Key Points
Question
Is cardiac resynchronization therapy associated with improvement in left ventricular function in patients with chemotherapy-induced cardiomyopathy?
Findings
In this uncontrolled, prospective, cohort study that included 26 patients with chemotherapy-induced cardiomyopathy treated with cardiac resynchronization therapy and follow-up data at 6 months, the mean left ventricular ejection fraction changed from 28% to 39%, a difference that was statistically significant.
Meaning
Cardiac resynchronization therapy was significantly associated with improvement in left ventricular function, but the findings are limited by the small sample size, short follow-up, and absence of a control group.
Abstract
Importance
The incidence of chemotherapy-induced cardiomyopathy is increasing and is associated with poor clinical outcomes.
Objective
To assess the association of cardiac resynchronization therapy (CRT) with improvement in cardiac function, as well as clinical improvement in patients with chemotherapy-induced cardiomyopathy.
Design, Setting, and Participants
The Multicenter Automatic Defibrillator Implantation Trial–Chemotherapy-Induced Cardiomyopathy was an uncontrolled, prospective, cohort study conducted between November 21, 2014, and June 21, 2018, at 12 tertiary centers with cardio-oncology programs in the United States. Thirty patients were implanted with CRT owing to reduced left ventricular ejection fraction (LVEF≤35%), New York Heart Association class II-IV heart failure symptoms, and wide QRS complex, with established chemotherapy-induced cardiomyopathy and were followed up for 6 months after CRT implantation. The date of final follow-up was February 6, 2019.
Exposures
CRT implantation according to standard of care.
Main Outcomes and Measures
The primary end point was change in LVEF from baseline to 6 months after initiating CRT. Secondary outcomes included all-cause mortality and change in left ventricular end-systolic volume and end-diastolic volume.
Results
Among 30 patients who were enrolled (mean [SD] age, 64 [11] years; 26 women [87%]; 73% had a history of breast cancer; 20% had a history of lymphoma or leukemia), primary end point data were available for 26 patients and secondary end point data were available for 23 patients. Patients had nonischemic cardiomyopathy with left bundle branch block, median LVEF of 29%, and a mean QRS duration of 152 ms. Patients with CRT experienced a statistically significant improvement in mean LVEF at 6 months from 28% to 39% (difference, 10.6% [95% CI, 8.0%-13.3%]; P < .001). This was accompanied by a reduction in LV end-systolic volume from 122.7 to 89.0 mL (difference, 37.0 mL [95% CI, 28.2-45.8]) and reduction in LV end-diastolic volume from 171.0 to 143.2 mL (difference, 31.9 mL [95% CI, 22.1-41.6]) (both P < .001). Adverse events included a procedure-related pneumothorax (1 patient), a device pocket infection (1 patient), and heart failure requiring hospitalization during follow-up (1 patient).
Conclusions and Relevance
In this preliminary study of patients with chemotherapy-induced cardiomyopathy, CRT was associated with improvement in LVEF after 6 months. The findings are limited by the small sample size, short follow-up, and absence of a control group.
Trial Registration
ClinicalTrials.gov Identifier: NCT02164721
This cohort study examines the association of cardiac resynchronization therapy with change in left ventricular ejection fraction (LVEF)among patients with chemotherapy-induced cardiomyopathy.
Introduction
With the advent of new therapies and an increasing number of long-term cancer survivors, the incidence of chemotherapy-induced cardiomyopathy (CHIC) has been increasing. Patients with CHIC have presented with heart failure several months to years after the administration of chemotherapy, especially anthracyclines.1 Data from several studies inclusive of these patients from 1988 through 2014 have demonstrated left ventricular (LV) dysfunction, with 5% presenting with overt symptomatic heart failure.2,3,4
CHIC is a progressive condition that is associated with a decreased quality of life and poor clinical outcomes.5 When this form of cardiomyopathy is accompanied by abnormal electrical activation of the ventricles manifested by left bundle branch block (LBBB), the prospects of clinical recovery are minimal. Cardiac resynchronization therapy (CRT) could potentially improve cardiac function and outcomes in this clinical setting.6 Despite the magnitude of this clinical problem, this patient group has not been evaluated for their response to CRT in a systematic manner. The study hypothesis was that CHIC is a distinct pathophysiologic entity and that CRT may improve clinical outcomes in these patients.
