The key inflammatory cytokine, interleukin-1β (IL-1β), is a prime mediator of the inflammatory response and it upregulates IL-18, a pro-inflammatory chemokine belonging to the CXC chemokine family [15–18].
The present study evaluated the expressions of inflammatory cytokines in VSMCs. We observed uric acid-induced NLRP3 activation, which leads to an increase of inflammatory markers such as IL-1β and IL-18 expressions in VSMCs. Upon treatment with the inhibitor of NLRP3 (MCC950), the modulated levels of IL-1β and IL-18 expressions were observed. Our results suggest a novel role of the NLRP3-inflammosome in the development of inflammation, and indicate that inhibition of NLRP3 signaling might serve as a therapeutic target in management of hypertension.
Material and Methods
Vascular smooth muscle cell (VSMCs) culture
VSMCs were obtained from the heart and confirmed with α-smooth muscle actin staining. VSMC initially were grown in growth medium with 5% FBS. At 60–70% cell confluence, the medium was changed to serum-free medium. VSMCs were co-cultured with conditioned medium from monocytes, and co-culture experiments performed with RPMI1640 in 10% FBS during the experiments [19].
Cell viability detection
VSMCs (5.0×103/well) were seeded in 96-well plates with various concentrations of uric acid (0, 6, 9, 12 mg/dl) for 24 h, 48 h, and 72 h. Then, Cell Counting Kit-8 (CCK-8) agent (Beyotime, China) was added to the cells, and the absorbance was measured at 450 nm by an ELISA reader (BioTek, USA) according to the manufacturer’s protocol.
Colony formation assay
We combined 1.5 ml 2×VSMC growth media with 1.5 ml of 1% bact-agar solution, plated onto 60-mm Petri dishes and allowed to solidify. Then, 1×104 cells in 1.5 ml 2×growth media was combined with 1.5 ml of 0.7% agarose solution and plated on top of the bottom layer. After 2 weeks, plates were stained with crystal violet for 3 h for visualization and counting.
Immunofluorescence
Cells were placed on collagen-coated coverslips in 24-well multi-chamber slides (7.5×104 cells/cm2) and were treated with the indicated reagents. Cells were washed with PBS and fixed with 1.5% paraformaldehyde, and F-actin was stained for 15 min with 5×10−6 mol/L tetramethylrhodamine isothiocyanate (TRITC)-phalloidin (Sigma Chemical Co., St. Louis, MO) in PBS containing 0.01% Nonidet. For staining cells in collagen gels, fluorescence staining for Alpha-smooth muscle actin (α-SMA) and β-actin was conducted for monolayer cultures as described previously [20].
Western blotting
Cells were washed once with cold phosphate-buffered saline (PBS). Cells were lysed in lysis buffer (20 mM Tris-HCl, pH 7.4, 150 mM NaCl, 0.5% NP-40, 10% Glycerol, 1 mM DTT and complete protease inhibitor cocktail) for 10 min on ice and centrifuged at 20 000 rpm for 10 min. Protein was detected on the clarified lysates, and equal amounts of protein were then processed for immunblotting. The membranes were scanned using the Li-COR system. Primary antibodies (Abcam, Cambridge, UK) were used at 1: 500 dilutions and secondary antibodies (Santa Cruz, CA, USA) were used at 1: 10 000 dilutions [21].
Reverse transcription polymerase chain reaction (RT-PCR)
Total RNAs were extracted using TRIzol (Invitrogen) according to the manufacturer’s protocol. The cDNA was synthesized and amplified with a Multiplex polymerase chain reaction (PCR) kit (TaKaRa, Japan). Quantitative PCR was performed using SYBR Green (TaKaRa) and the ABI 7500 real-time PCR system (Applied Biosystems). The primers used were:
NLRP3 forward, 5′-CTAGGCAACAACGACTTGGG-3′,
reverse, 5′-ACCGAGAAGGCTCA AAGACA-3′;
ASC forward, 5′-ACTCATTGCCAGGGTCACAGAAGTG-3′,
reverse, 5′-GCTTCCTCATCTTGTCTTGGCTGGT-3′;
Caspase-1 forward, 5′-CGTCTTGCCCTCATTATCTG-3′,
reverse, 5′-TCACCTCTTTCACCATCTCC-3′;
GAPDH forward, 5′-CTTTGGTATCGTGGAAGGACTC-3′ and
reverse, 5′-GTAGAGGCAGGGATGATGTTCT-3′.
