Abstract
Objectives:
Heart transplantation (HT) in children with heterotaxy may be affected by anomalous cardiac position, venous return, and splenic function. Outcomes are not well described. We compared post-HT outcomes, including survival and resource utilization, among recipients with congenital heart disease (CHD) in the presence and absence of heterotaxy.
Methods:
Using linked Pediatric Health Information System and Scientific Registry of Transplant Recipients data (2001–2016), we identified 177 HT recipients with heterotaxy. We compared post-HT outcomes to 1,202 non-heterotaxy recipients with CHD in multivariable regression models. Length of stay (LOS) and cost from HT to discharge were also compared.
Results:
Heterotaxy HT recipients were older (median age 5.1 vs. 1.6 years, p<0.001) and more often Black, Asian, Hispanic, or “other” non-Caucasian (54 vs. 32%, p<0.001). Heterotaxy was independently associated with increased mortality (HR 1.61, [95% CI 1.20–2.15], p=0.001), even among 6-month survivors (HR 2.00 [1.17–3.43], p=0.012). Heterotaxy recipients more commonly required dialysis (OR 2.64 [1.52–4.56], p=0.001) and cardiac reoperation (OR 2.36 [1.29–3.41], p=0.003) prior to discharge. They had longer ischemic times (19 additional minutes [10.4–27.6], p<0.001, adjusted for organ procurement distance), ICU LOS (16 vs. 13 days, p=0.012), and hospital LOS (median 26 vs. 23 days, p=0.005). Post-HT hospitalization costs were also greater ($448,000 vs. $381,000, p=0.001).
Conclusions:
Heterotaxy is associated with increased post-operative complications, length of stay, costs, and mortality after HT. While increased surgical complexity can account for many of these differences, inferior late survival is not well explained and deserves further study.
Central message:
Heart transplantation in heterotaxy syndrome is associated with increased post-operative complications and inferior early and late survival compared to other forms of congenital heart disease.
Perspective statement:
Heart transplantation in heterotaxy is underreported and affected by anomalous cardiac position, venous return, and splenic function. This novel linked-registry analysis is the largest report of post-HT outcomes for children with heterotaxy. Providers should recognize that although surgical complexity contributes to increased early mortality, mechanisms of inferior late survival deserve more attention.
1. INTRODUCTION
Heterotaxy is a syndrome characterized by organ laterality defects and complex congenital heart disease (CHD)[1]. Survival in patients with heterotaxy has historically been poor compared to other forms of CHD, particularly early after cardiac surgery due to the technical difficulties posed by anomalies of situs[2, 3]. Heart transplantation (HT) has been reported for patients with heterotaxy and anomalies of cardiac situs in case reports[4] and single center series[5]. However, due to the lack of an indicator variable in the major transplant registries there has been no large, multicenter analysis of outcomes and complications for this important group of pediatric HT recipients; and thus limited information to guide providers, patients, and families.
In this study we used a novel linkage between the Scientific Registry of Transplant Recipients (SRTR) and the Pediatric Health Information System (PHIS) databases to describe survival, length of stay, and hospitalization costs following HT for children and young adults with heterotaxy. We hypothesized that HT recipients with heterotaxy would have increased early post-HT mortality, greater length of stay (LOS), and higher post-HT hospital costs compared to recipients with CHD in the absence of heterotaxy. Following the early-post HT phase, we hypothesized that survival for heterotaxy patients would be similar to non-heterotaxy recipients.
2. METHODS
2.1. Database Linkage and Patient Selection
Linkage of the SRTR and PHIS databases at the patient level using indirect identifiers was performed and validated as previously described [6]. The SRTR data system includes data on all donors, wait-listed candidates, and transplant recipients in the US, submitted by the members of the Organ Procurement and Transplantation Network (OPTN). The Health Resources and Services Administration (HRSA), U.S. Department of Health and Human Services provides oversight to the activities of the OPTN and SRTR contractors. [7]. PHIS is an administrative database of ICD-9/10 codes, hospital charges, and resource utilization for hospital-based patient encounters across more than 45 US children’s hospitals[8]. For this analysis ICD-10 codes were mapped to ICD-9 using the AHRQ MapIT Tool[9](supplemental table 1).
The linked database contained 3,062 records of transplant and follow-up data from November 2001 to November 2016. After exclusions for retransplantation (n=179), unavailable cost data (n=151), and non-CHD diagnosis (n=1,353), analysis was performed on patients who underwent primary HT for CHD (n=1,379, 45%). Heterotaxy was defined as any patient with an SRTR diagnosis of CHD plus ≥1 of the following ICD-9/10 codes recorded in their entire PHIS record: 759.3/Q89.3 (situs inversus); 759.0/Q89.01, Q89.09 (polysplenia/asplenia); or 746.87/Q24.0 (dextrocardia). Primary HT recipients that did not meet these criteria formed the comparison group of CHD recipients without heterotaxy (non-heterotaxy CHD).
2.2. Data Sources and Collection
From SRTR we collected recipient sex, race, diagnosis (CHD/non-CHD), history of sternotomy and/or thoracic surgery, and HT date; age, UNOS waitlist urgency status, patient location (ICU/in-hospital/out-hospital), and use of inotropic support, mechanical ventilation, VAD, and ECMO at HT; dialysis while listed; most recent pre-HT serum creatinine; donor-specific crossmatch result (DSXM); ischemic time; and donor and recipient organ procurement organizations (OPOs). The following events were collected from SRTR: treatment for infection or acute rejection, cardiac reoperation, and post-transplant dialysis during the HT admission; latest vital status (alive/deceased/retransplanted) with date; cause of death; infection or rejection requiring rehospitalization; and occurrence of post-transplant lymphoproliferative disease (PTLD) and coronary allograft vasculopathy (CAV).
PHIS data were used to characterize the underlying CHD phenotypes in the study population; characterize surgeries, procedures, and infections that occurred after HT during the HT hospitalization; quantify the durations of post-HT mechanical ventilation, and post-HT ICU and post-HT hospital LOS; and quantify inpatient costs. All costs were adjusted for inflation to 2016 US Dollars using the medical component of the consumer price index and were calculated from charges using hospital and year-specific cost-to-charge ratios.