The Multicenter Automatic Defibrillator Implantation Trial–Chemotherapy-Induced Cardiomyopathy (MADIT-CHIC) was an uncontrolled, prospective, cohort study in cancer survivors with CHIC designed to assess the association of CRT with LV reverse remodeling and clinical improvement.
Methods
Trial Design and Oversight
From November 21, 2014, through June 21, 2018, patients were enrolled from 12 tertiary centers with cardio-oncology programs in the United States and implanted with CRT. All enrolling sites had the study protocol (Supplement 1) approved by the local institutional review board. All patients provided written informed consent. In an attempt to determine whether there were any race/ethnic differences in response to CRT, this information was collected at the time of enrollment. Race/ethnicity was self-reported into fixed categories. The primary hypothesis was that CRT is associated with an improvement in LV ejection fraction (LVEF) from baseline to 6-month follow-up. Follow-up ended at 6 months following CRT implantation. An independent data and safety monitoring board regularly reviewed adverse events and outcomes for safety.
Recruitment and Follow-up
Patients enrolled in the study had a diagnosis of CHIC, defined as a history of exposure to chemotherapy known to cause LV dysfunction, no evidence of heart failure prior to chemotherapy initiation, and the development of clinical heart failure with systolic dysfunction at least 6 months after the completion of their cancer treatment, without other evident cause of the cardiomyopathy as ascertained by the enrolling physician.
The study included men or women aged 18 to 80 years of age with either a class I or class II indication for CRT according to current US guidelines (Box 1 in Supplement 2).7 Class I indication included patients with LVEF of 35% or less, sinus rhythm, LBBB, QRS of 150 ms or more, and New York Heart Association (NYHA) class II, III, or IV symptoms on guideline-directed medical therapy. Class IIa indication included patients with LVEF of 35% or less, LBBB, QRS of 120 to 149 ms, and NYHA class II, III, or IV symptoms on guideline-directed medical therapy (class IIa1) or those with LVEF of 35% or less, non-LBBB, and QRS of 150 ms or more (class IIa2). Exclusion criteria are listed in Box 2 in Supplement 2. Patients in the study were followed up by telephone 3 months after CRT implantation and had a 6-month clinic visit including clinical evaluation, device testing, and echocardiogram.
Therapy
Patients enrolled in the study were implanted with a commercially available CRT–defibrillator/pacemaker generator (CRT-pacemaker was implanted in 4 patients), with the device implant procedure performed by a qualified physician per local standard-of-care guidelines. All device components used in the study were market-released products. CRT-pacemaker consists of a biventricular pacemaker, while CRT-defibrillator consists of a biventricular pacemaker and automatic defibrillator. Devices were programmed according to a prespecified programming protocol to maximize biventricular pacing for optimal outcomes.
Echocardiographic Studies
Brigham and Women’s Hospital, Boston, Massachusetts, served as the central echocardiography core laboratory with assessment of the echocardiography images without the knowledge of clinical characteristics or outcomes. Two-dimensional echocardiography was performed at baseline and at the 6-month follow-up to assess changes in LV volumes and ejection fraction. Complete measurement of baseline and 6-month LV volumes were available in paired samples for analysis. Volumes were estimated by averaging those derived from the 2-chamber and 4-chamber views according to the Simpson method,8 and the LVEF was calculated using the Simpson biplane formula.8
Study End Points
The primary end point of the study was change in LVEF from baseline to 6 months after initiating CRT. Secondary outcomes were all-cause mortality and changes in LV end-systolic volume (LVESV) and LV end-diastolic volume (LVEDV) at 6 months following CRT. Tertiary end points included changes in NYHA functional class and change in left atrial size from baseline to 6 months. Heart failure events were also reported as a post hoc analysis, defined as an event requiring an inpatient hospitalization with augmented treatment for heart failure per center investigators.
Statistical Analysis
Continuous variables are expressed as mean (SD). Categorical data are summarized as frequencies and percentages. Clinical characteristics are shown for the total patient population.
Study Power and Sample Size Calculation
According to similar analyses in 749 patients from the Multicenter Automatic Defibrillator Implantation Trial–Cardiac Resynchronization Therapy (MADIT-CRT), but comparing 1-year LVEF with baseline, the SD of the improvement in LVEF was approximately 5 percentage points.9 Using this estimate of SD and comparison with t tests using a 2-sided .05 significance level, it was estimated that 80% power to detect effect sizes as small as 2 percentage points could be achieved with 65 to 75 enrolled patients (assuming a dropout rate of 20%-30%) and that detection of effect sizes of 5 or more points could be achieved enrolling 15 or fewer patients.