Enzyme-linked immunoassay (ELISA)
Cytokine levels of IL-18 and IL-1β were measured by ELISA according the manufacturer’s instructions. The mature IL-1β ELISA used the monoclonal antibody (mAb) and rabbit polyclonal mature IL-1βAb (raised against entire 17-kDa mature IL-1β) as capture and detection antibodies, respectively. For the measurement of IL-18, ELISA kits from R&D Systems were used [19].
Statistical analysis
We used the t test for statistical analysis of qPCR and ELISA results. Data are represented as mean ±SEM of triplicate independent sets of experiments. Statistical significance is indicated as an asterisk (*) (P<0.05).
Results
High level of Uric acid promoted the proliferation of VSMC
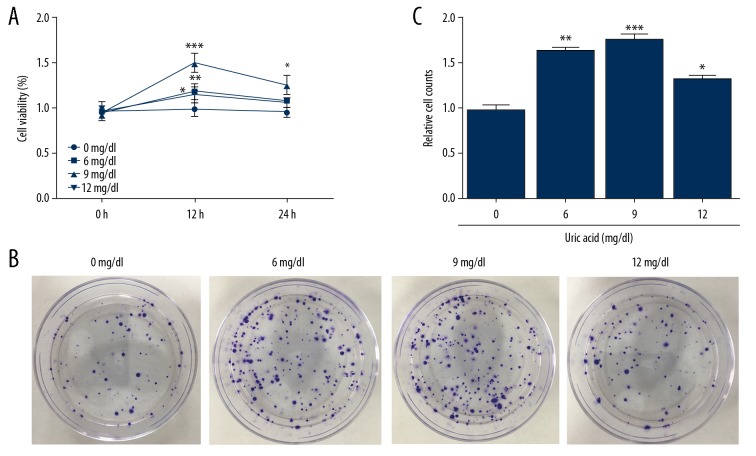
The doses of uric acid (0, 6, 9, 12 mg/dl) were added to VSMC and their proliferation ability was tested by CCK-8 and colony formation assay. Since the proliferation of VSMCs leads to intimal thickening in restenosis and other CVDs, we first examined the cell proliferative ability of VSMCs at elevated uric acid levels. The results of CCK-8 assay showed that with the increase of the dose to 9 mg/dl, cell proliferation was maximally induced, and after 12 mg/dl, the cell proliferation ability declined but was still higher than in the control group. Colony formation assay also showed similar results (Figure 1), indicating that uric acid affects the proliferation of VSMCs. When uric acid was at 9 mg/dl, the proliferative ability of VSMCs was increased.
Figure 1.
High level of uric acid promoted the proliferation of VSMC. Different doses of uric acid (0, 6, 9, 12 mg/dl) were added to VSMC. The cell viability of VSMCs was determined by CCK-8 at 12 h and 24 h (A). Colony formation assay was performed to detect cell proliferation (B) and for quantification of colony formation numbers (C). Experiments were performed at least 3 times. Data are expressed as mean ±SD. * p<0.05, ** p<0.01, *** p<0.001 vs. cells treated with 0 mg/ml uric acid.