To characterize the spectrum of underlying CHD phenotypes we identified the following cardiovascular morphology ICD-9/10 diagnosis codes applied during the transplant hospitalization in PHIS: ICD-9 codes beginning with 745 (Bulbus cordis anomalies and anomalies of cardiac septal closure), 746 (Other congenital anomalies of the heart), and 747 (Other congenital anomalies of the circulatory system), and ICD-10 codes Q20-Q28 (Congenital malformations of the circulatory system). Similarly, cardiovascular surgeries and procedures that occurred after HT during the transplant hospitalization were assessed by identifying ICD-9 procedure codes beginning with 35 through 39 (Operations on the cardiovascular system) and ICD-10 procedure codes beginning with 02 (Heart and Great Vessels) after HT. To explore differences in encapsulated bacterial infections, we identified the relevant ICD-9/10 diagnosis codes assigned during the transplant admission (supplemental table 2). Duplicated codes were only counted once per subject.
2.3. Statistical Analysis
Data are described as median (interquartile range), mean ± standard deviation, or count (%) as appropriate. Odds and hazards ratios are reported with 95% CI [lower bound- upper bound]. Categorical data were compared using the chi-squared or Fisher’s exact test, as appropriate. Continuous data were compared using the Wilcoxon rank-sum test. Post-HT procedures were compared between the heterotaxy and non-heterotaxy CHD groups by calculating the odds ratio of procedure with Fisher exact 95% confidence limits. Univariate survival was assessed with the Kaplan-Meier estimator and compared using the log-rank test. Because death competes with other time-to-event outcomes (infection, rejection, PTLD, and CAV after HT discharge) these outcomes were analyzed by competing risk analyses[10]. Significant differences in outcomes between heterotaxy and non-heterotaxy CHD groups in univariate comparison were adjusted for covariates using multivariable Cox proportional hazards, logistic, and linear regression models, as appropriate. To build the models the following covariates were tested for a univariate effect on outcome: race, age, year of HT, prior sternotomy, dialysis while on waitlist, DSXM result, waitlist urgency status, patient location, inotropic support, mechanical ventilation, VAD, and ECMO. For analysis of post-HT renal failure requiring dialysis, we also included pre-HT dialysis, glomerular filtration rate (eGFR) <40 ml/min/1.73m2, and ischemic time >3.5 hours. eGFR was estimated using the modified bedside Schwartz formula[11], capped at 200 mL/min/1.73 m2. Procurement distance was estimated by computing the geodesic distance between OPO zip codes[12]. Covariates with p≤0.1 in univariate comparisons were included in a multivariable model with the main variable of interest. Covariates with Wald test p>0.1 in the full multivariable model were then dropped from the analysis to produce the final models. The proportional hazards assumption was assessed for the final Cox models by visualization of log-log survival plots and with regression of the scaled Schoenfeld residuals on the identity function of time [13]. Covariates that violated the PH assumption were stratified in the Cox models. Presence of significant interaction terms were assessed with the likelihood ratio test.
Incomplete data were compared across groups (supplemental table 3). For pre-HT VAD and dialysis, missing data were rare (<3%) and imputed as absence of the feature. For DSXM, prior sternotomy, and prior univentricular CHD repair surgery, missing data were assumed to be missing at random and imputed using multiple imputation. Subjects with missing outcomes data were excluded for analysis of that outcome.
2.4. Sensitivity Analyses
To address potential misclassification based on the non-specificity of the dextrocardia and situs inversus ICD codes, subjects were reclassified using stricter inclusion criteria of only asplenia/polysplenia ICD codes and the survival analysis was repeated. To address the potential for bias from inadequate/incomplete selection of regression model covariables, we conducted propensity score matching of heterotaxy recipients where the probability of a subject being classified into the heterotaxy group was determined by logistic regression on characteristics listed in supplemental table 4. Missing data was coded as a categorical dummy variable for each covariate, allowing patients with missing data to be matched. Matching was 1:2 using a matching caliper of one-fifth the standard deviation of the logit of the propensity scores. Covariate balance was assessed through descriptive statistics of matching covariates as in the main analysis.
All analyses were performed using Stata13 (StataCorp, College Station, TX) with two-sided p<0.05 considered statistically significant. This research was approved by the Vanderbilt University and University of Pittsburgh Institutional Review Boards, PHIS, and SRTR.
3. RESULTS
3.1. Group Characteristics
Of 1,379 primary HT recipients with underlying CHD, 177 (13%) were categorized as heterotaxy and 1,202 (87%) as non-heterotaxy. Characteristics of the groups at transplant are shown in table 1. The heterotaxy group was older (median age 5.1 vs. 1.6 years, p<0.001) and more often of African-American, Asian, Hispanic, and “other” non-Caucasian race (54 vs. 32%, p<0.001). A higher proportion in the non-heterotaxy group had reduced eGFR (9 vs 3%, p=0.004). We found no other significant differences between groups, including proportion with prior sternotomy (68 vs. 61%, p=0.130).
Table 1:
Group Characteristics at HT
| Non-Heterotaxy CHD (n=1254) |
Heterotaxy (n=186) |
p-value† | |
|---|---|---|---|
| Age (years) | 1.6 [0.3–9.0] | 5.1 [0.8–11.5] | <0.001 |
| Female | 509 (41) | 80 (43) | 0.576 |
| Weight (kg)* | 9 [4.1–22.0] | 15.2 [6.4–30.0] | <0.001 |
| Height (cm)* | 76 [54–119] | 98 [63–138] | <0.001 |
| Creatinine (mg/dL)* | 0.4 [0.3–0.6] | 0.4 [0.3–0.6] | 0.296 |
| eGFR <40 mL/min/1.73 m2 * | 111/1223 (9) | 5/182 (3) | 0.004 |
| Race: Caucasian | 852 (68) | 89 (46) | <0.001‡ |
| African-American | 166 (13) | 36 (19) | |
| Asian | 20 (2) | 10 (5) | |
| Hispanic | 188 (15) | 42 (23) | |
| Other | 28 (2) | 9 (5) | |
| Year of Transplant | 2011 [2007–2014] | 2011 [2008–2014] | 0.267 |
| Listing Status: 1A | 1023 (82) | 146 (78) | 0.261‡ |
| 1B | 156 (12) | 31 (17) | |
| 2 | 75 (6) | 9 (5) | |
| Location: ICU | 637 (51) | 85 (46) | 0.357‡ |
| Hospitalized, non-ICU | 209 (17) | 37 (20) | |
| Not hospitalized | 408 (33) | 64 (34) | |
| Inotrope Support | 645 (51) | 97 (52) | 0.875 |
| VAD support | 86 (7) | 13 (8) | 0.878 |
| Dialysis* | 28 (2) | 6 (3) | 0.434 |
| ECMO | 94 (7) | 8 (5) | 0.206 |
| Mechanical Ventilation | 276 (22) | 36 (20) | 0.769 |
| Positive DSXM* | 203/1004 (20) | 22/149 (15) | 0.207 |
| Prior sternotomy and/or surgery for CHD* | 512/826 (62) | 94/137 (69) | 0.152 |
| If prior sternotomy and/or surgery for CHD, underwent univentricular repair* | 314/461 (68) | 63/83 (76) | 0.196 |
| Distance between donor and recipient OPO | 494 [200–638] | 328 [103–579] | 0.047 |
Data presented as n(%) or median[IQR];
missing data present;
continuous data compared with rank-sum test, binary outcomes compared using chi-squared test of proportions,
tested across all groups
Abbreviations: eGFR: estimated glomerular filtration rate; ICU: intensive Care Unit; VAD: Ventricular assist device; ECMO: Extracorporeal Membrane Oxygenation; DXSM: Donor-specific Crossmatch result; CHD: Congenital Heart Disease; OPO: Organ Procurement Organization.