Primary End Point
The prespecified primary end point analysis on LVEF improvement at 6 months was planned to be analyzed using an analysis of covariance model with covariate adjustments. Missing data in the study were relatively infrequent, with 4 data points missing for LVEF improvement and 7 data points missing for LVEDV and LVESV improvement, and therefore, multiple imputation was not performed. Patients with missing data were excluded from the analyses. Baseline LVEF was tested in the model, but it had a P value greater than .2, and therefore it was omitted per the statistical analysis section of the study protocol. Further, the final analysis did not include regression variables for disease etiology or study centers because of the homogeneity of etiology (all but 1 patient had nonischemic cardiomyopathy) and the small number of centers in this study. All participants with complete data on the primary end point were included in the primary analysis.
Secondary and Tertiary End Points
The secondary and tertiary end point analyses were not prespecified in the protocol and are therefore exploratory. Changes in echocardiography variables and NYHA functional class from baseline to 6 months were tested for normality using the Kolmogorov-Smirnov test.
Post Hoc Subgroup Analyses
Exploratory (post hoc) analyses of changes in the primary end point in relevant subgroups that were identified following completion of data collection were performed.
There were no missing data on subgroup determinations. Because there were no adjustment covariates, normally distributed variables were tested using paired t tests; nonnormally distributed variables were tested with the Wilcoxon signed rank test. All statistical tests were 2-sided and a P < .05 was considered statistically significant. Because of the potential for type I error due to multiple comparisons, findings for analyses of secondary end points should be interpreted as exploratory. Analyses were carried out with SAS software (version 9.4, SAS Institute). All analyses used version 1.0 of the database, which was released on March 30, 2019.
Results
Study Population
Thirty patients were prospectively enrolled from 12 tertiary centers with cardio-oncology programs between November 21, 2014, and June 21, 2018. In the overall study, the mean (SD) age was 64 (11) years. A total of 26 patients (87%) were women, 21 (75%) were white, and all but 1 patient had a confirmed diagnosis of nonischemic cardiomyopathy, with a median LVEF of 29% (Table 1). All patients had LBBB and all except 1 patient had sinus rhythm at the time of enrollment. A total of 8 patients (27%) had a history of atrial arrhythmias, and 3 patients (10%) had a history of ventricular arrhythmias. Most patients had a history of breast cancer (73%), 6 patients (20%) had a history of lymphoma or leukemia, and 2 patients (7%) had a diagnosis of sarcoma; of these patients, 24 (83%) received anthracycline therapy (mean equivalent cumulative doxorubicin dose, 307 mg/m2). Besides anthracyclines, 3 patients received cyclophosphamide; 1, trastuzumab; 1, dasatinib; and 1, docetaxel. All patients had symptomatic heart failure, with 17 patients (57%) in NYHA class II and 13 patients (43%) in NYHA class III. The median age at cancer diagnosis was 52 years, with a median heart failure diagnosis time of 13.8 years from the last cancer diagnosis. Guideline-directed medical therapy at enrollment included the use of β-blockers in 93% of the patients, angiotensin-converting enzyme inhibitors or angiotensin receptor blocker in 77%, and loop diuretics in 93% of the patients (Table 1). Four patients had CRT pacemakers and 26 had CRT defibrillators implanted.
Table 1. Baseline Clinical Characteristics.