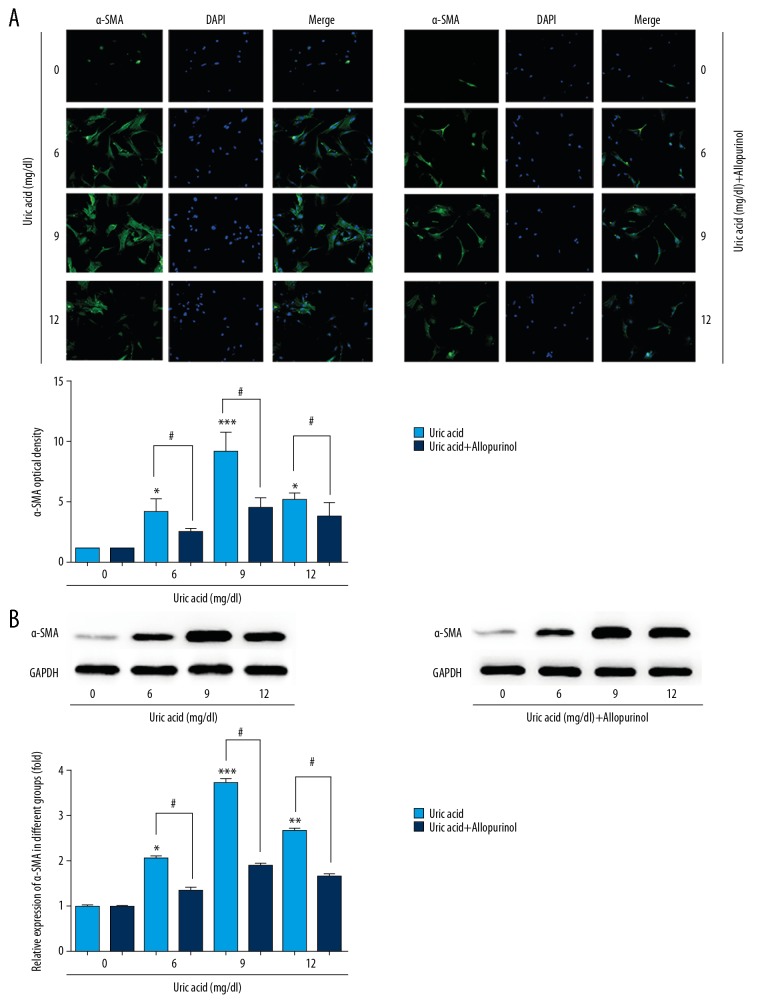
Allopurinol inhibited uric acid-induced high expression of α-SMA in VSMCs
VSMCs were pretreated with uric acid (0, 6, 9, 12 mg/dl), with or without allopurinol treatment, then expression level of α-SMA was observed by immunofluorescence and Western blot (Figure 2). The expression level of α-SMA as determined by immunofluorescence was quantified according to the method described in a previous study [22]. The immunofluorescence of the protein level of α-SMA increased compared to the control group, and the α-SMA level decreased after 12 mg/dl but was still higher than in the control group, consistent with its effect on VSMCs proliferation, whereas allopurinol inhibited the expression of α-SMA in VSMCs. Western blot analysis (Figure 2B) also showed continuous changes in α-SMA expression under different concentrations of uric acid as detected by immunofluorescence, and protein expression of α-SMA was also decreased by treatment with allopurinol.
Figure 2.
Allopurinol inhibited uric acid-induced high expression of α-SMA in VSMCs. The VSMCs were pretreated with uric acid (0, 6, 9, 12 mg/dl), with or without treatment of allopurinol. Then, the expression level of α-SMA was determined by immunofluorescence (A) and Western blot (B). Experiments were performed at least 3 times. Data are expressed as mean ±SD. * p<0.05, ** p<0.01, *** p<0.001 vs. cells treated with 0 mg/ml uric acid. # p<0.05, ## p<0.01 vs. cells only treated with uric acid.