ICD codes used to define the heterotaxy group were distributed as follows: dextrocardia alone (37%); asplenia/polysplenia alone (18%); asplenia/polysplenia with dextrocardia (18%); asplenia/polysplenia with situs inversus (10%); asplenia/polysplenia, dextrocardia, and situs inversus (8%); dextrocardia with situs inversus (7%); and situs inversus alone (3%). Supplemental table 5 demonstrates the spectrum of underlying CHD phenotypes described by ICD diagnosis codes. The heterotaxy group was enriched for codes for abnormal endocardial cushion development, common ventricle, pulmonary outflow tract obstruction, disorders of pulmonary and systemic venous return, truncus abnormalities, and conduction abnormalities, whereas the non-heterotaxy CHD group was enriched for hypoplastic left heart syndrome, coronary artery anomalies, and secundum atrial septal defects.
3.2. Survival After Transplantation
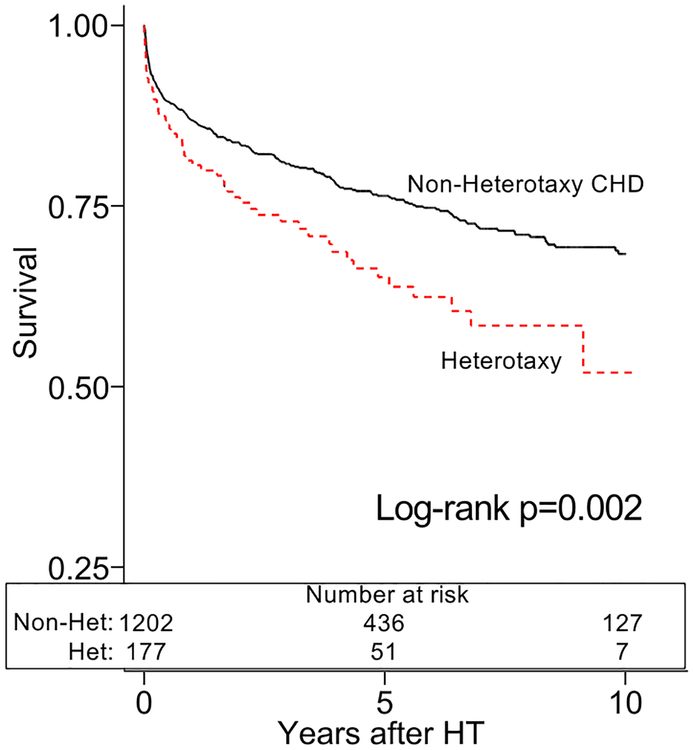
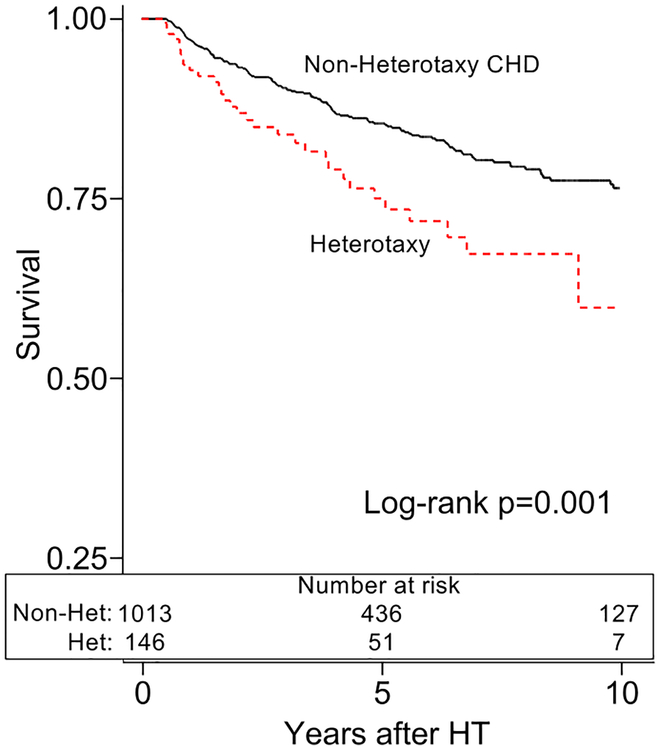
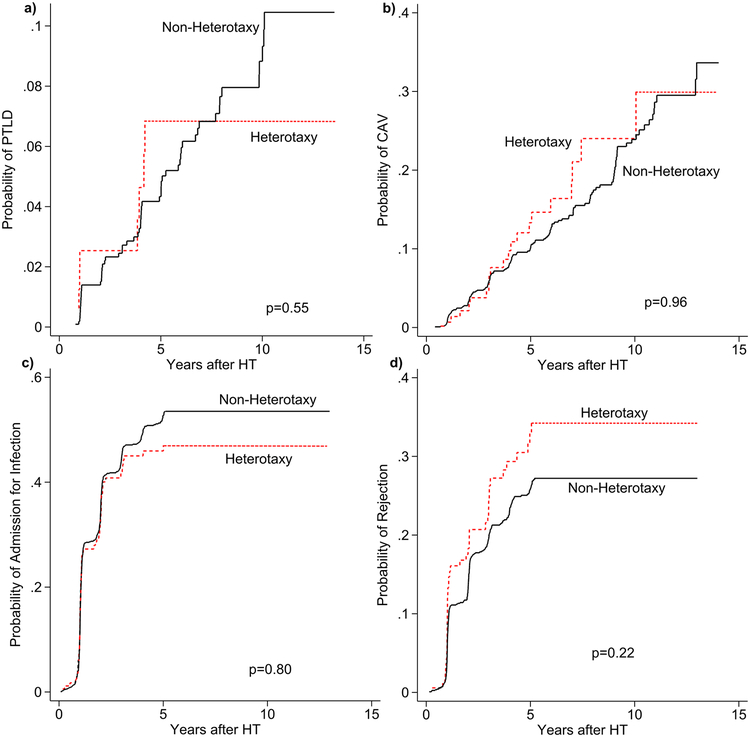
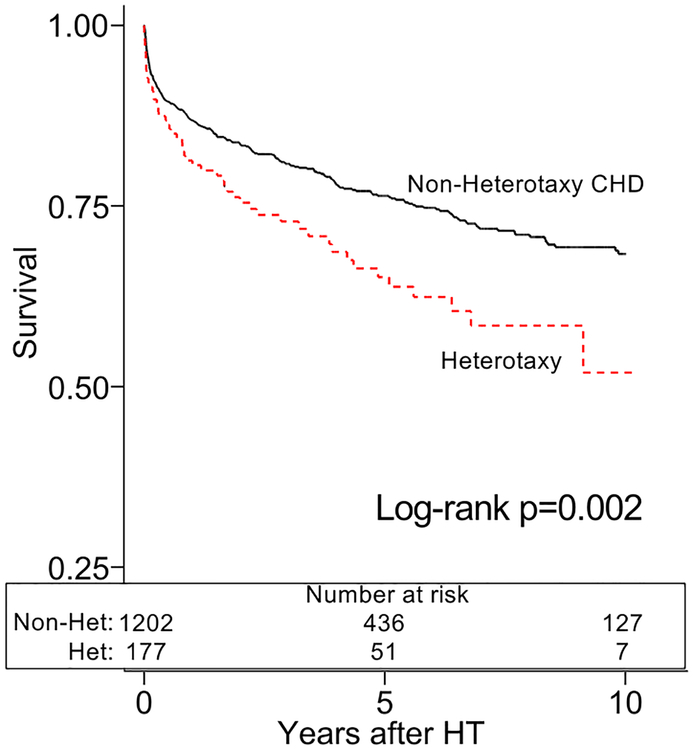
The heterotaxy group demonstrated inferior post-HT survival in unadjusted analysis (figure 1a). After adjustment for African-American race, calendar year of HT, and pre-HT ECMO, heterotaxy was associated with an increased mortality compared to non-heterotaxy CHD (HR 1.61, [1.20–2.15], p=0.001). These findings persisted amongst 6-month post-HT survivors in unadjusted (figure 1b) and adjusted analyses (HR 2.00, [1.17–3.43], p=0.012; adjusted for age and African-American race). There were no significant differences in cause of death between the groups (table 2). We also found no differences in the cumulative incidence of PTLD, CAV, admission for infection, or admission for rejection between the groups (figure 2a–d).
Figure 1a:
Survival after heart transplantation with heterotaxy syndrome
Caption: Patients with heterotaxy (dotted line) have inferior survival after heart transplant compared to other patients with congenital heart disease (Non-Heterotaxy CHD; solid line).
Figure 1b:
Survival after heart transplantation with heterotaxy syndrome, conditional upon survival to 6-months