| Clinical Characteristic | No. (%)a |
|---|---|
| Demographics | |
| Sex | |
| Women | 26 (87) |
| Men | 4 (13) |
| Age at enrollment, median (IQR), y | 66 (54-72) |
| Race and ethnicity, No./No. (%) | |
| White | 21/28 (75) |
| Black/African American | 7/28 (25) |
| Hispanic | 4/25 (16) |
| Nonischemic cardiomyopathy, No./No. (%) | 29/29 (100) |
| QRS after consent, median (IQR), ms | 152 (142-160) |
| LBBB after consent | 30 (100) |
| Sinus rhythm after consent | 29 (97) |
| Comorbidities, Patient History | |
| Diabetes at baseline | 6 (20) |
| Hypertension at baseline | 17 (57) |
| History of ventricular arrhythmias | 3 (10) |
| History of atrial arrhythmias | 8 (27) |
| Anthracycline history, No./No. (%) | 24/29 (83) |
| Time since cancer diagnosis, median (IQR), y | 13.8 (12.7) |
| Age at cancer diagnosis, median (IQR), y | 52 (40-59) |
| History of breast cancer | 22 (73) |
| History of leukemia or lymphoma | 6 (20) |
| History of sarcomas | 2 (7) |
| Physiologic and Laboratory Parameters at Baseline | |
| Blood urea nitrogen, median (IQR) [No. of patients], mg/dL | 20 (15-27) [27] |
| Creatinine, median (IQR) [No. of patients], mg/dL | 0.95 (0.80-1.25) [28] |
| New York Heart Association classb | |
| II | 17 (57) |
| III | 13 (43) |
| Blood pressure, median (IQR), mm Hg | |
| Systolic | 121.5 (116-133) |
| Diastolic | 70.5 (60-77) |
| Medical Therapy Use at Baseline | |
| β-Blocker | 28 (93) |
| ACEI or ARB | 23 (77) |
| Loop diuretic | 28 (93) |
| Aldosterone antagonist | 15 (50) |
Abbreviations: ACEI, angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blocker; IQR, interquartile range; LBBB, left bundle-branch block.
SI conversion factors: To convert creatinine to μmol/L, multiply by 88.4; urea nitrogen to mmol/L, multiply by 0.357.
Unless otherwise stated.
The New York Heart Association Classification provides a simple way of classifying the severity of heart failure. It categorizes patients in 1 of 4 classes based on their symptoms or limitations during physical exertion. Class I is least severe and class IV is most severe.
Primary End Point
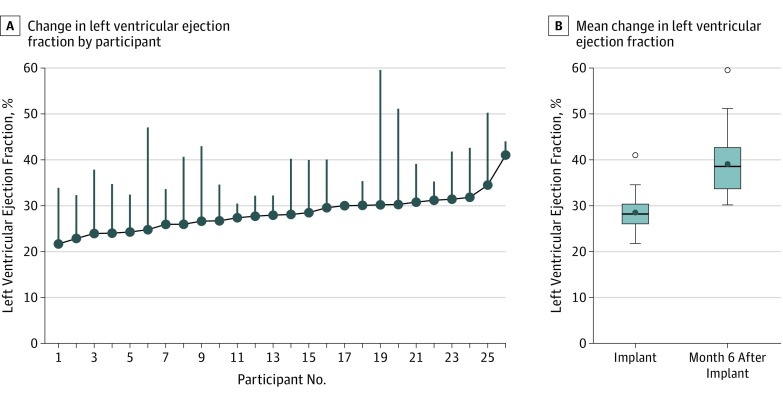
Of the 30 enrolled patients, 26 complete paired echocardiography data sets were available for analysis. One patient had insufficient quality of echocardiography images, 1 patient moved away, 1 patient had echocardiography done at a different center and images could not be obtained, and 1 patient had echocardiography performed at 3 months instead of the 6-month visit per physician discretion. Patients with CRT experienced a statistically significant improvement in mean LVEF at 6 months from 28% to 39% (difference, 10.6% [95% CI, 8.0%-13.3%]; P < .001) (Figure 1).
Figure 1. Change in the Primary End Point of Left Ventricular Ejection Fraction at 6 Months Following Cardiac Resynchronization Therapy Implantation .
A, Each participant is presented in order, from left to right, of their baseline left ventricular ejection fraction value. The vertical lines show the change from their baseline to 6-month left ventricular ejection fraction value. B, Boxplots represent the change in left ventricular ejection fraction from the time of the implant to 6 months after cardiac resynchronization therapy. The open circles represent outlying maximum values (defined as 1.5 times the interquartile range above the 75th percentile value [interquartile range = 75th percentile - 25th percentile]).
Secondary End Points
There was a reduction in LVESV from 122.7 to 89.0 mL (difference, 37.0 mL [95% CI, 28.2-45.8]) and reduction in LVEDV from 171.0 to 143.2 mL (difference, 31.9 mL [95% CI, 22.1-41.6]) (both P < .001) (Table 2). During the 6-month follow-up, there were no deaths.