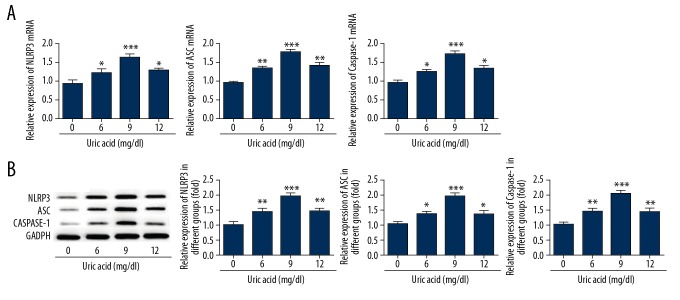
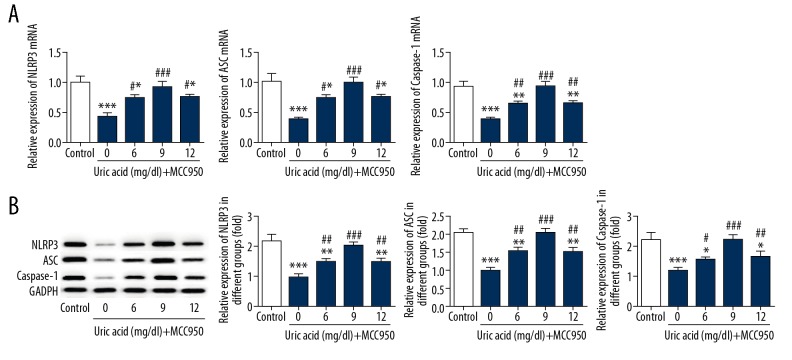
Inhibition of NLRP3 suppressed uric acid-induced high expression of the NLRP3-inflammasome in VSMCs
After VSMCs were treated with uric acid (0, 6, 9, 12 mg/dl) for 24 h, the protein and gene expressions of NLRP3 were analyzed. The levels of NLRP3, ASC (apoptosis-associated speck-like protein containing a CARD) and caspase-1 were significantly increased compared to the control cells (Figure 3). After VSMCs were treated with uric acid (0, 6, 9, 12 mg/dl), the inhibitor of NLRP3-MCC950 was added into cells. After 1 day, the expression level of NLRP3 was detected by Western blot and real-time PCR. We observed that MCC950 downregulated the expression of NLRP3, ASC, and caspase-1 in VSMCs (Figure 4), indicating that inhibition of NLRP3 protects against hypertension.
Figure 3.
Uric acid increased the expression of the NLRP3-inflammasome in VSMCs. After VSMCs were treated with uric acid (0, 6, 9, 12 mg/dl) for 24 h, the expressions of NLRP3, ASC, and caspase-1 were analyzed by RT-PCR (A) and Western blot (B). Experiments were performed at least 3 times. * p<0.05, ** p<0.01, *** p<0.001 vs. cells treated with 0 mg/ml uric acid.
Figure 4.
Inhibition of NLRP3 suppressed the expression of the NLRP3-inflammasome in VSMCs. VSMCs were pretreated with uric acid (0, 6, 9, 12 mg/dl) and were treated with NLRP3 inhibitor-MCC950. The expressions of NLRP3, ASC, and caspase-1 were analyzed by RT-PCR (A) and Western blot (B). Experiments were performed at least 3 times. * p<0.05, ** p<0.01, *** p<0.001 vs. control. # p<0.05, ## p<0.01, ### p<0.001 vs. cells treated with MCC950 only.