Caption: Amongst patients that live to 6-months post-heart transplant, patients with heterotaxy (dotted line) have decreased survival after heart transplant compared to other patients with congenital heart disease (Non-Heterotaxy CHD; solid line).
Table 2:
Cause of Death
| Non-Heterotaxy CHD (n=291) | Heterotaxy (n=59) | p-value* | |
|---|---|---|---|
| Cardiovascular | 70 (24) | 12 (20) | 0.615 |
| Cerebrovascular | 21 (7) | 6 (10) | 1 |
| Graft Failure | 83 (29) | 16 (27) | 0.875 |
| Hemorrhage | 12 (4) | 3 (5) | 1 |
| Infection | 29 (10) | 8 (14) | 0.725 |
| Malignancy | 6 (2) | 3 (5) | 0.180 |
| Pulmonary | 28 (10) | 10 (17) | 0.109 |
| Other | 48 (16) | 8 (14) | 0.698 |
| Unknown | 26 (9) | 4 (7) | 0.592 |
Presented as n(%);
Fisher’s exact test of proportions; CHD: Congenital Heart Disease
Figure 2:
Cumulative incidence of development of common post-HT co-morbidities
Caption: Cumulative incidence of post-transplant lymphoproliferative disease (PTLD; 2a), coronary artery vasculopathy (CAV; 2b), first hospitalization for infection (2c), and first hospitalization for rejection (2d). Patients with heterotaxy syndrome (dotted line) are compared to other patients with congenital heart disease (solid line). Cumulative incidence is calculated for each group with the competing outcome of death/retransplant (these curves are not illustrated for clarity). Statistical comparison of curves performed as described by Pepe and Mori.
3.3. Post-transplant Complications, Length of Stay, and Cost
Perioperative complications recorded in the SRTR are shown in table 3. Cardiac reoperation (adjusted OR 2.10, p=0.003) and renal failure requiring dialysis (adjusted OR 2.63, p=0.001) were increased among heterotaxy recipients prior to discharge following HT. Heterotaxy recipients were more commonly treated for infection prior to discharge following HT (adjusted OR 1.61, p=0.015); however, we found no difference in PHIS-recorded encapsulated bacterial infection ICD codes between the groups (2.9 vs. 2.3%, p=0.638). There were similar proportions with acute rejection during the HT hospitalization (17 vs. 16%, p=0.716).