Table 2. Secondary and Tertiary Echocardiography End Points at Baseline and 6 Months Following Cardiac Resynchronization Therapy Implantation.
| Variable | Baseline | 6 Months | No. of Patients With Baseline and 6-mo Results | Absolute Change, Mean (SD) | P Valuea | ||
|---|---|---|---|---|---|---|---|
| No. of Patients | Mean (SD) | No. of Patients | Mean (SD) | ||||
| Left ventricular end-diastolic volume, mL | 27 | 171.0 (47.2) | 25 | 143.2 (41.2) | 23 | −31.9 (22.5) | <.001 |
| Left ventricular end-systolic volume, mL | 27 | 122.7 (37.1) | 25 | 89.0 (32.2) | 23 | −37.0 (20.4) | <.001 |
| Left ventricular ejection fraction, % | 29 | 28.5 (3.8) | 26 | 39.1 (7.1) | 26 | 10.6 (6.6) | <.001 |
| Left atrial volume, mL | 29 | 60.3 (15.2) | 26 | 47.9 (11.6) | 26 | −12.6 (9.6) | <.001 |
P value is derived from paired t tests examining absolute change from baseline to 6 months when normality assumptions were met.
Tertiary End Points
CRT was associated with a reduction in left atrial volume from 60.3 mL to 47.9 mL at 6 months (P < .001) (Table 2). Overall, NYHA functional status was improved for 41% of patients at 6 months, with improvements seen in 19% of the class II patients and 69% of the class III patients. Accordingly, at 6-month follow-up, 24 patients (83%) had no or mild heart failure symptoms.
Post Hoc Subgroup Analyses
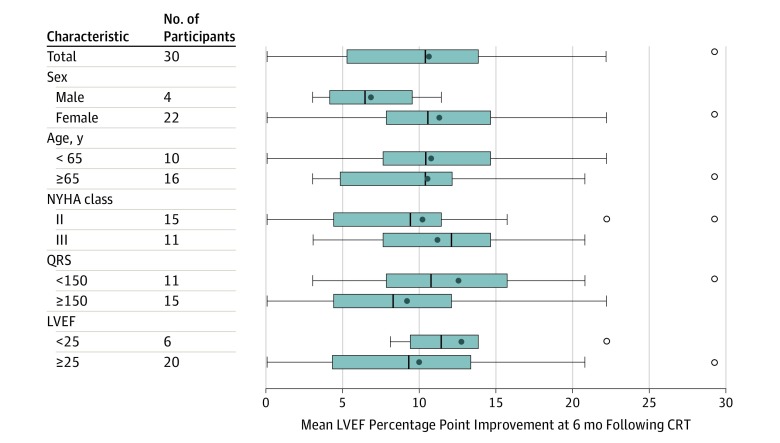
Improvements in LVEF across a number of relevant subgroups, including those with age younger or older than 65 years, NYHA class II or III at baseline, QRS duration below or above 150 ms, and LVEF below or above 25% are shown in Figure 2.
Figure 2. Subgroup Analysis for the Primary End Point of Left Ventricular Ejection Fraction (LVEF) Change at 6 Months Following Cardiac Resynchronization Therapy (CRT) Implantation.
Boxplots represent mean LVEF change across different subgroups at 6 months following CRT. The New York Heart Association (NYHA) Classification provides a simple way of classifying the severity of heart failure. It categorizes patients in 1 of 4 classes based on their symptoms or limitations during physical exertion. NYHA class I is least severe and NYHA class IV is most severe. The open circles represent outlying maximum values (defined as 1.5 times the interquartile range above the 75th percentile value [interquartile range = 75th percentile - 25th percentile]).
Post Hoc End Points
Adverse events included 1 patient in the study developing a procedure-related pneumothorax and another developing a pocket infection. During the 6-month follow-up, 1 patient had a heart failure event requiring hospitalization (Table 3).
Table 3. Outcomes and Procedure-Related Adverse Events.
| Outcome | No. (%) |
|---|---|
| Death from any cause | 0 |
| Heart failure events | 1 (3) |
| Device infection | 1 (3) |
| Bleeding requiring intervention (procedure related) | 1 (3) |
| Pneumothorax | 1 (3) |
Discussion
In this preliminary study of 26 patients with CHIC, CRT was associated with improvement in LVEF after 6 months. Findings are limited by the small sample size, short follow-up, and absence of a control group.