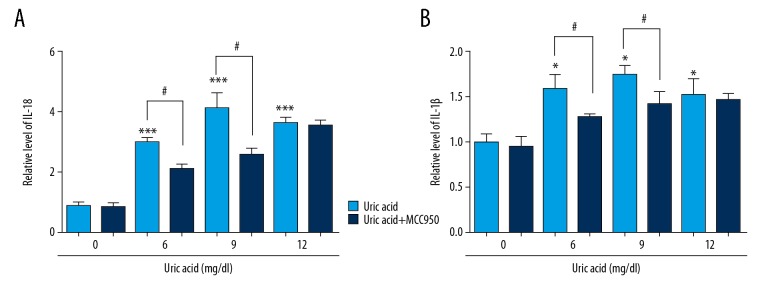
Inhibition of NLRP3 suppressed the downstream signaling of IL-1β and IL-18 activated by uric acid
Cells were pretreated with uric acid (0, 6, 9, 12 mg/dl) for 24 h, with or without MCC950, and the production of the inflammatory cytokines IL-18 and IL-1β was measured using ELISA. When cells were treated with uric acid only, the expressions of IL-18 and IL-1β were increased in a dose-dependent manner up to 9 mg/dl, and decreased under 12 mg/dl but were still higher than in the control group, consistent with the previous results. MCC950 significantly decreased the expression of IL-18 and IL-1β in VSMCs when cells were treated with uric acid at 6 mg/dl and 9 mg/dl (Figure 5).
Figure 5.
Inhibition of NLRP3 suppressed the downstream signaling of IL-1β and IL-18 activated by uric acid. In cells pretreated with uric acid (0, 6, 9, 12 mg/dl) for 24 h, with or without MCC950, the levels of IL-1β (A) and IL-18 (B) were detected by ELISA. Data are expressed as mean ±SD. * p<0.05, *** p<0.001 vs. cells treated with 0 mg/ml uric acid. # p<0.05 vs. cells only treated with uric acid.
Discussion
Uric acid, the final product of the purine metabolism, has been a focus of research because of its association with various inflammatory diseases and high mortality in hyperuricemic patients. A large body of evidence suggests that elevated uric acid levels are strongly associated with the occurrence and development of hypertension [23,24]. Because hyperuricemia is a risk factor for hypertension, it is important to be aware of the mechanisms involved in the production of uric acid [25].
VSMCs can infiltrate into the vascular intima and undergo remodeling to ultimately transform into an inflammatory phenotype of atherosclerosis [26]. In addition, VSMCs are the main source of foam cells as well as macrophages, and can include residual cholesterol in human arteriosclerotic diseases [27]. Our results show that increasing uric acid promotes the proliferation of VSMCs. Furthermore, the protein level of α-SMA is increased by uric acid stimulation. α-SMA is commonly used as a marker of myofibroblast formation. Allopurinol, a specific inhibitor of xanthine oxidase, is the pro-drug of oxypurinol and is mainly used for the prevention of gout and tumor lysis syndrome with either disease- or therapy-related hyperuricemia [28]. Whitman et al. [29] found that allopurinol could protect endothelial cell function in patients with coronary heart disease. In particular, allopurinol was also reported to efficiently block uric acid generation [28]. In the present study, allopurinol treatment significantly decreased the protein level of α-SMA induced by uric acid, consistent with previous reports, indicating that allopurinol could be used to treat cardiovascular diseases [30].
NLRP3-inflammasome-mediated inflammation is critically involved in myocardial injury, with a prominent role for IL-1β, which acts as an early mediator of inflammation [31,32]. The inflammasome is a multiprotein complex that affects activation of caspase-1, which activates secretion of the proinflammatory cytokines IL-18 and IL-1β [33]. Upon activation, NLRP3 undergoes conformational changes along with self-oligomerization binding to a ligand. In this case, receptor oligomerization leads to the recruitment of ASC, an inflammasome adaptor composed of C-terminal CARD and N-terminal PYD, thus promoting the formation of an ASC-PYD filament [34,35]. Then, the homotypic CARDs interact with each other, recruiting the immature form of the inflammasome effector caspase-1. On the ASC filament, pro-caspase-1 oligomerization causes maturation of caspase-1. Cleaved caspase-1 in turn forms an active heterotetramer, which ultimately produces the mature form of the highly inflammatory cytokines IL-1β and IL-18 [36,37]. Previous data showed that uric acid is a main activator for the NLRP3 inflammasome in pseudogout and in gout [38]. In this study, we surprisingly found that uric acid elevated the expression of the NLRP3-inflammasome in VSMCs. We also found that MCC950, the NLRP3 inhibitor, can rescue uric acid-induced α-SMA expression. However, although these results show that elevated α-SMA expression induced by uric acid activates the NLRP3-inflammasome pathway, the protein level of α-SMA regulated by NLRP3 in vitro and in vivo are still unclear. Further studies are needed to clarify whether uric acid is involved in the development of CVDs and to identify possible methods for the management of CVDs.