Table 3:
Peri-operative outcomes
| Outcome | Non-Heterotaxy CHD | Heterotaxy | Unadjusted OR | Unadjusted p-value | Adjusted OR | Adjusted p-value |
|---|---|---|---|---|---|---|
| Infection during HT admission | 294/974 (30%) | 57/145 (49%) | 1.50 [1.04–2.15] | 0.030 | 1.54 [1.06–2.25]* | 0.015* |
| Cardiac Reoperation | 97/979 (10%) | 27/143 (19%) | 2.12 [1.32, 3.38] | 0.002 | 1.91 [1.17–3.11] † | 0.010† |
| Post-HT Renal Failure Requiring Dialysis | 77/1253 (6%) | 23/186 (12%) | 2.16 [1.32, 3.53] | 0.002 | 2.58[1.51–4.42] ‡ | 0.001‡ |
| Mechanically Ventilated > 1 week | 429/1202 (36%) | 78/177 (44%) | 1.42 [1.3–1.95] | 0.032 | 1.70 [1.20–2.40] § | 0.003§ |
| Rejection during HT admission | 187/1124 (17%) | 29/137 (17%) | 1.02 [0.67,1.57] | 0.916 | -- | -- |
Source: SRTR database; CHD: Congenital heart disease; HT: Heart transplant; OPO: Organ Procurement Organization;
Data displayed as n(%), Odds ratio [95% CI]
Infection during HT admission adjusted for: Year, pre-HT ECMO pre-HT, Mechanical Ventilation (MV) pre-HT, home location at HT, positive DSXM, history of prior univentricular CHD repair
Cardiac reoperation adjusted for: patient race, VAD and MV pre-HT
Post-HT Renal Failure Requiring Dialysis failure adjusted for: Pre-HT dialysis, eGFR <40 mL/min/1.73 m2, ischemic time>3.5hours, ECMO, ICU location at HT, female race
Mechanical Ventilation >1 week adjusted for: MV pre-HT, pre-HT ECMO, Age
As shown in table 4, select ICD cardiac procedure codes were more common among heterotaxy recipients prior to discharge after HT, including cardiac catheterization (OR 1.48, p=0.01), placement of non-coronary, non-drug eluting stents (OR 2.85, p=0.016), and hemodialysis (OR 2.67, p=0.013). We also found the duration of mechanical ventilation after HT was greater for heterotaxy recipients (median 5 vs. 4 days, p=0.039; table 5), with a greater proportion of heterotaxy recipients requiring mechanical ventilation for >1 week after HT (44 vs. 36%, adjusted OR 1.70, [1.20–2.40], p=0.003). Ischemic times were longer for the heterotaxy group (mean 249 ± 74 vs. 234 ± 10 minutes, p=0.009), despite a shorter median distance between donor and recipient OPOs (328 (103–579) vs. 407 ± (208–656) miles, p=0.023). After adjustment for significant covariates (distance between donor and recipient OPOs, Caucasian race, outpatient location at HT, and history of prior univentricular CHD repair) in a linear regression model, heterotaxy was associated with 19 additional minutes of ischemic time (95% CI [10.4–27.6], p<0.001). Recipients with heterotaxy had longer post-HT ICU (median 16 vs. 13 days; p=0.012) and total hospital LOS (median 26 vs. 23 days, p=0.005) conditional on survival to discharge. Post-HT hospitalization costs were also greater for heterotaxy recipients (median $448,000 vs. $381,000; p=0.001).
Table 4:
Post-HT ICD procedure codes
| ICD-9 Description | ICD-9 code* | Heterotaxy (n=177) | Non-Heterotaxy CHD (n= 1199) | OR [95% CI]† | p-value† |
|---|---|---|---|---|---|
| Other revision of vascular procedure | 39.49 | 4 (2.3) | 9 (0.8) | 3.06 [0.99–9.47] | 0.074 |
| Insertion of non-drug-eluting peripheral (non-coronary) vessel stent(s) | 39.9 | 8 (4.5) | 19 (1.6) | 2.94 [1.29–6.68] | 0.016 |
| Hemodialysis | 39.95 | 11 (6.2) | 29 (2.4) | 2.67 [1.33–5.39] | 0.013 |
| Venous cutdown | 38.94 | 5 (2.8) | 14 (1.2) | 2.46 [0.91–6.66] | 0.086 |
| Venous catheterization for renal dialysis | 38.95 | 8 (4.5) | 23 (1.9) | 2.42 [1.09–5.39] | 0.050 |
| Angioplasty of other non-coronary vessel(s) | 39.5 | 18 (10.2) | 79 (6.6) | 1.60 [0.94–2.74] | 0.085 |
| Venous catheterization, not elsewhere classified | 38.93 | 47 (26.6) | 215 (17.9) | 1.65 [1.15–2.38] | 0.010 |
| No Post-Tx procedure performed | none | 44 (24.9) | 378 (31.5) | 0.72 [0.50–1.03] | 0.081 |
Source: PHIS database, ICD-9 Procedure codes. CHD: Congenital Heart Disease;
Displayed as n(%), Odds ratio (95% CI)
ICD-10 procedure codes were translated to ICD-9
Fisher’s Exact 95% CI
Table 5:
Post Heart Transplant Resource utilization and LOS
| Outcome | Non-Heterotaxy CHD | Heterotaxy | p-value† |
|---|---|---|---|
| Ventilation Days | 4 [1–14] | 5 [2–21] | 0.039 |
| iNO days | 2 [0–5] | 2[0–5] | 0.176 |
| ECMO post-HT | 172/1030 (14) | 22/177 (12) | 0.564 |
| ICU LOS after HT(days)* | 13 [0–458] | 16 [0–190] | 0.012 |
| Hospital LOS after HT(days)* | 23 [14–42] | 26 [16–49] | 0.005 |
| Cost after HT (2016 USD, Thousands) | $381 [262–580] | $448 [320–709] | 0.001 |
Source: PHIS database; Displayed as n(%) or median [IQR];
Conditional upon survival to discharge;
Binary data compared with Fisher’s exact test, continuous data with Wilcoxon rank-sum test; HT: heart transplant; iNO: inhaled nitric oxide; LOS: length of stay
3.4. Sensitivity Analyses
After reclassification of patients meeting the definition of heterotaxy solely on the basis of asplenia, heterotaxy remained an independent predictor of mortality (HR 1.56, [1.06–2.29], p=0.025 after adjustment for African-American race, year of transplant, and ECMO use at HT; see supplemental figure 1). In propensity score-matched cohorts, 177 recipients with heterotaxy were matched to 351 non-heterotaxy CHD HT recipients (supplemental table 4 and supplemental figure 2); heterotaxy was associated with increased mortality (HR 1.52, [1.06–2.17];p=0.022).
4. DISCUSSION
In this analysis we provide the most extensive report of post-HT outcomes for children with heterotaxy from a large sample of pediatric HT centers. To date, knowledge about HT outcomes for heterotaxy patients has been limited to anecdotal experience, case reports, and small, single center series. Jacobs et al [14] reported suboptimal outcomes in 5 HT recipients with heterotaxy compared to non-heterotaxy CHD patients, while Cohen et al[15] reported survival to 32 and 33 months in 2 heterotaxy recipients. In the largest previous report of 29 heterotaxy recipients at a single-center, Larsen et al[5] did not find a statistically significant difference in survival compared to controls with dilated cardiomyopathy, though the analysis may have been underpowered. Our main findings are that patients with heterotaxy syndrome have decreased survival after HT and accrue greater costs for care with longer LOS after HT relative to other recipients who underwent HT for CHD. Our data support increased surgical complexity and a greater risk of surgical complications, as evidenced by longer ischemic times and increased need for catheterization and peripheral vessel stenting after HT, as possible explanations for inferior survival and greater resource utilization among heterotaxy recipients.