Advances in cancer treatment have significantly improved the survival rates in a variety of hematological and solid-organ malignancies. Concomitant with this improvement in survival, a major adverse event that can accompany the antineoplastic effect is an increase in the incidence of heart failure in patients surviving cancer.3,4,5 Anthracyclines remain the mainstay of therapy for many lymphomas, soft-tissue sarcomas, and breast cancers, which comprised the majority of patients in this study. The downside to the antineoplastic effect of anthracyclines is the inherent cardiotoxicity that significantly affects clinical outcomes leading to heart failure in a substantial proportion of these patients. CHIC, secondary to anthracyclines, has now become a well-recognized entity. While most patients demonstrate cardiac dysfunction within the first year of receiving treatment,3 some patients may have a much more delayed presentation especially if there are no obvious symptoms and they are not receiving regular cardiac surveillance.
Historically, reports have suggested progressive heart failure and mortality rates as high as 50% within 2 years of diagnosis. More contemporary studies suggest similar long-term outcomes when CHIC is compared with other nonischemic causes of heart failure.2,10,11,12 Also, a substantial number of patients will develop cardiomyopathy and heart failure with evidence to suggest that CHIC is a progressive condition with relatively poor clinical outcomes if not identified early.5
While anthracyclines are most commonly associated with the development of cardiomyopathy, many other therapies, including tyrosine kinase inhibitors and immunotherapies, are also linked to left ventricular dysfunction, and the natural history of these cardiotoxicities and response to therapy may differ.13,14 In this study, most of the patients were exposed to anthracyclines, providing a better understanding of CRT effects in this specific cohort.
CRT is a guideline-based therapy that corrects mechanical dyssynchrony through electrical resynchronization, thereby improving the contractility of the heart. In appropriately selected patients, CRT is associated with not only an improvement in hemodynamic stress, cardiac perfusion, and metabolism, but also molecular changes that accompany the reverse remodeling process.15 These physiological effects in turn have been shown to be associated with improved functional capacity and quality of life, reduced heart failure hospitalization, and improved long-term survival.9,16,17,18,19,20
A retrospective analysis of 4 patients with CHIC provided the first proof of concept of the clinical benefit from CRT in this cohort, which led to the design of this prospective, multicenter study.6 An uncontrolled, prospective, cohort study design was selected because CHIC with a wide QRS is a progressive condition and portends a poor prognosis, and CRT is currently indicated in patients with low LVEF and wide QRS. Therefore, the ethics of deferring therapy in the control group would have been questionable.
In this study, there was only 1 heart failure event reported and no deaths occurred; therefore, the small sample precludes any definitive conclusions regarding clinical outcomes. Prior research has, however, suggested that normalization of LVEF in Adriamycin-induced cardiomyopathy is associated with a significant improvement in clinical outcomes, and hence, CRT may have an important role in improving LVEF and outcomes.21
When echocardiography outcomes in this study are compared with CRT patients with nonischemic cardiomyopathy and LBBB in the previously published MADIT-CRT, a similar degree of LVEF improvement (10.6% in MADIT-CHIC vs 12.6% in MADIT-CRT) and LV volume reduction (LVESV percentage change, −30% in MADIT-CHIC and −37% in MADIT-CRT) was observed.9,17 Echocardiography outcomes were assessed at 6 months in MADIT-CHIC and at 12 months in MADIT-CRT. Linde et al19 previously suggested that the degree of LV reverse remodeling increases up to 2 years following CRT after which it plateaus. Furthermore, favorable reverse remodeling has been associated with improved clinical outcomes.9
Even though the evolving discipline of cardio-oncology provides a multidisciplinary approach to this growing population of patients, it is still limited by the lack of data on therapeutic strategies incorporating device treatment, such as CRT, to manage this vulnerable population and mitigate overall risk. There is now some evidence that the early initiation of cardioprotective medications, such as angiotensin-converting enzyme inhibitors and β-blockers, may prevent and/or reverse cardiac dysfunction.3,22 However, most patients with cardiomyopathy and accompanying conduction tissue disease leading to left ventricular dyssynchrony and heart failure do not respond to pharmacological therapy alone. The concomitant presence of a conduction defect (ie, LBBB) and the resulting mechanical dyssynchrony worsens the mechanoenergetic efficiency within the failing heart beyond the direct myopathic effect of the chemotherapy. The primary benefit of CRT in this cohort of patients could be due to the direct reversal of mechanical dyssynchrony and the associated improved remodeling of the heart. To our knowledge, the MADIT-CHIC study is the first prospective, multicenter clinical study evaluating the role of CRT in patients with CHIC.