Conclusions
We provide evidence that uric acid promotes VSMCs proliferation through NLRP3-inflammasome activation. Our results suggest a new role of the NLRP3-inflammasome, which can modulate the inflammatory cytokines in uric acid-stimulated VSMCs. Inhibition of NLRP3 signaling might serve as a therapeutic target in the management of hypertension, and provide a new strategy for the treatment of hypertension.
Footnotes
Source of support: Departmental sources
Conflict of interest
None.
References
- 1.Kutzing MK, Firestein BL. Altered uric acid levels and disease states. J Pharmacol Exp Ther. 2008;324(1):1–7. doi: 10.1124/jpet.107.129031. [DOI] [PubMed] [Google Scholar]
- 2.Abeles AM. Hyperuricemia, gout, and cardiovascular disease: An update. Curr Rheumatol Rep. 2015;17(3):13. doi: 10.1007/s11926-015-0495-2. [DOI] [PubMed] [Google Scholar]
- 3.Wannamethee SG, Papacosta O, Lennon L, Whincup PH. Serum uric acid as a potential marker for heart failure risk in men on antihypertensive treatment: The British Regional Heart Study. Int J Cardiol. 2018;252:187–92. doi: 10.1016/j.ijcard.2017.11.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kanbay M, Segal M, Afsar B, et al. The role of uric acid in the pathogenesis of human cardiovascular disease. Heart. 2013;99(11):759–66. doi: 10.1136/heartjnl-2012-302535. [DOI] [PubMed] [Google Scholar]
- 5.Sanchez-Lozada LG. The pathophysiology of uric acid on renal diseases. Contrib Nephrol. 2018;192:17–24. doi: 10.1159/000484274. [DOI] [PubMed] [Google Scholar]
- 6.Yoshino S, Tabata H, Miyata M, et al. Hyperuricemia is associated with endothelial dysfunction and morphological abnormalities of neointima after 2nd generation drug-eluting stents deplpyment assessed by optical coherence tomography. J Am Coll Cardiol. 2018;71(11 Suppl):A1250. [Google Scholar]
- 7.Choi HK, Atkinson K, Karlson EW, et al. Purine-rich foods, dairy and protein intake, and the risk of gout in men. N Engl J Med. 2004;350(11):1093–103. doi: 10.1056/NEJMoa035700. [DOI] [PubMed] [Google Scholar]
- 8.Miao Z, Li C, Chen Y, et al. Dietary and lifestyle changes associated with high prevalence of hyperuricemia and gout in the Shandong coastal cities of Eastern China. J Rheumatol. 2008;35(9):1859–64. [PubMed] [Google Scholar]
- 9.Rodenbach KE, Schneider MF, Furth SL, et al. Hyperuricemia and progression of CKD in children and adolescents: The chronic kidney disease in children (CKiD) Cohort Study. Am J Kidney Dis. 2015;66(6):984–92. doi: 10.1053/j.ajkd.2015.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Viazzi F, Bonino B, Ratto E, et al. [Hyperuricemia, diabetes and hypertension]. G Ital Nefrol. 2015;32(Suppl 62) pii: gin/32.S62.10 [in Italian] [PubMed] [Google Scholar]