The late survival difference we observed in the conditional 6-month survival cohorts, controlled for age and race, is not well explained by our analysis. We did not find differences in cumulative incidence of common causes of late post-HT mortality such as rejection, infection, PTLD, and CAV. We also did not find significant differences in cause of death between the groups. Though the heterotaxy group was older and disproportionately non-white, both known risk factors for inferior survival after pediatric HT[16], the late survival difference persisted after controlling for age and race. Potentially the degree of medical/surgical complexity or accrued pre-HT comorbidities for heterotaxy recipients persists after HT and influences late survival but is not well captured in this analysis. While we did not find significant differences in the proportion of prior sternotomies between the groups, these data were incompletely captured and do not convey number, timing, or complexity of cardiac surgeries prior to HT. Similarly, although we found no difference in time-to-event for common post HT morbidities, we do not have details about event severity. For example, rejection associated with hemodynamic compromise has a significantly higher risk of death than rejection without hemodynamic compromise[17, 18]. Likewise, PTLD and CAV severity impact survival [19, 20]. The broad, multisystem involvement of heterotaxy syndrome[21], including the potential for overwhelming encapsulated bacterial sepsis from asplenia/functional asplenia, may also contribute to the observed survival difference. In a recently reported neonatal heterotaxy syndrome cohort, extracardiac causes were implicated in 18% of deaths[22].
The primary strength of our analysis is the ability to identify and analyze patients with heterotaxy in a large, multicenter cohort, thereby providing novel insights into post-HT survival as well as peri-operative complications, procedures, and resource utilization for the HT hospitalization via analysis of ICD codes captured by PHIS. We hypothesized that patients with heterotaxy have worse perioperative outcomes due to increased surgical complexity related to anomalies of cardiac position and venous return [4, 23–26]. Indeed, we have found evidence of this. Adjusted ischemic time was increased by nearly 20 minutes in the heterotaxy group. With shorter procurement distances for heterotaxy recipients, this increased ischemic time likely reflects greater surgical complexity with longer cardiopulmonary bypass durations. Increases in ischemic and bypass times have been reported by others for HT in heterotaxy[5], and both are risk factors for mortality in children undergoing cardiac surgery[14, 27]. Also consistent with our hypothesized increased surgical complexity, we found that heterotaxy recipients underwent cardiac reoperation about twice as frequently as non-heterotaxy CHD recipients, and they more commonly underwent cardiac catheterization and peripheral vascular stent placement after HT prior to discharge from the HT hospitalization. Unfortunately, we cannot capture clinical details about the indications for cardiac reoperation and catheterization, or the location of peripheral vascular stent placement to further characterize these post-HT complications.
Infection, renal failure requiring dialysis, and longer duration of mechanical ventilation were also more common post-operatively in heterotaxy recipients. The occurrence of one likely contributes to the need for another (e.g. renal failure with volume overload may drive the need for continued mechanical ventilation.) Unfortunately, we cannot disentangle these overlapping associations in our dataset. While asplenia predisposes to bacteremia and sepsis, particularly with encapsulated bacteria,[28] we did not find differences in the presence of ICD codes for encapsulated bacterial infections between the groups. The lack of observed difference may be due to the relatively short observation window, analysis of both asplenic and polysplenic patients in the heterotaxy group (which cannot be unbundled with ICD-9 coding), inability to identify causative organisms, or insufficient coding. Heterotaxy has been associated with prolonged mechanical ventilation and increased odds of tracheostomy after cardiac surgery,[29] which has been attributed to an increased prevalence of ciliary dyskinesia in these patients[30]. Although ciliary dyskinesia has been implicated in polycystic kidney disease[31], and heterotaxy syndrome is associated with genitourinary anomalies, we found no reports of predisposition toward renal failure in heterotaxy. Given that longer bypass times are an independent risk factor for acute kidney injury and dialysis in adults undergoing HT [32] and children undergoing surgery for CHD repair [32–34], a relationship between these in heterotaxy recipients is possible.
It is not surprising that post-HT ICU and total LOS and costs were greater for heterotaxy recipients given the increased post-HT complications observed. The 10–15% increase in LOS and cost observed in heterotaxy patients is within the expected variation in cost of HT for individuals with other complicating features, such as requirement for mechanical circulatory support[35] or allosensitization[36], and thus would not likely influence decision-making around the appropriateness of HT for individuals with heterotaxy.
Despite the novelty of our analysis, there are important limitations. Our definition of heterotaxy is reliant on ICD codes, which can be inherently ambiguous. Errors and incomplete ICD coding may also exist. Our methodology does not allow us to distinguish between left- and right-isomeric forms of heterotaxy, making our population clinically heterogeneous. Furthermore, the diagnosis of heterotaxy can be clinically difficult to categorize, even with access to more detailed anatomic information than was available[37]. However, the racial distribution of our heterotaxy cohort mirrors other reports of increased prevalence in African-American, Asian, and Hispanic individuals[38–41]. Furthermore, our heterotaxy cohort is enriched for ICD diagnoses common in heterotaxy, such as anomalous pulmonary and systemic venous return, pulmonary outflow anomalies, and congenital heart block. These observations support the validity of our cohort. Still, it is possible that some non-heterotaxy patients in our primary analysis were misclassified as heterotaxy, particularly those who met criteria based on having the ICD-9 diagnosis codes of dextrocardia and/or situs inversus without polysplenia. These codes may have been applied when there is not atrial isomerism, but rather when the heart alone is malpositioned or in patients with situs inversus totalis. However, when we included only patients with ICD codes for asplenia/polysplenia, our primary survival outcome was unchanged.
Another potential limitation is the validity of our control group as a comparison. Though we utilized a data-driven model selection technique to identify the relevant covariates to include in our regression models, some potentially important variables such as age or prior univentricular repair did not meet the preset covariate inclusion criteria. However, when we locked these clinically relevant covariates into the multivariable model, we still observed increased mortality in heterotaxy patients (HR 1.57, [1.16–2.11], p=0.003). Also when we utilized an entirely different technique to control for possible selection bias (i.e., propensity score matching), we still found increased mortality for recipients with heterotaxy. Together, these sensitivity analyses suggest that our conclusions are robust to various model building techniques and selection of controls, adding validity to our conclusions.
Our sample is limited only to HT recipients at PHIS-member hospitals, primarily children and young adults. Although HT in adults with heterotaxy is reported[42–47], our findings should not be generalized to this population. We were unable to capture some post-discharge events (e.g. encapsulated bacterial infection, intervention for vascular stenosis) because PHIS does not capture outpatient encounters or track patients across hospitals. Thus, our analysis of post-discharge complications is limited to those reported at yearly intervals in SRTR. As the Pediatric Heart Transplant Study[48] dataset collects greater detail on some of these events, it may be a good choice for linkage to PHIS to further explore associations of post-discharge infection, rejection, PTLD, and CAV on late survival in heterotaxy syndrome.