Limitations
This study has several limitations. First, this study had a small number of patients enrolled and did not have long-term outcome data. Second, the lack of a control group precludes comparing CRT vs medical therapy and does not allow estimation of the potential effect size independent of spontaneous improvement or random variation in LVEF. Third, diagnosis of CHIC was ascertained by center investigators, and it is plausible that the cause of the cardiomyopathy may not have been directly related to chemotherapy in some patients. Fourth, the measurement tool used for the study, 2-dimensional echocardiography, has a greater measurement error than 3-dimensional echocardiography, which further limits the interpretability of the data.
Conclusions
In this preliminary study of patients with CHIC, CRT was associated with improvement in LVEF after 6 months. The findings are limited by the small sample size, short follow-up, and absence of a control group.
Trial Protocol
Box 1. Clinical Inclusion Criteria
Box 2. Clinical Exclusion Criteria
Data Sharing Statement
References
- 1.Armstrong GT, Liu Q, Yasui Y, et al. . Late mortality among 5-year survivors of childhood cancer: a summary from the Childhood Cancer Survivor Study. J Clin Oncol. 2009;27(14):2328-2338. doi: 10.1200/JCO.2008.21.1425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Swain SM, Whaley FS, Ewer MS. Congestive heart failure in patients treated with doxorubicin: a retrospective analysis of three trials. Cancer. 2003;97(11):2869-2879. doi: 10.1002/cncr.11407 [DOI] [PubMed] [Google Scholar]
- 3.Cardinale D, Colombo A, Bacchiani G, et al. . Early detection of anthracycline cardiotoxicity and improvement with heart failure therapy. Circulation. 2015;131(22):1981-1988. doi: 10.1161/CIRCULATIONAHA.114.013777 [DOI] [PubMed] [Google Scholar]
- 4.Baech J, Hansen SM, Lund PE, et al. . Cumulative anthracycline exposure and risk of cardiotoxicity: a Danish nationwide cohort study of 2440 lymphoma patients treated with or without anthracyclines. Br J Haematol. 2018;183(5):717-726. doi: 10.1111/bjh.15603 [DOI] [PubMed] [Google Scholar]
- 5.Armenian SH, Lacchetti C, Barac A, et al. . Prevention and monitoring of cardiac dysfunction in survivors of adult cancers: American Society of Clinical Oncology clinical practice guideline. J Clin Oncol. 2017;35(8):893-911. doi: 10.1200/JCO.2016.70.5400 [DOI] [PubMed] [Google Scholar]
- 6.Ajijola OA, Nandigam KV, Chabner BA, et al. . Usefulness of cardiac resynchronization therapy in the management of doxorubicin-induced cardiomyopathy. Am J Cardiol. 2008;101(9):1371-1372. doi: 10.1016/j.amjcard.2007.12.037 [DOI] [PubMed] [Google Scholar]
- 7.Epstein AE, DiMarco JP, Ellenbogen KA, et al. ; American College of Cardiology Foundation; American Heart Association Task Force on Practice Guidelines; Heart Rhythm Society . 2012 ACCF/AHA/HRS focused update incorporated into the ACCF/AHA/HRS 2008 guidelines for device-based therapy of cardiac rhythm abnormalities: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol. 2013;61(3):e6-e75. doi: 10.1016/j.jacc.2012.11.007 [DOI] [PubMed] [Google Scholar]
- 8.Lang RM, Bierig M, Devereux RB, et al. ; Chamber Quantification Writing Group; American Society of Echocardiography’s Guidelines and Standards Committee; European Association of Echocardiography . Recommendations for chamber quantification: a report from the American Society of Echocardiography’s Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr. 2005;18(12):1440-1463. doi: 10.1016/j.echo.2005.10.005 [DOI] [PubMed] [Google Scholar]
- 9.Solomon SD, Foster E, Bourgoun M, et al. ; MADIT-CRT Investigators . Effect of cardiac resynchronization therapy on reverse remodeling and relation to outcome: multicenter automatic defibrillator implantation trial: cardiac resynchronization therapy. Circulation. 2010;122(10):985-992. doi: 10.1161/CIRCULATIONAHA.110.955039 [DOI] [PubMed] [Google Scholar]