- 11.Chen M, Lu X, Lu C, et al. Soluble uric acid increases PDZK1 and ABCG2 expression in human intestinal cell lines via the TLR4-NLRP3 inflammasome and PI3K/Akt signaling pathway. Arthritis Res Ther. 2018;20(1):20. doi: 10.1186/s13075-018-1512-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jin M, Yang F, Yang I, et al. Uric acid, hyperuricemia and vascular diseases. Front Biosci (Landmark Ed) 2012;17:656–69. doi: 10.2741/3950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Villani AC, Lemire M, Fortin G, et al. Common variants in the NLRP3 region contribute to Crohn’s disease susceptibility. Nat Genet. 2009;41(1):71–76. doi: 10.1038/ng285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wen H, Ting JP, O’Neill LA. A role for the NLRP3 inflammasome in metabolic diseases – did Warburg miss inflammation? Nat Immunol. 2012;13(4):352–57. doi: 10.1038/ni.2228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Calixto JB, Campos MM, Otuki MF, Santos AR. Anti-inflammatory compounds of plant origin. Part II. modulation of pro-inflammatory cytokines, chemokines and adhesion molecules. Planta Med. 2004;70(2):93–103. doi: 10.1055/s-2004-815483. [DOI] [PubMed] [Google Scholar]
- 16.Schroder K, Zhou R, Tschopp J. The NLRP3 inflammasome: A sensor for metabolic danger? Science. 2010;327(5963):296–300. doi: 10.1126/science.1184003. [DOI] [PubMed] [Google Scholar]
- 17.Haneklaus M, Gerlic M, Kurowska-Stolarska M, et al. Cutting edge: miR-223 and EBV miR-BART15 regulate the NLRP3 inflammasome and IL-1beta production. J Immunol. 2012;189(8):3795–99. doi: 10.4049/jimmunol.1200312. [DOI] [PubMed] [Google Scholar]
- 18.Cullen SP, Kearney CJ, Clancy DM, Martin SJ. Diverse activators of the NLRP3 inflammasome promote IL-1beta secretion by triggering necrosis. Cell Rep. 2015;11(10):1535–48. doi: 10.1016/j.celrep.2015.05.003. [DOI] [PubMed] [Google Scholar]
- 19.Sarkar A, Mitra S, Mehta S, et al. Monocyte derived microvesicles deliver a cell death message via encapsulated caspase-1. PLoS One. 2009;4(9):e7140. doi: 10.1371/journal.pone.0007140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Arora PD, Narani N, McCulloch CA. The compliance of collagen gels regulates transforming growth factor-beta induction of alpha-smooth muscle actin in fibroblasts. Am J Pathol. 1999;154(3):871–82. doi: 10.1016/s0002-9440(10)65334-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Prasad EM, Mopuri R, Islam MS, Kodidhela LD. Cardioprotective effect of Vitex negundo on isoproterenol-induced myocardial necrosis in wistar rats: A dual approach study. Biomed Pharmacother. 2017;85:601–10. doi: 10.1016/j.biopha.2016.11.069. [DOI] [PubMed] [Google Scholar]
- 22.Eggenhofer E, Steinmann JF, Renner P, et al. Mesenchymal stem cells together with mycophenolate mofetil inhibit antigen presenting cell and T cell infiltration into allogeneic heart grafts. Transpl Immunol. 2011;24(3):157–63. doi: 10.1016/j.trim.2010.12.002. [DOI] [PubMed] [Google Scholar]
- 23.Zhang JX, Zhang YP, Wu QN, Chen B. Uric acid induces oxidative stress via an activation of the renin-angiotensin system in 3T3-L1 adipocytes. Endocrine. 2015;48(1):135–42. doi: 10.1007/s12020-014-0239-5. [DOI] [PubMed] [Google Scholar]
- 24.Kirca M, Oguz N, Cetin A, et al. Uric acid stimulates proliferative pathways in vascular smooth muscle cells through the activation of p38 MAPK, p44/42 MAPK and PDGFRbeta. J Recept Signal Transduct Res. 2017;37(2):167–73. doi: 10.1080/10799893.2016.1203941. [DOI] [PubMed] [Google Scholar]