In summary, pediatric HT recipients with heterotaxy syndrome experience increased mortality, longer LOS, and greater cost of care from HT surgery to discharge relative to other HT recipients with CHD. Early mortality and resource utilization differences are likely due to increased early post-operative complications, including more frequent cardiac reoperations and vascular stenting; however, the reason(s) for the difference in late survival is unclear. Further study of this small but important pediatric HT group to determine why there is a late difference in survival is warranted.
Supplementary Material
Supplemental Figure 1: Sensitivity analysis of survival after heart transplant for patients meeting stricter inclusion criteria for heterotaxy syndrome
Caption: Stricter inclusion criteria defined as ICD code for asplenia/polysplenia only (dotted line) compared to all other patients with CHD (solid line)
Supplemental Figure 2: Sensitivity analysis of survival for patients with heterotaxy syndrome compared to a propensity score matched group of non-heterotaxy patients with CHD.
Caption: Patients with heterotaxy (dotted line) have inferior survival after heart transplant compared to a propensity matched group of patients with congenital heart disease (Non-Heterotaxy CHD; solid line).
Central Picture Legend:
Heterotaxy has inferior post-heart transplant survival compared to other forms of CHD
Abbreviations:
- HT
Heart Transplant/transplantation
- SRTR
Scientific Registry of Transplant Recipients
- PHIS
Pediatric Health Information System
- CHD
Congenital Heart Disease
- DSXM
Donor-specific crossmatch
- OPO
Organ Procurement Organization
- PTLD
Post-transplant Lymphoproliferative Disease
- CAV
Coronary Allograft Vasculopathy
- VAD
Ventricular Assist Device
- ECMO
Extracorporeal Membrane Oxygenation
Footnotes
Disclosures:
The data reported here have been supplied by the Minneapolis Medical Research Foundation (MMRF) as the contractor for the Scientific Registry of Transplant Recipients (SRTR). The interpretation and reporting of these data are the responsibility of the author(s) and in no way should be seen as an official policy of or interpretation by the SRTR or the U.S. Government.
REFERENCES
- 1.Loomba RS, et al. , Manifestations of bodily isomerism. Cardiovasc Pathol, 2016. 25(3): p. 173–80. [DOI] [PubMed] [Google Scholar]
- 2.Landis BJ, Cooper DS, and Hinton RB, CHD associated with syndromic diagnoses: peri-operative risk factors and early outcomes. Cardiol Young, 2016. 26(1): p. 30–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jonas RA, Surgical management of the neonate with heterotaxy and long-term outcomes of heterotaxy. World J Pediatr Congenit Heart Surg, 2011. 2(2): p. 264–74. [DOI] [PubMed] [Google Scholar]
- 4.Deuse T and Reitz BA, Heart transplantation in situs inversus totalis. J Thorac Cardiovasc Surg, 2010. 139(2): p. 501–3. [DOI] [PubMed] [Google Scholar]
- 5.Larsen RL, et al. , Usefulness of cardiac transplantation in children with visceral heterotaxy (asplenic and polysplenic syndromes and single right-sided spleen with levocardia) and comparison of results with cardiac transplantation in children with dilated cardiomyopathy. Am J Cardiol, 2002. 89(11): p. 1275–9. [DOI] [PubMed] [Google Scholar]
- 6.Godown J, et al. , A unique linkage of administrative and clinical registry databases to expand analytic possibilities in pediatric heart transplantation research. Am Heart J, 2017. 194: p. 9–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.SRTR. The SRTR Database: Overview. December/01/2017]; Available from: https://www.srtr.org/about-the-data/the-srtr-database/.
- 8.PHIS. Overview. December/1/2017]; Available from: https://www.childrenshospitals.org/programs-and-services/data-analytics-and-research/pediatric-analytic-solutions/pediatric-health-information-system.
- 9.AHRQ, AHRQ Map It Tool. 2015, Agency for Healthcare Research and Quality. [DOI] [PubMed] [Google Scholar]
- 10.Pepe MS and Mori M, Kaplan—meier, marginal or conditional probability curves in summarizing competing risks failure time data? Statistics in Medicine, 1993. 12(8): p. 737–751. [DOI] [PubMed] [Google Scholar]
- 11.Schwartz GJ, et al. , New equations to estimate GFR in children with CKD. J Am Soc Nephrol, 2009. 20(3): p. 629–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nichols A, VINCENTY: Stata module to calculate distances on the Earth’s surface. 2007.
- 13.Grambsch PM and Therneau TM, Proportional hazards tests and diagnostics based on weighted residuals. Biometrika, 1994. 81(3): p. 515–526. [Google Scholar]
- 14.Jacobs JP, et al. , Lessons learned from 119 consecutive cardiac transplants for pediatric and congenital heart disease. Ann Thorac Surg, 2011. 91(4): p. 1248–54; discussion 1254–5. [DOI] [PubMed] [Google Scholar]
- 15.Cohen MS, et al. , Heterotaxy syndrome with functional single ventricle: does prenatal diagnosis improve survival? Ann Thorac Surg, 2006. 82(5): p. 1629–36. [DOI] [PubMed] [Google Scholar]
- 16.Rossano JW, et al. , The Registry of the International Society for Heart and Lung Transplantation: Twentieth Pediatric Heart Transplantation Report-2017; Focus Theme: Allograft ischemic time. J Heart Lung Transplant, 2017. 36(1557–3117 (Electronic)): p. 1060–1069. [DOI] [PubMed] [Google Scholar]
- 17.Everitt MD, et al. , Rejection with hemodynamic compromise in the current era of pediatric heart transplantation: a multi-institutional study. (1557–3117 (Electronic)). [DOI] [PubMed]
- 18.Pahl E, et al. , Death after rejection with severe hemodynamic compromise in pediatric heart transplant recipients: a multi-institutional study. J Heart Lung Transplant, 2001. 20(3): p. 279–87. [DOI] [PubMed] [Google Scholar]
- 19.Kindel SJ, et al. , Improved Detection of Cardiac Allograft Vasculopathy: A Multi-Institutional Analysis of Functional Parameters in Pediatric Heart Transplant Recipients. J Am Coll Cardiol, 2015. 66(5): p. 547–57. [DOI] [PubMed] [Google Scholar]
- 20.Webber SA, et al. , Lymphoproliferative disorders after paediatric heart transplantation: a multi-institutional study. Lancet, 2006. 367(9506): p. 233–9. [DOI] [PubMed] [Google Scholar]