- 10.Goorin AM, Borow KM, Goldman A, et al. . Congestive heart failure due to adriamycin cardiotoxicity: its natural history in children. Cancer. 1981;47(12):2810-2816. doi: [DOI] [PubMed] [Google Scholar]
- 11.Felker GM, Thompson RE, Hare JM, et al. . Underlying causes and long-term survival in patients with initially unexplained cardiomyopathy. N Engl J Med. 2000;342(15):1077-1084. doi: 10.1056/NEJM200004133421502 [DOI] [PubMed] [Google Scholar]
- 12.Fornaro A, Olivotto I, Rigacci L, et al. . Comparison of long-term outcome in anthracycline-related versus idiopathic dilated cardiomyopathy: a single centre experience. Eur J Heart Fail. 2018;20(5):898-906. doi: 10.1002/ejhf.1049 [DOI] [PubMed] [Google Scholar]
- 13.Slamon D, Eiermann W, Robert N, et al. ; Breast Cancer International Research Group . Adjuvant trastuzumab in HER2-positive breast cancer. N Engl J Med. 2011;365(14):1273-1283. doi: 10.1056/NEJMoa0910383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hall PS, Harshman LC, Srinivas S, Witteles RM. The frequency and severity of cardiovascular toxicity from targeted therapy in advanced renal cell carcinoma patients. JACC Heart Fail. 2013;1(1):72-78. doi: 10.1016/j.jchf.2012.09.001 [DOI] [PubMed] [Google Scholar]
- 15.Spragg DD, Kass DA. Pathobiology of left ventricular dyssynchrony and resynchronization. Prog Cardiovasc Dis. 2006;49(1):26-41. doi: 10.1016/j.pcad.2006.05.001 [DOI] [PubMed] [Google Scholar]
- 16.Bristow MR, Saxon LA, Boehmer J, et al. ; Comparison of Medical Therapy, Pacing, and Defibrillation in Heart Failure (COMPANION) Investigators . Cardiac-resynchronization therapy with or without an implantable defibrillator in advanced chronic heart failure. N Engl J Med. 2004;350(21):2140-2150. doi: 10.1056/NEJMoa032423 [DOI] [PubMed] [Google Scholar]
- 17.Moss AJ, Hall WJ, Cannom DS, et al. ; MADIT-CRT Trial Investigators . Cardiac-resynchronization therapy for the prevention of heart-failure events. N Engl J Med. 2009;361(14):1329-1338. doi: 10.1056/NEJMoa0906431 [DOI] [PubMed] [Google Scholar]
- 18.Goldenberg I, Kutyifa V, Klein HU, et al. . Survival with cardiac-resynchronization therapy in mild heart failure. N Engl J Med. 2014;370(18):1694-1701. doi: 10.1056/NEJMoa1401426 [DOI] [PubMed] [Google Scholar]
- 19.Linde C, Gold MR, Abraham WT, et al. ; REsynchronization reVErses Remodeling in Systolic left vEntricular dysfunction Study Group . Long-term impact of cardiac resynchronization therapy in mild heart failure: 5-year results from the REsynchronization reVErses Remodeling in Systolic left vEntricular dysfunction (REVERSE) Study. Eur Heart J. 2013;34(33):2592-2599. doi: 10.1093/eurheartj/eht160 [DOI] [PubMed] [Google Scholar]
- 20.Tang AS, Wells GA, Talajic M, et al. ; Resynchronization-Defibrillation for Ambulatory Heart Failure Trial Investigators . Cardiac-resynchronization therapy for mild-to-moderate heart failure. N Engl J Med. 2010;363(25):2385-2395. doi: 10.1056/NEJMoa1009540 [DOI] [PubMed] [Google Scholar]
- 21.Cardinale D, Colombo A, Lamantia G, et al. . Anthracycline-induced cardiomyopathy: clinical relevance and response to pharmacologic therapy. J Am Coll Cardiol. 2010;55(3):213-220. doi: 10.1016/j.jacc.2009.03.095 [DOI] [PubMed] [Google Scholar]
- 22.Gulati G, Heck SL, Ree AH, et al. . Prevention of cardiac dysfunction during adjuvant breast cancer therapy (PRADA): a 2 × 2 factorial, randomized, placebo-controlled, double-blind clinical trial of candesartan and metoprolol. Eur Heart J. 2016;37(21):1671-1680. doi: 10.1093/eurheartj/ehw022 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocol
Box 1. Clinical Inclusion Criteria
Box 2. Clinical Exclusion Criteria
Data Sharing Statement