- 25.Kuwabara M, Niwa K, Hisatome I, et al. Asymptomatic hyperuricemia without comorbidities predicts cardiometabolic diseases: Five-year Japanese cohort study. Hypertension. 2017;69(6):1036–44. doi: 10.1161/HYPERTENSIONAHA.116.08998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Orekhov AN, Andreeva ER, Krushinsky AV, et al. Intimal cells and atherosclerosis. Relationship between the number of intimal cells and major manifestations of atherosclerosis in the human aorta. Am J Pathol. 1986;125(2):402–15. [PMC free article] [PubMed] [Google Scholar]
- 27.Allahverdian S, Chehroudi AC, McManus BM, et al. Contribution of intimal smooth muscle cells to cholesterol accumulation and macrophage-like cells in human atherosclerosis. Circulation. 2014;129(15):1551–59. doi: 10.1161/CIRCULATIONAHA.113.005015. [DOI] [PubMed] [Google Scholar]
- 28.Riegersperger M, Covic A, Goldsmith D. Allopurinol, uric acid, and oxidative stress in cardiorenal disease. Int Urol Nephrol. 2011;43(2):441–49. doi: 10.1007/s11255-011-9929-6. [DOI] [PubMed] [Google Scholar]
- 29.Whitman SC, Miller DB, Wolfe BM, et al. Uptake of type III hypertriglyceridemic VLDL by macrophages is enhanced by oxidation, especially after remnant formation. Arterioscler Thromb Vasc Biol. 1997;17(9):1707–15. doi: 10.1161/01.atv.17.9.1707. [DOI] [PubMed] [Google Scholar]
- 30.Zhu X, Guo J, He C, et al. Ultrasound triggered image-guided drug delivery to inhibit vascular reconstruction via paclitaxel-loaded microbubbles. Sci Rep. 2016;6:21683. doi: 10.1038/srep21683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pomerantz BJ, Reznikov LL, Harken AH, Dinarello CA. Inhibition of caspase 1 reduces human myocardial ischemic dysfunction via inhibition of IL-18 and IL-1beta. Proc Natl Acad Sci USA. 2001;98(5):2871–76. doi: 10.1073/pnas.041611398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yellon DM, Hausenloy DJ. Myocardial reperfusion injury. N Engl J Med. 2007;357(11):1121–35. doi: 10.1056/NEJMra071667. [DOI] [PubMed] [Google Scholar]
- 33.Franchi L, Eigenbrod T, Munoz-Planillo R, Nunez G. The inflammasome: A caspase-1-activation platform that regulates immune responses and disease pathogenesis. Nat Immunol. 2009;10(3):241–47. doi: 10.1038/ni.1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lu A, Magupalli VG, Ruan J, et al. Unified polymerization mechanism for the assembly of ASC-dependent inflammasomes. Cell. 2014;156(6):1193–206. doi: 10.1016/j.cell.2014.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Srinivasula SM, Poyet JL, Razmara M, et al. The PYRIN-CARD protein ASC is an activating adaptor for caspase-1. J Biol Chem. 2002;277(24):21119–22. doi: 10.1074/jbc.C200179200. [DOI] [PubMed] [Google Scholar]
- 36.Latz E, Xiao TS, Stutz A. Activation and regulation of the inflammasomes. Nat Rev Immunol. 2013;13(6):397–411. doi: 10.1038/nri3452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Broderick L, De Nardo D, Franklin BS, et al. The inflammasomes and autoinflammatory syndromes. Annu Rev Pathol. 2015;10:395–424. doi: 10.1146/annurev-pathol-012414-040431. [DOI] [PubMed] [Google Scholar]
- 38.Martinon F, Petrilli V, Mayor A, et al. Gout-associated uric acid crystals activate the NALP3 inflammasome. Nature. 2006;440(7081):237–41. doi: 10.1038/nature04516. [DOI] [PubMed] [Google Scholar]