- 21.Kothari SS, Non-cardiac issues in patients with heterotaxy syndrome. Ann Pediatr Cardiol, 2014. 7(3): p. 187–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gottschalk I, et al. , Extracardiac anomalies in prenatally diagnosed heterotaxy syndrome. Ultrasound Obstet Gynecol, 2016. 47(4): p. 443–9. [DOI] [PubMed] [Google Scholar]
- 23.Chang YL, et al. , Cardiac transplantation in situs inversus: two cases reports. Transplant Proc, 2008. 40(8): p. 2848–51. [DOI] [PubMed] [Google Scholar]
- 24.Beiras-Fernandez A, et al. , Challenging venous reconstruction and heart transplantation in a patient with viscero-atrial situs inversus and complex congenital heart disease with Fontan circulation. J Heart Lung Transplant, 2007. 26(3): p. 290–2. [DOI] [PubMed] [Google Scholar]
- 25.Rubay JE, et al. , Orthotopic heart transplantation in situs inversus. Ann Thorac Surg, 1995. 60(2): p. 460–2. [DOI] [PubMed] [Google Scholar]
- 26.Michler RE and Sandhu AA, Novel approach for orthotopic heart transplantation in visceroatrial situs inversus. Ann Thorac Surg, 1995. 60(1): p. 194–7. [PubMed] [Google Scholar]
- 27.Ford MA, et al. , Association of graft ischemic time with survival after heart transplant among children in the United States. J Heart Lung Transplant, 2011. 30(11): p. 1244–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Prendiville TW, et al. , Heterotaxy syndrome: defining contemporary disease trends. Pediatr Cardiol, 2010. 31(7): p. 1052–8. [DOI] [PubMed] [Google Scholar]
- 29.Swisher M, et al. , Increased postoperative and respiratory complications in patients with congenital heart disease associated with heterotaxy. J Thorac Cardiovasc Surg, 2011. 141(3): p. 637–44, 644.e1–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nakhleh N, et al. , High prevalence of respiratory ciliary dysfunction in congenital heart disease patients with heterotaxy. Circulation, 2012. 125(18): p. 2232–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kathem SH, Mohieldin AM, and Nauli SM, The Roles of Primary cilia in Polycystic Kidney Disease. AIMS molecular science, 2014. 1(1): p. 27–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Boyle JM, et al. , Risks and outcomes of acute kidney injury requiring dialysis after cardiac transplantation. Am J Kidney Dis, 2006. 48(5): p. 787–96. [DOI] [PubMed] [Google Scholar]
- 33.Pedersen KR, et al. , Risk factors for acute renal failure requiring dialysis after surgery for congenital heart disease in children. Acta Anaesthesiol Scand, 2007. 51(10): p. 1344–9. [DOI] [PubMed] [Google Scholar]
- 34.Chan K. l., et al. , Peritoneal dialysis after surgery for congenital heart disease in infants and young children. The Annals of Thoracic Surgery, 2003. 76(5): p. 1443–1449. [DOI] [PubMed] [Google Scholar]
- 35.Godown JSA, Thurm C, Hall M, Dodd DA, Soslow JH, Mettler BA, Bearl D, Feingold B, Mechanical circulatory support costs in children bridged to heart transplantation - Analysis of a linked database. Am Heart J, 2018(In Press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.West SC, et al. , Charges and resource utilization for pediatric heart transplantation across a positive virtual and/or cytotoxicity crossmatch. Pediatric Transplantation, 2018. 22(1): p. e13095–n/a. [DOI] [PubMed] [Google Scholar]
- 37.Yim D, et al. , Disharmonious Patterns of Heterotaxy and Isomerism: How Often Are the Classic Patterns Breached? Circ Cardiovasc Imaging, 2018. 11(2): p. e006917. [DOI] [PubMed] [Google Scholar]
- 38.Gatrad AR, Read AP, and Watson GH, Consanguinity and complex cardiac anomalies with situs ambiguus. Arch Dis Child, 1984. 59(3): p. 242–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kim SJ, Heterotaxy syndrome. Korean Circ J, 2011. 41(5): p. 227–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kim SJ, et al. , Outcome of 200 patients after an extracardiac Fontan procedure. J Thorac Cardiovasc Surg, 2008. 136(1): p. 108–16. [DOI] [PubMed] [Google Scholar]
- 41.Lin AE, et al. , Laterality defects in the national birth defects prevention study (1998–2007): birth prevalence and descriptive epidemiology. Am J Med Genet A, 2014. 164A(10): p. 2581–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Matsuda H, et al. , Orthotropic heart transplantation for adult congenital heart disease: a case with heterotaxy and dextrocardia. Gen Thorac Cardiovasc Surg, 2017. 65(1): p. 47–51. [DOI] [PubMed] [Google Scholar]
- 43.Gonzalez-Lopez MT, et al. , Orthotopic Heart Transplantation in an Adult Patient with Heterotaxy Syndrome: Surgical Implications. J Card Surg, 2015. 30(12): p. 910–2. [DOI] [PubMed] [Google Scholar]
- 44.Bang JH, et al. , Heart Transplantation in a Patient with Left Isomerism. Korean J Thorac Cardiovasc Surg, 2015. 48(4): p. 277–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Vallabhajosyula P, et al. , Combined heart-liver transplant in a situs-ambiguous patient with failed Fontan physiology. J Thorac Cardiovasc Surg, 2013. 145(4): p. e39–41. [DOI] [PubMed] [Google Scholar]
- 46.Munoz-Guijosa C, et al. , Orthotopic heart transplantation in a patient with situs invs, transposition of the great arteries and Mustard operation. Eur J Cardiothorac Surg, 2008. 34(1): p. 219–21. [DOI] [PubMed] [Google Scholar]
- 47.Berdat PA, et al. , Successful heart transplantation in a patient with Ivemark syndrome combined with situs inversus, single atrium and ventricle after total cavo-pulmonary connection. Eur J Cardiothorac Surg, 1998. 14(6): p. 631–4. [DOI] [PubMed] [Google Scholar]
- 48.Dipchand AI, et al. , Ten yr of pediatric heart transplantation: A report from the Pediatric Heart Transplant Study. Pediatric Transplantation, 2013. 17(2): p. 99–111. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1: Sensitivity analysis of survival after heart transplant for patients meeting stricter inclusion criteria for heterotaxy syndrome
Caption: Stricter inclusion criteria defined as ICD code for asplenia/polysplenia only (dotted line) compared to all other patients with CHD (solid line)
Supplemental Figure 2: Sensitivity analysis of survival for patients with heterotaxy syndrome compared to a propensity score matched group of non-heterotaxy patients with CHD.
Caption: Patients with heterotaxy (dotted line) have inferior survival after heart transplant compared to a propensity matched group of patients with congenital heart disease (Non-Heterotaxy CHD; solid line).